-
PDF
- Split View
-
Views
-
Cite
Cite
Xiu-Qing Li, Dapeng Zhang, Gene Expression Activity and Pathway Selection for Sucrose Metabolism in Developing Storage Root of Sweet Potato, Plant and Cell Physiology, Volume 44, Issue 6, 15 June 2003, Pages 630–636, https://doi.org/10.1093/pcp/pcg080
Close - Share Icon Share
Abstract
Development of sweet potato (Ipomoea batatas) storage root coincides with starch accumulation made using cleaved products of imported photoassimilate sucrose. The genes and pathways are predominantly active for sucrose metabolism in developing storage root were unknown. In this study, we used both an expressed sequence tag (EST) approach and a reverse transcription–polymerase chain reaction (RT-PCR) approach to answer this question. Sucrose synthase (SuSy) was found to be significantly more frequent in storage root ESTs than in fibrous root ESTs. SuSy was the most abundant carbohydrate-metabolism gene in the storage-root ESTs. RT-PCR results confirmed this by showing that invertase was active in fibrous roots but rapidly decreased to an undetectable level during storage root development while SuSy became predominant. Invertase expression was also detectable in young immature storage root and shoot tips, suggesting an involvement in cell formation. SuSy expression pattern showed considerable similarity to that of ADP-glucose pyrophosphorylase, an essential enzyme for starch synthesis. The results indicated that (i) SuSy was the most actively expressed enzyme in sucrose metabolism in developing storage root and was correlated with sink strength, and (ii) whereas invertase was active at cell formation stages, SuSy pathway was predominant for sucrose cleavage related to starch-accumulation.
(Received February 25, 2003; Accepted April 15, 2003)
Introduction
Sweet potato is the seventh most important food crop in the world, and has great potential in terms of the contribution it could make to the improvement of food security in developing countries. The content of dry matter, mainly starch, in the storage roots varies greatly in sweet potato germplasm, ranging from 18 to 42%. Starch in the plant storage organs was synthesized using cleaved products of sucrose that is imported as the main photoassimilate from photosynthetic organs. Genes that affect sucrose cleavage and related metabolisms may play a central role in the control of storage root development, storage sink strength, and photosynthate partitioning.
Extensive knowledge of carbohydrate metabolism, at the molecular biology level, has been obtained by scientific investigation using the potato as a model plant (Fernie and Willmitzer 2001, Fernie et al. 2002). A potato tuber is a modified stem, while the sweet potato storage organ is a modified root. However, sweet potato and potato are both starch accumulating crops. Their starch storage organs are of relatively large volume, non-photosynthetic, and undesiccated. The documentation of information concerned with carbohydrate-metabolism gene expression in both potato and sweet potato may provide insights into both common and specific mechanisms of the carbohydrate metabolism of root and tuber plants.
There are two possible pathways for cleaving sucrose in the cytosol (Fernie et al. 2002). One is the conversion of sucrose to glucose and fructose by invertase; the glucose is then converted to phosphorylated glucose by hexokinase. The second possible pathway is the conversion of sucrose to UDP-glucose and fructose by sucrose synthase (SuSy). The UDP-glucose formed is then converted to glucose-1-phosphate by UDP-glucose pyrophosphorylase (UGPase), for use in subsequent reactions related to starch synthesis. SuSy gene activity was reported to be correlated with sink strength in tomato fruit (Wang et al. 1993). It is the most active of the genes involved in carbohydrate metabolism in immature potato tubers (Li 2001).
Invertase was found to play also an important role in cleaving sucrose in sweet potato storage roots when freshly harvested (Takahata et al. 1996) and during storage at different temperatures (Huang et al. 1999). However, high enzymatic activities of SuSy (Saitou et al. 1997, Yatomi et al. 1996) and transcriptional expression of SuSy (Saitou et al. 1997) was observed in sweet potato storage root. Since both studies (Saitou et al. 1997, Yatomi et al. 1996) concerning SuSy studied only a single stage of the storage root, strong activity of SuSy could be interpreted in two ways: (i) associated with sink strength, or (ii) caused by cell expansion or other functions of the storage root but may not necessarily be correlated to sink strength during storage root development. This second possibility cannot be eliminated without analyzing the SuSy gene expression at different stages of storage root development.
Although both invertase and SuSy have been found to be active in sweet potato suspension cells (Wang et al. 2000), to the best of our knowledge, so far there are no reports comparing the invertase pathway and SuSy pathway for the storage roots either at single stage or different stages. There are no reports on SuSy gene sequence of sweet potato storage roots despite the release of two expressed sequence tags (ESTs) (BM878716, BM878759) from in vitro plantlets. Therefore, there is no evidence to date for whether the invertase pathway or the SuSy-UGPase pathway is the primary one for the rapid accumulation of starch in sweet potato storage roots. To our knowledge no study exists on the overall evaluation of gene expression of sucrose metabolism in sweet potato storage root.
EST frequency, starting with 515 ESTs, was successfully used in profiling the genes with enhanced expression in specific tissues of either humans or plants; for example, for human brain cells (Adams et al. 1992), guard cells of Brassica campestris (Kwak et al. 1997), human prostate cells (Vasmatzis et al. 1998), petunia petal protoplast cells (Yu et al. 1999), inner bark of Cryptomeria japonica (Ujino et al. 2000), mature whole tuber of potato cultivar Kuras (Crookshanks et al. 2001), and rose petals (Channeliere et al. 2002). The efficiency of either cDNA synthesis and or the transformation of E. coli was not found to significantly change the EST frequency in these reports. This might be because the length of the majority of cDNAs is far within the limited size for transforming E. coli and that a non full-length cDNA would still represent a transcript molecule as long as the short cDNA fraction is not manually dropped off before ligating the cDNAs into phagemids.
In the current study on sweet potato we used both a random-EST approach and a reverse transcription–polymerase chain reaction RT-PCR approach: (i) to develop an overview of the relative activity of the sucrose metabolism genes at a rapid enlargement stage in storage roots of a high dry matter variety; (ii) to determine whether the most active gene’s expression is correlated with starch accumulation and (iii) to determine the major pathway for cleaving sucrose to phosphoglucoses at the stage at which rapid accumulation of starch occurs.
Results
Root morphology and dry matter determination
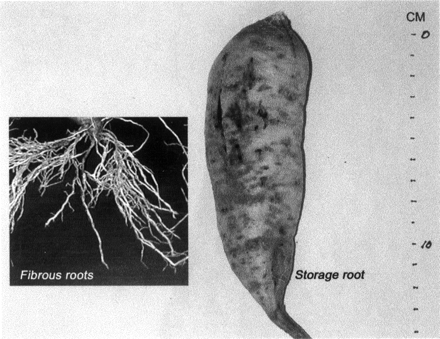
Fig. 1 shows the morphology of both the fibrous roots and the immature storage root of the high dry matter genotype Kyukei-63 used in the cDNA library construction. The enlarged area of the immature storage root was 13.5 cm, about one third the volume of the mature tubers. This medium-sized stage is the stage at which the rapid accumulation of starch and strong sink strength are expected. The dry matter content of the immature storage roots was 33.25% (w/w) at the stage at which RNA preparation was undertaken. Both leaves and roots were vigorous at the time of RNA preparation. The plant showed no obvious sign of stress.
EST frequency
Out of 1,002 EST sequences, 500 ESTs were from fibrous roots (Fig. 1 left panel) and 502 from storage roots at the enlargement stage (Fig. 1 right panel). EST frequency of sucrose metabolism genes is shown in Table 1. The size, the GenBank accessory numbers and the groups of the ESTs are listed in Table 2.
EST-based estimation of invertase gene expression
There were no ESTs homologous to invertase genes, hexokinase, or glucose phosphorylase genes. This indicates that the invertase pathway was not very active in either the fibrous roots or the immature storage roots for the stage as shown in Fig. 1. Therefore, invertase was unlikely to be the major player in cleaving sucrose for starch synthesis at the storage-root stage studied.
EST-based estimation of sucrose synthesis-related genes
Genes involved in the synthesis of sucrose, such as sucrose phosphate synthase and sucrose phosphate phosphatase, were not observed in this first set of ESTs. This suggests that roots do not need to synthesize much sucrose because sucrose is mainly transported from the plant’s leaves. Several other enzymes, such as fructokinase and phosphohexoseisomerase, which are thought to be essential enzymes for the cells, were not observed. This was not surprising since, statistically, the first sets of ESTs can only pick up those extremely active genes that have many more copies of transcripts than do those genes with an average level of expression activity. The absence of ESTs for these enzymes in the first sets may mean that the genes were not as active as the observed ones.
EST-based estimation of sucrose synthase gene expression
SuSy was extremely active in the storage roots studied and was active in the fibrous roots studied. Six ESTs from the immature storage roots and one EST from the fibrous roots were highly homologous with the potato SuSy gene (gb|M18745). The nucleotide sequence identity between ESTs with the potato sucrose gene was found to range from 82 to 88%.
One EST of UGPase was observed in storage roots; none was observed in fibrous roots. Although the presence of only one EST does not necessarily mean that this gene was more active than those genes that did not show up in the first sets of ESTs, its presence supports the possibility of a strong expression of the SuSy-UGPase pathway in the storage roots of sweet potato.
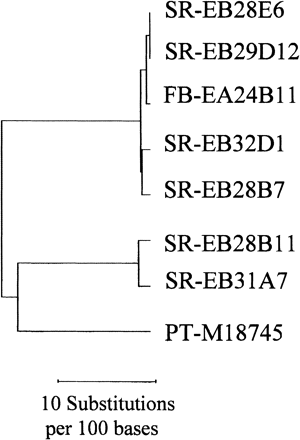
The seven SuSy ESTs formed two groups on the phylogenetic tree (Fig. 2). Five of the ESTs formed the first group, and the remaining two formed the second. It is unknown at present whether the two groups of SuSy genes in sweet potato correspond to two different loci. Each group may represent a locus, because of the divergence that exists between the two groups and because of the high degree of identity in the coding regions within each group. It is known that potato SuSy genes are mapped on two chromosomes in potato (Chen et al. 2001).
The coding regions of the SuSy ESTs in the present report are nearly identical between members of each group, suggesting that the nucleotide sequences are of a high quality.
The fact that six ESTs for sucrose synthase and none for invertase were identified in these 1002 ESTs suggests that SuSy pathway was the predominant pathway in cleaving imported sucrose in the developing storage roots studied.
RT-PCR analysis
RT-PCR approach was used to verify the key results learned from the EST-based study and to extend the analysis from the stage of enlarging storage tuber to different stages of storage root development. The fibrous root, young shoot, and leaf of the same plants were also used in the comparison. Four pairs of primers were designed and are presented in Table 3. One pair was specific to SuSy Group 1, one pair to SuSy Group 2, one pair to the large subunit of AGPase, and one pair for acid invertase.
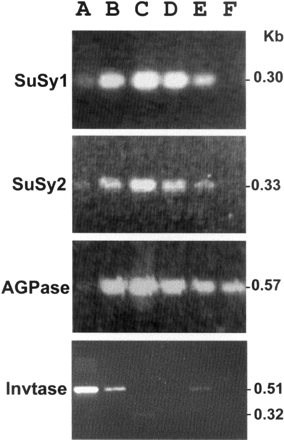
The RT-PCR analysis results are presented in Fig. 3. RT-PCR reactions for all the four genes produced RT-PCR products at expected size. The overall expression patterns of the two genes showed significant similarity. For both AGPase and the two groups of SuSy, the strongest expression occurred in the medium-sized immature storage root. Expression was very low in fibrous roots, while relatively good expression occurred in tiny, young shoots. The main difference occurred with regard to expression in leaves: the RT-PCR analysis detected relatively active expression in the case of AGPase but not in the case of the SuSy genes studied. The results indicated that both Group 1 and Group 2 of SuSy demonstrated enhanced expression in storage root and were most active at the enlarging stage of the immature storage root at which rapid accumulation of starch is expected.
Invertase showed an expression pattern basically opposite to those of SuSy and AGPase (bottom panel of Fig. 3). Invertase showed the strongest expression in fibrous root and rapidly reduced to an undetectable level during storage root development. Very young shoot tips of aboveground plants also showed detectable expression activity of the invertase gene. A RT-PCR product of 0.32 kb, much smaller than the expected size, was slightly visible in the medium-sized immature storage roots. Presently we do not know what this could mean. In general, the invertase activity appears to be associated with cell formation but negatively correlated to sink strength.
Discussion
The present report provides the first root DNA sequence for the regions covered by the ESTs of different alleles of SuSy genes in sweet potato. The conserved regions of SuSy have also been isolated by PCR amplification followed by partial sequencing, but the sequence information was not released (Saitou et al. 1997). The partial cloning of UGPase in the present study is the first reported in sweet potato. The sequence information provided here would be useful for further genetic mapping, cloning, and characterization of the genes.
The data obtained in the present study on gene EST frequency clearly indicated that SuSy is the most active gene at the enlarging, starch-accumulating stage of sweet potato storage roots. The RT-PCR analysis added information about the expression activity changes in different stage of roots and different organs. However, we should point out that SuSy may not necessarily be the most active genes at the initiation and maturation stages of sweet potato storage roots, for which we do not have EST information. For the enlarging stage of storage root, SuSy gene expression activity was significantly higher than that of any other enzyme in carbohydrate metabolism. The molecular mechanism behind this phenomenon requires further investigation.
Although transcript analysis such as EST and RT-PCR gives direct information only at transcriptional level, it does not have the common artifacts associated with the enzymatic difference between in vivo and in vitro such as the artifact indicated by Florova et al. (2002) for the enzymatic activity testing protocols of polyketide synthase.
The reason for using RT-PCR approach, rather than Northern blotting, to verify the EST results is that RT-PCR is considerably more sensitive than Northern blot hybridization for the detection of gene transcriptional expression. PCR can successfully detect DNA sequence from a single cell (Li et al. 1988). RT-PCR analysis can effectively detect the transcripts of weakly expressed genes, whereas Northern blot hybridization cannot (Tanaka et al. 1998). Because of this extremely high sensitivity of RT-PCR in detecting gene expression, the failure of both EST and RT-PCR experiments to detect the acid invertase transcripts at the storage-root stage of rapid accumulation of starch allows us to conclude that acid invertase pathway is not the predominant sucrose-cleavage pathway for starch accumulation.
SuSy gene expression was compared with that of AGPase in the RT-PCR analysis of this study because AGPase is the enzyme responsible for forming ADP-glucose, the substrate for starch synthesis. Inhibition of AGPase gene expression, using an antisense AGPase gene, significantly reduced starch accumulation in potato tubers (Mueller-Rober et al. 1992). Overexpressing a mutated E. coli AGPase gene glgC16 in transgenic potato plants increased the plants’ starch synthesis rate (Stark et al. 1992). AGPase is a key enzyme for starch synthesis. It is the essential component of sink strength for starchy crops.
In the present study the significant similarity in gene expression patterns between SuSy and AGPase at different developmental stages and in different organs of storage roots suggests that SuSy activity is positively correlated to sink strength at normal growth conditions of sweet potato. The results clearly indicate that the SuSy activity in storage roots is not an unrelated coincidence to a particular developmental stage but is indeed correlated with sink strength during the storage root development; therefore, we confirmed the suggested hypothesis based on analysis of one stage of storage root by Yatomi et al. (1996) and Saitou et al. (1997) that SuSy activity is associated with sink strength in sweet potato.
It was reported that there is a switch between invertase and SuSy activities during potato tuber development (Appeldoorn et al. 2002, Fernie et al. 2002). Invertase activity is high during the early stage of tuber initiation whereas SuSy dominates in the developing tubers. In addition to the potato, the current study on both SuSy and invertase indicates that a similar phenomenon exists in the sweet potato, another crop that stores starch in underground organs. This similarity between the two crops offers an insight into the evolutionary mechanisms for the biochemical pathways in storage-root and tuber crops.
Whether SuSy is correlated with starch or dry matter accumulation appears to be a controversial matter, in that findings differ for different plants: SuSy was found to be correlated with sink strength or dry weight accumulation in tomato (Wang et al. 1993), potato (Hajirezaei et al. 2000), radish (Usuda et al. 1999), and sweet potato (present study; Yatomi et al. 1996). Although SuSy plays a role in the development of the taproot of carrot, invertase was found to be essential for the carrot sink activity (Sturm et al. 1999). SuSy transcript also predominates in the taproot of sugar beet under normal growth conditions (Hesse and Willmitzer 1996), though the physiological role of SuSy is not likely for sucrose cleavage. In potato, antisense inhibition of SuSy was found to lead to a strong accumulation of reducing sugar and to an inhibition of starch accumulation, accompanied by a decrease in total tuber dry weight and by a reduction in the amount of soluble tuber protein present (Zrenner et al. 1995). However, transgenic tomato plants with decreased SuSy are unaltered in terms of the accumulation of starch and sugar in their fruit (Chengappa et al. 1999). Acid invertase is the enzyme primarily responsible for sucrose catabolism in the expanding common oak leaf (Alaoui et al. 1996) and was highly correlated with dry weight gain in most of the flower organs in lily (Ramwala and Miller 1998). However, the same was not true in radish (Usuda et al. 1999). In beans, invertase activity was found to be associated with pod elongation, while SuSy was correlated with dry matter accumulation (Sung et al. 1994). These observations taken together pointed out that both invertase and SuSy are important for plant development and that more research effort is required for understanding why plants need the coexistence of these two systems for sucrose cleavage.
Our present study found that invertase is preferred in cell-forming stages of different organs but SuSy is preferred for starch synthesis. This finding provided insight into understanding the coexistence of the two systems of sucrose cleavage. SuSy expression is strongest in all the sucrose metabolism genes and correlates with sink strength in sweet potato storage root. These results of relative activity between SuSy and invertase indicate that the mechanism of sucrose metabolism in the storage root of sweet potato and the tuber of potato is markedly similar, regardless of the difference in botanical origin between storage root (modified root) and tuber (modified stem) in these two starch producing crops.
Materials and Methods
Plant materials and sample preparation
Terminal cuttings of a high dry matter breeding genotype Kuykei-63 sweet potato [Ipomoea batatas (L.) Lam.] were planted in the nursery at the International Potato Center (CIP)’s San Ramon experimental station, San Ramon, Peru. This is a Peruvian tropical mid-elevation site, positioned at 11° 06′ S, 75° 18′ W, and 800 m above sea level. The plants were watered regularly after they were transplanted. Care was taken to avoid drought or overwatering. Samples were taken 50 d after transplanting; the dry matter content of the sampled roots was determined by desiccation in an oven.
RNA extraction
After they were detached from the mother plants, the roots were surface sterilized for 1 min in 1% hypochlorite solution. They were then rinsed twice with autoclaved double-distilled water, and then used immediately in the RNA extraction procedure. The roots were ground in liquid nitrogen, according to the protocol for total RNA extraction previously described by Li et al. (1996).
cDNA library construction
Total RNA was extracted from storage roots and non-storage roots (i.e. fibrous roots). The cDNA library was constructed using the lambda Uni-ZAP XR vector (Stratagene, Vancouver, Canada). All manipulation followed the instruction manual that came with the kit. The first strain of cDNA was synthesized using oligo dT20 as primer. After in vitro excision, cDNA clones were in form of pBluescript phagemid. There was no “normalization” or probe hybridization-based selection of the cDNA library.
DNA sequencing and analysis
The clones that were to be sequenced were randomly picked from the cDNA library. Double-stranded plasmid DNA was prepared using the plasmid miniprep kit (Cat. # 27106, Qiagen, Mississauga, Canada). Single DNA sequencing reactions were performed using the T3 primer derived from the pBluescript SK- vector (Stratagene, GenBank X52324). Nucleotide sequencing was conducted using the Applied Biosystems BigDye Ready Reaction (Cat. # 4303152, California, U.S.A.), then processed with ABI DNA sequencers. Sequences were processed into FASTA files. The vector sequence was removed. BLASTn and BLASTX searches were conducted using the most recent releases of GenBank by National Center for Biotechnology Information. Phylogeny clustering was done with the software PHYLIP version 3.5.
RT-PCR analysis
RNA was extracted from young shoot tips, fully expanded leaves, and from different stages of the root using the protocol of Li et al. (1996). The conversion of RNA to cDNA, before RT-PCR, was with the kit (Cat. # 11146–016) “ThermoSript RT-PCR System of Life Technology, Invitrogen”. Before conducting RT-PCR experiments, the cDNA concentration of cDNA samples was made to be identical by dilutions based on the DNA amount analyses with “BioRad Gel Doc Documentation System” and its DNA analysis software “Quantity One”.
The PCR reaction mix consisted of 1.5 mM magnesium chloride, 0.2 mM dNTPs, 0.2 µM of each forward and reverse primer, 0.5 units of Platinum Taq polymerase (Cat. # 10966–034, Invitrogen, Carlsbad, U.S.A.), and 1× PCR reaction buffer supplied with the taq polymerase by the company. The reaction was conducted using the following protocol: 94°C for 2 min; 35 cycles at a temperature of 94°C for 30 s, annealing at 53°C (for SuSy group 1) or 55°C (for SuSy Group 2, AGPase, and invertase) for 30 s, 68°C for 1 min; final-elongation occurred at 68°C for 5 min. All the PCR experiments were repeated one to three times.
Acknowledgments
The authors wish to thank Birt Stevens and Muhammad Haroon for the technical assistance they provided. We would also like to thank the plant nursery team at CIP for their high quality management of the sweet potato plants used in this study and DNA Landmarks Inc. (Montreal, Canada) for sequencing part of the ESTs. This research was supported by a collaborative project between the International Potato Center (CIP) and the Potato Research Centre of Agriculture and Agri-Food Canada.
Corresponding author: E-mail, lixq@agr.gc.ca; Fax, +1-506-452-3316.
Fig. 1 Fibrous roots and an immature storage root of the sweet potato used in the construction of the cDNA library. The fibrous roots clearly indicate a normal growth of the plant.
Fig. 2 Phylogram of sweet potato sucrose synthase ESTs in comparison with a potato homologue that was the closest gene based on BLASTn search. SR, storage roots; FR, fibrous roots; PT, potato. The ESTs formed two groups, and both were homologous to the potato SuSy.
Fig. 3 RT-PCR analysis of gene expression of SuSy, AGPase and acid invertase. (A) fibrous root; (B) tiny young immature storage root; (C) medium-sized immature storage root; (D) mature-sized storage root; (E) tiny young shoot; and (F) leaves. SuSy, sucrose synthase; AGPase, ADP-glucose pyrophosphorylase; Invtase, acid invertase. Note that the invertase was most actively expressed in the fibrous roots, but both SuSy and AGPase gave the strongest expression in the medium-sized, immature storage roots at the stage involving rapid accumulation of starch.
Frequency of ESTs a for enzymes involved in sucrose metabolism and its immediate downstream biochemical reactions
| Enzyme | EST in storage roots b | EST in fibrous roots |
| Sucrose phosphate synthase | 0 | 0 |
| Sucrose phosphate phosphatase | 0 | 0 |
| Invertase | 0 | 0 |
| Sucrose synthase (SuSy) | 6 | 1 |
| UDP-glucose pyrophosphorylase (UGPase) | 1 | 0 |
| Hexokinase | 0 | 0 |
| Fructokinase | 0 | 0 |
| Phosphoglucomutase | 0 | 0 |
| Phosphohexoseisomerase | 0 | 0 |
| Sucrose transporter | 0 | 0 |
| Fructose-1,6-bisphosphatase | 0 | 0 |
| Glucose phosphorylase | 0 | 0 |
| Enzyme | EST in storage roots b | EST in fibrous roots |
| Sucrose phosphate synthase | 0 | 0 |
| Sucrose phosphate phosphatase | 0 | 0 |
| Invertase | 0 | 0 |
| Sucrose synthase (SuSy) | 6 | 1 |
| UDP-glucose pyrophosphorylase (UGPase) | 1 | 0 |
| Hexokinase | 0 | 0 |
| Fructokinase | 0 | 0 |
| Phosphoglucomutase | 0 | 0 |
| Phosphohexoseisomerase | 0 | 0 |
| Sucrose transporter | 0 | 0 |
| Fructose-1,6-bisphosphatase | 0 | 0 |
| Glucose phosphorylase | 0 | 0 |
a The ESTs were from a high dry matter sweet potato clone Kyukei-63.
b The immature storage roots were at enlarging stage of about 14 cm. The EST frequency clearly indicated that SuSy gene group is the most actively expressed at transcriptional level for the sucrose metabolism enzymes.
Frequency of ESTs a for enzymes involved in sucrose metabolism and its immediate downstream biochemical reactions
| Enzyme | EST in storage roots b | EST in fibrous roots |
| Sucrose phosphate synthase | 0 | 0 |
| Sucrose phosphate phosphatase | 0 | 0 |
| Invertase | 0 | 0 |
| Sucrose synthase (SuSy) | 6 | 1 |
| UDP-glucose pyrophosphorylase (UGPase) | 1 | 0 |
| Hexokinase | 0 | 0 |
| Fructokinase | 0 | 0 |
| Phosphoglucomutase | 0 | 0 |
| Phosphohexoseisomerase | 0 | 0 |
| Sucrose transporter | 0 | 0 |
| Fructose-1,6-bisphosphatase | 0 | 0 |
| Glucose phosphorylase | 0 | 0 |
| Enzyme | EST in storage roots b | EST in fibrous roots |
| Sucrose phosphate synthase | 0 | 0 |
| Sucrose phosphate phosphatase | 0 | 0 |
| Invertase | 0 | 0 |
| Sucrose synthase (SuSy) | 6 | 1 |
| UDP-glucose pyrophosphorylase (UGPase) | 1 | 0 |
| Hexokinase | 0 | 0 |
| Fructokinase | 0 | 0 |
| Phosphoglucomutase | 0 | 0 |
| Phosphohexoseisomerase | 0 | 0 |
| Sucrose transporter | 0 | 0 |
| Fructose-1,6-bisphosphatase | 0 | 0 |
| Glucose phosphorylase | 0 | 0 |
a The ESTs were from a high dry matter sweet potato clone Kyukei-63.
b The immature storage roots were at enlarging stage of about 14 cm. The EST frequency clearly indicated that SuSy gene group is the most actively expressed at transcriptional level for the sucrose metabolism enzymes.
ESTs of sucrose synthase (SuSy) and UGPase of immature storage roots from a high dry matter sweet potato genotype Kyukei-63
| EST | GenBank DbEST Id a | GenBank accessory a | Gene | Length (bp) | Group | Closest known genes | Degree of homology |
| EA24B11F | 15130251 | CA409457 | SuSy | 567 | SuSy1 | Potato, gb|M18745 | 186 bits, Expect = 9e-45 |
| EB28B7F | 15130252 | CA409458 | SuSy | 246 | SuSy1 | Potato, gb|M18745 | 107 bits, Expect = 3e-21 |
| EB28B11F | 15130253 | CA409459 | SuSy | 673 | SuSy2 | Potato, gb|M18745 | 315 bits, Expect = 2e-83 |
| EB28E6F | 15130254 | CA409460 | SuSy | 374 | SuSy1 | Potato, gb|M18745 | 127 bits, Expect = 5e-27 |
| EB29D12F | 15130255 | CA409461 | SuSy | 613 | SuSy1 | Potato, gb|M18745 | 196 bits, Expect = 1e-47 |
| EB31A7F | 15130256 | CA409462 | SuSy | 624 | SuSy2 | Potato, gb|M18745 | 216 bits, Expect = 1e-53 |
| EB32D1F | 15130257 | CA409463 | SuSy | 470 | SuSy1 | Potato, gb|M18745 | 270 bits, Expect = 6e-70 |
| EB29E8F | 15130258 | CA409464 | UGPase | 477 | UGPase1 | Potato, emb|Z18924 | 77.8 bits, Expect = 5e-12 |
| EST | GenBank DbEST Id a | GenBank accessory a | Gene | Length (bp) | Group | Closest known genes | Degree of homology |
| EA24B11F | 15130251 | CA409457 | SuSy | 567 | SuSy1 | Potato, gb|M18745 | 186 bits, Expect = 9e-45 |
| EB28B7F | 15130252 | CA409458 | SuSy | 246 | SuSy1 | Potato, gb|M18745 | 107 bits, Expect = 3e-21 |
| EB28B11F | 15130253 | CA409459 | SuSy | 673 | SuSy2 | Potato, gb|M18745 | 315 bits, Expect = 2e-83 |
| EB28E6F | 15130254 | CA409460 | SuSy | 374 | SuSy1 | Potato, gb|M18745 | 127 bits, Expect = 5e-27 |
| EB29D12F | 15130255 | CA409461 | SuSy | 613 | SuSy1 | Potato, gb|M18745 | 196 bits, Expect = 1e-47 |
| EB31A7F | 15130256 | CA409462 | SuSy | 624 | SuSy2 | Potato, gb|M18745 | 216 bits, Expect = 1e-53 |
| EB32D1F | 15130257 | CA409463 | SuSy | 470 | SuSy1 | Potato, gb|M18745 | 270 bits, Expect = 6e-70 |
| EB29E8F | 15130258 | CA409464 | UGPase | 477 | UGPase1 | Potato, emb|Z18924 | 77.8 bits, Expect = 5e-12 |
a ID and accessory numbers of the ESTs presented; SuSy, sucrose synthase; UGPase, UDP-glucose pyrophosphorylase.
ESTs of sucrose synthase (SuSy) and UGPase of immature storage roots from a high dry matter sweet potato genotype Kyukei-63
| EST | GenBank DbEST Id a | GenBank accessory a | Gene | Length (bp) | Group | Closest known genes | Degree of homology |
| EA24B11F | 15130251 | CA409457 | SuSy | 567 | SuSy1 | Potato, gb|M18745 | 186 bits, Expect = 9e-45 |
| EB28B7F | 15130252 | CA409458 | SuSy | 246 | SuSy1 | Potato, gb|M18745 | 107 bits, Expect = 3e-21 |
| EB28B11F | 15130253 | CA409459 | SuSy | 673 | SuSy2 | Potato, gb|M18745 | 315 bits, Expect = 2e-83 |
| EB28E6F | 15130254 | CA409460 | SuSy | 374 | SuSy1 | Potato, gb|M18745 | 127 bits, Expect = 5e-27 |
| EB29D12F | 15130255 | CA409461 | SuSy | 613 | SuSy1 | Potato, gb|M18745 | 196 bits, Expect = 1e-47 |
| EB31A7F | 15130256 | CA409462 | SuSy | 624 | SuSy2 | Potato, gb|M18745 | 216 bits, Expect = 1e-53 |
| EB32D1F | 15130257 | CA409463 | SuSy | 470 | SuSy1 | Potato, gb|M18745 | 270 bits, Expect = 6e-70 |
| EB29E8F | 15130258 | CA409464 | UGPase | 477 | UGPase1 | Potato, emb|Z18924 | 77.8 bits, Expect = 5e-12 |
| EST | GenBank DbEST Id a | GenBank accessory a | Gene | Length (bp) | Group | Closest known genes | Degree of homology |
| EA24B11F | 15130251 | CA409457 | SuSy | 567 | SuSy1 | Potato, gb|M18745 | 186 bits, Expect = 9e-45 |
| EB28B7F | 15130252 | CA409458 | SuSy | 246 | SuSy1 | Potato, gb|M18745 | 107 bits, Expect = 3e-21 |
| EB28B11F | 15130253 | CA409459 | SuSy | 673 | SuSy2 | Potato, gb|M18745 | 315 bits, Expect = 2e-83 |
| EB28E6F | 15130254 | CA409460 | SuSy | 374 | SuSy1 | Potato, gb|M18745 | 127 bits, Expect = 5e-27 |
| EB29D12F | 15130255 | CA409461 | SuSy | 613 | SuSy1 | Potato, gb|M18745 | 196 bits, Expect = 1e-47 |
| EB31A7F | 15130256 | CA409462 | SuSy | 624 | SuSy2 | Potato, gb|M18745 | 216 bits, Expect = 1e-53 |
| EB32D1F | 15130257 | CA409463 | SuSy | 470 | SuSy1 | Potato, gb|M18745 | 270 bits, Expect = 6e-70 |
| EB29E8F | 15130258 | CA409464 | UGPase | 477 | UGPase1 | Potato, emb|Z18924 | 77.8 bits, Expect = 5e-12 |
a ID and accessory numbers of the ESTs presented; SuSy, sucrose synthase; UGPase, UDP-glucose pyrophosphorylase.
Primers used for RT-PCR analysis of SuSy and AGPase
| Gene | Primer | Primer sequence | Length | Note | Reference |
| SuSy | SU5132F | 5′-GAA ACG TAT CCA GGA GAA GTA CA-3′ | 23mer | Conserved for both Group 1 and Group 2 | This study |
| SuSy group 1 | SU5300R1 | 5′-CAA AAC AAA CGA GAA TCA TTC G | 22mer | Conserved for Group 1 SuSy | This study |
| SuSy group 2 | SU5166R2 | 5′-TGA CAA CTC GAG GGT GAC CAA C | 22mer | Conserved for Group 2 SuSy | This study |
| AGPase | SP9AGPF | 5′-CCC AAA CAC AAG GGG AAA CGG GGA TGA AG | 29mer | This study; Z79636 and AF068260 | |
| SP9AGPR | 5′-CAG TTA GGG CCA AGT TAG CGT CG | 23mer | This study; AF068260 | ||
| Invertase | SPINVF | 5′-GGA CTT TAG GGA CCC CAC CAC TGC | 24mer | This study; AF017082 | |
| SPINVR | 5′-TCG CCT GAA GAG ATG CCC AAC CT | 23mer |
| Gene | Primer | Primer sequence | Length | Note | Reference |
| SuSy | SU5132F | 5′-GAA ACG TAT CCA GGA GAA GTA CA-3′ | 23mer | Conserved for both Group 1 and Group 2 | This study |
| SuSy group 1 | SU5300R1 | 5′-CAA AAC AAA CGA GAA TCA TTC G | 22mer | Conserved for Group 1 SuSy | This study |
| SuSy group 2 | SU5166R2 | 5′-TGA CAA CTC GAG GGT GAC CAA C | 22mer | Conserved for Group 2 SuSy | This study |
| AGPase | SP9AGPF | 5′-CCC AAA CAC AAG GGG AAA CGG GGA TGA AG | 29mer | This study; Z79636 and AF068260 | |
| SP9AGPR | 5′-CAG TTA GGG CCA AGT TAG CGT CG | 23mer | This study; AF068260 | ||
| Invertase | SPINVF | 5′-GGA CTT TAG GGA CCC CAC CAC TGC | 24mer | This study; AF017082 | |
| SPINVR | 5′-TCG CCT GAA GAG ATG CCC AAC CT | 23mer |
Notes: RT-PCR product is expected to be 298 bp for SU5132F with SU5300R1 (specific to SuSy group 1), 326 bp for SU5132F with SU5166R2 (specific to SuSy group 2), 576 bp for SP29AGPF with SP29AGPR, and 515 bp for SPINVF with SPINVR.
Primers used for RT-PCR analysis of SuSy and AGPase
| Gene | Primer | Primer sequence | Length | Note | Reference |
| SuSy | SU5132F | 5′-GAA ACG TAT CCA GGA GAA GTA CA-3′ | 23mer | Conserved for both Group 1 and Group 2 | This study |
| SuSy group 1 | SU5300R1 | 5′-CAA AAC AAA CGA GAA TCA TTC G | 22mer | Conserved for Group 1 SuSy | This study |
| SuSy group 2 | SU5166R2 | 5′-TGA CAA CTC GAG GGT GAC CAA C | 22mer | Conserved for Group 2 SuSy | This study |
| AGPase | SP9AGPF | 5′-CCC AAA CAC AAG GGG AAA CGG GGA TGA AG | 29mer | This study; Z79636 and AF068260 | |
| SP9AGPR | 5′-CAG TTA GGG CCA AGT TAG CGT CG | 23mer | This study; AF068260 | ||
| Invertase | SPINVF | 5′-GGA CTT TAG GGA CCC CAC CAC TGC | 24mer | This study; AF017082 | |
| SPINVR | 5′-TCG CCT GAA GAG ATG CCC AAC CT | 23mer |
| Gene | Primer | Primer sequence | Length | Note | Reference |
| SuSy | SU5132F | 5′-GAA ACG TAT CCA GGA GAA GTA CA-3′ | 23mer | Conserved for both Group 1 and Group 2 | This study |
| SuSy group 1 | SU5300R1 | 5′-CAA AAC AAA CGA GAA TCA TTC G | 22mer | Conserved for Group 1 SuSy | This study |
| SuSy group 2 | SU5166R2 | 5′-TGA CAA CTC GAG GGT GAC CAA C | 22mer | Conserved for Group 2 SuSy | This study |
| AGPase | SP9AGPF | 5′-CCC AAA CAC AAG GGG AAA CGG GGA TGA AG | 29mer | This study; Z79636 and AF068260 | |
| SP9AGPR | 5′-CAG TTA GGG CCA AGT TAG CGT CG | 23mer | This study; AF068260 | ||
| Invertase | SPINVF | 5′-GGA CTT TAG GGA CCC CAC CAC TGC | 24mer | This study; AF017082 | |
| SPINVR | 5′-TCG CCT GAA GAG ATG CCC AAC CT | 23mer |
Notes: RT-PCR product is expected to be 298 bp for SU5132F with SU5300R1 (specific to SuSy group 1), 326 bp for SU5132F with SU5166R2 (specific to SuSy group 2), 576 bp for SP29AGPF with SP29AGPR, and 515 bp for SPINVF with SPINVR.
Abbreviations
- AGPase
ADP-glucose pyrophosphorylase
- CIP
International Potato Center
- dNTP
deoxynucleoside triphosphate
- EST
expressed sequence tag
- RT-PCR
reverse transcription–polymerase chain reaction
- SuSy
sucrose synthase
- UGPase
UDP-glucose pyrophosphorylase.
The nucleotide sequences reported in this paper have been submitted to GenBank under accession numbers: CA409457, CA409458, CA409459, CA409460, CA409461, CA409462, CA409463 and CA409464.
References
Adams, M.D., Dubnick, M., Kerlavage, A.R., Moreno, R., Kelley, J.M., Utterback, T.R., Nagle, J.W., Field, C. and Venter, J.C. (
Alaoui, S.B., Richard, S., Barnola, P. and Dizengremel, P. (
Appeldoorn, N.J., Sergeeva, L., Vreugdenhil, D., Van Der Plas, L.H. and Visser, R.G. (
Channeliere, S., Riviere, S., Scalliet, G., Szecsi, J., Jullien, F., Dolle, C., Vergne, P., Dumas, C., Bendahmane, M., Hugueney, P. and Cock, J.M. (
Chen, X., Salamini, F. and Gebhardt, C. (
Chengappa, S., Guilleroux, M., Phillips, W. and Shields, R. (
Crookshanks, M., Emmersen, J., Welinder, K.G. and Nielsen, K.L. (
Fernie, A.R. and Willmitzer, L. (
Fernie, A.R., Willmitzer, L. and Trethewey, R.N. (
Florova, G., Kazanina, G. and Reynolds, K.A. (
Hajirezaei, M.R., Takahata, Y., Trethewey, R.N., Willmitzer, L. and Sonnewald U. (
Hesse, H. and Willmitzer, L. (
Huang, Y.H., Picha, D.H., Kilili, A.W. and Johnson, C.E. (
Kwak, J.M., Kim, S.A., Hong, S.W. and Nam, H.G. (
Li, H., Glyllensten, U.B., Cui, X., Saiki R.K., Erlich, H.A. and Arnheim, N. (
Li, X.-Q. (
Li, X.-Q., Zhang, Z.M. and Brown G.G. (
Mueller-Rober, B., Sonnewald, U. and Willmitzer, L. (
Ramwala, A.P. and Miller, W.P. (
Saitou, K., Araki, T., Agata, W., Kubota, F. and Nakayama K. (
Stark, D.M., Timmerman, K.P., Barry, G.F., Peiss, J. and Kishore, G.M. (
Sturm, A., Lienhard, S., Schatt, S. and Hardegger, M. (
Sung, S.S., Sheih, W.J., Geiger, D.R. and Black, C.C. (
Takahata, Y., Noda, T. and Sato, T. (
Tanaka, N., Yamakawa, M. and Yamashita, I. (
Ujino, I.T., Yoshimura, K., Ugawa, Y., Yoshimaru, H., Nagasaka, K. and Tsumura, Y. (
Usuda, H., Demura, T., Shimogawara, K. and Fukuda, H. (
Vasmatzis, G., Essand, M., Brinkmann, U., Lee, B. and Pastan, I. (
Wang, F., Sanz, A., Brenner, M.L. and Smith, A. (
Wang, H.L., Lee, P.D., Chen, W.L., Huang, D.J. and Su, J.C. (
Yatomi, M., Kubota, F., Saito, K. and Agata, W. (
Yu, H.J., Moon, M.S., Lee, H.S., Mun, J.H., Kwon, Y.M. and Kim, S.G. (