-
PDF
- Split View
-
Views
-
Cite
Cite
Howard V. Davies, Louise V. T. Shepherd, Michael M. Burrell, Fernando Carrari, Ewa Urbanczyk-Wochniak, Andrea Leisse, Robert D. Hancock, Mark Taylor, Roberto Viola, Heather Ross, Diane McRae, Lothar Willmitzer, Alisdair R. Fernie, Modulation of Fructokinase Activity of Potato (Solanum tuberosum) Results in Substantial Shifts in Tuber Metabolism, Plant and Cell Physiology, Volume 46, Issue 7, July 2005, Pages 1103–1115, https://doi.org/10.1093/pcp/pci123
Close - Share Icon Share
Abstract
Potato plants (Solanum tuberosum L. cvs Desiree and Record) transformed with sense and antisense constructs of a cDNA encoding the potato fructokinase StFK1 exhibited altered transcription of this gene, altered amount of protein and altered enzyme activities. Measurement of the maximal catalytic activity of fructokinase revealed a 2-fold variation in leaf (from 90 to 180% of wild type activity) and either a 10- or 30-fold variation in tuber (from 10 or 30% to 300% in Record and Desiree, respectively) activity. The comparative effect of the antisense construct in leaf and tuber tissue suggests that this isoform is only a minor contributor to the total fructokinase activity in the leaf but the predominant isoform in the tuber. Antisense inhibition of the fructokinase resulted in a reduced tuber yield; however, its overexpression had no impact on this parameter. The modulation of fructokinase activity had few, consistent effects on carbohydrate levels, with the exception of a general increase in glucose content in the antisense lines, suggesting that this enzyme is not important for the control of starch synthesis. However, when metabolic fluxes were estimated, it became apparent that the transgenic lines display a marked shift in metabolism, with the rate of redistribution of radiolabel to sucrose markedly affected by the activity of fructokinase. These data suggest an important role for fructokinase, acting in concert with sucrose synthase, in maintaining a balance between sucrose synthesis and degradation by a mechanism independent of that controlled by the hexose phosphate-mediated activation of sucrose phosphate synthase.
Introduction
Fructokinase (FK; EC 2.7.1.4) efficiently catalyses the phosphorylation of fructose to fructose 6-phosphate. Hexose phosphorylation is especially important in plants since the use of free phosphates is particularly complex (Kruger 1997). However, although there have been many reports on the more generic enzyme hexokinase (Jang et al. 1997, Martinez-Barajas and Randall 1998, Sindelar et al. 1998, Dai et al. 1999, Veramendi et al. 1999, Veramendi et al. 2002, Moore et al. 2003, Roessner-Tunali et al. 2003), the role of FK is much less well characterized (Pego and Smeekens 2000). This is somewhat surprising since in vitro kinetic studies of FK isoforms from potato were carried out earlier than (Gardener et al. 1992) or simultaneously with those of hexokinase (Renz and Stitt 1993, Renz et al. 1993). Similarly, the gene for potato FK was cloned prior to any for hexokinase (Taylor et al. 1995) and specifically for those for potato hexokinase (Veramendi et al. 1999, Veramendi et al. 2002).
Although several gene functional studies have been carried out to assess the role of hexokinase, to date the majority of assessments of plant FKs have been carried out in heterologous expression systems (Kanayama et al. 1998; Jiang et al. 2003, Zhang et al. 2003). The exception to this statement is the analysis of FKs from tomato, performed in some detail by Bennett and co-workers (Kanayama et al. 1998, Dai et al. 2002a, Odanaka et al. 2002, German et al. 2003). In an initial study, they established that the two major tomato isoforms differed in both their regulation by substrate and their spatial location, suggesting that the highly regulated form (FKII) was most probably involved in starch metabolism (Kanayama et al. 1998). These suggestions were largely supported by studies in which the expression of the isoforms was independently repressed by antisense inhibition revealing distinct phenotypes. The results from this study collectively indicated that flowering time is specifically promoted by FKI and that FKII plays specific roles in contributing to stem and root growth and to seed development; however, neither modulation had any dramatic effect on fruit starch content (Dai et al. 2002b, Odanaka et al. 2002). Further experimentation indicated that the modulated stem growth may well result from a reduction in the active xylem of the tomato stem, implying that FKII may have a role in the development of this important transport conduit (German et al. 2003). Thus there is growing evidence of the importance of FK for normal plant development. However, to date, there has been very little assessment of the direct metabolic role of the enzyme in plants.
In the potato, a fairly complete characterization of the sucrose to starch transition of the tuber has been carried out over the last few years (Stitt and Sonnewald 1995, Kossmann and Lloyd 2000, Fernie et al. 2002). In addition, recent estimates of mass action ratios, derived from the analysis of subcellular metabolite pool sizes by non-aqueous fractionation (Farre et al. 2001, Tiessen et al. 2002), were able to yield important insights into possible sites of metabolic regulation within this pathway. The regulatory sites identified in these studies included the reaction catalysed by FK. When the results of these earlier studies were analysed further within the framework of metabolic control analysis, it became clear that much of the control of this pathway is vested in the transport of ATP to the plastid and the first two steps of plastidial starch synthesis (Geigenberger et al. 2004a). However, given that this route from sucrose to starch is non-linear, it is impossible to use the summation theory (Kacser and Burns 1973) to predict the relative importance of steps in the pathway for which transgenics have not been created. One such reaction step is that catalysed by FK. When considered alongside the fact that FK is well characterized as a regulatory enzyme (Renz et al. 1993, Kanayama et al. 1998), this gives ample rationale for studying the enzyme via the reverse genetics approach. In this study, we describe the generation and characterization of both overexpressing and antisense lines of potato FK in the cvs Desiree and Record. We discuss the impact of these manipulations with respect to plant growth, tuber yield and metabolism, paying particular attention to tuber carbohydrate metabolism.
Results
Preparation and selection of transgenic plants modified in expression of StFK1
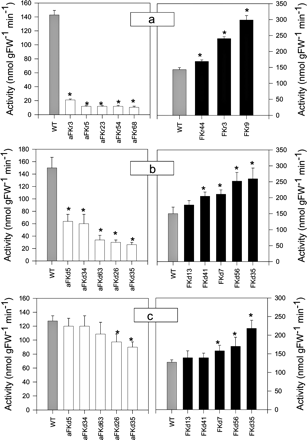
The gene encoding a fructokinase (StFK1) from potato (Taylor et al. 1995) was cloned in sense and antisense orientations into the plant transformation vectors pBIN19 and pBINARkan between either the 35S cauliflower mosiac virus (CaMV) promoter and the CaMV terminator or between the 35S CaMV promoter and ocs terminator, for transformation into Solanum tuberosum cvs Record and Desiree, respectively (Fig. 1A). Wild-type potato leaves were transformed with one of these constructs using an Agrobacterium-mediated protocol (Rocha-Sosa et al. 1989), and approximately 80 transgenic lines were transferred to the greenhouse for each genotype for screening at the level of transgene expression. Following the initial screening (Fig. 1B; data shown only from Desiree cv.), up to five lines per genotype were chosen that exhibited appreciable expression. These lines were amplified and replicates from each line were grown in the greenhouse in 2 liters pots to confirm the expression of the transgene. Immunoblots using an antibody raised against pea seed FK (D. Randall, personal communication) also indicated that the FK protein content was depressed in the antisense and elevated in the overexpression lines (Fig. 1C). Before selecting the lines for further experimentation, we next analysed the activity of FK in the transgenic lines using assays optimized for potato leaf and tuber samples (Fig. 2A–C). Interestingly, the greatest range of hexokinase activity was found in tubers of the Record cv. which exhibited between 6 and 211% of the activity found in wild type; tubers of the Desiree cv. also exhibited a wide range of activities (20–177%), whereas that observed in leaves of the Desiree plants was relatively low (75–175%). Reassuringly, the modification of enzymatic activity was specific to fructose phosphorylation since no significant differences were observed in hexokinase activity (data not shown).
Phenotypic characterization of StFK plants
The transgenic lines exhibited a mild aerial growth phenotype, with the antisense lines appearing stunted with respect to the wild type whilst the overexpressors were, if anything slightly bigger than the wild-type plants (lines aFKd26 and aFKd34 exhibited a total aerial biomass of 188 ± 7 and 191 ± 5, respectively, whilst lines FKd26 and FKd34 exhibited a total aerial biomass of 218 ± 8 and 211 ± 14 respectively, in contrast to the 206 ± 12 exhibited by wild type; all values were determined in six independent plants after 10 weeks of growth and represent mean g FW ± SE. This effect was noted in both the Record and Desiree backgrounds; however, the data presented are for Desiree only. Given that transgenic tomato plants also displayed a stunted growth pattern (German et al. 2003), we took care to observe if the plants created here shared any other phenotypic traits. However, in contrast to their findings, we were not able to observe wilting in young potato leaves.
With regard to tuber yield and phenotype, field trials carried out in two successive years produced generally consistent results (Table 1 and data not shown). In 1998, the field trial revealed a decrease in tuber number in the antisense lines (significantly so in the case of lines aFKr3, aFKr 23 and aFKr54). This finding was replicated in the case of line aFKr3 in the previous year’s field trial. However, the other results were less consistent, and one of the sense lines, FKr44, also exhibited reduced tuber number in this harvest. Interestingly when the percentage dry matter content was measured (a parameter indicative of starch yield), this was also reduced in those antisense lines which show a reduced number of tubers and increased in the sense line FKr3. When the total tuber yield per plant is assessed, it is clear that line aFKr3 displays a reduced yield; however, this is the only line to do so consistently across the yield trials. When the plants were grown in the greenhouse, there was an apparent trend in the specific gravity of the tubers (another marker for tuber starch content) and again in their percentage dry matter, suggesting a correlation between FK activity and tuber starch content.
Measurement of activities of key enzymes of metabolism
Since the effect on the aerial phenotype was quite dramatic, especially given the fact that the changes in the activity of FK were relatively mild in the leaf, we next assayed a range of key enzymes of carbohydrate metabolism. We performed these analyses in extracts of leaf material harvested from healthy fully expanded 10-week-old leaves (of the Desiree plants) in the middle of the light period. Analysis of ADPglucose pyrophosphorylase (AGPase), sucrose synthase, UDPglucose pyrophosphorylase, phosphoglucose isomerase, phosophoglucomutase, phosphofructokinase, pyruvate kinase, enolase and aldolase revealed no significant changes in the activities of these enzymes in the transformants, with the exception of a 30% increase in phosphoglucomutase in line aFKd5, 25 and 30% increases in pyruvate kinase and aldolase, respectively, in line aFKd26 and a 28% decrease in the activity of enolase in line FKd35 with respect to wild type.
Determination of leaf metabolite content
Given that there was a notable aerial phenotype in the transformants (despite the fact that changes in enzyme activity were largely confined to the minor changes observed in FK activity), we next determined the levels of a wide range of metabolites using an established gas chromatography–mass spectrometry (GC-MS) profiling method. In addition, we measured the leaf starch content. The rationale was to identify metabolic changes diagnostic of modified photosynthesis (Stitt 1997, Lytovchenko et al. 2002a, Lytovchenko et al. 2002b). For this purpose, we analysed the metabolite content in samples from the two antisense lines aFKd26 and aFKd34 and the two overexpression lines FKd13 and FKd41 alongside their wild-type control cv. Desiree (Supplementary Table 1). The starch content of leaves of the overexpressing lines was unaltered with respect to wild type (3.5 ± 0.9, 2.9 ± 0.5 and 4.3 ± 0.8 µmol g FW–1 for FKd13, FKd41 and wild type, respectively), whilst that of the antisense lines was significantly decreased (1.3 ± 0.02 and 1.7 ± 0.3 for aFKd26 and aFKd34, respectively). This pattern is partially mirrored by the levels of phosphorylated intermediates in these lines (Table 2), given that the antisense lines display mild decreases in the levels of hexose phosphates. However, it is important to note that these changes are in no instance statistically significant. There was also little change in the levels of leaf metabolites, with only line aFKd26 exhibiting significant changes in the levels of amino acids (decreasing in the case of arginine and increasing in the case of proline). Other changes that were documented in the transformants were an increase in the levels of dehydroascorbate in the antisense lines, a reduction in the level of malate and phosphoric acid in line FKd41 and a reduction in benzoate in lines aFKd26 and aFKd34. In addition to the scarcity of significant changes in the individual metabolite pools, it is interesting to note that there are also no patterns of change in the leaf metabolite pools that correlate with the FK activity.
Determination of tuber carbohydrate content
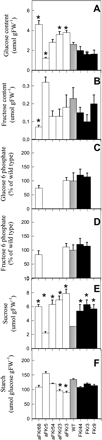
Having established that there was little effect of the manipulation of FK activity in leaf metabolism, we next turned our attention to the tuber. Given that the yield trials indicated, if anything, a decrease in starch content with FK down-regulation, we first analysed the tuber carbohydrate content in developing tubers of both the Record and the Desiree transformants (Fig. 3, 4) and in mature tubers of the Record plants (data not shown). In the case of the Record transformants, there is a reasonable correlation between the amount of glucose and the FK activity, with the sense lines tending to contain less glucose and the antisense lines tending to contain more (although it should be noted that line aFKr5 contains significantly less glucose than the wild type). Levels of fructose are largely unaltered in the transgenic lines, with the exception of line aFKr68 which contains significantly lower fructose. There was a trend toward increases in glucose 6-phosphate and fructose 6-phosphate with increasing FK activity; however, these changes were significant neither in the antisense nor in the overexpression lines. With the exception of line aFKr5, which seems somewhat anomalous, both sense and antisense lines contain higher levels of sucrose than the wild type. Consistent with the early tuber density measurements, however, the starch content of both sense and antisense lines seems somewhat lower than the wild type, significantly so in the case of lines aFKr23 and aFKr3. These changes were mirrored in mature tubers harvested from the same lines.
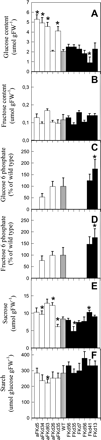
Analysis of the Desiree tubers revealed similar changes in the carbohydrate content of the transformants. Four of the five antisense lines exhibited significant increases in the glucose level, whilst one of the overexpressors, line FKd41, displayed a decreased glucose level. As was observed with cv. Record plants, the fructose levels of the transformants were relatively unaltered in the Desiree background. The levels of glucose 6-phosphate and fructose 6-phodphate correlated fairly well to the activity of FK displayed by the transformants; however, these differences were only significant in the case of the overexpressing lines. Sucrose levels were generally increased in the antisense lines (with the exception of the significant decrease in line aFKd35), but were not reproducibly altered in the overexpression lines with respect to wild type. Starch content in these lines appears to be positively correlated with respect to FK activity but, with respect to wild type, these differences were only significant for the decrease observed in line aFKd63.
Determination of tuber metabolite content
Studies were expanded to evaluate the levels of a wider range of metabolites of primary metabolism. For this purpose, we assessed the relative metabolite levels of two sense and two antisense lines in both Desiree and Record cvs utilizing an established GC-MS protocol (Supplementary Tables 2 and 3, respectively). Similar to the situation that was observed in leaves and following manipulation of hexokinase activity in potato tubers (A.R. Fernie, unpublished results), there were relatively few changes in the levels either of organic acids or amino acids or of the minor carbohydrates of the potato tuber. Furthermore, many of those that we observed either did not seem to correlate with the expression level or were not conserved between the genetic backgrounds. There are, however, several changes that are similar across the data sets. For example, there appears to be clear positive correlations of dehydroascorbate and arabinose levels with FK activity. The other differences observed were confined to a single genetic background. In cv. Record, overexpression of fructokinase also leads to increases in alanine, asparagine, ornithine and proline as well as saccharate, quinate, citrate, fucose and glycerol 1-phosphate, whereas its repression leads to increases in γ-aminobutyric acid (GABA), glutamine, lysine, tyrosine, valine, fumarate, malate and quinate and decreased maltose. In cv. Desiree, fewer changes were noted, with antisense lines characterized by elevated malate, galactose and mannose and depressed levels of β-alanine, tyramine, saccharate and shikimate, whereas overexpression led to decreased levels of glycine, tyramine, tyrosine and succinate.
Redistribution of radiolabel following incubation of tuber discs in labelled substrate
In order to gain a deeper insight into the changes in tuber metabolism, radiolabelling experiments were performed with the characterized cv. Record sense line FKr3 and antisense line aFKr3. These lines did not display the most extreme changes in the level of FK and showed a similar metabolic phenotype to the other Record transformants. Excised tuber discs were incubated with either radiolabelled fructose or sucrose. It should be noted that line aFKr3 was characterized by a reduction in tuber yield; however, given that our experiments were carried out on tuber discs of equivalent mass, this should not overly influence the observations reported here. Following incubation in [U14C]fructose, line FKr3 exhibited an elevated rate of fructose uptake and consequently an elevated rate of [U14C]fructose metabolism. This line also exhibited a greater proportional redistribution of label into carbon dioxide and starch which is most probably indicative of a higher rate of plastidial metabolism. In contrast, the relative radiolabel redistribution to sucrose, amino acids and unidentified insolubles was decreased with respect to wild type. The situation in the antisense line, aFKr3, is largely the converse: this line exhibits decreased rates of uptake and metabolism and a decreased redistribution of radiolabel to glucose, unidentified sugars, amino acids, unidentified insolubles and starch, and a dramatic increase in redistribution of radiolabel to sucrose. The concentrations of fructose remaining in the medium at the end of the experiment were 3.25, 2.86 and 3.28 mM in wild type, FKr3 and aFKr3, respectively, indicating that these observations were unlikely to be influenced by substrate availability. However, since interpretation of these results may be complicated by the varying levels of dilution of the specific activity in pools of the metabolic intermediates (Geigenberger et al. 1997), we determined the relative fluxes of glycolysis and starch synthesis, and glycolysis and sucrose synthesis in these lines. Flux through glycolysis was estimated by summation of the radiolabel recovered in carbon dioxide, organic acids and amino acids (as described previously; Fernie et al. 2001a). Given that all three pathways share common intermediates, i.e. the hexose phosphate pool, this calculation effectively removes any bias imposed by the fact that the different genotypes may display different specific activities of the hexose phosphate pool.
The relative fluxes deduced in this manner suggest that line FKr3 is characterized by a decreased rate of glycolysis with respect to starch synthesis, whereas the line aFKr3 is characterized by an increased rate of glycolysis with respect to starch synthesis but a dramatically reduced rate of glycolysis with respect to sucrose synthesis.
Following incubation in [U14C]sucrose, a similar pattern emerged, with line FKr3 again showing increased uptake and metabolism of the [U14C]sucrose. As would be expected, the label recovered in fructose was significantly lower in this overexpressing line as was that accumulating in glucose. Similarly to the situation observed following the feeding of fructose, the proportion of radiolabel redistributed to organic and amino acids was also diminished in this line, whilst again that redistributed to starch was dramatically increased. In contrast to the situation observed following the feeding of fructose, the release of 14CO2 from line FKr3 was not significantly higher than the wild type following incubation in [U14C]sucrose. The pattern of metabolism of [U14C]sucrose in line aFKr3 also fairly closely resembled that described above for the radiolabelled fructose. In contrast to the situation observed in line FKr3, radiolabel was dramatically increased in fructose with respect to the wild type. Moreover, there was a large increase in the relative redistribution of radiolabel in organic acids in this line. However, these changes were again accompanied by a large decrease in the redistribution of radiolabel into amino acids, starch and other insoluble compounds. As for the fructose experiment, the measurements of sucrose concentrations at the end of the experiment (4.25, 3.71 and 3.61 mM in wild type, FKr3 and aFKr3, respectively) indicate that these observations were unlikely to be influenced by substrate availability. Moreover, when the relative rates of glycolysis and starch synthesis were calculated, as defined above, the metabolic shifts in the transgenics were very similar to those estimated from the [U14C]fructose data.
Discussion
Genetic manipulation of StFKI in potato plants
Despite the fact that StFK1 was cloned at a relatively early time point (Taylor et al. 1995) and that it has been pinpointed as a potential regulatory point by a combination of allosteric, kinetic and theoretical factors (Renz et al. 1993, Viola 1996, Geigenberger et al. 2004b), the role of this enzyme in the potato has not yet been addressed via direct molecular means. Whilst the role of FK has been the subject of intensive recent investigation in the closely related species tomato (Solanum lycopersicum; Kanayama et al. 1997, Odanaka et al. 2002, German et al. 2003), the profound differences in metabolism in these species (see Fernie and Willmitzer 2004 for details) led us to address the role of FK in potato. For this purpose, we generated transgenic potato plants in two cultivars of potato—Record and Desiree that expressed StFK1 in both the sense and antisense orientation. The identification and preliminary characterization of these lines revealed that the pattern of expression of StFK1 is similar to that previously reported for the tomato FRKII, with seemingly much higher relative expression in sink tissues (on the basis of enzyme activity measurements, the antisense effect being much more predominant in the tuber than in leaf tissue). This is perhaps unsurprising given that these two FKs share 97% homology at the amino acid and 93% homology at the nucleotide level (Dai et al. 1997).
Effect of manipulation of fructokinase activity on plant growth
Suppression of tomato FK resulted in growth inhibition of both stems and roots and a reduction in the number of flowers, and consequently fruits, in addition to a reduced number of seeds per fruit (Odanaka et al. 2002). The results of a second independent experiment also implied a role for FK in xylem development, with antisense plants being characterized by a wilting phenotype in young, but not mature, leaves as well as displaying increased stomatal resistance coupled to a deceased rate of photosynthesis (German et al. 2003). However, care must be taken in interpreting these observations since tomato plants displaying increased FK activity also displayed these traits, suggesting that they may be secondary, pleiotropic effects of the genetic manipulation. That said, the fact that the growth phenotype was observed in independent studies using two distinct tomato cultivars provides strong support for a vital role for this enzyme in source to sink assimilate partitioning. Intriguingly, the antisense repression of StFK1 in potato, described here, also resulted in decreased aerial growth in both cultivars, and antisense transformants were furthermore generally characterized by a reduced rate of tuberization and a reduced total tuber yield. When taken together, these data suggest that the importance of FK in the maintenance of assimilate partitioning is conserved across cultivated species of the Solanaceous family. These data are as such consistent with observations that the antisense inhibition of sucrose synthase, one of the two known routes for the provision of substrate to the FK, had severe growth effects in both potato (Zrenner et al. 1995) and tomato (D’Aoust et al. 1999). The absence of such growth effects on the inhibition of either isoform of potato hexokinase (Veramendi et al. 1999, Veramendi et al. 2002) strengthens support for the notion that the sucrose synthase route of sucrose degradation is important for the maintenance of sink strength in the potato tuber. The overexpression of FK did not, however, result in elevation in the rate of tuberization or an increased tuber yield, suggesting that this enzyme is not normally limiting for growth in the potato. That said, the percentage dry matter content, which is indicative of the starch content, suggests a positive correlation between the FK activity and starch accumulation, which is in accordance with earlier studies hinting at the possible importance of FK in starch synthesis within the potato tuber (discussed in detail below; Ross et al. 1994, Viola 1996, Geigenberger et al. 2004b).
Effect of manipulation of fructokinase activity on leaf metabolism
Since we observed a decreased aerial growth in the antisense lines, we decided to perform a preliminary characterization of leaf metabolism. For this purpose, we analysed FK activities and metabolite levels in source leaves of the Desiree transgenics. Strikingly, the antisense inhibition of StFK1 had dramatic effects on the activity of tuber FK but much less severe effects on that of the leaf FK; this is, however, consistent with the pattern of expression of this gene and suggests that StFK1 is the minor form of FK of the leaf. This observation suggests that, as in the tomato (Odanaka et al. 2002), the different isoforms of FK are differentially expressed in the potato. Despite the fact that the range of FK activities was relatively small, we observed a clear trend in the level of hexose phosphates in the transformants, with the antisense plants displaying reduced contents whereas the overexpressors displayed increased content. However, it must be noted that these changes were, in no instance, significant. Similarly few changes were apparent when the levels of metabolites less intimately associated with the FK reaction were analysed by the use of an established GC-MS-based metabolite profiling method. This observation thus suggests that, despite the minor effect on aerial growth, the modification of FK activity has relatively minor effects on photosynthetic metabolism in the potato. Whilst redundancy of the FK may explain the lack of effects in the antisense plants, the fact that its overexpression had little effect is perhaps more surprising especially since the overexpression of a mammalian ketohexokinase, which catalyses the phosphorylation of the C1 atom of fructose, had dramatic consequences on leaf morphology and metabolism (Geigenberger et al. 2004b). The overexpression of the mammalian enzyme resulted in dramatic repression of photosynthesis and hypoxic conditions developing in the leaf veins. However, in this previous study, we postulated that this was most likely to be due to elevated production of additional triose phosphates and a direct inhibition of photosynthesis by glyceraldehydes, since fructose 1-phosphate did not accumualate in these plants. The lack of major effects on the overexpression of the potato FK is in keeping with these previous conclusions.
Effect of manipulation of fructokinase activity on tuber metabolism
Although the effect of modulating FK was relatively minor in the leaf, it led to much more severe effects in the tuber (summarized in Fig. 5). Activity measurements in the antisense lines suggest that this isoform is clearly the major FK activity within the tuber. Potato plants exhibiting reduced FK were characterized by a reduced tuber number and a reduced tuber yield. This phenotype is similar to that observed previously on the antisense inhibition of the major isoform of sucrose synthase in the tuber (Zrenner et al. 1995) and as such supports earlier claims that this route of sucrose degradation is an important determinant of sink strength. However, given that there was no clear link between the tuber number and tuber yield in the overexpression lines, it would appear that the FK activity resident in potato tubers does not limit tuber formation or growth. When the steady-state carbohydrate levels are considered in both the Record and Desiree transformants, a couple of interesting features are conserved: notably the fructose content of the tubers is independent of the FK activity, despite the fact that the glucose content appears to be inversely correlated to the FK activity. Moreover, the starch content is significantly decreased in a few of the antisense lines, whist sucrose levels are generally elevated in the antisense lines (but also in some of the sense lines). In addition, the hexose phosphates are tendentially increased in the sense lines and decreased in the antisense lines. At first glance, the lack of change in fructose content in the antisense lines is surprising; however, it is worth bearing in mind that sucrose synthase is feedback inhibited by fructose and is therefore potentially operating at a slower rate in the antisense lines. As mentioned earlier, FK is one of the few reaction steps of the sucrose to starch transition for which the control coefficient has not yet been calculated. However, from the data presented in Figs. 3 and 4, it can be surmised that, despite the mild decrease in starch in some of the antisense lines, FK exhibits very little control over this pathway. Although physiologically very distinct, it is interesting to compare these data with those reported for transgenic tomato in which the antisense inhibition of the tomato homolog of StFK1 was carried out (Dai et al. 2002a). The repression of FK in the tomato also did not have an adverse effect on starch yield, and increases of starch were even reported in young fruits of the FK-deficient lines. These findings are the converse of suggestions made on the basis of earlier studies (Viola et al. 1991, Schaffer and Petreikov 1997), in that they suggest that FK activity is not limiting for starch synthesis in Solanaceous species. This is perhaps not entirely surprising given that the concentration of fructose available for the enzyme is tightly regulated by allosteric properties of the sucrose synthase reaction (Doehlert 1987, Geigenberger and Stitt 1993).
Despite the lack of change in starch levels, radiolabel feeding experiments revealed that the transgenic tubers were characterized by a marked shift in metabolism. These flux experiments revealed highly interesting changes, with overexpression of FK stimulating uptake and metabolism of both fructose and sucrose. Moreover, the overexpression of FK resulted in a decrease in partitioning of label toward sucrose, whereas, conversely, its antisense resulted in a massive increase in partitioning of fructose toward sucrose. This is intriguing since previous experiments have documented that even mild increases in hexose phosphates stimulate sucrose cycling via activation of sucrose phosphate synthesis (Sweetlove et al. 1999, Fernie et al. 2001b). However, here the pattern of change in hexose phosphate content is opposite to that in redistribution of radiolabel to sucrose, and (given that the changes in partitioning are much larger than those in the hexose phosphate pool size) presumably the rate of sucrose synthesis. This suggests that the changes in sucrose synthesis are largely conferred by an alteration of the equilibrium position of sucrose synthase to favour sucrose formation.
These observations are entirely consistent with the allosteric properties of sucrose synthase discussed above. That this regulation can seemingly over-ride that occurring at sucrose phosphate synthase is highly interesting. However, it is important to note that the steady-state levels of sucrose within the tuber do not correlate with FK activity, being elevated in both sense and antisense lines. Although it could be argued that the data presented here could merely be reflective of shifts in the endogenous pool sizes of the sugar pools, the fact that flux estimates from both the fructose and sucrose experiments are in good agreement with one another suggests that this is not the case. That said, more detailed studies are likely to be required to understand fully the involvement of the FK reaction in the regulation of sucrose degradation and (re)synthesis within the tuber. In addition to the changes seen in sucrose metabolism, there was an apparent elevated starch synthesis in the overexpressors but an apparent reduction in this rate in the antisense plants. Given that the hexose phosphates are the direct precursors for starch synthesis, this is probably not too surprising; however, it is once again the converse of the steady-state observations. There is, however, relatively little effect on the redistribution toward glycolytic products, which is consistent with the fact that there is little variance within the metabolites determined by GC-MS (Defernez et al. 2004, this study).
In conclusion, these studies suggest that StFKI, like sucrose synthase, plays an important role in sink strength, most probably due to its ability to modulate sucrose uptake and metabolism, but is not of great importance in leaf photosynthetic metabolism. They show that the major tuber isoform of FK has little impact on glycolysis and subsequently on metabolites downstream of glycolysis. They also reveal that in vivo, FK has little influence on starch synthesis, although modification of FK can have a large effect on partitioning of radiolabel to starch in well oxygenated tuber discs. To summarize, FK appears to be able to act in concert with sucrose synthase to maintain a balance between sucrose synthesis and degradation by a mechanism independent of that controlled by the hexose phosphate-mediated activation of sucrose phosphate synthase; however, further studies are required to understand the exact mechanisms by which this is achieved.
Materials and Methods
Plant material
Solanum tuberosum L. cv. Desiree was obtained from Saatzucht Lange (Bad Schwartau, Germany), whilst cv. Record was obtained from cultivar stocks at the Scottish Crop Research Institute (SCRI). Plants were maintained in tissue culture with a 16 h light/8 h dark regime on MS medium (Murashige and Skoog 1962) containing 2% sucrose. In the greenhouse, plants were grown under the same light regime with a minimum of 250 µmol photons m–2 s–1 at 22°C. Transgenic plants of cv. Record were also grown in the field at SCRI. In 1997 and 1998, 10 tubers of each transgenic and control line were planted in rows with a 30 cm spacing between plants. Standard potato fertilizer regimes were applied, and plants were allowed to grow to full maturity. Each plant was then harvested separately for determination of tuber numbers, fresh weight yield and specific gravity (percentage dry matter).
Chemicals
All chemicals were purchased from Sigma-Aldrich Chemie GmbH (Deisenhofen, Germany), with the exception of the starch determination kit and biochemical enzymes from Boehringer Mannheim (Mannheim, Germany), MSTFA which was purchased from Macherey-Nagel GmbH & Co. KG (Düren, Germany) and radiolabel which was from Amersham International (Braunschweig, Germany).
Generation and selection of transgenic lines
For transformation, the potato cDNA encoding FK (GenBank accession number: Z12823; Smith et al. 1993) was subcloned in sense (1143 bp PstI–SalI fragment) or antisense (1143 bp HindIII–BamHI fragment) orientation into cvs Record and Desiree using standard molecular procedures (Sambrook et al. 1989) into the promoter-terminator cassette pJIT60 (Guerineau and Mullineaux 1993) between the double 35S CaMV promoter (Sanders et al. 1987) and the CaMV terminator. For both the sense and antisense constructs, the promoter-cDNA-terminator was excised as a KpnI–XhoI fragment and cloned into the binary vector pBIN19 (Bevan 1984). These constructs were then introduced into potato cv. Record via an Agrobacterium-mediated transformation protocol (Rocha-Sosa et al. 1989). Transgenic plants were then selected on kanamycin-containing medium (Dietze et al. 1995). Initial screening of around 80 lines was performed at the transcript level in leaves of plants grown in 2 liters pots under greenhouse conditions. A second screen was then carried out at the transcript, protein and enzyme activity levels with tubers (and in the case of the Desiree transformant leaves) of the pre-selected lines.
Extraction of RNA and Northern blot experiments
Total RNA was isolated from 2 g of fresh weight of tuber tissue as described by Logemann et al. (1987). Standard conditions were used for the transfer of RNA to membranes and for the subsequent hybridization.
Immunoblots
Soluble proteins from parenchyma tissue of developing tubers were denatured in buffer containing SDS (Laemmli 1970). A 30 µg aliquot of soluble protein was then separated on a 10% polyacrylamide gel [37.5 : 1 (w/w) acrylamide : bis-acrylamide] containing 1 g l–1 SDS. The proteins were blotted on a nitrocellulose membrane and the blots incubated at room temperature for 4 h with a polyclonal antibody (1 : 1,000 dilution) raised against pea seed fructokinase (FK-1) (obtained from Professor D. D. Randall, University of Missouri, CO, USA). The blots were washed three times for 5 min in 1× phosphate-buffered saline (PBS) then incubated overnight at room temperature with goat anti-rabbit immunoglobulin conjugate labelled with alkaline phosphatase (Sigma Éü-8025) made to a dilution of 1 : 10,000. The blots were washed twice for 5 min in 1× PBS containing 0.1% Tween-20, followed by a 5 min wash in 1× PBS containing 0.1% Tween-20 and 1 M NaCl, then three 5 min washes in 1× PBS containing 0.1% Tween-20, and a 5 min wash in 1× PBS. The blots were finally washed twice for 5 min in sterile dH2O. Cross-reacting bands were identified by NBT/BCIP developing solution (Bio Rad; catalogue no 1706432).
Enzyme analysis
Enzyme extracts were carried out as described by Tauberger et al. (2000). AGPase, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), phosphofructokinase, phosphoglucose isomerase, phosphoglycerate kinase and triose phosphate isomerase were determined as described in Fernie et al. (2001a), whilst UGPase was determined as defined by Sweetlove et al. (1996), FK as detailed by Gardener et al. (1992) and hexokinase as detailed by Veramendi et al. (2002).
Determination of starch and soluble sugars
Leaf or tuber parenchyma samples were rapidly frozen in liquid N2 and extracted in either ethanol or trichloroacetic acid. Starch and sugars were determined spectrophotometrically as described in Fernie et al. (2001a). Hexose phosphates were also determined spectrophotometrically as described by Regierer et al. (2002). The recovery of each metabolite through the extraction, storage and assay process has been documented previously (Fernie et al. 2001a).
Extraction, derivatization and analysis of potato leaf and tuber metabolites using gas chromatography-mass spectrometry
Metabolite analysis by GC-MS was carried out exactly as described by Roessner et al. (2001a), Lytovchenko et al. (2002a) and Lytovchenko et al. (2002b) in the cases of tuber parenchyma and leaf samples, respectively. Both chromatograms and mass spectra were evaluated using the MASSLAB program (ThermoQuest, Manchester, UK) and the resulting data were prepared and presented as described in Roessner et al. (2001b).
Labelling experiments
Developing tubers (∼50 g fresh weight each) from field-grown plants were washed under tap water, and 5 mm diameter, 1 mm thick discs excised with a cork borer. Discs were immediately transferred to 50 mM MES pH 6.5, 300 mM mannitol and washed twice to remove excess starch. Accurately weighed tissue samples (∼500 mg) were then transferred to 2 cm sealed glass vials of high surface area : volume ratio in order to minimize the possibility of hypoxia containing 650 µl 50 mM MES pH 6.5, 300 mM mannitol and 0.148 MBq of d-[U-14C]fructose or 0.148 MBq of [U-14C]sucrose (specific activity 45.5 MBq mmol–1). To ensure that substrate availability was not compromised during the experiment, the incubation medium was evaluated by scintillation counting following the experiment. Vials were sealed with a rubber stopper which held a paper filter moistened with 200 µl of 10% (w/v) KOH to trap 14CO2. Incubation was allowed to proceed for 3 h prior to extraction, as described below. The oxygenation state of the experiment was assessed by comparing the metabolic fate of the radiolabel observed in wild-type tuber slices obtained here with that observed under suboptimal oxygen partial pressures (Geigenberger et al. 2000, Bologa et al. 2003). For both the fructose and sucrose feeding experiments, the metabolic fate of the radiolabel was consistent with normoxia.
Fractionation of 14C-labelled tissue extracts
Material was washed three times for 5 min in 5 ml of 50 mM MES pH 6.5, 300 mM mannitol prior to successive extraction in 5 ml of 80% (v/v) ethanol, 2 ml of 50% (v/v) ethanol, 2 ml of 20% (v/v) ethanol, 2 ml of H2O and finally 2 ml of 80% (v/v) ethanol. The supernatants were combined and dried down under vacuum; the ethanol-soluble components were resuspended in 2 ml of H2O and separated into neutral/basic and anionic fractions as described by Souleyre et al. (2004). Sucrose, glucose and fructose were separated by high-performance liquid chromatography (HPLC) using a Metacarb 87C 300×7.8 mm column (MetaChem Technologies Inc., Torrance, CA) with 0.6 ml min–1 ultrapure water as the mobile phase at a temperature of 70°C. Radiolabelled sugars were detected using a Reeve 9701 radiodetector, identified by co-chromatography with authentic standards and the radioactivity in each sugar estimated from standard curves using known quantities of label. The insoluble plant material was separated into starch and non-starch components as described in Runquist and Kruger (1999). The recovery of radioactivity during fractionation was typically between 90 and 105% of that applied.
Statistical analyses
The t-tests have been performed using the algorithm embedded into Microsoft Excel (Microsoft Corporation, Seattle, WA, USA). The term significant is used in the text only when the change in question has been confirmed to be significant (P < 0.05) with the t-test.
Supplementary material
Supplementary material mentioned in the article is available to online subscribers at the journal website www.pcp.oupjournals.org.
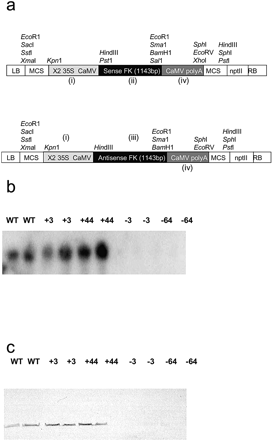
Fig. 1 (a–c) Fructokinase expression constructs, Northern and Western blots of transgenic lines. (a) Construction of a chimeric gene for expression of Solanum tuberosum fructokinase (StFK1) in the sense or antisense orientation: (i) an ∼700 bp fragment encoding the CaMV 35S promoter in duplicate; (ii) a 1,143 bp fragment encoding the full-length StFK1 cDNA in the sense orientation; (iii) a 1,143 bp fragment encoding the full-length StFK1 cDNA in the antisense orientation; and (iv) the polyadenylation signal of the CaMV gene. The basic vector used in cloning was pBIN19. (B) Northern blot analysis of transgenic potato plants (cv. Desiree) expressing StFK1 in the sense or antisense orientation. RNA was extracted from fully expanded leaves of 6-week-old plants. The filter was hybridized with the full coding region of StFK4. (C) Western blot analysis of transgenic potato plants (cv. Record) was carried out using an antibody that recognizes potato fructokinase (pea seed FK-1; D. Randall, personal communication).
Fig. 2 (a–d) Fructokinase activity in transgenic potato (Solanum tuberosum) plants. (a) Fructokinase activity in developing tubers harvested from 10-week-old plants of cv. Record. (b) Fructokinase activity in developing tubers harvested from 10-week-old plants of cv. Desiree. (c) Fructokinase activity in fully expanded source leaves harvested from 6-week-old plants of cv. Desiree. Values are presented as means ± SE of measurements on six plants per line; those marked by an asterisk were determined to be significantly different from wild type (P < 0.05) by the performance of t-tests.
Fig. 3 (a–f) Metabolite contents of tubers from 10-week-old potato (Solanum tuberosum cv. Record) plants. (a) Glucose, (b) fructose, (c) glucose 6-phosphate, (d) fructose 6-phosphate, (e) sucrose, (f) starch. Values are presented as means ± SE of measurements on six plants per line; those marked by an asterisk were determined to be significantly different from wild type (P < 0.05) by the performance of t-tests. Values for the hexose phosphates are given relative to wild type since they were determined as analytes during our metabolite profiling described in Supplementary Table 2.
Fig. 4 (a–f) Metabolite contents of tubers from 10-week-old potato (Solanum tuberosum cv. Desiree) plants. (a) Glucose, (b) fructose, (c) glucose 6-phosphate, (d) fructose 6-phosphate, (e) sucrose, (f) starch. Values are presented as means ± SE of measurements on six plants per line; those marked by an asterisk were determined to be significantly different from wild type (P < 0.05) by the performance of t-tests. Values for the hexose phosphates are given relative to wild type since they were determined as analytes during our metabolite profiling described in Supplementary Table 3.
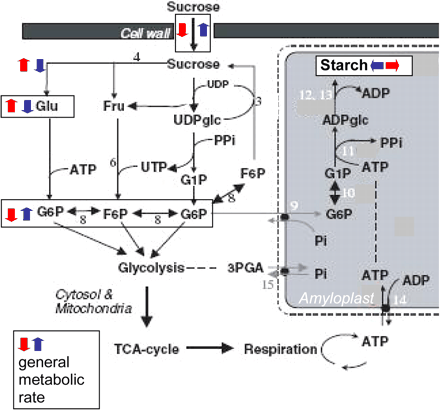
Fig. 5 Model of metabolic shifts observed in the tubers of the transgenic plants. Differences observed in the antisense plants are represented in red, whereas those in the antisense plants are represented in blue; symbols next to metabolites indicate changes in steady-state metabolite levels, whilst symbols next to metabolic reactions represent changes in assimilate partitioning as assessed by radiolabelled feeding experiments. Sucrose synthase (1), UDPglucose pyrophosphorylase (2), sucrose phosphate synthase (3), invertase (4), hexokinase (5), fructokinase (FK) (6), cytosolic phosphoglucose mutase (7), cytosolic phosphoglucose isomerase (8), hexose phosphate translocator (9), plastidial phosphoglucose mutase (10), ADPglucose pyrophosphorylase (11) soluble starch synthase (12), starch branching enzyme (13), amyloplastidial adenylate transporter (14), triose phosphate transporter (15). G1P, glucose 1-phosphate; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; 3PGA, 3-phosphoglycerate; Pi, inorganic phosphate; PPi, pyrophosphate.
Tuber numbers, yield (kg FW plant–1) and percentage dry matter (deduced from specific gravity) of transgenic tubers of cv. Record
| Lines\parameter | Tuber number per plant | % Dry matter | Tuber kg FW plant–1 |
| Summer 1998 field trial | |||
| Wild type (Record) | 15.0 ± 0.3 | 24.4 ± 0.2 | 2.00 ± 0.09 |
| aFKr3 | 7.0 ± 0.7 | 21.6 ± 0.1 | 0.62 ± 0.16 |
| aFKr 5 | 16.0 ± 0.5 | 24.8 ± 0.1 | 2.20 ± 0.13 |
| aFKr 23 | 12.0 ± 0.6 | 22.0 ± 0.2 | 1.80 ± 0.16 |
| aFKr54 | 10.0 ± 0.6 | 22.1 ± 0.1 | 1.50 ± 0.09 |
| aFKr68 | 20.0 ± 0.9 | 23.9 ± 0.3 | 1.60 ± 0.19 |
| FKr 3 | 15.0 ± 0.7 | 25.8 ± 0.2 | 2.00 ± 0.08 |
| FKr44 | 20.0 ± 0.8 | 24.5 ± 0.2 | 1.80 ± 0.13 |
| FKr9 | 15.0 ± 0.5 | 26.1 ± 0.1 | 1.65 ± 0.13 |
| Summer 1997 field trial | |||
| Wild type (Record) | 20.0 ± 0.7 | 23.7 ± 0.2 | 2.26 ± 0.16 |
| AFKr3 | 17.0 ± 0.6 | 21.1 ± 0.1 | 1.20 ± 0.09 |
| AFKr 5 | 23.0 ± 0.8 | 23.2 ± 0.1 | 1.90 ± 0.13 |
| AFKr 23 | 20.0 ± 0.6 | 23.1 ± 0.2 | 1.50 ± 0.22 |
| AFKr54 | 21.0 ± 1.5 | 22.8 ± 0.2 | 2.00 ± 0.25 |
| AFKr68 | 24.0 ± 1.7 | 21.2 ± 0.2 | 1.72 ± 0.19 |
| FKr 3 | 22.0 ± 0.9 | 25.1 ± 0.2 | 1.82 ± 0.25 |
| FKr44 | 17.0 ± 1.2 | 24.5 ± 0.3 | 1.78 ± 0.09 |
| FKr9 | 19.0 ± 1.1 | 24.2 ± 0.4 | 1.90 ± 0.25 |
| Lines\parameter | Tuber number per plant | % Dry matter | Tuber kg FW plant–1 |
| Summer 1998 field trial | |||
| Wild type (Record) | 15.0 ± 0.3 | 24.4 ± 0.2 | 2.00 ± 0.09 |
| aFKr3 | 7.0 ± 0.7 | 21.6 ± 0.1 | 0.62 ± 0.16 |
| aFKr 5 | 16.0 ± 0.5 | 24.8 ± 0.1 | 2.20 ± 0.13 |
| aFKr 23 | 12.0 ± 0.6 | 22.0 ± 0.2 | 1.80 ± 0.16 |
| aFKr54 | 10.0 ± 0.6 | 22.1 ± 0.1 | 1.50 ± 0.09 |
| aFKr68 | 20.0 ± 0.9 | 23.9 ± 0.3 | 1.60 ± 0.19 |
| FKr 3 | 15.0 ± 0.7 | 25.8 ± 0.2 | 2.00 ± 0.08 |
| FKr44 | 20.0 ± 0.8 | 24.5 ± 0.2 | 1.80 ± 0.13 |
| FKr9 | 15.0 ± 0.5 | 26.1 ± 0.1 | 1.65 ± 0.13 |
| Summer 1997 field trial | |||
| Wild type (Record) | 20.0 ± 0.7 | 23.7 ± 0.2 | 2.26 ± 0.16 |
| AFKr3 | 17.0 ± 0.6 | 21.1 ± 0.1 | 1.20 ± 0.09 |
| AFKr 5 | 23.0 ± 0.8 | 23.2 ± 0.1 | 1.90 ± 0.13 |
| AFKr 23 | 20.0 ± 0.6 | 23.1 ± 0.2 | 1.50 ± 0.22 |
| AFKr54 | 21.0 ± 1.5 | 22.8 ± 0.2 | 2.00 ± 0.25 |
| AFKr68 | 24.0 ± 1.7 | 21.2 ± 0.2 | 1.72 ± 0.19 |
| FKr 3 | 22.0 ± 0.9 | 25.1 ± 0.2 | 1.82 ± 0.25 |
| FKr44 | 17.0 ± 1.2 | 24.5 ± 0.3 | 1.78 ± 0.09 |
| FKr9 | 19.0 ± 1.1 | 24.2 ± 0.4 | 1.90 ± 0.25 |
Growth conditions, sampling and analysis are as described in Materials and Methods. Data are presented as means ± SE of 10 individual plants per line. Values given in bold were determined to be significantly different from wild type (P < 0.05) by the performance of t-tests.
Tuber numbers, yield (kg FW plant–1) and percentage dry matter (deduced from specific gravity) of transgenic tubers of cv. Record
| Lines\parameter | Tuber number per plant | % Dry matter | Tuber kg FW plant–1 |
| Summer 1998 field trial | |||
| Wild type (Record) | 15.0 ± 0.3 | 24.4 ± 0.2 | 2.00 ± 0.09 |
| aFKr3 | 7.0 ± 0.7 | 21.6 ± 0.1 | 0.62 ± 0.16 |
| aFKr 5 | 16.0 ± 0.5 | 24.8 ± 0.1 | 2.20 ± 0.13 |
| aFKr 23 | 12.0 ± 0.6 | 22.0 ± 0.2 | 1.80 ± 0.16 |
| aFKr54 | 10.0 ± 0.6 | 22.1 ± 0.1 | 1.50 ± 0.09 |
| aFKr68 | 20.0 ± 0.9 | 23.9 ± 0.3 | 1.60 ± 0.19 |
| FKr 3 | 15.0 ± 0.7 | 25.8 ± 0.2 | 2.00 ± 0.08 |
| FKr44 | 20.0 ± 0.8 | 24.5 ± 0.2 | 1.80 ± 0.13 |
| FKr9 | 15.0 ± 0.5 | 26.1 ± 0.1 | 1.65 ± 0.13 |
| Summer 1997 field trial | |||
| Wild type (Record) | 20.0 ± 0.7 | 23.7 ± 0.2 | 2.26 ± 0.16 |
| AFKr3 | 17.0 ± 0.6 | 21.1 ± 0.1 | 1.20 ± 0.09 |
| AFKr 5 | 23.0 ± 0.8 | 23.2 ± 0.1 | 1.90 ± 0.13 |
| AFKr 23 | 20.0 ± 0.6 | 23.1 ± 0.2 | 1.50 ± 0.22 |
| AFKr54 | 21.0 ± 1.5 | 22.8 ± 0.2 | 2.00 ± 0.25 |
| AFKr68 | 24.0 ± 1.7 | 21.2 ± 0.2 | 1.72 ± 0.19 |
| FKr 3 | 22.0 ± 0.9 | 25.1 ± 0.2 | 1.82 ± 0.25 |
| FKr44 | 17.0 ± 1.2 | 24.5 ± 0.3 | 1.78 ± 0.09 |
| FKr9 | 19.0 ± 1.1 | 24.2 ± 0.4 | 1.90 ± 0.25 |
| Lines\parameter | Tuber number per plant | % Dry matter | Tuber kg FW plant–1 |
| Summer 1998 field trial | |||
| Wild type (Record) | 15.0 ± 0.3 | 24.4 ± 0.2 | 2.00 ± 0.09 |
| aFKr3 | 7.0 ± 0.7 | 21.6 ± 0.1 | 0.62 ± 0.16 |
| aFKr 5 | 16.0 ± 0.5 | 24.8 ± 0.1 | 2.20 ± 0.13 |
| aFKr 23 | 12.0 ± 0.6 | 22.0 ± 0.2 | 1.80 ± 0.16 |
| aFKr54 | 10.0 ± 0.6 | 22.1 ± 0.1 | 1.50 ± 0.09 |
| aFKr68 | 20.0 ± 0.9 | 23.9 ± 0.3 | 1.60 ± 0.19 |
| FKr 3 | 15.0 ± 0.7 | 25.8 ± 0.2 | 2.00 ± 0.08 |
| FKr44 | 20.0 ± 0.8 | 24.5 ± 0.2 | 1.80 ± 0.13 |
| FKr9 | 15.0 ± 0.5 | 26.1 ± 0.1 | 1.65 ± 0.13 |
| Summer 1997 field trial | |||
| Wild type (Record) | 20.0 ± 0.7 | 23.7 ± 0.2 | 2.26 ± 0.16 |
| AFKr3 | 17.0 ± 0.6 | 21.1 ± 0.1 | 1.20 ± 0.09 |
| AFKr 5 | 23.0 ± 0.8 | 23.2 ± 0.1 | 1.90 ± 0.13 |
| AFKr 23 | 20.0 ± 0.6 | 23.1 ± 0.2 | 1.50 ± 0.22 |
| AFKr54 | 21.0 ± 1.5 | 22.8 ± 0.2 | 2.00 ± 0.25 |
| AFKr68 | 24.0 ± 1.7 | 21.2 ± 0.2 | 1.72 ± 0.19 |
| FKr 3 | 22.0 ± 0.9 | 25.1 ± 0.2 | 1.82 ± 0.25 |
| FKr44 | 17.0 ± 1.2 | 24.5 ± 0.3 | 1.78 ± 0.09 |
| FKr9 | 19.0 ± 1.1 | 24.2 ± 0.4 | 1.90 ± 0.25 |
Growth conditions, sampling and analysis are as described in Materials and Methods. Data are presented as means ± SE of 10 individual plants per line. Values given in bold were determined to be significantly different from wild type (P < 0.05) by the performance of t-tests.
Hexose phosphate contents (nmol g FW–1) of extracts made from healthy fully expanded leaves harvested in the middle of the light period from 8-week-old plants of sense FKd and antisense aFKd lines in the Desiree background
| Line | |||||
| Metabolite level | Wild type | FKd13 | FKd41 | aFKd26 | aFKd34 |
| Glucose 6P | 147 ± 15 | 161 ± 29 | 167 ± 21 | 138 ± 13 | 130 ± 7 |
| Glucose 1P | 17 ± 4 | 21 ± 3 | 23 ± 4 | 16 ± 3 | 14 ± 4 |
| Fructose 6P | 49 ± 6 | 57 ± 4 | 54 ± 9 | 42 ± 4 | 46 ± 7 |
| Ratio | |||||
| Glc 6P/Glc 1P | 8.6 ± 1.3 | 7.7 ± 0.9 | 7.3 ± 0.8 | 8.6 ± 1.1 | 9.3 ± 0.8 |
| Glc 6P/Fru 6P | 3.0 ± 0.4 | 2.8 ± 0.3 | 3.0 ± 0.2 | 3.2 ± 0.3 | 3.0 ± 0.4 |
| Line | |||||
| Metabolite level | Wild type | FKd13 | FKd41 | aFKd26 | aFKd34 |
| Glucose 6P | 147 ± 15 | 161 ± 29 | 167 ± 21 | 138 ± 13 | 130 ± 7 |
| Glucose 1P | 17 ± 4 | 21 ± 3 | 23 ± 4 | 16 ± 3 | 14 ± 4 |
| Fructose 6P | 49 ± 6 | 57 ± 4 | 54 ± 9 | 42 ± 4 | 46 ± 7 |
| Ratio | |||||
| Glc 6P/Glc 1P | 8.6 ± 1.3 | 7.7 ± 0.9 | 7.3 ± 0.8 | 8.6 ± 1.1 | 9.3 ± 0.8 |
| Glc 6P/Fru 6P | 3.0 ± 0.4 | 2.8 ± 0.3 | 3.0 ± 0.2 | 3.2 ± 0.3 | 3.0 ± 0.4 |
Hexose phosphates were determined in exactly the same samples used for the GC-MS analysis presented in Supplementary data 1. Values are the mean ± SE of determinations on six individual plants per line.
Hexose phosphate contents (nmol g FW–1) of extracts made from healthy fully expanded leaves harvested in the middle of the light period from 8-week-old plants of sense FKd and antisense aFKd lines in the Desiree background
| Line | |||||
| Metabolite level | Wild type | FKd13 | FKd41 | aFKd26 | aFKd34 |
| Glucose 6P | 147 ± 15 | 161 ± 29 | 167 ± 21 | 138 ± 13 | 130 ± 7 |
| Glucose 1P | 17 ± 4 | 21 ± 3 | 23 ± 4 | 16 ± 3 | 14 ± 4 |
| Fructose 6P | 49 ± 6 | 57 ± 4 | 54 ± 9 | 42 ± 4 | 46 ± 7 |
| Ratio | |||||
| Glc 6P/Glc 1P | 8.6 ± 1.3 | 7.7 ± 0.9 | 7.3 ± 0.8 | 8.6 ± 1.1 | 9.3 ± 0.8 |
| Glc 6P/Fru 6P | 3.0 ± 0.4 | 2.8 ± 0.3 | 3.0 ± 0.2 | 3.2 ± 0.3 | 3.0 ± 0.4 |
| Line | |||||
| Metabolite level | Wild type | FKd13 | FKd41 | aFKd26 | aFKd34 |
| Glucose 6P | 147 ± 15 | 161 ± 29 | 167 ± 21 | 138 ± 13 | 130 ± 7 |
| Glucose 1P | 17 ± 4 | 21 ± 3 | 23 ± 4 | 16 ± 3 | 14 ± 4 |
| Fructose 6P | 49 ± 6 | 57 ± 4 | 54 ± 9 | 42 ± 4 | 46 ± 7 |
| Ratio | |||||
| Glc 6P/Glc 1P | 8.6 ± 1.3 | 7.7 ± 0.9 | 7.3 ± 0.8 | 8.6 ± 1.1 | 9.3 ± 0.8 |
| Glc 6P/Fru 6P | 3.0 ± 0.4 | 2.8 ± 0.3 | 3.0 ± 0.2 | 3.2 ± 0.3 | 3.0 ± 0.4 |
Hexose phosphates were determined in exactly the same samples used for the GC-MS analysis presented in Supplementary data 1. Values are the mean ± SE of determinations on six individual plants per line.
Metabolism of either 5 mM [U-14C]fructose or 5 mM [U14C]sucrose by 10 mm tuber discs isolated from developing tubers of wild type and transgenic plants
| Potato line | |||||||
| [U14C]fructose | [U14C]sucrose | ||||||
| Wild type | FKr3 | aFKr3 | Wild type | FKr3 | aFKr3 | ||
| Uptake (kdpm) | 849 ± 197 | 1,164 ± 105 | 653 ± 45 | 1,998 ± 16 | 2,825 ± 97 | 1,742 ± 42 | |
| Metab. (kdpm) | 69 ± 1 | 1,091 ± 101 | 520 ± 34 | 1,759 ± 16 | 2,605 ± 93 | 1,340 ± 31 | |
| Fraction | Redistribution of radiolabel (% total metabolized) | ||||||
| Carbon dioxide | 5.3 ± 0.1 | 10.9 ± 0.8 | 7.7 ± 0.6 | 5.5 ± 0.1 | 6.0 ± 0.4 | 6.6 ± 0.6 | |
| Fructose | – | – | – | 2.6 ± 1.6 | 1.0 ± 1.3 | 6.7 ± 1.2 | |
| Glucose | 4.4 ± 0.4 | 5.1 ± 0.4 | 0.0 ± 0.0 | 10.0 ± 0.6 | 5.0 ± 1.3 | 18.5 ± 1.2 | |
| Sucrose | 8.7 ± 0.1 | 6.9 ± 0.8 | 60.2 ± 1.4 | – | – | – | |
| Other sugars | 15.4 ± 2.0 | 11.8 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Organic acids | 1.6 ± 0.3 | 1.2 ± 0.1 | 3.2 ± 1.5 | 16.8 ± 1.8 | 9.3 ± 0.4 | 21.0 ± 1.1 | |
| Amino acids | 18.5 ± 0.6 | 9.7 ± 0.5 | 6.3 ± 0.2 | 10.6 ± 1.3 | 9.2 ± 0.3 | 8.0 ± 0.9 | |
| Starch | 43.3 ± 1.8 | 53.5 ± 0.7 | 22.0 ± 2.8 | 53.3 ± 1.2 | 68.9 ± 4.3 | 38.6 ± 2.5 | |
| Other insolubles | 2.9 ± 0.4 | 0.9 ± 0.11 | 0.5 ± 0.3 | 1.5 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.2 | |
| Relative partitioning of radiolabel | |||||||
| Glycolysis: starch synthesis | 0.58 ± 0.04 | 0.41 ± 0.01 | 0.79 ± 0.13 | 0.62 ± 0.01 | 0.36 ± 0.03 | 0.92 ± 0.07 | |
| Glycolysis: sucrose synthesis | 2.93 ± 0.08 | 3.10 ± 0.54 | 0.29 ± 0.22 | – | – | – | |
| Potato line | |||||||
| [U14C]fructose | [U14C]sucrose | ||||||
| Wild type | FKr3 | aFKr3 | Wild type | FKr3 | aFKr3 | ||
| Uptake (kdpm) | 849 ± 197 | 1,164 ± 105 | 653 ± 45 | 1,998 ± 16 | 2,825 ± 97 | 1,742 ± 42 | |
| Metab. (kdpm) | 69 ± 1 | 1,091 ± 101 | 520 ± 34 | 1,759 ± 16 | 2,605 ± 93 | 1,340 ± 31 | |
| Fraction | Redistribution of radiolabel (% total metabolized) | ||||||
| Carbon dioxide | 5.3 ± 0.1 | 10.9 ± 0.8 | 7.7 ± 0.6 | 5.5 ± 0.1 | 6.0 ± 0.4 | 6.6 ± 0.6 | |
| Fructose | – | – | – | 2.6 ± 1.6 | 1.0 ± 1.3 | 6.7 ± 1.2 | |
| Glucose | 4.4 ± 0.4 | 5.1 ± 0.4 | 0.0 ± 0.0 | 10.0 ± 0.6 | 5.0 ± 1.3 | 18.5 ± 1.2 | |
| Sucrose | 8.7 ± 0.1 | 6.9 ± 0.8 | 60.2 ± 1.4 | – | – | – | |
| Other sugars | 15.4 ± 2.0 | 11.8 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Organic acids | 1.6 ± 0.3 | 1.2 ± 0.1 | 3.2 ± 1.5 | 16.8 ± 1.8 | 9.3 ± 0.4 | 21.0 ± 1.1 | |
| Amino acids | 18.5 ± 0.6 | 9.7 ± 0.5 | 6.3 ± 0.2 | 10.6 ± 1.3 | 9.2 ± 0.3 | 8.0 ± 0.9 | |
| Starch | 43.3 ± 1.8 | 53.5 ± 0.7 | 22.0 ± 2.8 | 53.3 ± 1.2 | 68.9 ± 4.3 | 38.6 ± 2.5 | |
| Other insolubles | 2.9 ± 0.4 | 0.9 ± 0.11 | 0.5 ± 0.3 | 1.5 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.2 | |
| Relative partitioning of radiolabel | |||||||
| Glycolysis: starch synthesis | 0.58 ± 0.04 | 0.41 ± 0.01 | 0.79 ± 0.13 | 0.62 ± 0.01 | 0.36 ± 0.03 | 0.92 ± 0.07 | |
| Glycolysis: sucrose synthesis | 2.93 ± 0.08 | 3.10 ± 0.54 | 0.29 ± 0.22 | – | – | – | |
Values are presented as means ± SE (n =3); those denoted in bold type were assessed to be significantly different from the wild type (P < 0.05) by performance of t-tests. Metab., metabolized.
Metabolism of either 5 mM [U-14C]fructose or 5 mM [U14C]sucrose by 10 mm tuber discs isolated from developing tubers of wild type and transgenic plants
| Potato line | |||||||
| [U14C]fructose | [U14C]sucrose | ||||||
| Wild type | FKr3 | aFKr3 | Wild type | FKr3 | aFKr3 | ||
| Uptake (kdpm) | 849 ± 197 | 1,164 ± 105 | 653 ± 45 | 1,998 ± 16 | 2,825 ± 97 | 1,742 ± 42 | |
| Metab. (kdpm) | 69 ± 1 | 1,091 ± 101 | 520 ± 34 | 1,759 ± 16 | 2,605 ± 93 | 1,340 ± 31 | |
| Fraction | Redistribution of radiolabel (% total metabolized) | ||||||
| Carbon dioxide | 5.3 ± 0.1 | 10.9 ± 0.8 | 7.7 ± 0.6 | 5.5 ± 0.1 | 6.0 ± 0.4 | 6.6 ± 0.6 | |
| Fructose | – | – | – | 2.6 ± 1.6 | 1.0 ± 1.3 | 6.7 ± 1.2 | |
| Glucose | 4.4 ± 0.4 | 5.1 ± 0.4 | 0.0 ± 0.0 | 10.0 ± 0.6 | 5.0 ± 1.3 | 18.5 ± 1.2 | |
| Sucrose | 8.7 ± 0.1 | 6.9 ± 0.8 | 60.2 ± 1.4 | – | – | – | |
| Other sugars | 15.4 ± 2.0 | 11.8 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Organic acids | 1.6 ± 0.3 | 1.2 ± 0.1 | 3.2 ± 1.5 | 16.8 ± 1.8 | 9.3 ± 0.4 | 21.0 ± 1.1 | |
| Amino acids | 18.5 ± 0.6 | 9.7 ± 0.5 | 6.3 ± 0.2 | 10.6 ± 1.3 | 9.2 ± 0.3 | 8.0 ± 0.9 | |
| Starch | 43.3 ± 1.8 | 53.5 ± 0.7 | 22.0 ± 2.8 | 53.3 ± 1.2 | 68.9 ± 4.3 | 38.6 ± 2.5 | |
| Other insolubles | 2.9 ± 0.4 | 0.9 ± 0.11 | 0.5 ± 0.3 | 1.5 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.2 | |
| Relative partitioning of radiolabel | |||||||
| Glycolysis: starch synthesis | 0.58 ± 0.04 | 0.41 ± 0.01 | 0.79 ± 0.13 | 0.62 ± 0.01 | 0.36 ± 0.03 | 0.92 ± 0.07 | |
| Glycolysis: sucrose synthesis | 2.93 ± 0.08 | 3.10 ± 0.54 | 0.29 ± 0.22 | – | – | – | |
| Potato line | |||||||
| [U14C]fructose | [U14C]sucrose | ||||||
| Wild type | FKr3 | aFKr3 | Wild type | FKr3 | aFKr3 | ||
| Uptake (kdpm) | 849 ± 197 | 1,164 ± 105 | 653 ± 45 | 1,998 ± 16 | 2,825 ± 97 | 1,742 ± 42 | |
| Metab. (kdpm) | 69 ± 1 | 1,091 ± 101 | 520 ± 34 | 1,759 ± 16 | 2,605 ± 93 | 1,340 ± 31 | |
| Fraction | Redistribution of radiolabel (% total metabolized) | ||||||
| Carbon dioxide | 5.3 ± 0.1 | 10.9 ± 0.8 | 7.7 ± 0.6 | 5.5 ± 0.1 | 6.0 ± 0.4 | 6.6 ± 0.6 | |
| Fructose | – | – | – | 2.6 ± 1.6 | 1.0 ± 1.3 | 6.7 ± 1.2 | |
| Glucose | 4.4 ± 0.4 | 5.1 ± 0.4 | 0.0 ± 0.0 | 10.0 ± 0.6 | 5.0 ± 1.3 | 18.5 ± 1.2 | |
| Sucrose | 8.7 ± 0.1 | 6.9 ± 0.8 | 60.2 ± 1.4 | – | – | – | |
| Other sugars | 15.4 ± 2.0 | 11.8 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Organic acids | 1.6 ± 0.3 | 1.2 ± 0.1 | 3.2 ± 1.5 | 16.8 ± 1.8 | 9.3 ± 0.4 | 21.0 ± 1.1 | |
| Amino acids | 18.5 ± 0.6 | 9.7 ± 0.5 | 6.3 ± 0.2 | 10.6 ± 1.3 | 9.2 ± 0.3 | 8.0 ± 0.9 | |
| Starch | 43.3 ± 1.8 | 53.5 ± 0.7 | 22.0 ± 2.8 | 53.3 ± 1.2 | 68.9 ± 4.3 | 38.6 ± 2.5 | |
| Other insolubles | 2.9 ± 0.4 | 0.9 ± 0.11 | 0.5 ± 0.3 | 1.5 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.2 | |
| Relative partitioning of radiolabel | |||||||
| Glycolysis: starch synthesis | 0.58 ± 0.04 | 0.41 ± 0.01 | 0.79 ± 0.13 | 0.62 ± 0.01 | 0.36 ± 0.03 | 0.92 ± 0.07 | |
| Glycolysis: sucrose synthesis | 2.93 ± 0.08 | 3.10 ± 0.54 | 0.29 ± 0.22 | – | – | – | |
Values are presented as means ± SE (n =3); those denoted in bold type were assessed to be significantly different from the wild type (P < 0.05) by performance of t-tests. Metab., metabolized.
Abbreviations
- AGPase
ADPglucose pyrophosphorylase
- CaMV
cauliflower mosaic virus
- FK
fructokinase
- GC-MS
gas chromatography–mass spectrometry
- PBS
phosphate-buffered saline
References
Bevan, M. (
Bologa, K.L., Fernie, A.R., Leisse, A., Ehlers Loureiro, M. and Geigenberger, P. (
Dai, N., German, M.A., Matsevitz, T., Hanael, R., Swartzberg, D., Yeselson, Y., Petreikov, M., Schaffer, A.A. and Granot, D. (
Dai, N., Kandel, M., Petreikov, M., Levine, I., Ricard, B., Rothan, C., Schaffer, A.A. and Granot, D. (
Dai, N., Schaffer, A., Petreikov, M. and Granot, D. (
Dai, N., Schaffer, A., Petreikov, M., Shahak, Y., Giller, Y., Ratner, K., Levine, A. and Granot, D. (
D’Aoust, M.A., Yelle, S. and Nguyen-Quoc, B. (
Defernez, M., Gunning, Y.M., Parr, A.J., Shepherd, L.V.T., Davies, H.V. and Colquhoun, I.L. (
Dietze, J., Blau, A. and Willmitzer, L. (
Doehlert, D.C. (
Farre, E.M., Tiessen, A., Roessner, U., Geigenberger, P., Trethewey, R.N. and Willmitzer, L. (
Fernie, A.R., Roessner, U., Trethewey, R.N. and Willmitzer, L. (
Fernie, A.R., Roscher, A., Ratcliffe, R.G. and Kruger, N.K. (
Fernie, A.R. and Willmitzer, L. (
Fernie, A.R., Willmitzer, L. and Trethewey, R.N. (
Gardener, A., Davies, H.V. and Burch, L.R. (
Geigenberger, P. and Stitt, M. (
Geigenberger, P., Reimholz, R., Geiger, M., Merlo, L., Canale, V. and Stitt, M. (1) Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit.
Geigenberger, P., Fernie, A.R., Gibon, Y., Christ, M. and Stitt, M. (
Geigenberger, P., Regierer, B., Lytovchenko, A., Leisse, A., Schauer, N., Springer, F., Kossmann, J. and Fernie, A.R. (
Geigenberger, P., Stitt, M. and Fernie, A.R. (
German, M.A., Dai, N., Matsevitz, T., Hanael. R., Petreikov, M., Bernstein, N., Ioffe, M., Shahak, Y., Schaffer, A.A. and Granot, D. (
Guerineau, F. and Mullineaux, P. (
Jang, J.C., Leon, P., Zhou, L. and Sheen, J. (
Jiang, H.W., Dian, W.M., Liu, F.Y. and Wu, P. (
Kanayama, Y., Dai, N., Granot, D., Petreikov, M., Schaffer, A. and Bennett, A.B. (
Kanayama, Y., Granot, D., Dai, N., Petreikov, M., Schaffer, A., Powell, A. and Bennett, A.B. (
Kossmann, J. and Lloyd, J.R. (
Kruger, N.J. (
Laemmli, U.K. (
Logemann, J., Schell, J. and Willmitzer, L. (
Lytovchenko, A., Bieberich, K., Willmitzer, L. and Fernie, A.R. (
Lytovchenko, A., Sweetlove, L.J., Pauly, M. and Fernie, A.R. (
Martinez-Barajas, E. and Randall, D.D. (
Moore, B., Zhou, L., Rolland, F., Hall, Q., Cheng, W.H., Liu, Y.X., Hwang, I., Jones, T. and Sheen, J. (
Murashige, T. and Skoog, F. (
Odanaka, S., Bennett, A.B. and Kanayama, Y. (
Pego, J.V. and Smeekens, S.C.M. (
Regierer, B., Fernie, A.R., Springer, F., Perez-Melis, A., Leisse, A., Koehl, K., Willmitzer, L., Geigenberger, P. and Kossmann, J. (
Renz, A., Merlo, L. and Stitt, M. (
Renz, A. and Stitt, M. (
Rocha-Sosa, M., Sonnewald, U., Frommer, W.B., Stratmann, M., Schell, J. and Willmitzer, L. (
Roessner, U., Luedemann, A., Brust, D., Fiehn, O., Linke, T., Willmitzer, L. and Fernie, A.R. (
Roessner, U., Willmitzer, L., Fernie, A.R. (
Roessner-Tunali, U., Hegemann, B., Lytovchenko, A., Carrari, F., Bruedigam, C., Granot, D. and Fernie, A.R. (
Ross, H.A., Davies, H.V., Burch, L.P., Viola, R. and McDae, D. (
Runquist, M. and Kruger, N.J. (
Sambrook, J., Fritsch, E.F., Maniatis, T. (
Sanders, P.R., Winter, J.A., Barnason, A.R. and Fraley, R. (
Schaffer, A.A. and Petreikov, M. (
Sindelar, L., Sindelarova, M. and Burketova, L. (
Smith, S.B., Taylor, M.A., Burch, L.R. and Davies, H.V. (
Souleyre, E.J.F., Iannetta, P.P.M., Ross, H.A., Hancock, R.D., Shepherd, L.V.T., Viola, R., Taylor, M.A. and Davies, H.V. (
Stitt, M. (
Stitt, M. and Sonnewald, U. (
Sweetlove, L.J., Burrell, M.M. and ap Rees, T. (
Sweetlove, L.J., Müller-Röber, B., Willmitzer, L. and Hill, S.A. (
Tauberger, E., Fernie, A.R., Emmermann, M., Renz, A., Kossmann, J., Willmitzer, L. and Trethewey, R.N. (
Taylor, M.A., Ross, H.A., Gardner, A. and Davies, H.V. (
Tiessen, A., Hendricks, J.H.M., Stitt, M., Branscheid, A., Gibon, Y., Farre, E.M. and Geigenberger, P. (
Veramendi, J., Roessner, U., Renz, A., Willmitzer, L. and Trethewey, R.N. (
Veramendi, J., Fernie, A.R., Leisse, A., Willmitzer, L. and Trethewey, R.N. (
Viola, R., Davies, H.V. and Chudeck, A.R. (
Viola, R. (
Viola, R., Roberts, A.G., Haupt, S., Gazzani, S., Hancock, R.D., Mamiroli, N., Machray, G.C. and Oparka, K.J. (
Zhang, S.R., Nichols, S.E. and Dong, J.G. (