-
PDF
-
Views
-
Cite
Cite
Sara B. Connolly, Joshua P. Prager, R. Norman Harden, A Systematic Review of Ketamine for Complex Regional Pain Syndrome, Pain Medicine, Volume 16, Issue 5, May 2015, Pages 943–969, https://doi.org/10.1111/pme.12675
Close - Share Icon Share
Abstract
This systematic review aims to examine the available literature and to synthesize published data concerning the treatment of Complex Regional Pain Syndrome (CRPS) with ketamine.
The search was conducted utilizing the databases Medline, Embase and the Cochrane Central Registry of Controlled Trials. All relevant articles were systematically reviewed.
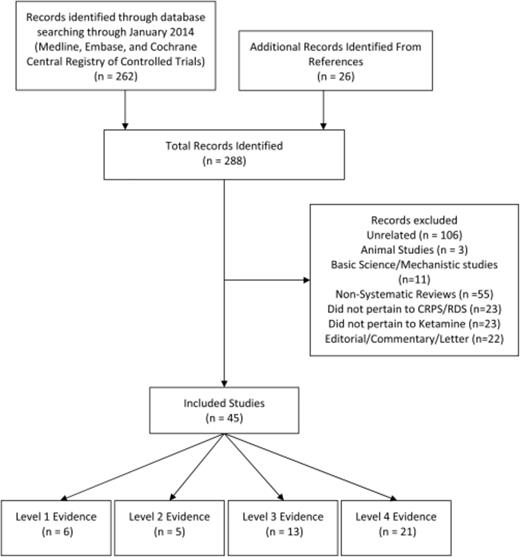
The search yielded 262 articles, 45 of which met the inclusion/exclusion criteria. Of those included, 6 were reviews, 5 were randomized placebo-controlled trials, 13 were observational studies, and 21 were case reports.
There is no high quality evidence available evaluating the efficacy of ketamine for CRPS and all manuscripts examined in this review were of moderate to low quality. Therefore, we conclude there is currently only weak evidence supporting the efficacy of ketamine for CRPS, yet there is clearly a rationale for definitive study.
Introduction
Ever since its introduction into human clinical anesthesia, ketamine has had a controversial history. Domino first introduced ketamine in the literature 50 years ago as a “dissociative anesthetic” producing potent analgesia without loss of consciousness . After Food and Drug Administration (FDA) approval in 1970, it was first used by forward medical units in the US/Vietnam war and is still used as a “battlefield anesthetic” . It was used in psychiatric research and “recreationally” by academics in the 70s . It is commonly used in the initiation and maintenance of general anesthesia, and is now more frequently used in intensive care, emergency medicine, battlefield medicine, treatment of certain psychiatric conditions, migraine, and pediatric procedures. It is also a drug of abuse, called “special K” on the street .
Ketamine (2,2 Chlorophenyl 2 methylamino cyclohexanone) is predominantly a N-methyl-D-aspartate (NMDA) receptor antagonist, but also at high dose has monamine, muscarinic, mu 2 opioid, and voltage-gated calcium effects . It is also considered to have effects on immune function . It exists in two optimal isomers (S(+) and R(−) ketamine) with similar pharmacokinetic profiles. S(+) ketamine has a higher affinity for the receptor, and as a result it is the more physiologically active enantiomenter. Ketamine has been formulated for oral, parenteral (SQ, IM, and IV), topical, intranasal, intrathecal, and intrarectal use .
In 1987, Davis and Lodge found that NMDA receptors were involved with the wind-up response to repeated noxious stimulation . It is now accepted that glutamate is involved in acute and chronic pain pathways and that activation of these pathways leads to the activity at the NMDA receptor. This contributes to increased excitatory transmission along afferent pain pathways in the dorsal horn of the spinal cord, and accentuates the responsiveness of nociceptive neurons, (or amplifies the pain signal); a phenomenon known as central sensitization .
This discovery led researchers to evaluate the efficacy of NMDA antagonists as a treatment option for pain. The first clinical report of ketamine use for pain was in 1989 . Since that time, ketamine has established a place in the perioperative management of acute pain, where it has been shown to be effective in reducing morphine requirements in the first 24 hours after surgery . Ketamine has also shown short term efficacy in treating phantom limb pain and has recently shown antidepressant effects . There are many case series and retrospective reports, and a few randomized control trials, which suggest ketamine may prove useful for chronic pain syndromes that involve central sensitization, including postherpetic neuralgia, migraine, burns, fibromyalgia, neuropathies, and Complex Regional Pain Syndrome (CRPS) .
The Budapest consensus group defined CRPS as “a syndrome characterized by a continuing (spontaneous and/or evoked) regional pain that is seemingly disproportionate in time or degree to the usual course of any known trauma or other lesion. The pain is regional (not in a specific nerve territory or dermatome) and usually has a distal predominance of abnormal sensory, motor, sudomotor, vasomotor, edema and/or trophic findings. The syndrome shows variable progression over time” . It can occur after a noxious event, or brain or spinal cord injury and it has a reported incidence rate of from 5.46 to 26.2 per 100,000 persons The pathophysiology of CRPS is still not fully understood, and is heterogeneous, involving interaction of four statistically distinct sign and symptom factors. One of the hallmarks of CRPS is central sensitization, making NMDA receptor antagonists such as ketamine an attractive potential treatment option .
As it becomes clear that NMDA receptors are likely participant in central sensitization , many believe a safe, tolerable, and effective NMDA antagonist treatment would be an important tool. Unlike opioids used to treat pain, ketamine does not produce respiratory depression. However, ketamine has a range of reported effects in humans including “dissociative” anesthesia, analgesia, elevated blood pressure (as opposed to most anesthetics), and bronchodilitation The desirable effects of ketamine are counterbalanced by side effects such as hallucinogenic and other psychomimetic effects as well as adverse events such as liver injury . There is little high level evidence that ketamine is safe, tolerated, and/or effective in chronic pain conditions in general (level I–II: see Table 0001 ); this article will examine the entire body of literature available to determine the efficacy and safety of ketamine in the specific treatment of CRPS (level I–III evidence will be discussed, and “stand out” level IV evidence will be reported in appendices).
Levels of evidence used in this review
| Level I: Meta-analysis or systematic reviews |
| Level II: One or more well-powered randomized, controlled trials |
| Level III: Retrospective studies, open-label trials, pilot studies |
| Level IV: Anecdotes, case reports, clinical experience, etc |
| Level I: Meta-analysis or systematic reviews |
| Level II: One or more well-powered randomized, controlled trials |
| Level III: Retrospective studies, open-label trials, pilot studies |
| Level IV: Anecdotes, case reports, clinical experience, etc |
Levels of evidence used in this review
| Level I: Meta-analysis or systematic reviews |
| Level II: One or more well-powered randomized, controlled trials |
| Level III: Retrospective studies, open-label trials, pilot studies |
| Level IV: Anecdotes, case reports, clinical experience, etc |
| Level I: Meta-analysis or systematic reviews |
| Level II: One or more well-powered randomized, controlled trials |
| Level III: Retrospective studies, open-label trials, pilot studies |
| Level IV: Anecdotes, case reports, clinical experience, etc |
Methods
This review aims to examine the literature and to synthesize the published data concerning the treatment of CRPS with ketamine. We systematically reviewed all relevant articles that provided data on the efficacy and utility of ketamine for CRPS. The search was conducted utilizing the databases Medline (1966–2010), Embase (1980–2010) and the Cochrane Central Registry of Controlled Trials and included all relevant articles through January 2014. We utilized 10 different search strategies, including combinations of the following (MeSH) search terms: “ketamine” or its scientific name, “2, 2 Chlorophenyl 2 methylamino cyclohexanone” AND “complex regional pain syndrome,” “reflex sympathetic dystrophy,” “causalgia,” “CRPS,” “RSD” OR “algodystrophy”. Reference lists of articles identified were screened for relevant articles that did not come up in the database search, and recent guidelines were consulted. Data from animal studies, abstracts, letters, “anecdotes” and single case reports were not included.
The methodological strength of each study was classified using a practical, easy-to-use “levels of evidence” scheme modeled after that used by Harden et al. (Table 0001 ) . Clinical trials, prospective, retrospective, and cross-sectional studies were all included in this review. The primary aim is to present a comprehensive and detailed review of all the relevant literature pertaining to the use of ketamine for CRPS. Due to a lack of high quality research in this area, the authors have chosen to include less rigorous preliminary research reports (supplemented by extensive empirical experience) to provide all literature that may be relevant so that the practitioner may better assess and analyze the modality under discussion.
Results
The search yielded 262 articles. The titles, abstracts, and the full text of these were screened by the primary author for relevance to this publication. Twenty-six additional articles were identified by reviewing reference lists and recently published journals. Of these, 6 articles represented Level I evidence of ketamine for CRPS, 5 articles Level II, 13 articles Level III, and 21 articles of Level IV were considered for this review. Only Levels I–III are mentioned in text. Level II, III, and IV evidence is reported in tables in Appendices A, B, and C. The third author screened any of the studies where it was unclear whether they met the inclusion/exclusion criteria and a decision was made by consensus. The screening process is depicted in a flow chart (Figure 0001 ).
Study selection: A summary of the results of our literature searches and the process of inclusion/exclusion of articles.
Level I Evidence
Only one systematic review was found specifically for the efficacy and safety of ketamine for patients with CRPS. Azari et al. evaluated 3 randomized, placebo-controlled trials, 7 observational studies, and 9 case studies/reports. The authors concluded that the current level of evidence is 2B (i.e., weak recommendation, moderate-quality evidence in that scheme), stating that “we do not have sufficient evidence to recommend routine use of ketamine in CRPS.” “Although ketamine demonstrates promise for safe and effective use in the treatment of CRPS, the need for large, well designed, randomized controlled trials is evident.”
One Cochrane Review evaluated “interventions for treating pain and disability in adults with complex regional pain syndrome” . The authors evaluated 3 randomized placebo-controlled trials (RCT) and 1 meta-analysis and found “low quality evidence that a course of IV ketamine may be effective for CRPS-related pain. However, the effects did not appear to be sustained beyond 4 to 11 weeks post-treatment and it is also associated with a variety of side effects…While this evidence, arising from 2 small trials (combined n = 79), is by no means conclusive, it does suggest that ketamine, and perhaps other NMDA receptor antagonists, might represent a promising therapy and target for future studies” .
A systematic review of the treatment of CRPS by Cossins et al. indicated “the review found moderate evidence for the efficacy of low-dose IV ketamine infusion in long-standing CRPS” evaluating only 2 RCTs. Cossins et al. also acknowledges that “the interpretation of these results has recently been made more complicated by reports about incidences of liver failure with prolonged or repeated treatment.” .
Several authors have published updated guidelines for the treatment of CRPS. The most recent by Harden et al. comments that ketamine has been “evaluated for neuropathic pain and for CRPS specifically, but toxicity at effective doses has generally been too high…Caution is indicated and independent confirmation of these studies is needed” . The authors do suggest NMDA receptor antagonists for refractory patients with significant allodynia/hyperalgesia. The UK guidelines evaluated only 1 high quality trial and 1 low quality trial and determined that there is “moderate evidence for IV ketamine (at low-dose, 2 trials, 1 days outpatient or 4.5 day continuous)” . Similarly, the Dutch guidelines published in 2010 by Perez et al. rated the evidence for this treatment as “moderate, or Level III.” The authors concluded that there are merely “indications that intravenous administration of a subanesthetic dose of ketamine reduces pain in CRPS-I patients,” but further research is needed .
Other recent reviews report conflicting views of ketamine for CRPS. Goebel states that efficacy of ketamine for CRPS has been demonstrated in his review. Yet, he also indicates that long-term safety or efficacy data are not available and a majority of patients transiently developed either hallucinations or inebriation . Gay's 2013 review considers ketamine an emerging treatment aimed at the etiology . A review by Collins et al (2010) stated no significant effect on pain reduction could be established for ketamine IV in CRPS, but significant effects were noted in postamputation pain. Additional RCTs are needed to make conclusions about the therapeutic potential of NMDA receptor antagonists in neuropathic pain . A review by the International Association for the Study of Pain Neuropathic Pain Special Interest Group did not recommend ketamine for neuropathic pain either, stating the “evidence of benefit in small RCTs, but administration protocol and long-term benefits and especially risks remain unclear” .
General reviews of ketamine indicate that there is little evidence for the use of oral ketamine, and it “may have a limited place as add-on therapy in complex chronic pain patients” . A comprehensive review of Intravenous infusions stated that ketamine is “the most effective and well-studied NMDA receptor antagonist, but it is routinely available only in an IV formulation.” It also has many obstacles, including “low oral bioavailability, a lack of any easily available formulation for chronic delivery, concerns over psychomimetic side effects, and mixed efficacy in clinical trials.” The authors concluded that ketamine shows promise, but further studies are needed .
Level II Evidence
Five articles were found evaluating the efficacy of ketamine for CRPS in randomized, placebo-controlled trials.
In a study by Sigtermans (2009), 60 CRPS1 patients received a 100 hour continuous IV infusion of subanaesthetic S(+)-ketamine. Pain scores (0–10 numeric rating scale (NRS) scores) for patients receiving ketamine were significantly lower for the first 11 weeks of the study compared with those receiving placebo, with the lowest pain score achieved at week 1 (ketamine, 2.68 ± 0.51, placebo 5.45 ± 0.48 P <0.001). The authors concluded that “a multiple day ketamine infusion resulted in significant pain relief,” despite the fact that significance was lost by week 12 of the study ( P = 0.07). Treatment relieved spontaneous pain, but did not cause functional improvement (i.e., no change in ability to use the affected limb, walking ability, active range of motion, or threshold for touch, skin temperature, or volume measurements) . A secondary analysis of Sigtermans' RCT of ketamine for CRPS-I evaluated the time dependent relationship between pain and motor function in 29 upper-limb CRPS-I patients. Movement parameters were assessed using a finger-tapping task. For the 29 subjects analyzed, significant pain relief was achieved for up to 6-week post infusion, but ketamine had no direct effect on motor function assessed over a 12-week follow-up period. The authors did find that motor function was significantly (inversely) related to pain intensity irrespective of whether patients had received ketamine or placebo . Data from this same study was later published by Dahan et al. Dahan et al. also reported on Pharmacokinetic-Pharmacodynamic (PK-PD) modeling of ketamine in CRPS1 patients. This report focused on the PK-PD profile of ketamine, finding that plasma concentrations decreased rapidly on termination of infusion .
In 2009, Schwartzman (2009) studied low dose multiday outpatient infusion of ketamine for 19 severe longstanding CRPS patients. Subjects were infused intravenously with normal saline with or without ketamine for 4 hours daily for 10 days. The study was powered for 20 subjects per arm, but was discontinued early at 10 subjects in the placebo group and nine in the ketamine group. The resulting pilot study showed that intravenous ketamine resulted in statistically significant reductions in weekly pain measures (MPQ and a seven question pain questionnaire) for the full 12 weeks of the study ( P < 0.05). The ketamine group also showed nonsignificant improvement in quantitative sensory testing (pressure evoked pain, brush allodynia, mechanical detection, cold pain, heat pain). Although activity levels showed no change after treatment, the activity watch revealed fewer nighttime pain awakenings as well as lower daily pain scores. Weekly quality of life scores showed no change after ketamine treatment .
Finch et al. ( ) aimed to determine the effects of topical ketamine on the sensory disturbances in 20 patients with CRPS. In this double-blind, placebo-controlled randomized crossover trial, patients received treatment with 10% ketamine (or placebo) on 2 occasions, at least 1week apart. Sensory tests were performed on the symptomatic and contralateral limb and on each side of the forehead before and 30 minutes after each topical ketamine treatment. The authors concluded topical ketamine does not lead to pain reduction in patients with CRPS, but does cause a reduction in allodynia .
Evidence for the efficacy of ketamine can be found in RCTs in related diagnoses. There are 2 additional studies worth noting. Carr 2004 reported on the efficacy and safety of intranasal ketamine for breakthrough pain (BTP) in 20 chronic pain patients (including one CRPS patient). In this randomized, double-blind, placebo-controlled crossover trial, subjects reported daily BTP episodes for 1 week prior to randomization. Treatment of breakthrough episodes with either ketamine or placebo occurred at two separate visits at least 48 hours apart. Patients reported significantly lower breakthrough pain intensity following intranasal ketamine than after placebo ( P < 0.0001), with pain relief occurring within 10 minutes of dosing and lasting for up to 60 minutes. No patient in the ketamine group required additional rescue medication for BTP episodes .
Pain and sensory thresholds were examined before and after intravenous administration of ketamine (0.15 mg/kg), morphine (0.075 mg/kg) or saline in 8 patients with postherpetic neuralgia in a randomized, double-blind, cross-over study by Eide et al. Ketamine (and not morphine) normalized abnormal heat pain sensations in 4 patients and produced significant relief of pain. Pain evoked by non-noxious stimulation of the skin (allodynia) was significantly inhibited by ketamine as well as by morphine. Neither treatment had an effect on thresholds for warm, cold, heat pain, or tactile sensation .
Level III Evidence
Thirteen prospective open label, retrospective or observational studies were found.
Only 2 studies have evaluated IV ketamine at anesthetic doses. Five open label studies and 4 retrospective analyses evaluated IV ketamine at “subanesthetic” doses. Two case series were found evaluating topical ketamine, and case reports are the only evidence for oral ketamine. There was only 1 study looking at subcutaneous ketamine, and another used ketamine as an adjuvant in sympathetic blocks.
IV Ketamine at Anesthetic Doses
In 2007, Koffler et al. first sought to evaluate the effects of ketamine in an open label prospective study evaluating the neurocognitive effects of ketamine at anesthetic doses. This study concluded that 5 days of anesthetic ketamine infusion therapy is an effective treatment for CRPS I, as indicated by significant reductions in both acute (present pain intensity) and overall pain 6 weeks following completion of the treatment. All patients had been withdrawn from narcotics and required no pain medicine at the 6 month follow-up. No adverse neurocognitive effects were found after “thorough psychological and neuropsychological” evaluation. Performances on nearly every test remained stable or improved (attention and processing speed) and only motor strength declined. However, no comment could be made regarding long-term effects past the 6 week follow-up date for the study .
Kiefer conducted a nonrandomized, open-label, phase II trial of a 5-day continuous ketamine infusion at anesthetic doses for 20 refractory CRPS patients. All 20 patients were deeply sedated for the duration of the treatment. The study showed a significant reduction in NRS from baseline in all patients 1 week after infusion (8.9 vs. 0.5, P =<=0.001) and 6 months after infusion (8.9 vs. 2.0, P < 0.001). “Complete remission from CRPS was observed at 1 month in all patients, at 3 months in 17, and at 6 months in 16 patients” . Subjects also reported a significant decrease in movement impairment, improved quality of life, and increased ability to work (at 6 months, only 2 were unable to work). The results of this study suggest an impressive effect of anesthetic ketamine in advanced and refractory CRPS. However, Kiefer et al. highlight that safety is the main concern. No life threatening complications occurred, but subjects reported psychotropic side effects, as well as difficulty sleeping, nightmares, and muscular weakness persisting for weeks after treatment. The majority also had infections associated with the intensive care nature of the treatment. The authors concluded that patients must be watched carefully during the infusion for these reasons .
IV Ketamine at Subanesthetic Doses
Fourty CRPS type I and II subjects in an open-label prospective study received 4-hour ketamine infusions daily for 10 days in a study by Goldberg, et al. Daily pain diaries indicated significant reduction of pain ( P = 0.001) with increased ability to initiate movement and tendency to decreased autonomic regulation. A total of 36 out of 40 subjects had pain relief for at least 2 weeks post treatment, while 8 subjects experienced pain relief for up to 12 weeks. Minimal side effects were reported .
Kiefer investigated the effects of S(+) ketamine on pain relief and somatosensory features (QST) in long-standing, generalized CRPS. Four subjects received continuous S(+) ketamine infusions gradually titrated over 10 days. Pain intensity and side effects were rated on visual analogue scale (VAS) scales. QST (thermal and mechanical detection, and pain thresholds) was analyzed at baseline and following treatment. Subanesthetic S(+)-ketamine showed no reduction of pain and effected no change in thermal and mechanical detection or pain thresholds .
An observational study by Sigtermans et al. sought to compare the effect of ketamine on acute versus chronic pain. Ten subjects received seven 5 minute IV ketamine infusions over 125 minutes. Spontaneous pain ratings (VAS) and VAS responses to experimental heat stimuli were obtained before and after each infusion and for 3-hours following the infusions. A significant reduction in spontaneous pain VAS score was achieved at 60 minutes, and pain relief was maintained for 3 hours post infusion. In contrast, evoked pain levels were significantly lowered during the infusion, but returned to baseline on termination of the infusion .
Noppers et al. conducted a single blind study in which 6 patients with CRPS-1 received two 100 hour continuous subanesthetic ketamine infusions separated by 16 days. Reductions in pain levels (NRS) were seen in 5/6 patients after the first infusion, but the trial was ended prematurely due to adverse events .
Sixteen subjects received 5-day ketamine infusion at a moderate dose in an open-label prospective study by Goldberg et al. Analysis of pain diaries revealed that pain scores decreased from (7.5 vs. 5.4, P = 0.001) by day 5 and a significant improvement in the ability to initiate movement by day 10 ( P = 0.012). Maximum pain relief correlated with maximum plasma levels of ketamine and norketamine. The 3 and 6 month evaluations showed pain relief was maintained for 60% and 40% of the subjects, respectively. The authors concluded that a 5 day ketamine infusion was “generally effective” for the treatment of severe CRPS .
Correll et al. conducted a retrospective chart review of 33 CRPS patients who received subanesthetic ketamine titrated to the highest tolerated dose. A total of 76% (25/33) of patients achieved complete pain relief initially from only one infusion, but some relapsed and received a second ( n = 12) or third ( n = 2) infusion. Patients receiving a second treatment of intravenous ketamine infusion were shown to have longer periods of pain relief than patients treated with a single infusion (i.e., 7/12 patients experienced relief at 1 year). Overall, 83% of patients achieved complete relief, 13% partial relief, and 4% no relief .
Webster (2006) was the first to evaluate the safety and efficacy of prolonged ketamine infusions in noncancer pain. In this retrospective chart review, 8 out of 13 subjects had CRPS. The ketamine infusions lasted 16.4 days on average (ranging from 5 to 55 days), but with no major adverse events and very few side effects reported. Overall, 11 of 13 patients (85%) reported a decrease in pain after treatment, and 7 out of 13 subjects still perceived improvement 1 month after the infusion .
In a 5 year retrospective chart review of 31 patients with refractory chronic pain, all patients reported a significant reduction in VAS score postinfusion, with significantly larger decreases in pain for the 18 CRPS patients. Per clinic protocol, infusions lasted between 30–165 minutes and were routinely scheduled every 3–4 weeks. There was no data on long-term pain relief, although follow-up phone calls revealed some patients received at least a few weeks of relief .
In a recent retrospective case series, ketamine was evaluated for inpatients with neuropathic pain from major limb injuries sustained in combat. Nineteen patients whose pain was inadequately controlled by multimodal analgesia received a 3-day infusion of IV subanesthetic ketamine. Results showed a significant reduction in PPI, improvement in GPR, and a decrease in mean opioid requirement. The authors concluded that low dose ketamine infusions for complex combat injury pain were safe and effective .
Topical Ketamine
Ushida (2002) found that repeated use of topical (10%) ketamine relieved pain and swelling in 4 out of 5 patients in the early dystrophic stage of CRPS-I. Two patients also showed a decrease in allodynia. No pain relief was found in long term CRPS (15 years) ( n = 1) or in CRPS II ( n = 2). The authors concluded that ketamine appears to be beneficial for the patients with acute early dystrophic stage of CRPS I .
Crowley (1998) conducted a case series of topical ketamine in which 5 patients diagnosed with “RSD” received a single application of topical ketamine. Patients reported pain reduction of 65% to 100% relative to pretreatment NAS. No side effects were reported up to 48 hours post infusion .
Adjuvant for Sympathetic Block
Sunder 2008 reported the use of ketamine as an adjuvant in sympathetic blocks for peripheral nerve injury in a case series. Three patients with peripheral nerve injury following gunshot wound injuries (thought to be CRPS-II) responded positively to the sympathetic blocks, and the addition of ketamine relieved symptoms of allodynia .
Subcutaneous
The study mentioned above by Webster (2006) first evaluated ketamine administered via subcutaneous route, but after administering to 5 patients, found it to be unsafe. Subjects developed irritation or sterile abscesses at the infusion site .
Oral
Only case reports evaluated oral ketamine for CRPS. (Villhauer-Perez and Furuhashi-Yonaha) .
Adverse Events and Side Effects
The trials also provide evidence of the potential adverse events that may occur from ketamine treatment. Notable adverse events from the trials above are detailed below.
A trial by Noppers et al published data regarding hepatotoxicity and anesthetic ketamine treatment . In this trial, 5 out of 6 patients were withdrawn from the trial, 1 due to hypertension, 1 due to psychotropic side effects, and 3 due to elevated liver enzymes (suspected drug induced liver injury). This occurred in 1 patient after a single course of treatment, and in 3 patients after a second course of treatment 16 days later. For those patients, it took 2 months for the liver enzyme levels returned to normal. Noppers et al. concluded that there is an increased risk with prolonged or repeated ketamine treatment in a short period of time . Sears and Bell published commentaries relaying their concern for the toxic effects of ketamine not only on the liver, but on other organs as well .
In Kiefer's trial of ketamine at anesthetic doses, no life threatening complications occurred. Subjects did report psychotropic side effects, as well as difficulty sleeping, nightmares, and muscular weakness persisting for weeks after treatment. The majority also had infections associated with the intensive care nature of the treatment. The authors concluded that patients must be watched carefully during the infusion for these reasons .
Three cases of transient blindness have been reported for ketamine administered as an anesthetic agent. All cases resolved. No cases have been reported for ketamine administered for treatment of CRPS .
Among the trials of sub anesthetic ketamine, severe headache, nausea, dizziness, feeling of inebriation, fatigue, and drug high were reported fairly often. Hallucinations and psychomimetic side effects were also reported in a few studies . No side effects were reported with topical applications of ketamine . Further descriptions of the adverse events for each study can be found in Appendices A, B, and C.
Discussion
CRPS is difficult to treat effectively. Many therapies have been explored for CRPS, but numerous patients remain in pain. NMDA receptors have a solid rationale as treatment targets in CRPS because of the presumptive mechanism (in some cases) of central sensitization. Thus, many turn to ketamine as the most potent option, as other NMDA receptors (e.g., memantine and amantadine) are weaker and have failed to show beneficial effects alone .
There is no high quality evidence available evaluating the efficacy of ketamine for pain and all single studies in this review (Level II and III) were of moderate to low quality. Previous reviews (Level I evidence) similarly found the evidence to be of low quality and gave a weak recommendation at best. There are multiple difficulties encountered when attempting to evaluate the Level II and Level III evidence for ketamine. Investigators used different routes of administration, different doses, and different outcome measures. The studies have relatively low sample sizes, and various inclusion/exclusion criteria. There are methodological flaws found in the Level II studies that preclude definitive conclusions (i.e., early termination of trial), and nongeneralizable results to CRPS populations in general (i.e., intranasal breakthrough pain study). There is likely to be a large placebo response, making Level III evidence of questionable utility.
No conclusion can be made in regards to the most effective route of administration or the dose from the available literature. While ketamine is available for oral, parenteral (SQ, IM, and IV), topical, intranasal, intrathecal, and IR use, the literature shows that intrathecal administration of ketamine is toxic . Our literature search only revealed use of ketamine in intravenous, topical, intranasal, and oral form. The majority of the research shows efficacy of IV ketamine, but treatment durations range from 30 minutes to 8 weeks and doses ranging from 0.35mg/kg/hour to 7mg/kg/hour . Topical ketamine studies show wide variability in terms of dose and demonstrated mixed results in terms of efficacy. Webster attempted to study subcutaneous ketamine in a larger study, but discontinued that treatment arm due to adverse events . Only case reports are available for oral ketamine. Good evidence for intranasal and other routes of administration has only been reported in other types of pain .
With such small sample sizes, the profile of the patients included in these studies is crucial to understanding the results. While some studies found there was no difference based on factors such as age, gender, or pain duration, others found ketamine to be effective in relatively new CRPS patients, but ineffective in refractory patients with a long disease history. This result is counterintuitive considering ketamine is intended to treat the central sensitization of the pain, which would be more developed in long-term patients. The preponderance of the research found used older diagnostic criteria (IASP), so comparison to modern studies is difficult. Some have called into question whether these subjects would be included had researchers used the stricter Budapest criteria, which could produce different results .
The utility of ketamine is limited by its side effect profile. Hallucinogenic and other psychomimetic effects are of greatest concern when using ketamine and often discourage use. Ketamine also induces feelings of inebriation, nausea, headaches, vomiting, and elevated blood pressure. There is evidence that repeated use causes hepatic dysfunction, not only from a study by Noppers, et al. , but also from the reports of liver toxicity found in ketamine abusers . Concern has been raised regarding effects on cognitive function, although one study has shown no residual cognitive effects 6 weeks after IV ketamine infusion . Such effects in conjunction with risks of abuse and dependence recommend caution. Domino and Zsigmond suggested “taming the ketamine tiger” by reducing its emergence delirium with Diazepam. Now, shorter acting agents such as midazolam, propofol and clonidine are used more frequently. In our review, studies that coadminister such agents generally reported fewer serious adverse events (see appendices) .
One must also consider the practicality of use of ketamine. Studies report short-term efficacy, so repeated dosing would be needed. Infusion protocols require hospitalization and close observation, especially when ketamine is given at anesthetic doses. There is inherent risk in any general anesthetic setting and this risk is greatly increased with prolonged anesthesia . Intensive care is associated with risks of infections, and infectious complications are the main source of morbidity and mortality in modern intensive care medicine. Such protocols will have a cost implication, and to date, no cost–benefit analysis has been published .
Down-regulation of central sensitization mediated by the NMDA-receptor blockade might explain in part the analgesic effects of ketamine, but the mechanism that leads to long-term pain relief is not understood. Research has been conducted to develop a pharmacokinetic model that reflects the plasma profiles of ketamine and its metabolites, and to construct preliminary pharmacodynamic models of ketamine to guide the development of future clinical studies. Studies have shown that the metabolite norketamine produces similar effects to ketamine, while less is known about the other downstream metabolites . A study by Olofsen et al. showed significant differences in the pharmacokinetic estimates of S(+) ketamine between study groups, with larger concentrations found in healthy volunteers compared to CRPS-1 patients . Dahan et al. found that, although plasma concentrations of S(+)-ketamine and its metabolite decrease rapidly on the termination of infusion, pain relief outlasts the treatment period by about 50 days . Patients report dreams 24 hours later, recreational users report dissociative effects for days after drug use, and chronic pain patients have reported relief lasting even longer . Dahan et al. hypothesizes that ketamine initiates a cascade of events, which lead to pain relief that lasts but slowly abates when ketamine molecules were no longer present . Some believe this may be due to synaptic plasticity; there is a change in the brain alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate/NMDA receptor ratio .
Although most of the attention to the effects of ketamine have been directed to the role ketamine plays at the NMDA receptor, one must also consider its role in the attenuation of secretion of the proinflammatory cytokines IL-6 and TNF-alpha. The effect of intravenous immunoglobulin on CRPS has been studied . Thus, ketamine's role as an immunomodulator in this neuroinflammatory syndrome requires further investigation. Ketamine acts at numerous other receptors (including opiate and sigma receptors) as well as indirect interactions with several neurotransmitters and one cannot attribute any efficacy of ketamine to NMDA receptor activity alone .
A better understanding of these events will lead to more effective use of ketamine for pain relief.
Conclusion
Based on the literature identified and the extent of evidence found for ketamine for CRPS, we find the evidence to date to be inconclusive. The quality of the research to date is low, in small “n” studies, with methodological flaws; thus there we conclude there is only weak evidence for the efficacy of ketamine for CRPS, and it cannot be considered a first line option. The work to date is preliminary, and some would argue compelling, yet clearly there is a crucial and compelling need for better, better designed and more definitive research. One guideline considers it anesthetic dose a “last resort” intervention because of the weak evidence and toxicity (especially of anesthetic doses) , but the question of dose (anesthetic versus subanesthetic) is particularly relevant in treatment decisions. Nonetheless, CRPS is a significant clinical problem with limited therapeutic options, and therefore, any intervention able to produce improvements should be studied properly, and our review suggests subanesthetic dose ketamine holds promise. There is a critical need for “high quality, randomized, controlled trials with larger numbers of patients and standardized, clinically relevant routes of administration” .
Recommendations
In future trials, randomization will be necessary. There is no further need for open label trials at this point in the development of the drug. Blinding depends on the design, formulation, dose and comparator. Administration of any formulation is technically easily blinded, yet “clinical blinding” is more difficult with drugs such as ketamine, either for subjects or researchers. Active controls are preferred, particularly in moderate to higher doses where psychomimetic effects are expected. In naïve subjects blinding is less difficult, but in experienced ketamine patients blinding is more complicated; benzodiazepines are excellent active controls in that they reproduce some of the psychomimetic effects of ketamine, and may control aspects of “the delirium” . In the extreme, intractable cases where IV ketamine would normally be considered, the prolonged use of “control” groups must consider the suffering involved, and more analgesic controls may be considered. In certain brief paradigms (i.e., an acute trial) placebo can be justly considered. Prolonged wait listing/historical controls runs into other ethical issues such as the ongoing extreme pain and the tradition that early treatment is critical. Blinding the operator raises some safety issues, but can be accomplished with safety being monitored by a separate (unblinded) physician or practitioner. Blinded data entry and analysis of psychometric or psychophysical data makes for a “half-blind.”
Side effects must be thoroughly assessed, acutely and over time. For acute paradigms single shot, intranasal and rectal administrations are intriguing. In chronic pain, the risk to benefit ratio would suggest topical, continuous SQ administration and low dose outpatient administration. Given the apparent risk:benefit issues, it is questionable that high dose protocols are worth pursuing, although practitioners in the area suggest it is significantly (though anecdotally) better. If we are to continue with high dose experiments, we must only do this in the context of Institutional Review Board approved, properly designed trials .
References
Appendix A
Evaluation of Randomized Placebo-Controlled Trials of Ketamine For Complex Regional Pain Syndrome (CRPS)
Evaluation of Randomized Placebo-Controlled Trials of Ketamine For Complex Regional Pain Syndrome (CRPS)
Appendix B
Evaluation of Observational Studies of Ketamine For Complex Regional Pain Syndrome (CRPS)
| Author | Year | Study Type | Diagnosis | Diagnostic Criteria | Pain Duration | N | Route | Dose | Results | Adverse Events/Side Effects |
| Polomano, RC, et al. | 2013 | Retrospective Chart Review | Neuropathic Pain From Major Limb Injuries | N/A | Post Traumatic Injury | 19 | 3-Day Infusion Of IV Sub-Anesthetic Ketamine With Multimodal Analgesia | Sub-Anesthetic | Significant Reduction In PPI (Present Pain Intensity), Improvement In GPR (Global Pain Relief), And A Decrease In Mean Opioid Requirement. | OveralLTherapy Was Well Tolerated. Patients Reported Drowsiness (4), Hallucinations (1), Vivid Dreams (1), Slight Decrease In Blood Pressure (2), Nausea (1) |
| Webster, L.R. And MJ. Walker | 2006 | Retrospective Chart Review | Neuropathic Pain (8/13 CRPS, 1 Migraine 3 Neuropathy, 1 Phantom Limb) | N/A | Unknown | 13 | IV Or Subcutaneous Ketamine | Low Dose Continuous Infusion. Mean Duration: 16.4 Days (Range 5-55) Carefully Titrated To The Minimum Effective Level. Average Dose = 0.12 Mg/Kg/H (Range O.Olmg/Kg/H, - 0.25 Mg/Kg/H). | VAS Decreased Significantly From 7.7+-1.8 To 4.8+-2.3. Patients Showed Improvement In Global Pain Relief (8/13 Patients Reported Good Pain Relief). In Interviews, 12/13 Perceived An Improvement In Pain. 7/13 Still Perceived Improvement 1 Month After Infusion Treatment Ended. Overall, 11/13 Patients Reported 85% Decrease In Pain From Start Of Infusion To End | Adverse Events Due To Problems With Subq Delivery (5/5). This Route Was Discontinued. For IV Ketamine, Patients Reported Fatigue (4), Dizziness (3), Confusion (2), And Spinal Pain (2). No Hallucinations Were Reported. |
| Goldberg, ME | 2005 | Pilot, Open-Label Study | Refractory CRPS Type 1 And II | IASP | Range 3 Months-3years | 40 | Low Dose IV Ketamine | 4 Hour Ketamine Infusion Escalated From 40-80 Mg Daily For 10 Days + Clonidine + Midazolam | Pain Decreased Significantly From 7.54 To 5.44 On Average. Pain Diaries Showed Significant Reduction In The “Worst Pain Of The Day” And “Least Daily Pain”. Significant Reduction In “Punishing Pain”. Patients Showed Increased Ability To Initiate Movement. | Side Effects Were Minimal-4/10 And 5/40 Reported Headaches And Restlessness. |
| Correll, GE | 2004 | Retrospective Chart Review | CRPS Type 1 And Type 11 | Official Diagnosis With Confirmati on From The Senior Anesthesiol ogist | Ave=28.1 Months. Range= 0.25-240 Mo. | 33 (25M 8F) | IV Sub-Anesthetic Ketamine | IV Ketamine Started At lOmg/Hr, Increased As Tolerated Until Feelings Of Inebriation Onset Of CNS Symptoms. (Highest Tolerated Dose Producing Analgesia (Average=23.4 Mg/Hr Range 15-50mg/Hr). Duration Averaged 4.7 Days, 0.75-20 Days). 21 Received Treatment Only Once, 10 Received Two Courses Of Treatment, And 2 Received Three Courses. | 76% (25/33) Got 100% Pain Relief Initially, 6/33 Had Partial Relief, And 2 None After 1st Infusion. After 1st Infusion, 54% Relief Lasted >3 Months, And 31% >6 Months. After The Second Infusion, 100% Pain Relief In All Patients. For 10.58% Of Patients, Relief Lasted 1 Year, And For 33% Relief Lasted 3 Years. Overall, 83% With Complete Relief, 13% With Partial Relief, And 2 With No Relief (4%) | 4 Patients Had Elevated Hepatic Enzyme Profiles, Which Resolved After Discontinuation.. CNS Side Effects Were Common, Including Inebriation, Dizziness, Blurred Vision And Nausea. Hallucinations Occurred In 6/33 Patients. |
| Crowley, KLP | 1998 | Case Series | CRPS Type 1 And Type II | None | Range 1-7 Years | 5 | Topical | Single Dose Application Of Topical Ketamine. Dose Ranging From lOmgTo 700mg Per Single Application | Results Showed A Significant Reduction Relative To The Pretreatment NAS Of 65% To 100%. Initial Response Was Within 20 Seconds To 3 Minutes. | No Reported Side Effects Occurred |
| Koffler SP, et al. | 2007 | Pilot, Open-Label Study | CRPS-1 | lASPAnd 1999 Modified Research Criteria | Average= 55.67+-24.75 Months Since Injury | 9 (8F, 1 M) | IV Anesthetic Ketamine | Anesthetic Dose- 250-300ug/DI For At Least 4.5 Days | There Were Significant Reductions In MPQ, Acute Pain (PPI) And Overall Pain (PRI). All Patients Had Been Withdrawn From Narcotics And Required No Pain Medicine At The 6-Month Follow-Up. One Patient Reported Slightly More Pain And Higher Depression And Anxiety Following Treatment. Attention, Learning And Memory, And Mood Remain Unchanged Post Treatment. Processing Speed Improved Significantly, While Motor Strength Was Reduced But Motor Speed Remained Stable. | Muscle Weakness, Dizziness, Fatigue, Episodes Of Hyperhidrosis, And Feelings Hot And Slightly Anxious Were Reported. Effects Were Resolved Maximally Within 2-4 Weeks. 2 Patients Had Mild Unsettling Flashbacks At 4 Weeks. They Were Treated With Ativan (4mg) And Did Not Reoccur. |
| Kiefer RT, et al. | 2008 | Pilot, Open-Label Study | Refractory CRPS | lASPAnd 1999 Modified Research Criteria | Average= 58 (+- 20) Months | 4(F) | IV Sub Anesthetic S(+)-Ketamine | Continuous S(+)-Ketamine-Infusions Gradually Titrated (50 Mg/Day-500 Mg/Day) And Increased By lOOmg/Day Over A 10-Day Period. 3/4 Up Titrated To 500mg/Day. One UptitratedTo 300mg/Day Due To Dizziness. Clonidine Administered To Alleviate SE. | At Baseline, The Average Pain Was 81.7(+-4.5) Mm, Peak Pain Was 86+-3.2mm, And Least Pain Was 74.2(+-4.7). After 10 Days Of Treatment, Average Pain Was 85.1(+-1.9mm), Peak Pain Was 90.0(+-1.9) Mm, And Least Pain Was 78.7(+-3) Mm. There Was A Slight Insignificant Increase In Mechanosensory Detection Thresholds In All Patients. 2/10 Showed Slight Increase In Mechanosensory Pain Thresholds. There Was A Minor Reduction In Touch Allodynia And A Decrease In Cold Pain Thresholds. No Significant Changes In Warm And Cold Detection. | No Significant Side Effects Occurred. Some Side Effects Were Already Observed At Baseline Due To Current Medications. During Infusion, Intensity Of General Symptoms Decreased (Nausea, Tiredness, Insomnia Headaches And Weakness). Insignificant Increase In Euphoria Disorientation. No Change In Hallucinations/Nightmare s. |
| Sigtermans M, et al. | 2010 | Observational Study | CRPS-1 | IASP Criteria | 8.4+- 6.1 Yrsfl.l-20.7) | 10(10 F) | IV Sub Anesthetic S(+)-Ketamine | Seven Intravenous 5-Min Low-Dose S(+)-Ketamine Infusions Every 20 Minutes With Increasing Doses At Each 20-Min Interval. Total Infusion Time= 125 Minutes. Dose= 1st lnfusion= 1.5mg/70kg. Last lnfusion= 10.5 Mg/70kg. Increased In 1.5mg/70kg Increments | There Was A Significant Decrease In VAS After Treatment (Baseline 6.2+-0.2cm). The Lowest VAS Score Was At T=125min (VAS =0.4+-0.3 Cm). Pain Rating Remained More Than 50% Below Baseline For 3 Hours Post Infusion. Evoked Pain At Baseline Was 7.7(+-0.3)Cm. Significant Decreases Were Seen In VAS At T=80min. Maximum Reduction Was At T=125 Min (VAS=3.1+-1.4cm) In Between Infusions, Evoked Pain VAS Returned To Baseline. | No Specific Adverse Events Were Reported. “Drug High” Rated On VAS Gradually Increased Post Infusion As Dosing Increased. Peak Was Reached At T=125 Min And Averaged 9.7+-0.2. Side Effect Rapidly Dissipated In The Elimination Phase (Nearly Gone 60 Min Post Infusion). |
| Ushida T, et al. | 2002 | Case Series | CRPS Type 1 And Type II | None | Range 4 Months To 15 Years | 7 | Topical | Ointment Containing KET (0.257o-1.5%) Used 3 Times A Day | Significant Reduction In VAS Score And Decrease In Swelling Was Reported In 4/7 Patients. No Apparent Changes Were Seen In One CRPS-I Patient And Both CRPS-II Patients. | No Reported Side Effects Occurred |
| Kiefer RT, et al. | 2008 | Pilot, Open-Label Study | Refractory CRPS | lASPAnd 1999 Modified Research Criteria | Average= 49.4 (+-25) Months, Range 6-84 Months | 20 | IV Anesthetic Ketamine | Anesthetic Dosage Over 5 Days Up To 7mg/Kg/Hour And Coadministed Midazolam And Clonidine | Significant Pain Relief Was Observed At 1, 3, And 6 Months Following Treatment (93.5, 11.1%; 89.4, 17.0%; 79.3, 25.3%; P < 0.001). Complete Remission From CRPS Was Observed At 1 Month In All Patients, At 3 Months In 17, And At 6 Months In 16 Patients. If Relapse Occurred, Significant Pain Relief Was Still Attained At 3 And 6 Months. Quality Of Life, The Associated Movement Disorder, And The Ability To Work Significantly Improved In The Majority Of Patients At 3 And 6 Months. | No Life Threatening Complications Occurred, But Subjects Reported PsychotropicSide Effects, As Well As Difficulty Sleeping, Nightmares, And Muscular Weakness Persisting For Weeks After Treatment. The Majority Also Had Infections Associated With The Intensive Care Nature Of The Treatment. The Authors Concluded That Patients Must Be Watched Carefully During The Infusion ForThese Reasons. |
| Patil S, & Anitescu M. | 2012 | Retrospective Chart Review | Refractory Pain ( CRPS, Low Back Pain And Headaches) | N/A | More Than 6 Months | 31, (18 CRPS) | IV Sub-Anesthetic Ketamine | Infusion Started At .5mg/Kg To Highest Tolerated Dose. Average Does= 0.9 Mg/Hr, Median = 38.3 Min. Average Number Of Infusions = 4 (CRPS=5.5). Scheduled Routinely Every 3-4 Weeks Per Pain Protocol. Subjects Were Pretreated With Midazolam And Ondansetron. | All Patients Reported A Significant Reduction In VAS Score. For CRPS Patients, Pain Was Reduced By 7.2 On 10 Point NRS Scale. Data From Follow-Up Calls Showed That 78% Said Pain Relief Lasted Only 1-2 Days, 27% Said Hours, 38% Said Pain Lasted Up To 3 Weeks. | 35 Nonserious AE Reported By 23 Patients (46.9). Hypertension And Sedation Were Most Common. Higher Incidence Of Hallucination And Confusion In Non-CRPS Patients. In All Cases SE Were Minimal. |
| Author | Year | Study Type | Diagnosis | Diagnostic Criteria | Pain Duration | N | Route | Dose | Results | Adverse Events/Side Effects |
| Polomano, RC, et al. | 2013 | Retrospective Chart Review | Neuropathic Pain From Major Limb Injuries | N/A | Post Traumatic Injury | 19 | 3-Day Infusion Of IV Sub-Anesthetic Ketamine With Multimodal Analgesia | Sub-Anesthetic | Significant Reduction In PPI (Present Pain Intensity), Improvement In GPR (Global Pain Relief), And A Decrease In Mean Opioid Requirement. | OveralLTherapy Was Well Tolerated. Patients Reported Drowsiness (4), Hallucinations (1), Vivid Dreams (1), Slight Decrease In Blood Pressure (2), Nausea (1) |
| Webster, L.R. And MJ. Walker | 2006 | Retrospective Chart Review | Neuropathic Pain (8/13 CRPS, 1 Migraine 3 Neuropathy, 1 Phantom Limb) | N/A | Unknown | 13 | IV Or Subcutaneous Ketamine | Low Dose Continuous Infusion. Mean Duration: 16.4 Days (Range 5-55) Carefully Titrated To The Minimum Effective Level. Average Dose = 0.12 Mg/Kg/H (Range O.Olmg/Kg/H, - 0.25 Mg/Kg/H). | VAS Decreased Significantly From 7.7+-1.8 To 4.8+-2.3. Patients Showed Improvement In Global Pain Relief (8/13 Patients Reported Good Pain Relief). In Interviews, 12/13 Perceived An Improvement In Pain. 7/13 Still Perceived Improvement 1 Month After Infusion Treatment Ended. Overall, 11/13 Patients Reported 85% Decrease In Pain From Start Of Infusion To End | Adverse Events Due To Problems With Subq Delivery (5/5). This Route Was Discontinued. For IV Ketamine, Patients Reported Fatigue (4), Dizziness (3), Confusion (2), And Spinal Pain (2). No Hallucinations Were Reported. |
| Goldberg, ME | 2005 | Pilot, Open-Label Study | Refractory CRPS Type 1 And II | IASP | Range 3 Months-3years | 40 | Low Dose IV Ketamine | 4 Hour Ketamine Infusion Escalated From 40-80 Mg Daily For 10 Days + Clonidine + Midazolam | Pain Decreased Significantly From 7.54 To 5.44 On Average. Pain Diaries Showed Significant Reduction In The “Worst Pain Of The Day” And “Least Daily Pain”. Significant Reduction In “Punishing Pain”. Patients Showed Increased Ability To Initiate Movement. | Side Effects Were Minimal-4/10 And 5/40 Reported Headaches And Restlessness. |
| Correll, GE | 2004 | Retrospective Chart Review | CRPS Type 1 And Type 11 | Official Diagnosis With Confirmati on From The Senior Anesthesiol ogist | Ave=28.1 Months. Range= 0.25-240 Mo. | 33 (25M 8F) | IV Sub-Anesthetic Ketamine | IV Ketamine Started At lOmg/Hr, Increased As Tolerated Until Feelings Of Inebriation Onset Of CNS Symptoms. (Highest Tolerated Dose Producing Analgesia (Average=23.4 Mg/Hr Range 15-50mg/Hr). Duration Averaged 4.7 Days, 0.75-20 Days). 21 Received Treatment Only Once, 10 Received Two Courses Of Treatment, And 2 Received Three Courses. | 76% (25/33) Got 100% Pain Relief Initially, 6/33 Had Partial Relief, And 2 None After 1st Infusion. After 1st Infusion, 54% Relief Lasted >3 Months, And 31% >6 Months. After The Second Infusion, 100% Pain Relief In All Patients. For 10.58% Of Patients, Relief Lasted 1 Year, And For 33% Relief Lasted 3 Years. Overall, 83% With Complete Relief, 13% With Partial Relief, And 2 With No Relief (4%) | 4 Patients Had Elevated Hepatic Enzyme Profiles, Which Resolved After Discontinuation.. CNS Side Effects Were Common, Including Inebriation, Dizziness, Blurred Vision And Nausea. Hallucinations Occurred In 6/33 Patients. |
| Crowley, KLP | 1998 | Case Series | CRPS Type 1 And Type II | None | Range 1-7 Years | 5 | Topical | Single Dose Application Of Topical Ketamine. Dose Ranging From lOmgTo 700mg Per Single Application | Results Showed A Significant Reduction Relative To The Pretreatment NAS Of 65% To 100%. Initial Response Was Within 20 Seconds To 3 Minutes. | No Reported Side Effects Occurred |
| Koffler SP, et al. | 2007 | Pilot, Open-Label Study | CRPS-1 | lASPAnd 1999 Modified Research Criteria | Average= 55.67+-24.75 Months Since Injury | 9 (8F, 1 M) | IV Anesthetic Ketamine | Anesthetic Dose- 250-300ug/DI For At Least 4.5 Days | There Were Significant Reductions In MPQ, Acute Pain (PPI) And Overall Pain (PRI). All Patients Had Been Withdrawn From Narcotics And Required No Pain Medicine At The 6-Month Follow-Up. One Patient Reported Slightly More Pain And Higher Depression And Anxiety Following Treatment. Attention, Learning And Memory, And Mood Remain Unchanged Post Treatment. Processing Speed Improved Significantly, While Motor Strength Was Reduced But Motor Speed Remained Stable. | Muscle Weakness, Dizziness, Fatigue, Episodes Of Hyperhidrosis, And Feelings Hot And Slightly Anxious Were Reported. Effects Were Resolved Maximally Within 2-4 Weeks. 2 Patients Had Mild Unsettling Flashbacks At 4 Weeks. They Were Treated With Ativan (4mg) And Did Not Reoccur. |
| Kiefer RT, et al. | 2008 | Pilot, Open-Label Study | Refractory CRPS | lASPAnd 1999 Modified Research Criteria | Average= 58 (+- 20) Months | 4(F) | IV Sub Anesthetic S(+)-Ketamine | Continuous S(+)-Ketamine-Infusions Gradually Titrated (50 Mg/Day-500 Mg/Day) And Increased By lOOmg/Day Over A 10-Day Period. 3/4 Up Titrated To 500mg/Day. One UptitratedTo 300mg/Day Due To Dizziness. Clonidine Administered To Alleviate SE. | At Baseline, The Average Pain Was 81.7(+-4.5) Mm, Peak Pain Was 86+-3.2mm, And Least Pain Was 74.2(+-4.7). After 10 Days Of Treatment, Average Pain Was 85.1(+-1.9mm), Peak Pain Was 90.0(+-1.9) Mm, And Least Pain Was 78.7(+-3) Mm. There Was A Slight Insignificant Increase In Mechanosensory Detection Thresholds In All Patients. 2/10 Showed Slight Increase In Mechanosensory Pain Thresholds. There Was A Minor Reduction In Touch Allodynia And A Decrease In Cold Pain Thresholds. No Significant Changes In Warm And Cold Detection. | No Significant Side Effects Occurred. Some Side Effects Were Already Observed At Baseline Due To Current Medications. During Infusion, Intensity Of General Symptoms Decreased (Nausea, Tiredness, Insomnia Headaches And Weakness). Insignificant Increase In Euphoria Disorientation. No Change In Hallucinations/Nightmare s. |
| Sigtermans M, et al. | 2010 | Observational Study | CRPS-1 | IASP Criteria | 8.4+- 6.1 Yrsfl.l-20.7) | 10(10 F) | IV Sub Anesthetic S(+)-Ketamine | Seven Intravenous 5-Min Low-Dose S(+)-Ketamine Infusions Every 20 Minutes With Increasing Doses At Each 20-Min Interval. Total Infusion Time= 125 Minutes. Dose= 1st lnfusion= 1.5mg/70kg. Last lnfusion= 10.5 Mg/70kg. Increased In 1.5mg/70kg Increments | There Was A Significant Decrease In VAS After Treatment (Baseline 6.2+-0.2cm). The Lowest VAS Score Was At T=125min (VAS =0.4+-0.3 Cm). Pain Rating Remained More Than 50% Below Baseline For 3 Hours Post Infusion. Evoked Pain At Baseline Was 7.7(+-0.3)Cm. Significant Decreases Were Seen In VAS At T=80min. Maximum Reduction Was At T=125 Min (VAS=3.1+-1.4cm) In Between Infusions, Evoked Pain VAS Returned To Baseline. | No Specific Adverse Events Were Reported. “Drug High” Rated On VAS Gradually Increased Post Infusion As Dosing Increased. Peak Was Reached At T=125 Min And Averaged 9.7+-0.2. Side Effect Rapidly Dissipated In The Elimination Phase (Nearly Gone 60 Min Post Infusion). |
| Ushida T, et al. | 2002 | Case Series | CRPS Type 1 And Type II | None | Range 4 Months To 15 Years | 7 | Topical | Ointment Containing KET (0.257o-1.5%) Used 3 Times A Day | Significant Reduction In VAS Score And Decrease In Swelling Was Reported In 4/7 Patients. No Apparent Changes Were Seen In One CRPS-I Patient And Both CRPS-II Patients. | No Reported Side Effects Occurred |
| Kiefer RT, et al. | 2008 | Pilot, Open-Label Study | Refractory CRPS | lASPAnd 1999 Modified Research Criteria | Average= 49.4 (+-25) Months, Range 6-84 Months | 20 | IV Anesthetic Ketamine | Anesthetic Dosage Over 5 Days Up To 7mg/Kg/Hour And Coadministed Midazolam And Clonidine | Significant Pain Relief Was Observed At 1, 3, And 6 Months Following Treatment (93.5, 11.1%; 89.4, 17.0%; 79.3, 25.3%; P < 0.001). Complete Remission From CRPS Was Observed At 1 Month In All Patients, At 3 Months In 17, And At 6 Months In 16 Patients. If Relapse Occurred, Significant Pain Relief Was Still Attained At 3 And 6 Months. Quality Of Life, The Associated Movement Disorder, And The Ability To Work Significantly Improved In The Majority Of Patients At 3 And 6 Months. | No Life Threatening Complications Occurred, But Subjects Reported PsychotropicSide Effects, As Well As Difficulty Sleeping, Nightmares, And Muscular Weakness Persisting For Weeks After Treatment. The Majority Also Had Infections Associated With The Intensive Care Nature Of The Treatment. The Authors Concluded That Patients Must Be Watched Carefully During The Infusion ForThese Reasons. |
| Patil S, & Anitescu M. | 2012 | Retrospective Chart Review | Refractory Pain ( CRPS, Low Back Pain And Headaches) | N/A | More Than 6 Months | 31, (18 CRPS) | IV Sub-Anesthetic Ketamine | Infusion Started At .5mg/Kg To Highest Tolerated Dose. Average Does= 0.9 Mg/Hr, Median = 38.3 Min. Average Number Of Infusions = 4 (CRPS=5.5). Scheduled Routinely Every 3-4 Weeks Per Pain Protocol. Subjects Were Pretreated With Midazolam And Ondansetron. | All Patients Reported A Significant Reduction In VAS Score. For CRPS Patients, Pain Was Reduced By 7.2 On 10 Point NRS Scale. Data From Follow-Up Calls Showed That 78% Said Pain Relief Lasted Only 1-2 Days, 27% Said Hours, 38% Said Pain Lasted Up To 3 Weeks. | 35 Nonserious AE Reported By 23 Patients (46.9). Hypertension And Sedation Were Most Common. Higher Incidence Of Hallucination And Confusion In Non-CRPS Patients. In All Cases SE Were Minimal. |
Evaluation of Observational Studies of Ketamine For Complex Regional Pain Syndrome (CRPS)
| Author | Year | Study Type | Diagnosis | Diagnostic Criteria | Pain Duration | N | Route | Dose | Results | Adverse Events/Side Effects |
| Polomano, RC, et al. | 2013 | Retrospective Chart Review | Neuropathic Pain From Major Limb Injuries | N/A | Post Traumatic Injury | 19 | 3-Day Infusion Of IV Sub-Anesthetic Ketamine With Multimodal Analgesia | Sub-Anesthetic | Significant Reduction In PPI (Present Pain Intensity), Improvement In GPR (Global Pain Relief), And A Decrease In Mean Opioid Requirement. | OveralLTherapy Was Well Tolerated. Patients Reported Drowsiness (4), Hallucinations (1), Vivid Dreams (1), Slight Decrease In Blood Pressure (2), Nausea (1) |
| Webster, L.R. And MJ. Walker | 2006 | Retrospective Chart Review | Neuropathic Pain (8/13 CRPS, 1 Migraine 3 Neuropathy, 1 Phantom Limb) | N/A | Unknown | 13 | IV Or Subcutaneous Ketamine | Low Dose Continuous Infusion. Mean Duration: 16.4 Days (Range 5-55) Carefully Titrated To The Minimum Effective Level. Average Dose = 0.12 Mg/Kg/H (Range O.Olmg/Kg/H, - 0.25 Mg/Kg/H). | VAS Decreased Significantly From 7.7+-1.8 To 4.8+-2.3. Patients Showed Improvement In Global Pain Relief (8/13 Patients Reported Good Pain Relief). In Interviews, 12/13 Perceived An Improvement In Pain. 7/13 Still Perceived Improvement 1 Month After Infusion Treatment Ended. Overall, 11/13 Patients Reported 85% Decrease In Pain From Start Of Infusion To End | Adverse Events Due To Problems With Subq Delivery (5/5). This Route Was Discontinued. For IV Ketamine, Patients Reported Fatigue (4), Dizziness (3), Confusion (2), And Spinal Pain (2). No Hallucinations Were Reported. |
| Goldberg, ME | 2005 | Pilot, Open-Label Study | Refractory CRPS Type 1 And II | IASP | Range 3 Months-3years | 40 | Low Dose IV Ketamine | 4 Hour Ketamine Infusion Escalated From 40-80 Mg Daily For 10 Days + Clonidine + Midazolam | Pain Decreased Significantly From 7.54 To 5.44 On Average. Pain Diaries Showed Significant Reduction In The “Worst Pain Of The Day” And “Least Daily Pain”. Significant Reduction In “Punishing Pain”. Patients Showed Increased Ability To Initiate Movement. | Side Effects Were Minimal-4/10 And 5/40 Reported Headaches And Restlessness. |
| Correll, GE | 2004 | Retrospective Chart Review | CRPS Type 1 And Type 11 | Official Diagnosis With Confirmati on From The Senior Anesthesiol ogist | Ave=28.1 Months. Range= 0.25-240 Mo. | 33 (25M 8F) | IV Sub-Anesthetic Ketamine | IV Ketamine Started At lOmg/Hr, Increased As Tolerated Until Feelings Of Inebriation Onset Of CNS Symptoms. (Highest Tolerated Dose Producing Analgesia (Average=23.4 Mg/Hr Range 15-50mg/Hr). Duration Averaged 4.7 Days, 0.75-20 Days). 21 Received Treatment Only Once, 10 Received Two Courses Of Treatment, And 2 Received Three Courses. | 76% (25/33) Got 100% Pain Relief Initially, 6/33 Had Partial Relief, And 2 None After 1st Infusion. After 1st Infusion, 54% Relief Lasted >3 Months, And 31% >6 Months. After The Second Infusion, 100% Pain Relief In All Patients. For 10.58% Of Patients, Relief Lasted 1 Year, And For 33% Relief Lasted 3 Years. Overall, 83% With Complete Relief, 13% With Partial Relief, And 2 With No Relief (4%) | 4 Patients Had Elevated Hepatic Enzyme Profiles, Which Resolved After Discontinuation.. CNS Side Effects Were Common, Including Inebriation, Dizziness, Blurred Vision And Nausea. Hallucinations Occurred In 6/33 Patients. |
| Crowley, KLP | 1998 | Case Series | CRPS Type 1 And Type II | None | Range 1-7 Years | 5 | Topical | Single Dose Application Of Topical Ketamine. Dose Ranging From lOmgTo 700mg Per Single Application | Results Showed A Significant Reduction Relative To The Pretreatment NAS Of 65% To 100%. Initial Response Was Within 20 Seconds To 3 Minutes. | No Reported Side Effects Occurred |
| Koffler SP, et al. | 2007 | Pilot, Open-Label Study | CRPS-1 | lASPAnd 1999 Modified Research Criteria | Average= 55.67+-24.75 Months Since Injury | 9 (8F, 1 M) | IV Anesthetic Ketamine | Anesthetic Dose- 250-300ug/DI For At Least 4.5 Days | There Were Significant Reductions In MPQ, Acute Pain (PPI) And Overall Pain (PRI). All Patients Had Been Withdrawn From Narcotics And Required No Pain Medicine At The 6-Month Follow-Up. One Patient Reported Slightly More Pain And Higher Depression And Anxiety Following Treatment. Attention, Learning And Memory, And Mood Remain Unchanged Post Treatment. Processing Speed Improved Significantly, While Motor Strength Was Reduced But Motor Speed Remained Stable. | Muscle Weakness, Dizziness, Fatigue, Episodes Of Hyperhidrosis, And Feelings Hot And Slightly Anxious Were Reported. Effects Were Resolved Maximally Within 2-4 Weeks. 2 Patients Had Mild Unsettling Flashbacks At 4 Weeks. They Were Treated With Ativan (4mg) And Did Not Reoccur. |
| Kiefer RT, et al. | 2008 | Pilot, Open-Label Study | Refractory CRPS | lASPAnd 1999 Modified Research Criteria | Average= 58 (+- 20) Months | 4(F) | IV Sub Anesthetic S(+)-Ketamine | Continuous S(+)-Ketamine-Infusions Gradually Titrated (50 Mg/Day-500 Mg/Day) And Increased By lOOmg/Day Over A 10-Day Period. 3/4 Up Titrated To 500mg/Day. One UptitratedTo 300mg/Day Due To Dizziness. Clonidine Administered To Alleviate SE. | At Baseline, The Average Pain Was 81.7(+-4.5) Mm, Peak Pain Was 86+-3.2mm, And Least Pain Was 74.2(+-4.7). After 10 Days Of Treatment, Average Pain Was 85.1(+-1.9mm), Peak Pain Was 90.0(+-1.9) Mm, And Least Pain Was 78.7(+-3) Mm. There Was A Slight Insignificant Increase In Mechanosensory Detection Thresholds In All Patients. 2/10 Showed Slight Increase In Mechanosensory Pain Thresholds. There Was A Minor Reduction In Touch Allodynia And A Decrease In Cold Pain Thresholds. No Significant Changes In Warm And Cold Detection. | No Significant Side Effects Occurred. Some Side Effects Were Already Observed At Baseline Due To Current Medications. During Infusion, Intensity Of General Symptoms Decreased (Nausea, Tiredness, Insomnia Headaches And Weakness). Insignificant Increase In Euphoria Disorientation. No Change In Hallucinations/Nightmare s. |
| Sigtermans M, et al. | 2010 | Observational Study | CRPS-1 | IASP Criteria | 8.4+- 6.1 Yrsfl.l-20.7) | 10(10 F) | IV Sub Anesthetic S(+)-Ketamine | Seven Intravenous 5-Min Low-Dose S(+)-Ketamine Infusions Every 20 Minutes With Increasing Doses At Each 20-Min Interval. Total Infusion Time= 125 Minutes. Dose= 1st lnfusion= 1.5mg/70kg. Last lnfusion= 10.5 Mg/70kg. Increased In 1.5mg/70kg Increments | There Was A Significant Decrease In VAS After Treatment (Baseline 6.2+-0.2cm). The Lowest VAS Score Was At T=125min (VAS =0.4+-0.3 Cm). Pain Rating Remained More Than 50% Below Baseline For 3 Hours Post Infusion. Evoked Pain At Baseline Was 7.7(+-0.3)Cm. Significant Decreases Were Seen In VAS At T=80min. Maximum Reduction Was At T=125 Min (VAS=3.1+-1.4cm) In Between Infusions, Evoked Pain VAS Returned To Baseline. | No Specific Adverse Events Were Reported. “Drug High” Rated On VAS Gradually Increased Post Infusion As Dosing Increased. Peak Was Reached At T=125 Min And Averaged 9.7+-0.2. Side Effect Rapidly Dissipated In The Elimination Phase (Nearly Gone 60 Min Post Infusion). |
| Ushida T, et al. | 2002 | Case Series | CRPS Type 1 And Type II | None | Range 4 Months To 15 Years | 7 | Topical | Ointment Containing KET (0.257o-1.5%) Used 3 Times A Day | Significant Reduction In VAS Score And Decrease In Swelling Was Reported In 4/7 Patients. No Apparent Changes Were Seen In One CRPS-I Patient And Both CRPS-II Patients. | No Reported Side Effects Occurred |
| Kiefer RT, et al. | 2008 | Pilot, Open-Label Study | Refractory CRPS | lASPAnd 1999 Modified Research Criteria | Average= 49.4 (+-25) Months, Range 6-84 Months | 20 | IV Anesthetic Ketamine | Anesthetic Dosage Over 5 Days Up To 7mg/Kg/Hour And Coadministed Midazolam And Clonidine | Significant Pain Relief Was Observed At 1, 3, And 6 Months Following Treatment (93.5, 11.1%; 89.4, 17.0%; 79.3, 25.3%; P < 0.001). Complete Remission From CRPS Was Observed At 1 Month In All Patients, At 3 Months In 17, And At 6 Months In 16 Patients. If Relapse Occurred, Significant Pain Relief Was Still Attained At 3 And 6 Months. Quality Of Life, The Associated Movement Disorder, And The Ability To Work Significantly Improved In The Majority Of Patients At 3 And 6 Months. | No Life Threatening Complications Occurred, But Subjects Reported PsychotropicSide Effects, As Well As Difficulty Sleeping, Nightmares, And Muscular Weakness Persisting For Weeks After Treatment. The Majority Also Had Infections Associated With The Intensive Care Nature Of The Treatment. The Authors Concluded That Patients Must Be Watched Carefully During The Infusion ForThese Reasons. |
| Patil S, & Anitescu M. | 2012 | Retrospective Chart Review | Refractory Pain ( CRPS, Low Back Pain And Headaches) | N/A | More Than 6 Months | 31, (18 CRPS) | IV Sub-Anesthetic Ketamine | Infusion Started At .5mg/Kg To Highest Tolerated Dose. Average Does= 0.9 Mg/Hr, Median = 38.3 Min. Average Number Of Infusions = 4 (CRPS=5.5). Scheduled Routinely Every 3-4 Weeks Per Pain Protocol. Subjects Were Pretreated With Midazolam And Ondansetron. | All Patients Reported A Significant Reduction In VAS Score. For CRPS Patients, Pain Was Reduced By 7.2 On 10 Point NRS Scale. Data From Follow-Up Calls Showed That 78% Said Pain Relief Lasted Only 1-2 Days, 27% Said Hours, 38% Said Pain Lasted Up To 3 Weeks. | 35 Nonserious AE Reported By 23 Patients (46.9). Hypertension And Sedation Were Most Common. Higher Incidence Of Hallucination And Confusion In Non-CRPS Patients. In All Cases SE Were Minimal. |
| Author | Year | Study Type | Diagnosis | Diagnostic Criteria | Pain Duration | N | Route | Dose | Results | Adverse Events/Side Effects |
| Polomano, RC, et al. | 2013 | Retrospective Chart Review | Neuropathic Pain From Major Limb Injuries | N/A | Post Traumatic Injury | 19 | 3-Day Infusion Of IV Sub-Anesthetic Ketamine With Multimodal Analgesia | Sub-Anesthetic | Significant Reduction In PPI (Present Pain Intensity), Improvement In GPR (Global Pain Relief), And A Decrease In Mean Opioid Requirement. | OveralLTherapy Was Well Tolerated. Patients Reported Drowsiness (4), Hallucinations (1), Vivid Dreams (1), Slight Decrease In Blood Pressure (2), Nausea (1) |
| Webster, L.R. And MJ. Walker | 2006 | Retrospective Chart Review | Neuropathic Pain (8/13 CRPS, 1 Migraine 3 Neuropathy, 1 Phantom Limb) | N/A | Unknown | 13 | IV Or Subcutaneous Ketamine | Low Dose Continuous Infusion. Mean Duration: 16.4 Days (Range 5-55) Carefully Titrated To The Minimum Effective Level. Average Dose = 0.12 Mg/Kg/H (Range O.Olmg/Kg/H, - 0.25 Mg/Kg/H). | VAS Decreased Significantly From 7.7+-1.8 To 4.8+-2.3. Patients Showed Improvement In Global Pain Relief (8/13 Patients Reported Good Pain Relief). In Interviews, 12/13 Perceived An Improvement In Pain. 7/13 Still Perceived Improvement 1 Month After Infusion Treatment Ended. Overall, 11/13 Patients Reported 85% Decrease In Pain From Start Of Infusion To End | Adverse Events Due To Problems With Subq Delivery (5/5). This Route Was Discontinued. For IV Ketamine, Patients Reported Fatigue (4), Dizziness (3), Confusion (2), And Spinal Pain (2). No Hallucinations Were Reported. |
| Goldberg, ME | 2005 | Pilot, Open-Label Study | Refractory CRPS Type 1 And II | IASP | Range 3 Months-3years | 40 | Low Dose IV Ketamine | 4 Hour Ketamine Infusion Escalated From 40-80 Mg Daily For 10 Days + Clonidine + Midazolam | Pain Decreased Significantly From 7.54 To 5.44 On Average. Pain Diaries Showed Significant Reduction In The “Worst Pain Of The Day” And “Least Daily Pain”. Significant Reduction In “Punishing Pain”. Patients Showed Increased Ability To Initiate Movement. | Side Effects Were Minimal-4/10 And 5/40 Reported Headaches And Restlessness. |
| Correll, GE | 2004 | Retrospective Chart Review | CRPS Type 1 And Type 11 | Official Diagnosis With Confirmati on From The Senior Anesthesiol ogist | Ave=28.1 Months. Range= 0.25-240 Mo. | 33 (25M 8F) | IV Sub-Anesthetic Ketamine | IV Ketamine Started At lOmg/Hr, Increased As Tolerated Until Feelings Of Inebriation Onset Of CNS Symptoms. (Highest Tolerated Dose Producing Analgesia (Average=23.4 Mg/Hr Range 15-50mg/Hr). Duration Averaged 4.7 Days, 0.75-20 Days). 21 Received Treatment Only Once, 10 Received Two Courses Of Treatment, And 2 Received Three Courses. | 76% (25/33) Got 100% Pain Relief Initially, 6/33 Had Partial Relief, And 2 None After 1st Infusion. After 1st Infusion, 54% Relief Lasted >3 Months, And 31% >6 Months. After The Second Infusion, 100% Pain Relief In All Patients. For 10.58% Of Patients, Relief Lasted 1 Year, And For 33% Relief Lasted 3 Years. Overall, 83% With Complete Relief, 13% With Partial Relief, And 2 With No Relief (4%) | 4 Patients Had Elevated Hepatic Enzyme Profiles, Which Resolved After Discontinuation.. CNS Side Effects Were Common, Including Inebriation, Dizziness, Blurred Vision And Nausea. Hallucinations Occurred In 6/33 Patients. |
| Crowley, KLP | 1998 | Case Series | CRPS Type 1 And Type II | None | Range 1-7 Years | 5 | Topical | Single Dose Application Of Topical Ketamine. Dose Ranging From lOmgTo 700mg Per Single Application | Results Showed A Significant Reduction Relative To The Pretreatment NAS Of 65% To 100%. Initial Response Was Within 20 Seconds To 3 Minutes. | No Reported Side Effects Occurred |
| Koffler SP, et al. | 2007 | Pilot, Open-Label Study | CRPS-1 | lASPAnd 1999 Modified Research Criteria | Average= 55.67+-24.75 Months Since Injury | 9 (8F, 1 M) | IV Anesthetic Ketamine | Anesthetic Dose- 250-300ug/DI For At Least 4.5 Days | There Were Significant Reductions In MPQ, Acute Pain (PPI) And Overall Pain (PRI). All Patients Had Been Withdrawn From Narcotics And Required No Pain Medicine At The 6-Month Follow-Up. One Patient Reported Slightly More Pain And Higher Depression And Anxiety Following Treatment. Attention, Learning And Memory, And Mood Remain Unchanged Post Treatment. Processing Speed Improved Significantly, While Motor Strength Was Reduced But Motor Speed Remained Stable. | Muscle Weakness, Dizziness, Fatigue, Episodes Of Hyperhidrosis, And Feelings Hot And Slightly Anxious Were Reported. Effects Were Resolved Maximally Within 2-4 Weeks. 2 Patients Had Mild Unsettling Flashbacks At 4 Weeks. They Were Treated With Ativan (4mg) And Did Not Reoccur. |
| Kiefer RT, et al. | 2008 | Pilot, Open-Label Study | Refractory CRPS | lASPAnd 1999 Modified Research Criteria | Average= 58 (+- 20) Months | 4(F) | IV Sub Anesthetic S(+)-Ketamine | Continuous S(+)-Ketamine-Infusions Gradually Titrated (50 Mg/Day-500 Mg/Day) And Increased By lOOmg/Day Over A 10-Day Period. 3/4 Up Titrated To 500mg/Day. One UptitratedTo 300mg/Day Due To Dizziness. Clonidine Administered To Alleviate SE. | At Baseline, The Average Pain Was 81.7(+-4.5) Mm, Peak Pain Was 86+-3.2mm, And Least Pain Was 74.2(+-4.7). After 10 Days Of Treatment, Average Pain Was 85.1(+-1.9mm), Peak Pain Was 90.0(+-1.9) Mm, And Least Pain Was 78.7(+-3) Mm. There Was A Slight Insignificant Increase In Mechanosensory Detection Thresholds In All Patients. 2/10 Showed Slight Increase In Mechanosensory Pain Thresholds. There Was A Minor Reduction In Touch Allodynia And A Decrease In Cold Pain Thresholds. No Significant Changes In Warm And Cold Detection. | No Significant Side Effects Occurred. Some Side Effects Were Already Observed At Baseline Due To Current Medications. During Infusion, Intensity Of General Symptoms Decreased (Nausea, Tiredness, Insomnia Headaches And Weakness). Insignificant Increase In Euphoria Disorientation. No Change In Hallucinations/Nightmare s. |
| Sigtermans M, et al. | 2010 | Observational Study | CRPS-1 | IASP Criteria | 8.4+- 6.1 Yrsfl.l-20.7) | 10(10 F) | IV Sub Anesthetic S(+)-Ketamine | Seven Intravenous 5-Min Low-Dose S(+)-Ketamine Infusions Every 20 Minutes With Increasing Doses At Each 20-Min Interval. Total Infusion Time= 125 Minutes. Dose= 1st lnfusion= 1.5mg/70kg. Last lnfusion= 10.5 Mg/70kg. Increased In 1.5mg/70kg Increments | There Was A Significant Decrease In VAS After Treatment (Baseline 6.2+-0.2cm). The Lowest VAS Score Was At T=125min (VAS =0.4+-0.3 Cm). Pain Rating Remained More Than 50% Below Baseline For 3 Hours Post Infusion. Evoked Pain At Baseline Was 7.7(+-0.3)Cm. Significant Decreases Were Seen In VAS At T=80min. Maximum Reduction Was At T=125 Min (VAS=3.1+-1.4cm) In Between Infusions, Evoked Pain VAS Returned To Baseline. | No Specific Adverse Events Were Reported. “Drug High” Rated On VAS Gradually Increased Post Infusion As Dosing Increased. Peak Was Reached At T=125 Min And Averaged 9.7+-0.2. Side Effect Rapidly Dissipated In The Elimination Phase (Nearly Gone 60 Min Post Infusion). |
| Ushida T, et al. | 2002 | Case Series | CRPS Type 1 And Type II | None | Range 4 Months To 15 Years | 7 | Topical | Ointment Containing KET (0.257o-1.5%) Used 3 Times A Day | Significant Reduction In VAS Score And Decrease In Swelling Was Reported In 4/7 Patients. No Apparent Changes Were Seen In One CRPS-I Patient And Both CRPS-II Patients. | No Reported Side Effects Occurred |
| Kiefer RT, et al. | 2008 | Pilot, Open-Label Study | Refractory CRPS | lASPAnd 1999 Modified Research Criteria | Average= 49.4 (+-25) Months, Range 6-84 Months | 20 | IV Anesthetic Ketamine | Anesthetic Dosage Over 5 Days Up To 7mg/Kg/Hour And Coadministed Midazolam And Clonidine | Significant Pain Relief Was Observed At 1, 3, And 6 Months Following Treatment (93.5, 11.1%; 89.4, 17.0%; 79.3, 25.3%; P < 0.001). Complete Remission From CRPS Was Observed At 1 Month In All Patients, At 3 Months In 17, And At 6 Months In 16 Patients. If Relapse Occurred, Significant Pain Relief Was Still Attained At 3 And 6 Months. Quality Of Life, The Associated Movement Disorder, And The Ability To Work Significantly Improved In The Majority Of Patients At 3 And 6 Months. | No Life Threatening Complications Occurred, But Subjects Reported PsychotropicSide Effects, As Well As Difficulty Sleeping, Nightmares, And Muscular Weakness Persisting For Weeks After Treatment. The Majority Also Had Infections Associated With The Intensive Care Nature Of The Treatment. The Authors Concluded That Patients Must Be Watched Carefully During The Infusion ForThese Reasons. |
| Patil S, & Anitescu M. | 2012 | Retrospective Chart Review | Refractory Pain ( CRPS, Low Back Pain And Headaches) | N/A | More Than 6 Months | 31, (18 CRPS) | IV Sub-Anesthetic Ketamine | Infusion Started At .5mg/Kg To Highest Tolerated Dose. Average Does= 0.9 Mg/Hr, Median = 38.3 Min. Average Number Of Infusions = 4 (CRPS=5.5). Scheduled Routinely Every 3-4 Weeks Per Pain Protocol. Subjects Were Pretreated With Midazolam And Ondansetron. | All Patients Reported A Significant Reduction In VAS Score. For CRPS Patients, Pain Was Reduced By 7.2 On 10 Point NRS Scale. Data From Follow-Up Calls Showed That 78% Said Pain Relief Lasted Only 1-2 Days, 27% Said Hours, 38% Said Pain Lasted Up To 3 Weeks. | 35 Nonserious AE Reported By 23 Patients (46.9). Hypertension And Sedation Were Most Common. Higher Incidence Of Hallucination And Confusion In Non-CRPS Patients. In All Cases SE Were Minimal. |
Evaluation of Case Reports of Ketamine for Complex Regional Pain Syndrome (CRPS)
| Author | Year | Diagnosis | Pain Duration | N | Route | Dose | Results | Adverse Events/Side Effects |
| Alvarado, R. et al. | 2012 | CRPS-1 | 12 Years | 1 | IV | Anesthetic Dose And Multidisciplinary Treatments | Dramatic Resolution Of Lymphedema And Significant Improvement In Pain, Functioning And Use Of Analgesic Medications | No Reported Adverse Events |
| Becerra, L. et al. | 2009 | CRPS | 8 Years | 1 | IV | Anesthetic Dose (Uptitrated From 3mg/Kg/H To 7mg/Kg/H) For 5 Days. Midazolam Was Coadministered | VAS Decreased From 7-9/10 To 0-1 Post Treatment. There Were Changes In The Cerebral Cortex And Subcortical Regions Such As The Caudate Nucleus And Cerebellum. | No Reported Adverse Events |
| Everett, A. et al. | 2009 | CRPS-1 | 1 | Parenteral Ketamine Infusion | Continuous Peripheral Nerve Block And Parenteral Ketamine Infusion (0.1 Mg/Kg/H To 0.6mg/Kg/H) For 4 Days | Complete Resolution Of Her Pain Symptoms (Reduced From 8/10 To 0/10). Patient Was Still Pain Free 6 Months Later | No Adverse Events Were Reported. | |
| Harbut R.E. And G.E. Correll | 2002 | CRPS-1 | 9 Years | 1 | IV | Continuous Infusion Of Sub-Anesthetic Ketamine Uptitrated From 10mg/Hr To 30mg/Hr For 7 Days | VAS Score Decreased To 0/10. At 1 Week Follow-Up, Patient Was Pain Free And Continued Narcotic Taper. Clonidine Was Continued For Withdrawal Symptoms (Insomnia, Restlessness, And Diarrhea). Patient Discontinued Narcotics And Clonidine At 1 Month, And Remained Pain Free 5 Months Later And Full Functionality Was Restored. | No Adverse Events Were Reported. The Patient Was Given Clonidine For Withdrawal Symptoms (Insomnia, Restlessness, And Diarrhea) |
| Keppel Hesselink, J.M. And D.J. Kopsky | 2013 | CRPS-1 | 13 Years | 1 | Topical | Palmitoylethanolamide And Topical Ketamine 10% Cream | 50% Decrease In Pain After 1 Month Of Treatment. Also A Decrease In Swelling And Skin Discoloration And She Could Walk Independently | No Reported Adverse Events |
| Kiefer, RT et al. | 2007 | CRPS-1 | 6 Months | 1 | IV | Anesthetic Dose Of Ketamine Supplemented With Midazolam For 5 Days In Gradually Increasing Doses. (3-5mg/Kg/H) | On Day 6 Symptoms Of Edema And Discoloration Were Completely Resolved And The Patient Emerged Pain Free. Analgesic Effect Has Lasted 8 Years. Her Motor Function Has Steadily Improved With Physiotherapy | No Serious Adverse Events. The Patient Experienced Psychomimetic Side Effects (Agitation, Anxiety, And Nightmares) Which Were Managed With The Concomitant Use Of Midazolam And Resolved Within 1 Month. |
| Kishimoto, N et al. | 1995 | RSD | 6 Months | 1 | Stellate Ganglion Blocks, Continuous Epidural Block, And Continuous IV Ketamine | 1 Infusion Of Subanesthetic Ketamine For Two Hours | Burning Pain And Hyperperspiration Were Treated With SGB And CEB, And Allodynia Disappeared After Ketamine Treatment | |
| Kopsky, D.J. And J.M. Keppel Hesselink | 2011 | CRPS-1 | 3 Years | 1 | Topical | Multimodal (10% Ketamine, 5% Amitriptyline, And 50% Dimethyl Sulfoxide) Cream Applied 3 Times Daily | The Pain Decreased From 9 To 1.5/10 On The NRS, 26 To 6/45 On The SF-MPQ, And From 44 To 12 Out Of 100 On The NPSI. Quality Of Life Increased Profoundly. Pain Relief Was Sustained For At Least 5 Months With Continued Use. | No Reported Adverse Events |
| Lin, TC et al. | 1998 | RSD | 3 Months And 5 Months | 2 | Lumbar Epidural Catheter 3 Times Per Day | Subanalgesic Doses Of Ketamine (7.5mg), Morphine (0.75mg) And 6 Ml Bupivacaine 0.1%. Case#l -Every 12 Hours For 9 Days, Overall Received 11 Courses Of Treatment. Case #2 - 7 Courses Of Treatment Overall. | After Several Courses Of Treatment Over 3 And 6 Months, Pain Relief Was Achieved; Both Patients Were Able To Walk With Slight Weight Bearing And With Further Improvement In Signs And Symptoms. VAS Scores Decreased From 9 To 1/10 And 9 To 2/10 Respectively. | No Reported Adverse Events |
| Maneksha, FR | 2000 | CRPS | 5 Months | 1 | IV | 250 Mg Lidocaine Through IV, 10 Mg Ketamine Infused Slowly, A Bolus Of Fentanyl 25 Mg IV | NRS Decreased From 10/10 To 7/10 At Discharge. Weight Bearing With Crutches Now Possible. Patient Continued Treatment With Pt And Pharmacotherapy (Gabapentin And Amitriptyline) And NRS Ranging From 3/10 To 4/10. | Lidocaine Produced Minor Symptoms Of Toxicity With Perioral Tingling And Some Slurring Of Speech, But No Change In Symptoms. Ketamine Caused Considerable Sedation But No Change In Pain Level. After Fentanyl Patient Seemed To Become More Uncomfortable, Developing Severe Spasms In Her Foot. |
| Moesker, A | 2009 | CRPS | 6 Years | 1 | IV | Uptitrated To 4.8 (Mu)/Kg/Min For 5 Days | VAS Pain Score Decreased To 0, And Pain Relief Lasted 3 Weeks. Pain Relief From Second Infusion Lasted 3 Months, And A Third Treatment For As Long As 6 Months | No Serious Side Effects Occurred |
| Nama Sharanya, BS. et al. | 2010 | CRPS-1 | 8 Years | 1 | IV | Subanesthetic Ketamine (100mcg/Kg/Hr)+ Dexmedetomidine For 19 Hours + Lorazepam To Minimize Side Effects | Complete Resolution Of Her Pain And Associated Symptoms. Pain Decreased From 10/10 To 0/10 NRS Scale With Elimination Of Burning And Allodynia | No Reported Adverse Events |
| Noppers, IM et al. | 2011 | Ranged 11 Months To 12 Years | IV | Subjects Received Two 100 Hour Infusions Of Ketamine Separated By 16 Days. Infusion Of Ketamine Started At 1.2 Mg/Kg/H And Was Increased In Steps Of 0.6 Mg/Kg/H 1 3 Times A Day Until Patient Had NRS Of 0. | Reductions In Pain Levels (NRS) Were Seen In 5/6 Patients After The First Infusion, But The Trial Was Ended Prematurely Due To Adverse Events | One Patient Developed Severe Hypertension, One Patient Developed Severe Psychotropic Side Effects, And 3 Developed Elevated Hepatotoxicity. The Trial Was Stopped For These 5 Patients. | ||
| Rasmussen, J et al. | 2013 | CRPS | 2 Years | 1 | Ketamine + Dexmedetomidine Coma (Ketamine 7 mg/Kg/Hr; Dexmedetomidine 1.5m cg/Kg/Hr; Midazolam 17 mg/Hr) | 100% Resolution Of Pain Without Analgesic Or Opioid Use For At Least 45 Days Post Treatment | Severe Hallucinations And Anxiety, Were Treated With Chlordiazepoxide Taper | |
| Ricke, AK, et al. | 2011 | RSD | Unknown | 1 | IV | Ketamine Titrated From 10-20 Mg/Hour Over 5 Days | Patient Reported Relief On Day 1, Considerable Pain On Day 2 Resulting In Restarting Duloxetine And Mirtazapine, And Significant Pain Relief On Day. | Subtle Behavior Changes Were Seen In The First 6 Days, With Notable Changes And Mania-Like Symptoms On Day 7. Ketamine Therapy Was Discontinued. On Psychiatric Examination, The Patient Was Manic, Emotionally Labile, And Noted To Have Pressured Speech, Racing Thoughts, Flight Of Ideas And Paranoia. |
| Sakamoto, E et al. | 2010 | CRPS-II | Unknown | 1 | IV | Repeated Low Dose Ketamine Infusions | Ketamine Infusions Alleviated Pain, But Trophic Changes Persisted | No Reported Adverse Events |
| Shirani, P et al. | 2008 | CRPS-1 | 6 Years | 1 | IV | 1st Infusion: 50mg IV Ketamine Over 30 Minutes. | NRS Decreased From 10 To 3/10 After The First Infusion. After The Third Infusion, The Edema, Discoloration, And Temperature Of The Affected Areas Normalized. The Patient Became Pain Free With No Pain At The One-Year Follow-Up. | Hypertension (Treated With Labetalol And Hydralazine, And Given Midazolam For Next Infusions), Euphoria, And Headaches. |
| Shrikhande, AA et al. | 2011 | CRPS-1 | 6 And 8 Years | 2 | Ketamine PCA, Lumbar Nerve Blocks, Epidural Injections, | Both Patients Received Functional Independence Upon Discharge | ||
| Takahashi, H et al. | 1998 | CRPS-II | Post Trauma And Post-Operative Neuropathic Pain (15 Days Post-Op) | 1 | Epidural | Continuous Infusion 25microg/Kg/Hr For 10 Days | Complete Pain Relief Was Achieved And Lasted For 8 Months Post Treatment | Side Effects Occurred At Higher Doses (Severe Headache, Nausea, And Discomfort Lasting 24 Hours). No Side Effects At Lower Doses |
| Tateyama, H et al. | 2000 | CRPS-1 | 2 Years | 1 | Instillation | Prostaglandin El (20mug/lhour 12 Times) And Ketamine (200mg/2hours 2 Times) And Silver Spike Point Stimulation | Decrease In VAS From 6 To 2/10 | No Reported Adverse Events |
| Villanueva-Perez, VL et al. | 2007 | CRPS | 1 Month | 1 | IV + Oral | 30 Mg Of Oral Ketamine (3 Ml Of Syrup) Every 8 Hours, Increasing Weekly In 5 Mg Increments Until A Maximum Dose Of 60 Mg/6 Hours Was Reached. | VAS Score Decreased From 10 To 3-4 Over 4-5 Months. | Nausea And Vomiting Which Were Controlled By Haloperidol (0.3-0.5 Mg/8 Hours). Progressed To Swelling Of Face And Neck, Left Exophthalmos And Sever Pain With Color Changes In Affected Areas. Patient Continues 240mg/Day Of Ketamine Orally. |
| White A et al. | 2012 | RSD/CRPS | 16 Years | 1 | IV | Ketamine-Benzodiazepine Coma (700mg/H +35 Mg/H Midazolam For 5 Days) | NRS Decreased From 10 To 2/10. | Patient Exhibited Cognitive Changes And Psychotic Symptoms, Visual Hallucinations, Anxiety, Insomnia And Emotional Liability For 2 Days Post-Treatment. SE Were Successfully Treated With lOOmg Quetiapine. |
| Wu, CT et al. | 2006 | CRPS-1 | lYear | 1 | Epidural Catheter | 7.5 Mg Ketamine Plus 0.75 Mg Morphine In 6 Ml Bupivacaine (0.1%) Were Administered 4 Times Per Day | NRS Decreased From 9 To 5 After 3 Months But Treatment Lost Effect Towards The End. Lumbar Sympathetic Block With 1% Lidocaine 10 Ml Every Other Week. NRS Decreased To 4 After Another 4 Months Of Treatment. After Another 2 Months, NRS Decreased To 3 And Swelling Diminished. | |
| Sunder, RA et al. | 2008 | CRPS-II | 8 Weeks - 3 Months | 3 | Sympathetic Blocks + Ketamine | 10ml 0.25% Bupivacaine + 0.5 mg/Kg Ketamine For 3 Days | VAS For Spontaneous Pain Reduced From 10 To 0/10 With Ketamine. The Addition Of Ketamine To The Sympathetic Blocks Elicited Resulted In Marked Relief In The Allodynia | Light Headedness, Mild Sedation And Euphoria |
| Author | Year | Diagnosis | Pain Duration | N | Route | Dose | Results | Adverse Events/Side Effects |
| Alvarado, R. et al. | 2012 | CRPS-1 | 12 Years | 1 | IV | Anesthetic Dose And Multidisciplinary Treatments | Dramatic Resolution Of Lymphedema And Significant Improvement In Pain, Functioning And Use Of Analgesic Medications | No Reported Adverse Events |
| Becerra, L. et al. | 2009 | CRPS | 8 Years | 1 | IV | Anesthetic Dose (Uptitrated From 3mg/Kg/H To 7mg/Kg/H) For 5 Days. Midazolam Was Coadministered | VAS Decreased From 7-9/10 To 0-1 Post Treatment. There Were Changes In The Cerebral Cortex And Subcortical Regions Such As The Caudate Nucleus And Cerebellum. | No Reported Adverse Events |
| Everett, A. et al. | 2009 | CRPS-1 | 1 | Parenteral Ketamine Infusion | Continuous Peripheral Nerve Block And Parenteral Ketamine Infusion (0.1 Mg/Kg/H To 0.6mg/Kg/H) For 4 Days | Complete Resolution Of Her Pain Symptoms (Reduced From 8/10 To 0/10). Patient Was Still Pain Free 6 Months Later | No Adverse Events Were Reported. | |
| Harbut R.E. And G.E. Correll | 2002 | CRPS-1 | 9 Years | 1 | IV | Continuous Infusion Of Sub-Anesthetic Ketamine Uptitrated From 10mg/Hr To 30mg/Hr For 7 Days | VAS Score Decreased To 0/10. At 1 Week Follow-Up, Patient Was Pain Free And Continued Narcotic Taper. Clonidine Was Continued For Withdrawal Symptoms (Insomnia, Restlessness, And Diarrhea). Patient Discontinued Narcotics And Clonidine At 1 Month, And Remained Pain Free 5 Months Later And Full Functionality Was Restored. | No Adverse Events Were Reported. The Patient Was Given Clonidine For Withdrawal Symptoms (Insomnia, Restlessness, And Diarrhea) |
| Keppel Hesselink, J.M. And D.J. Kopsky | 2013 | CRPS-1 | 13 Years | 1 | Topical | Palmitoylethanolamide And Topical Ketamine 10% Cream | 50% Decrease In Pain After 1 Month Of Treatment. Also A Decrease In Swelling And Skin Discoloration And She Could Walk Independently | No Reported Adverse Events |
| Kiefer, RT et al. | 2007 | CRPS-1 | 6 Months | 1 | IV | Anesthetic Dose Of Ketamine Supplemented With Midazolam For 5 Days In Gradually Increasing Doses. (3-5mg/Kg/H) | On Day 6 Symptoms Of Edema And Discoloration Were Completely Resolved And The Patient Emerged Pain Free. Analgesic Effect Has Lasted 8 Years. Her Motor Function Has Steadily Improved With Physiotherapy | No Serious Adverse Events. The Patient Experienced Psychomimetic Side Effects (Agitation, Anxiety, And Nightmares) Which Were Managed With The Concomitant Use Of Midazolam And Resolved Within 1 Month. |
| Kishimoto, N et al. | 1995 | RSD | 6 Months | 1 | Stellate Ganglion Blocks, Continuous Epidural Block, And Continuous IV Ketamine | 1 Infusion Of Subanesthetic Ketamine For Two Hours | Burning Pain And Hyperperspiration Were Treated With SGB And CEB, And Allodynia Disappeared After Ketamine Treatment | |
| Kopsky, D.J. And J.M. Keppel Hesselink | 2011 | CRPS-1 | 3 Years | 1 | Topical | Multimodal (10% Ketamine, 5% Amitriptyline, And 50% Dimethyl Sulfoxide) Cream Applied 3 Times Daily | The Pain Decreased From 9 To 1.5/10 On The NRS, 26 To 6/45 On The SF-MPQ, And From 44 To 12 Out Of 100 On The NPSI. Quality Of Life Increased Profoundly. Pain Relief Was Sustained For At Least 5 Months With Continued Use. | No Reported Adverse Events |
| Lin, TC et al. | 1998 | RSD | 3 Months And 5 Months | 2 | Lumbar Epidural Catheter 3 Times Per Day | Subanalgesic Doses Of Ketamine (7.5mg), Morphine (0.75mg) And 6 Ml Bupivacaine 0.1%. Case#l -Every 12 Hours For 9 Days, Overall Received 11 Courses Of Treatment. Case #2 - 7 Courses Of Treatment Overall. | After Several Courses Of Treatment Over 3 And 6 Months, Pain Relief Was Achieved; Both Patients Were Able To Walk With Slight Weight Bearing And With Further Improvement In Signs And Symptoms. VAS Scores Decreased From 9 To 1/10 And 9 To 2/10 Respectively. | No Reported Adverse Events |
| Maneksha, FR | 2000 | CRPS | 5 Months | 1 | IV | 250 Mg Lidocaine Through IV, 10 Mg Ketamine Infused Slowly, A Bolus Of Fentanyl 25 Mg IV | NRS Decreased From 10/10 To 7/10 At Discharge. Weight Bearing With Crutches Now Possible. Patient Continued Treatment With Pt And Pharmacotherapy (Gabapentin And Amitriptyline) And NRS Ranging From 3/10 To 4/10. | Lidocaine Produced Minor Symptoms Of Toxicity With Perioral Tingling And Some Slurring Of Speech, But No Change In Symptoms. Ketamine Caused Considerable Sedation But No Change In Pain Level. After Fentanyl Patient Seemed To Become More Uncomfortable, Developing Severe Spasms In Her Foot. |
| Moesker, A | 2009 | CRPS | 6 Years | 1 | IV | Uptitrated To 4.8 (Mu)/Kg/Min For 5 Days | VAS Pain Score Decreased To 0, And Pain Relief Lasted 3 Weeks. Pain Relief From Second Infusion Lasted 3 Months, And A Third Treatment For As Long As 6 Months | No Serious Side Effects Occurred |
| Nama Sharanya, BS. et al. | 2010 | CRPS-1 | 8 Years | 1 | IV | Subanesthetic Ketamine (100mcg/Kg/Hr)+ Dexmedetomidine For 19 Hours + Lorazepam To Minimize Side Effects | Complete Resolution Of Her Pain And Associated Symptoms. Pain Decreased From 10/10 To 0/10 NRS Scale With Elimination Of Burning And Allodynia | No Reported Adverse Events |
| Noppers, IM et al. | 2011 | Ranged 11 Months To 12 Years | IV | Subjects Received Two 100 Hour Infusions Of Ketamine Separated By 16 Days. Infusion Of Ketamine Started At 1.2 Mg/Kg/H And Was Increased In Steps Of 0.6 Mg/Kg/H 1 3 Times A Day Until Patient Had NRS Of 0. | Reductions In Pain Levels (NRS) Were Seen In 5/6 Patients After The First Infusion, But The Trial Was Ended Prematurely Due To Adverse Events | One Patient Developed Severe Hypertension, One Patient Developed Severe Psychotropic Side Effects, And 3 Developed Elevated Hepatotoxicity. The Trial Was Stopped For These 5 Patients. | ||
| Rasmussen, J et al. | 2013 | CRPS | 2 Years | 1 | Ketamine + Dexmedetomidine Coma (Ketamine 7 mg/Kg/Hr; Dexmedetomidine 1.5m cg/Kg/Hr; Midazolam 17 mg/Hr) | 100% Resolution Of Pain Without Analgesic Or Opioid Use For At Least 45 Days Post Treatment | Severe Hallucinations And Anxiety, Were Treated With Chlordiazepoxide Taper | |
| Ricke, AK, et al. | 2011 | RSD | Unknown | 1 | IV | Ketamine Titrated From 10-20 Mg/Hour Over 5 Days | Patient Reported Relief On Day 1, Considerable Pain On Day 2 Resulting In Restarting Duloxetine And Mirtazapine, And Significant Pain Relief On Day. | Subtle Behavior Changes Were Seen In The First 6 Days, With Notable Changes And Mania-Like Symptoms On Day 7. Ketamine Therapy Was Discontinued. On Psychiatric Examination, The Patient Was Manic, Emotionally Labile, And Noted To Have Pressured Speech, Racing Thoughts, Flight Of Ideas And Paranoia. |
| Sakamoto, E et al. | 2010 | CRPS-II | Unknown | 1 | IV | Repeated Low Dose Ketamine Infusions | Ketamine Infusions Alleviated Pain, But Trophic Changes Persisted | No Reported Adverse Events |
| Shirani, P et al. | 2008 | CRPS-1 | 6 Years | 1 | IV | 1st Infusion: 50mg IV Ketamine Over 30 Minutes. | NRS Decreased From 10 To 3/10 After The First Infusion. After The Third Infusion, The Edema, Discoloration, And Temperature Of The Affected Areas Normalized. The Patient Became Pain Free With No Pain At The One-Year Follow-Up. | Hypertension (Treated With Labetalol And Hydralazine, And Given Midazolam For Next Infusions), Euphoria, And Headaches. |
| Shrikhande, AA et al. | 2011 | CRPS-1 | 6 And 8 Years | 2 | Ketamine PCA, Lumbar Nerve Blocks, Epidural Injections, | Both Patients Received Functional Independence Upon Discharge | ||
| Takahashi, H et al. | 1998 | CRPS-II | Post Trauma And Post-Operative Neuropathic Pain (15 Days Post-Op) | 1 | Epidural | Continuous Infusion 25microg/Kg/Hr For 10 Days | Complete Pain Relief Was Achieved And Lasted For 8 Months Post Treatment | Side Effects Occurred At Higher Doses (Severe Headache, Nausea, And Discomfort Lasting 24 Hours). No Side Effects At Lower Doses |
| Tateyama, H et al. | 2000 | CRPS-1 | 2 Years | 1 | Instillation | Prostaglandin El (20mug/lhour 12 Times) And Ketamine (200mg/2hours 2 Times) And Silver Spike Point Stimulation | Decrease In VAS From 6 To 2/10 | No Reported Adverse Events |
| Villanueva-Perez, VL et al. | 2007 | CRPS | 1 Month | 1 | IV + Oral | 30 Mg Of Oral Ketamine (3 Ml Of Syrup) Every 8 Hours, Increasing Weekly In 5 Mg Increments Until A Maximum Dose Of 60 Mg/6 Hours Was Reached. | VAS Score Decreased From 10 To 3-4 Over 4-5 Months. | Nausea And Vomiting Which Were Controlled By Haloperidol (0.3-0.5 Mg/8 Hours). Progressed To Swelling Of Face And Neck, Left Exophthalmos And Sever Pain With Color Changes In Affected Areas. Patient Continues 240mg/Day Of Ketamine Orally. |
| White A et al. | 2012 | RSD/CRPS | 16 Years | 1 | IV | Ketamine-Benzodiazepine Coma (700mg/H +35 Mg/H Midazolam For 5 Days) | NRS Decreased From 10 To 2/10. | Patient Exhibited Cognitive Changes And Psychotic Symptoms, Visual Hallucinations, Anxiety, Insomnia And Emotional Liability For 2 Days Post-Treatment. SE Were Successfully Treated With lOOmg Quetiapine. |
| Wu, CT et al. | 2006 | CRPS-1 | lYear | 1 | Epidural Catheter | 7.5 Mg Ketamine Plus 0.75 Mg Morphine In 6 Ml Bupivacaine (0.1%) Were Administered 4 Times Per Day | NRS Decreased From 9 To 5 After 3 Months But Treatment Lost Effect Towards The End. Lumbar Sympathetic Block With 1% Lidocaine 10 Ml Every Other Week. NRS Decreased To 4 After Another 4 Months Of Treatment. After Another 2 Months, NRS Decreased To 3 And Swelling Diminished. | |
| Sunder, RA et al. | 2008 | CRPS-II | 8 Weeks - 3 Months | 3 | Sympathetic Blocks + Ketamine | 10ml 0.25% Bupivacaine + 0.5 mg/Kg Ketamine For 3 Days | VAS For Spontaneous Pain Reduced From 10 To 0/10 With Ketamine. The Addition Of Ketamine To The Sympathetic Blocks Elicited Resulted In Marked Relief In The Allodynia | Light Headedness, Mild Sedation And Euphoria |
Evaluation of Case Reports of Ketamine for Complex Regional Pain Syndrome (CRPS)
| Author | Year | Diagnosis | Pain Duration | N | Route | Dose | Results | Adverse Events/Side Effects |
| Alvarado, R. et al. | 2012 | CRPS-1 | 12 Years | 1 | IV | Anesthetic Dose And Multidisciplinary Treatments | Dramatic Resolution Of Lymphedema And Significant Improvement In Pain, Functioning And Use Of Analgesic Medications | No Reported Adverse Events |
| Becerra, L. et al. | 2009 | CRPS | 8 Years | 1 | IV | Anesthetic Dose (Uptitrated From 3mg/Kg/H To 7mg/Kg/H) For 5 Days. Midazolam Was Coadministered | VAS Decreased From 7-9/10 To 0-1 Post Treatment. There Were Changes In The Cerebral Cortex And Subcortical Regions Such As The Caudate Nucleus And Cerebellum. | No Reported Adverse Events |
| Everett, A. et al. | 2009 | CRPS-1 | 1 | Parenteral Ketamine Infusion | Continuous Peripheral Nerve Block And Parenteral Ketamine Infusion (0.1 Mg/Kg/H To 0.6mg/Kg/H) For 4 Days | Complete Resolution Of Her Pain Symptoms (Reduced From 8/10 To 0/10). Patient Was Still Pain Free 6 Months Later | No Adverse Events Were Reported. | |
| Harbut R.E. And G.E. Correll | 2002 | CRPS-1 | 9 Years | 1 | IV | Continuous Infusion Of Sub-Anesthetic Ketamine Uptitrated From 10mg/Hr To 30mg/Hr For 7 Days | VAS Score Decreased To 0/10. At 1 Week Follow-Up, Patient Was Pain Free And Continued Narcotic Taper. Clonidine Was Continued For Withdrawal Symptoms (Insomnia, Restlessness, And Diarrhea). Patient Discontinued Narcotics And Clonidine At 1 Month, And Remained Pain Free 5 Months Later And Full Functionality Was Restored. | No Adverse Events Were Reported. The Patient Was Given Clonidine For Withdrawal Symptoms (Insomnia, Restlessness, And Diarrhea) |
| Keppel Hesselink, J.M. And D.J. Kopsky | 2013 | CRPS-1 | 13 Years | 1 | Topical | Palmitoylethanolamide And Topical Ketamine 10% Cream | 50% Decrease In Pain After 1 Month Of Treatment. Also A Decrease In Swelling And Skin Discoloration And She Could Walk Independently | No Reported Adverse Events |
| Kiefer, RT et al. | 2007 | CRPS-1 | 6 Months | 1 | IV | Anesthetic Dose Of Ketamine Supplemented With Midazolam For 5 Days In Gradually Increasing Doses. (3-5mg/Kg/H) | On Day 6 Symptoms Of Edema And Discoloration Were Completely Resolved And The Patient Emerged Pain Free. Analgesic Effect Has Lasted 8 Years. Her Motor Function Has Steadily Improved With Physiotherapy | No Serious Adverse Events. The Patient Experienced Psychomimetic Side Effects (Agitation, Anxiety, And Nightmares) Which Were Managed With The Concomitant Use Of Midazolam And Resolved Within 1 Month. |
| Kishimoto, N et al. | 1995 | RSD | 6 Months | 1 | Stellate Ganglion Blocks, Continuous Epidural Block, And Continuous IV Ketamine | 1 Infusion Of Subanesthetic Ketamine For Two Hours | Burning Pain And Hyperperspiration Were Treated With SGB And CEB, And Allodynia Disappeared After Ketamine Treatment | |
| Kopsky, D.J. And J.M. Keppel Hesselink | 2011 | CRPS-1 | 3 Years | 1 | Topical | Multimodal (10% Ketamine, 5% Amitriptyline, And 50% Dimethyl Sulfoxide) Cream Applied 3 Times Daily | The Pain Decreased From 9 To 1.5/10 On The NRS, 26 To 6/45 On The SF-MPQ, And From 44 To 12 Out Of 100 On The NPSI. Quality Of Life Increased Profoundly. Pain Relief Was Sustained For At Least 5 Months With Continued Use. | No Reported Adverse Events |
| Lin, TC et al. | 1998 | RSD | 3 Months And 5 Months | 2 | Lumbar Epidural Catheter 3 Times Per Day | Subanalgesic Doses Of Ketamine (7.5mg), Morphine (0.75mg) And 6 Ml Bupivacaine 0.1%. Case#l -Every 12 Hours For 9 Days, Overall Received 11 Courses Of Treatment. Case #2 - 7 Courses Of Treatment Overall. | After Several Courses Of Treatment Over 3 And 6 Months, Pain Relief Was Achieved; Both Patients Were Able To Walk With Slight Weight Bearing And With Further Improvement In Signs And Symptoms. VAS Scores Decreased From 9 To 1/10 And 9 To 2/10 Respectively. | No Reported Adverse Events |
| Maneksha, FR | 2000 | CRPS | 5 Months | 1 | IV | 250 Mg Lidocaine Through IV, 10 Mg Ketamine Infused Slowly, A Bolus Of Fentanyl 25 Mg IV | NRS Decreased From 10/10 To 7/10 At Discharge. Weight Bearing With Crutches Now Possible. Patient Continued Treatment With Pt And Pharmacotherapy (Gabapentin And Amitriptyline) And NRS Ranging From 3/10 To 4/10. | Lidocaine Produced Minor Symptoms Of Toxicity With Perioral Tingling And Some Slurring Of Speech, But No Change In Symptoms. Ketamine Caused Considerable Sedation But No Change In Pain Level. After Fentanyl Patient Seemed To Become More Uncomfortable, Developing Severe Spasms In Her Foot. |
| Moesker, A | 2009 | CRPS | 6 Years | 1 | IV | Uptitrated To 4.8 (Mu)/Kg/Min For 5 Days | VAS Pain Score Decreased To 0, And Pain Relief Lasted 3 Weeks. Pain Relief From Second Infusion Lasted 3 Months, And A Third Treatment For As Long As 6 Months | No Serious Side Effects Occurred |
| Nama Sharanya, BS. et al. | 2010 | CRPS-1 | 8 Years | 1 | IV | Subanesthetic Ketamine (100mcg/Kg/Hr)+ Dexmedetomidine For 19 Hours + Lorazepam To Minimize Side Effects | Complete Resolution Of Her Pain And Associated Symptoms. Pain Decreased From 10/10 To 0/10 NRS Scale With Elimination Of Burning And Allodynia | No Reported Adverse Events |
| Noppers, IM et al. | 2011 | Ranged 11 Months To 12 Years | IV | Subjects Received Two 100 Hour Infusions Of Ketamine Separated By 16 Days. Infusion Of Ketamine Started At 1.2 Mg/Kg/H And Was Increased In Steps Of 0.6 Mg/Kg/H 1 3 Times A Day Until Patient Had NRS Of 0. | Reductions In Pain Levels (NRS) Were Seen In 5/6 Patients After The First Infusion, But The Trial Was Ended Prematurely Due To Adverse Events | One Patient Developed Severe Hypertension, One Patient Developed Severe Psychotropic Side Effects, And 3 Developed Elevated Hepatotoxicity. The Trial Was Stopped For These 5 Patients. | ||
| Rasmussen, J et al. | 2013 | CRPS | 2 Years | 1 | Ketamine + Dexmedetomidine Coma (Ketamine 7 mg/Kg/Hr; Dexmedetomidine 1.5m cg/Kg/Hr; Midazolam 17 mg/Hr) | 100% Resolution Of Pain Without Analgesic Or Opioid Use For At Least 45 Days Post Treatment | Severe Hallucinations And Anxiety, Were Treated With Chlordiazepoxide Taper | |
| Ricke, AK, et al. | 2011 | RSD | Unknown | 1 | IV | Ketamine Titrated From 10-20 Mg/Hour Over 5 Days | Patient Reported Relief On Day 1, Considerable Pain On Day 2 Resulting In Restarting Duloxetine And Mirtazapine, And Significant Pain Relief On Day. | Subtle Behavior Changes Were Seen In The First 6 Days, With Notable Changes And Mania-Like Symptoms On Day 7. Ketamine Therapy Was Discontinued. On Psychiatric Examination, The Patient Was Manic, Emotionally Labile, And Noted To Have Pressured Speech, Racing Thoughts, Flight Of Ideas And Paranoia. |
| Sakamoto, E et al. | 2010 | CRPS-II | Unknown | 1 | IV | Repeated Low Dose Ketamine Infusions | Ketamine Infusions Alleviated Pain, But Trophic Changes Persisted | No Reported Adverse Events |
| Shirani, P et al. | 2008 | CRPS-1 | 6 Years | 1 | IV | 1st Infusion: 50mg IV Ketamine Over 30 Minutes. | NRS Decreased From 10 To 3/10 After The First Infusion. After The Third Infusion, The Edema, Discoloration, And Temperature Of The Affected Areas Normalized. The Patient Became Pain Free With No Pain At The One-Year Follow-Up. | Hypertension (Treated With Labetalol And Hydralazine, And Given Midazolam For Next Infusions), Euphoria, And Headaches. |
| Shrikhande, AA et al. | 2011 | CRPS-1 | 6 And 8 Years | 2 | Ketamine PCA, Lumbar Nerve Blocks, Epidural Injections, | Both Patients Received Functional Independence Upon Discharge | ||
| Takahashi, H et al. | 1998 | CRPS-II | Post Trauma And Post-Operative Neuropathic Pain (15 Days Post-Op) | 1 | Epidural | Continuous Infusion 25microg/Kg/Hr For 10 Days | Complete Pain Relief Was Achieved And Lasted For 8 Months Post Treatment | Side Effects Occurred At Higher Doses (Severe Headache, Nausea, And Discomfort Lasting 24 Hours). No Side Effects At Lower Doses |
| Tateyama, H et al. | 2000 | CRPS-1 | 2 Years | 1 | Instillation | Prostaglandin El (20mug/lhour 12 Times) And Ketamine (200mg/2hours 2 Times) And Silver Spike Point Stimulation | Decrease In VAS From 6 To 2/10 | No Reported Adverse Events |
| Villanueva-Perez, VL et al. | 2007 | CRPS | 1 Month | 1 | IV + Oral | 30 Mg Of Oral Ketamine (3 Ml Of Syrup) Every 8 Hours, Increasing Weekly In 5 Mg Increments Until A Maximum Dose Of 60 Mg/6 Hours Was Reached. | VAS Score Decreased From 10 To 3-4 Over 4-5 Months. | Nausea And Vomiting Which Were Controlled By Haloperidol (0.3-0.5 Mg/8 Hours). Progressed To Swelling Of Face And Neck, Left Exophthalmos And Sever Pain With Color Changes In Affected Areas. Patient Continues 240mg/Day Of Ketamine Orally. |
| White A et al. | 2012 | RSD/CRPS | 16 Years | 1 | IV | Ketamine-Benzodiazepine Coma (700mg/H +35 Mg/H Midazolam For 5 Days) | NRS Decreased From 10 To 2/10. | Patient Exhibited Cognitive Changes And Psychotic Symptoms, Visual Hallucinations, Anxiety, Insomnia And Emotional Liability For 2 Days Post-Treatment. SE Were Successfully Treated With lOOmg Quetiapine. |
| Wu, CT et al. | 2006 | CRPS-1 | lYear | 1 | Epidural Catheter | 7.5 Mg Ketamine Plus 0.75 Mg Morphine In 6 Ml Bupivacaine (0.1%) Were Administered 4 Times Per Day | NRS Decreased From 9 To 5 After 3 Months But Treatment Lost Effect Towards The End. Lumbar Sympathetic Block With 1% Lidocaine 10 Ml Every Other Week. NRS Decreased To 4 After Another 4 Months Of Treatment. After Another 2 Months, NRS Decreased To 3 And Swelling Diminished. | |
| Sunder, RA et al. | 2008 | CRPS-II | 8 Weeks - 3 Months | 3 | Sympathetic Blocks + Ketamine | 10ml 0.25% Bupivacaine + 0.5 mg/Kg Ketamine For 3 Days | VAS For Spontaneous Pain Reduced From 10 To 0/10 With Ketamine. The Addition Of Ketamine To The Sympathetic Blocks Elicited Resulted In Marked Relief In The Allodynia | Light Headedness, Mild Sedation And Euphoria |
| Author | Year | Diagnosis | Pain Duration | N | Route | Dose | Results | Adverse Events/Side Effects |
| Alvarado, R. et al. | 2012 | CRPS-1 | 12 Years | 1 | IV | Anesthetic Dose And Multidisciplinary Treatments | Dramatic Resolution Of Lymphedema And Significant Improvement In Pain, Functioning And Use Of Analgesic Medications | No Reported Adverse Events |
| Becerra, L. et al. | 2009 | CRPS | 8 Years | 1 | IV | Anesthetic Dose (Uptitrated From 3mg/Kg/H To 7mg/Kg/H) For 5 Days. Midazolam Was Coadministered | VAS Decreased From 7-9/10 To 0-1 Post Treatment. There Were Changes In The Cerebral Cortex And Subcortical Regions Such As The Caudate Nucleus And Cerebellum. | No Reported Adverse Events |
| Everett, A. et al. | 2009 | CRPS-1 | 1 | Parenteral Ketamine Infusion | Continuous Peripheral Nerve Block And Parenteral Ketamine Infusion (0.1 Mg/Kg/H To 0.6mg/Kg/H) For 4 Days | Complete Resolution Of Her Pain Symptoms (Reduced From 8/10 To 0/10). Patient Was Still Pain Free 6 Months Later | No Adverse Events Were Reported. | |
| Harbut R.E. And G.E. Correll | 2002 | CRPS-1 | 9 Years | 1 | IV | Continuous Infusion Of Sub-Anesthetic Ketamine Uptitrated From 10mg/Hr To 30mg/Hr For 7 Days | VAS Score Decreased To 0/10. At 1 Week Follow-Up, Patient Was Pain Free And Continued Narcotic Taper. Clonidine Was Continued For Withdrawal Symptoms (Insomnia, Restlessness, And Diarrhea). Patient Discontinued Narcotics And Clonidine At 1 Month, And Remained Pain Free 5 Months Later And Full Functionality Was Restored. | No Adverse Events Were Reported. The Patient Was Given Clonidine For Withdrawal Symptoms (Insomnia, Restlessness, And Diarrhea) |
| Keppel Hesselink, J.M. And D.J. Kopsky | 2013 | CRPS-1 | 13 Years | 1 | Topical | Palmitoylethanolamide And Topical Ketamine 10% Cream | 50% Decrease In Pain After 1 Month Of Treatment. Also A Decrease In Swelling And Skin Discoloration And She Could Walk Independently | No Reported Adverse Events |
| Kiefer, RT et al. | 2007 | CRPS-1 | 6 Months | 1 | IV | Anesthetic Dose Of Ketamine Supplemented With Midazolam For 5 Days In Gradually Increasing Doses. (3-5mg/Kg/H) | On Day 6 Symptoms Of Edema And Discoloration Were Completely Resolved And The Patient Emerged Pain Free. Analgesic Effect Has Lasted 8 Years. Her Motor Function Has Steadily Improved With Physiotherapy | No Serious Adverse Events. The Patient Experienced Psychomimetic Side Effects (Agitation, Anxiety, And Nightmares) Which Were Managed With The Concomitant Use Of Midazolam And Resolved Within 1 Month. |
| Kishimoto, N et al. | 1995 | RSD | 6 Months | 1 | Stellate Ganglion Blocks, Continuous Epidural Block, And Continuous IV Ketamine | 1 Infusion Of Subanesthetic Ketamine For Two Hours | Burning Pain And Hyperperspiration Were Treated With SGB And CEB, And Allodynia Disappeared After Ketamine Treatment | |
| Kopsky, D.J. And J.M. Keppel Hesselink | 2011 | CRPS-1 | 3 Years | 1 | Topical | Multimodal (10% Ketamine, 5% Amitriptyline, And 50% Dimethyl Sulfoxide) Cream Applied 3 Times Daily | The Pain Decreased From 9 To 1.5/10 On The NRS, 26 To 6/45 On The SF-MPQ, And From 44 To 12 Out Of 100 On The NPSI. Quality Of Life Increased Profoundly. Pain Relief Was Sustained For At Least 5 Months With Continued Use. | No Reported Adverse Events |
| Lin, TC et al. | 1998 | RSD | 3 Months And 5 Months | 2 | Lumbar Epidural Catheter 3 Times Per Day | Subanalgesic Doses Of Ketamine (7.5mg), Morphine (0.75mg) And 6 Ml Bupivacaine 0.1%. Case#l -Every 12 Hours For 9 Days, Overall Received 11 Courses Of Treatment. Case #2 - 7 Courses Of Treatment Overall. | After Several Courses Of Treatment Over 3 And 6 Months, Pain Relief Was Achieved; Both Patients Were Able To Walk With Slight Weight Bearing And With Further Improvement In Signs And Symptoms. VAS Scores Decreased From 9 To 1/10 And 9 To 2/10 Respectively. | No Reported Adverse Events |
| Maneksha, FR | 2000 | CRPS | 5 Months | 1 | IV | 250 Mg Lidocaine Through IV, 10 Mg Ketamine Infused Slowly, A Bolus Of Fentanyl 25 Mg IV | NRS Decreased From 10/10 To 7/10 At Discharge. Weight Bearing With Crutches Now Possible. Patient Continued Treatment With Pt And Pharmacotherapy (Gabapentin And Amitriptyline) And NRS Ranging From 3/10 To 4/10. | Lidocaine Produced Minor Symptoms Of Toxicity With Perioral Tingling And Some Slurring Of Speech, But No Change In Symptoms. Ketamine Caused Considerable Sedation But No Change In Pain Level. After Fentanyl Patient Seemed To Become More Uncomfortable, Developing Severe Spasms In Her Foot. |
| Moesker, A | 2009 | CRPS | 6 Years | 1 | IV | Uptitrated To 4.8 (Mu)/Kg/Min For 5 Days | VAS Pain Score Decreased To 0, And Pain Relief Lasted 3 Weeks. Pain Relief From Second Infusion Lasted 3 Months, And A Third Treatment For As Long As 6 Months | No Serious Side Effects Occurred |
| Nama Sharanya, BS. et al. | 2010 | CRPS-1 | 8 Years | 1 | IV | Subanesthetic Ketamine (100mcg/Kg/Hr)+ Dexmedetomidine For 19 Hours + Lorazepam To Minimize Side Effects | Complete Resolution Of Her Pain And Associated Symptoms. Pain Decreased From 10/10 To 0/10 NRS Scale With Elimination Of Burning And Allodynia | No Reported Adverse Events |
| Noppers, IM et al. | 2011 | Ranged 11 Months To 12 Years | IV | Subjects Received Two 100 Hour Infusions Of Ketamine Separated By 16 Days. Infusion Of Ketamine Started At 1.2 Mg/Kg/H And Was Increased In Steps Of 0.6 Mg/Kg/H 1 3 Times A Day Until Patient Had NRS Of 0. | Reductions In Pain Levels (NRS) Were Seen In 5/6 Patients After The First Infusion, But The Trial Was Ended Prematurely Due To Adverse Events | One Patient Developed Severe Hypertension, One Patient Developed Severe Psychotropic Side Effects, And 3 Developed Elevated Hepatotoxicity. The Trial Was Stopped For These 5 Patients. | ||
| Rasmussen, J et al. | 2013 | CRPS | 2 Years | 1 | Ketamine + Dexmedetomidine Coma (Ketamine 7 mg/Kg/Hr; Dexmedetomidine 1.5m cg/Kg/Hr; Midazolam 17 mg/Hr) | 100% Resolution Of Pain Without Analgesic Or Opioid Use For At Least 45 Days Post Treatment | Severe Hallucinations And Anxiety, Were Treated With Chlordiazepoxide Taper | |
| Ricke, AK, et al. | 2011 | RSD | Unknown | 1 | IV | Ketamine Titrated From 10-20 Mg/Hour Over 5 Days | Patient Reported Relief On Day 1, Considerable Pain On Day 2 Resulting In Restarting Duloxetine And Mirtazapine, And Significant Pain Relief On Day. | Subtle Behavior Changes Were Seen In The First 6 Days, With Notable Changes And Mania-Like Symptoms On Day 7. Ketamine Therapy Was Discontinued. On Psychiatric Examination, The Patient Was Manic, Emotionally Labile, And Noted To Have Pressured Speech, Racing Thoughts, Flight Of Ideas And Paranoia. |
| Sakamoto, E et al. | 2010 | CRPS-II | Unknown | 1 | IV | Repeated Low Dose Ketamine Infusions | Ketamine Infusions Alleviated Pain, But Trophic Changes Persisted | No Reported Adverse Events |
| Shirani, P et al. | 2008 | CRPS-1 | 6 Years | 1 | IV | 1st Infusion: 50mg IV Ketamine Over 30 Minutes. | NRS Decreased From 10 To 3/10 After The First Infusion. After The Third Infusion, The Edema, Discoloration, And Temperature Of The Affected Areas Normalized. The Patient Became Pain Free With No Pain At The One-Year Follow-Up. | Hypertension (Treated With Labetalol And Hydralazine, And Given Midazolam For Next Infusions), Euphoria, And Headaches. |
| Shrikhande, AA et al. | 2011 | CRPS-1 | 6 And 8 Years | 2 | Ketamine PCA, Lumbar Nerve Blocks, Epidural Injections, | Both Patients Received Functional Independence Upon Discharge | ||
| Takahashi, H et al. | 1998 | CRPS-II | Post Trauma And Post-Operative Neuropathic Pain (15 Days Post-Op) | 1 | Epidural | Continuous Infusion 25microg/Kg/Hr For 10 Days | Complete Pain Relief Was Achieved And Lasted For 8 Months Post Treatment | Side Effects Occurred At Higher Doses (Severe Headache, Nausea, And Discomfort Lasting 24 Hours). No Side Effects At Lower Doses |
| Tateyama, H et al. | 2000 | CRPS-1 | 2 Years | 1 | Instillation | Prostaglandin El (20mug/lhour 12 Times) And Ketamine (200mg/2hours 2 Times) And Silver Spike Point Stimulation | Decrease In VAS From 6 To 2/10 | No Reported Adverse Events |
| Villanueva-Perez, VL et al. | 2007 | CRPS | 1 Month | 1 | IV + Oral | 30 Mg Of Oral Ketamine (3 Ml Of Syrup) Every 8 Hours, Increasing Weekly In 5 Mg Increments Until A Maximum Dose Of 60 Mg/6 Hours Was Reached. | VAS Score Decreased From 10 To 3-4 Over 4-5 Months. | Nausea And Vomiting Which Were Controlled By Haloperidol (0.3-0.5 Mg/8 Hours). Progressed To Swelling Of Face And Neck, Left Exophthalmos And Sever Pain With Color Changes In Affected Areas. Patient Continues 240mg/Day Of Ketamine Orally. |
| White A et al. | 2012 | RSD/CRPS | 16 Years | 1 | IV | Ketamine-Benzodiazepine Coma (700mg/H +35 Mg/H Midazolam For 5 Days) | NRS Decreased From 10 To 2/10. | Patient Exhibited Cognitive Changes And Psychotic Symptoms, Visual Hallucinations, Anxiety, Insomnia And Emotional Liability For 2 Days Post-Treatment. SE Were Successfully Treated With lOOmg Quetiapine. |
| Wu, CT et al. | 2006 | CRPS-1 | lYear | 1 | Epidural Catheter | 7.5 Mg Ketamine Plus 0.75 Mg Morphine In 6 Ml Bupivacaine (0.1%) Were Administered 4 Times Per Day | NRS Decreased From 9 To 5 After 3 Months But Treatment Lost Effect Towards The End. Lumbar Sympathetic Block With 1% Lidocaine 10 Ml Every Other Week. NRS Decreased To 4 After Another 4 Months Of Treatment. After Another 2 Months, NRS Decreased To 3 And Swelling Diminished. | |
| Sunder, RA et al. | 2008 | CRPS-II | 8 Weeks - 3 Months | 3 | Sympathetic Blocks + Ketamine | 10ml 0.25% Bupivacaine + 0.5 mg/Kg Ketamine For 3 Days | VAS For Spontaneous Pain Reduced From 10 To 0/10 With Ketamine. The Addition Of Ketamine To The Sympathetic Blocks Elicited Resulted In Marked Relief In The Allodynia | Light Headedness, Mild Sedation And Euphoria |
Funding sources: The primary author's work on this publication was supported by the Reflex Sympathetic Dystrophy Syndrome Association.
Conflict of interest: Sara Connolly received payment for work on this review from the Reflex Sympathetic Dystrophy Syndrome Association. R. Norman Harden is a member of the board of directors of the Reflex Sympathetic Dystrophy Syndrome Association.