-
PDF
- Split View
-
Views
-
Cite
Cite
Jieqing Ping, Yunfeng Liu, Lianjun Sun, Meixia Zhao, Yinghui Li, Maoyun She, Yi Sui, Feng Lin, Xiaodong Liu, Zongxiang Tang, Hanh Nguyen, Zhixi Tian, Lijuan Qiu, Randall L. Nelson, Thomas E. Clemente, James E. Specht, Jianxin Ma, Dt2 Is a Gain-of-Function MADS-Domain Factor Gene That Specifies Semideterminacy in Soybean , The Plant Cell, Volume 26, Issue 7, July 2014, Pages 2831–2842, https://doi.org/10.1105/tpc.114.126938
Close - Share Icon Share
Abstract
Similar to Arabidopsis thaliana, the wild soybeans (Glycine soja) and many cultivars exhibit indeterminate stem growth specified by the shoot identity gene Dt1, the functional counterpart of Arabidopsis TERMINAL FLOWER1 (TFL1). Mutations in TFL1 and Dt1 both result in the shoot apical meristem (SAM) switching from vegetative to reproductive state to initiate terminal flowering and thus produce determinate stems. A second soybean gene (Dt2) regulating stem growth was identified, which, in the presence of Dt1, produces semideterminate plants with terminal racemes similar to those observed in determinate plants. Here, we report positional cloning and characterization of Dt2, a dominant MADS domain factor gene classified into the APETALA1/SQUAMOSA (AP1/SQUA) subfamily that includes floral meristem (FM) identity genes AP1, FUL, and CAL in Arabidopsis. Unlike AP1, whose expression is limited to FMs in which the expression of TFL1 is repressed, Dt2 appears to repress the expression of Dt1 in the SAMs to promote early conversion of the SAMs into reproductive inflorescences. Given that Dt2 is not the gene most closely related to AP1 and that semideterminacy is rarely seen in wild soybeans, Dt2 appears to be a recent gain-of-function mutation, which has modified the genetic pathways determining the stem growth habit in soybean.
INTRODUCTION
Soybean (Glycine max) stem growth habit is a key adaptation and agronomic trait that directly affects plant height, flowering time and duration, node production, leaf morphology, root architecture, maturity, water use efficiency, abiotic stress tolerance, and, ultimately, soybean yield (Bernard, 1972; Specht et al., 2001; Heatherly and Smith, 2004). Based on the timing of the termination of apical stem growth, most soybean cultivars can be classified into two categories of stem architecture, commonly known as determinate and indeterminate types. A determinate stem arises when apical stem growth abruptly ceases at the onset of floral induction. This generally produces a thick stem because latitudinal growth in stem girth continues after apical growth in stem length has ceased. An indeterminate stem tip continues terminal growth, as does its lateral growth, though both cease at the onset of seed filling, thus producing a stem that is tapered in thickness from base to tip. Despite this simple classification, the abruptness of stem termination varies among soybean accessions in the USDA Soybean Germplasm Collection, with phenotypic scores ranging from 1 (very determinate) to 5 (very indeterminate). Scores of <2.0 are generally classified as determinate, scores equal to or greater than 2.0 and less than 2.5 as semideterminate and scores of 2.5 or greater as indeterminate (http://www.ars-grin.gov/npgs/descriptors/soybean).
In the US and China, most of the soybean cultivars grown in the north are indeterminate types, which allow for more overlap of vegetative growth with reproductive development, providing better adaptation to a shorter growing season. In contrast, most of the cultivars grown in the south are determinate types, which have distinctly separate vegetative and reproductive stages (Heatherly and Elmore, 2004). Semideterminate cultivars are also useful in the north, and while they usually produce fewer stem nodes than indeterminate cultivars, they do not require a dense seeding rate to achieve yields like determinate cultivars. Moreover, the semideterminate cultivars are somewhat shorter than indeterminate cultivars, which provide some degree of lodging resistance (Chang et al., 1982), similar to that achieved by the “green revolution” gene in cereals (Peng et al., 1999). In the past decade, more semideterminate cultivars have been developed for use, particularly in high-yield, lodging-prone environments where short stature is desirable; for example, NE3001 is one such semideterminate cultivar that performs extremely well in irrigated production systems (Setiyono et al., 2007). Actually, semideterminate cultivars produce even more pods per plant than indeterminate cultivars if they do not lodge (Setiyono et al., 2007). Hence, it was deemed worthwhile to explore soybean yield potential by modifying genes affecting stem architecture and optimizing management practices.
Classical genetic analyses demonstrated that soybean stem growth habit was regulated by an epistatic interaction between two major genes, Dt1 and Dt2 (Bernard, 1972). In Dt1Dt1 genetic backgrounds, Dt2Dt2 genotypes produce semideterminate phenotypes, whereas dt2dt2 genotypes produce indeterminate phenotypes. However, in dt1dt1 genetic backgrounds, the phenotype is determinate, indicating an epistatic effect of the dt1 allele on the expression of the Dt2/dt2 locus. Because Dt1 is incompletely dominant over dt1, Dt1/dt1 heterozygotes are also semideterminate, whereas Dt2 is completely dominant over dt2; a dihybrid (Dt1dt1;Dt2dt2) produces progeny with an F2 phenotypic ratio of 1 indeterminate:11 semideterminate:4 determinate. Recent studies showed that Dt1 was a functionally conserved ortholog of Arabidopsis thaliana TERMINAL FLOWER1 (TFL1) (Liu et al., 2010; Tian et al., 2010), a floral suppressor gene primarily expressed in shoot apical meristems (SAMs) (Shannon and Meeks-Wagner, 1991; Bradley et al., 1997), and that the transition from indeterminate phenotype to determinate phenotype was caused by independent artificial selection of four point mutations in the Dt1 gene during soybean domestication (Tian et al., 2010). The Dt1 locus is located on chromosome 19 (Liu et al., 2007; Tian et al., 2010). The Dt2 locus was inferentially localized to the distal end of chromosome 18 because of its linkage to a gene governing the isozyme mannose-6-phosphate isomerase (MPI) that was mapped there (Muehlbauer et al., 1989).
Semideterminate stem termination was also observed and genetically investigated in tomato (Solanum lycopersicum) (Elkind et al., 1991; Pnueli et al., 1998; Fridman et al., 2002) and two legume species, pigeon pea (Cajanus cajan) (Gupta and Kapoor, 1991), and chickpea (Cicer arietinum) (Hegde, 2011). In tomato, the stem growth habit was found to be regulated by two genes, SELF-PRUNING (SP), the TFL1/Dt1 equivalent, and the Sdt/sdt locus responsible for semideterminacy. However, unlike in soybean, semideterminacy (sdtsdt) in tomato is recessive, which is suppressed in the Sp- genotypes, leading to a dominant epistasis (i.e., 12 indeterminate:3 determinate:1 semideterminate individuals in F2 progeny derived from a dihybrid [Spsp;Sdtsdt]). This ratio has also been found in pigeon pea and chickpea. Because semideterminacy is dominant in soybean but recessive in the other three species, it is worthwhile to examine the evolutionary novelty of the genetic mechanism underlying semideterminacy in soybean.
Here, we report the isolation and characterization of the Dt2 gene by an integrated approach that involved linkage mapping, target gene association analysis, interspecific genetic and genomic comparison, profiling of gene expression, and complementation test. The research findings, coupled with the previous elucidation of Dt1, have laid the foundation for further dissection of the molecular mechanisms by which these genes and other factors act to determine soybean stem architecture.
RESULTS
Molecular Mapping of the Dt2 Locus to a Genomic Region Near the End of the Short Arm of Chromosome 18
To map the Dt2 gene, a cross between a semideterminate soybean cultivar NE3001 (Dt2Dt2;Dt1Dt1) and an indeterminate soybean cultivar IA3023 (dt2dt2;Dt1Dt1) (Setiyono et al., 2007) was made to generate an F2 population comprising 681 individual F2 plants. Each of the F2 plants were advanced to the F2:3 progenies, which were then used to deduce the genotypes of individual F2 plants. Based on high-confidence phenotyping data from the 679 F2:3 families, 156 F2 individuals were deduced as semideterminate homozygotes (Dt2Dt2), 350 F2 individuals as semideterminate heterozygotes (Dt2dt2), and 173 F2 individuals as indeterminate homozygotes (dt2dt2). These data confirmed the reported single-gene inheritance pattern of dominant semideterminacy versus recessive indeterminacy (3:1; χ2 test, P = 0.77). The observed genotypic segregation pattern also fits the expected 1:2:1 ratio (χ2 test, P = 0.47).
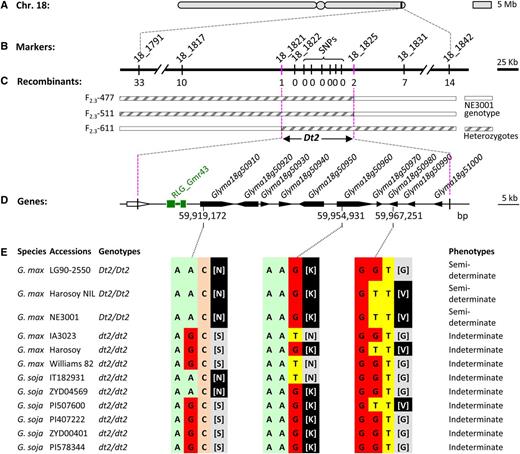
A previous linkage analysis with 20 F2 plants demonstrated that Dt2 was linked at ∼17% recombination units from the gene MPI (Muehlbauer et al., 1989), which is located at 61.7 Mb, a position that is only ∼0.6 Mb from the distal end of the short arm of chromosome 18 (Schmutz et al., 2010). Given this information, we then randomly selected simple sequence repeat (SSR) markers (Song et al., 2010) distributed in the 4 Mb (58 to 62 Mb) genomic segment located at the end of chromosome 18 to genotype the 679 F2 individuals and mapped the Dt2 locus to a 1.5-centimorgan region between SSR_18_1791 and SSR_18_1842, which spans 263 kb, according to the reference genome sequence (Figure 1B). Subsequently, polymorphic markers SSR_18_1821, SSR_18_1822, and SSR_18_1825 were used to search for recombinants identifiable between SSR_18_1791 and SSR_18_1842, and among the 47, we discovered from the 679 F2 individuals, 1, 0, and 2 recombination events were detected in a 81-kb region bounded by SSR_18_1821 and SSR_18_1825 (Figure 1C). In an attempt to further narrow the region encompassing the presumptive Dt2 gene, we next developed six single nucleotide polymorphism (SNP) markers within the 81-kb region by sequencing DNA fragments from genes adjacent to the boundaries of the region in the two parents (Figure 1B; Supplemental Table 1). These markers were used to genotype the three recombinants detected by SSR_18_1821 and SSR_18_1825, but no additional recombination events were identified.
Map-Based Cloning of the Dt2 Locus and Target Gene Association Analysis.
(A) Physical location of the Dt2 regions in the Williams 82 reference genome. The bars indicate two arms of chromosome 18, and the circle indicates approximate position of the centromeric region.
(B) Physical locations of molecular markers defining the Dt2 region.
(C) Graphical genotypes of recombinants carrying crossovers in the Dt2 region determined by molecular markers and phenotypes of individual recombinants.
(D) Genes predicted in the defined Dt2 region according to annotation of the reference genome and an LTR-retrotransposon located 2.7 kb upstream of Glyma18g50910.
(E) Comparison of the coding sequences of the three genes in the mapped Dt2 region between two parental lines NE3001 and IA3023 and among additional semideterminate and indeterminate soybean accessions. In each of the three genes, the trinucleotide differences between semideterminate NE3001 and indeterminate IA3023 that resulted in a single amino acid difference (shown in square brackets) were not consistently associated with those two stem termination types in other accessions.
Sequence Comparison between Semideterminate and Indeterminate Soybean Lines
According to the Williams 82 reference genome, 10 genes were predicted in the defined 81-kb Dt2 region (Figure 1D; Supplemental Table 2). In an attempt to pinpoint the candidate gene for Dt2, we amplified and sequenced the coding regions of the 10 genes in the two parents NE3001 and IA3023. In each of the three genes, Glyma18g50910, Glyma18g50960, and Glyma18g50980, a single nucleotide variant (SNV) in the predicted coding region was observably different between the two parents, and each of the SNVs altered an amino acid (Figure 1E). Then, the coding regions of these three genes in the semideterminate near isogenic lines (NILs) of Harosoy L62-364, the semideterminate soybean variety LG90-2550, and the indeterminate Harosoy were sequenced and compared with the coding sequences of these genes from six highly diverged Glycine soja (the progenitor species of cultivated soybeans) accessions (Kim et al., 2010; Li et al., 2014). Each of the G. soja accessions contained the Dt1 allele and exhibited an indeterminate phenotype. As shown in Figure 1E, none of the three SNVs detected as differing between the mapping population parents NE3001 and IA3023 were able to distinguish the semideterminate accessions from the indeterminate ones examined (Figure 1E).
We further investigated the SNV detected in the coding sequence of Glyma18g50910 in a population including in 20 G. soja accessions and 17 soybean landraces (Hyten et al., 2006), which were phenotyped as “indeterminate” (Tian et al., 2010). Eleven and five were found to have the same nucleotide as NE3001 and the respective remaining ones were found to have the same nucleotide as IA3023 at this SNV site (Supplemental Table 3). Glyma18g50930, contained an ∼1334-bp deletion in NE3001 compared with IA3023 and Williams 82 and appeared to be a pseudogene (null mutation) in the former. For the remaining six of the 10 genes in the 81-kb segment, the coding sequences between the two parents were identical. These observations suggest that it was most likely that the allelic difference between the Dt2 and dt2 alleles responsible for the phenotypic difference in stem growth habit could be attributed to the gene's noncoding sequences or the flanking regulatory elements.
Prediction of the Dt2 Candidate by Interspecific Comparison of Homologous Genes
The 10 genes in the 81-kb Dt2 region were next compared with the whole set of genes annotated in the Arabidopsis genome (Arabidopsis Genome Initiative, 2000) by BLAST searches and analysis of interspecific syntenic genomic regions as described previously (Tian et al., 2010). Glyma18g50910 was found to be the only soybean gene in the mapped Dt2 region that had a significant match with the Arabidopsis genes involved in the Arabidopsis flowering networks (Liu et al., 2009; Yant et al., 2009; Fornara et al., 2010). The best matches of Glyma18g50910 in Arabidopsis were the three floral homeotic MADS domain factor genes, which were the fruit tissue identity gene FRUITFUL (FUL) (Gu et al., 1998), the floral meristem identity gene APETALA1 (AP1) (Gustafson-Brown et al., 1994; Liljegren et al., 1999), and the floral regulatory gene CAULIFLOWER (CAL) (Kempin et al., 1995) (Figure 2; Supplemental Figure 1). It has been demonstrated that AP1 and another floral identity gene, LEAFY (LFY; Weigel et al., 1992), antagonize TFL1, the functional ortholog of the soybean Dt1, to regulate the fate of lateral meristems at the inflorescence apex in Arabidopsis (Bradley et al., 1997; Liljegren et al., 1999; Ratcliffe et al., 1999; Liu et al., 2009, 2013). These findings, along with all of our foregoing observations, including the deduced interaction between Dt2 and Dt1 and the mapping of Dt2, suggest that Glyma18g50910 was most likely to be the candidate for the Dt2 locus.
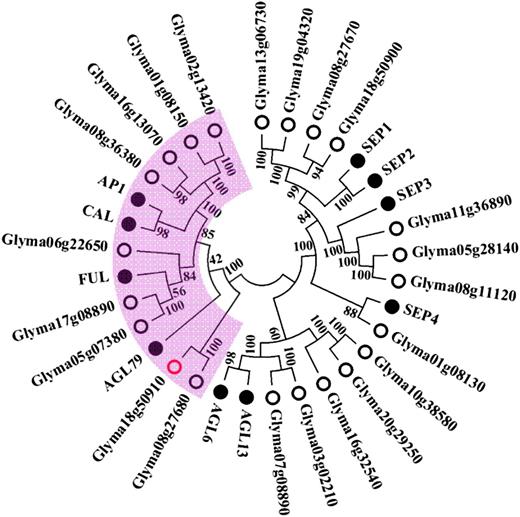
Phylogenetic Relationship of Closely Related Homologs of the Dt2 Candidate Gene in Soybean and Arabidopsis.
The predicted full length of amino acid sequences of the genes was used to construct the neighbor-joining tree. Numbers adjacent to nodes indicating bootstrap values from the test of 1000 replicates. Pink (shaded region) includes all gene homologs identified in the Arabidopsis and soybean that belong to the AP1/SQUA subfamily.
[See online article for color version of this figure.]
Extrapolation of the Dt2 Candidate by Analysis of Gene Expression
Given that none of the nucleotide changes in the 10 genes in the mapped Dt2 genomic region that resulted in amino acid changes could explain the phenotypic difference in stem growth habit between the semideterminate and indeterminate accessions examined (Figure 1E), the development of stem growth habit in soybean is very likely related to differential allelic expression at the Dt2/dt2 locus, and if this is the case, then the expression of Dt1 would be strongly downregulated by Dt2 and not regulated, or upregulated, by dt2.
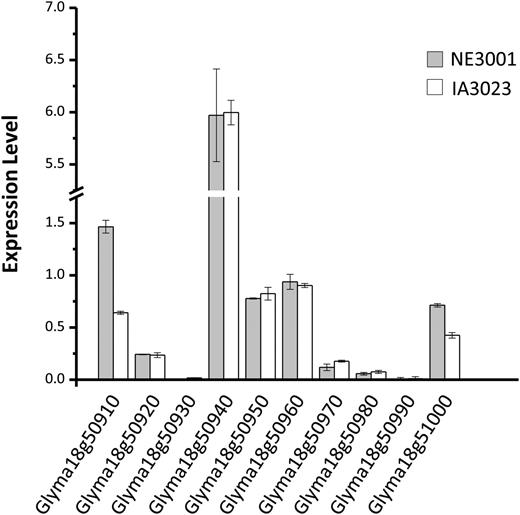
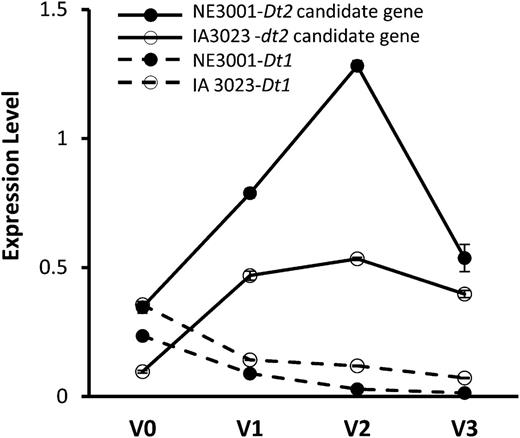
To test this postulation, we first examined the expression patterns of the ten genes in the mapped Dt2 region of NE3001 in various tissues and at various developmental stages before the transition of vegetative growth to reproductive growth of main stem tips by quantitative real-time PCR (qRT-PCR). It was documented that, at the V2 stage, when the first trifoliate leaflets at node 2 are fully expanded but the second trifoliate leaflets at node 3 are not yet unfolded, floral induction occurs in all meristems (apical and lateral), abruptly in the case of the determinants, less abruptly in the case of semideterminants, but not in the terminal apical meristems in indeterminate types (Wilkerson et al., 1989). As expected, the Dt2 candidate gene Glyma18g50910 transcripts were found to be the most abundant in apical stem tips collected at the V2 stage (Supplemental Figure 2). Subsequently, we compared the expression patterns of these 10 genes in apical stem tips at this developmental stage between NE3001 and IA3023 and found that Glyma18g50910 exhibited considerably higher levels of expression in NE3001 than in IA3023 (Figure 3; Supplemental Figure 2). Another gene showing higher level of expression in NE3001 than in IA3023 was the F-box domain gene Glyma18g51000, but its expression level was relatively low and did not show obvious difference between the Dt2 and dt2 NILs L62-364 and Harosoy (Figure 3; Supplemental Figure 3). None of the other eight genes in the mapped Dt2 region showed differential expression between NE3001 and IA3023 (Figure 3). These observations suggested Glyma18g50910 as the only candidate for Dt2. Overall, the expression level of Glyma18g50910 in NE3001 is higher than that of dt2 in IA3023 in apical stem tips from the V0 stage (when the cotyledons at node 0 are fully extended but the unifoliate leaflets at node 1 are not yet unrolled) to the V3 stage (when the second trifoliate leaflets are fully expanded but before the third trifoliate leaflets are still unrolled). By contrast, Dt1 was mainly expressed in main stem tips at the V0 stage (Figure 4). Photoperiod induction is known to begin at the V0 stage (Wilkerson et al., 1989), which converts all existing vegetative meristems to inflorescence meristems except the main stem apex in indeterminate Dt1Dt1;dt2dt2 genotypes, which remains vegetative.
Expression of 10 Genes in the Mapped Dt2 Region in Apical Stem Tips of NE3001 and IA3023 at V2 Stage Detected by qRT-PCR.
The y axis indicates the expression levels of individual genes (x axis) relative to expression of Cons4. Expression levels were shown as means ± standard errors of the means from four replicates.
Expression of Dt1 or dt1 and the Dt2/dt2 Candidate Gene Glyma18g50910 in NE3001 and/or IA3023 Detected by qRT-PCR.
The y axis indicates expression of the Dt2 candidate gene or Dt1/dt1 relative to expression of Cons4 in apical stem tips collected at four developmental stages from V0 to V3 (V3, the stage begins when the 2nd trifoliate leaflets are fully expanded but before the 3rd trifoliate leaflets are still unrolled). Expression levels were shown as means ± standard errors of the means from four replicates.
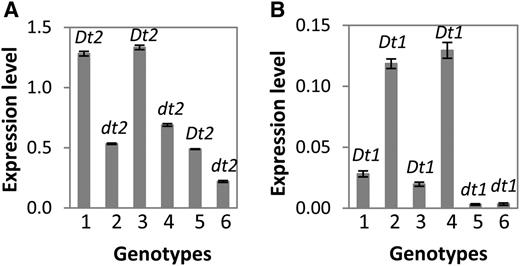
We also monitored the expression patterns of Glyma18g50910 and Dt1 in apical stem tips at the V2 stage in Harosoy and the three Harosoy NILs. As shown in Figure 5, the expression level of Glyma18g50910 in the Harosoy NIL L62-364 (Dt2Dt2;Dt1Dt1) is similar to that in NE3001 (Dt2Dt2;Dt1Dt1), the expression level of Glyma18g50910 in Harosoy (dt2dt2;Dt1Dt1) is similar to that in IA3023 (dt2dt2;Dt1Dt1), and overall Glyma18g50910 was expressed at higher level in the semideterminate lines than in the indeterminate lines. By contrast, the expression level of Dt1 in the Dt2Dt2;Dt1Dt1 semideterminate genotypes was lower than that in the dt2dt2;Dt1Dt1 indeterminate genotypes. These data, at the given transcription levels, suggest that dominant Glyma18g50910 in the semideterminate lines downregulates the expression of Dt1, or inversely, that recessive Glyma18g50910 in the indeterminate lines upregulates the expression of Dt1. The two Harosoy NILs homozygous for dt1dt1 were found to be expressed at minimal levels, irrespective of a Dt2Dt2 or dt2dt2 background. Together, these expression analyses suggest that Glyma18g50910 was the candidate gene for the Dt2/dt2 locus and that the semideterminacy was regulated by the transcriptional activity of this gene. As expected for a MADS domain factor, the protein of this candidate Dt2 gene was localized to the nucleus (Supplemental Figure 4).
Expression of Dt1/dt1 and the Dt2/dt2 Candidate Gene in Apical Stem Tips of Different Genotypes Detected by qRT-PCR.
(A) Expression levels of Dt2 or dt2 relative to expression of Cons4.
(B) Expression levels of Dt1 or dt1 relative to expression of Cons4.
1 to 6 are NE3001 (Dt2/Dt2;Dt1/Dt1), IA3023 (dt2/dt2;Dt1/Dt1), L62-364 (Dt2/Dt2;Dt1/Dt1), Harosoy (dt2/dt2;Dt1/Dt1), L67-3256 (Dt2/Dt2;dt1/dt1), and L62-973 (dt2/dt2;dt1/dt1). Expression levels were shown as means ± standard errors of the means from four replicates.
Validation of the Dt2 Candidate by Complementation Test
To validate the candidacy of Glyma18g50910 for the Dt2 locus, we introduced this candidate gene amplified from NE3001 into Thorne (McBlain et al., 1993), an indeterminate cultivar (dt2dt2;Dt1Dt1) routinely used in Agrobacterium tumefaciens–mediated soybean transformation experiments. In this study, two constructs were made: one harboring a Glyma18g50910 cassette regulated by the cauliflower mosaic virus 35S promoter, the coding sequence (CDS) of Glyma18g50910 from NE3001, coupled with a 35S terminator (dubbed 35S:CDS-Dt2). The other genetic element consisted of the Glyma18g50910 cassette regulated by the putative endogenous promoter that resides ∼2.5 kb upstream of the CDS and terminated with ∼1.5 kb downstream of Glyma18g50910 from NE3001 (dubbed Pro-Dt2:CDS-Dt2).
A total of nine independent events carrying the 35S:CDS-Dt2 expression and six independent events harboring the Pro-Dt2:CDS-Dt2 transgenic allele were obtained. Progeny (T1) plants from each event were advanced to T3 in the greenhouse and subsequent T3 lineages were phenotyped for stem growth habit under field conditions. As shown in Supplemental Table 4, in all nine 35S:CDS-Dt2 events, transgenic plants with semideterminate stems were observed. In addition, indeterminate plants were also observed in the T3 progenies derived from all nine transgenic events, and this phenotypic segregation was perfectly associated with the presence and absence of the Dt2 transgene (Supplemental Table 4). The semideterminacy varied among different events, which was largely reflected by the plants’ height and node numbers of the main stems (Figures 6E and 6F). Generally, the plants with similar expression levels to the Dt2 in NE3001 showed similar degrees of stem termination (Figure 6E; Supplemental Figure 5). The expression levels of the transgene measured by qRT-PCR were negatively associated with both the node numbers and the heights of the main stems (Supplemental Table 5). Moreover, these transgenic plants flowered earlier than Thorne, the recipient line of the transgene (Figures 6A and 6B), similar to that observed between the semideterminate Dt2 Harosoy NIL and the indeterminate Harosoy NIL. These observations, together with other evidence described above, indicate that Glyma18g50910 in NE3001 was the Dt2 gene.
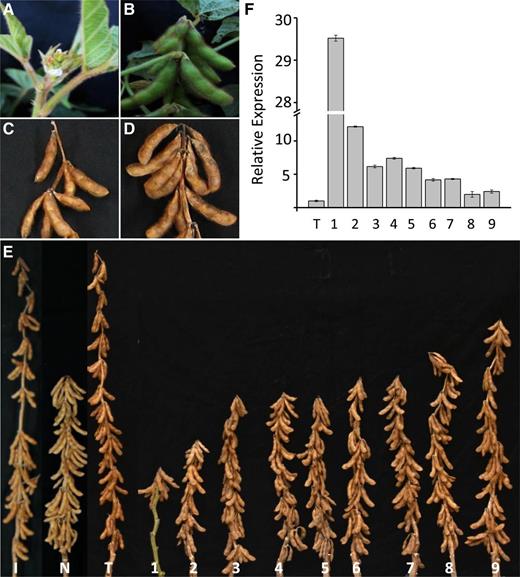
Overexpression of Transgene Dt2 in an Indeterminate Cultivar Resulting in Phenotypic Changes from Indeterminate to Semideterminate Types.
(A) Apical stem tip of immature Thorne showing indeterminate stem growth and late flowering.
(B) Apical stem tip of an immature Thorne 35S:CDS-Dt2 transgenic plant showing semideterminate stem growth and early flowering.
(C) Apical stem tip of mature Thorne showing indeterminate stem growth.
(D) Apical stem tip of a mature Thorne 35S:CDS-Dt2 transgenic plant showing semi-indeterminate stem growth.
(E) Photograph of IA3012 (I), NE3001 (N), Thorne (T), and nine T3 Thorne 35S:CDS-Dt2 transgenic plants derived from nine independent transformation events, which show different degrees of apical stem termination and heights.
(F) Overexpression of transgene Dt2 in nine T3 Thorne 35S:CDS-Dt2 transgenic plants (1 to 9), each derived from an independent transformation event, relative to expression of dt2 in Thorne in stem tips at the V2 stages (with two sets of unfolded trifoliate leaves). Values were shown as mean ± standard errors of the means from four replicates normalized to expression of dt2 in Thorne, which was set as 1.0. Cons4 was used as an endogenous control for gene expression analysis.
By contrast, none of the six Pro-Dt2:CDS-Dt2 events produced any semideterminate plants. We speculated that it was likely that some of the regulatory components essential for the expression of Dt2 were not included in the native-Dt2 construct. To test this possibility, we designed PCR primers that can specifically amplify the transcripts of the transgene and the Dt2/dt2. As expected, high levels of expression of Dt2 in NE3001, 35S:CDS-Dt2 transgene in semideterminate transgenic plants were detected in apical stem tips by qRT-PCR but were not detected in the Pro-Dt2:CDS-Dt2 transgenic plants (Figure 6F; Supplemental Figures 5 and 6).
Nucleotide Variation in Dt2 and Its Upstream and Downstream Sequences between Semideterminate and Indeterminate Soybean Lines
To shed light on potential causative mutation(s) at the Dt2 locus that led to differential allelic expression responsible for the phenotypic variation in soybean stem growth habit, we compared genomic sequences of the Dt2 locus and its flanking intergenic spaces that cover an ∼22-kb region from the SSR marker 18-1821 to the 3′ untranslated region of the adjacent gene Glyma18g50920 (Figures 1D and 7A). The NE3001 sequences from this region were generated by PCR amplification and sequenced and were then compared with corresponding sequences from the Williams 82 reference genome. Subsequently, the forms of sequence variations, including SNPs and insertions/deletions (Indels) detected between these two soybean lines, in additional indeterminate soybean lines were determined using the available genome resequencing data and/or de novo genome sequencing data from IA3023 and six G. soja accessions (Kim et al., 2010; Li et al., 2014; www.soybase.org). As shown in Figure 7, a total of 37 SNPs that each distinguished NE3001 from the eight indeterminate accessions were identified, and all of these indeterminate lines shared the same nucleotide at each of the 37 SNP sites (Figure 7B). Of these SNPs, three were found in the 2.5-kb sequence upstream of the CDS of the Dt2 locus, 23 (62%) in the first intron of the Dt2 locus, one in the second intron of the Dt2 locus, and one in the 1.5-kb downstream of the CDS of the Dt2 locus. However, because there are only a limited number of elite cultivars with clearly defined semideterminate phenotypes available, and because it is difficult to distinguish semideterminate phenotypes from determinate phenotypes of plants with diverged genetic background, further identification of causative mutations by association analysis in the targeted region may not be very effective, or perhaps completely ineffective.
Nucleotide Differences in Dt2 and Its Flanking Intergenic Spaces That Distinguish NE3001 from IA3023 and Additional Indeterminate Varieties Examined.
(A) Distribution of 37 SNPs in an ∼22-kb genomic region surrounding Dt2. Green boxes connected by a green bar indicate a long terminal repeat retrotransposon. Gray boxes and black boxes indicate untranslated regions and exons of Glyma18g50910, respectively. A cluster of high density of SNPs (from 16 to 33) within the first exon of the gene was illustrated on a magnified scale above the gene.
(B) Nucleotides at the 37 SNP positions, as shown in the (A), in the examined soybean accessions. Dashes indicate unknown nucleotides.
DISCUSSION
Evolutionary Relationship and Novelty of the Dt2 Gene Homologs among Soybean and Other Species
Members of MADS domain gene family play essential roles in various aspects of plant development, such as root, flower, seed, and fruit development (Smaczniak et al., 2012). Among the 104 MADS domain genes predicted in the Arabidopsis genome (Martinez-Castilla and Alvarez-Buylla, 2003), SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1, AP1, FUL, CAL, and AGAMOUS-LIKE24 have been found to be activators involved in floral induction, a process that transforms the SAM, which forms leaves, into an inflorescence meristem, on which flowers form and develop (Liu et al., 2009; Yant et al., 2009; Fornara et al., 2010). However, despite their close phylogenetic relationships and functional similarities, none of the FUL, AP1, and CAL regions exhibited syntenic relationships with the Dt2 region (Shu et al., 2013). Indeed, Dt2 was not the gene most closely related to these three Arabidopsis genes based on their phylogeny (Figure 2; Supplemental Figure 1 and Supplemental Data Set 1). Hence, it remains unclear which Arabidopsis gene is the functional counterpart of Dt2.
If the established phylogenetic relationships of the MADS domain gene homologs within and between the Arabidopsis and soybean genomes did reflect the orders and timeframes within which these genes were diverged and generated, then Dt2 should have more functionally diverged from AP1 than the four soybean genes Glyma02g13420, Glyma01g08150, Glyma16g13070, and Glyma08g36380, that were more closely related to AP1 (Figure 2). This speculation appears to be echoed by the observation that the proteins encoded by these four AP1-homologous genes of soybean all contain the conserved eudicot AP1-like (euAP1) motif present in the Arabidopsis AP1 (Rijpkema et al., 2007; Shan et al., 2007) at their C termini, whereas Dt2 contains the conserved paleoAP1 motif at its C terminus. Indeed, a previous study demonstrated that Gm-AP1 (i.e., Glyma16g13070) was most likely to be the functional homolog of the Arabidopsis AP1 in soybean, which is involved in flower development (Chi et al., 2011), although it remains unclear whether additional soybean genes closely related to AP1, such as Glyma02g13420, Glyma01g08150, and Glyma08g36380, also function like AP1 in Arabidopsis.
In Arabidopsis, TFL1 is primarily expressed in the center of SAMs at stem apexes, where the TFL1 protein is produced to repress the expression of AP1 (Shannon and Meeks-Wagner, 1991; Bradley et al., 1997). Such an interaction prevents the conversion of the vegetative SAMs there to a reproductive inflorescence meristem and thus inhibits terminal flowering. If the indeterminate soybean employs a mechanism similar to that observed in Arabidopsis, in maintaining vegetative growth at the stem apexes, then Dt2 is unlikely to be the functional counterpart of AP1. Given that the semideterminate and determinate stem growth habit phenotypes are rarely observed in G. soja (Ting, 1946; Nagata, 1950), it would be reasonable to speculate that Dt2 is a gain-of-function mutation, which occurred after the domestication events of the cultivated soybeans. Because the Dt2Dt2;Dt1Dt1 genotype produces semideterminate stems, it is highly likely that the terminal flowering in plants of such a genotype is initiated by the repression of Dt1 expression in SAMs directly or indirectly by Dt2.
In addition to the distinction in the deduced digenic epistatic interactions underlying the stem growth habit and the inverse patterns of dominance-recessiveness of indeterminacy over semideterminacy in other species, the genes interacting with Dt1 in soybean, SP in tomato, and their functional counterparts in chickpea and pigeon pea, as revealed by classical genetic analyses (Bernard, 1972; Elkind et al., 1991; Gupta and Kapoor, 1991; Hegde, 2011), appear to be different. Although Sdt (i.e., PW9-2-5) has not been isolated, it was found to cosegregate with SP-9D (no recombinants among 4029 gametes), the closest paralog of SP (Fridman et al., 2002), suggesting that Sdt may be a functionally diverged SP homolog. Given such similar inheritance patterns of the stem growth habit among tomato, chickpea, and pigeon pea, it is possible that the functional counterparts of Sdt1 in these two legume species are also the TFL1/Dt1/SP homologs. By contrast, Dt2 is neither a Dt1 homolog nor located in the Dt1 paralogous regions (Tian et al., 2010).
It has been documented that two genes, DETERMINATE (Det) and VEGETATIVE1 (Veg1), regulate the stem growth habit in pea (Pisum sativum). Det appears to be the functional equivalent of TFL1/Dt1 (Foucher et al. 2003), while Veg1 is an Arabidopsis AGAMOUS-LIKE79 (AGL79)-like MADS box gene and specifies secondary inflorescence meristem identity (Berbel et al., 2012). In the DetDet genetic backgrounds, Veg1Veg1 genotypes produce indeterminate phenotypes, whereas veg1veg1 genotypes produce plants that never flower. However, in the detdet genetic backgrounds, the phenotype is determinate, indicating an epistatic effect of the det allele on the expression of the Veg1/veg1 locus, similar to the effect of dt1 on the expression of the Dt2/dt2 locus. Because the pea genome has not been sequenced, whether Dt2 and Veg1 are orthologs has not been firmly established by comparison of genome sequences. Nevertheless, comparative mapping in pea and Medicago truncatula located the putative Veg1 ortholog Medtr7g016630 (i.e., Mt-FULc) (Berbel et al., 2012) to a position at the top of Medicago chromosome 7 that corresponds to the position of Veg1 at the bottom of the pea linkage group V (Hecht et al., 2005; Zhu et al., 2005) and the Medicago genomic region surrounding FULc appears to be orthologous to the Dt2 (i.e., Gm-FULc described in Berbel et al., 2012) genomic region (Supplemental Table 6). As seen in Dt2, Veg1 also contains the paleoAP1 motif at its C terminus. Together, these observations suggest that Veg1 and Dt2 may be orthologous genes. However, given the fact that Dt2, in the presence of Dt1, is responsible for the conversion of apical stems from vegetative growth to reproductive growth to produce semideterminate phenotypes, whereas Veg1, in the presence of Det, appears to be essential for development of second-order inflorescence (I2) in the indeterminate pea plants (Singer et al., 1999; Berbel et al., 2012), the functional divergence between Dt2 and Veg1 is expected. Alternatively, because the Dt1dt1 genotypes produce semideterminate phenotypes due to incomplete dominance of Dt1 to dt1, similar to those produced by Dt1Dt1 in the presence of Dt2, the lack of semideterminate phenotypes in pea could be the explained by strong, complete dominance of Det over det (Singer et al., 1999), which may lead Veg1 to be hypostatic to DetDet or Detdet to produce indeterminate phenotypes. Further examination and comparison of expression patterns of Dt1 versus Det and Dt2 versus Veg1 in the primary (I1) and I2 inflorescence meristems of soybean and pea may help to elucidate functional similarity and/or divergence between Dt2 and Veg1.
Causative Mutation(s) and Differential Allelic Expression
Accumulating evidence has revealed regulatory roles of intronic sequences in gene expression. The introns of LFY are known to be critical for proper expression of LFY in monocots (Prasad et al., 2003; Bomblies and Doebley, 2005; Rao et al., 2008). A more recent study revealed that the intron sequences, particularly the first introns of AP1 and FUL, were bound by the microRNA-targeted transcription factor SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 (SPL3), and both intron and exon sequences of LFY were bound by SPL3 to activate the expression of these genes (Yamaguchi et al., 2009). If one accepts the thesis that the regulation of Dt2 in the semideterminate soybean varieties did need such cis-regulatory elements, particularly the intron sequences, bound by regulatory factors, as AP1 and FUL did in Arabidopsis, the lack of, or reduction in expression of the Pro-Dt2:CDS-Dt2 transgene and, thus, the failure in recovering the expected phenotypes versus overexpression of the 35S:CDS-Dt2 cassette and the observed phenotypic switch of the transgenic plants from indeterminate type to semideterminate type would be explained by the lack of intron sequences in the Pro-Dt2:CDS-Dt2 cassette.
Unfortunately, the first intron of Glyma18g50910 in NE3001 is composed of a 4483-bp sequence enriched with T/A (72.4%), arranged in long strings of Ts, As, ATs, and/or TAs, and technical difficulties occurred when amplifying desirable and large genomic fragments from this portion of the gene for cloning. As a result, constructs with the complete genomic sequence of the gene were not successfully made. Finer-scale linkage mapping would be able to define the causative mutation(s) to a smaller region. However, given that 37 SNPs were found within in an ∼22-kb region surrounding Dt2, with 24 SNPs in the first intron of the gene (Figure 7), it remains challenging to pinpoint causative mutations, if located with this intron, by fine mapping with a manageable number of F2:3 families. Further effort that perhaps involves genetic and molecular approaches is needed toward the discovery of the cis-regulatory components responsible for the Dt2 activity.
Although Dt2 was expressed at a significantly higher level than dt2 in the apical stem tip, as measured by qRT-PCR, the expression of dt2 in both Dt1Dt1 and dt1dt1 genetic backgrounds was still substantial (Figure 5). One might have expected a qualitative expression difference (i.e., presence versus absence in Dt2 versus dt2 expression). However, a quantitative difference in expression was observed, indicating that it was sufficient for the complete dominance of Dt2 over dt2 in downregulating Dt1 expression or vice versa. Because the proportion of SAM tissue in the apical stem tip is relatively small, differential expression between Dt2 and dt2 in SAM may not be fully reflected by the qRT-PCR experiment. Nevertheless, a lower level of Dt1 expression was detected in a Dt2Dt2 background than in a dt2dt2 background, demonstrating the regulatory role that Dt2 has on Dt1. Given that Dt1 is incompletely dominant to dt1 (Woodworth, 1932; Williams, 1950), such an interacting level of differential expression between Dt2 and dt2, and Dt1 expression may underlie the phenotypic difference between the Dt2Dt2;Dt1Dt1 and dt2dt2;Dt1Dt1 genotypes.
METHODS
Plant Materials
The mapping population was derived from the cross between the soybean (Glycine max) cultivars NE3001 (Dt2Dt2;Dt1Dt1) and IA3023 (dt2dt2;Dt1Dt1). The three NILs of the recurrent parent Harosoy (PI 548573) were L62-364 (PI 547681), L62-973 (PI 547687), and L67-3256 (PI547703). Seed of these four lines were obtained from the USDA Soybean Germplasm Collection. Transgenic soybean lines were phenotyped and advanced to T2 in the greenhouse from November 2012 to April 2013 and phenotyped in the field at West Lafayette, IN, in October 2013.
DNA Isolation, PCR, RNA Isolation, RT-PCR, and Sequencing
Genomic DNA isolation, PCR primer design, PCR amplification, PCR fragment purification, total RNA isolation, cDNA synthesis by RT-PCR, sequencing PCR, and RT-PCR fragments were conducted as previously described (Tian et al., 2010). Primers used for PCR, RT-PCR, and sequencing are listed in Supplemental Data Set 2.
Molecular Mapping
Because the environment in which soybean plants grow can have large effects on stem growth habit (Bernard, 1972; Specht et al., 2001; Heatherly and Smith, 2004; Setiyono et al., 2007), accurate genotyping of individual F2 plants is not always possible. We thus advanced the 681 F2 population to the F3 generation; subsequently, ∼50 F3 plants grown from each F2 plant were scored for abruptness of stem termination in a field nursery located in Lincoln, NE, where NE3001 was developed. The stem termination phenotype of each F3 plant in each F2:3 progeny row was examined to determine if the F3 plants in a given row were all semideterminate, all indeterminate, or segregating in a ratio of 3:1 for semideterminate to indeterminate. This F2:3 progeny phenotyping resulted in an accurate deduction of a respective of dt2dt2, Dt2Dt2, and Dt2dt2 genotype for nearly all of the F2 plant progenitors. An equal amount of leaf tissue was collected from the ∼15 to 20 F3 plants tracing to each F2 plant and was used for molecular marker assays that provided molecular genotypes for each F2 progenitor plant. Of the 681 F2 individuals, two produced too few F3 plants for a reliable inference of the F2 phenotypes and thus were excluded in linkage analysis. SSR markers for mapping were selected based on their physical locations on chromosome 18 (Song et al., 2010). SNP markers were designed based on genic sequence variation in the mapped regions between two parental lines. Genotyping of recombinants with SNP markers was performed either by direct sequencing of PCR fragments or using the cleaved amplified polymorphic sequence markers (Supplemental Table 1).
Sequence Alignments, Comparison, and Phylogenetic Analysis
BLASTP was used to search the soybean Dt2 candidate gene against the Arabidopsis thaliana protein database (www.arabidopsis.org) to identify homologous genes showing high levels of sequence similarity and then the identified Arabidopsis genes were used to identify homologous genes in soybean. A group of homologous genes between soybean and Arabidopsis that include all genes belonging to the AP1/SQUA subfamily in Arabidopsis, i.e., AP1, CAL, FUL, and AGL79 (Rijpkema et al., 2007; Shan et al., 2007), and all their homologs in soybean were identified. The full length of predicted protein sequences from these genes was aligned using MUSCLE (V3.6) with default parameters (Supplemental Data Set 1). The phylogenetic tree was generated using neighbor-joining method integrated in MEGA (V6.06) with a bootstrap of 1000 replicates, Poisson model for amino acid substitution, and pairwise deletion of gaps.
Plasmid Construction and Transformation
The 2.5-kb upstream sequence from the start codon (ATG) of Glyma18g50910, the 1.5-kb downstream sequence of the gene from the stop codon (TAG), and the CDS of the gene from the semideterminate cultivar NE3001 were obtained by PCR and RT-PCR with primers shown in Supplemental Data Set 2. The PCR fragments were ligated to pCR2.1-TOPO TA vector (Life technologies) and then sequenced. Selected clones with verified inserts by sequencing were used to make two Dt2 constructs: 35S promoter + the CDS of the Dt2 candidate + the 35S terminator (dubbed 35S:CDS-Dt2 construct) and the 2.5-kb upstream sequence + the CDS + the 1.5-kb downstream sequences of the Dt2 candidate gene (dubbed Pro-Dt2:CDS-Dt2 construct).
To make the 35S:CDS-Dt2 construct, a pCR2.1-TOPO clone carrying the CDS of Glyma18g50910 was digested with NcoI/BamHI and with BamHI/XbaI, respectively, to isolate a 348-bp fragment and a 414-bp fragment from the CDS of the gene. Simultaneously, the pRTL2 vector was digested with NcoI/XbaI to generate a linearized plasmid. These three restriction fragments were purified separately and then ligated to form the 35S-Dt2 construct using T4 DNA ligase (Promega), which was then transformed into competent Escherichia coli cells. To make the Pro-Dt2:CDS-Dt2 construct, selected pCR2.1-TOPO clones carrying the verified 2.5-kb upstream sequence and the 1.5-kb downstream sequence of Glyma18g50910 were digested with HindIII/XhoI and XbaI/HindIII respectively, to isolate the 2.5- and 1.5-kb fragments. The assembled and verified 35S:CDS-Dt2 construct (designated pPTN1171) was digested with XhoI/XbaI to isolate the CDS of the gene. Simultaneously, the pPTN200 vector was digested with HindIII to generate a linearized plasmid. These four restriction fragments were purified and then ligated to form the Pro-Dt2:CDS-Dt2 construct, designated pPTN1178.
Both the 35S:CDS-Dt2 and Pro-Dt2:CDS-Dt2 constructs were confirmed by digestion with relevant restriction enzymes and by sequencing. Two confirmed constructs were introduced into Agrobacterium tumefaciens separately and subsequently transferred into the indeterminate soybean cultivar Thorne following a protocol as described previously (Clemente et al., 2000). The presence of the constructs in recovered transgenic plants was confirmed by PCR with primers specific to the cloning vectors (Supplemental Data Set 2).
Subcellular Localization
Subcellular localization of Dt2 was performed using coding sequence of a green fluorescent protein fused in-frame to the Dt2 coding sequence. The fusion plasmids were under the control of the cauliflower mosaic virus 35S promoter and introduced into leaf epidermal cells of 3- to 4-week-old Nicotiana benthamiana plants by Agrobacterium infiltration. The transformed leaf cells were observed and photographed through a microscope.
qRT-PCR
qRT-PCR was performed using SYBR Green PCR Master Mix (Life Technologies) as described previously (Tian et al., 2010). The soybean ATP binding cassette transporter gene (Glyma12g02310), dubbed Cons4 (Libault et al., 2008), was used as a control. Three biological replicates were analyzed to quantify the levels of gene expression in NE3001, IA3023, and the four NILs. Three technical replicates were analyzed to measure gene expression in Throne and transgenic lines. Primers used for qRT-PCR are listed in Supplemental Data Set 2.
Accession Numbers
Sequence data from this article were submitted to the National Center for Biotechnology Information under accession numbers KF908014 to KF908015.
Supplemental Data
The following materials are available in the online version of the article.
Supplemental Figure 1. Alignment of Predicted MADS Box Domains of the Dt2 Candidate Gene Homologs in Soybean and Arabidopsis.
Supplemental Figure 2. Expression of the Dt2 Candidate Genes in the Semideterminate Soybean Cultivar NE3001 Detected by qRT-PCR.
Supplemental Figure 3. Expression of Glyma18g51000 in Apical Stem Tips of NILs L62-364 and Harosoy at V2 Stage Detected by qRT-PCR.
Supplemental Figure 4. Subcellular Localization of the Dt2 Protein in Tobacco Epidermal Cells.
Supplemental Figure 5. Expression of Endogenous Dt2/dt2 and/or the Transgenic Dt2 in Parental and Transgenic Lines Determined by qRT-PCR.
Supplemental Figure 6. Expression of Thorne Endogenous dt2 and the Transgenic Dt2 in the Pro-Dt2:CDS-Dt2 Transgenic Lines Determined by qRT-PCR.
Supplemental Table 1. Molecular Markers Used for Mapping of the Dt2 Gene.
Supplemental Table 2. Genes in the Defined Dt2 Region According to the Williams 82 Reference Genome.
Supplemental Table 3. Polymorphisms of a Single Nucleotide Variant in the Coding Region of the Dt2 Candidate Gene in a Natural Population Previously Described.
Supplemental Table 4. Phenotypic Segregation for Stem Growth Habit of the T3 Progenies from Individual T2 Plants Derived from Nine Independent Transformation Events.
Supplemental Table 5. Correlation between Expression Level of the Transgenes and Phenotypic Variation among Transgenic Plants.
Supplemental Table 6. Genes Surrounding Dt2 in Soybean and Their Putative Orthologs in Medicago truncatula.
Supplemental Data Set 1. Alignment Information Used for Phylogenetic Tree Construction.
Supplemental Data Set 2. Primers Used for Amplification of Gene Fragments by PCR, RT-PCR, qRT-PCR, Gene Cloning and Verification, and Sequencing.
Acknowledgments
This work was mainly supported by soybean checkoff funds from the United Soybean Board (project numbers 0217 and 1420-532-5617) and partially supported by Purdue Agricultural Research Program, Purdue Research Foundation, and “Partnership for Research & Education in Plant Breeding and Genetics” program funded by the USDA's National Institute of Food and Agriculture and corporate partners Ag Alumni Seed, AgReliant Genetics, Beck's Hybrids, ConAgraFoods, Dow AgroSciences, Indiana Crop Improvement Association, and Pioneer Hi-Bred International.
Glossary
- SAM
shoot apical meristem
- SSR
simple sequence repeat
- SNP
single nucleotide polymorphism
- SNV
single nucleotide variant
- NIL
near isogenic line
- qRT-PCR
quantitative real-time PCR
- CDS
coding sequence
AUTHOR CONTRIBUTIONS
J.E.S., T.E.C., and J.M. designed the research. J.P., Y.L., L.S., M.Z., Y.L., M.S., Y.S., F.L., X.L., H.N., and Z.T. performed the research. J.P., Y.L., L.S., L.Q., R.L.N., T.E.C., J.E.S., and J.M. analyzed the data. R.L.N., T.E.C., J.E.S., and J.M. wrote the article.
References
Heatherly, L.G., and Elmore, R.W. (2004). Managing inputs for peak production. In Soybeans: Improvement, Production, and Uses, 3rd ed, Agronomy Monograph 16, H.R. Boerma and J.E. Specht, eds (Madison, WI: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America), pp.
Nagata, T. (1950). Studies on the Characteristics of Soybean Varieties. (Tokyo: Japan Soybean Association).
Author notes
These authors contributed equally to this work.
Current address: Institute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Current address: Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100101, China.
Address correspondence to maj@purdue.edu.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jianxin Ma (maj@purdue.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
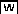
Online version contains Web-only data.