-
PDF
- Split View
-
Views
-
Cite
Cite
Tyler A Churchward-Venne, Philippe J M Pinckaers, Joop J A van Loon, Luc J C van Loon, Consideration of insects as a source of dietary protein for human consumption, Nutrition Reviews, Volume 75, Issue 12, December 2017, Pages 1035–1045, https://doi.org/10.1093/nutrit/nux057
Close - Share Icon Share
Abstract
Consumption of sufficient dietary protein is fundamental to muscle mass maintenance and overall health. Conventional animal-based protein sources such as meat (ie, beef, pork, lamb), poultry, fish, eggs, and dairy are generally considered high-quality sources of dietary protein because they meet all of the indispensable amino-acid requirements for humans and are highly digestible. However, the production of sufficient amounts of conventional animal-based protein to meet future global food demands represents a challenge. Edible insects have recently been proposed as an alternative source of dietary protein that may be produced on a more viable and sustainable commercial scale and, as such, may contribute to ensuring global food security. This review evaluates the protein content, amino-acid composition, and digestibility of edible insects and considers their proposed quality and potential as an alternative protein source for human consumption.
INTRODUCTION
Consumption of sufficient amounts of dietary protein is fundamental to human growth and overall health. Dietary protein plays an important role in improving diet quality, promoting healthy aging, supporting bodyweight management, improving body composition, regulating appetite, and maintaining/increasing skeletal muscle mass in certain populations (ie, elderly, athletes).1 Therefore, protein consumption plays an important role in managing current public health issues such as obesity and age-related muscle loss (ie, sarcopenia). Conventional animal-based, protein-dense foods such as meat, eggs, and milk are considered high-quality protein sources because they meet all of the current indispensable amino-acid (IAA) requirements and are easily digested and absorbed (defined in terms of the balance of amino acids across the small intestine).2,3 However, with the global population projected to reach approximately 9.6 billion by 2050,4 the production of sufficient amounts of conventional animal-based, protein-dense foods to meet global dietary demands may no longer be desired nor feasible.5
With increases in the global population and per capita income, the demand for animal-based, protein-rich food is expected to increase. Specifically, meat consumption per capita is expected to increase by 29%, from 40.0 kg in the year 2013 to 51.5 kg in 2050.5 Accordingly, global meat production will need to reach 494 million tons by 2050, an increase of 206 million tons from 2013.5 Global demands for other animal-based, protein-rich foods are also expected to increase, with dairy and egg production projected to reach 1043 and 102 million tons globally by 2050.6 The worldwide production of agricultural commodities such as maize, rice, wheat, and soy, which represent key sources of plant-based dietary protein, may also need to increase by approximately 60%–110% by 2050 to meet global demands.7 The inability to meet global demands for protein could exacerbate the chronic inadequate protein intake and protein energy malnutrition that currently affect approximately 1 billion people worldwide.5
Insects have been proposed as an alternative protein source4,8 that may assist in meeting global demands for food protein, thereby contibuting to global food security.9 Although entomophagy, or consumption of insects, is a relatively new concept in North America and Europe, approximately 2 billion people worldwide, mostly residing in Africa, Asia, and South America, habitually consume insects as part of their traditional diet.8 To date, more than 2000 species of insects have been reported to be used as food by humans,10 including beetles (Coleoptera), caterpillars (larvae of butterflies and moths; Lepidoptera), bees, wasps, and ants (Hymenoptera), and locusts, grasshoppers, and crickets (Orthoptera).9 The majority of insects for human consumption are harvested from wild populations in nature where they have been an important source of dietary protein for millennia.4
Recently there has been a surge of interest, particularly in North America and Europe, in the mass production of insects as an environmentally and economically viable protein-dense food source. Edible insects and insect-based food products, including insect powders, flours, protein bars, pasta, burgers, nuggets, and spreads, are currently available on the market for consumers. However, data on the protein quality of insect-derived proteins, defined in terms of their protein content, IAA content, and digestibility are required to better evaluate their potential in meeting protein and amino-acid requirements in humans. The present review evaluates the protein content, amino-acid composition, and digestibility of proteins from edible insects. The capacity of insect-derived proteins to meet amino-acid requirements for humans based on the current World Health Organization, Food and Agriculture Organization of the United Nations, and the United Nations University (WHO/FAO/UNU) guidelines11 is examined and comparisons are drawn among edible insects and some conventional animal- and plant-based, protein-dense foods. The majority of data available on the protein and amino-acid content of edible insects has been obtained from insects collected in the wild or from insects reared for use as animal feed.8,12–14 Therefore, the crude protein content and amino-acid composition of 7 commercially available, edible insect flours/powders and 1 arachnid flour obtained from facility-reared/farmed insects were analyzed to gain additional insight into the nutritional composition of commercially available, edible insect products sold for human consumption.
INSECTS AS A MORE SUSTAINABLE SOURCE OF PROTEIN
With an increase in the global population, alternative protein sources, in addition to the conventional animal- and plant-based proteins, will be required to meet global demands for dietary protein and ensure global food security. Insects have been proposed as an alternative protein-dense food source that may be produced on a more viable and sustainable commercial scale.4,8,12 For example, on a fresh-weight basis, the protein content (g/100 g) of edible insects ranges from 7% to 48%,4 which is comparable with the fresh-weight protein content of beef (19%–26%), tilapia (16%–19%), and shrimp (13%–27%). In addition to representing a protein-dense food source, insects may possess environmental, economic, and agricultural advantages when compared with conventional livestock. These advantages include a higher efficiency of converting feed into body mass (feed conversion efficiency)15; a higher percent edible weight (≈80% for crickets) than conventional livestock (≈55% for chicken and pork; ≈40% for cattle)9; a lower contribution to greenhouse gas emissions and ammonia than cattle16; a substantially reduced requirement for land17 and water18 compared with cattle; and a higher capacity to produce offspring (crickets lay ≈1500 eggs over a 1-month period).15 Although available life-cycle assessments on Yellow mealworms (Tenebrio molitor L.)17 and fly species (Diptera)19,20 indicate that their mass production may offer an environmentally sustainable alternative source of dietary protein, additional life-cycle assessments on other edible insect species are required to gain more insight into the sustainability of insect production systems.
DIETARY PROTEIN QUALITY
Approaches to assess dietary protein quality aim to determine the capacity of a protein to meet protein and IAA requirements (ie, meet metabolic requirements for amino acids and nitrogen). The need for IAAs is defined by the requirement pattern presented in the most recent WHO/FAO/UNU report11 and is based on the level of intake necessary to meet metabolic requirements in different age groups as well as requirements associated with different physiological conditions, including growth, pregnancy, and lactation.
Protein quality has been evaluated using different approaches, including protein efficiency ratio, biological value, net protein utilization, chemical score, and the protein digestibility-corrected amino acid score (PDCAAS). The PDCAAS was the standard index for evaluating protein quality for a number of years; however, the currently recommended method for evaluating protein quality for application in human nutrition is the digestible indispensable amino acid score (DIAAS).21 The PDCAAS determined protein quality based on the profile and the relative amounts of dietary IAAs in the test protein and crude protein digestibility based on fecal nitrogen content, expressed relative to a profile of amino-acid requirements.22 With the PDCAAS, a score of 1.0 meant that 100% of the minimal requirements for IAA intake would be met if 0.66 g/kg/day of the test protein source was ingested. Protein sources with scores over 1.0 were not considered to contribute any additional benefits in humans22 and were therefore artificially truncated to a score of 1.0. However, truncating a score to 1.0 has meant that certain sources of dietary protein have been nutritionally undervalued by the PDCAAS. With the DIAAS, protein quality is determined based on the amino-acid requirement pattern and the ileal digestibility of each individual IAA as assessed in humans, growing pigs, or growing rats.21 Furthermore, protein sources are not truncated with the DIAAS, permitting differences in higher quality sources of protein to be assessed. A high-quality protein is one that meets at least 100% of all IAA requirements if 0.66 g/kg/day of the protein source is ingested.3 In general, animal-derived (dairy products, egg, meat, poultry, seafood, and other) protein sources are of higher quality than most plant-derived (beans, cereals, corn, nuts, peas, potato, rice, seeds, soy products, vegetables, and wheat) proteins. Certain plant-based proteins (cereals) are limited in IAAs such as lysine, threonine, and tryptophan, whereas others (legumes) are limited in cysteine and methionine. Therefore, whereas the DIAAS for milk, eggs, and beef are well above 100%, plant-derived proteins generally score well below 80%, with the exception of soy protein isolate, which has a score of above 100%.3
PROTEIN CONTENT OF EDIBLE INSECTS
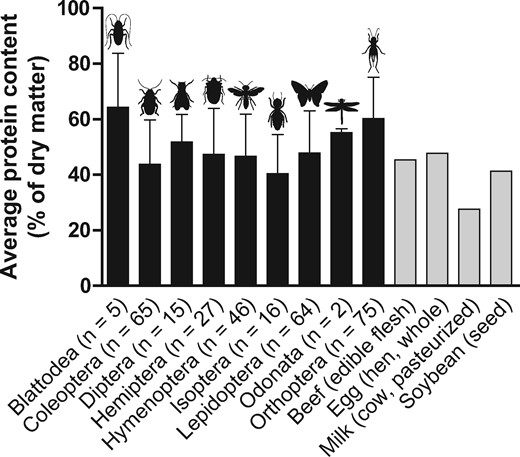
The average (mean ± SD) protein content (percentage of protein based on dry matter) of edible insects across 9 different insect orders is shown in Figure 1. Protein contents of individual insects (n = 315) from species within each order, including information on their country of origin and cultivation method (reared vs wild), are presented in Table S1 in the Supporting Information online. Figure 1 shows that average protein content of edible insects differs among insect order but is generally comparable in density to that of conventional high-quality animal- (beef, eggs, and milk) and plant-based (soy) protein-dense foods when assessed on a dry-matter basis. Mean protein content ranges from 40% ± 14% for insects from the order Isoptera (termites) to 64% ± 20% for insects from the order Blattodea (cockroaches) when assessed on a dry matter basis (Figure 1). In addition to insect order, the protein content of edible insects also varies among insect species belonging to the same order (Table S1 in the Supporting Information online). For example, within the order Orthoptera (crickets, grasshoppers, locusts), the species Melanoplus mexicanus was reported to possess a protein content of approximately 77%,23 whereas Brachytrupes spp have been reported to contain approximately 6% protein when assessed on a dry-matter basis.24 However, the 6% protein content reported for Brachytrupes spp24 is 3.6 standard deviations outside the mean protein content of 60.2% for the order Orthoptera (Figure 1;Table S1 in the Supporting Information online). Finally, in some cases there is substantial variability in the reported protein content of insects belonging to the same species (Table S1 in the Supporting Information online).
Protein content (mean ± SD) expressed as a percentage of dry matter of various edible insects arranged based on insect order. Data for insects were obtained from Table S1 in the Supporting Information online. The protein content (percentage of dry matter) of some conventional protein-dense foods, including beef (edible flesh), egg (hen, whole), milk (cow, pasteurized), and soybean (seed) are shown for comparison and were adapted from the Food and Agriculture Organization of the United Nations.25 Values between parentheses indicate the number of insect samples per order. Note that the most recent taxonomic insights have included the Isoptera in the order Blattodea. Adapted from Rumpold and Schluter (2013).12
Factors that may contribute to the variation in protein content of edible insects of the same species include differences in the diet or feed (ie, leaves vs seeds), developmental stage of the insect (ie, eggs, larvae, pupae, or adults), location and season of insect collection, and insect processing prior to analysis (ie, evaluation of whole insect vs insect with inedible parts removed).26 For example, insects fed bran were reported to have almost double the protein content as the same insects fed with maize.4 In general, adult insects and insects processed to remove inedible parts such as wings and legs generally contain higher protein content.4 Other factors that may explain the variability reported in the literature include the limited data (small sample size) available for some orders and species12 and differences in analytical techniques used to assess protein content. The majority of studies listed in Table S1 in the Supporting Information online assessed crude protein content based on measured nitrogen using the Kjeldahl method and the generalized nitrogen-to-protein conversion factor of 6.25 to estimate the protein content from the nitrogen content. However, the exoskeleton of insects contains chitin, which is a nitrogen-containing polysaccharide. Finke27 estimated the chitin content of several commercially raised insect species to range from 2.7 to 49.8 mg per kg fresh-weight and from 11.6 to 137.2 mg per kg dry matter. Therefore, chitin can contribute to the measured nitrogen content of edible insects such that assessment of crude protein (measured nitrogen × 6.25) may slightly overestimate true protein content (ie, the sum of all amino acids). In addition, compounds such as choline, creatine, purines, pyrimidines, uric acid, and free amino acids represent other sources of nonprotein nitrogen that are present in edible insects and may overestimate true protein content and consequently, underestimate amino-acid contents per unit weight of protein.
Overall, the protein content of edible insects is relatively high, ranging from approximately 40% for insects belonging to the order Isoptera (termites) and Coleoptera (beetles) to approximately 60% for insects from the order Blattodea (cockroaches) and Orthoptera (crickets, grasshoppers, locusts). These protein contents are comparable to conventional high-quality animal- (beef, eggs, milk) and plant-based (soy) protein-dense foods on a dry-matter basis.
AMINO-ACID COMPOSITION OF EDIBLE INSECTS
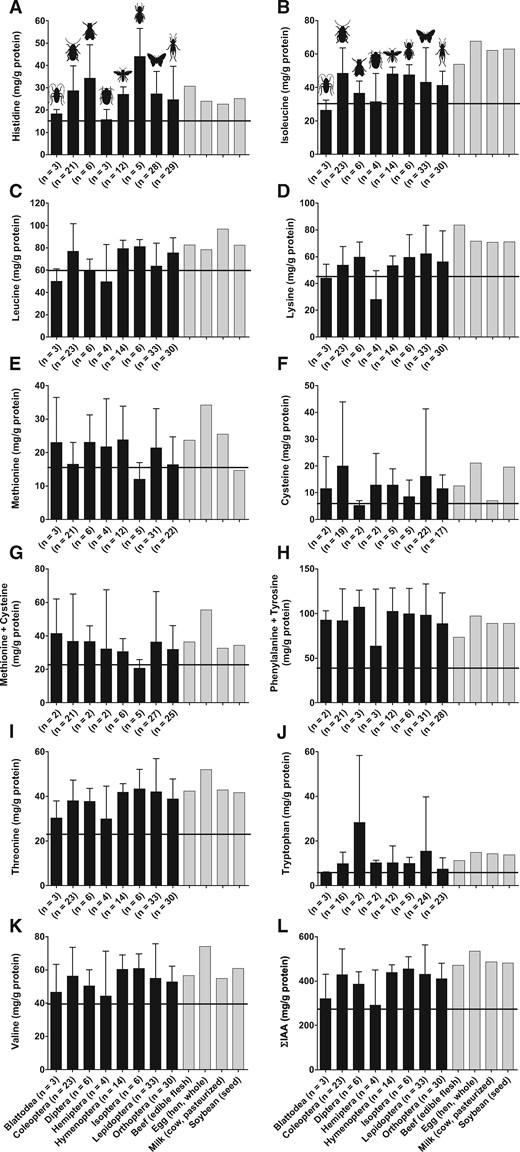
The average IAA contents (mg/g protein) of edible insects across 8 different insect orders relative to the most recently published WHO/FAO/UNU requirements11 are presented in Figure 2. Individual IAA and dispensable amino-acid data of all insect species (n = 119) are shown in Table S2 in the Supporting Information online. As for protein content, there is large variability in the IAA (Figure 2;Table S2 in the Supporting Information online) and dispensable amino-acid (Table S2 in the Supporting Information online) content of edible insects, likely due in part to the factors outlined in the previous section. However, insects from the orders Coleoptera, Hymenoptera, Lepidoptera, and Orthoptera on average meet or exceed current IAA requirements for adults, with IAA contents being comparable to beef, eggs, milk, and soy (Figure 2). Alternatively, insects from the orders Blattodea, Diptera, Hemiptera, and Isoptera seem to be deficient in at least one of the amino acids isoleucine, leucine, lysine, methionine, and/or cysteine (Figure 2). Previous studies have identified tryptophan, cysteine, and lysine as the first limiting amino acids in most edible insects; however, limiting amino acids vary widely among the various insect species.28 Although tryptophan, cysteine, and lysine are limiting amino acids in most edible insects, they are found in sufficient quantities in certain insects, including palm weevil larvae, aquatic insects, and a number of caterpillars.4 The termite species Macrotermes bellicosus is also rich in tryptophan and lysine and has been proposed to represent a useful protein source to supplement cereal-based diets that tend to be low in these amino acids.29 Bauserman et al.30 evaluated the potential of a caterpillar-enriched cereal as a complementary food product for infants and young children in the Democratic Republic of Congo and concluded that the caterpillar had appropriate macro- and micronutrient contents for complementary feeding. Because many children living in the developing world, where growth stunting is a major issue, have a diet that is limiting in the IAA lysine, lysine-rich insect species could play a role in combating growth stunting in developing countries, where many insects already form an integral component of the traditional diet.4
Indispensable amino-acid content (mean ± SD) expressed in milligrams per gram of protein for the amino acids histidine (A), isoleucine (B), leucine (C), lysine (D), methionine (E), cysteine (F), methionine + cysteine (G), phenylalanine + tyrosine (H), threonine (I), tryptophan (J), valine (K), and the sum total of indispensable amino acids (ƩIAA) (L) of various edible insects arranged based on insect order and shown relative to current adult requirements (horizontal line). Data for insects were obtained from Table S2 in the Supporting Information online. The amino-acid content (mg/g protein) of some conventional protein-dense foods, including beef (edible flesh), egg (hen, whole), milk (cow, pasteurized), and soybean (seed) are shown for comparison and were adapted from the Food and Agriculture Organization of the United Nations.25 Values between parentheses indicate the number of insect samples per order. Abbreviation: ƩIAA, sum total of indispensable amino acids.
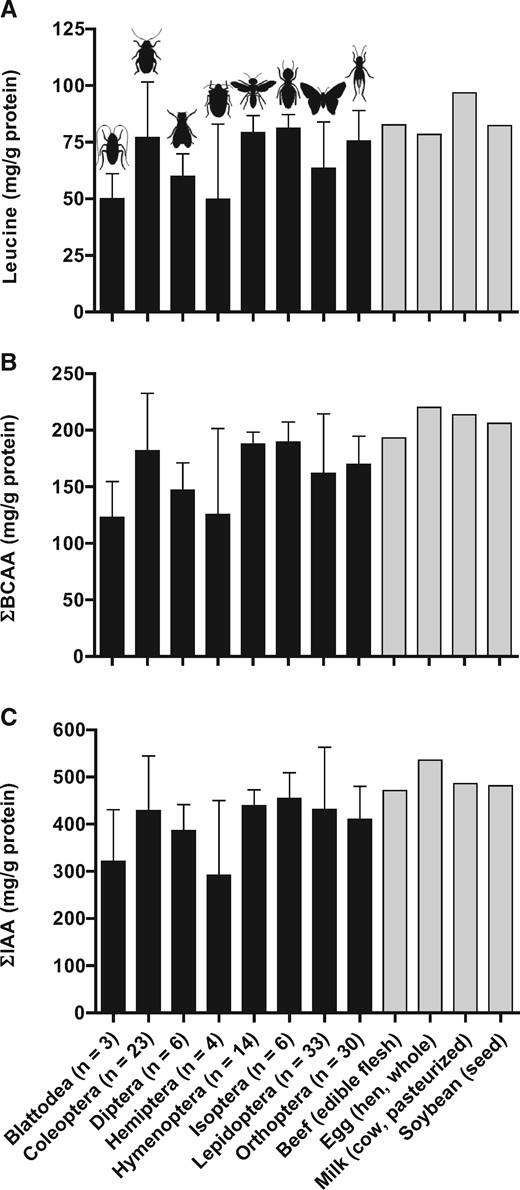
An important point on requirement levels for IAA as currently defined11 is that they reflect the minimum level of required intake of each amino acid and should not be taken to represent “optimal” levels of amino-acid intake. The PDCAAS and DIAAS approach to assessing protein quality have been criticized because they do not take into account 1) physiological needs of IAA for specific tissue (eg, skeletal muscle) protein synthesis or function and 2) the roles of synthesizable amino acids (eg, dispensible amino acids) in tissue protein turnover.31 For example, the amino acid leucine is unique among IAA in its capacity to stimulate muscle protein synthesis.32 As recently discussed by Phillips,33 if the postprandial stimulation of muscle protein synthesis and the accretion of muscle protein are the desired outcome, it may be important to emphasize the available leucine content of a protein source, in addition to considering the total IAA content. As seen in Table S2 in the Supporting Information online and Figure 3, insects from the orders Colepotera, Hymenoptera, Isoptera, and Orthoptera possess on average a leucine content of 77.0 (range, 37.4–129.5), 79.2 (range, 69.7–93.0), 81.2 (range, 73.0–90.6), and 75.6 (range, 42.5–100) mg/g protein, a content greater than that reported for soy protein isolate (62 mg/g protein)34 and comparable to that reported for skim milk powder (77 mg/g protein)34 and micellar casein (82 mg/g protein).34 Given the range of leucine content among edible insects, future studies should identify those species with the highest leucine as well as IAA content and should evaluate their capacity to stimulate postprandial muscle protein synthesis rates after ingestion by human study participants. Such information will provide insight regarding the quality of insect-derived proteins when compared with other animal- and plant-derived protein sources to support muscle tissue health.
Leucine (A), sum total of the branched chain amino acids (B), and sum total of the indispensable amino acids (C) content (mean ± SD) expressed in milligram per gram protein of various edible insects arranged based on insect order. Data for insects were obtained from Table S2 in the Supporting Information online. The amino-acid content (mg/g protein) of some conventional protein-dense foods, including beef (edible flesh), egg (hen, whole), milk (cow, pasteurized), and soybean (seed) are shown for comparison and were adapted from the Food and Agriculture Organization of the United Nations.25 Values between parentheses indicate the number of insect samples per order. Abbreviations: ƩBCAA, sum total of the branched chain amino acids; ƩIAA, sum total of indispensable amino acids.
DIGESTIBILITY OF EDIBLE INSECT–DERIVED PROTEINS
Indices of protein digestibility from insects across 7 different insect orders are shown in Table S3 in the Supporting Information online. Protein digestibility (generally defined in terms of the balance of amino acids across the small intestine) represents a key component of protein quality becuase it dictates the postprandial availability of protein-derived amino acids. Because human and/or animal studies to determine true protein digestibility (ileal amino-acid digestibility) are expensive and difficult to perform, several authors have applied multienzyme in vitro systems to assess the “digestibility” of insect-derived proteins (Table S3 in the Supporting Information online). Ramos-Elorduy et al35 determined that the degree of protein digestibility of several species of edible insects ranged from approximately 77%–98%. Furthermore, in vitro gastroduodenal digestibility of proteins extracted from Tenebrio molitor differed between fractions; the value for hemolymph proteins (80%) was higher than for muscle proteins (50%).36 Alternatively, Phelps et al37 reported that the digestibility of termite protein was approximately 60% of the digestibility of casein protein when assessed in vivo in rats (51% vs 84%, respectively, for the same amount of protein). One of the factors that may modulate the digestibility of insect protein is the presence of chitin in the sample, an insoluble fiber and component of the insect exoskeleton. Removal of chitin from whole dried insects has been reported to enhance their protein quality based on measures of digestibility, amino-acid availability, net protein utilization, and the protein efficiency ratio when assessed in weaning rats.38 Therefore, protein from insect species with lower amounts of chitin or processed insect-derived protein powders lacking chitin may have higher digestibility and prove to be of better nutritional value.
Overall, information on the digestibility of insect-based proteins is limited. Most studies to date have assessed the digestibility of insect-derived proteins using an in vitro model, although a few studies have applied various methods to assess in vivo digestibility in rats,37–41 poultry,42,43 and pigs.44,45 Future studies should examine ileal dietary amino-acid digestibility, preferably in humans, to permit evaluation of the quality of insect-derived proteins using relevant indexes such as the DIAAS. In addition, studies are warranted to evaluate in vivo postprandial protein digestion and amino-acid absorption kinetics following the ingestion of insect-derived proteins because the rate of protein digestion has been shown to regulate whole-body protein metabolism in response to the ingestion of different sources of dietary protein.46,47 Such studies are necessary to gain a more comprehensive understanding of the nutritional quality and functional characteristics of edible insect-derived proteins.
COMMERCIALLY PRODUCED EDIBLE INSECTS FOR HUMAN CONSUMPTION
Currently, insect-rearing facilities and farms are being developed in many countries due to growing interest in various species of edible insects as an alternative protein-rich food source suitable for human consumption. Insects produced in these facilities for human consumption are first cleaned, then typically euthanized by freeze-drying. Freeze-dried insects can be packaged and consumed whole or subjected to further processing in the form of roasting, cooling, and grinding to produce a fine powder or flour from whole insects prior to packaging. Defatted insect powder is also available as a more concentrated source of insect-based protein that may serve as an alternative to dairy-based protein powders used in exercise and sports nutrition.
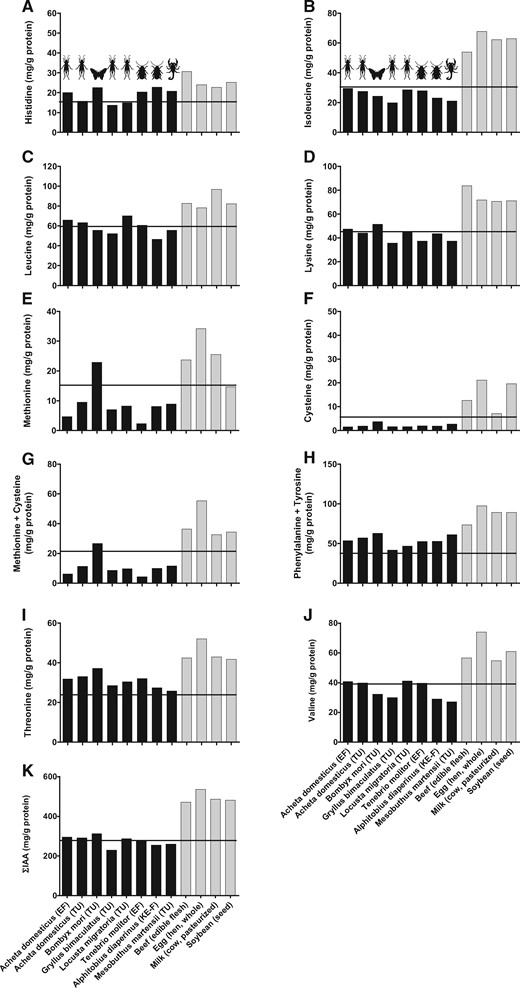
Protein from commercially produced edible insects may be expected to have more consistent protein and amino-acid content than insects collected from the wild due to standardized feed and environmental conditions. However, limited data on the protein content of commercially produced edible insects sold for human consumption are available. As such, the crude protein (Table 1) and amino-acid content (Table 2) of 7 insect flours/powders and 1 arachnid flour obtained from facility-reared/farmed insects and commercially available for human consumption were evaluated. Acheta domesticus (house cricket) and Tenebrio molitor (yellow mealworm) powders were obtained from ENTOMO Farms, Canada. Acheta domesticus (house cricket), Gryllus bimaculatus (2-spotted cricket), Bombyx mori (silkworm), Locusta migratoria (African migratory locust), and Mesobuthus martensii (scorpion) flours were obtained from Thailand Unique, Thailand. Alphitobius diaperinus (lesser mealworm) defatted protein concentrate was obtained from Kreca Ento-Food BV, The Netherlands. Nitrogen content was analyzed using a Vario MAX CN elemental analyzer (Elementar Analysesysteme GmbH, Langenselbold, Germany). Crude protein was calculated as Nitrogen × 6.25. To determine amino-acid content, approximately 6 mg of each product was hydrolyzed in 3 mL of 6 M hydrochloric acid for 12 hours at 110 °C. After hydrolysis, the samples were cooled to 4 °C to stop the hydrolyzation process. Hydrochloric acid was then evaporated under a nitrogen stream to obtain the dry amino acids for amino-acid analysis. Amino-acid concentrations were determined using ultra-performance liquid chromatography tandem mass spectrometry. Crude protein content of the analyzed products ranged from 44.4% for Mesobuthus martensii (scorpion) powder to 70.0% for defatted protein concentrate obtained from Alphitobius diaperinus (lesser mealworm) on an as-is basis. Despite the relatively high protein content of the products, the protein from each product did not meet adult requirements for each IAA because each product was deficient in at least 1 IAA (Figure 4). Therefore, the protein within the specific edible insect/arachnid products from the batches that were examined would be expected to be of lower nutritional value than that reported for beef, eggs, milk, and soy (Figure 4).
Nitrogen and protein content of commercially available edible insect– and edible arachnid–based products on an as-is basis
| Protein source . | Company . | Product . | Nitrogen (%) . | Protein (%)a . |
|---|---|---|---|---|
| Edible insect | ||||
| Acheta domesticus | ENTOMO Farms | Cricket protein powder 2050 | 10.4 | 65.0 |
| Acheta domesticus | Thailand Unique | Cricket flour | 9.2 | 57.5 |
| Bombyx mori | Thailand Unique | Silkworm flour | 8.6 | 53.8 |
| Gryllus bimaculatus | Thailand Unique | Cricket flour | 9.5 | 59.4 |
| Locusta migratoria | Thailand Unique | Locust flour | 11.1 | 69.4 |
| Tenebrio molitor | ENTOMO Farms | Mealworm protein powder 2050 | 9.3 | 58.1 |
| Alphitobius diaperinus | Kreca Ento-Food BV | EntoPure sports protein concentrate | 11.2 | 70.0 |
| Edible arachnid | ||||
| Mesobuthus martensii | Thailand Unique | Scorpion powder | 7.1 | 44.4 |
| Protein source . | Company . | Product . | Nitrogen (%) . | Protein (%)a . |
|---|---|---|---|---|
| Edible insect | ||||
| Acheta domesticus | ENTOMO Farms | Cricket protein powder 2050 | 10.4 | 65.0 |
| Acheta domesticus | Thailand Unique | Cricket flour | 9.2 | 57.5 |
| Bombyx mori | Thailand Unique | Silkworm flour | 8.6 | 53.8 |
| Gryllus bimaculatus | Thailand Unique | Cricket flour | 9.5 | 59.4 |
| Locusta migratoria | Thailand Unique | Locust flour | 11.1 | 69.4 |
| Tenebrio molitor | ENTOMO Farms | Mealworm protein powder 2050 | 9.3 | 58.1 |
| Alphitobius diaperinus | Kreca Ento-Food BV | EntoPure sports protein concentrate | 11.2 | 70.0 |
| Edible arachnid | ||||
| Mesobuthus martensii | Thailand Unique | Scorpion powder | 7.1 | 44.4 |
Protein was calculated using a nitrogen-to-protein conversion factor of 6.25.
Nitrogen and protein content of commercially available edible insect– and edible arachnid–based products on an as-is basis
| Protein source . | Company . | Product . | Nitrogen (%) . | Protein (%)a . |
|---|---|---|---|---|
| Edible insect | ||||
| Acheta domesticus | ENTOMO Farms | Cricket protein powder 2050 | 10.4 | 65.0 |
| Acheta domesticus | Thailand Unique | Cricket flour | 9.2 | 57.5 |
| Bombyx mori | Thailand Unique | Silkworm flour | 8.6 | 53.8 |
| Gryllus bimaculatus | Thailand Unique | Cricket flour | 9.5 | 59.4 |
| Locusta migratoria | Thailand Unique | Locust flour | 11.1 | 69.4 |
| Tenebrio molitor | ENTOMO Farms | Mealworm protein powder 2050 | 9.3 | 58.1 |
| Alphitobius diaperinus | Kreca Ento-Food BV | EntoPure sports protein concentrate | 11.2 | 70.0 |
| Edible arachnid | ||||
| Mesobuthus martensii | Thailand Unique | Scorpion powder | 7.1 | 44.4 |
| Protein source . | Company . | Product . | Nitrogen (%) . | Protein (%)a . |
|---|---|---|---|---|
| Edible insect | ||||
| Acheta domesticus | ENTOMO Farms | Cricket protein powder 2050 | 10.4 | 65.0 |
| Acheta domesticus | Thailand Unique | Cricket flour | 9.2 | 57.5 |
| Bombyx mori | Thailand Unique | Silkworm flour | 8.6 | 53.8 |
| Gryllus bimaculatus | Thailand Unique | Cricket flour | 9.5 | 59.4 |
| Locusta migratoria | Thailand Unique | Locust flour | 11.1 | 69.4 |
| Tenebrio molitor | ENTOMO Farms | Mealworm protein powder 2050 | 9.3 | 58.1 |
| Alphitobius diaperinus | Kreca Ento-Food BV | EntoPure sports protein concentrate | 11.2 | 70.0 |
| Edible arachnid | ||||
| Mesobuthus martensii | Thailand Unique | Scorpion powder | 7.1 | 44.4 |
Protein was calculated using a nitrogen-to-protein conversion factor of 6.25.
Amino-acid content of commercially available edible insect– and edible arachnid–based products on an as-is basis
| Amino acids . | His . | Ile . | Leu . | Lys . | Met . | Cys . | Met + Cys . | Phe . | Tyr . | Phe + Tyr . | Thr . | Val . | Arg . | Ser . | Pro . | Ala . | Gly . | GluA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Edible insect | ||||||||||||||||||
| Acheta domesticus | 20.1 | 29.6 | 66.0 | 47.4 | 4.7 | 1.5 | 6.2 | 30.7 | 22.8 | 53.5 | 31.8 | 40.8 | 53.4 | 42.6 | 52.7 | 80.7 | 44.6 | 107.1 |
| Acheta domesticus | 15.7 | 27.5 | 63.3 | 44.0 | 9.5 | 1.8 | 11.3 | 28.7 | 28.3 | 57.0 | 33.1 | 39.8 | 50.3 | 36.7 | 55.4 | 94 | 44.3 | 90.2 |
| Bombyx mori | 22.6 | 24.3 | 55.6 | 51.5 | 22.9 | 3.7 | 26.6 | 38.5 | 24.5 | 63.0 | 37.2 | 32.2 | 43.9 | 41.1 | 34.9 | 46.9 | 33.8 | 90.2 |
| Gryllus bimaculatus | 13.7 | 19.8 | 52.2 | 35.6 | 7.0 | 1.6 | 8.6 | 22.6 | 19 | 41.6 | 28.5 | 29.9 | 44.8 | 37.7 | 47 | 71.7 | 38.7 | 78.1 |
| Locusta migratoria | 14.9 | 28.5 | 70.2 | 45.0 | 8.2 | 1.6 | 9.8 | 23.2 | 23.5 | 46.7 | 30.4 | 41.1 | 49.0 | 33.1 | 60.8 | 105.6 | 48.0 | 95.8 |
| Tenebrio molitor | 20.4 | 27.9 | 60.7 | 37.2 | 2.3 | 2.0 | 4.3 | 28.3 | 24.1 | 52.4 | 32.1 | 39.6 | 37.5 | 41.3 | 56.6 | 74.8 | 45.1 | 103.6 |
| Alphitobius diaperinus | 22.8 | 23.1 | 46.6 | 43.6 | 8.1 | 1.9 | 10.0 | 29.7 | 23.2 | 52.9 | 27.4 | 29.1 | 38.0 | 28.6 | 43.1 | 52.9 | 31.5 | 90.7 |
| Edible arachnid | ||||||||||||||||||
| Mesobuthus martensii | 20.7 | 21.1 | 55.5 | 37.2 | 8.9 | 2.7 | 11.6 | 31.7 | 29.4 | 61.1 | 25.8 | 27.1 | 42.8 | 31.5 | 33.3 | 47.8 | 61.3 | 72.4 |
| Amino acids . | His . | Ile . | Leu . | Lys . | Met . | Cys . | Met + Cys . | Phe . | Tyr . | Phe + Tyr . | Thr . | Val . | Arg . | Ser . | Pro . | Ala . | Gly . | GluA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Edible insect | ||||||||||||||||||
| Acheta domesticus | 20.1 | 29.6 | 66.0 | 47.4 | 4.7 | 1.5 | 6.2 | 30.7 | 22.8 | 53.5 | 31.8 | 40.8 | 53.4 | 42.6 | 52.7 | 80.7 | 44.6 | 107.1 |
| Acheta domesticus | 15.7 | 27.5 | 63.3 | 44.0 | 9.5 | 1.8 | 11.3 | 28.7 | 28.3 | 57.0 | 33.1 | 39.8 | 50.3 | 36.7 | 55.4 | 94 | 44.3 | 90.2 |
| Bombyx mori | 22.6 | 24.3 | 55.6 | 51.5 | 22.9 | 3.7 | 26.6 | 38.5 | 24.5 | 63.0 | 37.2 | 32.2 | 43.9 | 41.1 | 34.9 | 46.9 | 33.8 | 90.2 |
| Gryllus bimaculatus | 13.7 | 19.8 | 52.2 | 35.6 | 7.0 | 1.6 | 8.6 | 22.6 | 19 | 41.6 | 28.5 | 29.9 | 44.8 | 37.7 | 47 | 71.7 | 38.7 | 78.1 |
| Locusta migratoria | 14.9 | 28.5 | 70.2 | 45.0 | 8.2 | 1.6 | 9.8 | 23.2 | 23.5 | 46.7 | 30.4 | 41.1 | 49.0 | 33.1 | 60.8 | 105.6 | 48.0 | 95.8 |
| Tenebrio molitor | 20.4 | 27.9 | 60.7 | 37.2 | 2.3 | 2.0 | 4.3 | 28.3 | 24.1 | 52.4 | 32.1 | 39.6 | 37.5 | 41.3 | 56.6 | 74.8 | 45.1 | 103.6 |
| Alphitobius diaperinus | 22.8 | 23.1 | 46.6 | 43.6 | 8.1 | 1.9 | 10.0 | 29.7 | 23.2 | 52.9 | 27.4 | 29.1 | 38.0 | 28.6 | 43.1 | 52.9 | 31.5 | 90.7 |
| Edible arachnid | ||||||||||||||||||
| Mesobuthus martensii | 20.7 | 21.1 | 55.5 | 37.2 | 8.9 | 2.7 | 11.6 | 31.7 | 29.4 | 61.1 | 25.8 | 27.1 | 42.8 | 31.5 | 33.3 | 47.8 | 61.3 | 72.4 |
Amino-acid content was measured in milligrams per gram of protein. Tryptophan was not measured.
Abbreviations: Ala, alanine; Arg, arginine; GluA, glutamic acid; Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine; Met, methionine; Cys, cysteine; Met+Cys, methionine + cysteine; Phe, phenylalanine; Phe + Tyr, phenylalanine + tyrosine; Pro, proline; Ser, serine; Thr, threonine; Tyr, tyrosine; Val, valine.
Amino-acid content of commercially available edible insect– and edible arachnid–based products on an as-is basis
| Amino acids . | His . | Ile . | Leu . | Lys . | Met . | Cys . | Met + Cys . | Phe . | Tyr . | Phe + Tyr . | Thr . | Val . | Arg . | Ser . | Pro . | Ala . | Gly . | GluA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Edible insect | ||||||||||||||||||
| Acheta domesticus | 20.1 | 29.6 | 66.0 | 47.4 | 4.7 | 1.5 | 6.2 | 30.7 | 22.8 | 53.5 | 31.8 | 40.8 | 53.4 | 42.6 | 52.7 | 80.7 | 44.6 | 107.1 |
| Acheta domesticus | 15.7 | 27.5 | 63.3 | 44.0 | 9.5 | 1.8 | 11.3 | 28.7 | 28.3 | 57.0 | 33.1 | 39.8 | 50.3 | 36.7 | 55.4 | 94 | 44.3 | 90.2 |
| Bombyx mori | 22.6 | 24.3 | 55.6 | 51.5 | 22.9 | 3.7 | 26.6 | 38.5 | 24.5 | 63.0 | 37.2 | 32.2 | 43.9 | 41.1 | 34.9 | 46.9 | 33.8 | 90.2 |
| Gryllus bimaculatus | 13.7 | 19.8 | 52.2 | 35.6 | 7.0 | 1.6 | 8.6 | 22.6 | 19 | 41.6 | 28.5 | 29.9 | 44.8 | 37.7 | 47 | 71.7 | 38.7 | 78.1 |
| Locusta migratoria | 14.9 | 28.5 | 70.2 | 45.0 | 8.2 | 1.6 | 9.8 | 23.2 | 23.5 | 46.7 | 30.4 | 41.1 | 49.0 | 33.1 | 60.8 | 105.6 | 48.0 | 95.8 |
| Tenebrio molitor | 20.4 | 27.9 | 60.7 | 37.2 | 2.3 | 2.0 | 4.3 | 28.3 | 24.1 | 52.4 | 32.1 | 39.6 | 37.5 | 41.3 | 56.6 | 74.8 | 45.1 | 103.6 |
| Alphitobius diaperinus | 22.8 | 23.1 | 46.6 | 43.6 | 8.1 | 1.9 | 10.0 | 29.7 | 23.2 | 52.9 | 27.4 | 29.1 | 38.0 | 28.6 | 43.1 | 52.9 | 31.5 | 90.7 |
| Edible arachnid | ||||||||||||||||||
| Mesobuthus martensii | 20.7 | 21.1 | 55.5 | 37.2 | 8.9 | 2.7 | 11.6 | 31.7 | 29.4 | 61.1 | 25.8 | 27.1 | 42.8 | 31.5 | 33.3 | 47.8 | 61.3 | 72.4 |
| Amino acids . | His . | Ile . | Leu . | Lys . | Met . | Cys . | Met + Cys . | Phe . | Tyr . | Phe + Tyr . | Thr . | Val . | Arg . | Ser . | Pro . | Ala . | Gly . | GluA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Edible insect | ||||||||||||||||||
| Acheta domesticus | 20.1 | 29.6 | 66.0 | 47.4 | 4.7 | 1.5 | 6.2 | 30.7 | 22.8 | 53.5 | 31.8 | 40.8 | 53.4 | 42.6 | 52.7 | 80.7 | 44.6 | 107.1 |
| Acheta domesticus | 15.7 | 27.5 | 63.3 | 44.0 | 9.5 | 1.8 | 11.3 | 28.7 | 28.3 | 57.0 | 33.1 | 39.8 | 50.3 | 36.7 | 55.4 | 94 | 44.3 | 90.2 |
| Bombyx mori | 22.6 | 24.3 | 55.6 | 51.5 | 22.9 | 3.7 | 26.6 | 38.5 | 24.5 | 63.0 | 37.2 | 32.2 | 43.9 | 41.1 | 34.9 | 46.9 | 33.8 | 90.2 |
| Gryllus bimaculatus | 13.7 | 19.8 | 52.2 | 35.6 | 7.0 | 1.6 | 8.6 | 22.6 | 19 | 41.6 | 28.5 | 29.9 | 44.8 | 37.7 | 47 | 71.7 | 38.7 | 78.1 |
| Locusta migratoria | 14.9 | 28.5 | 70.2 | 45.0 | 8.2 | 1.6 | 9.8 | 23.2 | 23.5 | 46.7 | 30.4 | 41.1 | 49.0 | 33.1 | 60.8 | 105.6 | 48.0 | 95.8 |
| Tenebrio molitor | 20.4 | 27.9 | 60.7 | 37.2 | 2.3 | 2.0 | 4.3 | 28.3 | 24.1 | 52.4 | 32.1 | 39.6 | 37.5 | 41.3 | 56.6 | 74.8 | 45.1 | 103.6 |
| Alphitobius diaperinus | 22.8 | 23.1 | 46.6 | 43.6 | 8.1 | 1.9 | 10.0 | 29.7 | 23.2 | 52.9 | 27.4 | 29.1 | 38.0 | 28.6 | 43.1 | 52.9 | 31.5 | 90.7 |
| Edible arachnid | ||||||||||||||||||
| Mesobuthus martensii | 20.7 | 21.1 | 55.5 | 37.2 | 8.9 | 2.7 | 11.6 | 31.7 | 29.4 | 61.1 | 25.8 | 27.1 | 42.8 | 31.5 | 33.3 | 47.8 | 61.3 | 72.4 |
Amino-acid content was measured in milligrams per gram of protein. Tryptophan was not measured.
Abbreviations: Ala, alanine; Arg, arginine; GluA, glutamic acid; Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine; Met, methionine; Cys, cysteine; Met+Cys, methionine + cysteine; Phe, phenylalanine; Phe + Tyr, phenylalanine + tyrosine; Pro, proline; Ser, serine; Thr, threonine; Tyr, tyrosine; Val, valine.
Indispensable amino-acid content (mean) expressed in milligrams per gram of protein for the amino acids histidine (A), isoleucine (B), leucine (C), lysine (D), methionine (E), cysteine (F), methionine + cysteine (G), phenylalanine + tyrosine (H), threonine (I), valine (J), and the sum total of indispensable amino acids (ƩIAA) (K) of 7 commercially available edible insect and 1 edible arachnid food product and shown relative to current adult requirements (horizontal line). Note that tryptophan was not measured. Data for insects were obtained from Table 2. The amino-acid content (mg/g protein) of some conventional protein-dense foods, including beef (edible flesh), egg (hen, whole), milk (cow, pasteurized), and soybean (seed) are shown for comparison and were adapted from the Food and Agriculture Organization of the United Nations.25Abbreviations: EF, ENTOMO Farms; KE-F, Kreca Ento-Food BV; TU, Thailand Unique; ƩIAA, sum total of indispensable amino acids.
PROTEIN QUALITY OF EDIBLE INSECTS FOR HUMAN CONSUMPTION
Data on the quality of insect-derived proteins evaluated using contemporary approaches such as the DIAAS are currently lacking. However, the PDCAAS of some species of edible insects have been reported.48 Yang et al48 reported that the beetle Holotrichia parallela had a PDCAAS of 0.89 based on net protein content and in vitro assessment of protein digestibility, while the silkworm Samia ricinii was reported to have a score of 0.86 when assessed in the diet of rats.41 These scores are superior to many plant-derived proteins such as wheat, oats, and pea (PDCAAS: 0.45, 0.57, and 0.67, respectively) but inferior to animal-derived proteins such as milk, eggs, and beef (PDCAAS: 1.00, 1.00, and 0.92, respectively).2 In line with these assessments of protein quality, some insect proteins have been reported to be comparable to soy49 but inferior to casein37 in their capacity to support growth in weaning rats.
An important component of dietary protein quality not captured in the PDCAAS or DIAAS is the capacity of a protein source to increase postprandial skeletal muscle protein synthesis rates and, as such, support skeletal muscle mass maintenance. Although conventional animal-based protein sources such as egg, beef, milk, whey, and casein all represent high-quality sources of dietary protein, the matched ingestion of some of these proteins (ie, whey and casein) has been associated with a divergent postprandial protein synthetic response (for a comprehensive review on the impact of protein source on skeletal muscle protein metabolism, see Witard et al50). This suggests that protein sources of similar quality, when evaluated based only on their respective PDCAAS or DIAAS, can differ substantially in their functional capacity (eg, ability to stimulate a postprandial anabolic response). Future studies should assess the DIAAS of various edible insect species and evaluate their capacity to stimulate postprandial protein synthesis rates as compared with more conventional animal-based proteins (eg, milk or beef).
CONCLUSION
Although insect-based food products (eg, insect powders, flours, protein bars, pasta, burgers, nuggets, and spreads) are available on the market, limited data are currently available on the protein content, amino-acid composition, and digestibility of protein from insects that are commercially produced and sold for human consumption. The majority of the information available comes from insects collected from wild populations. These data indicate that the protein content of insects from some orders is comparable to conventional high-quality animal and plant-derived protein-dense food sources such as beef, eggs, milk, and soy. In terms of their amino-acid content, insects belonging to the orders Coleoptera, Hymenoptera, Lepidoptera, and Orthoptera on average meet or even exceed IAA requirements for adults. However, the protein and amino-acid content of edible insects is quite variable, probably due in part to lack of standardization of diet or feed, developmental stage at time of analysis, location and season of insect collection, and processing procedures prior to analysis. Currently, most information on the digestibility of insect-based proteins has been obtained from in vitro assessments of digestibility. However, calculation of the DIAAS, the currently recommended approach for protein quality evaluation in humans, is based on assessment of ileal amino-acid digestibility in humans, pigs, or rats. In the absence of ileal digestibility data, it is difficult to evaluate the quality of insect-derived proteins based on contemporary approaches to protein quality assessment in humans. Furthermore, dietary protein quality may also relate to the capacity of a protein source to stimulate postprandial protein synthesis. Therefore, characterization of protein quality by contemporary approaches such as the DIAAS, and assessment of the capacity of insect-based proteins to stimulate postprandial protein synthesis rates in vivo are necessary to gain comprehensive insight into the functional characteristics and quality of these proteins, as compared with more conventional animal- and plant-based protein sources.
Acknowledgments
Author contributions. T.A.C-V and L.J.C.vL conceived of the idea of the manuscript. T.A.C-V and P.J.M.P compiled the articles and extracted the data. T.A.C-V wrote the manuscript. P.J.M.P, J.J.A.vL, and L.J.C.vL critically revised the manuscript. All authors read and approved the final manuscript.
Funding/support. No external funding was received for this work.
Declaration of interest. J.J.A.vL. is on the Board of Advisors for Proti-Farm. Proti-Farm produces large quantities of sustainable, high-quality insect ingredients for the food and pharmaceutical industry and is based in Ermelo, The Netherlands. L.J.C.vL has received grants from the Top Institute Food and Nutrition, PepsiCo, and Friesland Campina to study the impact of protein feeding on protein metabolism in human skeletal muscle tissue, which are all unrelated to the topic of this review. L.J.C.vL has received consulting fees from Nutricia Research and Friesland Campina, and speaking honoraria from Friesland Campina, Nutricia, Nestle, and PepsiCo. The other authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1Protein content (%) of edible insects (based on dry matter)
Table S2Amino-acid content (mg/g protein) of edible insects
Table S3Protein digestibility of edible insects
References
World Health Organization, Food and Agriculture Organization of the United Nations, United Nations University. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation. WHO Technical Report Series 935. Geneva, Switzerland: World Health Organization;
Food and Agriculture Organization of the United Nations [FAO]. Report of an FAO expert consultation. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation. Rome, Italy: FAO;