-
PDF
- Split View
-
Views
-
Cite
Cite
Yasuhiro Kadota, Tatsuaki Goh, Hajime Tomatsu, Ryosuke Tamauchi, Katsumi Higashi, Shoshi Muto, Kazuyuki Kuchitsu, Cryptogein-Induced Initial Events in Tobacco BY-2 Cells: Pharmacological Characterization of Molecular Relationship among Cytosolic Ca2+ Transients, Anion Efflux and Production of Reactive Oxygen Species, Plant and Cell Physiology, Volume 45, Issue 2, 15 February 2004, Pages 160–170, https://doi.org/10.1093/pcp/pch020
Close - Share Icon Share
Abstract
Ion fluxes and the production of reactive oxygen species (ROS) are early events that follow elicitor treatment or microbial infection. However, molecular mechanisms for these responses as well as their relationship have been controversial and still largely unknown. We here simultaneously monitored the temporal sequence of initial events at the plasma membrane in suspension-cultured tobacco cells (cell line BY-2) in response to a purified proteinaceous elicitor, cryptogein, which induced hypersensitive cell death. The elicitor induced transient rise in cytosolic Ca2+ concentration ([Ca2+]cyt) showing two distinct peaks, followed by biphasic (rapid/transient and slow/prolonged) Cl– efflux and H+ influx. Pharmacological analyses suggested that the two phases of the [Ca2+]cyt response correspond to Ca2+ influx through the plasma membrane and an inositol 1,4,5-trisphophate-mediated release of Ca2+ from intracellular Ca2+ stores, respectively, and the [Ca2+]cyt transients and the Cl– efflux were mutually dependent events regulated by protein phosphorylation. The elicitor also induced production of ROS including •O2– and H2O2, which initiated after the [Ca2+]cyt rise and required Ca2+ influx, Cl– efflux and protein phosphorylation. An inhibitor of NADPH oxidase, diphenylene iodonium, completely inhibited the elicitor-induced production of •O2– and H2O2, but did not affect the [Ca2+]cyt transients. These results suggest that cryptogein-induced plasma membrane Ca2+ influx is independent of ROS, and NADPH oxidase dependent ROS production is regulated by these series of ion fluxes.
(Received August 20, 2003; Accepted December 6, 2003)
Introduction
Plants respond to attacks from pathogens by activating a variety of defense mechanisms, including the synthesis of phytoalexins and hypersensitive cell death, which restricts growth of pathogens at the site of infection (Jones and Dangl 1996). These responses require recognition between the host and pathogen mediated by signaling molecules called elicitors derived from pathogens or plants and their specific receptors (Shibuya et al. 1993, Heath 2000).
Upon recognition of pathogenic signals, plant cells initiate activation of a complexed signal transduction network that release second messengers and trigger inducible defense responses. Characteristic early events include an influx of Ca2+ and H+, Cl– efflux, membrane depolarization and production of reactive oxygen species (ROS) (Kuchitsu et al. 1993, Kuchitsu et al. 1995, Kuchitsu et al. 1997, Levine et al. 1994, Kikuyama et al. 1997, Jabs et al. 1997, Pugin et al. 1997, Blume et al. 2000). These initial responses are followed by the production of phytoalexins, transcriptional activation of defense genes and hypersensitive cell death. These downstream events are inhibited by some blockers for Ca2+ channels (Ebel et al. 1995, Tavernier et al. 1995, Binet et al. 2001, Lecourieux et al. 2002) and anion channels (Ebel et al. 1995, Levine et al. 1996, Jabs et al. 1997, Wendehenne et al. 2002), suggesting that the initial ion fluxes are crucial for the induction of defense responses.
Since individual initial events have been analyzed independently, however, the relationship among various responses is still largely unknown. In addition, the characteristics of initial events have been reported differently in different systems. Particularly the relationship between elicitor-induced changes in cytosolic Ca2+ concentrations ([Ca2+]cyt) and ROS production has been controversial. Mithöfer et al. (2001) argued that β-glucan-induced H2O2 synthesis in soybean is independent of [Ca2+]cyt changes. By contrast, Chandra et al. (1997) showed that a change in [Ca2+]cyt is essential for the initiation of oligogalacturonide-induced ROS production in tobacco cells. Cessna and Low (2001) reported that fungal elicitor-induced oxidative burst depends on the release of Ca2+ from intracellular stores and is independent of the influx of extracellular Ca2+. Furthermore, application of H2O2 induces [Ca2+]cyt changes (Price et al. 1994, Levine et al. 1996, Takahashi et al. 1998, Kawano and Muto 2000) and H2O2 is suggested to play an important role in [Ca2+]cyt rise in abscisic acid (ABA) signaling in guard cells (Pei et al. 2000, Kwak et al. 2003). Lecourieux et al. (2002) suggested that ROS might also affect [Ca2+]cyt in elicitor signaling.
To elucidate the relationship among these various initial responses, it is important to simultaneously monitor and compare the responses under the same conditions. In the present study, we concomitantly analyzed various initial events including plasma membrane ion fluxes and ROS production, to compare the temporal sequence of these events. We also applied pharmacological manipulations to elucidate their underlying mechanisms.
A 10-kDa proteinaceous elicitor, cryptogein, produced by a pathogenic fungus Phytophthora cryptogea induces hypersensitive response in planta as well as in cell suspensions of tobacco (Ricci et al. 1989, Binet et al. 2001). Ca2+ and anion channel inhibitors inhibit cryptogein-induced cell death in Nicotiana tabacum cv. Xanthi cells, suggesting that cryptogein induced the cell death via a specific signal transduction pathway of the cells (Binet et al. 2001, Lecourieux et al. 2002).
Tobacco BY-2 cell line has been used as one of the ideal model plant cells for cell and molecular biological research (Nagata et al. 1992). We have analyzed cryptogein-induced cell death in BY-2 cells as a model system for hypersensitive cell death. We here show that the elicitor induced an influx of extracellular Ca2+, followed by inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release from intracellular stores. The Ca2+ influx and Cl– efflux are mutually dependent and both responses are regulated by protein phosphorylation. The elicitor-induced ROS production was shown not to be involved in the induction of [Ca2+]cyt rise but to be induced downstream of Ca2+ influx, Cl– efflux and protein phosphorylation.
Results
Cryptogein induced transient cytosolic Ca2+ rise showing two distinct peaks
A proteinaceous elicitor cryptogein induced cell death in suspension-cultured tobacco BY-2 cells. Evans blue assay (Turner and Novacky 1974) showed that in the cell suspension 3 d after subculture cell death was induced 10–15 h after the application of cryptogein (500 nM, data not shown). To analyze various ion fluxes in defense signaling, we applied apoaequorin-expressing BY-2 cells to monitor cryptogein-induced changes in [Ca2+]cyt. Cryptogein also caused cell death in apoaequorin-expressing cell cultures that was indistinguishable from the non-transformed control. After incubation of the cells with coelenterazine for 6 h in the normal growth medium to reconstruct aequorin, chemiluminescence was monitored with a luminometer while the cell suspension was continuously shaken for aeration. Cryptogein induced characteristic two transient peaks in aequorin luminescence reflecting [Ca2+]cyt increase (Fig. 1A). After an apparent lag of 64±4 s (SE, n = 15), the elicitor caused a rapid increase in [Ca2+]cyt (first response peak), which reached a maximum level at 137±5 s of treatment. Thereafter, [Ca2+]cyt decreased until the second rise in [Ca2+]cyt (second response peak) occurred, which reached maximum at 362±16 s. Subsequent to this, [Ca2+]cyt decreased gradually to the basal (pre-treatment) levels.
Effects of nutrition and aeration on [Ca2+]cyt responses
The temporal pattern of changes in [Ca2+]cyt was greatly affected by the culture conditions, especially by nutrition and the degree of aeration. When the extracellular growth medium was replaced by a suspension buffer containing 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, and 2 mM HEPES-HCl (pH 5.75), incubated with coelenterazine for 6 h and luminescence was measured with shaking, the cryptogein-induced [Ca2+]cyt response showed 4-fold larger than that measured in the growth medium (Fig. 1B). After an apparent lag of 71±3 s (n = 3), cryptogein caused a rapid increase in [Ca2+]cyt, which reached a maximum level at 173±7 s of treatment. This peak appeared the composite of the two phases of the [Ca2+]cyt change.
When the cells were kept in the growth medium and luminescence was measured without shaking, cryptogein-induced [Ca2+]cyt changes became smaller (Fig. 1C). The [Ca2+]cyt changes also became smaller in the cells kept in the above buffer solution and measured without shaking (Fig. 1D). This [Ca2+]cyt changes were characterized by a rapid and transient increase which occurred after a lag of 56±15 s peaked at 255±50 s (n = 3), followed by a decline to an elevated level that was sustained. These results indicate that the elicitor-induced Ca2+ responses are affected depending on the conditions of nutrition and aeration. Since the conditions used in Fig. 1A reflect the physiological status of the regular culture conditions, we carried out the following experiments in the growth medium with shaking except otherwise stated.
Plasma membrane fluxes of H+ and Cl–, and ROS production
Extracellular pH and Cl– concentration ([Cl–]) were simultaneously monitored with ion-selective electrodes in the growth medium with shaking as shown in Fig. 1A (Fig. 2A). After 116±5 s (n = 6) lag following the application of cryptogein, the pH of the extracellular medium exhibited a biphasic change, namely a rapid and transient increase peaked at 488±19 s, followed by a slow and prolonged period of alkalinization. Extracellular [Cl–] also showed a biphasic increase after a lag of 120±6 s (n = 9), but the rapid transient peak was not apparent.
Production of superoxide anion (•O2–) and H2O2 were monitored with chemiluminescence assays using 2-methyl-6-[p-methoxyphenyl]-3,7-dihydroimidazo[1,2-a]pyrazin-3-one (MCLA) (Uehara et al. 1993) and luminol, respectively (Fig. 2B, C). Due to the pH-dependency of luminol chemiluminescence, the luminol assay for H2O2 was carried out after replacing the growth medium with a buffer solution (pH 7.0) and adding 5 mM K-phosphate buffer (pH 7.0, see Materials and Methods). Cryptogein induced a biphasic transient •O2– production after a lag of 124±6 s (n = 7) (Fig. 2B), which was clearly after the increase in [Ca2+]cyt in the same culture under the same conditions. H2O2 production occurred later (268±14 s (n = 7) after cryptogein application; peaked at 594±41 s) and subsequently decreased to the basal levels (Fig. 2C).
In summary, concomitant analyses revealed the following time sequence of the initial events after cryptogein application: [Ca2+]cyt change starts at 64±4 s (n = 15), changes of extracellular pH and [Cl–] at 116±5 s (n = 6) and 120±6 s (n = 9), respectively then •O2– production at 124±6 s (n = 7) and H2O2 production at 268±14 s (n = 7).
Origins of Ca2+ for the two distinct Ca2+ transients
To elucidate the molecular mechanisms underlying the elicitor-induced changes in [Ca2+]cyt, we tested the effects of various pharmacological reagents. To prevent the side effects of the inhibitors, cytotoxicity of all inhibitors was tested using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) and Evans blue (Campling et al. 1988). Only those showed no cytotoxity was applied for further experiments (data not shown).
A Ca2+ chelator, 1,2-bis-(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA), inhibited both peaks of the [Ca2+]cyt transients (Fig. 3A), suggesting that increases in [Ca2+ ]cyt are due to the influx of extracellular Ca2+. Phospholipase C, an enzyme producing IP3, is inhibited by neomycin and U-73122 (Zhang et al. 2002). Neither of these inhibitors affected the first peak of the [Ca2+]cyt transients, while both inhibited the second peak (Fig. 3B, C), suggesting that the second peak in the [Ca2+]cyt response is due to the release of Ca2+ from intracellular Ca2+ stores via IP3-dependent pathway. Application of BAPTA during the second peak caused decrease in aequorin chemiluminescence (data not shown), suggesting that IP3-dependent Ca2+ release from intracellular stores might require a continuous influx of extracellular Ca2+.
Roles of protein phosphorylation and anion efflux on [Ca2+]cyt responses
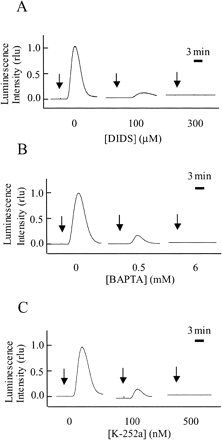
A protein kinase inhibitor, K-252a, inhibited both of the [Ca2+]cyt response peaks in a dose-dependent manner and completely abolished the both peaks at 1 µM, suggesting that protein phosphorylation is crucial for the cryptogein-induced [Ca2+]cyt response (Fig. 4A).
To examine the relationship between the increase in [Ca2+]cyt and Cl– efflux, we tested the effects of various anion channel blockers. Niflumic acid and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) induced extracellular alkalinization and showed cytotoxicity (data not shown), whereas 4,4′-diisothiocyanostilbene-2,2′-disulfonate (DIDS) had no cytotoxic effects at least up to 15 h (data not shown). DIDS inhibited [Ca2+]cyt responses in a concentration-dependent manner (Fig. 4B), suggesting that an efflux of anions is crucial for the induction of the [Ca2+]cyt response. However, in contrast to the inhibitory effect of K-252a, the inhibition by DIDS was always incomplete, even at concentrations high enough to cause cytotoxicity (1.5 mM, data not shown).
Cryptogein-induced changes in [Ca2+]cyt and efflux of Cl– are interdependent
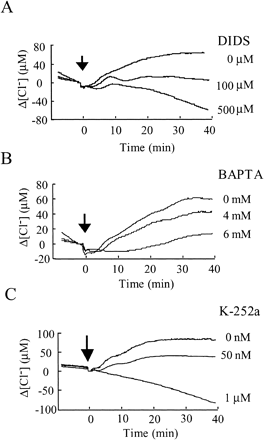
Inhibition of [Ca2+]cyt responses by DIDS suggested that anion efflux is important in Ca2+ signaling. Indeed, the cryptogein-induced Cl– efflux was inhibited both by DIDS (Fig. 5A) and BAPTA (Fig. 5B), suggesting that the Cl– efflux requires an influx of extracellular Ca2+ through the plasma membrane. Thus the elicitor-induced increase in [Ca2+]cyt and the Cl– efflux appear to be interdependent. The finding that K-252a inhibited changes both in [Ca2+]cyt and [Cl–] (Fig. 4A, 5C) suggests that protein phosphorylation regulates the activation of Ca2+- and Cl–-permeable ion channels.
The initial increase in [Ca2+]cyt is independent of •O2– and H2O2
To examine the relationship between the elicitor-induced ROS production and [Ca2+]cyt responses, we examined the effect of diphenylene iodonium (DPI), an inhibitor of mammalian and plant NADPH oxidases (Sagi and Fluhr 2001). Even though 2–5 µM DPI was sufficient to completely inhibit •O2– and H2O2 production (Fig. 6A, B), 15 µM DPI failed to inhibit the cryptogein-induced increase in [Ca2+]cyt (Fig. 6C). The second part of the [Ca2+]cyt response appeared to be rather enhanced by DPI. DPI did not show any effect on cryptogein-induced [Ca2+]cyt changes, suggesting that this inhibitor does not show toxic effect at least during measuring time (30 min). Furthermore, Evans blue assay showed that 15 µM DPI did not affect cell viability at least for 1 h after application (data not shown). These results indicate that •O2– and H2O2 do not directly participate in the elicitor-induced [Ca2+]cyt transients.
ROS production requires protein phosphorylation and transmembrane ion fluxes
DIDS, BAPTA and K-252a independently inhibited H2O2 production, suggesting that the production of H2O2 depends upon an efflux of anions, Ca2+ influx and protein phosphorylation (Fig. 7). Since these inhibitors themselves affected MCLA chemiluminescence, it was impossible to analyze effects of these inhibitors on •O2– production. In conclusion, the elicitor-induced ROS production occurs downstream of the ion fluxes and protein phosphorylation.
Discussion
Though ion fluxes and ROS production induced by pathogenic signals and elicitors are suggested to be important for activation of defense responses, their molecular mechanisms were largely unknown. In the present study, we have simultaneously monitored cryptogein-induced various initial responses including fluxes of Ca2+, H+ and Cl– as well as ROS production to elucidate their molecular mechanisms and interrelationships.
Culture conditions affect [Ca2+]cyt responses
Changes in [Ca2+]cyt were greatly affected by culture conditions, especially nutrition and degree of aeration (Fig. 1). Replacement of the extracellular growth medium by the buffer solution caused 4-fold increase of the elicitor-induced [Ca2+]cyt change (Fig. 1B), which might be due to changes in intracellular Ca2+ homeostasis. ATP synthesis in the cells suspended in the buffer without sucrose or any other carbon source is expected to be low. A low ATP level would in turn lower the activities of Ca2+-ATPase, H+-ATPase and subsequently Ca2+/H+ antiporters all of which are important to keep [Ca2+]cyt low (Sanders et al. 2002). In fact, treatment of the cells with an inhibitor for Ca2+-ATPase, 2,5-di-(t-butyl)-1,4-hydroquinone (BHQ), enhanced the cryptogein-induced [Ca2+]cyt rise (data not shown).
Plant cells are generally sensitive to oxygen. Oxygen supply greatly affected cytoplasmic pH and plasma membrane potential. Sufficient aeration is crucial for noninvasive in vivo measurements of ion fluxes under physiological conditions (Kuchitsu et al. 1997, Kikuyama et al. 1997). When the aequorin luminescence was monitored without shaking the cell suspension, the [Ca2+]cyt changes were much smaller than those were measured with shaking (Fig. 2C, D), suggesting that aeration (oxygen supply to the cells) could also be an indispensable factor for in vivo measurement of [Ca2+]cyt under physiological conditions. These results strongly indicate the importance of careful examination of experimental conditions for [Ca2+]cyt measurements. Furthermore, these results also suggest that interpretation of previously reported [Ca2+]cyt changes should be reconsidered according to the experimental conditions.
Temporal pattern of cryptogein-induced [Ca2+]cyt rise
Noninvasive in vivo monitoring of [Ca2+]cyt revealed that the elicitor triggered two transient peaks in [Ca2+]cyt in the normal growth medium with constant aeration. Recently, Lecourieux et al. (2002) reported cryptogein-induced [Ca2+]cyt changes in cell suspensions of Nicotiana plumbaginifolia. They showed transient increase in [Ca2+]cyt peaked at 5 min after cryptogein application, followed by a sustained [Ca2+]cyt increase, which peaked at 30 min and failed to return to the baseline even after 2.5 h after elicitation. This temporal pattern was different from our observation with tobacco BY-2 cells in which no sustained increase of [Ca2+]cyt was observed and [Ca2+]cyt rather decreased to the basal levels (Fig. 1A).
These different signatures of Ca2+ response patterns could be explained in part by differences in the conditions for [Ca2+]cyt measurements. Lecourieux et al. (2002) incubated the cells with the suspension buffer similar to that used in the experiments shown in Fig. 1B and 1D, at least for 6 h prior to monitoring luminescence with a luminometer not equipped with shaking apparatus. When the cellular chemiluminescence was monitored without shaking after incubation of the cells in the buffer solution, which mimicked the experimental conditions used by Lecourieux et al. (2002), the [Ca2+]cyt signature (Fig. 1D; rapid and transient increase followed by prolonged rise) also resembled the pattern reported with Nicotiana plumbaginifolia. We decided to monitor [Ca2+]cyt in the normal growth medium with shaking, which mimicked the regular growth conditions, to further characterize the physiological events underlying the elicitor-induced [Ca2+]cyt transient peaks.
The two peaks in [Ca2+]cyt are due to extracellular Ca2+ influx and IP3-mediated release of Ca2+ from intracellular stores
Two inhibitors for phospholipase C, neomycin and U73122, are reported to abolish IP3-mediated Ca2+ release in mammalian and plant cells (Franklin-Tong et al. 1996, Takahashi et al. 2001). In the present study, these inhibitors predominantly inhibited the second peak of the cryptogein-induced [Ca2+]cyt transients (Fig. 3B, C). Since the cryptogein-induced [Ca2+]cyt response requires entry of extracellular Ca2+ (Fig. 3A), the first peak of the response is likely due to an influx of extracellular Ca2+, whereas the second peak is due to a release of Ca2+ into the cytoplasm from intracellular stores through IP3-dependent signaling pathway. Phospholipase C is also activated by Ca2+ in plants as well as in animals (Miyakawa et al. 2001, Zhang et al. 2002). The Ca2+ influx during the first peak of the response may participate in the activation of phospholipase C. Interestingly, oligosaccharide elicitors such as β-glucan, chitoheptaose and oligogalacturonides induce similar [Ca2+]cyt transient peaks (Mithöfer et al. 1999, Lecourieux et al. 2002), suggesting that signals from different kinds of elicitors are mediated by similar Ca2+ signaling pathways.
Cryptogein-induced ion channel cascade
An efflux of anions is suggested to be essential for the induction of defense responses and hypersensitive cell death (Ebel et al. 1995, Wendehenne et al. 2002). To clarify the relationship between anion efflux and changes in [Ca2+]cyt, we analyzed the effects of anion channel inhibitors on the induction of [Ca2+]cyt responses by cryptogein. We found that DIDS specifically inhibited the [Ca2+]cyt transients as well as Cl– efflux in a dose dependent manner (Fig. 4B, 5A), suggesting important roles of anion efflux in induction of the [Ca2+]cyt responses. [Ca2+]cyt changes induced by other elicitors are shown to be inhibited by anthracene-9-carboxylic acid, and NPPB (Blume et al. 2000, Mithöfer et al. 2001) and by niflumate (Cessna and Low 2001). Although these inhibitors showed cytotoxicity in tobacco BY-2 cells, these observations indicate that the anion efflux is important for [Ca2+]cyt mobilization induced by various elicitors.
The Cl– efflux was also inhibited by BAPTA and K-252a (Fig. 5B, C), suggesting that the cryptogein-induced Cl– efflux requires both extracellular Ca2+ and protein phosphorylation. DIDS did not inhibit the [Ca2+]cyt responses completely, even at concentrations high enough to cause cytotoxicity. This implies that at least part of the Ca2+ influx is independent of the Cl– efflux. Since the Cl– efflux requires Ca2+ entry (Fig. 5B), the Cl– efflux-independent Ca2+ influx might be responsible for initiating the Cl– efflux. Indeed, the initial Ca2+ influx (at 64±4 s (n = 15)) occurred before the Cl– efflux (at 120±6 s, (n = 9)) (Fig. 1A, 2A). Therefore, we surmise that cryptogein-induced rapid Ca2+ influx activates a Cl– efflux, and that these two ion fluxes together induce a further influx of Ca2+.
Cl– efflux might induce membrane depolarization in cryptogein signaling. In stomatal guard cells, an anion channel plays a major role in ABA-induced plasma-membrane depolarization and stomatal closing (Ward et al. 1995). ABA-regulated plasma membrane Ca2+ influx and Cl– efflux are regulated by protein phosphatases and kinases (Pei et al. 1997, Allen et al. 1999, Li et al. 2000, Kwak et al. 2002). Molecular functional analyses for the role of plasma membrane Ca2+ influx and Cl– efflux in membrane depolarization and signal transduction would be an important future research subject. Recently, the gene encoding a putative voltage-dependent Ca2+-permeable channel, AtTPC1, was cloned from Arabidopsis (Furuichi et al. 2001). AtTPC1 is ubiquitously expressed in the whole plant. Such voltage-dependent Ca2+-permeable channel may be involved in the elicitor-induced Ca2+ influx.
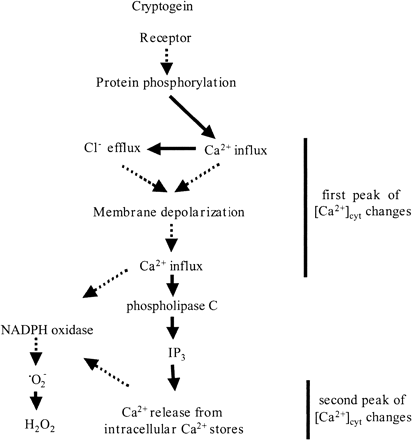
Cryptogein also triggered complexed pattern of H+ flux (Fig. 2A). Characteristic for the first peak is similar to the elicitor-induced transient cytoplasmic acidification and extracellular alkalinization (Kuchitsu et al. 1997) and may be due to a plasma membrane H+ influx. Though pathogenic signal-induced external alkalinization is observed in many experimental systems, its molecular mechanism is still unknown. The pH change started after the initiation of the Ca2+ influx and was inhibited by BAPTA, DIDS and K-252a (Kadota et al. unpublished results), suggesting that the pH changes are downstream events of the initial Ca2+ influx, Cl– efflux and protein phosphorylation. Plasma membrane H+ influx may be regulated by Ca2+, membrane depolarization and protein phosphorylation. The present simultaneous measurements and pharmacological analyses of various ion fluxes revealed that cryptogein induces a series of plasma membrane ion fluxes mediated by various ion channels (“ion channel cascade”) summarized in Fig. 8.
NADPH oxidase-dependent ROS production occurs downstream of the [Ca2+]cyt transients
Several recent reports have suggested that H2O2 triggers the changes in [Ca2+]cyt (Price et al. 1994, Levine et al. 1996, Takahashi et al. 1998, Kawano and Muto 2000, Pei et al. 2000). It has also been also suggested that H2O2 produced upon elicitation activates [Ca2+]cyt changes. Lecourieux et al. (2002) showed that 10 µM DPI, an inhibitor of NADPH oxidase, partially inhibited [Ca2+]cyt changes. Yeast elicitors and chitosan stimulate an NADPH-dependent, hyperpolarization-activated Ca2+ current (ICa) in Arabidopsis guard cells. Since this current is also activated by ROS (Pei et al. 2000), elicitor-induced ROS may activate ICa (Klüsener et al. 2002).
However, the present results suggest that ROS do not directly participate in the cryptogein-induced [Ca2+]cyt transient peaks and that the ROS production occurs downstream of the initial [Ca2+]cyt responses. This notion is supported by the following observations. Firstly, cryptogein-induced [Ca2+]cyt responses occurred prior to the production of ROS (Fig. 2B, C). The changes in [Ca2+]cyt occurred 64±4 s (n = 15) after cryptogein application, while •O2– and H2O2 production started at 124±6 s (n = 7) and 268±14 s (n = 7), respectively. Secondly, 2–5 µM DPI inhibited ROS production completely (Fig. 6A, B), but did not affect the initial [Ca2+]cyt increase triggered by cryptogein (Fig. 6C). Finally, cryptogein-induced H2O2 production was inhibited by DIDS, BAPTA and K-252a (Fig. 7), suggesting that the production of ROS requires Ca2+ influx, Cl– efflux and protein phosphorylation.
Overexpression of antisense RNA for NtrbohD, a homologue of the flavocytochrome of the neutrophil NADPH oxidase, resulted in repression of cryptogein-induced ROS production but did not affect the extracellular alkalinization (Simon-Plas et al. 2002). Considering that extracellular alkalinization requires an increase in [Ca2+]cyt (Tavernier et al. 1995, Kadota et al. unpublished results), cryptogein-induced initial [Ca2+]cyt increase may be intact even when the elicitor-induced ROS production is repressed by the antisense RNA. These results are consistent with the present finding that cryptogein-induced ROS production is not directly involved in the [Ca2+]cyt transients, and instead is triggered downstream of the fluxes of Ca2+ and Cl– as well as protein phosphorylation.
Concluding remarks
Based on the effects of various pharmacological inhibitors and the response lag times observed in the present study, we hypothesize that cryptogein-induced signaling pathway comprises the following processes summarized in Fig. 8. Cryptogein is recognized by plasma membrane-bound receptors (Wendehenne et al. 1995). Protein phosphorylation then induces a rapid Ca2+ influx that activates an efflux of Cl–. These ion fluxes could induce membrane depolarization, which would cause a further influx of Ca2+ via voltage-dependent Ca2+ channels (Ward et al. 1995). The Ca2+ influx could activate phospholipase C, which in turn would cause Ca2+ to be released from intracellular Ca2+ stores. We propose that these series of ion fluxes (“ion channel cascade”) play indispensable roles in defense signaling and regulation of downstream events including oxidative burst. These ion channels may be present as a complex together with other signaling components such as receptors and protein kinases/phosphatases (Yamazaki et al. 2003). Future molecular genetic identification and functional analyses of these ion channels should be one of the most important targets to understand the molecular mechanisms for recognition of pathogens and to manipulate defense signaling to improve disease resistance.
Materials and Methods
Plant material
Tobacco BY-2 (Nicotiana tabacum L. cv. Bright Yellow 2) cell suspensions and transgenic tobacco cell cultures that express apoaequorin protein specifically in the cytosol (Takahashi et al. 1997) were maintained by weekly dilution (1/100 and 1/50, respectively) of cells with fresh Linsmaier and Skoog (LS) medium modified according Nagata et al. (1992). Cells were maintained at 28°C with aeration (shaking at 100 rpm) in the dark.
Expression and purification of cryptogein
Pichia pastoris (strain GS115) bearing the plasmid pLEP3 was used for cryptogein production. Cryptogein was expressed according to O’Donohue et al. (1996) and was dissolved in distilled water. Cryptogein concentration was determined using UV spectroscopy employing extinction coefficients of 8,306 M–1 cm–1 at 277 nm (O’Donohue et al. 1995).
Chemicals
BAPTA, DIDS and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) were obtained from Dojindo Laboratories (Kumamoto, Japan). U73122 was purchased from Calbiochem (La Jolla, CA, U.S.A.). Coelenterazine and MCLA were obtained from Molecular Probes (Eugene, OR, U.S.A.). K-252a, neomycin and luminol were purchased from Wako Pure Chemical (Osaka, Japan).
Measurement of changes in [Ca2+]cyt
The apoaequorin-expressing BY-2 cell suspension (3 d after subculture) was incubated with 1 µM coelenterazine for at least 6 h at 28°C. Cell suspension (250 µl) was transferred to the culture tube (1.1 cm in diameter), and set in a luminometer (Lumicounter 2500, Microtech Nition, Chiba, Japan). In this luminometer, the culture tube rotates 17 revolutions every 3 s in the clockwise and counterclockwise in turn, and agitate the cells. Ca2+-dependent aequorin luminescence was measured after incubation for 15 min to stabilize the cells.
Measurement of pH and [Cl–]
Aliquots of cells (30 g fresh weight) that had been subcultured for 3 d were transferred to 30 ml of fresh culture medium that lacked KH2PO4 and CaCl2, but contained CaSO4. The cells were incubated in open 100-ml vials with shaking on a gyratory shaker at 160 rpm. The pH and [Cl–] of the culture medium were measured simultaneously with a combination electrode that was sensitive to H+ and Cl– (Models 9620 10-D & 8002, Horiba, Kyoto, Japan). Analog signals were converted to a digital signal with an A–D converter (power Lab/L800, AD Instruments, Castle Hill, Australia) and the data were analyzed with appropriate software (Chart v3.6.8, AD Instruments).
Measurement of ROS (•O2– and H2O2)
The apoaequorin-expressing BY-2 cell suspension (3 d after subculture) was used for measurement of •O2– and H2O2. After treatment the cells in normal growth medium with 20 µM MCLA, •O2–-dependent luminescence was measured with a luminometer (Lumicounter 2500, Microtech Nition, Chiba, Japan) as the same condition to the measurement of [Ca2+]cyt.
To monitor H2O2, cells were washed and resuspended in a 5 mM HEPES buffer (pH 7.0) containing 175 mM mannitol, 3 mM CaCl2, and 0.5 mM K2SO4. After a 3 h equilibration period on a gyratory shaker (100 rpm, 28°C), 0.5 mM luminol and 5 mM K-phosphate buffer (pH 7.0) was added. Fifteen minutes after the luminol application, H2O2-dependent chemiluminescence was monitored with a luminometer (Lumicounter 2500, Microtech Nition, Chiba, Japan) under the same condition as the measurement of [Ca2+]cyt.
Acknowledgments
The authors are grateful to Dr. Munehiro Kikuyama for technical support for the real time measurement of ion fluxes. We thank Dr. Toyoki Amano for valuable suggestions and Prof. Jean-Claude Pernollet for providing us with cryptogein. This work was supported in part by a grant from the Research for the Future Program of the Japan Society for the Promotion of Science and a grant for scientific research in priority areas from the Ministry of Education, Science, Culture, Sports and Technology, Japan. This work was supported in part by a Grant-in-Aid for the Research for the Future Program and Scientific Research (B) (no. 14340251) from the Japan Society for the Promotion of Science to K. K. and S. M., respectively, and Grants-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Science, Culture, Sports, and Technology, Japan to K. K. (No. 13039015) and S. M. (No. 13039008).
The authors would like to dedicate this article to the memory of Dr. Muto, who died on 23 January 2004.
Corresponding author: E-mail, kuchitsu@rs.noda.tus.ac.jp; Fax, +81-4-7123-9767.
Fig. 1 Apoaequorin-expressing cells exhibit different [Ca2+]cyt responses to cryptogein in different culture conditions. [Ca2+]cyt-dependent luminescence of reconstituted aequorin was monitored in tobacco cells treated with cryptogein (500 nM, solid lines) or DW (broken lines). (A) Apoaequorin-expressing BY-2 cells cultured in normal growth medium were incubated with 1 µM coelenterazine for 6 h. [Ca2+]cyt responses to cryptogein or DW application (arrows) were measured during shaking of the samples. Data are from one representative of 15 experiments. (B) Apoaequorin-expressing BY-2 cells were transferred to a suspension buffer containing 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, and 2 mM HEPES-HCl (pH 5.75) and incubated with 1 µM coelenterazine for 6 h. Cryptogein-induced [Ca2+]cyt changes were measured with shaking. Data are from one representative of three experiments. (C) Apoaequorin-expressing BY-2 cells cultured in the normal growth medium were incubated with 1 µM coelenterazine for 6 h. Cryptogein-induced [Ca2+]cyt changes were measured without shaking. Data are from one representative of three experiments. (D) Apoaequorin-expressing BY-2 cells were transferred to the suspension buffer and incubated with 1 µM coelenterazine for 6 h. Cryptogein-induced [Ca2+]cyt changes were measured without shaking. Data are from one representative of three experiments.
Fig. 2 Cryptogein-induced changes in pH, [Cl–] and ROS (•O2– and H2O2) production. BY-2 cells were treated with cryptogein (500 nM, solid line) or DW (broken line). (A) Extracellular pH and [Cl–] were monitored simultaneously. Data are from one representative of 6 experiments. (B) •O2– production in apoaequorin-expressing cells measured by MCLA luminescence. The cryptogein-induced [Ca2+]cyt changes in the same culture are also indicated. Data are from one representative of seven experiments. (C) H2O2 production in apoaequorin-expressing cells measured by luminol luminescence. The arrows correspond to cryptogein application. Data are from one representative of seven experiments.
Fig. 3 The two peaks in [Ca2+]cyt consist of extracellular Ca2+ influx and subsequent IP3-mediated Ca2+ release from intracellular Ca2+ stores. Effect of the Ca2+ chelator, BAPTA (A) and the phoshopholipase C inhibitors, neomycin (B) and U73122 (C) on cryptogein-induced [Ca2+]cyt responses (relative luminescence). Each inhibitor was applied 15 min before the application of cryptogein (arrows). The maximum luminescence value of the control cells was expressed as 1.0. Data are from one representative experiment of five.
Fig. 4 The [Ca2+]cyt transients may be regulated by protein phosphorylation and Cl– efflux. Effects of the protein kinase inhibitor, K-252a (A), and the Cl– channel blocker, DIDS (B) on cryptogein-induced [Ca2+]cyt responses (relative luminescence). Each inhibitor was applied 15 min before the application of cryptogein (arrows). The maximum luminescence value of the control cells was expressed as 1.0. Data are from one representative experiment of five.
Fig. 5 Efflux of Cl– is inhibited by DIDS and regulated by protein phosphorylation and Ca2+ influx. Effect of DIDS (A), BAPTA (B) and K-252 (C) on the cryptogein-induced Cl– efflux (relative luminescence). Each inhibitor was applied 15 min before the application of cryptogein (arrows). Data are from one representative experiment of three.
Fig. 6 The relationship between ROS (•O2– and H2O2) production and changes in [Ca2+]cyt. DPI, an inhibitor of NADPH oxidase, was applied to apoaequorin-expressing BY-2 cells and the effect on cryptogein-induced •O2– production (A), H2O2 production (B) and [Ca2+]cyt responses (C) was analyzed. Each inhibitor was applied 15 min before the application of cryptogein (arrows). The maximum luminescence value of the control cells was expressed as 1.0. Data are from one representative experiment of four.
Fig. 7 H2O2 production is inhibited by DIDS, BAPTA and K-252a. Effect of DIDS (A), BAPTA (B) and K-252 (C) on cryptogein-induced H2O2 production (relative luminescence) in apoaequorin-expressing BY-2 cells. Each inhibitor was applied 15 min before the application of cryptogein (arrows). The maximum luminescence value of the control cells was expressed as 1.0. Data are from one representative experiment of four.
Fig. 8 A model of cryptogein-induced ion fluxes and ROS production. The model was based on the effects of inhibitors (K-252a, BAPTA, U-73122, neomycin, DIDS, and DPI) and response lag times. Unbroken and broken arrows indicate established and hypothetical links, respectively.
Abbreviations
- BAPTA
1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- [Ca2+]cyt
cytosolic free Ca2+ concentration
- [Cl–]
Cl– concentration
- DIDS
4,4′-diisothiocyanostilbene-2,2′-disulfonate
- DPI
diphenylene iodonium
- IP3
inositol 1,4,5-trisphosphate
- MCLA, 2-methyl-6-[p-methoxyphenyl]-3,7-dihydroimidazo[1,2-a]pyrazin-3-one
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide
- NPPB
5-nitro-2-(3-phenylpropylamino)benzoic acid
- •O2–
superoxide anion
- ROS
reactive oxygen species.
References
Allen, G.J., Kuchitsu, K., Chu, S.P., Murata, Y. and Schroeder, J.I. (
Binet, M., Humbert, C., Lecourieux, D., Vantard, M. and Pugin, A. (
Blume, B., Nürnberger, T., Nass, N. and Scheel, D. (
Campling, B.G., Pym, J., Galbraith, P.R. and Cole, S.P. (
Cessna, S.G. and Low, P.S. (
Chandra, S., Stennis, M. and Low, P.S. (
Ebel, J., Bhagwat, A.A., Cosio, E.G., Feger, M., Kissel, U., Mithöfer, A. and Waldmüller, T. (
Franklin-Tong, V.E., Drobak, B.K., Allan, A.C., Watkins, P.A.C. and Trewavas, A.J. (
Furuichi, T., Cunningham, K.W. and Muto, S. (
Jabs, T., Tschöpe, M., Colling, C., Hahlbrock, K. and Scheel, D. (
Jones, A.M. and Dangl, J.L. (
Kawano, T. and Muto, S. (
Kikuyama, M., Kuchitsu, K., Shibuya, N. (
Klüsener, B., Young, J.J., Murata, Y., Allen, G.J., Mori, I.C., Hugouvieux, V. and Schroeder, J.I. (
Kuchitsu, K., Kikuyama, M. and Shibuya, N. (
Kuchitsu, K., Kosaka, H., Shiga, T. and Shibuya, N. (
Kuchitsu, K., Yazaki, Y., Sakano, K. and Shibuya, N. (
Kwak, J.M., Moon, J.-H., Murata, Y., Kuchitsu, K., Leonhardt, N., DeLong, A. and Schroeder, J.I. (
Kwak, J.M., Mori, I.C., Pei, Z.M., Leonhardt, N., Torres, M.A., Dangl, J.L., Bloom, R.E., Bodde, S., Jones, J.D. and Schroeder, J.I. (
Lecourieux, D., Mazars, C., Pauly, N., Ranjeva, R. and Pugin, A. (
Levine, A., Tenhaken, R., Dixon, R. and Lamb, C. (
Levine, A., Pennell, R.I., Alvarez, M.E., Palmer, R. and Lamb, C. (
Li, J., Wang, X.Q., Watson, M.B. and Assmann, S.M. (
Mithöfer, A., Ebel, J., Bhagwat, A.A., Boller, T. and Neuhaus-Url, G. (
Mithöfer, A., Fliegmann, J., Daxberger, A., Ebel, C., Neuhaus-Url, G., Bhagwat, A.A., Keister, D.L. and Ebel, J. (
Miyakawa, T., Mizushima, A., Hirose, K., Yamazawa, T., Bezprozvanny, I., Kurosaki, T. and Iino, M. (
Nagata, T., Nemoto, Y. and Hasezawa, S. (
O’Donohue, M.J., Boissy, G., Huet, J.C., Nespoulous, C., Brunie, S. and Pernollet, J.C. (
O’Donohue, M.J., Gousseau, H., Huet, J.C., Tepfer, D. and Pernollet, J.C. (
Pei, Z.-M., Kuchitsu, K., Ward, J.M., Schwarz, M. and Schroeder, J.I. (
Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G.J., Grill, E. and Schroeder, J.I. (
Price, A.H., Taylor, A., Ripley, S.J., Griffith, A., Trewavas, A.J. and Knight, M.R. (
Pugin, A., Frachisse, J.M., Tavernier, E., Bligny, R., Gout, E., Douce, R. and Guern, J. (
Ricci, P., Bonnet, P., Huet, J.C., Sallantin, M., Beauvais-Cante, F., Bruneteau, M., Billard, V., Michel, G. and Pernollet, J.C. (
Sagi, M. and Fluhr, R. (
Sanders, D., Pelloux, J., Brownlee, C. and Harper, J.F. (
Shibuya, N., Kaku, H., Kuchitsu, K. and Maliarik, M.J. (
Simon-Plas, F., Elmayan, T. and Blein, J.P. (
Takahashi, K., Isobe, M., Knight, M.R., Trewavas, A.J. and Muto, S. (
Takahashi, K., Isobe, M. and Muto, S. (
Takahashi, S., Katagiri, T., Hirayama, T., Yamaguchi-Shinozaki, K. and Shinozaki, K. (
Tavernier, E., Wendehenne, D., Blein, J.P. and Pugin, A. (
Turner, J.G. and Novacky, A. (
Uehara, K., Maruyama, N., Huang, C.K. and Nakano, M. (
Ward, J.M., Pei, Z.M. and Schroeder, J.I. (
Wendehenne, D., Binet, M.N., Blein, J.P., Ricci, P. and Pugin, A. (
Wendehenne, D., Lamotte, O., Frachisse, J.M., Barbier-Brygoo, H. and Pugin, A. (
Yamazaki, D., Yoshida, S., Asami, T and Kuchitsu, K. (



![Fig. 1 Apoaequorin-expressing cells exhibit different [Ca2+]cyt responses to cryptogein in different culture conditions. [Ca2+]cyt-dependent luminescence of reconstituted aequorin was monitored in tobacco cells treated with cryptogein (500 nM, solid lines) or DW (broken lines). (A) Apoaequorin-expressing BY-2 cells cultured in normal growth medium were incubated with 1 µM coelenterazine for 6 h. [Ca2+]cyt responses to cryptogein or DW application (arrows) were measured during shaking of the samples. Data are from one representative of 15 experiments. (B) Apoaequorin-expressing BY-2 cells were transferred to a suspension buffer containing 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, and 2 mM HEPES-HCl (pH 5.75) and incubated with 1 µM coelenterazine for 6 h. Cryptogein-induced [Ca2+]cyt changes were measured with shaking. Data are from one representative of three experiments. (C) Apoaequorin-expressing BY-2 cells cultured in the normal growth medium were incubated with 1 µM coelenterazine for 6 h. Cryptogein-induced [Ca2+]cyt changes were measured without shaking. Data are from one representative of three experiments. (D) Apoaequorin-expressing BY-2 cells were transferred to the suspension buffer and incubated with 1 µM coelenterazine for 6 h. Cryptogein-induced [Ca2+]cyt changes were measured without shaking. Data are from one representative of three experiments.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/45/2/10.1093_pcp_pch020/2/m_pch02001.gif?Expires=1716529959&Signature=UXLGNjELt1VEQCCsT2iVS3VfBJtUtnh9Tl0AHpIvWXBRH~BXaoIkZD-IV9H1dXUf3Ug1edDZ5APVynudQMZK~w2Z9BrjqgoGRo~~Hz6DloK0e8kPO9~jdHjglcUuhhTAWE2wxIdn9G5tOmyCWAFlHNE0TDpBF5SFoz5EM7v0y3epnxvzmkOcXvaZ1ZYvFfxvDGH0YRiji2y5nWzGFWTmFu9f0KbY5gW9eUAHmwi3VGv2V8-eBmrE3HI70tA0PJJLdUFUr1k2QO2IIRjb8rY0nbofM0ir1WNUk6pBY4UY~soL7mij~5oIWDblPRfwHPwTED8tlSrl6LYjI7y5ad2D3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2 Cryptogein-induced changes in pH, [Cl–] and ROS (•O2– and H2O2) production. BY-2 cells were treated with cryptogein (500 nM, solid line) or DW (broken line). (A) Extracellular pH and [Cl–] were monitored simultaneously. Data are from one representative of 6 experiments. (B) •O2– production in apoaequorin-expressing cells measured by MCLA luminescence. The cryptogein-induced [Ca2+]cyt changes in the same culture are also indicated. Data are from one representative of seven experiments. (C) H2O2 production in apoaequorin-expressing cells measured by luminol luminescence. The arrows correspond to cryptogein application. Data are from one representative of seven experiments.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/45/2/10.1093_pcp_pch020/2/m_pch02002.gif?Expires=1716529959&Signature=N7NnBJ-gxs7nREsI3DQLZ~ekkX1ydD7D7bZCoy6OWO-sKSRQH2gooPgqUxeZwCmKB1Cgtdqape7lq1jUKinnBWIBJKtCsixP4NuC45syW1A8X2rhTMaw11qdAMn0wUkHgMSoYH4JdI6Idg01qFRVTQsSyAtKBMJpWhWYzROxLEqFPmMwcdoLYtCdAL-Ky0j~Be27iKIp43Xo2U0aMwonfZS2iKPcQ-8fZDAhNzhAv8drbcos9oOSZGYZyH9s-9zoy-cXcyNF5qK6yqSZXQNkFV2wXeEvt~hVoKO34yzobJ~RmGUDy1gOYU~zAI1M5DbJYmoQ73WITsN9l0iQZ5bhgw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3 The two peaks in [Ca2+]cyt consist of extracellular Ca2+ influx and subsequent IP3-mediated Ca2+ release from intracellular Ca2+ stores. Effect of the Ca2+ chelator, BAPTA (A) and the phoshopholipase C inhibitors, neomycin (B) and U73122 (C) on cryptogein-induced [Ca2+]cyt responses (relative luminescence). Each inhibitor was applied 15 min before the application of cryptogein (arrows). The maximum luminescence value of the control cells was expressed as 1.0. Data are from one representative experiment of five.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/45/2/10.1093_pcp_pch020/2/m_pch02003.gif?Expires=1716529959&Signature=A11rqtKpbg3N7swqLubRQ9Y-7t9rHAhL77Z3GRtVCXD1t5fO269Ca~AmuOefsmX9UMq4ESJyy1HCxlM0FroW8etES5QQHKSFnxwkJPjS2g6IG4tipbdYI-~XCTgsLeOHjIq8gfTk2oDRUbxkPNZf~5d6mIiuTtrQWJmhFTyjuR-wpmnFJyJrbiOxfuEbI1t4ibJerwrRQK3Ykon-nfEnJNnLL3BBy0jACaFhWG-PJWaot9HJez-aKLTzd1dXg5FU7Eti9mAB9UCti5gKQG0MKs~uORIYSEF0ny9~9ev1nVd6sb1mxUW0GEiLDyOt7~04N6QCv6TCHR8wXEPIt1rJcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4 The [Ca2+]cyt transients may be regulated by protein phosphorylation and Cl– efflux. Effects of the protein kinase inhibitor, K-252a (A), and the Cl– channel blocker, DIDS (B) on cryptogein-induced [Ca2+]cyt responses (relative luminescence). Each inhibitor was applied 15 min before the application of cryptogein (arrows). The maximum luminescence value of the control cells was expressed as 1.0. Data are from one representative experiment of five.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/45/2/10.1093_pcp_pch020/2/m_pch02004.gif?Expires=1716529959&Signature=Ngd0RodFVc7aAIUPQE9CycEhPv5b2IPAt62dqu~BRg-ATx8I4SQH2kR6xjMiikFLQgqttN9nkN0IO6pBQxsJPTS2o9hwp2CTCpdmDzRMCJDqqUEtIExrEZ8NAOZk-ebtxKzN8UhfrDlIBxPo9Gh-HICp9Ee4pVoe8Wp29UkywiQsOrT54wxCHuDfeSS7lIgJUSZJ8TvOIp5Fiktu7S6OgW9q1JSbBCkiIJ3a6EMu-9jZV3eA52Y8y8WlrN3~eGJ5umrFrs2F6pkmNBnzB6xeXfoJWfPVVJo0uzuCq1867aPzX7uKEYB63ebBhI0Wmr7m9MN-XKu6~JXZHoPHSWj52A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6 The relationship between ROS (•O2– and H2O2) production and changes in [Ca2+]cyt. DPI, an inhibitor of NADPH oxidase, was applied to apoaequorin-expressing BY-2 cells and the effect on cryptogein-induced •O2– production (A), H2O2 production (B) and [Ca2+]cyt responses (C) was analyzed. Each inhibitor was applied 15 min before the application of cryptogein (arrows). The maximum luminescence value of the control cells was expressed as 1.0. Data are from one representative experiment of four.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/45/2/10.1093_pcp_pch020/2/m_pch02006.gif?Expires=1716529959&Signature=IpUnSBF0XQVjvAuRTw5AbqdA6ZzUKwfvIPIJQJm6ALE-CCXxcld3bz57BLhuyCqtfkbgauG-wgCVn3doGosLZpzcEmIIUJS3lHZheYskEfvqt07otTIXrC0m5ArbSy9hiV3OpCaguibvyKDqBHyMS2StSvyqsYM~Yy9HlvAvHmno1N3uYmgZ4gS~BCiBrdojTMA7BGsXKe9jsNQgKfTLIYCZe8A638ITr4HgX7r04pcv3npdlLP7K1grVFm-kbj71ZcoD72E-qzyYtnN3OLFQUZiX8Ti~rJ7zsJXea1Q69lCpHwwGQI4NQK3GutzVcdVg-j9qCzVSRp9L7zWdMwfVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)