-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshihisa Ikeda, Hiroharu Banno, Qi-Wen Niu, Stephen H. Howell, Nam-Hai Chua, The ENHANCER OF SHOOT REGENERATION 2 gene in Arabidopsis Regulates CUP-SHAPED COTYLEDON 1 at the Transcriptional Level and Controls Cotyledon Development , Plant and Cell Physiology, Volume 47, Issue 11, November 2006, Pages 1443–1456, https://doi.org/10.1093/pcp/pcl023
Close - Share Icon Share
Abstract
Cytokinins stimulate shoot regeneration in tissue culture but the genes required for this developmental process are not well understood. Here we show that the Arabidopsis gene, ENHANCER OF SHOOT REGENERATION2 ( ESR2 ), encoding an AP2-domain transcriptional factor, functions in the regeneration of shoots in tissue culture. ESR2 overexpression conferred cytokinin-independent shoot regeneration from cre1/ahk4 mutant explants, suggesting that CRE1 is not required for ESR2 -mediated shoot regeneration. ESR2 directly targeted CYCLIN D1;1, ARABIDOPSIS PHOSPHOTRANSMITTER 6 ( AHP6 ) and CUP-SHAPED COTYLEDON 1 ( CUC1 ) in assays involving the translocation of ESR2–ER (estradiol receptor) fusions to the nucleus. Knock-down of ESR2 expression by RNA interference (RNAi) reduced CUC1 expression and resulted in altered cotyledon phenotypes at a low frequency, including partially fused cotyledons and triple cotyledons. Phenotypes of induced ESR2 expression in a cuc1-1 mutant background were suppressed. Our results suggest that ESR2 plays a role in shoot regeneration through transcriptional regulation of CUC1 .
Introduction
Plants have the remarkable ability to regenerate in tissue culture conditions under the influence of plant hormones, such as cytokinin and auxin (Skoog and Miller 1957 ). We are interested in the molecular events underlying the regeneration of shoots and the role of cytokinin in that process (Cary et al. 2002 , Che et al. 2002 , Che et al. 2006 ).
Considerable progress has been made in identifying genes involved in cytokinin biosynthesis and signaling (Kakimoto 2001 , Takei et al. 2001 , Hwang et al. 2002 , Jasinski et al. 2005 , Yanai et al. 2005 ); however, less is known about control of downstream developmental events activated by cytokinin. Using an enhancer activation tagging system, Kakimoto ( 1996 ) discovered CYTOKININ-INDEPENDENT 1 ( CKI1 ), which conferred cytokinin-independent shoot regeneration or callus growth. CKI1 encodes a receptor-like histidine kinase that does not appear to bind cytokinin. Recent work showed that CKI1 might function in cytokinin signaling independently from hormonal stimuli (Hwang and Sheen 2001 ).
Cytokinin signaling is mediated by a ‘two-component’ phosphorelay transfer system comprising sensor histidine kinases ( AHKs ), histidine-containing phosphotransmitters ( AHPs ) and response regulators ( ARRs ) (Hutchison and Kieber 2002 , Oka et al. 2002 ). CRE1/AHK4/WOL, AHK2 and AHK3 encode sensor histidine kinases that function as cytokinin receptors (Mahonen et al. 2000 , Inoue et al. 2001 , Ueguchi et al. 2001a , Ueguchi et al. 2001b ). Whereas some B-type ARRs are capable of directly activating cytokinin-responsive genes, A-type ARRs may serve as repressors to down-regulate cytokinin responses (Hwang and Sheen 2001 , Sakai et al. 2001 , To et al. 2004 , Leibfried et al. 2005 ). Some A-type ARRs are primary response genes that are rapidly induced by cytokinin (Sakai et al. 2001 ). Other components of the cytokinin signaling system for shoot formation are unknown.
To identify genes involved in shoot regeneration, we performed a functional screen and characterized a gene, ENHANCER OF SHOOT REGENERATION 1 ( ESR1 ), encoding a transcription factor of the AP2/ERF family (Banno et al. 2001 ). Although inducible overexpression of ESR1 conferred cytokinin-independent shoot regeneration on roots, addition of cytokinins significantly increased regeneration efficiency. In contrast, constitutive ESR1 overexpression led to the formation of dark green calli and inhibited shoot regeneration, suggesting that ESR1 is involved in the transition from vegetative growth to organogenic development, or the maintenance of the organogenic cell identity. Nonetheless, how the gene executes this function and the molecular mechanism by which ESR1 promotes shoot regeneration remain unknown.
To investigate further the control of shoot regeneration, we characterized the molecular function of a gene ESR2 , which is more active than ESR1 in promoting shoot regeneration in tissue culture. Attempts to characterize genes directly activated by ESR2–estrogen receptor (ER) nuclear translocation led to the identification of CUC1 as one of the candidates. The link between the two transcriptional factors was further confirmed by the observation that knock-down expression of ESR2 [in plants bearing RNA an inteference (RNAi) construct] phenocopied the cuc1 single mutant (Aida et al. 1997 ). The cuc1 mutation is sufficient to block ESR2 -mediated shoot phenotypes in seedlings, suggesting that CUC1 acts downstream of ESR2 and plays an important role in this pathway.
Results
Identification and characterization of ESR2
The Arabidopsis genome probably encodes genes with functions redundant to those of ESR1 , because single mutations in ESR1 do not show any obvious phenotype (Banno et al. 2001 ). The Arabidopsis genome contains a gene ( At1g24590 ) with high sequence similarity to ESR1 (Kirch et al. 2003 ). This gene was named ESR2 and corresponds to the DORNRÖSCHEN-LIKE ( DRN-like ) gene published by Kirch et al. ( 2003 ). Both ESR1 and ESR2 genes share significant sequence similarity within the AP2/ERF-encoding domain and are likely to be paralogs, since the location of two other genes flanking both ESR1 and ESR2 is conserved. A phylogenetic analysis shows that ESR1 and ESR2 are the most closely related Arabidopsis AP2 domain-containing proteins (Alonso et al. 2003 ).
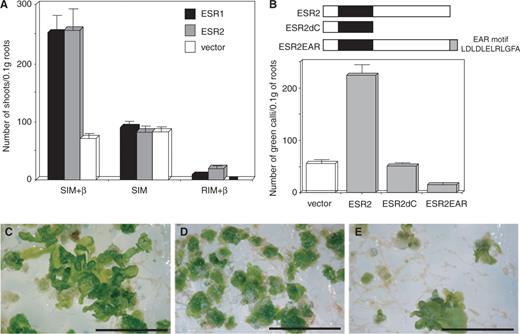
Using an estradiol-inducible (XVE) system (Zuo et al. 2000 ), we demonstrated that ESR2 enhanced shoot regeneration ( Fig. 1 A). In the presence of cytokinin [shoot induction medium + β-estradiol (SIM + β)], ESR2 induction increased shoot formation ∼3-fold, comparable with the enhancement resulting from ESR1 alone. In the absence of cytokinin [root induction medium + β-estradiol (RIM + β)], shoots developed very infrequently; however, ESR2 was nearly three times more effective in inducing shoot formation than ESR1 . No shoot formation was observed with an empty vector, pER10 ( Fig. 1 A).
Effects of ESR2 on shoot regeneration. (A) Numbers of regenerated shoots from 0.1 g fresh weight of root culture obtained by pER10- ESR1 , pER10- ESR2 and pER10 empty vector. Shoots were regenerated on shoot induction medium plus β-estradiol as an inducer (SIM + β), SIM without an inducer (SIM) and root induction medium plus β-estradiol (RIM + β). Each value represents the average of three independent experiments, bars indicate standard deviations. (B) Number of green calli developing from 0.1 g fresh weight of root culture 2 weeks after transfer onto SIM. ESR2 constructs are shown in the top panel. Solid boxes indicate the AP2 DNA-binding domain. The ESR2dC construct contains the N-terminal 133 amino acids. The ESR2EAR construct contains the entire coding sequence fused in-frame with the EAR motif, whose amino acid sequence is shown. Open bar represents the number of green calli obtained with the empty vector. In the bar graph, each value represents the average of three measurements from independent experiments, and bars indicate standard deviations. (C–E) Four-week-old root explants grown on SIM, transformed with empty vector alone (C), 35S :: ESR2 (D), and 35S :: ESR2EAR (E). Bars = 1 cm.
As previously seen with 35S :: ESR1 transformants (Banno et al. 2001 ), 35S :: ESR2 transformants produced rapidly growing, dark green calli, even in the absence of cytokinin; however, organogenesis was inhibited (data not shown). In the presence of cytokinin, 35S::ESR2 transformants produced green calli 4-fold more efficiently than vector control ( Fig. 1 B). A truncated form of ESR2 ( ESR2dC ) was also expressed to determine whether it would act in a dominant negative manner; however, the truncated construct also did not affect shoot regeneration efficiency. We attempted to convert ESR2 into a dominant transcriptional repressor by using the CREST methodology (Hiratsu et al. 2003 ) of creating a translational fusion of ESR2 with an EAR repressor (ERF-associated amphiphilic repression) motif from type II ERFs (Ohta et al. 2001 ). Compared with vector controls, the 35S :: ESR2EAR construct produced fewer green calli ( Fig. 1 B).
Four weeks after the transformation, whereas empty vector transformants developed lateral organs from green calli ( Fig. 1 C), this developmental process was inhibited by expression of the 35S::ESR2 construct ( Fig. 1 D). On the other hand, 35S::ESR2EAR transformants developed lateral organs ( Fig. 1 E). These results indicate that ectopic expression of a repressive form of ESR2 can interfere with both green calli formation and inhibition of lateral organ differentiation.
Pattern of ESR2 gene expression
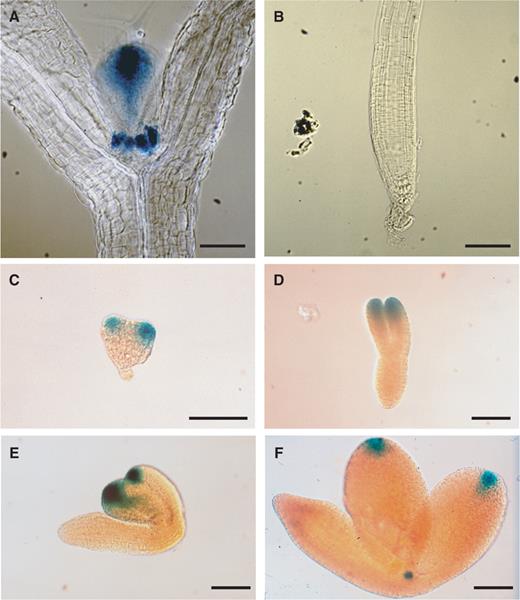
To determine the expression pattern of ESR2 , we fused a GUS (β-glucuronidase) coding sequence to a 2.5 kb upstream region of ESR2 . In seedlings grown on MS agar plates, GUS expression was observed in leaf primordia ( Fig. 2 A). No GUS staining was observed in the root apical meristem ( Fig. 2 B). Expression of the ESR2 promoter:: GUS transgene was readily observed during embryonic development in young cotyledons during the heart stage ( Fig. 2 C), and at the tip of the cotyledon from late heart stage to early walking stick stage ( Fig. 2 D, E). At the late walking stick stage, expression was restricted to cotyledon tips and occasionally in the shoot apical meristem (SAM) region ( Fig. 2 F).
Expression pattern of ESR2 . (A) Expression of the ESR2 promoter: :GUS transgene in a 10-day-old seedling. (B) No GUS activity was detectable in the root. (C) In heart stage embryos, GUS activity was observed in cotyledon primordia. (D–E) GUS expression was restricted to cotyledon tips in torpedo stage (D), and early walking stick stage embryos (E). (F) At the late walking stick stage, GUS activity was occasionally observed in the SAM, as well as the tips of cotyledons. At least 10 independent transgenic lines were examined with one representative line photographed. Bars = 20 μm.
Induced ESR expression can rescue cre1/ahk4 mutants
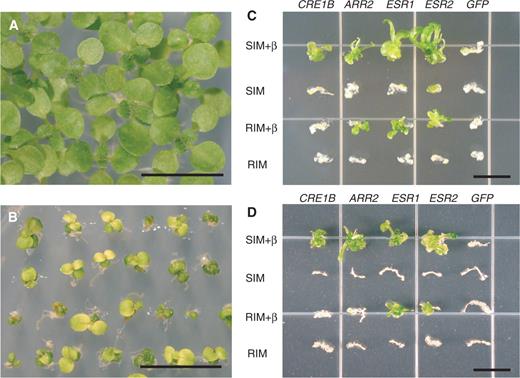
Transgenic plants constitutively expressing ESR2 ( 35S :: ESR2 ) were frequently growth impaired. Therefore, we used the β-estradiol-responsive XVE system to investigate the effects of induced expression of ESR2 . The β-estradiol treatment itself did not affect plant morphology ( Fig. 3 A). The phenotypes of induced XVE- ESR2 transgenic plants ( Fig. 3 B) were similar to those of mutants overproducing cytokinins (Chaudhury et al. 1993 , Tantikanjana et al. 2001 , Catterou et al. 2002 ), or to those of transgenic plants overexpressing the cytokinin biosynthetic gene, ISOPENTENYL TRANSFERASE ( IPT ) (Zubko et al. 2002 , Sun et al. 2003 ). The cytokinin-related phenotypes include anthocyanin accumulation, cytokinin-independent shoot regeneration, as well as inhibition of hypocotyl and root elongation in the dark. Not only does the induced expression of ESR1 produce cytokinin-related phenotypes, the ESR1 gene itself was also induced by cytokinin application (Banno et al. 2001 ). This suggests that ESR gene expression may cause an increase in endogenous cytokinin levels through induction of cytokinin biosynthetic genes as STM does (Jasinski et al. 2005 , Yanai et al. 2005 ). Alternatively, ESR proteins may be involved in, or may directly influence cytokinin signaling.
Phenotypes of estradiol-induced ESR expression. (A) Ten-day-old wild-type seedlings grown on MS medium containing β-estradiol. (B) Ten-day-old transgenic seedlings harboring XVE- ESR2 grown on MS medium containing β-estradiol. (C) Hypocotyl segments of the ahk4 mutant were transformed with XVE- CRE1B , XVE- ARR2 , XVE- ESR1 , XVE- ESR2 or XVE- GFP and cultured for 3 weeks on either SIM or RIM, with or without β-estradiol inducer (β). One hypocotyl segment representative of the response at each condition was selected and arranged to create a composite photograph for each line. (D) Root explants of the ahk4 mutant were transformed with the above-mentioned constructs and cultured under the same conditions as in (C). One root explant representative of the response at each condition was selected and arranged to create a composite photograph for each line. The same results were obtained with the cre1-1 mutant. Bars = 1 cm.
To address whether ESR1 and ESR2 are involved in cytokinin production or signaling, we asked whether inducible expression of these genes or ARR2 could rescue the cytokinin receptor mutant, cre1/ahk4 . Other groups had shown that ectopic expression of ARR2 elicits cytokinin responses in a tissue culture system (Hwang and Sheen 2001 ). In hypocotyl transformation experiments, induced expression of ESR1, ESR2 and ARR2 in the cre1/ahk4 mutant promoted shoot regeneration independently of cytokinin ( Fig. 3 C). CRE1 also complemented the mutant phenotype but only in the presence of cytokinin. The induced expression of GFP (green fluorescent protein) served as a negative control. In root transformation experiments, induced expression of either ESR1 or ESR2 in cre1/ahk4 also promoted shoot regeneration independently of added cytokinin ( Fig. 3 D). However, induced expression of either ARR2 or CRE1 led to shoot regeneration only in the presence of cytokinin. Cytokinin signaling, possibly mediated by CRE1, may be essential for ARR2 activation in roots. Because induced ESR1 or ESR2 expression rescued cre1/ahk4 mutants, it is unlikely that the two ESR proteins are involved in cytokinin biosynthesis, unless other cytokinin receptors come into play. Rather, we propose that the two ESR genes act downstream of the primary cytokinin signaling pathway or operate in an unrelated, but compensatory, way.
ESR2 alters the expression of cytokinin-responsive genes, cell cycle genes and homeodomain transcription factors
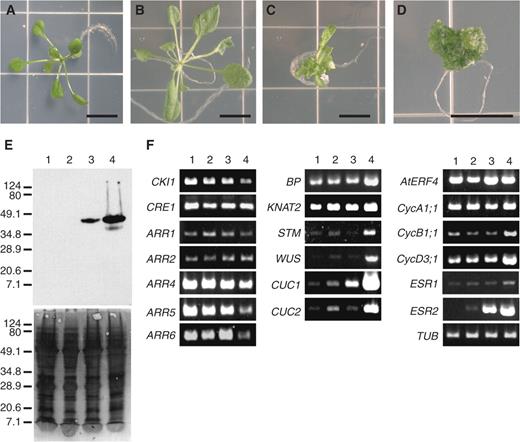
To examine the effects of ESR2 overexpression on plant development, fertile transgenic plants carrying a 35S :: ESR2myc transgene were analyzed. Transgenic plants harboring an empty vector were used as a negative control ( Fig. 4 A). T 2 progeny showed a variety of phenotypes, ranging from undetectable to disorganized cell proliferation ( Fig. 4 B–D). The phenotypic severity of these transgenic lines was correlated with endogenous ESR2myc expression levels ( Fig. 4 E).
Gene expression in 35S :: ESR2Myc transgenic plants with different phenotypes. (A) Homozygous transgenic plant harboring an empty vector. (B–D) T 2 progeny from a 35S :: ESR2Myc transgenic line with mild (B), moderate (C), or severe phenotypes (D). Bars = 1 cm. (E) ESR2 protein expression levels correlated with the severity of the phenotype. ESR2Myc protein levels were determined by Western blotting (upper panel). The lower panel shows the Coomassie brilliant blue staining pattern of the PVDF membrane. Lanes 1–4 contained protein extracts of plants in (A) to (D), respectively. Relative molecular masses (kDa) of the standard samples are indicated on the left. (F) Semi-quantitative analysis of gene expression by RT–PCR. Lanes 1–4 contained RNA samples of plants in (A) to (D), respectively. At least 10 independent seedlings were collected for each phenotype. Varying numbers of PCR cycles were examined for each gene, and two biological replicates were carried out. TUBULIN ( TUB ) was used as an internal control.
If ESR2 is indeed involved in the cytokinin primary response pathway, A-type ARR expression might be correlated with ESR2 expression levels. Surprisingly, compared with control plants ( Fig. 4 F, lane 1), the expression of three A-type ARRs ( ARR4, ARR5 and ARR6 ) analyzed was greatly reduced in transgenic line D ( Fig. 4 F, lane 4). Previous analysis had shown that the expression of A-type ARRs was positively regulated by B-type ARRs (Hwang and Sheen 2001 , Sakai et al. 2001 , Tajima et al. 2004 ). However, no significant differences in B-type ARRs ( ARR1 and ARR2 ) and CRE1 expression levels were observed among the transgenic lines ( Fig. 4 F). Another histidine kinase relative, CKI1 , is known to confer cytokinin-independent shoot regeneration when overexpressed (Kakimoto 1996 ). We examined the possibility that the effect of ESR2 on cytokinin-independent shoot regeneration is mediated by the enhanced expression of CKI1 . However, the CKI1 expression level was not altered.
The sequence similarity between the two ESR proteins and the other ERF family members prompted us to determine whether an elevation in ESR2 expression might influence expression of ethylene-responsive genes, such as AtERF4 (Fujimoto et al. 2000 ). However, no significant change in AtERF4 expression was observed in any of the transgenic lines, suggesting that ESR2 does not function as an ERF. No significant changes in ESR1 expression were observed, suggesting that all of the above events were ESR2 dependent.
Other studies with cytokinin-overproducing plants have shown that high endogenous cytokinin levels impact the regulation of cell division activity in the SAM and young leaves (Chaudhury et al. 1993 , Rupp et al. 1999 ). We found that the CycA1;1 expression level in all the transgenic lines was comparable with that of the controls. In contrast, expression of both CycB1;1 and CycD3;1 was increased in the transgenic plant line D ( Fig. 4 F, lane 4). This result indicated that increased CYCD3;1 levels were correlated with unlimited cell growth of plant line D and that of the tumorous tissues in which cell cycle progression was unchecked.
Cytokinin activates the expression of a number of genes involved in meristem specification or maintenance such as the class I KNOX homeobox genes, BREVIPEDICELLUS ( BP ) and SHOOT MERISTEMLESS ( STM ) (Rupp et al. 1999 ), although it is likely that their activation may be several steps away from the primary response pathway. In transgenic line D, expression of the class I KNOX genes was up-regulated, as well as that of WUSCHEL ( WUS ), a distantly related homeodomain transcription factor required for specification and maintenance of the shoot meristem stem cell niche (Mayer et al. 1998 , Baurle and Laux 2005 ). Many of the above-mentioned genes were significantly up-regulated in line D. Expression of CUC1 , a NAC domain transcriptional factor known to promote adventitious shoot formation (Daimon et al. 2003 , Hibara et al. 2003 ), was up-regulated in an ESR2 -dependent manner. However, a related gene, CUC2 , was only moderately induced by ESR2 . In summary, our results showed that ESR2 up-regulates genes involved in cell division, meristem specification or maintenance, and genes known to be activated by cytokinins, with the exception of A-type response regulators.
Identification of direct target genes by an estradiol receptor-mediated nuclear translocation system
The hormone-binding domain of the glucocorticoid receptor (GR) can be used to identify direct target genes for various transcription factors (Sablowski and Meyerowitz 1998 , Wagner et al. 1999 , Samach et al. 2000 , Sakai et al. 2001 , William et al. 2004 ). We attempted to identify genes directly activated by ESR2 fused to the hormone-binding domain of the ER. The hormone-binding domain of ER facilitates import of a fusion protein to the nucleus in response to β-estradiol. The CaMV 35S promoter was used to express the ESR2 fusion protein ( 35S :: ESR2-ER ). GFP was also fused to the ER and used as a negative control ( 35S :: GFP-ER ). Transgenic plants were treated with cycloheximide to prevent new protein synthesis and β-estradiol was applied to promote nuclear translocation of the chimeric transcription factor. After 1 h treatment, RNA transcripts were profiled using root explants grown on B5 hormone-free agar medium after callus induction medium (CIM) pre-incubation. Pre-incubation on CIM enhances ESR1 expression after transfer onto SIM (Banno et al. 2001 ). Total RNA from treated root explants was hybridized to Affymetrix Arabidopsis ATH1 GeneChips. The microarray experiments were conducted in a random block design with three biological replicates. The estimated means of the MAS5 normalized signals were sorted by fold change for samples with P -values <0.01 for the comparison between β-estradiol-treated 35S :: ESR2-ER root explants and similarly treated 35S :: GFP-ER explants. Fifty-one genes up-regulated >2-fold are listed in Table 1 . About 14% of these ESR2 targeted genes (7/51) belonged to the transcription factor families.
Genes directly up-regulated by estradiol induction of ESR2–ER nuclear translocation a
| Gene ID . | FC b . | SE c . | P -value d . | Description . |
|---|---|---|---|---|
| At5g09970 | 13.00 | 0.673 | 0.0072628 | CYP78A7, cytochrome P450 family protein |
| At4g23760 | 11.07 | 0.398 | 0.0014310 | Expressed protein |
| At3g55720 | 9.92 | 0.210 | 0.0077948 | Expressed protein |
| At5g67430 | 9.01 | 0.223 | 0.0046690 | GCN5-related N -acetyltransferase (GNAT) |
| At4g33730 | 8.60 | 0.545 | 0.0071309 | Glycosyl hydrolase family 9 protein |
| At1g71380 | 7.13 | 0.216 | 0.0047063 | Glycosyl hydrolase family 9 protein |
| At2g46990 | 6.34 | 0.191 | 0.0030819 | IAA20, auxin-induced protein |
| At1g80100 | 5.53 | 0.198 | 0.0042271 | AHP6, histidine phosphotransfer protein |
| At1g15150 | 5.33 | 0.284 | 0.0028840 | MATE efflux family protein |
| At4g20470 | 5.13 | 0.317 | 0.0046953 | Expressed protein |
| At3g48520 | 4.93 | 0.343 | 0.0001759 | CYP94B3, cytochrome P450 family protein |
| At4g27590 | 4.57 | 0.156 | 0.0000857 | Copper-binding protein |
| At1g70210 | 4.26 | 0.189 | 0.0062934 | Cyclin D1:1 |
| At3g56600 | 4.24 | 0.143 | 0.0001165 | Phosphatidylinositol 3- and 4-kinase |
| At1g55290 | 4.15 | 0.097 | 0.0045459 | Encodes a protein whose sequence is similar to oxidoreductase, 2OG-Fe(II) oxygenase |
| At3g50410 | 4.14 | 0.184 | 0.0068715 | OBP1, Dof-type zinc finger domain-containing protein |
| At2g45460 | 4.07 | 0.472 | 0.0093709 | Forkhead-associated (FHA) domain-containing protein |
| At4g14980 | 3.58 | 0.171 | 0.0077684 | DC1 domain-containing protein |
| At1g69880 | 3.52 | 0.168 | 0.0042381 | Thioredoxin (TRX-H-2), putative |
| At3g63440 | 3.45 | 0.118 | 0.0004572 | AtCKX7, cytokinin oxidase/dehydrogenase |
| At3g18610 | 3.38 | 0.175 | 0.0038292 | Nucleolin, putative |
| At5g66350 | 3.26 | 0.135 | 0.0040557 | Zinc finger protein, putative (SHI) |
| At1g07260 | 3.16 | 0.162 | 0.0014200 | UDP-glucoronosyl/UDP-glucosyl transferase family protein |
| At1g28360 | 3.09 | 0.185 | 0.0091796 | AtERF12 |
| At3g15170 | 3.07 | 0.129 | 0.0008111 | CUC1, cup-shaped cotyledon1 protein |
| At1g49620 | 3.03 | 0.228 | 0.0063879 | ICK5, putative cyclin-dependent kinase inhibitor |
| At2g32970 | 3.03 | 0.100 | 0.0007628 | Expressed protein |
| At5g01880 | 2.78 | 0.204 | 0.0041282 | Zinc finger (C3HC4-type RING finger) family protein |
| At3g50310 | 2.68 | 0.057 | 0.0000044 | MAPKKK20, member of MEKK subfamily |
| At5g53320 | 2.62 | 0.132 | 0.0031698 | Leucine-rich repeat transmembrane protein kinase, putative |
| At2g42280 | 2.60 | 0.179 | 0.0085707 | Basic helix–loop–helix (bHLH) family protein |
| At1g34210 | 2.60 | 0.168 | 0.0048316 | SERK2, plasma membrane LRR receptor-like serine threonine kinase |
| At1g47380 | 2.56 | 0.153 | 0.0028532 | Protein phosphatase 2C-related |
| At1g64700 | 2.54 | 0.183 | 0.0066078 | Expressed protein |
| At1g55200 | 2.54 | 0.107 | 0.0010749 | Protein kinase family protein |
| At1g77450 | 2.51 | 0.216 | 0.0092566 | No apical meristem (NAM) family protein |
| At1g73880 | 2.45 | 0.135 | 0.0060142 | UDP-glucoronosyl/UDP-glucosyl transferase family protein |
| At4g30560 | 2.45 | 0.135 | 0.0046448 | Cyclic nucleotide-regulated ion channel, putative |
| At2g17850 | 2.44 | 0.166 | 0.0059527 | Senescence-associated family protein |
| At3g54820 | 2.37 | 0.136 | 0.0055065 | Aquaporin, putative |
| At1g14260 | 2.36 | 0.195 | 0.0079047 | Zinc finger (C3HC4-type RING finger) family protein |
| At1g25320 | 2.35 | 0.127 | 0.0019168 | Leucine-rich repeat transmembrane protein kinase, putative |
| At3g54320 | 2.26 | 0.181 | 0.0087026 | WRI1, WRINKLED1 encodes transcription factor of the AP2/ERWEBP class |
| At1g48870 | 2.23 | 0.116 | 0.0036226 | WD-40 repeat family protein |
| At4g13345 | 2.19 | 0.156 | 0.0071155 | TMS membrane family protein/tumor differentially expressed (TDE) family protein |
| At5g25460 | 2.15 | 0.138 | 0.0004704 | Expressed protein |
| At1g08800 | 2.14 | 0.138 | 0.0092632 | Expressed protein, weak similarity to SP|Q02455 myosin-like protein MLP1 |
| At1g14687 | 2.11 | 0.097 | 0.0012244 | Zinc finger homeobox family protein |
| At1g75640 | 2.10 | 0.133 | 0.0073310 | Leucine-rich repeat family protein/protein kinase family protein |
| At5g66440 | 2.06 | 0.123 | 0.0032094 | Expressed protein |
| At4g20500 | 2.02 | 0.063 | 0.0008199 | Expressed protein |
| At1g78860 | 2.01 | 0.164 | 0.0095841 | Curculin-like (mannose-binding) lectin family protein, low similarity to Ser/Thr protein kinase |
| Gene ID . | FC b . | SE c . | P -value d . | Description . |
|---|---|---|---|---|
| At5g09970 | 13.00 | 0.673 | 0.0072628 | CYP78A7, cytochrome P450 family protein |
| At4g23760 | 11.07 | 0.398 | 0.0014310 | Expressed protein |
| At3g55720 | 9.92 | 0.210 | 0.0077948 | Expressed protein |
| At5g67430 | 9.01 | 0.223 | 0.0046690 | GCN5-related N -acetyltransferase (GNAT) |
| At4g33730 | 8.60 | 0.545 | 0.0071309 | Glycosyl hydrolase family 9 protein |
| At1g71380 | 7.13 | 0.216 | 0.0047063 | Glycosyl hydrolase family 9 protein |
| At2g46990 | 6.34 | 0.191 | 0.0030819 | IAA20, auxin-induced protein |
| At1g80100 | 5.53 | 0.198 | 0.0042271 | AHP6, histidine phosphotransfer protein |
| At1g15150 | 5.33 | 0.284 | 0.0028840 | MATE efflux family protein |
| At4g20470 | 5.13 | 0.317 | 0.0046953 | Expressed protein |
| At3g48520 | 4.93 | 0.343 | 0.0001759 | CYP94B3, cytochrome P450 family protein |
| At4g27590 | 4.57 | 0.156 | 0.0000857 | Copper-binding protein |
| At1g70210 | 4.26 | 0.189 | 0.0062934 | Cyclin D1:1 |
| At3g56600 | 4.24 | 0.143 | 0.0001165 | Phosphatidylinositol 3- and 4-kinase |
| At1g55290 | 4.15 | 0.097 | 0.0045459 | Encodes a protein whose sequence is similar to oxidoreductase, 2OG-Fe(II) oxygenase |
| At3g50410 | 4.14 | 0.184 | 0.0068715 | OBP1, Dof-type zinc finger domain-containing protein |
| At2g45460 | 4.07 | 0.472 | 0.0093709 | Forkhead-associated (FHA) domain-containing protein |
| At4g14980 | 3.58 | 0.171 | 0.0077684 | DC1 domain-containing protein |
| At1g69880 | 3.52 | 0.168 | 0.0042381 | Thioredoxin (TRX-H-2), putative |
| At3g63440 | 3.45 | 0.118 | 0.0004572 | AtCKX7, cytokinin oxidase/dehydrogenase |
| At3g18610 | 3.38 | 0.175 | 0.0038292 | Nucleolin, putative |
| At5g66350 | 3.26 | 0.135 | 0.0040557 | Zinc finger protein, putative (SHI) |
| At1g07260 | 3.16 | 0.162 | 0.0014200 | UDP-glucoronosyl/UDP-glucosyl transferase family protein |
| At1g28360 | 3.09 | 0.185 | 0.0091796 | AtERF12 |
| At3g15170 | 3.07 | 0.129 | 0.0008111 | CUC1, cup-shaped cotyledon1 protein |
| At1g49620 | 3.03 | 0.228 | 0.0063879 | ICK5, putative cyclin-dependent kinase inhibitor |
| At2g32970 | 3.03 | 0.100 | 0.0007628 | Expressed protein |
| At5g01880 | 2.78 | 0.204 | 0.0041282 | Zinc finger (C3HC4-type RING finger) family protein |
| At3g50310 | 2.68 | 0.057 | 0.0000044 | MAPKKK20, member of MEKK subfamily |
| At5g53320 | 2.62 | 0.132 | 0.0031698 | Leucine-rich repeat transmembrane protein kinase, putative |
| At2g42280 | 2.60 | 0.179 | 0.0085707 | Basic helix–loop–helix (bHLH) family protein |
| At1g34210 | 2.60 | 0.168 | 0.0048316 | SERK2, plasma membrane LRR receptor-like serine threonine kinase |
| At1g47380 | 2.56 | 0.153 | 0.0028532 | Protein phosphatase 2C-related |
| At1g64700 | 2.54 | 0.183 | 0.0066078 | Expressed protein |
| At1g55200 | 2.54 | 0.107 | 0.0010749 | Protein kinase family protein |
| At1g77450 | 2.51 | 0.216 | 0.0092566 | No apical meristem (NAM) family protein |
| At1g73880 | 2.45 | 0.135 | 0.0060142 | UDP-glucoronosyl/UDP-glucosyl transferase family protein |
| At4g30560 | 2.45 | 0.135 | 0.0046448 | Cyclic nucleotide-regulated ion channel, putative |
| At2g17850 | 2.44 | 0.166 | 0.0059527 | Senescence-associated family protein |
| At3g54820 | 2.37 | 0.136 | 0.0055065 | Aquaporin, putative |
| At1g14260 | 2.36 | 0.195 | 0.0079047 | Zinc finger (C3HC4-type RING finger) family protein |
| At1g25320 | 2.35 | 0.127 | 0.0019168 | Leucine-rich repeat transmembrane protein kinase, putative |
| At3g54320 | 2.26 | 0.181 | 0.0087026 | WRI1, WRINKLED1 encodes transcription factor of the AP2/ERWEBP class |
| At1g48870 | 2.23 | 0.116 | 0.0036226 | WD-40 repeat family protein |
| At4g13345 | 2.19 | 0.156 | 0.0071155 | TMS membrane family protein/tumor differentially expressed (TDE) family protein |
| At5g25460 | 2.15 | 0.138 | 0.0004704 | Expressed protein |
| At1g08800 | 2.14 | 0.138 | 0.0092632 | Expressed protein, weak similarity to SP|Q02455 myosin-like protein MLP1 |
| At1g14687 | 2.11 | 0.097 | 0.0012244 | Zinc finger homeobox family protein |
| At1g75640 | 2.10 | 0.133 | 0.0073310 | Leucine-rich repeat family protein/protein kinase family protein |
| At5g66440 | 2.06 | 0.123 | 0.0032094 | Expressed protein |
| At4g20500 | 2.02 | 0.063 | 0.0008199 | Expressed protein |
| At1g78860 | 2.01 | 0.164 | 0.0095841 | Curculin-like (mannose-binding) lectin family protein, low similarity to Ser/Thr protein kinase |
a As analyzed by Affymetrix Arabidopsis DNA chips (ATH1). Experiment was conducted with three biological replicates.
b Fold change for the comparison of the estimated means between β-estradiol-treated 35S::ESR2-ER root explants and similarly treated 35S::GFP-ER explants.
c Standard error for the above comparison.
dP -value for the above comparison.
Genes directly up-regulated by estradiol induction of ESR2–ER nuclear translocation a
| Gene ID . | FC b . | SE c . | P -value d . | Description . |
|---|---|---|---|---|
| At5g09970 | 13.00 | 0.673 | 0.0072628 | CYP78A7, cytochrome P450 family protein |
| At4g23760 | 11.07 | 0.398 | 0.0014310 | Expressed protein |
| At3g55720 | 9.92 | 0.210 | 0.0077948 | Expressed protein |
| At5g67430 | 9.01 | 0.223 | 0.0046690 | GCN5-related N -acetyltransferase (GNAT) |
| At4g33730 | 8.60 | 0.545 | 0.0071309 | Glycosyl hydrolase family 9 protein |
| At1g71380 | 7.13 | 0.216 | 0.0047063 | Glycosyl hydrolase family 9 protein |
| At2g46990 | 6.34 | 0.191 | 0.0030819 | IAA20, auxin-induced protein |
| At1g80100 | 5.53 | 0.198 | 0.0042271 | AHP6, histidine phosphotransfer protein |
| At1g15150 | 5.33 | 0.284 | 0.0028840 | MATE efflux family protein |
| At4g20470 | 5.13 | 0.317 | 0.0046953 | Expressed protein |
| At3g48520 | 4.93 | 0.343 | 0.0001759 | CYP94B3, cytochrome P450 family protein |
| At4g27590 | 4.57 | 0.156 | 0.0000857 | Copper-binding protein |
| At1g70210 | 4.26 | 0.189 | 0.0062934 | Cyclin D1:1 |
| At3g56600 | 4.24 | 0.143 | 0.0001165 | Phosphatidylinositol 3- and 4-kinase |
| At1g55290 | 4.15 | 0.097 | 0.0045459 | Encodes a protein whose sequence is similar to oxidoreductase, 2OG-Fe(II) oxygenase |
| At3g50410 | 4.14 | 0.184 | 0.0068715 | OBP1, Dof-type zinc finger domain-containing protein |
| At2g45460 | 4.07 | 0.472 | 0.0093709 | Forkhead-associated (FHA) domain-containing protein |
| At4g14980 | 3.58 | 0.171 | 0.0077684 | DC1 domain-containing protein |
| At1g69880 | 3.52 | 0.168 | 0.0042381 | Thioredoxin (TRX-H-2), putative |
| At3g63440 | 3.45 | 0.118 | 0.0004572 | AtCKX7, cytokinin oxidase/dehydrogenase |
| At3g18610 | 3.38 | 0.175 | 0.0038292 | Nucleolin, putative |
| At5g66350 | 3.26 | 0.135 | 0.0040557 | Zinc finger protein, putative (SHI) |
| At1g07260 | 3.16 | 0.162 | 0.0014200 | UDP-glucoronosyl/UDP-glucosyl transferase family protein |
| At1g28360 | 3.09 | 0.185 | 0.0091796 | AtERF12 |
| At3g15170 | 3.07 | 0.129 | 0.0008111 | CUC1, cup-shaped cotyledon1 protein |
| At1g49620 | 3.03 | 0.228 | 0.0063879 | ICK5, putative cyclin-dependent kinase inhibitor |
| At2g32970 | 3.03 | 0.100 | 0.0007628 | Expressed protein |
| At5g01880 | 2.78 | 0.204 | 0.0041282 | Zinc finger (C3HC4-type RING finger) family protein |
| At3g50310 | 2.68 | 0.057 | 0.0000044 | MAPKKK20, member of MEKK subfamily |
| At5g53320 | 2.62 | 0.132 | 0.0031698 | Leucine-rich repeat transmembrane protein kinase, putative |
| At2g42280 | 2.60 | 0.179 | 0.0085707 | Basic helix–loop–helix (bHLH) family protein |
| At1g34210 | 2.60 | 0.168 | 0.0048316 | SERK2, plasma membrane LRR receptor-like serine threonine kinase |
| At1g47380 | 2.56 | 0.153 | 0.0028532 | Protein phosphatase 2C-related |
| At1g64700 | 2.54 | 0.183 | 0.0066078 | Expressed protein |
| At1g55200 | 2.54 | 0.107 | 0.0010749 | Protein kinase family protein |
| At1g77450 | 2.51 | 0.216 | 0.0092566 | No apical meristem (NAM) family protein |
| At1g73880 | 2.45 | 0.135 | 0.0060142 | UDP-glucoronosyl/UDP-glucosyl transferase family protein |
| At4g30560 | 2.45 | 0.135 | 0.0046448 | Cyclic nucleotide-regulated ion channel, putative |
| At2g17850 | 2.44 | 0.166 | 0.0059527 | Senescence-associated family protein |
| At3g54820 | 2.37 | 0.136 | 0.0055065 | Aquaporin, putative |
| At1g14260 | 2.36 | 0.195 | 0.0079047 | Zinc finger (C3HC4-type RING finger) family protein |
| At1g25320 | 2.35 | 0.127 | 0.0019168 | Leucine-rich repeat transmembrane protein kinase, putative |
| At3g54320 | 2.26 | 0.181 | 0.0087026 | WRI1, WRINKLED1 encodes transcription factor of the AP2/ERWEBP class |
| At1g48870 | 2.23 | 0.116 | 0.0036226 | WD-40 repeat family protein |
| At4g13345 | 2.19 | 0.156 | 0.0071155 | TMS membrane family protein/tumor differentially expressed (TDE) family protein |
| At5g25460 | 2.15 | 0.138 | 0.0004704 | Expressed protein |
| At1g08800 | 2.14 | 0.138 | 0.0092632 | Expressed protein, weak similarity to SP|Q02455 myosin-like protein MLP1 |
| At1g14687 | 2.11 | 0.097 | 0.0012244 | Zinc finger homeobox family protein |
| At1g75640 | 2.10 | 0.133 | 0.0073310 | Leucine-rich repeat family protein/protein kinase family protein |
| At5g66440 | 2.06 | 0.123 | 0.0032094 | Expressed protein |
| At4g20500 | 2.02 | 0.063 | 0.0008199 | Expressed protein |
| At1g78860 | 2.01 | 0.164 | 0.0095841 | Curculin-like (mannose-binding) lectin family protein, low similarity to Ser/Thr protein kinase |
| Gene ID . | FC b . | SE c . | P -value d . | Description . |
|---|---|---|---|---|
| At5g09970 | 13.00 | 0.673 | 0.0072628 | CYP78A7, cytochrome P450 family protein |
| At4g23760 | 11.07 | 0.398 | 0.0014310 | Expressed protein |
| At3g55720 | 9.92 | 0.210 | 0.0077948 | Expressed protein |
| At5g67430 | 9.01 | 0.223 | 0.0046690 | GCN5-related N -acetyltransferase (GNAT) |
| At4g33730 | 8.60 | 0.545 | 0.0071309 | Glycosyl hydrolase family 9 protein |
| At1g71380 | 7.13 | 0.216 | 0.0047063 | Glycosyl hydrolase family 9 protein |
| At2g46990 | 6.34 | 0.191 | 0.0030819 | IAA20, auxin-induced protein |
| At1g80100 | 5.53 | 0.198 | 0.0042271 | AHP6, histidine phosphotransfer protein |
| At1g15150 | 5.33 | 0.284 | 0.0028840 | MATE efflux family protein |
| At4g20470 | 5.13 | 0.317 | 0.0046953 | Expressed protein |
| At3g48520 | 4.93 | 0.343 | 0.0001759 | CYP94B3, cytochrome P450 family protein |
| At4g27590 | 4.57 | 0.156 | 0.0000857 | Copper-binding protein |
| At1g70210 | 4.26 | 0.189 | 0.0062934 | Cyclin D1:1 |
| At3g56600 | 4.24 | 0.143 | 0.0001165 | Phosphatidylinositol 3- and 4-kinase |
| At1g55290 | 4.15 | 0.097 | 0.0045459 | Encodes a protein whose sequence is similar to oxidoreductase, 2OG-Fe(II) oxygenase |
| At3g50410 | 4.14 | 0.184 | 0.0068715 | OBP1, Dof-type zinc finger domain-containing protein |
| At2g45460 | 4.07 | 0.472 | 0.0093709 | Forkhead-associated (FHA) domain-containing protein |
| At4g14980 | 3.58 | 0.171 | 0.0077684 | DC1 domain-containing protein |
| At1g69880 | 3.52 | 0.168 | 0.0042381 | Thioredoxin (TRX-H-2), putative |
| At3g63440 | 3.45 | 0.118 | 0.0004572 | AtCKX7, cytokinin oxidase/dehydrogenase |
| At3g18610 | 3.38 | 0.175 | 0.0038292 | Nucleolin, putative |
| At5g66350 | 3.26 | 0.135 | 0.0040557 | Zinc finger protein, putative (SHI) |
| At1g07260 | 3.16 | 0.162 | 0.0014200 | UDP-glucoronosyl/UDP-glucosyl transferase family protein |
| At1g28360 | 3.09 | 0.185 | 0.0091796 | AtERF12 |
| At3g15170 | 3.07 | 0.129 | 0.0008111 | CUC1, cup-shaped cotyledon1 protein |
| At1g49620 | 3.03 | 0.228 | 0.0063879 | ICK5, putative cyclin-dependent kinase inhibitor |
| At2g32970 | 3.03 | 0.100 | 0.0007628 | Expressed protein |
| At5g01880 | 2.78 | 0.204 | 0.0041282 | Zinc finger (C3HC4-type RING finger) family protein |
| At3g50310 | 2.68 | 0.057 | 0.0000044 | MAPKKK20, member of MEKK subfamily |
| At5g53320 | 2.62 | 0.132 | 0.0031698 | Leucine-rich repeat transmembrane protein kinase, putative |
| At2g42280 | 2.60 | 0.179 | 0.0085707 | Basic helix–loop–helix (bHLH) family protein |
| At1g34210 | 2.60 | 0.168 | 0.0048316 | SERK2, plasma membrane LRR receptor-like serine threonine kinase |
| At1g47380 | 2.56 | 0.153 | 0.0028532 | Protein phosphatase 2C-related |
| At1g64700 | 2.54 | 0.183 | 0.0066078 | Expressed protein |
| At1g55200 | 2.54 | 0.107 | 0.0010749 | Protein kinase family protein |
| At1g77450 | 2.51 | 0.216 | 0.0092566 | No apical meristem (NAM) family protein |
| At1g73880 | 2.45 | 0.135 | 0.0060142 | UDP-glucoronosyl/UDP-glucosyl transferase family protein |
| At4g30560 | 2.45 | 0.135 | 0.0046448 | Cyclic nucleotide-regulated ion channel, putative |
| At2g17850 | 2.44 | 0.166 | 0.0059527 | Senescence-associated family protein |
| At3g54820 | 2.37 | 0.136 | 0.0055065 | Aquaporin, putative |
| At1g14260 | 2.36 | 0.195 | 0.0079047 | Zinc finger (C3HC4-type RING finger) family protein |
| At1g25320 | 2.35 | 0.127 | 0.0019168 | Leucine-rich repeat transmembrane protein kinase, putative |
| At3g54320 | 2.26 | 0.181 | 0.0087026 | WRI1, WRINKLED1 encodes transcription factor of the AP2/ERWEBP class |
| At1g48870 | 2.23 | 0.116 | 0.0036226 | WD-40 repeat family protein |
| At4g13345 | 2.19 | 0.156 | 0.0071155 | TMS membrane family protein/tumor differentially expressed (TDE) family protein |
| At5g25460 | 2.15 | 0.138 | 0.0004704 | Expressed protein |
| At1g08800 | 2.14 | 0.138 | 0.0092632 | Expressed protein, weak similarity to SP|Q02455 myosin-like protein MLP1 |
| At1g14687 | 2.11 | 0.097 | 0.0012244 | Zinc finger homeobox family protein |
| At1g75640 | 2.10 | 0.133 | 0.0073310 | Leucine-rich repeat family protein/protein kinase family protein |
| At5g66440 | 2.06 | 0.123 | 0.0032094 | Expressed protein |
| At4g20500 | 2.02 | 0.063 | 0.0008199 | Expressed protein |
| At1g78860 | 2.01 | 0.164 | 0.0095841 | Curculin-like (mannose-binding) lectin family protein, low similarity to Ser/Thr protein kinase |
a As analyzed by Affymetrix Arabidopsis DNA chips (ATH1). Experiment was conducted with three biological replicates.
b Fold change for the comparison of the estimated means between β-estradiol-treated 35S::ESR2-ER root explants and similarly treated 35S::GFP-ER explants.
c Standard error for the above comparison.
dP -value for the above comparison.
We focused on genes involved in signal transduction pathways such as ethylene, cytokinin, auxin and cell cycle control. AHP6 expression increased >5 fold following β-estradiol treatment on B5 medium ( Table 1 ). AHP6 is an unusual phosphotransmitter because it lacks a critical histidine residue that mediates the histidine to aspartate phosphorelay, suggesting that it probably does not transfer phosphoryl groups. None of the other functional AHPs was induced by ESR2. A gene ( At5g67430 ) related to HOOKLESS1 ( HLS1 ), which encodes an N -acetyl transferase and which has been shown to be ethylene responsive (Lehman et al. 1996 ), was up-regulated 9.0-fold. IAA20 expression was up-regulated 6.3-fold. Unlike other Aux/IAA protein members that were rapidly degraded by auxin treatment, IAA20, which lacks conserved domain II and a conserved lysine residue, was shown to be long-lived, regardless of auxin (Dreher et al. 2006 ). The expression of CUC1 was greatly up-regulated in 35S :: ESR2 transgenic plants ( Fig. 4 F) and induced by β-estradiol by 3.0-fold on B5 medium in the estradiol-mediated translocation system ( Table 1 ). These results suggest that the effect of ESR2 on CUC1 induction is direct. All of the genes described above contain the GCCGCC sequence in their promoters, which may be an ESR2-binding element. However, further promoter analysis is required to determine whether these sites are indeed responsible for ESR2 -induced up-regulation.
CUC1 acts downstream of ESR2
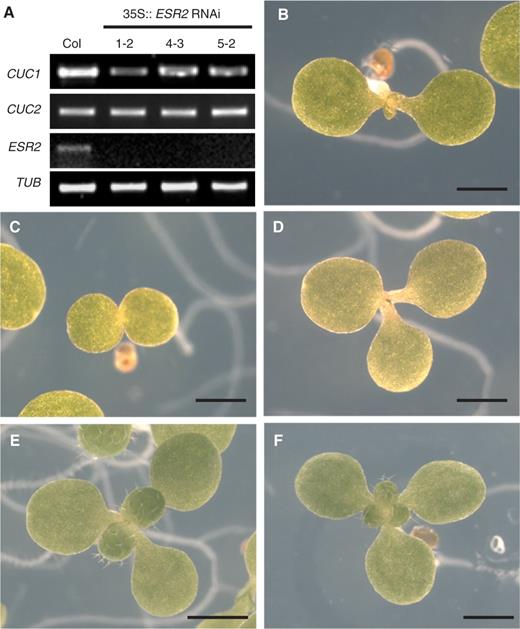
To understand better the function of ESR2 and its relationship to CUC1 , we generated transgenic plants expressing an ESR2 -specific RNAi construct ( 35S::ESR2 RNAi) and examined three independent lines. The endogenous expression of ESR2 was significantly suppressed to undetectable levels by reverse transcription–PCR (RT–PCR) ( Fig. 5 A).
Phenotypes of ESR2 knock-down seedlings. (A) Semi-quantitative RT–PCR analysis of the expression of CUC1, CUC2, ESR2 and TUBULIN (control) in wild-type (Col) and 35S :: ESR2 RNAi seedlings. Root explants at 6 d after transfer onto SIM were used for RNA preparation. (B) Seven-day-old wild-type seedling grown on MS agar medium. (C and D) Seven-day-old ESR2 RNAi seedlings with incompletely fused cotyledons in (C), and with triple cotyledons in (D). (E and F) Ten-day-old ESR2 RNAi seedlings grown on MS medium either with two true leaves in (E), or with three true leaves in (F). Bars = 1 mm.
When compared to wild-type seedlings cotyledons of ESR2 RNAi seedlings were partially fused ( Fig. 5 C). Seedlings with triple cotyledons were also observed at 0.25% frequency ( Fig. 5 D, Table 2 ). The phyllotaxy of first leaf development was also altered, such that either double or triple true leaves emerged ( Fig. 5 E, F). Approximately 0.5% of homozygous ESR2 RNAi seedlings displayed altered cotyledon phenotypes ( Table 2 ). This phenotype is reminiscent of cuc1 single mutant seedlings that display heart-shaped cotyledons at 0.5% frequency (Aida et al. 1997 ). We evaluated the expression of two CUC genes at 6 d after transfer onto SIM in the root tissue culture system, because they are up-regulated at this time (Cary et al. 2002 ). The expression of CUC1 , but not CUC2 , was reduced by ESR2 RNAi, suggesting that ESR2 only regulates CUC1 expression ( Fig. 5 A).
Analysis of 35S::ESR2 RNAi seedlings
| . | No. of seedlings with partially fused cotyledons . | No. of seedlings with triple cotyledons . | Total no. of seedlings a . | Frequency (%) . |
|---|---|---|---|---|
| Wild-type | 0 | 0 | 1,760 | 0 |
| 1-2T3 ESR2 RNAi | 2 | 3 | 1,178 | 0.42 |
| 4-3T3 ESR2 RNAi | 2 | 2 | 964 | 0.41 |
| 5-2T3 ESR2 RNAi | 4 | 3 | 1,172 | 0.597 |
| . | No. of seedlings with partially fused cotyledons . | No. of seedlings with triple cotyledons . | Total no. of seedlings a . | Frequency (%) . |
|---|---|---|---|---|
| Wild-type | 0 | 0 | 1,760 | 0 |
| 1-2T3 ESR2 RNAi | 2 | 3 | 1,178 | 0.42 |
| 4-3T3 ESR2 RNAi | 2 | 2 | 964 | 0.41 |
| 5-2T3 ESR2 RNAi | 4 | 3 | 1,172 | 0.597 |
a Seven-day-old seedlings grown on MS medium were analyzed.
Analysis of 35S::ESR2 RNAi seedlings
| . | No. of seedlings with partially fused cotyledons . | No. of seedlings with triple cotyledons . | Total no. of seedlings a . | Frequency (%) . |
|---|---|---|---|---|
| Wild-type | 0 | 0 | 1,760 | 0 |
| 1-2T3 ESR2 RNAi | 2 | 3 | 1,178 | 0.42 |
| 4-3T3 ESR2 RNAi | 2 | 2 | 964 | 0.41 |
| 5-2T3 ESR2 RNAi | 4 | 3 | 1,172 | 0.597 |
| . | No. of seedlings with partially fused cotyledons . | No. of seedlings with triple cotyledons . | Total no. of seedlings a . | Frequency (%) . |
|---|---|---|---|---|
| Wild-type | 0 | 0 | 1,760 | 0 |
| 1-2T3 ESR2 RNAi | 2 | 3 | 1,178 | 0.42 |
| 4-3T3 ESR2 RNAi | 2 | 2 | 964 | 0.41 |
| 5-2T3 ESR2 RNAi | 4 | 3 | 1,172 | 0.597 |
a Seven-day-old seedlings grown on MS medium were analyzed.
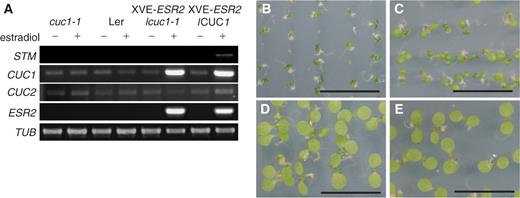
Transgenic plants harboring a single XVE- ESR2 transgene were crossed to cuc1-1 to examine the effects of CUC1 mutation on ESR2 -mediated cytokinin-related phenotypes. Equal induction of the ESR2 transgene was confirmed by RT–PCR ( Fig. 6 A). The levels of the wild-type CUC1 and mutant cuc1-1 transcripts were significantly elevated in an ESR2 -dependent manner; however, no induction of CUC2 was observed. The cytokinin-related phenotypes caused by ESR2 overexpression were greatly diminished in the cuc1-1 background (compare Fig. 6 B with C), suggesting that CUC1 acts downstream of ESR2 and plays an important role in the ESR2 -mediated cytokinin-related phenotypes in seedlings. β-Estradiol treatment itself did not alter plant morphology ( Fig. 6 D, E). We also found STM induction only in a wild-type background ( Fig. 6 A), consistent with the previous notion that CUC1 acts upstream of STM (Takada et al. 2001 ).
CUC1 acts downstream of ESR2 . (A) Semi-quantitative analysis of gene expression by RT–PCR. Ten-day-old seedlings were used for RNA preparation. Varying numbers of PCR cycles were examined for each gene, and three biological replicates were carried out. Note that in the cuc1-1 mutant background, the mutant transcript of CUC1 was also induced by ESR2 . TUBULIN ( TUB ) transcript levels were used as an internal control. (B) Ten-day-old seedlings harboring XVE- ESR 2 grown on MS medium containing β-estradiol. (C) Ten-day-old cuc1-1 seedlings harboring XVE- ESR2 grown on MS medium containing β-estradiol. (D) Ten-day-old wild-type seedlings grown on MS medium containing β-estradiol. (E) Ten-day-old cuc1-1 seedlings grown on MS medium containing β-estradiol Bars = 1 cm.
Discussion
ESR2 promotes shoot regeneration
ESR2 appears to act in a similar manner to ESR1 , conferring a strong shoot regeneration phenotype in the presence of exogenous cytokinin and a weaker regeneration phenotype in its absence. In this study, we have identified candidate genes directly activated by ESR2 . They include genes, such as CUC1 , involved in meristem specification. Our present study suggests that AHK4/CRE1 cytokinin receptor is not required for ESR2 overexpression phenotypes and that ESR2 directly targets CUC1 at the transcriptional level. This does not mean that ESR2 is the sole regulator of CUC1 , because the promoters of the target genes may have complex interactions with multiple regulators.
Activation of the cell cycle by ESR2
To identify other genes directly activated by ESR2 , we assayed global gene expression by DNA chip analysis following β-estradiol induction of nuclear translocation of ESR2–ER fusions. A similar approach was used to identify 16 target genes of LEAFY ( LFY ) (William et al. 2004 ). Here, we identified 51 candidate gene targets, which were up-regulated ≥2-fold by β-estradiol-induced ESR2–ER nuclear translocation ( Table 1 ). ESR2 overexpression results in cellular proliferation, suggesting the up-regulation of genes involved in cell cycle regulation ( Fig. 4 F). This is supported by the identification of CycD1;1 as another direct target of ESR2 . ESR2 may up-regulate CycD1;1 expression by affecting a candidate ESR-binding sequence (GCCGCC) located in the first intron of the CycD1;1 gene. This is reminiscent of AGAMOUS ( AG ) activation by LFY and WUS, in which the LFY- and WUS-binding sites are adjacent to one another in the second intron of AG (Lohmann et al. 2001 , Sharma et al. 2003 ). However, neither LFY nor WUS alone appear to be sufficient to activate AG , because neither factor alone can activate an AG reporter construct in vitro or in yeast cells (Lohmann et al. 2001 , Sharma et al. 2003 ). Analogous to AG , it is possible that in addition to ESR2, one or more unknown factors may be needed to activate CycD1;1 at the seedling stage, where we have not observed up-regulation of this gene (unpublished data). Rashotte et al. ( 2003 ) have shown that the steady-state level of CycD1;1 expression was reduced in wol and cre1-1 mutants. This is consistent with our finding that cytokinin can act synergistically with ESR activation (Banno et al. 2001 , this study). In a similar manner, AINTEGUMENTA ( ANT ), another member of the AP2 transcriptional factor family, extends the period of CycD3 expression, probably by inactivating cell cycle inhibitors such as p27 kip (Mizukami and Fischer 2000 ).
ESR overexpression can complement the cre1/ahk4 mutant
To examine the role of ESR2 in shoot formation, we asked whether the gene is involved in cytokinin biosynthesis or cytokinin signaling. The induction of shoots in cre1/ahk4 mutants by ESR genes suggests that the genes can provide functions normally supplied by cytokinin signaling. Previous studies showed that both CRE1/WOL/AHK4 and ARR2 genes are predominantly expressed in roots (Sakai et al. 1998 , Mahonen et al. 2000 , Ueguchi et al. 2001a ), and that signaling from CRE1 might be necessary for ARR2 activation at the post-transcriptional level. This probably explains why ectopic expression of full-length ARR2 does not promote shoot development in cre1 root explants. In contrast, induced expression of either ESR1 or ESR2 promoted shoot regeneration from mutant root explants, suggesting that ESR activity can complement this aspect of the cre1 phenotype.
How the expression of the two ESR genes is regulated is currently unknown. Even though ESR1 and ESR2 promoters contain four and six putative B-type ARR-binding sites (AGATT) (Sakai et al. 2000 ), respectively (data not shown), the ESR genes do not appear to be direct targets of the B-type response regulator, ARR1 , because induction of 35S:: Δ DDK ARR1-GR in transgenic plants did not activate expression of the ESR gene either in seedlings or in root explants (T. Aoyama personal communication and our unpublished data). Other B-type ARR genes, that are expressed in shoots, or other transcription factors induced by the cytokinin primary pathway may be necessary to induce ESR expression.
Negative regulation of the primary cytokinin pathway by ESR2
Given that several A-type ARRs are rapidly up-regulated by cytokinin (Brandstatter and Kieber 1998 , D'Agostino et al. 2000 , Hwang and Sheen 2001 , Sakai et al. 2001 , Hutchison and Kieber 2002 , Rashotte et al. 2003 , To et al. 2004 ), it was surprising to find that the expression of three A-type ARRs is suppressed in 35S :: ESR2 -overexpressing plants, especially in plants with severe phenotypes. It has been proposed that A-type ARRs may down-regulate cytokinin primary responses and the activity of B-type ARRs (Hwang and Sheen 2001 ). Recently, AHP6 was isolated as an extragenic suppressor of wol by a genetic suppressor screen, and the AHP6 protein was shown to act as an inhibitor of the cytokinin primary pathway by interacting with the phosphorelay machinery (Mahonen et al. 2006 ). In this study, we found that AHP6 is a likely direct target of ESR2 . We also found that the gene encoding cytokinin oxidase ( AtCKX7 ) was directly induced by 3.5-fold ( Table 1 ), which might also explain the reduced expression of type-A ARRs in 35S :: ESR2 transgenic plants. It is also possible that the increased expression of WUS led to the repression of some type-A ARR genes (Leibfried et al. 2005 ).
Determination of shoot apical meristem identity through CUC1 activation
It remains unclear how the two ESR genes promote shoot regeneration at the molecular level. Recent studies have shown that microRNAs target mRNAs of certain transcription factors and that post-transcriptional mechanisms play an important role in their regulation, as in the case of CUC1 and CUC2 (Rhoades et al. 2002 , Laufs et al. 2004 , Mallory et al. 2004 ). Our present study proposes that ESR2 is capable of regulating CUC1 at the transcriptional level, but not its closely related gene, CUC2 . This is also consistent with the genetic evidence that, when compared with either esr2 or cuc2 single mutants, the frequency of fused or single/triple cotyledons is significantly higher in the esr2cuc2 double mutant (J. Chandler and W. Werr personal communication), suggesting that ESR2 and CUC1 act in the same pathway and that both ESR2 and CUC2 genes share functional redundancy in cotyledon development. ESR2 may indirectly activate STM through CUC1 . Other studies have demonstrated that ectopic expression of CUC1 could promote adventitious shoot formation from calli through STM activation (Daimon et al. 2003 , Hibara et al. 2003 ).
We have shown that the ESR2 RNAi transgene has low penetrance in producing an altered cotyledon phenotype. ESR2 RNAi transgenic seedlings with fused cotyledons develop normal true leaves and flowers without any fused floral organs (data not shown), suggesting that the SAM in these transgenic plants retains normal function throughout vegetative and reproductive development. This is consistent with the expression pattern of ESR2 reported here, where no GUS signal was detected in the SAM after germination. Although both CUC genes are structurally and functionally similar to each other, their expression seems to be regulated differently. It has been previously suggested that CUC2 , but not CUC1 , is positively influenced by PIN FORMED1 ( PIN1 ), which encodes a presumptive auxin efflux transporter (Aida et al. 2002 ). Our results suggest that ESR2 regulates only CUC1 gene expression, and its induction may be in part responsible for the adventitious shoot formation. We are looking into the possibility that other ESR2 -targeted genes might also be major regulators of shoot regeneration.
Materials and Methods
Plant materials and growth conditions
Seeds of cre1-1 (L er ) and ahk4 (Ws) were provided by T. Kakimoto (Osaka University, Japan) and C. Ueguchi (Nagoya University, Japan), respectively. Seeds of cuc1-1 (CS3869) were obtained from Ohio State University Arabidopsis Biological Resource Center. Plants were grown in a greenhouse at 20°C with a photoperiod of 16 h light and 8 h dark.
Cloning and construction of transgenes
The protein-coding regions of ESR2 and its truncated derivatives were amplified by PCR from Arabidopsis genomic DNA (Col). CRE1B and ARR2 cDNA clones were kindly provided by Drs. T. Kakimoto and T. Aoyama, respectively. The coding sequences of GFP and the hormone-binding domain of ESTROGEN RECEPTOR (ER) were amplified from pGFP-2 and pER10 (Zuo et al. 2000 ), respectively. The primer sequences used for these constructions are described in Supplementary Table 1 . The resultant DNAs were cloned into either pSKM36, derivatives of pSK34, or pER10. The vector, pSKM36, permits fusion of four tandemly repeated MYC sequences to the C-terminus of a protein.
For ESR2 RNAi construction, the 3′ region specific for ESR2 was amplified by PCR with the primers listed in Supplementary Table 1 . The ESR2 -specific PCR products were digested with either Eco RI and Spe I or Cla I and Apa I, and cloned sequentially into the corresponding restriction enzyme sites of the pSK-Int vector that contains an ACTIN2 intron flanked by multiple cloning sites. The resulting products were digested with Bss HII and Not I, and cloned into the pSK34 vector at the Asc I and Not I sites. The inverted ESR2 -specific sequence with the ACTIN2 intron loop is driven by the 35S promoter.
The resulting constructs were introduced into Agrobacterium tumefaciens strain EHA105, which was used to generate transgenic plants by the floral dip method and root transformation. Shoot regeneration by transformation of root explants was described previously (Banno et al. 2001 ). The Arabidopsis thaliana Columbia ecotype was used to generate transgenic plants, unless indicated otherwise.
Analysis of XVE-ESR transgenic plants
Transgenic plants (L er ) harboring a single copy of pER10- ESR2 were selected by kanamycin resistance segregation and crossed to the cuc1-1 mutant (L er ). F 3 plants homozygous for the transgene and for cuc1-1 were confirmed by segregation of the kanamycin resistance and dCAPS markers for cuc1-1 as described elsewhere (Takada et al. 2001 ). F 3 plants were grown on MS agar medium with or without 10 μM estradiol (Sigma Chemical Co., St Louis, MO, USA).
GUS assay
A 2.5 kb fragment of genomic DNA upstream from the ESR2 translation start site was amplified by PCR. PCR products were digested with Sal I and Xba I and cloned into the Sal I and Xba I sites of pBI101 (Clonetech, Basingstoke, UK). At least 10 independent transgenic plants harboring the above construct were examined for GUS staining, and most of them showed similar results (data not shown). The GUS assay was performed as described elsewhere (Daimon et al. 2003 ).
Complementation of the cre1/ahk4 mutant
Arabidopsis thaliana cre1-1 (L er ) and ahk4 mutant seedlings (Ws) were used as recipients for transformation. Hypocotyl transformation of cre1/ahk4 was carried out as previously described (Inoue et al. 2001 ). For root transformation, seeds were sterilized and sown on MS agar medium and grown for 7 d at 22°C under continuous light. Seedlings were transferred to B5 liquid medium and incubated for 15 d with shaking at 125 r.p.m. Aerial parts were removed from the hydroponic culture and cultured roots were cut into 3–6 mm segments before being used for Agrobacterium -mediated transformation. Coding sequences of CRE1B, ARR2, ESR1, ESR2 and GFP were cloned at Asc I and Spe I sites of the pER10 vector, before being introduced into A. tumefaciens strain EHA105. Compositions of the CIM, the SIM and the RIM were as follows. (i) CIM: Gamborg's B5 salts, 2% glucose, Gamborg's B5 vitamins, 0.5 mg l −1 2,4-D, 0.5 mg l −1 kinetin and 0.25% Phytagel. (ii) SIM: MS salts, 1% sucrose, Gamborg's B5 vitamins, 0.3 mg l −1 indole butyric acid (IBA), 0.5 mg l −1trans -zeatin, 50 mg l −1 kanamycin and 0.25% Phytagel. (iii) RIM: SIM without trans -zeatin. To induce transcription of the transgene, 10 μM estradiol was included in the SIM and RIM.
RT–PCR analysis
Aerial parts of T 2 progeny harboring the 35S :: ESR2Myc construct with different phenotypes were used as the source of RNA. Purification of RNA and first strand synthesis were carried out as described elsewhere (Semiarti et al. 2001 ). cDNA synthesized from approximately 100 ng of total RNA was used as PCR templates. The reaction was cycled at 94°C for 25 s, 55°C for 25 s and 72°C for 2 min (16–30 cycles depending on the gene). For semi-quantification of mRNAs, we examined the amount of PCR products at three varying numbers of PCR cycles and confirmed that each PCR product was amplified quantitatively under the conditions we examined. The sequences of primer pairs are given in Supplementary Table 1 . Primer pairs for BP, KNAT2, STM, WUS and TUB have been described (Semiarti et al. 2001 ), as were those for CUC1 and CUC2 (Takada et al. 2001 ). Amplified DNA fragments were separated on 2% agarose gel and stained with ethidium bromide.
RNA sample preparation and microarray analysis
Root explants from four independent T 3 transgenic lines harboring either the 35S :: ESR2-ER or 35S :: GFP-ER transgene were prepared as described previously (Banno et al. 2001 ). The mixture of four independent T 3 transgenic lines harboring either of the above constructs was used. RNA transcripts were profiled on B5 hormone-free medium for 3 d after CIM pre-incubation for 4 d. Root explants were transferred onto the same B5 agar medium containing 10 μM estradiol and 30 μM cycloheximide (CHX), and were sprayed with the same medium solution containing the previously mentioned amounts of estradiol and CHX. After 1 h, total RNA was purified with the RNeasy Kit (Qiagen, Valencia, CA, USA), precipitated by LiCl and dissolved in diethylpyrocarbonate (DEPC)-treated water. Three biological replicates were carried out to normalize possible hybridization errors. Affymetrix Arabidopsis 22K GeneChips (ATH1) were used for microarray analysis. RNA samples were submitted to the GeneChip Facility at Iowa State University ( http://www.biotech.iastate.edu/facilities/genechip/Genechip.htm ). The subsequent procedures were performed as described (Che et al. 2002 ). Oligonucleotide array data was analyzed using the GeneChip Microarray Suite version 5.0. The microarray experiments were conducted in a random block design with three biological replicates. The estimated means of the MAS5 normalized signals were sorted by fold change for samples with P -values <0.01 for the comparison between β-estradiol-treated 35S :: ESR2-ER root explants and similarly treated 35S :: GFP-ER explants. P -values were calculated using EDGE software (version 1.1.208 from the Storey Lab at the University of Washington ( http://faculty.washington.edu/jstorey/edge/ ).
Aliquots of total RNA were also used for semi-quantitative RT–PCR analysis as described above.
Supplementary material
Supplementary material mentioned in the article is available to online subscribers at the journal website http://www.pcp.oxfordjournals.org/ .
Acknowledgments
We thank Takashi Aoyama for providing the ARR2 cDNA clone, communicating unpublished results and helpful suggestions, Chiharu Ueguchi for providing ahk4 mutant seeds, Tatsuo Kakimoto for cre1-1 mutant seeds and the CRE1B cDNA clone, John Chandler and Wolfgang Werr for sharing unpublished results, Dr. Jianru Zuo for suggestions, Ted Klein, Bill Gordon-Kamm, Enno Krebbes, Sheila Maddock, Masaki Ishikawa, and Kristiina Himanen for stimulating and helpful suggestions, Jiqing Peng and the GeneChip Facility in Iowa State University for helping with the microarray experiments, and Peter Hare and Misty Bliss for critical reading of the manuscript. This work was supported by a grant from Dupont/Pioneer Crop Genetics Research and by a grant from the National Science Foundation (IBN-0236060) to S.H.H.
References
Abbreviations:
- AHK
ARABIDOPSIS HISTIDINE KINASE
- AHP
ARABIDOPSIS HISTIDINE PHOSPHOTRANSMITTER
- ANT
AINTEGUMENTA
- ARR
ARABIDOPSIS RESPONSE REGULATOR
- BP
BREVIPEDICELLUS
- CHX
cycloheximide
- CIM
callus-inducing medium
- CUC
CUP-SHAPED COTYLEDON
- CKI1
CYTOKININ-INDEPENDENT 1
- CRE1
CYTOKININ RESPONSE 1
- CYC
CYCLIN
- DRN
DORNRÖSCHEN
- EAR
ERF-associated amphiphilic repression
- ERF
ETHYLENE RESPONSE FACTOR
- ER
estrogen receptor
- ESR
ENHANCER OF SHOOT REGENERATION
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- HLS
HOOKLESS
- IPT
isopentenyl transferase
- KNAT
KNOTTED-like from Arabidopsis thaliana
- PIN
PIN FORMED
- RIM
root induction medium
- RNAi
RNA interference
- RT–PCR
reverse transcription–PCR
- SAM
shoot apical meristem
- SIM
shoot induction medium
- STM
SHOOT MERISTEMLESS
- WOL
WOODEN LEG
- WUS
WUSCHEL
The nucleotide sequence data reported herein have been deposited in the EMBL/GenBank databases under the following accession numbers: ESR1, AF353577 (At1g12980); ESR2, AAN27907 (At1g24590).
Author notes
Present address: Umeå Plant Science Centre, Department of Forest Genetics and Plant Physiology, Swedish University of Agricultural Sciences, SE-901 83 Umeå, Sweden.
Present address: Department of Environmental Biology, School of Bioscience and Biotechnology, Chubu University, 1200 Matsumoto-cho, Kasugai, Aichi, 487-8501 Japan.