-
PDF
- Split View
-
Views
-
Cite
Cite
Chikahiro Miyake, Kuniaki Yonekura, Yoshichika Kobayashi, Akiho Yokota, Cyclic Electron Flow within PSII Functions in Intact Chloroplasts from Spinach Leaves, Plant and Cell Physiology, Volume 43, Issue 8, 15 August 2002, Pages 951–957, https://doi.org/10.1093/pcp/pcf113
Close - Share Icon Share
Abstract
Using thylakoid membranes, we previously demonstrated that accumulated electrons in the photosynthetic electron transport system induces the electron flow from the acceptor side of PSII to its donor side only in the presence of a pH gradient (ΔpH) across the thylakoid membranes. This electron flow has been referred to as cyclic electron flow within PSII (CEF-PSII) [Miyake and Yokota (2001)Plant Cell Physiol. 42: 508]. In the present study, we examined whether CEF-PSII operates in isolated intact chloroplasts from spinach leaves, by correlating the quantum yield of PSII [Φ(PSII)] with the activity of the linear electron flow [V(O2)]. The addition of the protonophore nigericin to the intact chloroplasts decreased Φ(PSII), but increased V(O2), and relative electron flux in PSII [Φ(PSII) × PFD] and V(O2) were proportional to one another. Φ(PSII) × PFD at a given V(O2) was much higher in the presence of ΔpH than that in its absence. These effects of nigericin on the relationship between Φ(PSII) × PFD and V(O2) are consistent with those previously observed in thylakoid membranes, indicating the occurrence of CEF-PSII also in intact chloroplasts. In the presence of ΔpH, CEF-PSII accounted for the excess electron flux in PSII that could not be attributed to photosynthetic linear electron flow. The activity of CEF-PSII increased with increased light intensity and almost corresponded to that of the water–water cycle (WWC), implying that CEF-PSII can dissipate excess photon energy in cooperation with WWC to protect PSII from photoinhibition under limited photosynthesis conditions.
(Received April 11, 2002; Accepted June 10, 2002)
Introduction
Electrons photoproduced in PSII of chloroplast thylakoid membranes are used as reductant for several metabolic pathways. Most of the electrons flow to the photosynthetic carbon-reduction (PCR) and photorespiratory carbon-oxidation (PCO) cycles in C3-plants. In these cycles, the reductants are driving force for the regeneration of ribulose-1,5-bisphosphate (RuBP), a substrate for RuBP carboxylase/oxygenase. Almost all of the electrons are utilized for RuBP regeneration when the rate of photosynthesis is limited by the supply of photon energy to chloroplasts. Under such conditions, electrons do not accumulate in the photosynthetic electron transport system and the system is kept in the oxidized state. However, when the rate of photosynthesis becomes saturated against light intensity or is suppressed, all of the electrons are not utilized in both the PCR and PCO cycles and the electrons accumulate in the photosynthetic electron transport system. These accumulated electrons start to flow to pathways other than the PCR and PCO cycles, in a process called alternative electron flow (AEF) (Krall and Edwards 1992).
AEF contains both O2-dependent and -independent flows (Miyake and Yokota 2000). The O2-dependent AEF is driven by the water–water cycle (WWC). In the WWC, electrons accumulated in the photosynthetic electron transport system flow to O2 via the photoreduction of O2 at PSI of the thylakoid membranes. The photoreduction of O2 produces O2–•. The O2–• is disproportionated to H2O2 and O2 by superoxide dismutase (SOD), and the H2O2 is reduced to water by ascorbate (Asc) peroxidase (APX). APX produces the primary oxidation product of Asc, monodehydroascorbate radical (MDA). The MDA is then photoreduced to Asc by the accumulated electrons at PSI. Therefore, the O2-dependent AEF driven by the WWC contains flows of electrons to both the production of O2–• and the regeneration of Asc (Asada 1999).
The O2-independent AEF observed in leaves is more active than the O2-dependent WWC (Miyake and Yokota 2000). A possible molecular mechanism for O2-independent AEF was found to be cyclic electron flow within PSII (CEF-PSII), in experiments done with thylakoid membranes (Miyake and Yokota 2001). The proposed pathway for CEF-PSII is as follows: P680 → PQH2 → Cyt b-559 → Chl z+ → P680+ (Arnon and Tang 1988, Heber et al. 1979, Meunier and Bendall 1993, Nedbal et al. 1992, Thompson and Brudvig 1988, Whitmarsh and Pakrasi 1996). For operation of CEF-PSII both ΔpH across thylakoid membranes and the reduced form of plastoquinone (PQ), PQH2 are required (Miyake and Yokota 2001). Low pH in the lumen of thylakoid membranes suppresses the transport of electrons from water to P680 in PSII (Krieger et al. 1992). This suppression is caused by a low pH-stimulated release of Ca2+ ions from the site of water oxidation in PSII and concomitant inhibition of the reaction. Limitation of the transfer of electrons from water to P680 would extend the lifetime of P680+ (Krieger et al. 1992), which can then accept electrons from PQH2 via Cyt b-559 and Chl z+ (Samson and Fork 1992, Thompson and Brudvig 1988). This sequential transfer of electrons within PSII, CEF-PSII, is thus dependent on ΔpH across thylakoid membranes. The electron flux through CEF-PSII increases with increased photosynthetic linear electron transport, which produces ΔpH across thylakoid membranes. The activity of CEF-PSII at steady state, observed with thylakoid membranes, can account for all of the O2-independent AEF observed in leaves (Miyake and Yokota 2001).
To understand the physiological function of CEF-PSII, we characterize it under conditions where intrinsic photosynthetic electron transport occurs in chloroplasts. In this study, we tried to detect the activity of CEF-PSII in intact chloroplasts under conditions that inhibit both the PCR and PCO cycles. Under these conditions, the activity of the WWC reaches its maximum (Asada 1996, Miyake and Yokota 2000). Since the WWC sustains ΔpH across thylakoid membranes, we considered the above conditions to be the best for characterizing CEF-PSII in intact chloroplasts. We measured the quantum yield of PSII and the activity of photosynthetic linear electron flow in isolated chloroplasts from spinach leaves. We found evidence for the operation of CEF-PSII in intact chloroplasts and discuss its physiological function.
Results
Effects of nigericin on Φ (PSII) and the rate of O2 photoreduction in intact chloroplasts
To detect the activity of CEF-PSII in intact chloroplasts, we did not add bicarbonate to the reaction mixture to suppress photosynthesis, because in CO2-fixing chloroplasts the photosynthetic linear electron flow driven by PCR cycle was inhibited in the absence of ΔpH and its activity cannot be correlated to Φ(PSII). As described below, we studied the effect of ΔpH on both Φ(PSII) and the activity of the linear electron flow. Under these conditions, we could not observe any light-dependent evolution of O2 (data not shown). Furthermore, glycolaldehyde, an inhibitor for photosynthesis, did not affect any of the results shown below (data not shown).
Under these conditions, the activity of the WWC reaches its maximum and sustains ΔpH across the thylakoid membranes (Asada 1999). This situation is a prerequisite for the expression of the activity of CEF-PSII, because WWC does not consume ATP (Miyake and Yokota 2001). Furthermore, the conditions we set up mimicked the stress imposed when photosynthesis is inhibited, allowing us to study how electrons produced in PSII are utilized under these conditions.
We measured Φ(PSII) in intact chloroplasts to study the effect of ΔpH on the electron flux in PSII [Je(PSII)]. Φ(PSII) [= (Fm′–Fs)/Fm′] was obtained from an analysis of the yield of Chl fluorescence (Genty et al. 1989, Schreiber et al. 1995). In intact chloroplasts, Φ(PSII) reflects Je(PSII) [Φ(PSII) = Je(PSII)/(α × PFD)] when the intensity of actinic light (PFD) and the molar ratio (α) of PSII to PSI in thylakoid membranes are constant. For example, a decrease in Φ(PSII) indicates a decrease in Je(PSII).
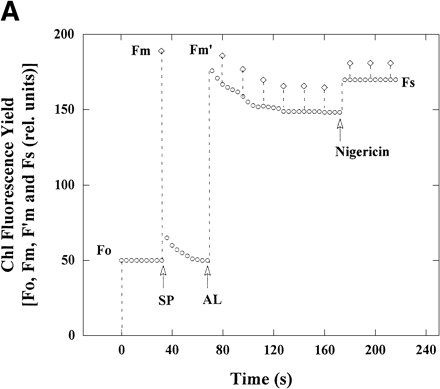
We added the protonophore nigericin to intact chloroplasts at steady state to abolish the ΔpH across the thylakoid membranes. The addition of nigericin to the intact chloroplasts at 50 µmol photon m–2 s–1 increased the yield of steady-state Chl fluorescence, Fs, with disappearance of NPQ (Fm/Fm′–1) (Fig. 1A). The disappearance of NPQ showed that the ΔpH across the thylakoid membranes was diminished (Horton and Ruban 1992). Steady state Φ(PSII) decreased from 0.10 in the absence of nigericin to 0.06 in its presence. The decrease in Φ(PSII) caused by addition of nigericin suggests that the electron flux in PSII was decreased. The decrease in Φ(PSII) was also observed on additions of 10 mM NH4Cl, 10 mM methylamine, 0.5 µM Gramicidin D and 0.5 µM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), other protonophores (data not shown).
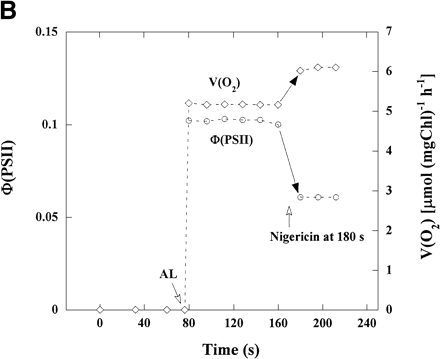
We then studied whether the addition of nigericin suppressed the activity of photosynthetic linear electron flow (Fig. 1B). As described below, the photosynthetic linear electron flow is driven mainly by WWC in intact chloroplasts that cannot photosynthesize. The rate of the linear electron flow in WWC is determined by the rate of O2 photoreduction at PSI of thylakoid membranes (Asada 2000). Thus, we measured the rate of O2 uptake due to O2 photoreduction as an indicator of the activity of photosynthetic linear electron flow. However, when WWC functions in intact chloroplasts, there is no net uptake of O2 (Asada and Badger 1984), as follows.
2H2O → O2 + 4H+ + 4e– (Evolution of O2 at PSII)
2O2 + 2e–→ 2O2–• (Photoreduction of O2 at PSI)
2O2–• + 2H+ → H2O2 + O2 (Disproportionation of O2– by SOD)
2Asc + H2O2 → 2MDA + 2H2O (Reduction of H2O2 by APX)
2MDA + 2e– + 2H+ → 2Asc (Regeneration of Asc at PSI)
Net exchange of O2 is zero.
As expected, little light-dependent oxygen exchange was observed in the present chloroplasts (data not shown).
The rate of O2 uptake by chloroplasts was, therefore, determined in the presence of KCN to inhibit the activities of CuZn-SOD and APX (Kanematsu and Asada 1978, Miyake and Asada 1992). In the presence of KCN, O2–• spontaneously disproportionates to H2O2 and O2, MDA is not produced, and then Asc is not regenerated. Under these conditions, we can observe O2 uptake by illuminated chloroplasts and determine the activity of the photosynthetic linear electron flow in WWC, as shown below.
H2O → 1/2O2 + 2H+ + 2e– (Evolution of O2 at PSII)
2O2 + 2e– → 2O2–• (Photoreduction of O2 at PSI)
2O2–• + 2H+ → H2O2 + O2 (Disproportionation of O2–)
H2O + 1/2O2 → H2O2 (Net exchange of O2)
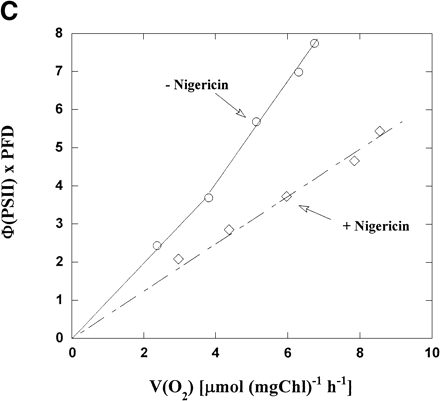
In the presence of KCN, illuminated chloroplasts showed the O2 uptake (Fig. 1B). The addition of nigericin to the reaction mixture caused an increase in the rate of O2 uptake [V(O2)] from 5.2 to 6.1 µmol (mg Chl)–1 h–1, about 1.2-fold, but a decrease in Φ(PSII), which are plotted from the data in Fig. 1A. These results show that V(O2) in the presence of nigericin, which is higher than that in its absence, is well accounted for by the electron flux in PSII shown as Φ(PSII) which is lower than that in its absence. Therefore, the electron flux in PSII in the absence of nigericin contains the excess electron flux not related to photosynthetic linear electron flow.
Dependence of both Φ(PSII) and V(O2) on light intensity in the presence or absence of nigericin
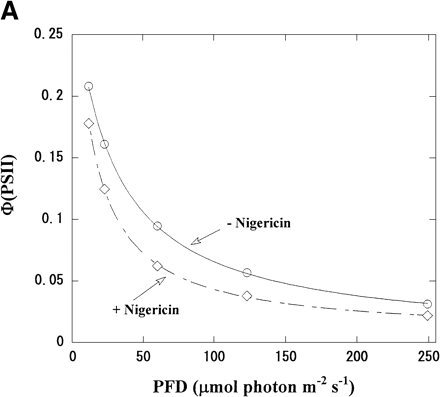
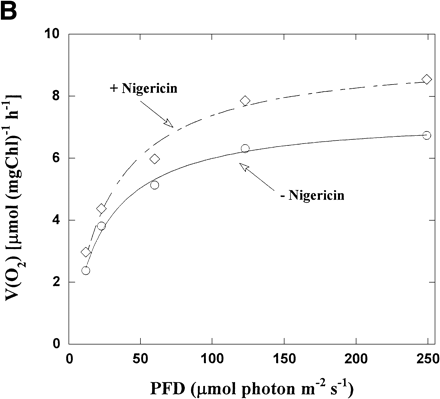
Φ(PSII) in intact chloroplasts was plotted against light intensity (Fig. 2A). Increasing the light intensity decreased Φ(PSII) from 0.21 to 0.03 in the absence of nigericin. The same relationship was seen in the presence of nigericin, but the values of Φ(PSII) were lower than those in its absence. On the contrary, V(O2) increased with increased light intensity in both the presence and absence of nigericin (Fig. 2B), but V(O2) in the presence of nigericin was larger than those in its absence. Thus, photosynthetic linear electron flow in intact chloroplasts was stimulated by nigericin at all light intensities, even though the electron flux in PSII was suppressed.
We plotted Φ(PSII) × PFD against V(O2) using the values in Fig. 2A and B. Φ(PSII) × PFD represents a relative electron flux in PSII, because α needed for the precise estimation of Je(PSII) was not determined in the present work. In the presence of nigericin, the plot of Φ(PSII) × PFD vs. V(O2) generated a straight line with a positive slope. Although the same relationship between Φ(PSII) × PFD and V(O2) was seen in the absence of nigericin, the slope was steeper and the Φ(PSII) × PFD at any given V(O2) was higher than that in the presence of nigericin (Fig. 2C). For example, Φ(PSII) × PFD was 7 when V(O2) was 6 µmol (mg Chl)–1 h–1, about two times higher than in the presence of nigericin. The deviation, observed in the absence of nigericin, from the linear relationship observed in its presence was also seen in the previous work with isolated thylakoid membranes (Miyake and Yokota 2001), which is attributed to the cyclic electron flow within PSII (CEF-PSII) producing excess electron flux against the photosynthetic linear electron flow. Thus, in the presence of ΔpH CEF-PSII functioned also in the intact chloroplasts at all the light intensities tested.
Estimation of the relative electron fluxes in PSII attributable to both WWC and CEF-PSII in intact chloroplasts
We estimated the relative electron fluxes in PSII attributable to WWC and CEF-PSII, as follows. The quantum yield of PSII at any light intensities in the presence of ΔpH, Φ(PSII)(+ΔpH), includes the electron fluxes in both WWC and CEF-PSII, Φ(PSII)(+ΔpH, WWC) and Φ(PSII)(+ΔpH, CEF-PSII) respectively. Therefore, Φ(PSII)(+ΔpH) is represented by equation (1).
Φ(PSII)(+ΔpH) = Φ(PSII)(+ΔpH, WWC) + Φ(PSII)(+ΔpH, CEF-PSII) (1)
In the absence of ΔpH, CEF-PSII is suppressed and all the electrons photoproduced in PSII flow to WWC. Then, the quantum yield of PSII, Φ(PSII)(–ΔpH), is represented by equation (2).
Φ(PSII)(–ΔpH) = Φ(PSII)(–ΔpH, WWC) (2)
Φ(PSII)(–ΔpH, WWC) is Φ(PSII) reflecting the electron flux in WWC in the absence of ΔpH. The rate of O2 photoreduction, V(O2), determines the electron flux in WWC, then a proportional relationship between WWC-dependent Φ(PSII) and V(O2) in both the presence and absence of ΔpH holds, as represented by equation (3).
Φ(PSII)(+ΔpH, WWC)/Φ(PSII)(–ΔpH, WWC) = V(O2)(+ΔpH)/V(O2)(–ΔpH) (3)
where V(O2)(+ΔpH) and V(O2)(–ΔpH) are the rates of O2 photoreduction in illuminated intact chloroplasts in the presence and absence of ΔpH, respectively. From equations (2) and (3), Φ(PSII)(+ΔpH, WWC) is derived as equation (4).
Φ(PSII)(+ΔpH, WWC) = V(O2)(+ΔpH)/V(O2)(–ΔpH) × Φ(PSII)(–ΔpH, WWC) = V(O2)(+ΔpH)/V(O2)(–ΔpH) × Φ(PSII)(–ΔpH) (4)
Furthermore, from equations (1) and (4), Φ(PSII)(+ΔpH, CEF-PSII) is derived as equation (5).
Φ(PSII)(+ΔpH, CEF-PSII) = Φ(PSII)(+ΔpH) – Φ(PSII)(+ΔpH, WWC) (5)
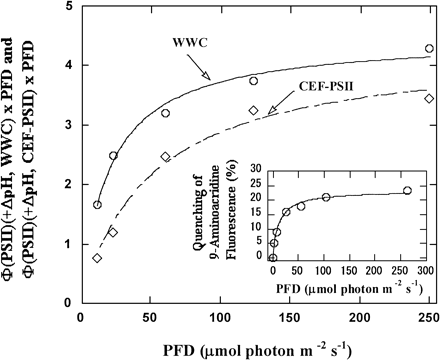
Therefore, the quantum yields of PSII reflecting both WWC and CEF-PSII in the presence of ΔpH can be calculated from four observable parameters: V(O2)(+ΔpH), V(O2)(–ΔpH), Φ(PSII)(–ΔpH), and Φ(PSII)(+ΔpH). We calculated and plotted the relative electron fluxes of both WWC and CEF-PSII, Φ(PSII)(+ΔpH, WWC) × PFD and Φ(PSII)(+ΔpH, CEF-PSII) × PFD, against light intensity, using the data in Fig. 2A and B (Fig. 3).
The relative electron fluxes of both WWC and CEF-PSII increased with increasing light intensity and saturated at about 100 µmol photon m–2 s–1 (Fig. 3). The quenching of 9-aminoacridine fluorescence also increased (Fig. 3). This shows the increase in ΔpH across thylakoid membranes. The dependence of the formation of ΔpH on light intensity corresponded well to the dependence of the relative electron flux of WWC. This suggests that WWC contributed to the formation of ΔpH. In the range of light intensities we used, the relative electron flux of CEF-PSII was lower than that of WWC. The ratio of CEF-PSII to WWC increased as light intensity increased. The relative electron flux of CEF-PSII was about 50% that of WWC at 10 µmol photon m–2 s–1 and about 80% at 250 µmol photon m–2 s–1. The affinity of CEF-PSII for photon was lower than that of WWC. The half-saturating light intensity was about 40 µmol photon m–2 s–1 for CEF-PSII, and about 10 µmol photon m–2 s–1for WWC.
Effects of oxygen on Φ(PSII) in intact chloroplasts
In the present work, we set up conditions where intact chloroplasts cannot photosynthesize and assumed that the photosynthetic linear electron flow in this situation is driven mainly by the WWC. We removed oxygen from the reaction mixture using the glucose/glucose oxidase/catalase system as described in Materials and Methods. Under these anaerobic conditions, Fs and even Fo approached Fm (data not shown). The values of Φ(PSII) were below the limit of detection, indicating little electron flux in PSII. The quenching of 9-aminoacridine fluorescence was also inhibited (data not shown), indicating that ΔpH across thylakoid membranes was not formed in the absence of oxygen. Furthermore, no effect of nigericin on the yield of the Chl fluorescence was observed (data not shown). These results showed that WWC is the main driver of photosynthetic linear electron flow in intact chloroplasts that cannot photosynthesize.
Discussion
Identification of cyclic electron flow within PSII in intact chloroplasts
In the present study, we examined whether cyclic electron flow within PSII functions in intact chloroplasts that cannot fix CO2. A ΔpH across the thylakoid membranes is required for CEF-PSII activity (Miyake and Yokota 2001). Nigericin, a protonophore, lowered the electron flux in PSII, but enhanced the linear electron flow in intact chloroplasts as determined by the photoreduction of O2 (Fig. 1B). In the absence of ΔpH, Φ(PSII) was directly proportional to V(O2). Although the same relationship was seen in the presence of ΔpH, Φ(PSII) at any given V(O2) was always larger in the presence of ΔpH than in its absence (Fig. 2C). These results were consistent with those observed in thylakoid membranes (Miyake and Yokota 2001). That is, in the presence of ΔpH, electron flow in PSII that is not part of the photosynthetic linear electron flow, namely CEF-PSII, occurs in intact chloroplasts. In CO2-fixing chloroplasts, we could not estimate the activity of CEF-PSII. Further development of the method to detect its activity is required for the characterization of CEF-PSII in photosynthesizing chloroplasts.
It is unlikely that there is another electron flow system, different from CEF-PSII, that produces the excess electron flux in PSII observed in the presence of ΔpH (Fig. 2C). The Φ(PSII) observed in Fig. 1A was almost constant during illumination with actinic light for at least 5 min in the presence of ΔpH (data not shown). This result means that the electron flux in PSII was constant. If there were another electron acceptor at PSI to produce the excess electron flux in PSII against the photosynthetic linear electron flow that was present in intact chloroplasts, the concentration of this electron acceptor should be larger than 300 mM in stroma, which was estimated from the electron flux in WWC. At 250 µmol photon m–2 s–1, the photoreduction rate of O2 was 8.5 µmol O2 (mg Chl)–1 h–1 (Fig. 2B). During 5 min, electron of 2.8 µmol e– (mg Chl)–1 from PSII was consumed for the O2-photoreduction. Thus, electron of 2×2.8 µmol e– (mg Chl)–1 flowed to WWC, as judged from its stoichiometry. The excess electron flux against the photosynthetic linear electron flow almost corresponded to WWC at 250 µmol photon m–2 s–1. This means that an electron acceptor should be present at the concentration of 280 mM as electron equivalent in stroma of chloroplasts, where the volume of stroma was assumed to be 20 µl (mg Chl)–1. Further, the electron acceptor should give Vmax, because its flux was constant during 5 min. That is, its concentration would be very high; more than 280 mM. Such an electron acceptor is unlikely to be present in stroma. Furthermore, if the electron acceptor accepted electrons at PSI, Φ(PSII) should be increased by the addition of nigericin, similar to the rate of O2 photoreduction. But Φ(PSII) was decreased by the addition of nigericin (Fig. 1, 2). Finally, no electron flux in PSII was detected under anaerobic conditions. If another electron acceptor different from O2 were present in intact chloroplasts, the electron flux in PSII driven by this acceptor should not depend on the presence of O2 and should be observable even under anaerobic conditions. In sum, the excess electron flux in PSII observed in this work largely depended on the activity of WWC and can be accounted for by the continuous electron flow system, CEF-PSII. The ΔpH produced and sustained by WWC would satisfy the conditions for operation of CEF-PSII.
Physiological function of cyclic electron flow within PSII of thylakoid membranes
The activity of CEF-PSII increased and had almost the same activity as WWC at high light intensity under conditions that suppressed photosynthesis (Fig. 3). That is, the electrons photoproduced in PSII were consumed by both WWC and CEF-PSII. This implies that CEF-PSII could dissipate excess photon energy in cooperation with WWC. WWC mainly drives photosynthetic linear electron flow and protects PSII from photoinhibition under conditions that limit photosynthesis, for example, drought, chilling, high temperature, high light, or low CO2 (Asada 2000, Biehler and Fock 1996, Cheeseman et al. 1997, Fryer et al. 1998, Lovelock and Winter 1996, Miyake and Yokota 2000, Park et al. 1996). When WWC cannot work, the reaction center chlorophyll (P680) excited in PSII cannot donate electrons to QA and QB via pheophytine because the shortage of electron acceptors for photosynthetic linear electron flow leads to over-reduction of QA and QB. As a result, excited P680 accumulates in PSII and deactivates to its triplet state, 3P680*. 3P680* easily transfers its excitation energy to O2, producing singlet oxygen, 1O2 (Hideg et al. 1994a, Hideg et al. 1994b, Macpherson et al. 1993, Mishra et al. 1994, Miyao 1994). 1O2 oxidizes amino acid residues in the D1 protein, triggering its proteolytic degradation and leading to photoinhibition of PSII. Like WWC, CEF-PSII would stimulate electron transfer from P680 to PQ by cycling electrons within PSII. The excess energy accumulated in P680 would be safely dissipated as heat by the sequential oxidoreductions in CEF-PSII.
Materials and Methods
Isolation of intact chloroplasts
Intact chloroplasts were isolated from spinach leaves, obtained from a local market, and purified by Percoll density gradient centrifugation as described previously (Asada et al. 1990). Isolated chloroplasts were suspended in a reaction buffer (50 mM HEPES-KOH, pH 7.6, 0.33 M sorbitol, 10 mM NaCl, 1 mM MgCl2, 2 mM EDTA and 0.5 mM KH2PO4) and used for experiments as the intact chloroplast preparation. The Percoll-purified chloroplasts were about 90–95% intact, as determined by the ferricyanide method (Heber and Santarius 1970). Concentrations of Chl were determined as described by Arnon (1949).
Measurement of oxygen uptake
Oxygen uptake was monitored with an oxygen electrode (Hansatech, King’s Lynn, U.K.). After incubation in darkness for 5 min under air-equilibrated conditions, the reaction mixture (2 ml) was illuminated with an iodine lamp at the indicated light intensities at 25°C.
Measurements of Chl fluorescence
The Chl fluorescence originating from PSII in intact chloroplasts was measured with a Chl fluorometer (PAM-101; Walz, Effeltrich, Germany). The steady-state fluorescence yield (Fs) was monitored at the indicated light intensities, and 900-ms pulses of saturating light (PFD, 5,000 µmol m–2 s–1) were supplied at intervals of 15 s for determination of maximum variable fluorescence (F′m). The protocol for measuring Chl fluorescence was similar to that described by Genty et al. (1989). The terminology used in this study of Chl fluorescence was that recommended by van Kooten and Snel (1990). The relative quantum yield of PSII [Φ(PSII)] at a steady state was defined as (F′m–Fs)/F′m, as proposed by Genty et al. (1989). The non-photochemical quenching coefficient (NPQ) was defined as Fm/Fm′–1. Anaerobic conditions were maintained by the addition of 10 mM glucose, 1 µM catalase, and 2 µM glucose oxidase to the reaction mixture with KCN omitted.
Measurement of quenching of 9-aminoacridine fluorescence
Fluorescence of 9-aminoacridine was measured with a Xe-PAM (Walz, Effeltrich, Germany), as described by Miyake et al. (1995). Quenching of 9-aminoacridine fluorescence represents the formation of ΔpH across thylakoid membranes (Heber et al. 1978) and is shown by percent of the fluorescence yield without actinic light.
Acknowledgments
The authors are grateful to Dr. K. Asada (Fukuyama University) both for his encouragement throughout the course of this study and for his careful reading of the original manuscript, and Dr. J. Mano (Yamaguchi University), Dr. T. Endo (Kyoto University) and Dr. A. Makino (Tohoku University) for their critical and stimulating discussions. This study was supported by the “Research for the Future” program (JSPS-00L01604) of the Japan Society for the Promotion of Science and by Grants-in-Aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan (13740458) to C.M.
Corresponding author: E-mail, cmiyake@brs.kyushu-u.ac.jp; Fax, +81-92-642-4424.
Fig. 1 The effect of nigericin on Chl fluorescence yield, Φ(PSII) and V(O2) in intact chloroplasts. (A) The reaction mixture (2 ml) contained 50 mM HEPES-KOH (pH 7.6), 0.33 M sorbitol, 10 mM NaCl, 1 mM MgCl2, 2 mM EDTA, 0.5 mM KH2PO4 and intact chloroplasts (28 µg Chl). Chl fluorescence yield was monitored with a weak, modulated measuring light. Measuring light was turned on at 0 s. The maximum yield of Chl fluorescence (diamonds) in the dark (Fm) was induced by 900 ms-pulses of saturating white light (SP). Illumination with continuous actinic red light (AL, >640 nm) at 50 µmol photons m–2 s–1 was started at the time shown by the AL arrow. Fm after illumination (Fm′, diamonds) was induced by SP at intervals of 15 s. Yield of steady-state Chl fluorescence (Fs) is shown as circles. Nigericin (0.67 µM) was added at 180 s after the start of illumination with measuring light. (B) Φ(PSII) was calculated from the data in (A). V(O2) in intact chloroplasts was determined under the same conditions as in (A), using separate samples from those used for measuring Chl fluorescence but in the presence of 0.1 mM KCN. Φ(PSII) and V(O2) were plotted against time after the start of illumination with measuring light. AL was turned on and nigericin was added at the times indicated.
Fig. 2 Dependence of both Φ(PSII) and V(O2) on light intensity in the presence or absence of nigericin. (A) The reaction mixture was the same as that described in the legend to Fig. 1. Φ(PSII) was determined under several light intensities in the presence (diamonds) and absence (circles) of nigericin, and plotted against light intensity. (B) V(O2) in intact chloroplasts was determined, as described in Fig. 1, in the presence (diamonds) and absence (circles) of nigericin under several light intensities, and plotted against light intensity. (C) Φ(PSII) × PFD and V(O2) were determined from the data in (A) and (B), and Φ(PSII) × PFD was plotted against V(O2). Diamonds, in the presence of nigericin; circles, in its absence.
Fig. 3 Dependence of the relative electron fluxes in PSII attributable to WWC and CEF-PSII on light intensity. The relative electron fluxes in PSII attributable to WWC and CEF-PSII, Φ(PSII)(+ΔpH, WWC) × PFD and Φ(PSII)(+ΔpH, CEF-PSII) × PFD, were calculated from the data in Fig. 2, as described in the text, and plotted against light intensity. Circles, Φ(PSII)(+ΔpH, WWC) × PFD; Diamonds, Φ(PSII) (+ΔpH, CEF-PSII) × PFD. The insert shows the dependence of the formation of ΔpH across thylakoid membranes on light intensity. The reaction mixture was the same as in Fig. 1A, except for the addition of 9-aminoacridine of 2 µM. Fluorescence of 9-aminoacridine was measured with excitation by xenon flashes at 16 Hz, and formation of ΔpH by actinic red light with several intensities was determined from its quenching.
Abbreviations
- AEF
alternative electron flow
- α
the molar ratio of PSII to PSI in thylakoid membranes
- APX
Asc peroxidase
- Asc
ascorbate
- CEF-PSII
cyclic electron flow within PSII
- ΔpH
a pH gradient across thylakoid membranes
- Φ(PSII)
the quantum yield of electron transport in PSII at steady state, defined as (F′m–Fs)/F′m
- Fm
maximal yield of Chl fluorescence after dark adaptation
- Fm′
Fm after illumination
- Fo
minimal Chl fluorescence after dark adaptation
- Fs
yield of steady-state Chl fluorescence
- Je(PSII)
the electron flux in PSII
- MDA
monodehydroascorbate radical
- NPQ
non-photochemical quenching coefficient of Chl fluorescence, defined as Fm/Fm′–1
- PCO cycle
photorespiratory carbon-oxidation cycle
- PCR cycle
photosynthetic carbon-reduction cycle
- PFD
photosynthetically active photon flux density
- PQ
plastoquinone
- PQH2
plastoquinol
- SOD
superoxide dismutase
- V(O2)
the rate of O2 uptake
- WWC
the water–water cycle.
References
Arnon, D.I. (
Arnon, D.I. and Tang, G.M.S. (
Asada, K. (
Asada, K. (
Asada, K. (
Asada, K. and Badger, M.R. (
Asada, K., Neubauer, C., Heber, U. and Schreiber, U. (
Biehler, K. and Fock, H. (
Cheeseman, J.M., Herendeen, L.B., Cheeseman, A.T. and Clough, B.F. (
Fryer, M.J., Andrews, J.R., Oxborough, K., Blowers, D.A. and Baker, N.R. (
Genty, B., Briantais, J.M. and Baker, N.R. (
Heber, U., Kirk, M.R. and Boardman, N.K. (
Heber, U. and Santarius, K.A. (
Heber, U., Egneus, H., Hank, U., Jensen, M. and Köster, S. (
Hideg, E., Spetea, C. and Vass, I. (
Hideg, E., Spetea, C. and Vass, I. (
Kanematsu, S. and Asada, K. (
Krall, J.P. and Edwards, G.E. (
Krieger, A., Moya, I. and Weis, E. (
Lovelock, C.E. and Winter, K. (
Macpherson, A.N., Telfer, A., Barber, J. and Truscott, T.G. (
Meunier, P.C. and Bendall, D.S. (
Mishra, N.P., Francke, C., Van Grokom, H.J. and Ghanotakis, D.F. (
Miyake, C. and Asada, K. (
Miyake, C., Schreiber, U. and Asada, K. (
Miyake, C. and Yokota, A. (
Miyake, C. and Yokota, A. (
Miyao, M. (
Nedbal, L., Samson, G. and Whitmarsh, J. (
Park, Y.I., Anderson, J.M. and Chow, W.S. (
Samson, G. and Fork, D.C. (
Schreiber, U., Hormann, H., Neubauer, C. and Klughammer, C. (
Thompson, L.K. and Brudvig, G.W. (
van Kooten, O. and Snel, J.F.H. (