-
PDF
- Split View
-
Views
-
Cite
Cite
Yasuhiro Kadota, Shinsuke Fujii, Yoko Ogasawara, Yutaka Maeda, Katsumi Higashi, Kazuyuki Kuchitsu, Continuous Recognition of the Elicitor Signal for Several Hours is Prerequisite for Induction of Cell Death and Prolonged Activation of Signaling Events in Tobacco BY-2 Cells, Plant and Cell Physiology, Volume 47, Issue 9, September 2006, Pages 1337–1342, https://doi.org/10.1093/pcp/pcj098
Close - Share Icon Share
Abstract
To provide insights into the mechanisms by which receptors for pathogenic elicitors activate defense signaling, we investigated the duration of cryptogein treatment required for induction of various defense responses including programmed cell death in synchronized tobacco BY-2 cells. Transient cryptogein treatment induced only a rapid and transient phase of oxidative burst and mitogen-activated protein kinase (MAPK) activation. Prolonged production of 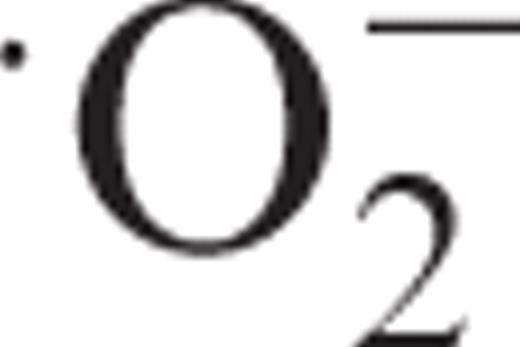 and prolonged activation of MAPKs, as well as accumulation of transcripts of defense-related genes and cell death, required continuous recognition of cryptogein for several hours. In contrast, desensitization was gradually induced in the absence of the elicitor.
and prolonged activation of MAPKs, as well as accumulation of transcripts of defense-related genes and cell death, required continuous recognition of cryptogein for several hours. In contrast, desensitization was gradually induced in the absence of the elicitor.
Plants respond to pathogenic invasion with a variety of defense mechanisms, including the synthesis of phytoalexins and hypersensitive cell death, which restrict the growth of pathogens at the infection site (Jones and Dangl 1996 ). These responses are thought to require recognition between the host and pathogen, mediated by pathogenic effector molecules called elicitors or pathogen-associated molecular proteins and their specific receptors (Shibuya et al. 1993 ). Several elicitor receptors have been suggested to be desensitized following elicitor recognition. Suspension-cultured tomato cells respond to chitin fragments with a rapid and transient alkalinization of the growth medium, but are refractory to a second treatment with the same stimulus (Felix et al. 1998 ). Once soybean cells are treated with α-1,4-linked oligogalacturonides, no changes in cytosolic free Ca 2+ concentration is induced in response to a second stimulus for up to 16 h after the first treatment (Navazio et al. 2002 ).
However, the period of time over which cells continue to recognize the elicitors and the duration of elicitor treatment necessary for induction of defense responses are totally unknown. When mature leaves or whole plants are treated with an elicitor, it is almost impossible to remove the elicitor completely from the intercellular space. Furthermore, a suitable experimental system in which cell death and defense responses are induced highly synchronously is required to analyze the duration of elicitor exposure.
A proteinaceous elicitor cryptogein derived from Phytophthora cryptogea elicits hypersensitive response (HR)-like cell death in leaves (Ricci et al. 1989 ) and suspension-cultured cells (Kadota et al. 2004a , Kadota et al. 2004b ) of tobacco. A recent study using synchronous cultured tobacco BY-2 cells revealed that cryptogein-induced defense responses depend on cell cycle phases (Kadota et al. 2004b , Kadota et al. 2005 ). Although elicitor recognition occurs at all phases of the cell cycle, cell death is only induced upon elicitor treatment during G 1 or S phase. Analyses of cryptogein signaling mechanisms would therefore be facilitated by studying responses in populations of cells synchronized at G 1 or S phase.
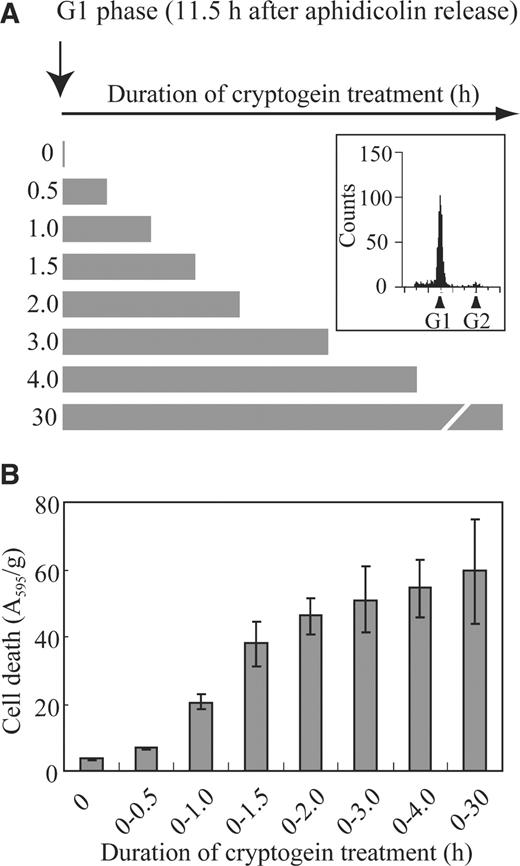
In the present study, we have analyzed the period of elicitor exposure necessary for induction of defense responses in synchronized cultured BY-2 cells. As cryptogein-induced cell death occurred only when the elicitor was recognized during G 1 or S phases (Kadota et al. 2004b ), BY-2 cells were synchronized using aphidicolin and treated with cryptogein during G 1 phase. Flow cytometric analysis showed that almost all cells were in G 1 phase at 11.5 h after aphidicolin release ( Fig. 1 ), when cryptogein was applied. Since 0.5–1 μM of cryptogein was sufficient to induce cell death (data not shown), we used 1 μM cryptogein in the following experiments. Cryptogein was removed by washing with excess fresh growth medium at the indicated time points (0, 0.5, 1, 2, 3 and 4 h after cryptogein application, Fig. 1 ), followed by cell death analyses at 30 h. Cryptogein treatment for 0.5 h during G 1 phase induced no cell death, and continuous treatment for at least 2–3 h was required to induce cell death ( Fig. 1 ). When cells were treated with cryptogein for longer than 3 h, approximately 40% of the cells were stained by Evans blue at 30 h. Continuous cryptogein treatment for 2–3 h was also required and sufficient to inhibit cell growth completely (data not shown).
A 2–3 h cryptogein treatment is prerequisite to induce cell death. Cells were synchronized at S phase using aphidicolin. Flow cytometric analysis (boxed) showed that almost all cells were in G 1 phase at 11.5 h after aphidicolin release (A). To analyze the minimum duration of cryptogein treatment necessary to induce cell death, cryptogein (1 μM) was applied at 11.5 h (G 1 phase) after aphidicolin release and, at the indicated time points (0, 0.5, 1, 2, 3 and 4 h), cryptogein was removed by washing with excess growth medium (B). Cell death rates were analyzed at 30 h after cryptogein application. These data represent the average of three independent experiments. Error bars indicate the standard error of the mean ( n = 3).
The duration of cryptogein treatment necessary for the activation of various defense signaling events was analyzed. Cryptogein-induced production of superoxide anion ( 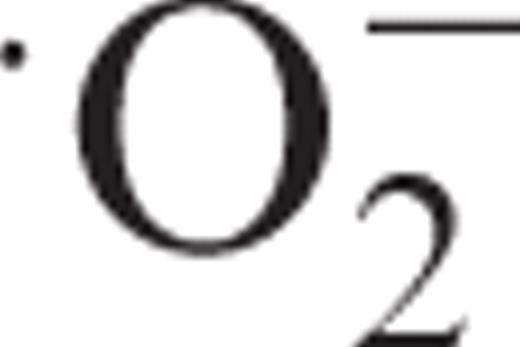 ) was monitored with an
) was monitored with an 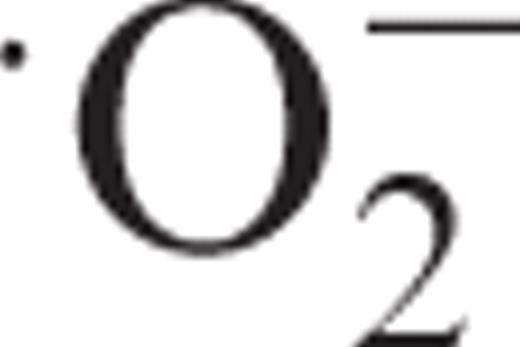 -specific chemiluminescence reagent, 2-methyl-6-[ p -methoxyphenyl]-3,7-dihydroimidazo [1,2-α]pyrazin-3-one (MCLA; Fig. 2 A). Since
-specific chemiluminescence reagent, 2-methyl-6-[ p -methoxyphenyl]-3,7-dihydroimidazo [1,2-α]pyrazin-3-one (MCLA; Fig. 2 A). Since 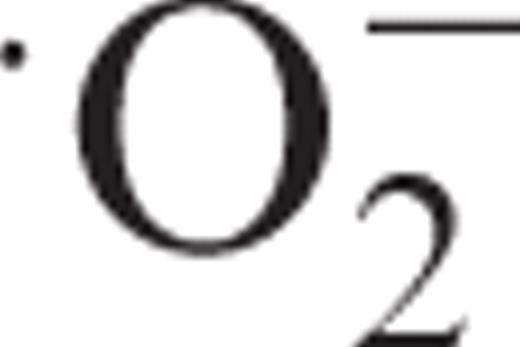 , produced by cellular enzymes such as NADPH oxidase, rapidly converts to H 2 O 2 possibly by cell wall-localized Cu/Zn superoxide dismutase (Kawano and Muto 2000 ), the endogenous level of
, produced by cellular enzymes such as NADPH oxidase, rapidly converts to H 2 O 2 possibly by cell wall-localized Cu/Zn superoxide dismutase (Kawano and Muto 2000 ), the endogenous level of 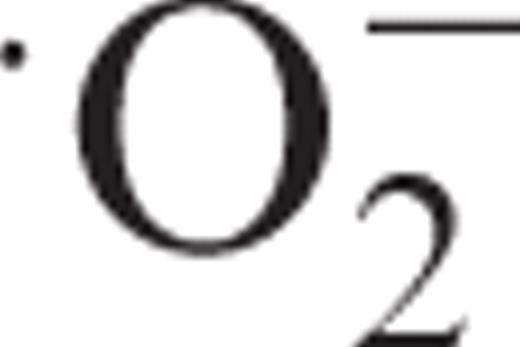 accumulated in the medium should be very low and MCLA is suggested to react only with the
accumulated in the medium should be very low and MCLA is suggested to react only with the 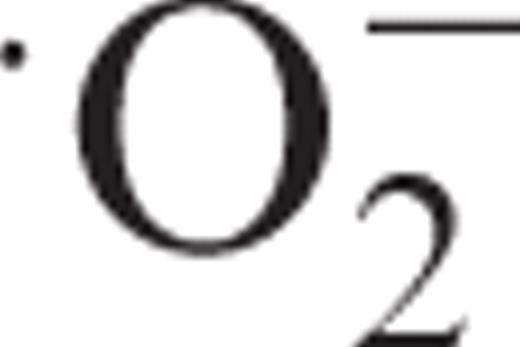 newly produced by the cells (Kadota et al. 2004a ). Cryptogein treatment at G 1 phase induced biphasic
newly produced by the cells (Kadota et al. 2004a ). Cryptogein treatment at G 1 phase induced biphasic 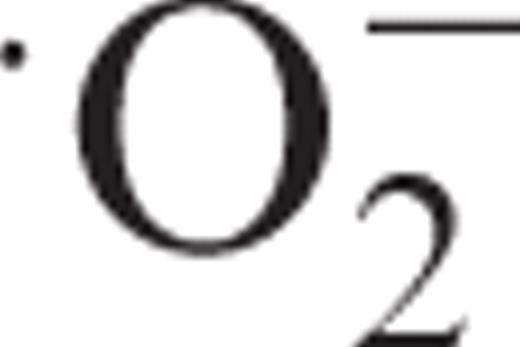 production; a rapid and transient increase peaked at around 10 min, followed by a slow and prolonged phase. The removal of cryptogein at 0.5, 1, 2 and 3 h by an extensive wash with the fresh medium caused the reduction in
production; a rapid and transient increase peaked at around 10 min, followed by a slow and prolonged phase. The removal of cryptogein at 0.5, 1, 2 and 3 h by an extensive wash with the fresh medium caused the reduction in 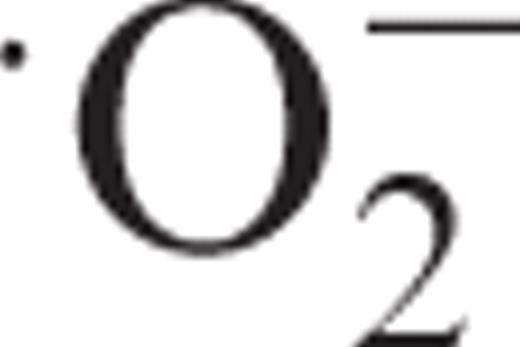 production ( Fig. 2 A). Re-treatment of the cells with cryptogein immediately after washing induced identical
production ( Fig. 2 A). Re-treatment of the cells with cryptogein immediately after washing induced identical 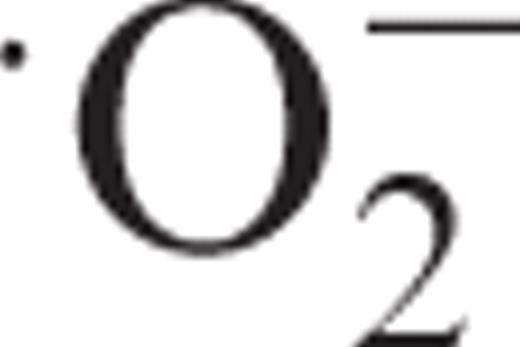 production to that induced by continuous elicitor treatment. These results suggest that prolonged
production to that induced by continuous elicitor treatment. These results suggest that prolonged 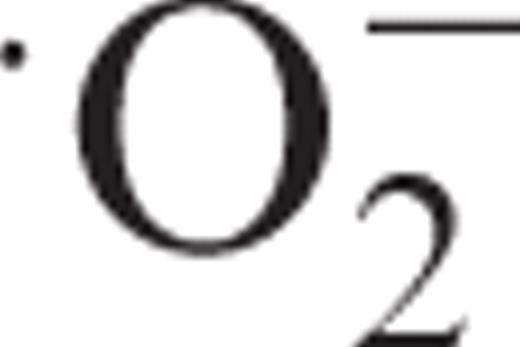 generation requires continuous exposure to the elicitor.
generation requires continuous exposure to the elicitor.
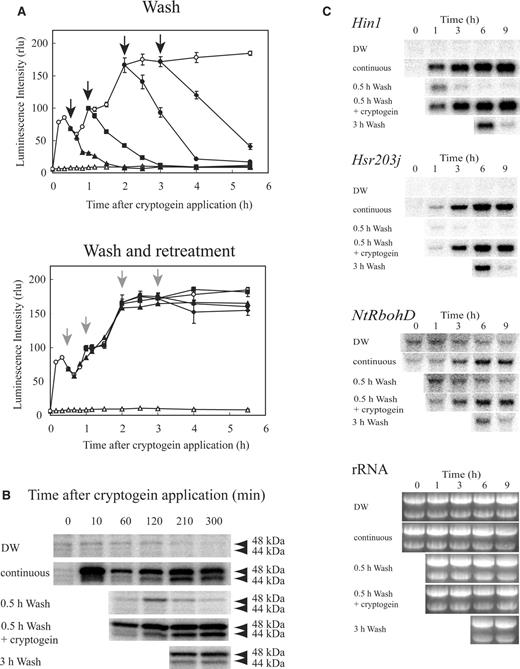
Prolonged 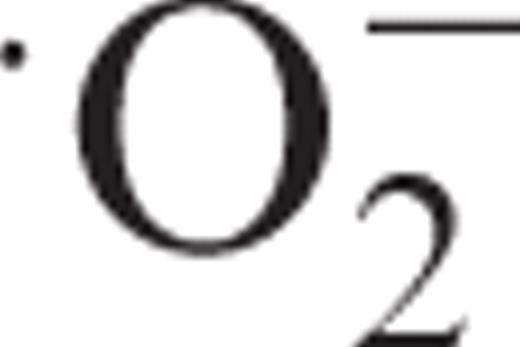 generation, activation of MAPKs and accumulation of transcripts of defense-related genes require continuous treatment with cryptogein. (A) Prolonged
generation, activation of MAPKs and accumulation of transcripts of defense-related genes require continuous treatment with cryptogein. (A) Prolonged 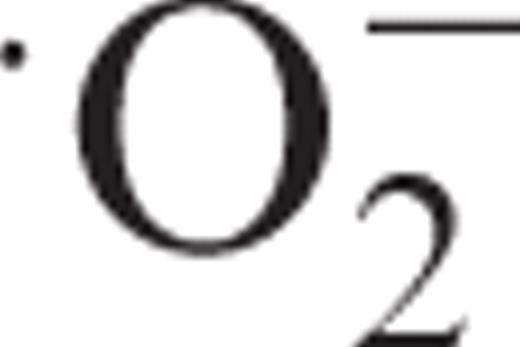 generation requires continuous treatment with cryptogein. BY-2 cells were synchronized at G 1 phase and treated with cryptogein (open circles) or distilled water (DW, open triangles) at 11.5 h after aphidicolin release.
generation requires continuous treatment with cryptogein. BY-2 cells were synchronized at G 1 phase and treated with cryptogein (open circles) or distilled water (DW, open triangles) at 11.5 h after aphidicolin release. 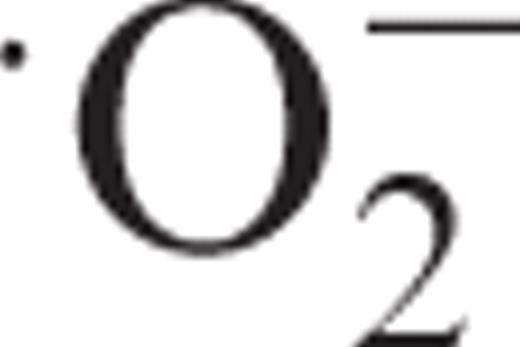 generation was monitored in both the cells from which cryptogein was removed and those re-treated just after removal at 0.5 h (filled triangles), 1 h (filled squares), 2 h (filled circles) and 3 h (filled diamonds). Black arrows and gray arrows indicate the removal of cryptogein and the removal and re-treatment with cryptogein, respectively. (B) Prolonged activation of MAPKs requires continuous treatment with cryptogein. Extracts containing 20 μg of protein in each samples were electrophoresed and the activation of MAPKs was monitored with an in-gel kinase assay. (C) Prolonged accumulation of transcripts of defense-related genes requires continuous treatment with cryptogein. The numbers of transcripts of defense-related genes ( Hin1 , Hsr203j and NtRbohD ) were estimated by RNA gel blot analysis. The results shown in this figure are from one experiment representative of three independent experiments.
generation was monitored in both the cells from which cryptogein was removed and those re-treated just after removal at 0.5 h (filled triangles), 1 h (filled squares), 2 h (filled circles) and 3 h (filled diamonds). Black arrows and gray arrows indicate the removal of cryptogein and the removal and re-treatment with cryptogein, respectively. (B) Prolonged activation of MAPKs requires continuous treatment with cryptogein. Extracts containing 20 μg of protein in each samples were electrophoresed and the activation of MAPKs was monitored with an in-gel kinase assay. (C) Prolonged accumulation of transcripts of defense-related genes requires continuous treatment with cryptogein. The numbers of transcripts of defense-related genes ( Hin1 , Hsr203j and NtRbohD ) were estimated by RNA gel blot analysis. The results shown in this figure are from one experiment representative of three independent experiments.
Cryptogein treatment induced biphasic activation of two mitogen-activated protein kinase (MAPK) homologs with apparent molecular masses of 48 and 44 kDa, identified as salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK), respectively (Zhang et al. 1998 ); rapid and transient activation followed by a slow and prolonged phase ( Fig. 2 B). Removal of cryptogein at 0.5 h after application caused rapid inactivation of both kinases. Re-application of cryptogein immediately after its removal at 0.5 h induced a pattern of activation identical to those observed in continuous treatment, indicating that prolonged activation of SIPK and WIPK requires continuous elicitor treatment. Removal of cryptogein at 3 h after application caused slow inactivation of these kinases, which were still active at 5 h.
The amounts of transcripts of several defense-related genes were analyzed by RNA gel blot analysis ( Fig. 2 C). The expression of the harpin-induced gene Hin1 correlated with the HR (Gopalan et al. 1996 ). Hsr203j , an HR-related (hsr) gene encoding a serine hydrolase with esterase activity, has been postulated as regulating either the establishment or the limitation of cell death (Pontier et al. 1998 ). A tobacco NOX (gp91 phox ) homolog ( NtRbohD ), a component of NADPH oxidase, is expressed in response to elicitors or avirulent pathogens and is suggested to be essential for the slow and prolonged production of reactive oxygen species (ROS) (Yoshioka et al. 2001 ). The accumulation of transcripts of these defense-related genes was induced by continuous cryptogein treatment, while it was severely inhibited upon removal of cryptogein at 0.5 or 3 h after application. Re-treatment with the elicitor immediately after its removal at 0.5 h induced a pattern of accumulation of transcripts for both genes identical to those observed for the continuous treatment. Similar results were also obtained for acidic chitinase (data not shown). These results indicate that accumulation of transcripts of defense-related genes requires continuous elicitor treatment for several hours. Both cryptogein-induced expression of NtRbohD and the slow and prolonged phase of 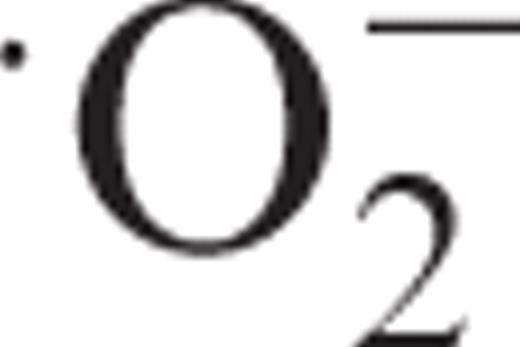 production required continuous elicitor treatment and showed a similar time course, suggesting that elicitor-induced expression of NtRbohD is responsible for the slow and prolonged phase of the oxidative burst.
production required continuous elicitor treatment and showed a similar time course, suggesting that elicitor-induced expression of NtRbohD is responsible for the slow and prolonged phase of the oxidative burst.
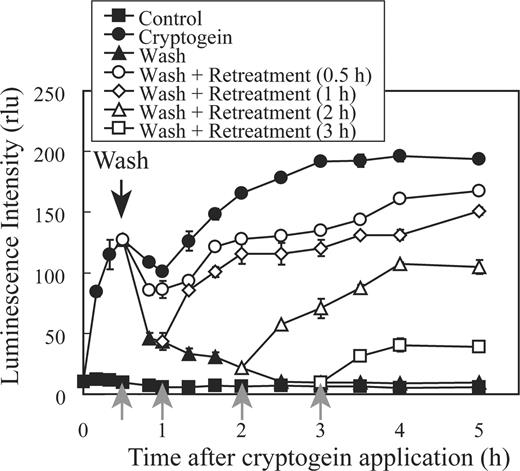
Re-treatment of the cells with cryptogein immediately after washing induced responses identical to those induced by continuous cryptogein treatment ( Fig. 2 ). In contrast, incubation of the cells without cryptogein for 80 min after the first elicitation resulted in a weaker response to the second elicitation (Binet et al. 1998 ). Therefore, desensitization to cryptogein may be gradually induced after removal of the elicitor. To test this hypothesis, cryptogein was applied to the cells in G 1 phase (11.5 h after aphidicolin release) followed by washing at 30 min after the elicitor treatment. Then cryptogein was re-applied at the indicated time points (0.5, 1, 2 and 3 h), and 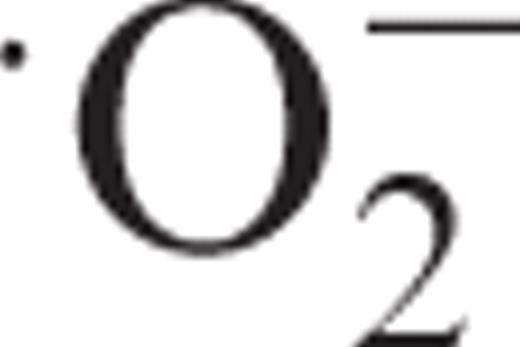 production was monitored using MCLA ( Fig. 3 ). Re-treatment with cryptogein at 0.5 or 1 h after removal induced a similar pattern of
production was monitored using MCLA ( Fig. 3 ). Re-treatment with cryptogein at 0.5 or 1 h after removal induced a similar pattern of 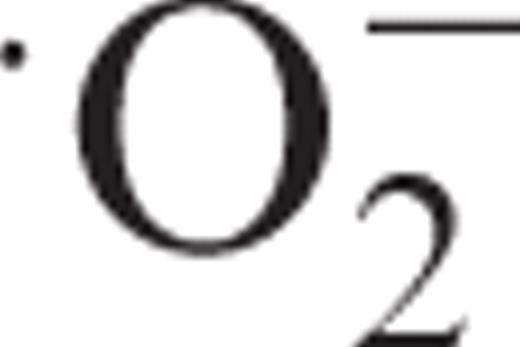 production to that induced by the continuous treatment. In contrast,
production to that induced by the continuous treatment. In contrast, 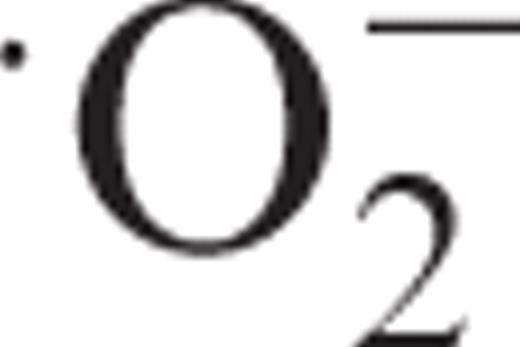 production induced by cryptogein re-treatment at 2 or 3 h after removal was greatly suppressed. These results indicate that desensitization is gradually induced after removal of cryptogein and takes several hours.
production induced by cryptogein re-treatment at 2 or 3 h after removal was greatly suppressed. These results indicate that desensitization is gradually induced after removal of cryptogein and takes several hours.
Desensitization is gradually induced after removal of cryptogein. Cryptogein (1 μM) or distilled water (control) was applied at 11.5 h after aphidicolin release, followed by removal of cryptogein by washing at 0.5 h after application. Then the cells were re-treated with cryptogein (1 μM) at 0.5, 1, 2 or 3 h, and 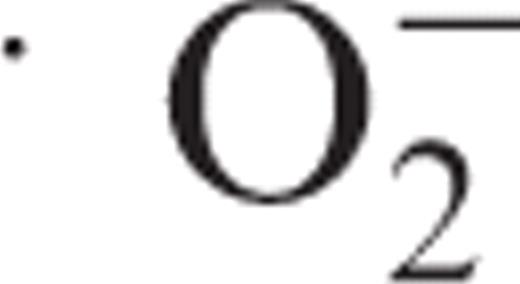 generation was monitored using MCLA. A black arrow indicates the removal of cryptogein, and gray arrows indicate the re-treatment with cryptogein. The results show one representative of three independent experiments.
generation was monitored using MCLA. A black arrow indicates the removal of cryptogein, and gray arrows indicate the re-treatment with cryptogein. The results show one representative of three independent experiments.
We showed here that continuous recognition of elicitor for several hours is prerequisite for induction of the full range of defense responses including cell death. All the experiments we showed here are performed using the cells in G 1 phase, but similar results were also obtained using the cells in S phase (data not shown). Washing the cells with 10 vols of fresh growth medium at 0.5 h after cryptogein application inhibited various responses, including ROS production, activation of MAPKs, accumulation of transcripts of defense-related genes ( Fig. 2 ) and cell death ( Fig. 1 ). These results indicate that washing with excess fresh growth medium eliminates the elicitor signal. Re-treatment with cryptogein after its removal induced identical responses to those induced by continuous treatment ( Fig. 2 ), confirming that the inhibition of cryptogein-induced responses by washing was not an artifact caused by the exchange of the growth medium, but was attributable to the removal of cryptogein.
Although a cryptogein receptor(s) has not been identified, high affinity binding sites and a putative binding protein for cryptogein localized in the plasma membrane of tobacco cells have been characterized by using 125 I-labeled cryptogein (Bourque et al. 1999 ). The binding was saturable and specific, and inhibited by unlabeled cryptogein, suggesting that the binding between cryptogein and receptor(s) is reversible. This is supported by the present results showing that washing the cells with excess fresh growth medium shut down the cryptogein signaling.
Signal desensitization has been reported for several kinds of elicitors such as chitin fragments and oligogalacturonides (Felix et al. 1998 , Küpper et al. 2001 , Navazio et al. 2002 ). Internalization of the flagellin receptor, FLS, into intracellular mobile vesicles followed by degradation is induced 1 h after application of the ligand peptide, flg22 in Arabidopsis (Robatzek et al. 2006 ). However, the present results show that BY-2 cells continue to recognize cryptogein for several hours, and the continuous recognition is prerequisite for the prolonged activation of defense responses. The rate for induction of elicitor desensitization may be different depending on the kinds of elicitors. Binet et al. ( 1998 ) showed that when cells were treated with oligogalacturonides for 40 min followed by further incubation without the elicitor for 80 min, cells become refractory to the second elicitation. In contrast, cells still responded to the second treatment with cryptogein under the same experimental conditions. In the present study, we showed that desensitization to cryptogein is gradually induced after removal of cryptogein ( Fig. 3 ). Signal desensitization for the elicitors that induce hypersensitive cell death may be induced more slowly than for the elicitors that do not induce cell death such as chitin fragments, oligogalacturonides and flagellin. Alternatively, the signal desensitization mechanism for cryptogein may be repressed during the continuous treatment with the elicitor. It is an important future research subject to compare the duration of elicitor recognition and the duration of elicitor recognition necessary for induction of defense responses among various kinds of elicitors.
Upon transient cryptogein treatment for 3 h, induction of cell death ( Fig. 1 ) as well as inactivation of mitochondrial reductases (data not shown) was comparable with those induced by continuous treatment for 30 h. In contrast, a 3 h transient treatment was insufficient for full induction of prolonged production of ROS, activation of MAPKs and accumulation of transcripts of defense-related genes ( Fig. 2 ). A 3 h transient cryptogein treatment is not sufficient for full induction of defense-related genes, but may be sufficient for synthesis of cell death-executing factors.
Plants frequently come into contact with various kinds of pathogens including virulent or avirulent ones. The requirement for a long duration of elicitor recognition for full induction of hypersensitive cell death and defense responses discovered in the present study may be crucial to distinguish the real infection of pathogens from accidental contact with microbes and to prevent plant cells from mistakenly triggering prolonged defense responses including cell death. Since cell proliferation and growth are inhibited in the process of defense responses (Kadota et al. 2004b , Gómez-Gómez and Boller 2002 ), such a determination mechanism for the cell fate to induce defense responses may be crucial for maintenance of maximum efficiency for defense and growth. The inter-relationship between molecular mechanisms for elicitor recognition and cell fate determination should be an emerging important topic for future studies.
Materials and Methods
A tobacco BY-2 ( Nicotiana tabacum L. cv. Bright Yellow 2) suspension was maintained by weekly dilution (1 : 100) of cells in modified Linsmaier and Skoog (LS) medium, as described by Nagata et al. ( 1992 ). The cell suspension was agitated on a rotary shaker at 100 r.p.m. at 28°C in the dark. A stationary culture of tobacco BY-2 was diluted 1 : 10 in fresh modified LS medium supplemented with 5 μg ml −1 aphidicolin (Wako Pure Chemical, Japan). After a 24 h culture period, aphidicolin was removed by extensive washing, and the cells were resuspended in fresh medium.
Pichia pastoris (strain GS115) bearing the plasmid pLEP3 was used for cryptogein production. Cryptogein was expressed according to methods described by O'Donohue et al. ( 1996 ) and was dissolved in distilled water.
Flow cytometric analysis was performed according to the manufacturer's protocol as described previously (Kadota et al. 2004b ). Fluorescence from artificially fragmented DNA produced during the extraction of the nuclei probably contributed to the observed fluorescence intensity in low fluorescence samples, and we therefore disregarded samples with fluorescence counts below a cut-off value equivalent to nuclei at the G 1 phase.
Cell death was analyzed by Evans blue assay (Turner and Novacky 1974 ) as described previously (Kadota et al. 2005 ).
For removal of cryptogein, BY-2 cells were filtrated and washed with 10 vols of fresh growth medium, and then resuspended in fresh medium.
A BY-2 cell suspension was washed and resuspended in fresh growth medium 30 min before measurement. An aliquot (250 μl) of the cell suspension was sampled at the indicated time and treated with 2 μM MCLA (Molecular Probes, Eugene, OR, USA); the 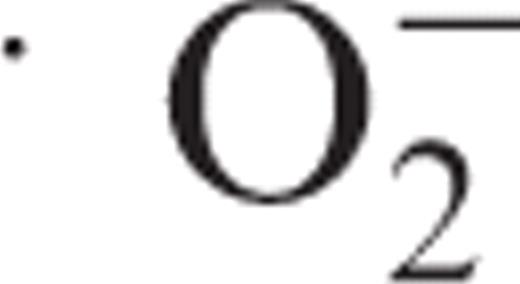 -dependent chemiluminescence was measured with a Lumicounter 2500 (Microtech Nition, Chiba, Japan).
-dependent chemiluminescence was measured with a Lumicounter 2500 (Microtech Nition, Chiba, Japan).
Extracts containing 20 μg of protein were electrophoresed on 10% SDS–polyacrylamide gels embedded with 0.25 mg ml −1 myelin basic protein in the separating gel as a substrate for the protein kinase. After electrophoresis, we performed an in-gel kinase assay as described previously (Kadota et al. 2005 ).
Total RNA was extracted from each frozen sample of cells using Trizol reagent according to the manufacturer's protocol (Invitrogen Co., Carlsbad, CA, USA). Northern blot analyses were performed as described previously (Kadota et al. 2004c ).
Acknowledgments
The authors thank Dr. Kaoru Suzuki for generous gifts of the cDNA clones of ACHN , Hin1 and Hsr203J , and for thoughtful discussions. We thank Dr. Toyoki Amano for valuable suggestions and Professor Jean-Claude Pernollet for providing us with a gene encoding cryptogein. We are also grateful to Mr. Takumi Higaki for critical reading of the manuscript. This work was supported in part by a Grant-in-Aid from the Research for the Future Program to K.K., Grants-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Science, Culture, Sports, and Technology of Japan to K.K. (No. 13039015) and a Scientific Research Grant (No. 06801) from the Japan Society for the Promotion of Science to Y.K.
References
Abbreviations:
- HR
Hypersensitive response
- MAPK
Mitogen-activated protein kinase
- MCLA
2-methyl-6-[ p -methoxyphenyl]-3,7-dihydroimidazo[1,2-α]pyrazin-3-one
superoxide anion
- ROS
reactive oxygen species.
Author notes
Present address: RIKEN Plant Science Center, 1-7-22 Suehirocho, Tsurumi-ku, Yokohama, 230-0045 Japan.
Present address: Department of Biological Sciences, Teikyo University of Science and Technology, Uenohara, Yamanashi, 409-0193 Japan.