-
PDF
- Split View
-
Views
-
Cite
Cite
Philippe P Hujoel, Tomotaka Kato, Isabel A Hujoel, Margaux L A Hujoel, Bleeding tendency and ascorbic acid requirements: systematic review and meta-analysis of clinical trials, Nutrition Reviews, Volume 79, Issue 9, September 2021, Pages 964–975, https://doi.org/10.1093/nutrit/nuaa115
Close - Share Icon Share
Abstract
The World Health Organization set the recommended daily vitamin C intake, henceforth referred to as ascorbic acid (AA), on the basis of scurvy prevention. Double-blind AA depletion-repletion studies suggest that this recommended AA dose may be too low to prevent microvascular fragility.
(1) To conduct a systematic review and meta-analysis of controlled clinical trials on whether AA supplementation leads to a reduced gingival bleeding tendency, a manifestation of microvascular fragility; and (2) to relate AA plasma levels to retinal hemorrhaging, another manifestation of microvascular fragility.
Data were reviewed from 15 trials conducted in 6 countries with 1140 predominantly healthy participants with measures of gingival bleeding tendency, and from the National Health and Nutrition Examination Survey (NHANES) III of 8210 US residents with measures of retinal hemorrhaging.
In clinical trials, AA supplementation reduced gingival bleeding tendency when estimated baseline AA plasma levels were < 28 μmol/L (standardized mean difference [SMD], −0.83; 95%CI, −1.16 to −0.49; P < 0.002). Supplementation with AA did not unequivocally reduce gingival bleeding tendency when baseline estimated AA plasma levels were >48 μmol/L or unknown (respective standardized mean differences: −0.23, 95%CI, −0.45 to −0.01, P < 0.05; and −0.56; 95%CI: −1.19 to 0.06, P < 0.08). In NHANES III, prevalence of both retinal hemorrhaging and gingival bleeding tendency increased when AA plasma levels were within the range that protects against scurvy (11–28 μmol/L; respective prevalence ratios adjusted for age and sex: 1.47; 95%CI: 1.22–1.77; and 1.64; 95%CI: 1.32–2.03; P < 0.001 for both).
Consistent evidence from controlled clinical trials indicates that setting human AA requirements based on scurvy prevention leads to AA plasma levels that may be too low to prevent an increased gingival bleeding tendency. Gingival bleeding tendency and retinal hemorrhaging coincide with low AA plasma levels and thus may be reflective of a systemic microvascular pathology that is reversible with an increased daily AA intake.
INTRODUCTION
There is a longstanding controversy about whether daily ascorbic acid (AA) intakes that are adequate to prevent scurvy are too low to prevent chronic and infectious diseases. This controversy is illustrated by the conflicting guidelines on recommended AA intakes published by the World Health Organization (WHO) and the National Academy of Medicine (NAM).1,2
Writing panels for the WHO and countries such as the United Kingdom, Australia, New Zealand, and India have concluded there is no consistent evidence indicating that AA provides health benefits other than scurvy prevention.1,3–5As a result, these writing panels set recommended daily AA intakes with the aim to prevent scurvy, a disease that has killed more people than any nutritional deficiency disease, save famine.6 The human AA requirement needed to prevent scurvy is low, with 10 mg/day being an often-quoted dose.7 To provide an adequate safety margin for scurvy prevention, the writing panels for these countries have recommended a daily AA intake of 40–45 mg, which leads, on average, to an AA plasma level of a little less than 20 µmol/L. Scurvy has become an extremely rare disease, implying that inadequate AA intake for scurvy prevention has also become rare.8
Writing panels for NAM and countries such as Japan, Germany, Switzerland, and Austria have concluded that recommending higher AA intakes may help prevent, in addition to scurvy, conditions such as cancer, cardiovascular disease, and some infectious diseases.2,9,10 These panels agree with WHO and like-minded groups that the evidence on these additional health benefits is inconsistent; instead, they base their recommendations on surrogates of those health benefits such as improved neutrophil antioxidant capacity.2 Writing panels for these countries have recommended daily AA intakes in the 75–110 mg range and plasma levels of approximately 50 µmol/L.2,9,10
These discrepancies in views on adequate daily AA intakes are of public health significance. Twelve percent of the global population and 29% of the South Asian population consume < 70 mg of AA daily.11 Twenty percent of the US population, 40% of the UK low-income population, and 89% of people ≥ 60 years old in north India have AA plasma levels < 28 µmol/L.12–14 If NAM is correct about the health benefits of higher AA plasma levels, then mistakenly recommending low daily AA intake could unnecessarily contribute to an increased risk for chronic and infectious diseases.
NAM reported an urgent need to resolve this dilemma and pointed to oral health markers as among the most promising functional biomarkers to achieve this goal.2 Specifically, NAM cited 2 AA depletion-repletion trials initiated by the US Department of Agriculture (USDA) that identified gingival bleeding tendency as a functional marker for the human AA requirement.15,16 The first aim of this systematic review was to determine whether controlled clinical trials (CCTs) on AA supplementation and gingival bleeding tendency consistently confirm the findings of these USDA AA depletion-repletion trials. Because gingival bleeding tendency is commonly viewed as a trivial health issue to be addressed with oral hygiene, gingival bleeding tendency in the US population was evaluated for its ability to predict retinal hemorrhaging, another microvascular pathology. We also evaluated whether these 2 microvascular pathologies are associated with the low AA plasma levels that are adequate for scurvy prevention.
METHODS
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed in the reporting of the methods we used and the results (Table S0 in the Supporting Information online).17
Study search and selection criteria
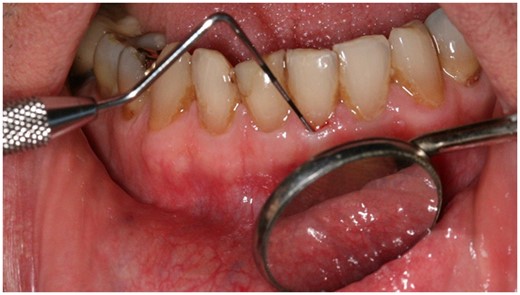
The PICOS criteria in this systematic review were whether increased AA intake, when compared with no increased AA intake and in the setting of controlled clinical trials (CCTs), reduced gingival bleeding tendency among individuals without reported signs of scurvy.15,16 Gingival bleeding tendency refers to those measures of gingival health that involve inserting a probe into the gingival cuff and observing subsequent gingival bleeding (Figure 1). This was the measure of gingival bleeding tendency used in the 2 USDA trials on the health effects of AA depletion and repletion.14,15 Gingival health measures other than gingival bleeding tendency are numerous and include the observation of spontaneous gingival bleeding, bleeding after massaging the gums, gingival color changes, or changes in tissue architecture (eg, edema, hyperplasia). WHO, for instance, defined gingival health in 1977 as bleeding after digital palpation.1 Gingival health measures other than gingival bleeding tendency also include self-reported symptoms, which are elicited with questions such as “do your gums bleed after brushing.” Findings of trials evaluating the latter endpoints (ie, measure of gingival health measures other than gingival bleeding tendency) are presented in the Supporting Information online.
The test for gingival bleeding tendency sensitive to ascorbic acid (AA) depletion and repletion consists of inserting a thin probe between the gingival cuff and the tooth at multiple sites and observing an onset of bleeding after retracting the probe. Such an increased gingival bleeding tendency can be common in patients who do not self-report bleeding after flossing or brushing their teeth. This measure of gingival bleeding tendency is referred to as bleeding on probing in the dental literature and was reported in 1940 as a sensitive diagnostic marker for a vitamin C deficiency.32 Other gingival measures such as bleeding on simple palpation of the gingiva or spontaneous gingival bleeding can be markers of advanced scurvy (i.e., severe vitamin C depletion) but not of mild to moderate vitamin C depletion
The PubMed, Embase, and Cochrane Controlled Clinical Trials databases were searched for applicable literature. The search strategies are detailed in Appendix S1 (in the Supporting Information online). References in identified articles and AA reference works18 were screened for CCTs not identified in the electronic search. The following studies were excluded: (1) AA repletion-depletion or saturation trials, (2) trials without non-AA supplemented control participants, (3) studies in which treatment assignment was determined by the participants, (4) trials limited to histological or biochemical endpoints, (5) trials on the topical effects of AA (eg, AA in a toothpaste or oral rinses), and (6) trials in which participants were described as having symptoms of scurvy consistent with depleted AA plasma levels.
Data collection process
Two reviewers (P.P.H. and T.K.) searched clinical trials and abstracted data on the basis of PICOS criteria (Table 1). Data abstraction for measures of gingival bleeding tendency was independently verified (I.A.H.). Missing standard deviations of mean changes in gingival health scores were imputed following Cochrane’s handbook method (see Supporting Information online).19
PICOS criteria for inclusion and exclusion to define research question
| Parameter . | Inclusion criteria . | Exclusion criteria . |
|---|---|---|
| Participants | Participants without serious comorbid conditions | Scorbutic participants |
| Intervention | Ascorbic acid supplementation | Topical ascorbic acid applications resulting in expectoration (eg, toothpaste) |
| Comparison | No or controlled lower ascorbic acid supplementation than intervention group | No control group |
| Outcome | Primary: gingival bleeding tendency defined as observing bleeding after the insertion of a thin probe into the gingival cuff (Figure 1) Secondary: Measures of gingival health other than gingival bleeding tendency | Histological endpoints Gingival biochemical markers |
| Study design | Controlled clinical trials | Cohort studies (self-selection of vitamin C intake)/case-control studies |
| Parameter . | Inclusion criteria . | Exclusion criteria . |
|---|---|---|
| Participants | Participants without serious comorbid conditions | Scorbutic participants |
| Intervention | Ascorbic acid supplementation | Topical ascorbic acid applications resulting in expectoration (eg, toothpaste) |
| Comparison | No or controlled lower ascorbic acid supplementation than intervention group | No control group |
| Outcome | Primary: gingival bleeding tendency defined as observing bleeding after the insertion of a thin probe into the gingival cuff (Figure 1) Secondary: Measures of gingival health other than gingival bleeding tendency | Histological endpoints Gingival biochemical markers |
| Study design | Controlled clinical trials | Cohort studies (self-selection of vitamin C intake)/case-control studies |
PICOS criteria for inclusion and exclusion to define research question
| Parameter . | Inclusion criteria . | Exclusion criteria . |
|---|---|---|
| Participants | Participants without serious comorbid conditions | Scorbutic participants |
| Intervention | Ascorbic acid supplementation | Topical ascorbic acid applications resulting in expectoration (eg, toothpaste) |
| Comparison | No or controlled lower ascorbic acid supplementation than intervention group | No control group |
| Outcome | Primary: gingival bleeding tendency defined as observing bleeding after the insertion of a thin probe into the gingival cuff (Figure 1) Secondary: Measures of gingival health other than gingival bleeding tendency | Histological endpoints Gingival biochemical markers |
| Study design | Controlled clinical trials | Cohort studies (self-selection of vitamin C intake)/case-control studies |
| Parameter . | Inclusion criteria . | Exclusion criteria . |
|---|---|---|
| Participants | Participants without serious comorbid conditions | Scorbutic participants |
| Intervention | Ascorbic acid supplementation | Topical ascorbic acid applications resulting in expectoration (eg, toothpaste) |
| Comparison | No or controlled lower ascorbic acid supplementation than intervention group | No control group |
| Outcome | Primary: gingival bleeding tendency defined as observing bleeding after the insertion of a thin probe into the gingival cuff (Figure 1) Secondary: Measures of gingival health other than gingival bleeding tendency | Histological endpoints Gingival biochemical markers |
| Study design | Controlled clinical trials | Cohort studies (self-selection of vitamin C intake)/case-control studies |
Data items, including endpoints and exposure, were as follows: (1) primary endpoint: measures of gingival bleeding tendency (Figure 1) and secondary endpoint of gingival health measures other than gingival bleeding tendency; (2) AA supplementation as a binary variable; and (3) estimated AA baseline plasma levels. Ascorbic acid supplementation was further classified as an AA supplement, an AA supplement with other active ingredients, an AA-rich fruit, and an AA-rich fruit combined with other active dietary interventions. Ascorbic acid baseline plasma levels were estimated on the basis of reported mean plasma levels or were imputed on the basis of dietary AA intake. These imputations were based on 6 depletion-repletion studies with confined participants.15,16,20–22 When no information was reported on either the baseline AA plasma level or typical AA intakes at baseline, AA plasma levels were listed as unknown.23 The estimated AA baseline plasma levels in all participants, or those participants in the control group,eg,24 were stratified on the basis of internationally established limits: very low (0–11 μmol/L), low (11–28 μmol/L), and normal (>28 μmol/L).12,25–27
Risk of bias in individual studies
The standard 6 criteria for the Cochrane risk of bias tool28 were abstracted. Two additional risks of bias were defined: baseline comparability and compliance. The CCT quality was also quantified using the Jadad scale, which ranges between 0 (poor-quality trial) and 5 (high-quality trial).29
Summary measure
Gingival health measures were reported as dichotomous (eg, a trial participant’s gingival bleeding worsened) or continuous (eg, a trial participant’s mean change in the prevalence of bleeding at multiple gingival sites). Odds ratios (calculated for dichotomous outcomes) and change scores (calculated for continuous outcomes from baseline to final examination19), and their respective standard errors were re-expressed as standardized mean differences (SMDs) and their standard errors (Appendix S2 in the Supporting Information online).19 Treatment effect estimates in cross-over trials were estimated following Cochrane handbook guidelines based on postintervention outcome only.19 Summary SMDs were calculated for CCTs that presented AA effectiveness for subgroups of trial participants.14 Data on nonrandomized periods of cross-over trials were not included in the analyses.19 Selected notes on data abstraction are provided in Appendix S3 in the Supporting Information online.
Statistical analyses
Robust variance estimation methods were used to account for the correlation of multiple AA treatment arms being compared to the same control group, or multiple AA effects each with their own control group being estimated in a split-mouth trial.30 The robust variance estimation methods were implemented using a small-sample correction.31 The AA effect sizes within trials were averaged (see Supporting Information online) and analyzed with DerSimonian-Laird models and meta-regression models with restricted maximum likelihood when the robust variance estimation methods were based on <4 degrees of freedom. Heterogeneity of effect sizes was quantified primarily with the I2 statistic and secondarily with the τ2 value.
Additional analyses
Robustness
An average AA-effect meta-analysis focused on calculating a weighted average of the AA effects for trials with multiple treatment arms. A least confounded meta-analysis focused on selecting the least confounded treatment arm within a trial (ie, the treatment arm with the largest, most accurately quantified AA dose or the longest supplementation period). The robustness of the conclusions was further assessed by evaluating the impact of other factors such as publication year, AA supplementation form (ie, AA as a single active systemic ingredient vs all other AA supplementation schemes), delivery of adjunctive dental treatments, and study quality as expressed by the Jadad score. The robustness of the results was evaluated by a variety of modeling techniques (see Supporting Information online).
Retinal hemorrhaging and gingival bleeding tendency as a function of AA plasma levels (NHANES III)
Individuals were classified as having retinal hemorrhaging when the variable labeled “hemorrhages or microaneurysms” scored a 1, 2, or 3 (and no retinal hemorrhaging when the same variable was scored a 0). Survey participants in NHANES III12 were classified as having a gingival bleeding tendency when ≥50% of the examined sites around their teeth exhibited a gingival bleeding tendency. Gingival bleeding tendency and retinal hemorrhaging were related to each other and to AA plasma levels using generalized estimating equation models with a log link and a binomial error, and adjusting for confounders.
Analysis of gingival health endpoints other than gingival bleeding tendency
This secondary endpoint was analyzed using the same statistical approaches as for the primary endpoint.
Relating AA intakes to AA plasma levels
Six US studies with confined participants related AA intakes ranging from 5 to 2500 mg to steady-state AA plasma levels.15,16,20–22 The AA plasma levels for a given dose were combined across studies using random-effects models. Missing values between 5 and 2500 mg were estimated using linear interpolation in a manner analogous to that used in a NAM report.2 These data formed the basis for imputing baseline plasma levels for those studies reporting average AA intakes at baseline, and for providing a point estimate of the estimated average requirement to reach a specific AA plasma concentration (ie, the requirement for half the healthy individuals). The recommended daily AA intake was calculated as the estimated average requirement multiplied by 1.2, as reported by NAM.2
RESULTS
Study selection and characteristics
We found 34 publications that reported on 36 trials with 49 treatment arms that measured gingival health endpoints (Figure S1 in the Supporting Information online). Fifteen trials with 17 treatment arms reported on the primary endpoint of measures of gingival bleeding tendency.24,32–44 Twenty-one trials with 32 treatment arms reported on the secondary endpoint of gingival health measures other than gingival bleeding tendency.
Fifteen trials reported on gingival bleeding tendency and were conducted in India, Indonesia, Italy, Germany, the United Kingdom, and United States (n = 1140 participants, with 97.4% apparently healthy participants and 2.6% of participants with newly diagnosed type 2 diabetes). The median AA supplementation dose and duration were 222 mg and 41 days, respectively. The control arm was compared to AA supplement in 9 arms, AA supplements plus other active ingredients in 3 arms, AA-rich fruits in 3 arms, and AA-rich fruits and other active dietary interventions in 2 arms. The median publication year was 2010.
Risk of bias within studies
Of the 8 risk-of-bias criteria, 6 scored well across the 15 studies (Table 2). Thirteen trials were at low risk for drop-out bias, and 11 trials were at low risk for lack of compliance. Of the 10 trials with estimated baseline AA plasma levels, 6 were at low risk for lack of baseline comparability. Thirteen of the 15 studies had blinded outcome assessment. Six of 11 trials with manufactured AA products (and for which placebos were possible) had participant blinding. Ten of the 15 studies were at low risk for selection bias. Two risk-of-bias criteria scored poorly. Ten trials were at high or unclear risk for allocation bias, and 13 trials were at unclear risk for selective reporting. The median Jadad score was 3 (range, 1–5).
Risk of bias in 15 controlled trials evaluating the impact of ascorbic acid supplementation on gingival bleeding tendency
| Reference . | Random sequence generation . | Allocation concealment . | Selective reporting . | Blinding of participants and personnel . | Blinding of outcome assessment . | Incomplete outcome data . | Baseline plasma AA comparability . | Compliance . |
|---|---|---|---|---|---|---|---|---|
| Roff, trial a32a |  |  |  |  |  |  |  |  |
| Roff, trial b32a |  |  |  |  |  |  |  |  |
| Graziani34 |  |  |  |  |  |  |  |  |
| Staudte35 |  |  |  |  |  |  |  |  |
| Amaliya24b |  |  |  |  |  |  |  |  |
| Gokhale36 |  |  |  |  |  |  |  |  |
| Chapple37 |  |  |  |  |  |  |  |  |
| Lingström, trial a40c |  |  |  |  |  |  |  |  |
| Lingström, trial b40c |  |  |  |  |  |  |  |  |
| Vogel41 |  |  |  |  |  |  |  |  |
| Woelber39 |  |  |  |  |  |  |  |  |
| Woelber38 |  |  |  |  |  |  |  |  |
| Munoz42 |  |  |  |  |  |  |  |  |
| Raghavendra44 |  |  |  |  |  |  |  |  |
| Sulaiman43 |  |  |  |  |  |  |  |  |
| Reference . | Random sequence generation . | Allocation concealment . | Selective reporting . | Blinding of participants and personnel . | Blinding of outcome assessment . | Incomplete outcome data . | Baseline plasma AA comparability . | Compliance . |
|---|---|---|---|---|---|---|---|---|
| Roff, trial a32a |  |  |  |  |  |  |  |  |
| Roff, trial b32a |  |  |  |  |  |  |  |  |
| Graziani34 |  |  |  |  |  |  |  |  |
| Staudte35 |  |  |  |  |  |  |  |  |
| Amaliya24b |  |  |  |  |  |  |  |  |
| Gokhale36 |  |  |  |  |  |  |  |  |
| Chapple37 |  |  |  |  |  |  |  |  |
| Lingström, trial a40c |  |  |  |  |  |  |  |  |
| Lingström, trial b40c |  |  |  |  |  |  |  |  |
| Vogel41 |  |  |  |  |  |  |  |  |
| Woelber39 |  |  |  |  |  |  |  |  |
| Woelber38 |  |  |  |  |  |  |  |  |
| Munoz42 |  |  |  |  |  |  |  |  |
| Raghavendra44 |  |  |  |  |  |  |  |  |
| Sulaiman43 |  |  |  |  |  |  |  |  |
Cochrane risk of bias was judged to be  low risk of bias,
low risk of bias,  high risk of bias, or
high risk of bias, or  unclear risk of bias. Follow-up smaller than 80% was labeled as high risk of bias. Trials with supervised AA intake, with reported compliance rates > 75%, or with statistically significant compliance measures, were classified to be at low risk for lack of compliance. Trials which reported a compliance rate of < 75% were classified as being at high risk for lack of compliance. Trials with no information on baseline comparability or compliance were classified as having an unclear risk for such bias. aTrial a and trial b refer to two clinical trials, one trial comparing “already dentally treated” to a control group, and another trial comparing new recruits to a different control group.32 bPlacebo for vitamin C supplement, no placebo possible for guava arm. 24 cTrial a and trial b refer to two clinical trials, one trial with vit C chewing gum versus a non-Vit C chewing gum, and another trial comparing vit C/carbamide chewing gum to no gum.40
unclear risk of bias. Follow-up smaller than 80% was labeled as high risk of bias. Trials with supervised AA intake, with reported compliance rates > 75%, or with statistically significant compliance measures, were classified to be at low risk for lack of compliance. Trials which reported a compliance rate of < 75% were classified as being at high risk for lack of compliance. Trials with no information on baseline comparability or compliance were classified as having an unclear risk for such bias. aTrial a and trial b refer to two clinical trials, one trial comparing “already dentally treated” to a control group, and another trial comparing new recruits to a different control group.32 bPlacebo for vitamin C supplement, no placebo possible for guava arm. 24 cTrial a and trial b refer to two clinical trials, one trial with vit C chewing gum versus a non-Vit C chewing gum, and another trial comparing vit C/carbamide chewing gum to no gum.40
Abbreviation: AA, ascorbic acid.
Risk of bias in 15 controlled trials evaluating the impact of ascorbic acid supplementation on gingival bleeding tendency
| Reference . | Random sequence generation . | Allocation concealment . | Selective reporting . | Blinding of participants and personnel . | Blinding of outcome assessment . | Incomplete outcome data . | Baseline plasma AA comparability . | Compliance . |
|---|---|---|---|---|---|---|---|---|
| Roff, trial a32a |  |  |  |  |  |  |  |  |
| Roff, trial b32a |  |  |  |  |  |  |  |  |
| Graziani34 |  |  |  |  |  |  |  |  |
| Staudte35 |  |  |  |  |  |  |  |  |
| Amaliya24b |  |  |  |  |  |  |  |  |
| Gokhale36 |  |  |  |  |  |  |  |  |
| Chapple37 |  |  |  |  |  |  |  |  |
| Lingström, trial a40c |  |  |  |  |  |  |  |  |
| Lingström, trial b40c |  |  |  |  |  |  |  |  |
| Vogel41 |  |  |  |  |  |  |  |  |
| Woelber39 |  |  |  |  |  |  |  |  |
| Woelber38 |  |  |  |  |  |  |  |  |
| Munoz42 |  |  |  |  |  |  |  |  |
| Raghavendra44 |  |  |  |  |  |  |  |  |
| Sulaiman43 |  |  |  |  |  |  |  |  |
| Reference . | Random sequence generation . | Allocation concealment . | Selective reporting . | Blinding of participants and personnel . | Blinding of outcome assessment . | Incomplete outcome data . | Baseline plasma AA comparability . | Compliance . |
|---|---|---|---|---|---|---|---|---|
| Roff, trial a32a |  |  |  |  |  |  |  |  |
| Roff, trial b32a |  |  |  |  |  |  |  |  |
| Graziani34 |  |  |  |  |  |  |  |  |
| Staudte35 |  |  |  |  |  |  |  |  |
| Amaliya24b |  |  |  |  |  |  |  |  |
| Gokhale36 |  |  |  |  |  |  |  |  |
| Chapple37 |  |  |  |  |  |  |  |  |
| Lingström, trial a40c |  |  |  |  |  |  |  |  |
| Lingström, trial b40c |  |  |  |  |  |  |  |  |
| Vogel41 |  |  |  |  |  |  |  |  |
| Woelber39 |  |  |  |  |  |  |  |  |
| Woelber38 |  |  |  |  |  |  |  |  |
| Munoz42 |  |  |  |  |  |  |  |  |
| Raghavendra44 |  |  |  |  |  |  |  |  |
| Sulaiman43 |  |  |  |  |  |  |  |  |
Cochrane risk of bias was judged to be  low risk of bias,
low risk of bias,  high risk of bias, or
high risk of bias, or  unclear risk of bias. Follow-up smaller than 80% was labeled as high risk of bias. Trials with supervised AA intake, with reported compliance rates > 75%, or with statistically significant compliance measures, were classified to be at low risk for lack of compliance. Trials which reported a compliance rate of < 75% were classified as being at high risk for lack of compliance. Trials with no information on baseline comparability or compliance were classified as having an unclear risk for such bias. aTrial a and trial b refer to two clinical trials, one trial comparing “already dentally treated” to a control group, and another trial comparing new recruits to a different control group.32 bPlacebo for vitamin C supplement, no placebo possible for guava arm. 24 cTrial a and trial b refer to two clinical trials, one trial with vit C chewing gum versus a non-Vit C chewing gum, and another trial comparing vit C/carbamide chewing gum to no gum.40
unclear risk of bias. Follow-up smaller than 80% was labeled as high risk of bias. Trials with supervised AA intake, with reported compliance rates > 75%, or with statistically significant compliance measures, were classified to be at low risk for lack of compliance. Trials which reported a compliance rate of < 75% were classified as being at high risk for lack of compliance. Trials with no information on baseline comparability or compliance were classified as having an unclear risk for such bias. aTrial a and trial b refer to two clinical trials, one trial comparing “already dentally treated” to a control group, and another trial comparing new recruits to a different control group.32 bPlacebo for vitamin C supplement, no placebo possible for guava arm. 24 cTrial a and trial b refer to two clinical trials, one trial with vit C chewing gum versus a non-Vit C chewing gum, and another trial comparing vit C/carbamide chewing gum to no gum.40
Abbreviation: AA, ascorbic acid.
Results of individual studies
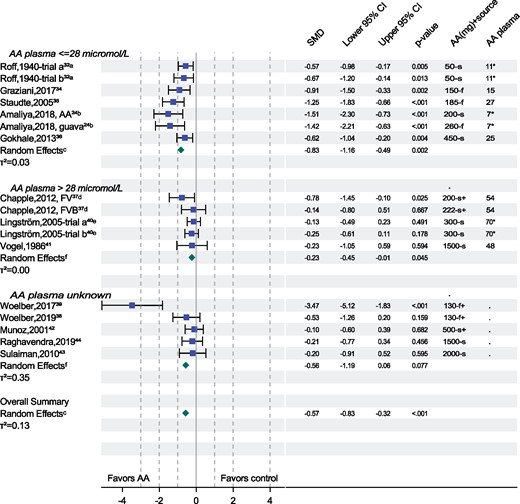
The results of individual studies included in this review are presented in the forest plot in Figure 2.
Forest plot displaying the effect of ascorbic acid (AA) supplementation on gingival bleeding tendency in 15 controlled clinical trials (n = 17 treatment arms). *, an imputed AA plasma level; f, a fruit rich in AA; f+, a fruit rich in AA combined with other dietary interventions; s, AA is the single active ingredient; s+, a supplement with AA and other active ingredients. aTrial a and trial b refer to two clinical trials, one trial comparing already dentally treated to a control group, and another trial comparing new recruits to a different control group. bTrial with two supplementation arms, one arm with AA, and one arm with guava fruit, both arms compared to the same control arm.24 cSummary estimates based on robust-variance estimation, dTrial with two supplementation arms, one arm with a fruit/vegetable product (FV), and one arm with a fruit/vegetable product with berry concentrate (FVB), both arms compared to the same control group; treatment effects were averaged to calculate summary estimate.37 eTrial a and trial b refer to two clinical trials, one trial with vit C chewing gum versus a non-Vit C chewing gum, and another trial comparing vit C/carbamide chewing gum to no gum.40 fSummary estimates are based on DerSimonian-Laird inverse-variance weighting estimation. Abbreviations: CI, confidence interval; SMD, standardized mean difference
Relating AA dietary intake to AA plasma levels in steady state
Data in Table S1 in the Supporting Information online were used to impute baseline AA plasma levels for studies reporting average AA dietary intakes and for relating AA plasma levels to AA dietary intake in the “Discussion.”
Synthesis of results stratified by estimated baseline AA plasma level
Low AA plasma levels (7–27 μmol/L)
Ascorbic acid supplementation was highly effective at reducing gingival bleeding tendency (SMD, –0.83; 95%CI, –1.16 to –0.49; P < 0.002; I2 = 31.0%).
Normal AA plasma levels (48–70 μmol/L)
Ascorbic acid supplementation in these trials was not unequivocally effective at reducing gingival bleeding tendency (SMD, –0.23; 95%CI, –0.45 to –0.01; P < 0.05; I2 = 0%). No trials studying individuals with plasma levels in the 28–47 μmol/L range were identified.
Baseline AA plasma levels as a continuous variable (7–70 μmol/L)
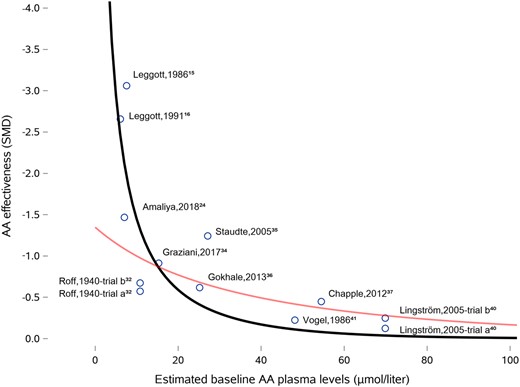
Regression of AA effectiveness on estimated baseline plasma levels showed a significant relationship (effectiveness of AA supplementation in decreasing gingival bleeding tendency increased 0.11 units for every 10 μmol/L decrease in baseline AA plasma level; 95%CI, 0.03–0.19; P < 0.03; Figure 3). Inclusion of the 2 USDA depletion-repletion trials changed the association between baseline AA levels and the effectiveness of AA supplementation moderately (effectiveness of AA supplementation in decreasing gingival bleeding tendency increased 0.16 units for every 10 μmol/L decrease in baseline AA plasma level; 95%CI, 0.03–0.29; P≤0.01).
Relationship between the effectiveness of ascorbic acid (AA) supplementation and estimated baseline AA plasma levels. The grey line reflects an exponential model fitted to AA supplementation studies only. The black line reflects an exponential model that included 2 double-blind AA depletion-repletion studies with confined participants.15,16 Effect sizes and standard errors in the 2 pre-post study designs with the first author of Leggott were calculated using the trial participant as their own controls
Baseline AA plasma levels unknown
When baseline AA plasma levels were unknown, the reduction in gingival bleeding tendency was not significant (SMD, –0.56; 95%CI, –1.19 to 0.06; P < 0.08; I2 = 74%).
Additional analyses
Robustness
Average and least-confounded AA-effects models provided results consistent with the main analysis presented here (Figures S1–S3 in the Supporting Information online). Ascorbic acid supplementation effectiveness in reducing gingival bleeding tendency was not associated with the Jadad score (P < 0.95) or with pure AA supplements vs other forms of AA supplementation (P < 0.14). Ascorbic acid supplementation was significantly less effective in studies with dental interventions such as periodontal cleanings or adjunctive home care (P < 0.02). The findings on the effectiveness of AA supplementation in trials with AA plasma levels < 28 μmol/L were robust with respect to statistical significance. The significance of the AA effectiveness estimates in trials with AA plasma levels > 28 μmol/L was not robust toward various small-sample-size approaches (Table S2 and Appendix S4 in the Supporting Information online).
Gingival bleeding tendency, retinal hemorrhaging, and AA plasma levels in NHANES III
The prevalence of retinal hemorrhaging in NHANES III participants aged ≥40 years was 7.7% (n = 8210). Low AA plasma levels (11–28 μmol/L), adjusted for age and sex, were associated with an increased prevalence of both retinal hemorrhaging (prevalence ratio, 1.47; 95%CI, 1.22–1.77; P < 0.001; n = 7619) and gingival bleeding tendency (prevalence ratio, 1.64; 95%CI, 1.32–2.03; P < 0.001; n = 6182). A diagnosis of increased gingival bleeding tendency, adjusted for age and sex, was associated with an increased the prevalence of retinal hemorrhaging (prevalence ratio, 1.66; 95%CI, 1.25–2.20; P < 0.004; n = 5,893). Models that included glycated hemoglobin levels and cotinine levels reduced the effect size of these associations, but statistical significance remained (Table S3 in the Supporting Information online).
Measures of gingival health not based on gingival bleeding tendency
Ascorbic acid supplementation was effective at reducing gingival health measures not based on gingival bleeding tendency (SMD, –0.33; 95%CI, –0.57 to –0.08; P < 0.02; τ2 = 0.17; I2 = 73%). No significant associations were identified between AA supplementation and reduced gingival health scores in 3 subgroups defined on the basis of estimated AA plasma levels at baseline (≤ 28 μmol/L, > 28 μmol/L, and unknown). See Figures S1–S3 and Table S4 in the Supporting Information online for results. No dose-response relationship was identified between AA effectiveness and baseline AA plasma levels (P < 0.70).
DISCUSSION
We conclude that AA supplementation is associated with a reduced gingival bleeding tendency when baseline AA plasma levels are < 28 μmol/L. The evidence supporting the causality of this association is substantial. The effectiveness of AA supplementation was consistent across clinical trials, consistent with double-blind AA depletion-repletion studies,15,16 and consistent with epidemiological studies.45,46 Causality is further supported by the evidence that low AA plasma levels are associated with retinal hemorrhaging and cerebral strokes,47–49 that retinal hemorrhaging and cerebral strokes are associated with an increased gingival bleeding tendency,50,51 and that AA supplementation reverses the retinal bleeding associated with low AA plasma levels.52,53 This body of evidence, largely published after NAM’s assessment in 2000, supports their statement that intraoral health markers are the most promising functional marker to set the human AA requirement.2
This systematic review identified remarkably consistent evidence that the low AA plasma levels that protect against scurvy do not protect against an increased gingival bleeding tendency. It should be stressed here that gingival bleeding tendency is viewed as a disease by WHO and leading dental organizations.54,55 All 6 trials conducted with participants with low baseline AA plasma levels (range, 7–27 μmol/L) consistently identified statistically significant benefits of AA supplementation on gingival bleeding tendency. Conversely, all 4 trials with participants with high AA plasma (> 48 μmol/L) levels did not identify such benefits. Like gingival bleeding tendency, retinal hemorrhaging is defined as a disease—a sign of retinopathy—and was also associated with low AA plasma levels. The association of both these conditions with low AA plasma levels suggests AA plasma levels that are sufficient to prevent scurvy may be too low to prevent systemic microvascular pathologies.
We also identified a significant dose-response relationship, which further highlights that the prevention of scurvy and gingival bleeding tendency have very different AA requirements (Figure 3). WHO states that 0 mg AA intake for 1 month, within the context of 45 mg daily AA supplementation, safely protects against scurvy.1 Experimental scurvy studies confirm this statement to be accurate.7 But an estimated 6.5 mg intake for 2 weeks led to dramatic increases in gingival bleeding tendency in a randomized trial.24 And a 5 mg intake for a month, within the context of 60 or 250 mg daily AA supplementation before starting the 5 mg AA dose, led to significant increases gingival bleeding tendency in 2 USDA depletion-repletion trials.15,16 The dose-response curve identified here thus suggests that a 0 mg AA intake for 1 month, within the context of a prior 45 mg daily AA intake, will lead to a most pronounced increase in gingival bleeding tendency which is reversible with AA supplementation. This provides strong evidence that preventing scurvy and preventing an increased gingival bleeding tendency have very different AA-intake requirements.
This systematic review adds weight to the argument that the inconsistent health benefits of AA in supplementation trials—the basis for some writing panels to recommend 40 to 45 mg daily AA intakes—is potentially due to inappropriate recruitment criteria and not to the questionable effectiveness of AA. Trials on gingival bleeding tendency, which, on average, recruited participants with AA plasma levels < 28 µmol/L, consistently showed significant benefits. Trials on gingival bleeding tendency, which, on average, recruited participants with AA plasma levels > 48 µmol/L, showed an absence of benefits. These findings thus confirm a hypothesis that has long been postulated: that participants recruited to AA supplementation trials need to have low AA plasma levels at baseline so supplementation will produce a clear difference in plasma and tissue AA concentrations across the compared groups.56–58 This has been referred to as the Goldilocks or “just right” principle for clinical trial design on AA supplementation.58 A recent systematic review of 35 randomized trials on AA supplementation showed that none of the identified trials limited recruitment to AA-deficient participants, indicating this “just right” principle has largely not been implemented thus far in AA trials evaluating the hard endpoints of morbidity and mortality.59
Current evidence suggests inadequate AA intake may cause microvascular fragility through 2 mechanistic pathways. Short-term suboptimal AA intakes may lead to immune-related pathologies, whereas long-term suboptimal AA intake may lead to additional collagen-related pathologies.
Short-term suboptimal AA intake may lead to immune-related pathologies, which may mechanistically explain how AA supplementation can improve gingival bleeding tendency within weeks.16,20,21,60 With healthy gums, approximately 30 000 leukocytes travel from the gingival microvasculature into the oral cavity every minute, a process called diapedesis.61 When the microbial mass on teeth increases, due to, for instance, sucrose consumption, an increased number of leukocytes travel into the oral cavity.61 This process involves chemotaxis and chemokinesis, which, at least for neutrophils, improve as intracellular AA concentrations increase. These intracellular concentrations and, consequently, diapedesis, can change significantly within weeks depending on AA dietary intake.62 And diapedesis has been linked to bleeding and periodontal disease.61,63–66 This immune-related causal pathway may explain why intracellular AA concentrations in leukocytes have a close temporal link to gingival bleeding tendency.15 As AA concentrations in leukocytes deplete slowly, gingival bleeding tendency increases slowly. And as AA concentrations in leukocytes replete quickly, gingival bleeding tendency decreases quickly. This immune-related causal pathway also explains why dental interventions such as oral hygiene and dental cleanings can partially mask the adverse symptoms caused by AA-deficient leukocytes. Removal of the microbial film, which drives the chemotaxis and chemokinesis, reduces diapedesis and thus reduces the bleeding caused by AA-deficient leukocytes.
Long-term suboptimal AA intake may lead to collagen-related pathologies, which may mechanistically explain why it takes 1–3 months for AA supplementation to improve retinal hemorrhaging.52,53,67 Experimental scurvy studies have shown that it takes, on average, 7 months for an AA intake of 0 mg to lead to impaired wound healing.7 Histological and biochemical studies pointed to impaired collagen metabolism as the mechanistic pathway to explain these clinical signs and symptoms, which led to the term collagen-related pathologies.7,68–73 Such collagen-related pathologies also weaken the structural integrity of blood vessel walls and thus provide an independent mechanism leading to hemorrhaging. It is this latter mechanism that most likely leads to the spontaneous gingival hemorrhaging characteristic of advanced scurvy. It has long been suspected that long-term suboptimal AA intakes lead to more subtle forms of collagen-related pathologies that contribute to systemic microvascular fragility. It is hypothesized here that the retinal hemorrhaging observed in the US population, as reported here, is caused by such long-term suboptimal AA intake and is a reflection of a collagen-related pathology, and not of an immune-related pathology, because the presence of the blood-retina barrier largely inhibits leukocyte migration.74
On a different note, the prevalence of retinal hemorrhaging attributable to low AA plasma levels was very low in the US population older than 40 years, suggesting the risk for collagen-related pathologies such as microvascular fragility is low. Such low risk would be consistent with the findings of experimental scurvy studies, which were mostly conducted in participants younger than 40 years, and which largely did not see an impact of AA depletion on the capillary fragility in the retina and other anatomic areas such as the skin, nailbed, or conjunctiva.7,75–77
Testing for gingival bleeding tendency may be a simple and inexpensive diagnostic method to rule in a diagnosis of low AA plasma levels. Ignoring such a potential diagnosis and instead promoting a “brush your teeth” message is not without risks. This systematic review identified trials in which AA supplementation reversed gingival bleeding tendency without dental interventions. The USDA depletion-repletion trials showed that good oral health and frequent flossing and brushing instructions were unable to prevent the increased gingival bleeding tendency resulting from AA depletion.15,16 A default prescription of oral hygiene and other periodontal interventions to “treat” microvascular pathologies, even if partially effective in reversing gingival bleeding as suggested in this meta-analysis, is risky because it does not address any potential morbidity and mortality associated with the systemic microvascular-related pathologies.
The lack of specificity and sensitivity of gingival health measures other than gingival bleeding tendency (for the detection of preclinical scurvy) may have confounded researchers for close to a century and may have led to a gradual dismissal of the once-dominant hypothesis that the oral cavity provides the earliest clinical signs of scurvy. In hindsight, it may be self-evident that gingival hemorrhaging measures, such as rubbing a finger over the gums and looking for subsequent bleeding, are insensitive toward assessing microvascular fragility. It is this insensitivity that led many gingival hemorrhaging measures to become largely abandoned in periodontal research, and that led organizations such as WHO to switch from “rubbing a finger across the gums” to the gingival bleeding tendency measures that are the primary focus here.78–80
In hindsight, it may also be evident that the clinical symptoms of scorbutic gums are not apparent in preclinical scurvy (ie, a lack of specificity). Gingival hyperplasia, sponginess, and necrosis are key clinical signs of scorbutic gums and become clinically apparent 30–38 weeks after being on an AA-free diet, after the appearance of the scurvy symptom of perifollicular hematomas.1 Such scorbutic gum symptoms did not manifest themselves in a subtler form during preclinical scurvy in the USDA depletion-repletion trials. Large sample sizes can overcome the lack of sensitivity and specificity of gingival health measures other than gingival bleeding tendency. This may explain why gingival health measures other than bleeding tendency, when summarized across all trials, showed a marginally significant improvement with AA supplementation, and why, for instance, 1 large, pivotal, randomized trial identified a significant impact of AA supplementation in a treatment arm.81
The key strength of the present study is that the reviewed controlled trial findings are consistent with 2 USDA depletion-repletion trials in which participants were in full-time residence, confined, and with strict control over all nutrient intake. Another strength of this study is the robustness of the findings; a simple vote count of significance shows that every controlled trial conducted in AA-deficient participants showed significant favorable effects resulting from supplementation. This systematic review on the primary endpoint of gingival bleeding tendency was weakened by the lack of individual participant data, the lack of platinum-standard randomized trials, the small number of available trials, the need to impute AA plasma levels on the basis of AA dietary intake for some trials, and the insufficient number of trials adhering to the Goldilocks principle. Additional weaknesses include the inability to rule out residual confounding in the retinal bleeding association with AA, and the lack of additional information, beyond what is already known, on how sex, obesity, and smoking influence human AA requirements.12,13,20,21,82,83
CONCLUSION
This systematic review and the USDA depletion-repletion studies provide strong and consistent clinical trial evidence that daily AA intakes of 30–60 mg, triple to sextuple of what is required for scurvy protection, are associated with an increased risk for microvascular hemorrhaging that is reversible with AA supplementation. This central finding suggests that leading health organizations currently advising daily intake of AA 40–45 mg daily could consider updating their recommendations to prevent microvascular hemorrhaging, which has been associated with the more serious outcome of stroke. The nonrobust finding of a reduction in gingival bleeding tendency observed when AA plasma levels were ≥ 50 µmol/L provide weak evidence that dietary intake may need to be approximately 110 mg/day.
Acknowledgements
Author contributions. P.P.H. conceived the hypothesis for conducting the systematic review and meta-analysis, conducted searches, abstracted data, conducted statistical analyses, was the principal writer, and bears responsibility for accuracy of data entry and accurateness of the statistical results presented. T.K. independently searched the literature, abstracted data, and discussed findings regularly with P.P.H. I.A.H. independently abstracted the risk-of-bias scores for the primary trials, edited the manuscript, and provided medical expertise. M.L.A.H. independently conducted statistical analyses, assessed the robustness of the findings, provided statistical expertise, and edited the manuscript.
Funding. No funding or sponsorship was provided for this research.
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Figure S1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram
Appendix S1 Search algorithm
Appendix S2 Statistical details
Appendix S3 Some notes on data abstraction of individual studies
Appendix S4 Sensitivity analysis of meta-analysis results for studies based on gingival bleeding tendency
Table S1 Depletion studies with confined subjects relating ascorbic acid (AA) intake to AA plasma levels
Table S2 Robustness of ascorbic acid (AA) effectiveness estimates in individuals with high or unknown baseline AA plasma levels
Table S3 Risk factors for gingival bleeding tendency and retinal hemorrhaging in National Health Examination Survey III in participants older than 40 years
Table S4 Risk of bias in 21 controlled trials evaluating the impact of ascorbic acid (AA) supplementation on gingival health endpoints other than gingival bleeding tendency
Figure S1 Forest plot displaying the effect of ascorbic acid (AA) supplementation on gingival health endpoints in 36 controlled clinical trials (49 treatment arms)
Figure S2 Forest plot displaying the impact of ascorbic acid (AA) supplementation on gingival health endpoints in the 36 least confounded treatments of 36 controlled clinical trials
Figure S3 Forest plot displaying the impact of ascorbic acid (AA) supplementation on gingival health endpoints in the 36 average AA treatment effects in 36 controlled clinical trials
References
Food and Agriculture Organization of the United Nations, World Health Organization, Status. . Human Vitamin and Mineral Requirements - report of a joint FAO/WHO expert consultation. Bangkok, Thailand;
Institute of Medicine. Panel on dietary antioxidants and related compounds. In:
Panel on Dietary Reference Values. Dietary reference values for food energy and nutrients for the United Kingdom.
National Health and Medical Research Council. Nutrient reference values for Australia and New Zealand.
National Institute of Nutrition. Dietary guidelines for Indians.
World Health Organization. Scurvy and its prevention and control in major emergencies. In: Department of Nutrition for Health and Development, ed.
National Institute of Health and Nutrition. Overview of dietary reference intakes for Japanese
German Nutrition Society.
World Health Organization. Oral health.