-
PDF
- Split View
-
Views
-
Cite
Cite
Kaijie Zhu, Xiongjie Zheng, Junli Ye, Yue Huang, Hongyan Chen, Xuehan Mei, Zongzhou Xie, Lixin Cao, Yunliu Zeng, Robert M. Larkin, Qiang Xu, Estela Perez-Roman, Manuel Talón, Cecilia Zumajo-Cardona, Eleanore T. Wurtzel, Xiuxin Deng, Regulation of carotenoid and chlorophyll pools in hesperidia, anatomically unique fruits found only in Citrus, Plant Physiology, Volume 187, Issue 2, October 2021, Pages 829–845, https://doi.org/10.1093/plphys/kiab291
Close - Share Icon Share
Abstract
Domesticated citrus varieties are woody perennials and interspecific hybrid crops of global economic and nutritional importance. The citrus fruit “hesperidium” is a unique morphological innovation not found in any other plant lineage. Efforts to improve the nutritional quality of the fruit are predicated on understanding the underlying regulatory mechanisms responsible for fruit development, including temporal control of chlorophyll degradation and carotenoid biosynthesis. Here, we investigated the molecular basis of the navel orange (Citrus sinensis) brown flavedo mutation, which conditions flavedo that is brown instead of orange. To overcome the limitations of using traditional genetic approaches in citrus and other woody perennials, we developed a strategy to elucidate the underlying genetic lesion. We used a multi-omics approach to collect data from several genetic sources and plant chimeras to successfully decipher this mutation. The multi-omics strategy applied here will be valuable in driving future gene discovery efforts in citrus as well as in other woody perennial plants. The comparison of transcriptomic and genomic data from multiple genotypes and plant sectors revealed an underlying lesion in the gene encoding STAY-GREEN (SGR) protein, which simultaneously regulates carotenoid biosynthesis and chlorophyll degradation. However, unlike SGR of other plant species, we found that the carotenoid and chlorophyll regulatory activities could be uncoupled in the case of certain SGR alleles in citrus and thus we propose a model for the molecular mechanism underlying the brown flavedo phenotype. The economic and nutritional value of citrus makes these findings of wide interest. The strategy implemented, and the results obtained, constitute an advance for agro-industry by driving opportunities for citrus crop improvement.
Introduction
The Citrus genus represents a wide-ranging group of economically and nutritionally valuable fruit crops of global importance, rich in vitamins and nutrients, including over 100 types of healthful carotenoids. Domesticated citrus varieties are interspecific hybrids derived from the complex hybridization of related species that originated eight million years ago (Wu et al., 2018). The reproductive novelty of apomixis or nucellar polyembryony, limited to only a few agricultural crops, facilitates asexual reproduction and maintains heterozygosity in most of the modern cultivated citrus species (Wang et al., 2017). However, the genetic makeup of citrus varieties represents a major challenge for map-based cloning necessary for gene discovery and elucidation of underlying molecular mechanisms. In a future where climate change and food security are drivers for crop improvement (Wurtzel et al., 2019), knowledge of molecular regulation in citrus is essential. Breeders have long been interested in carotenoid profiles because these plant-derived pigments are important for human health (Harjes et al., 2008; Wurtzel et al., 2012; Fiedor and Burda, 2014). Additionally, these pigments are vital for plant growth and development (Meier et al., 2011; Moise et al., 2014; Wurtzel, 2019). As citrus fruits mature, carotenoid levels increase in the flavedo, concomitantly with loss of chlorophyll (Zhu et al., 2017), although the mechanism coordinating these events is not well understood and thus limits further citrus improvement.
Fruit morphological types evolved multiple times in the evolution of many groups of flowering plants (Kliman, 2016), cases of convergent evolution which might or might not be explained by similar mechanisms. Investigation of the genetics, physiology, and biochemistry of fruit development has been extensively conducted in a variety of species (Bernardello, 1983; Fray and Grierson, 1993; Ferrándiz et al., 1999; Dorcey et al., 2009; Tafolla-Arellano et al., 2017; Ortiz‐Ramírez et al., 2019). However, are these taxa adequate for explaining the underlying mechanisms that support citrus fruit physiology and biochemistry? For example, immature tomato fruits (Solanum lycopersicum) are also green due to chlorophyll which later disappears as fruits mature and colored carotenoids accumulate (Figure 1). However, is the well-studied tomato a good model for citrus fruit development? Citrus species, members of the Rosid clade, and tomato species, members of the Asterid clade, actually represent two distantly related groups in the evolution of flowering plants (Chase et al., 2016). Furthermore, Citrus and Solanum manifest different fruit morphologies. Hence, it is difficult to extrapolate what is known from one species to another, since the molecular regulation of fruit development may not necessarily be conserved.
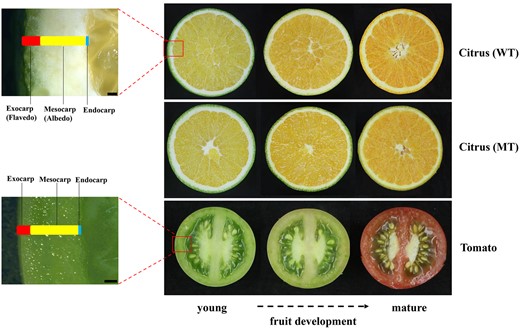
Anatomical differences between tomato and citrus. Right: Horizontal cross-sectioned fruits from “Lane Late” Navel Orange (WT), “Zong Cheng” (MT), and tomato at various developmental stages from young to mature. Left: Close-up of stereomicroscopy images showing pericarp of citrus and tomato for which exocarp, mesocarp, and endocarp layers are indicated. Bar = 1,000 μm.
Fruits are the result of extreme morpho-anatomical changes during the ontogenetic development of carpels after fertilization of ovules (Esau, 1965; Roth, 1977). The wall of the fruit, or pericarp, is formed of three distinct layers: the exocarp, which constitutes the outermost layer; the mesocarp, often consisting of multiple middle layers and vascular bundles and, the endocarp, which consists of one to several layers in contact with the developing seed (Esau, 1965). In tomato, the three layers of the pericarp are photosynthetically active and photosynthetic activity decreases during fruit development during which time green chloroplasts differentiate into red chromoplasts (Piechulla et al., 1987). Citrus fruit, although fleshy, constitutes a unique type among angiosperms, found only in the Rutaceae family. Known as hesperidium, this fruit is characterized by a thick, leathery rind proceeding from the exocarp and mesocarp, which are also called flavedo and albedo, respectively (Esau, 1965; Roth, 1977). The exocarp is the area where the chlorophyll-containing chloroplasts are located, while the plastids diminish toward the inside (Roth, 1977). Indeed, hormonal regulation of fruit development and ripening differs significantly between citrus, a nonclimacteric fruit, and tomato, a typical climacteric fruit (Matas et al., 2011; Ding et al., 2015; Chen et al., 2018; Fuentes et al., 2019). As shown in Figure 1, the tissues that accumulate and then later lose chlorophyll also differ; in citrus, chlorophyll accumulation is limited to the flavedo (exocarp), whereas in tomato, chlorophyll accumulates throughout the fruit tissues. The evolutionary, morphological, and physiological differences between citrus and tomato suggest that tomato fruit might not suffice for predicting regulatory features that govern citrus development and biochemical properties. Therefore, comprehensive elucidation of the underlying mechanisms that control citrus fruit development will yield the most predictive model to improve citrus fruit cultivars for enhanced nutritional qualities or resilience to biotic and abiotic stress. However, a thorough understanding of the dynamics controlling the underlying biochemical networks is limited by the inefficiency of map-based cloning in woody perennials such as citrus. Thus, alternate strategies are needed that capitalize on transcriptomic and genomic data, coupled with the unique genetic resources available in citrus.
Natural genetic variation is a valuable resource for elucidating the mechanisms that control biosynthesis and degradation of pigments as well as for identifying targets for plant breeding (Ahloowalia et al., 2004; Harjes et al., 2008; Vallabhaneni et al., 2009; Vallabhaneni and Wurtzel, 2009; Shumskaya et al., 2012). One of many such citrus mutants, Navel Orange (Citrus sinensis) Zong Cheng (MT), was discovered to have a brown flavedo phenotype, due to retention of chlorophyll which is otherwise lost during fruit maturation (Figure 2). We developed a strategy to overcome the limitations of map-based cloning in citrus. Using a multi-omics comparative approach, we identified the genetic lesion and function of the gene product. We discovered that coordinated regulation of fruit carotenoids and chlorophyll is conserved over a wide evolutionary distance, despite significant differences in fruit morphology, biochemistry, and physiology. However, allelic variation in citrus also revealed that in citrus, certain regulatory features found in tomato and other plants can be uniquely uncoupled.
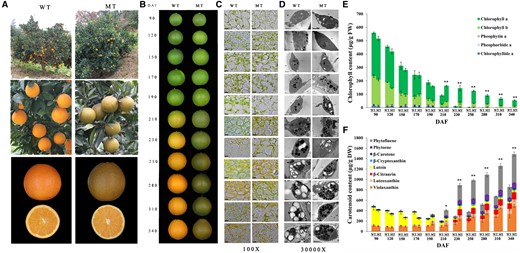
Phenotype and flavedo pigment content of WT and MT. A, WT and MT trees and fruits. The entire trees showing different fruit colors (top) and close-up view of orange WT fruits and brown MT fruits on the trees (middle) are shown. Close-up views of the orange flavedo and pulp in WT and the brown flavedo and orange pulp in MT (bottom) are shown. B, Phenotypes of WT and MT fruits at 11 developmental stages. The fruit images were digitally extracted for comparison. C, Light microscopy inspection of the flavedos from WT and MT at 11 developmental stages (magnified ×100). Bar = 10 μm. D, Transmission Electron Microscopy (TEM) show plastid ultrastructures from flavedos of WT and MT at 11 developmental stages (magnified ×30,000). Abbreviations and symbols are as follows: S, starch granule; P, plastoglobule; M, mitochondrion; T, thylakoid. Bar = 1 μm. E, Changes in the chlorophyll content in the flavedos of WT and MT at 11 developmental stages. F, Changes in the carotenoid content in the flavedos of WT and MT at 11 developmental stages. Results in (E) and (F) are means ± sd (Standard deviation) from three biological replicates. Asterisks indicate statistically significant differences compared with WT (Student’s t test P-value, *P < 0.05, **P < 0.01).
Results
Characterization of a citrus fruit mutant with a brown flavedo
“Zong Cheng” (MT) was discovered as a spontaneous bud mutant of the commercial variety Lane Late Navel Orange (WT; Citrus sinensis [L.] Osbeck). The fruits of MT have an abnormal brown-colored flavedo that is distinguishable from the orange-colored fruits of WT (Figure 2A). In contrast, the pulps of MT and WT are indistinguishable in color. To characterize the temporal onset of this variation in coloration, fruits were sampled from 90 to 340 d after flowering (DAF). Fruit phenotypes differed only after the breaker stage at 210 DAF (Figure 2B), as assessed by color values (L*, a*, and b*), levels of carotenoids and chlorophylls, and plastid ultrastructure. All color values for MT fruit decreased after the breaker stage, relative to WT (Supplemental Figure S1). Light microscope observations (Figure 2C) showed abundant chloroplasts in flavedos of the mature fruits of MT in contrast to WT, where chloroplasts were found to be diminished in number late in development. Transmission electron microscopy (TEM) also revealed chloroplasts in the flavedos of MT during the late stages of fruit development, at stages where chloroplasts were less visible in WT (Figure 2D). Fewer starch granules and more plastoglobules were found in the flavedo chloroplasts of MT at maturity as compared with the plastids of WT (280–340 DAF; Figure 2D). Chlorophyll levels gradually decreased until the breaker stage in both varieties, as a result of cell expansion in the flavedo (90–190 DAF; Figure 2E; Supplemental Table S1). Thereafter, chlorophyll levels decreased dramatically in WT, while decreases in chlorophyll levels were less apparent in MT (Figure 2E; Supplemental Figure S2A and Supplemental Table S1). After the breaker stage at 210 DAF, the carotenoid composition of WT and MT flavedos was not substantially different, but the carotenoid content in MT was significantly higher than that in WT (Figure 2F; Supplemental Figure S2B and Supplemental Table S2). These results suggest that the brown-colored flavedo of mature MT fruits is caused by the accumulation of green chlorophyll and orange carotenoid pigments as compared to WT fruit flavedo tissue which accumulates only the orange-colored carotenoids.
Identification of the genetic lesion underlying the MT phenotype
To identify the candidate gene responsible for the mutant phenotype, we cross-analyzed data from three sources that reflected transcript and genetic variation. First, we performed a transcriptome analysis of fruits from WT and MT citrus trees grown under similar conditions. Pairwise transcriptome comparisons between the flavedos of MT and WT fruits were made during five developmental stages (190, 210, 230, 250, and 280 DAF) to identify differentially expressed genes (DEGs; Supplemental Table S3). From this analysis, we identified 7,790 (4,597 nonredundant) DEGs, which included 86 genes that were differentially expressed at all five stages (Figure 3A). This dataset comprised Gene set 1A (Supplemental Table S4) and a heat map shows similar trends in expression patterns (Figure 3B, left). When the analysis was limited to the stages flanking the breaker stage (190, 210, and 230 DAF), 209 DEGs were identified (Supplemental Figure S3A). This latter dataset comprised Gene set 1B (Supplemental Table S5), and similar trends in expression patterns are shown by the heat map (Supplemental Figure S3B, left).
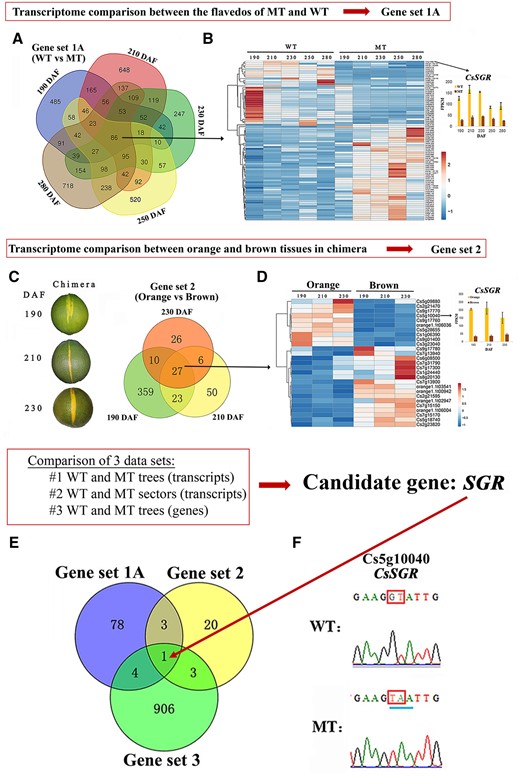
Multi-omics comparison of WT and MT. A, Venn diagram representation of the number of DEGs between WT and MT at five developmental stages (190–280 DAF). B, Left: Hierarchical cluster and heat map analysis illustrates expression profiles of the 86 genes that are differentially expressed at all stages as shown in (A); right: Fragments per kilobase million (FPKM) of the candidate gene CsSGR. Results are means ± sd from three biological replicates. The color bar indicates the expression levels (represented as FPKM means); red indicates high expression level; and blue indicates low expression level. C, Left: Phenotype of chimeras at three developmental stages from which orange and brown tissues were sampled for RNA-seq analysis; right: Venn diagram representing genes that are differentially expressed between orange and brown sectors at each of the three developmental stages (190, 210, and 230 DAF). D, Left: Hierarchical clustering and heat map analysis illustrating the expression profiles of the 27 genes that are differentially expressed at all developmental stages as shown in (C); right: FPKM of the candidate gene CsSGR. Results are means ± sd from three biological replicates. The color bar indicates the expression levels (represented as FPKM means); red indicates high expression level; and blue indicates low expression level. E, Venn diagram representing genes shared among Gene sets 1A, 2, and 3. F, Sections of sequencing chromatograms. The two mutations and stop codons in MT are indicated with red frames and a blue line, respectively.
Next, we analyzed data from chimeric tissue sectors that were present on many MT fruits where orange wild-type sectors intersected with tissue manifesting the brown mutant phenotype (Supplemental Figure S4). The advantage of analyzing this sectored material is that WT and MT sectors share more similar growth conditions as compared to WT and MT fruits harvested from neighboring trees. Pairwise transcriptome comparisons between the two tissue types were performed at stages flanking the breaker stage (190, 210, and 230 DAF) to identify DEGs between the orange and brown tissues (Supplemental Table S6). This comparison revealed 594 (501 nonredundant) DEGs of which 27 genes were differentially expressed at all three stages (Figure 3C). This dataset comprised Gene set 2 (Supplemental Table S7). The smaller number of DEGs of Gene set 2 (relative to Gene set 1B, which represented similar time points for fruits grown on different trees) likely reflected the homogeneity of growth conditions expected for tissues collected from the same fruit. The expression patterns of these 27 genes are shown by the heat map (Figure 3D, left).
Finally, we performed whole-genome sequencing (WGS) analysis of fruits from WT and MT plants. A total of 30,940 mutation sites were found by WGS (Supplemental Table S8). Next, we analyzed whether these mutation sites are in the coding sequence (CDS). We found mutations in 914 genes, which comprised Gene set 3 (Supplemental Table S9).
When we compared Gene sets 1A, 2, and 3 (Figure 3E), only one gene was found in common, Cs5g10040, which was annotated to encode a “senescence-inducible chloroplast STAY-GREEN protein”. The same result was obtained even if we created the Venn diagram using the 209 DEGs (Gene set 1B) from the comparison of WT and MT at only three developmental stages, in place of the 86 DEGs in Gene set 1A, which was based on five developmental stages (Supplemental Figure S3C). The STAY-GREEN (SGR) gene has been shown in other species to encode magnesium dechelatase, a key enzyme for chlorophyll degradation, which if missing contributes to delayed senescence (Matsuda et al., 2016; Shimoda et al., 2016). In the comparison of WT versus MT fruit transcriptomes, transcript levels of CsSGR were downregulated in MT as compared to WT at all five stages tested (Figure 3B, right). This result was further confirmed by reverse transcription quantitative PCR (RT-qPCR; Supplemental Figure S5A). Similarly, in comparisons of orange versus brown sectors of chimeric fruits, transcript levels of CsSGR were reduced in brown sectors relative to orange sectors (Figure 3D, right). We also used RT-qPCR to show that among the genes related to chlorophyll catabolism, only CsSGR was differentially expressed between the orange and brown sectors (Supplemental Figure S5B).
WGS analysis revealed point mutations in CsSGR at positions 6905076 and 6905077 on the fifth chromosome (Supplemental Table S10), which was validated by PCR-based sequencing (Figure 3F). The alteration from GT (Figure 3F; which corresponds to the reverse complemented AC in the genome sequence, Supplemental Table S10) to TA (Figure 3F; which corresponds to the reverse complemented TA in the genome sequence, Supplemental Table S10) introduced a premature stop codon (TAA) in the CsSGR CDS (Figure 3F). This stop codon likely explains the relatively reduced levels of SGR transcripts in MT compared with WT. Such aberrant transcripts would be targeted for nonsense-mediated mRNA decay, a mechanism known to eliminate mRNAs with premature termination codons to prevent the production of aberrant and potentially harmful proteins (Lykke-Andersen and Jensen, 2015). We also sequenced seven other WGS candidates (Figure 3E) that were identified by comparison between Gene set 3 and either Gene set 1A or Gene set 2, but CsSGR remained the only gene showing a difference between WT and MT (Supplemental Table S10). The other genes involved in chlorophyll degradation and carotenoid biosynthesis pathways, including their promoters, were analyzed by WGS, but no differences were found (Supplemental Tables S11 and S12). In addition, amplification and sequencing experiments, using genomic DNA as a template, showed that there are two CsSGR alleles in citrus (CsSGRa and CsSGRb) and that the two adjacent nucleotide mutations present in MT occurred 275-bp and 276-bp downstream of the translational start codon of CsSGRa (leading to the CsSGRaSTOP mutant allele in MT), while the sequence of CsSGRb in MT in this position matches the CsSGRb WT DNA sequence (Supplemental Figure S6A). Likewise, amplification and sequencing using cDNA as a template showed that the mutation in CsSGRa, referred to as CsSGRaSTOP, occurred 172-bp and 173-bp downstream of the translational start codon and that the sequence at this position for CsSGRb in MT matches the CsSGRb WT sequence (Supplemental Figure S6B). Consistent with these results, CsSGRa sequence in the brown sector of the chimera also carried the same 2-nt mutation (referred to as CsSGRaSTOP), while CsSGRa in the orange tissue was normal. In contrast with the CsSGRa of WT and CsSGRaSTOP in MT, there were no differences between WT and MT in their DNA or cDNA sequences for CsSGRb (Supplemental Figure S6). The protein encoded by CsSGRb in both WT and MT genotypes is predicted to be a truncated version of SGR as a result of a 3′-deletion in the gene in combination with mis-splicing of the mRNA resulting in a premature stop codon (Figure 4A; Supplemental Figure S7). However, the presence of CsSGRb in both WT and MT indicates that CsSGRb is not the primary cause of the MT brown flavedo phenotype. Instead, the mutation CsSGRaSTOP, found only in MT, is responsible for the mutant phenotype because the mutation encodes an internal stop codon which would also lead to a severely truncated CsSGRaSTOP protein due to premature termination of translation (Figure 4A; Supplemental Figure S7). In summary, both CsSGRaSTOP and CsSGRb are predicted to encode truncated peptides relative to CsSGRa.
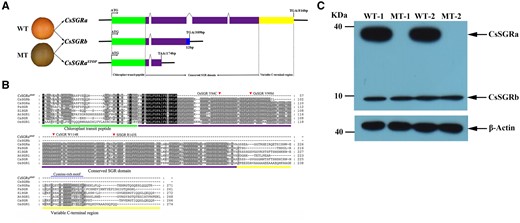
DNA sequence and protein differences among CsSGRa, CsSGRb, and CsSGRaSTOP. A, Schematic diagram showing the gene structure of CsSGRa, CsSGRb, and CsSGRaSTOP. B, Amino acid sequence alignments. The previously reported substitutions in highly conserved amino acids (rice, Y84C, and V99M; pepper, W114R; and tomato, R143S) are indicated with red triangles. A conserved cysteine-rich motif is marked by a blue line. The chloroplast transit peptide (green), the conserved SGR domain (purple), and the variable C-terminal region (yellow) are shown in the three panels. C, Western blot analysis of the proteins of CsSGRa and CsSGRb in the flavedo of WT (WT-1, WT-2) and MT (MT-1, MT-2) at the breaker stage (210 DAF). β-Actin protein is used as control.
SGR proteins contain a highly variable N-terminus including the predicted chloroplast transit peptide, a conserved central domain termed the SGR domain and variable C-terminal regions containing a cysteine-rich motif (Figure 4B; Sakuraba et al., 2014, 2015). CsSGRa is predicted to encode a 31-kD (271 residues) product, including a 4-kD (38 residues) transit peptide, which when cleaved upon import would yield a 26-kD (233 residues) plastid protein containing the SGR domain and the variable C-terminal domain (Figure 4B). CsSGRb is predicted to encode a 11-kD (102 residue) protein with 4 kD (38 residues) transit peptide, that if imported and cleaved into a chloroplast, would yield a mature protein of 7 kD (64 residues). CsSGRaSTOP is predicted to encode a 6-kD (57 residues) protein with 4 kD (38 residues) transit peptide, that if imported and cleaved into a chloroplast, would yield a mature protein of 2 kD (19 residues; Figure 4B). A citrus anti-SGR polyclonal antibody was developed (as described in the “Materials and methods”) to detect SGR antigens. As shown in Figure 4C, only WT showed a band predicted to correspond to SGRa, whereas SGRb and actin accumulated in WT and MT flavedo tissue. The CsSGRaSTOP transcript in MT likely undergoes nonsense-mediated RNA decay resulting in an undetectable protein, while the truncated, but longer, CsSGRb protein is detected (in MT and WT), along with CsSGRa (in WT only).
Phylogenetic and gene expression analysis of SGR genes in citrus
We performed a further phylogenetic analysis of SGR homologs in citrus. Two citrus SGR genes (Cs5g10040, Cs3g20450) retrieved from the C. sinensis genomic database (http://citrus.hzau.edu.cn/orange/; Xu et al., 2013) were compared with SGR protein sequences from other species (Supplemental Figure S8 and Supplemental Table S13). The phylogenetic tree clearly showed that Cs5g10040, the locus encoding CsSGRa and CsSGRb, belongs to the SGR subfamily (hereafter referred to as CsSGR) and that Cs3g20450 belongs to the SGR-LIKE (SGRL) subfamily (hereafter referred to as CsSGRL). Moreover, CsSGR was most closely related to SGR sequences from grape (Vitis vinifera) VvSGR1 and litchi (Litchi chinensis) LcSGR.
The expression levels of the CsSGR alleles and CsSGRL were evaluated in the flavedos and pulps of three citrus species at five developmental stages (Supplemental Figure S9). We found that the expression of CsSGRL was low and remained nearly constant among the different citrus fruit tissues, development stages, and species. In contrast, the expression levels of both CsSGR alleles increased during the ripening of citrus fruit. This trend was more pronounced in the flavedo than in the pulp. Additionally, the transcript levels of CsSGRa were substantially higher than for CsSGRb.
To further evaluate the tissue-specific and developmental expression of CsSGRa, we measured relative transcript levels in WT (Supplemental Figure S10A). CsSGRa was expressed predominantly in leaves and fruit tissues. During fruit development, the transcript levels were paralleled by CsSGR protein levels (Supplemental Figure S10B).
CsSGR function in chlorophyll degradation
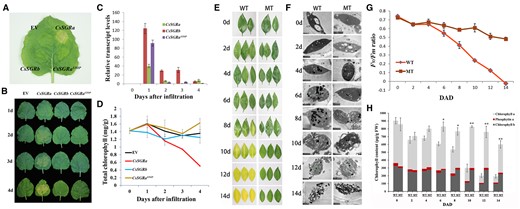
To confirm the role of CsSGR in chlorophyll degradation in vivo, we performed functional analyses of CsSGRa, CsSGRb, and CsSGRaSTOP using an Agrobacterium-based transient expression assay in leaves of Nicotiana benthamiana. Four days after infiltration of cloned cDNAs, significant loss of chlorophyll coloration was observed in the CsSGRa-overexpressing leaves (Figure 5, A and B), which was consistent with elevated transcript levels of the transgenes as confirmed using RT-qPCR (Figure 5C), and quantitative reduction in chlorophyll content (Figure 5D). In contrast, the leaves that overexpressed CsSGRb and CsSGRaSTOP and the leaves that were transiently transformed with the empty vector (EV) showed no obvious chlorophyll degradation. These results show that CsSGRa from WT could induce chlorophyll loss in contrast with the ineffective CsSGRaSTOP in MT and CsSGRb in both WT and MT.
Characterization of CsSGR in planta expression by transient overexpression of CsSGR alleles in N. benthamiana leaves and analysis of SGR phenotypes in citrus leaves of MT and WT. A, Phenotypes of leaves at the fourth day after infiltration with A. tumefaciens cultures containing plasmids that overexpress CsSGRa, CsSGRb, CsSGRaSTOP, and EV. B, Phenotypes of leaves from 1 to 4 d after infiltration with plasmids that overexpress CsSGRa, CsSGRb, CsSGRaSTOP, and EV. C, Relative transcript levels of CsSGRa, CsSGRb, and CsSGRaSTOP in leaves within respective CsSGRa-, CsSGRb-, and CsSGRaSTOP-overexpressing lines after infiltration as described in (B). NtActin was used as normalizer in RT-qPCR measurements. D, Analysis of total chlorophyll content in leaves after infiltration as described in (B). E, Dark-induced senescence phenotypes of WT and MT leaves. F, TEM of plastid ultrastructure of WT and MT leaves during the different periods shown in (E). Abbreviations and symbols are as follows: S, starch granule; P, plastoglobule; M, mitochondrion; T, thylakoid. Bar = 1 μm. G, Efficiencies of photosystem II (Fv/Fm) in WT and MT leaves during the periods shown in (E). DAD, days after darkness. H, Differences in chlorophyll content in WT and MT leaves during the periods shown in (E). All the above results are means ± sd from at least three biological replicates. Asterisks indicate statistically significant differences compared with WT (Student’s t test P-value, *P < 0.05, **P < 0.01). These experiments were repeated more than twice with similar results.
Next, we tested chlorophyll degradation activity of CsSGRa by in vitro assay of Mg-dechelatase, the SGR activity that catalyzes extraction of the magnesium in chlorophyll a to form pheophytin a (Shimoda et al., 2016). Recombinant CsSGRa protein produced in a wheat germ protein expression system was confirmed by immunoblot analysis (Supplemental Figure S11A). Mg-dechelatase reactions contained either recombinant CsSGRa, no added protein (No enzyme added), or heated-denatured CsSGRa (CsSGRa [heat-inactivated]). After incubation, pigments were analyzed using Ultra-performance liquid chromatography (UPLC). Chlorophyll and pheophytin have unique retention times and spectra as shown for the standards (Supplemental Figure S11B). The results revealed pheophytin a accumulation after incubation with CsSGRa, but not in the presence of the negative control (no enzyme) or heat-inactivated enzyme (Supplemental Figure S11, C and D). In addition, increasing concentrations of chlorophyll a substrate were accompanied by enhanced conversion to the pheophytin a product (Supplemental Figure S11E). These results demonstrated that CsSGRa is a bona fide SGR with Mg-dechelating activity responsible for the SGR-mediated chlorophyll degradation observed in vivo.
We also observed a difference in leaf senescence phenotypes of WT and MT leaves which could be explained by the differences in functional SGR (Supplemental Figure S12A). Further observation using a stereomicroscope showed that the withered leaves of MT were abnormally green (Supplemental Figure S12B). Previous studies in tomato (Solanum lycopersicum), pepper (Capsicum annuum), and rice (Oryza sativa) have shown that the SGR phenotype caused by mutations in SGR is not restricted to fruit but is also observed in senescent leaves (Park et al., 2007; Barry et al., 2008). Thus, a leaf senescence assay that uses dark treatment to induce leaf senescence was performed by incubating leaves in the dark for 14 d. A delay in chloroplast degradation was evident by leaf coloration and TEM analysis (Figure 5, E and F). The ratio Fv/Fm, which measures the maximum quantum efficiency of the photochemistry performed by photosystem II, decreased during the dark treatment in both materials, but the reduction was slower in MT relative to WT leaves (Figure 5G). Consistent with this result, the chlorophyll content was also higher in MT relative to WT leaves after prolonged treatment in the dark (Figure 5H). These results demonstrated that the mutations in CsSGRa inhibit the degradation of chlorophyll in both fruits and leaves of MT.
CsSGR function in regulating carotenoid biosynthesis
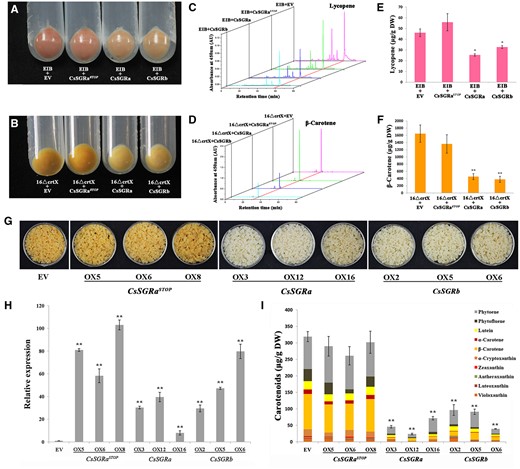
Phytoene synthase (PSY) is a rate-controlling enzyme for carotenoid biosynthesis (as reviewed in Wurtzel, 2019) and SGR can interact with PSY to reduce carotenoid biosynthesis in developing tomato fruits (Luo et al., 2013). While control of fruit development is not conserved between citrus and tomato, chlorophyll degradation and carotenoid biosynthetic pathways are highly conserved across the plant kingdom. Therefore, we tested whether SGR might also control carotenoid accumulation in citrus. We utilized two complementary approaches, a well-established heterologous bacterial platform and transgenic expression in citrus tissue culture. First, CsSGR alleles were introduced into strains of Escherichia coli that were engineered to produce carotenoids. In the first set of experiments, we tested the effect of CsSGR on bacterial PSY by using E. coli harboring one of the two gene clusters encoding bacterial carotenoid biosynthetic enzymes. Escherichia coli cells carrying one of the clusters were additionally transformed with pGEX-6p-1-EV, the EV control or with plasmids expressing either CsSGRaSTOP, CsSGRa, or CsSGRb. Escherichia coli transformants containing EV and CsSGRaSTOP showed accumulation consistent with the pink-colored lycopene (Figure 6A) or yellow-colored β-carotene (Figure 6B), whereas E. coli transformants containing CsSGRa or CsSGRb in combination with the carotenoid mini pathways were reduced in color. High-performance liquid chromatography (HPLC) analysis further confirmed the effects of CsSGR proteins on carotenoid content of these engineered strains (Figure 6, C and D). Indeed, lycopene and β-carotene levels in the dehydrated cell cultures were significantly reduced in strains overexpressing either CsSGRa or CsSGRb (Figure 6, E and F). In the second set of experiments (Supplemental Figure S13), we tested the effects of SGR on a plant PSY from citrus, CsPSY1. We began with E. coli that carried pACCRT-E, which produced the PSY precursor, geranylgeranyl pyrophosphate (GGPP), and transformed further with constructs including the citrus genes encoding CsPSY1, CsSGR, and/or EV controls. Protein expression was confirmed by SDS–PAGE (Supplemental Figure S13A). To test the effect of the various genes on phytoene accumulation, we used HPLC to separate phytoene isomers, which have characteristic spectra obtained with an in-line photodiode array detector (Supplemental Figure S13B). Escherichia coli cells encoding GGPPS (pACCRT-E) or with the addition of an EV (pACCRT-E+EV) produced only GGPP, which is not observable in the HPLC chromatogram as shown (Supplemental Figure S13B). When these strains were further transformed with bacterial PSY (pACCRT-EB) or the plant PSY from citrus (pACCRT-E+CsPSY1), phytoene was detected (Supplemental Figure S13B). The addition of either CsSGRa or CsSGRb in E. coli containing pACCRT-E+CsPSY1 significantly reduced phytoene content, while no significant change was observed when CsSGRaSTOP was introduced (Supplemental Figure S13, B and C). These results demonstrated that both CsSGRa and CsSGRb, but not CsSGRaSTOP, could inhibit carotenoid accumulation in the engineered E. coli system, which is caused by their effect on PSY, whether PSY originated from bacteria or plants.
Functional analysis of CsSGRa, CsSGRb, and CsSGRaSTOP in E. coli and transgenic citrus callus. Escherichia coli transformants harboring pACCRT-EIB for lycopene biosynthesis and pACCAR16ΔcrtX for β-carotene synthesis were co-transformed with pGEX-6p-1-EV, CsSGRa, CsSGRb, or CsSGRaSTOP. A, Escherichia coli transformants harboring plasmids pACCRT-EIB and expressing EV, CsSGRa, CsSGRb, or CsSGRaSTOP. B, Escherichia coli transformants harboring pACCAR16ΔcrtX and expressing EV, CsSGRa, CsSGRb, or CsSGRaSTOP. C, D, HPLC carotenoid profiles of E. coli cells transformed as described in (A, B). E, F, Lycopene and β-carotene content of E. coli cells transformed as described in (A, B). G, Phenotype of transgenic citrus callus. Transgenic callus lines refer to those overexpressing the EV, CsSGRa (OX3, OX12, and OX16), CsSGRb (OX2, OX5, and OX6), and CsSGRaSTOP (OX5, OX6, and OX8). H, Expression of CsSGRa, CsSGRb, or CsSGRaSTOP within respective transgenic citrus callus determined by RT-qPCR. Background expression of CsSGR including CsSGRa and CsSGRb were also detected in EV as the control. I, Carotenoid content and composition in transgenic citrus callus analyzed using HPLC. The various colored blocks represent carotenoid compounds as indicated in the key. All the above results are means ± sd from three biological replicates. Asterisks indicate statistically significant differences compared with EV (Student’s t test P-value, *P < 0.05, **P < 0.01).
To assess inhibition of carotenoid biosynthesis in plant cells, we overexpressed CsSGRa, CsSGRb, or CsSGRaSTOP, respectively, in citrus callus to compare with the EV control transformants that were orange in color. The transgenic citrus callus lines overexpressing CsSGRa and CsSGRb were white. However, the color of the transgenic lines overexpressing CsSGRaSTOP was indistinguishable from the EV transformants (Figure 6G). The overexpression of CsSGRa, CsSGRb, and CsSGRaSTOP in different transgenic lines was confirmed by RT-qPCR (Figure 6H). HPLC analysis indicated that the levels of all carotenoids declined significantly in the transgenic lines that overexpressed CsSGRa or CsSGRb in comparison with the EV control. However, no difference was found in the carotenoid levels of the transgenic lines that overexpressed CsSGRaSTOP relative to the EV control (Figure 6I). Taken together, these results demonstrated that CsSGRa and CsSGRb could negatively regulate the accumulation of carotenoids in transgenic citrus callus. The CsSGRaSTOP from MT appears to be an inactive allele, consistent with the observation that MT fruits exhibit increased levels of carotenoids compared with WT.
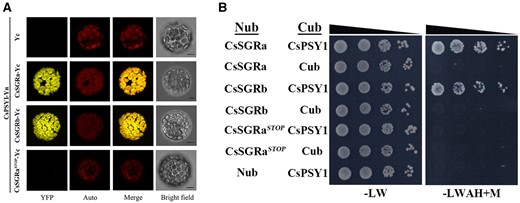
The effect of SGR on reducing carotenoid accumulation in bacteria could be explained if citrus SGR binds to PSY to inhibit PSY activity in vivo. To explore this possibility, we assessed co-expression, co-localization, and protein–protein interactions of PSY and SGR. First, we observed that CsPSY1 and CsSGR proteins showed similar co-expression patterns during the ripening of citrus fruit (Supplemental Figure S9). Next, we examined subcellular localization of GFP fusions of PSY and SGR proteins. CsSGRa-GFP, CsSGRb-GFP, CsSGRaSTOP-GFP, or CsPSY1-GFP were transiently expressed in citrus leaf protoplasts. We found that all of these proteins have a functional transit peptide that can direct GFP into chloroplasts of citrus leaf protoplasts (Supplemental Figure S14). These data suggest that CsSGR localizes to plastids where CsSGR can potentially regulate carotenoid formation by interacting with PSY. To test for interactions between SGR and PSY in citrus, we used bimolecular fluorescence complimentary (BiFC) and yeast two-hybrid (Y2H) assays to analyze the pairwise interactions between CsSGR proteins and CsPSY1. We found that when CsSGRa-YFPc or CsSGRb-YFPc were co-expressed with CsPSY1-yellow fluorescent protein (YFPn) in Arabidopsis mesophyll protoplasts and N. benthamiana leaves, chloroplasts emitted a yellow fluorescence as evidence of protein interaction (Figure 7A; Supplemental Figure S15A). In contrast, only weak background fluorescence was detected when the EV YFPc (Yc) or CsSGRaSTOP-YFPc were co-expressed with CsPSY1-YFPn. These results suggested that both CsSGRa and CsSGRb but not CsSGRaSTOP could interact with CsPSY1 in plant cells and that both SGRa and SGRb can localize to plastids. Additionally, we carried out Y2H analysis using a Y2H split-ubiquitin membrane system that proved suitable for studying the interactions between the membrane-associated PSY and other proteins (Obrdlik et al., 2004; Zhou et al., 2015). Yeast growth on the selective medium was observed only when cells carrying either Nub-CsSGRa or Nub-CsSGRb were mated with cells carrying CsPSY1-Cub (Figure 7B). These data indicated that CsSGRa and CsSGRb, but not CsSGRaSTOP, could physically interact with CsPSY1 in yeast. Similarly, using the Y2H assay, we found that both CsSGRa and CsSGRb but not CsSGRaSTOP interacted with AtPSY from Arabidopsis (Supplemental Figure S15B). Taken together, these data provide evidence that both CsSGRa and CsSGRb but not CsSGRaSTOP inhibit carotenoid biosynthesis by interacting directly with CsPSY1 in the plastid. Interestingly, CsSGRb, which encodes a truncated protein that lacks the chlorophyll-degradation activity, still maintains the ability to interact with PSY and inhibit carotenoid accumulation.
Testing interactions between CsSGRa, CsSGRb, or CsSGRaSTOP with CsPSY1. A, BiFC analysis. Arabidopsis mesophyll protoplasts were co-transformed with genes encoding CsSGRa (CsSGRa-Yc), CsSGRb (CsSGRb-Yc), or CsSGRaSTOP (CsSGRaSTOP-Yc) as C-terminal YFPn fusions and co-transformed with CsPSY1 as an N-terminal YFP fusion (CsPSY1-Yn). As a negative control, Arabidopsis mesophyll protoplasts were co-transformed with the Yc (EV) and CsPSY1-Yn. YFP indicates fluorescence from YFP. Merge indicates the digital addition of the Auto and YFP signals. The chlorophyll autofluorescence (Auto) was used to localize chloroplasts. Bars = 10 μm. B, Y2H analysis. Interactions between either CsSGRa, CsSGRb, or CsSGRaSTOP and CsPSY1 were examined by cotransforming yeast with genes encoding pairs of proteins fused to either the N-terminal (Nub) or C-terminal (Cub) ubiquitin moiety in yeast and spotting transformants onto either nonselective (−LW) or fully selective medium plates with 150 mM Met (−leucine, tryptophan, adenine, and histidine +M) in a series of 10-fold dilutions. EVs expressing Nub and Cub only were used as negative controls.
Discussion
Citrus breeding has produced a wealth of cultivars that include a wide range of nutritious food crops and genetic resources (Wu et al., 2018). Plant chimeras, including fruit sectors, are valuable isogenic resources that we used to generate comparative data in a uniform physiological background (Frank and Chitwood, 2016). The assessment of transcriptomic and genomic data provided an important means to overcome the general inefficiency of genetic analysis in citrus and other woody perennials. The molecular basis of the Zong Cheng brown flavedo phenotype in C. sinensis was thus elucidated by comparing multiple transcriptomic and genomic datasets. We discovered that allelic and DNA variation in the SGR gene was responsible for alteration in chlorophyll and carotenoid accumulation during the development of the flavedo in the fruit of the brown flavedo mutant. In plants, the key enzyme for chlorophyll degradation is magnesium dechelatase, encoded by the SGR gene (Matsuda et al., 2016; Shimoda et al., 2016), while the major rate-controlling enzyme for carotenoid biosynthesis is PSY (Wurtzel, 2019). We discovered that both MT and WT are heterozygous at the SGR locus. The SGRa allele in WT encodes a functional SGR which controls both carotenoid and chlorophyll levels, whereas MT plants or sectors encode SGRaSTOP, a dysfunctional protein. In addition, both MT and WT share alleles encoding SGRb, a truncated protein that retains the ability to negatively regulate carotenoid biosynthesis but is impaired in activity for chlorophyll degradation. We demonstrated that the truncated SGRb protein is imported into plastids where it can bind to PSY to reduce carotenoid biosynthesis. Thus, only the N-terminus of SGR is needed for PSY binding and inactivation, whereas chlorophyll degradation requires the entire peptide as suggested by mutations in rice, pepper, and tomato (Jiang et al., 2007; Park et al., 2007; Barry et al., 2008).
In tomato, it was shown that interaction between SGR and PSY leads to reduced fruit carotenoids and that SGR can coordinate biosynthesis of carotenoids coupled with degradation of chlorophyll (Luo et al., 2013). However, it was unknown whether SGR and PSY would co-localize in citrus plastids with the potential to interact, since PSY localization is dependent on plant species and PSY gene family member (Shumskaya et al., 2012; Shumskaya and Wurtzel, 2013). Indeed, we found that SGR and PSY do co-localize and interact, leading to reduced citrus carotenoids. However, the binding of SGR to PSY was not dependent on chlorophyll-degrading activity. Therefore, it appears that regulation of chlorophyll degradation and carotenoid biosynthetic pathways mediated by SGR in citrus hesperidium can be uncoupled, a phenomenon that has not been observed in other species.
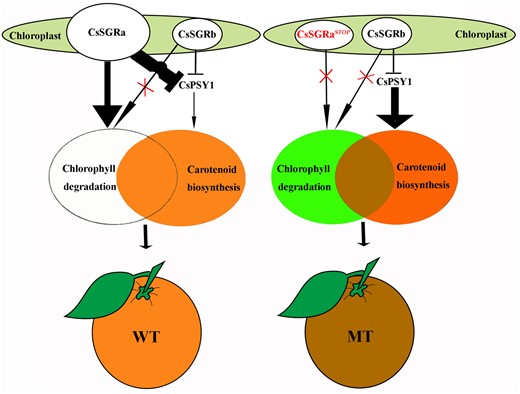
Based on the results presented, we propose a model for the molecular mechanism underlying the brown flavedo phenotype of MT (Figure 8). WT is heterozygous for SGRa/SGRb. The SGRa allele encodes a protein that can degrade chlorophyll and inhibit carotenoid biosynthesis by interacting directly with PSY. The SGRb allele encodes a protein that can also inhibit carotenoid biosynthesis by interacting directly with PSY but is unable to mediate chlorophyll degradation. However, MT carries the SGRaSTOP/SGRb genotype. The proteins encoded by SGRaSTOP and SGRb cannot degrade chlorophyll and therefore chlorophyll accumulates in the flavedos of MT fruits. In MT, only allele SGRb can inhibit carotenoid biosynthesis because SGRb binds to PSY to inhibit PSY activity. Given the absence of SGRa and the relatively lower expression of SGRb as compared to WT SGRa, higher levels of carotenoids accumulate in the flavedos of MT relative to WT. We suggest that this mechanism leads to the concurrent accumulation of chlorophylls and carotenoids, a higher carotenoid content in the flavedo and the development of brown fruit in MT.
Model for the phenotype of “Zong Cheng”. In WT (left), only CsSGRa can degrade chlorophyll. Both CsSGRa and CsSGRb can inhibit carotenoid biosynthesis by directly binding CsPSY1. The above activities produce an orange flavedo. In MT (right), neither CsSGRaSTOP nor CsSGRb can degrade chlorophyll. Only CsSGRb can inhibit carotenoid biosynthesis by directly binding CsPSY1. The above activities promote the retention of chlorophyll and the hyperaccumulation of carotenoids. Thus, the unique brown-colored flavedo of MT fruits is caused by the combined accumulation of green chlorophylls and orange carotenoids.
Citrus flavedo carotenoid accumulation is limited by SGR activity, an important finding for citrus breeding and efforts to enhance carotenoid phenotypes. In addition to SGRa, the SGRb allele can be considered a possible target for further improvement of flavedo carotenoid content, since PSY-binding activity will reduce carotenoid pools. Expression patterns of SGR and PSY among several citrus species suggest that the role of SGR is likely conserved in citrus fruit coloration. In other plants, SGR mutants manifest reduced chlorophyll degradation in senescing tissues, a valuable trait for maintaining leaf photosynthetic activity in late harvest times (Sakuraba et al., 2014). When tested in citrus, the brown flavedo mutation revealed a similar phenotype in senescing green tissues. Thus, the SGR mutation is a target for increasing photosynthetic activity in senescent citrus plants. In this and other late-maturing varieties, the phenotype could be especially useful for improving yields.
Conclusions
In summary, our findings indicate that the function of SGR evolved early in the evolution of flowering plants and preceded fruit morphological innovations in these species. However, unlike SGR of other plant species, we found that the carotenoid and chlorophyll regulatory activities could be uncoupled in the case of certain SGR alleles in citrus. These findings would not have been possible without the implementation of a multi-omics strategy to overcome the genetic heterogeneity of citrus, an approach that has great potential to drive gene discovery and plant improvement in citrus and other woody perennials of great economic importance.
Materials and methods
Plant materials and growth conditions
“Zong Cheng” (MT) was found in 2006 in a Lane Late Navel Orange (Citrus sinensis L. Osbeck; WT) orchard in Zigui County (Hubei Province, China). MT was propagated by grafting. The mutant phenotype was stable from 2008 to 2018. The fruits of MT were brown, and therefore readily distinguishable from the orange fruits of WT. The phenotypes of the leaves, flowers, and the phenological periods of MT and WT were not significantly different.
The trees of WT and MT were cultivated side-by-side. Fruit samples were collected at 11 different developmental stages from immature green to the fully colored stage: 90, 120, 150, 170, 190, 210, 230, 250, 280, 310, and 340 DAF. Red Tangerine (C. reticulata Blanco cv tangerine), Washington Navel Orange (C. sinensis [L.] Osbeck cv Washington Navel), and Cocktail Grapefruit (C. paradisi cv Cocktail) were collected from adult citrus trees grown at the National Center for Citrus Breeding, Huazhong Agricultural University. Fruits were obtained at five developmental stages that showed distinct fruit pigmentation: 120, 150, 180, 210, and 240 DAF. The flavedos and pulps were separated from the sampled fruits, immediately frozen in liquid nitrogen, and kept at −80°C until analysis. The leaf darkness treatment assay was performed on young and fully expanded citrus leaves. Leaves were excised and incubated on wet filter paper in total darkness at 30°C for various periods of time, as indicated.
Light and TEM, color index, and total chlorophyll content measurement
Light and TEM were described previously (He et al., 2018). Color measurements were performed with a color analyzer (KONICA MINOLTA CR-400, Japan) at three evenly distributed equatorial sites. Three color values L* (whiteness or brightness/darkness), a* (redness/greenness), and b* (yellowness/blueness) were measured. Total chlorophyll was extracted from the infiltrated region of Agrobacterium tumefaciens-infiltrated N. benthamiana leaves and quantified using a spectrophotometer, as described previously (Becker, 1994).
Carotenoid extraction and HPLC analysis
For carotenoid analysis, the samples from engineered bacteria and transgenic callus were ground into a powder after lyophilization with a lyophilizer (LABCONCO FreeZone®). Carotenoid pigment extraction and analysis were performed using reversed-phase (RP) HPLC, as previously described (Liu et al., 2007; Cao et al., 2012). Carotenoids were identified based on retention times and absorption spectra as compared to standards. Peak areas were recorded at 286, 348, 473, and 450 nm for phytoene, phytofluene, lycopene, and others, respectively (Xu et al., 2006). The carotenoid levels were quantified using calibration curves prepared with appropriate standards. At least three independent extractions were conducted per sample.
Extraction and UPLC analysis of chlorophylls and chlorophyll derivatives
Chlorophylls and their derivatives in the flavedos and leaves of WT and MT were extracted as described previously (Minguezmosquera and Garridofernandez, 1989). The separations and quantification of chlorophylls and chlorophyll derivatives were performed using an UPLC (Waters, H-Class) system with a C18 column (BEH C18, 50 × 2.1 mm i.d., 1.8 μm). Separation was performed using an elution gradient (0.4 mL min−1) with mobile phases A (water: ion pair reagent: methanol (1:1:8, v/v/v)) and B (methanol: acetone (1:1, v/v)). Ion pair reagent, 1 M ammonium acetate (CH3COONH4, CAS: 631-61-8, HPLC grade, purity ≥99.8%, Aladdin, Shanghai) and 0.05 M tetrabutylammonium acetate ((CH3CH2CH2CH2)4NOCH3, CAS: 10534-59-5, HPLC grade, purity ≥99.8%, Aladdin, Shanghai). The metabolites were eluted using the following program: (min, A%): (0, 75), (3, 35), (4, 35), (5, 25), (6, 16), (8, 0), (9, 75), (10, 75). The online UV-visible spectra were recorded from 350 to 750 nm using a photodiode array extended wavelength (eλ) detector. Detection was at 654 nm for Chl b and at 664 nm for Chl a and its derivatives. Data were collected and processed with the Empower 3 software. Chlorophylls and their derivatives were identified by comparing their retention time and spectral characteristics with authentic standards (Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai, China). The detailed methods were described previously (Zhu et al., 2020). At least three independent extractions and detections were performed for each sample.
Total RNA isolation, cDNA synthesis, and reverse transcription quantitative PCR analysis
Total RNA was extracted from citrus and transgenic callus tissues using a modified Trizol method described previously (Liu et al., 2007). Micro-spectrophotometer and agarose gel electrophoresis were used for detecting RNA concentration, integrity and purity. First-strand cDNA was synthesized from 1 μg of total RNA using the HiScript® II Q RT SuperMix for qPCR (+gDNA wiper; Vazyme). The gene-specific primers used in our RT-qPCR analyses (Supplemental Table S14) were designed using the Primer Premier 5 software. RT-qPCR was performed as previously reported (Zheng et al., 2019).
RNA-seq library construction, sequencing, and analysis
Six groups of sector tissue samples at 3 developmental stages from chimeric fruits and 10 groups of flavedo samples at five developmental stages from WT and MT were sequenced. For each group, three independent biological replicates were subjected to RNA-seq. RNA library construction and sequencing were conducted by Novogene Bioinformatics Technology (Wuhan) using Illumina HiSeq. The detailed methods were described previously (Lu et al., 2018). Hierarchical cluster and heat map analysis were implemented using ClustVis (https://biit.cs.ut.ee/clustvis/). Raw data were deposited in the NCBI database under accession number PRJNA573296.
WGS analysis
The Illumina reads of “Lane Late” Navel Orange (WT) and “Zong Cheng” (MT) were mapped to the reference genome of sweet orange by BWA (Li and Durbin, 2009). The duplicated mapping reads were then removed by SAMtools rmdup (Li and Durbin, 2009). IndelRealigner in the GATK package was used to perform local realignments around the InDels, and UnifiedGenotyper tool was finally used to identify single-nucleotide polymorphisms (SNPs; McKenna et al., 2010). An in-house Perl script was used to compare the genotypes among different species. The positions of SNPs in the different genotypes were identified. The details of these methods were described previously (Wang et al., 2018). Raw data were deposited in the NCBI database under accession number PRJNA568337.
SGR protein accession numbers and phylogenetic analysis
The sequence data described in this study were deposited in GenBank: CsSGRa, MN817581; CsSGRaSTOP, MN817582; CsSGRb, MN817583. Citrus sinensis CsSGR and CsSGRL sequences were obtained from the sweet orange genome database (http://citrus.hzau.edu.cn/orange/). The other SGRs and SGR-like proteins in plants, algae and bacteria were obtained from GenBank. The accession numbers of all proteins are listed in Supplemental Table S13. Amino acid sequence alignments were performed using ClustalX. The phylogenic trees were constructed using MEGA 6 with the neighbor-joining method and 1,000 bootstrap replicates.
Protein extraction, antibody generation, and western blotting
For western blotting assay, total flavedo proteins from WT and MT during different developmental stages were extracted and quantified as described previously (Cao et al., 2012). For generating an antibody that can detect both CsSGRa and CsSGRb proteins, the sequence shared by both CsSGRa and CsSGRb was identified (Supplemental Figure S16). A 621-bp dimer containing the SGRa and SGRb shared sequence separated by an adapter sequence and with ends compatible with BamHI and XhoI sites was commercially synthesized (Genewiz, Suzhou, China), then inserted into BamHI and XhoI sites downstream of a GST sequence and HRV 3C cleavage site in plasmid pGEX-6p-1. The recombinant construct was confirmed by sequencing and then transformed into E. coli strain BL21 (DE3). Cells were induced and the fusion protein with N-terminal GST tag was purified (Supplemental Figure S17). The GST tag was released by proteolytic cleavage at the HRV 3C cleavage site and the purified SGR antigen was injected into rabbits to generate the anti-SGR polyclonal antibodies (Frdbio, Wuhan, China). Actin, anti-β-Actin mouse mAb (Frdbio: RAB0100, Wuhan, China). Polyclonal anti-SGR was used in the western analysis of protein extracts (Cao et al., 2012) from either control cells carrying the EV (pGEX-6p-1) or cells expressing the anti-SGR protein fusion protein (pGEX-6p-SGR) and only the lane expressing anti-SGR showed a band of the predicted size (Supplemental Figure S18).
Transient expression in N. benthamiana leaves
For transient expression in N. benthamiana leaves, the full-length cDNAs from CsSGRa, CsSGRb, and CsSGRaSTOP were cloned into the overexpression vector pH7WG2D using the Gateway Cloning System (Invitrogen; See detailed information about constructs in Supplemental Table S15). Agrobacterium infiltration experiments were performed in N. benthamiana leaves with Agrobacterium strain GV3101 carrying the plasmids described above. The EV pH7WG2D was used as a control. The recombinant Agrobacterium strains were initially grown in Luria-Bertani solid medium at 28°C for 2 d and were scraped into the Agrobacterium infiltration buffer (10 mM MgCl2 and 40 mM acetosyringone). The concentration of bacteria was adjusted to an OD600 that ranged from 0.6 to 0.8 and then incubated at room temperature for 2 h. After the incubation step, the N. benthamiana leaves were infiltrated with the Agrobactrium infiltration buffer using a 1-mL syringe.
Fv/Fm measurments
Chlorophyll fluorescence measurements used to calculate Fv/Fm in citrus leaves were performed after a dark treatment and were obtained using a portable Li-6400XT photosynthesis system (Li-Cor, Lincoln, USA), according to the manufacturer’s instructions.
Mg-Dechelatase Assay
The CsSGRa CDS lacking the transit peptide was amplified (see primers listed in Supplemental Table S14) and a FLAG-tag was introduced at the C terminus. CsSGRa was synthesized using an in vitro transcription/translation system (TNT SP6 High-Yield Wheat Germ Protein Expression System, Promega), according to the manufacturer’s instructions. Monoclonal antibodies (Abcam) against the FLAG-tag were used in the western analysis of CsSGRa protein. The Mg-dechelatase assay (Shimoda et al., 2016) was conducted with CsSGRa protein diluted four-fold in a 50-μL reaction buffer having a final concentration of 50 mM Tris–HCl (pH 7.5), 100 mM NaCl, and 0.05% (v/v) polysorbate 20. We dissolved chlorophyll a (Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai, China) in 80% (v/v) acetone, and we added 0.8 μL of the acetone solution (375 μM substrate stock) to the reaction buffer (6 μM final concentration for the standard assay). Mixtures were incubated at 25°C in the dark for 60 min and then 9 volumes of acetone were added to stop the reaction. After centrifugation at 21,600g for 15 min at 4°C, the pigments were analyzed by UPLC as described above with detection at 410 nm. The pheophytin a standard was prepared by adding 0.75 mM HCl to chlorophyll a in 80% (v/v) acetone (Eijckelhoff and Dekker, 1997). Chlorophyll a and pheophytin a were identified by comparing their retention times and spectral characteristics with authentic standards (Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai, China). Chlorophyll a and pheophytin a were quantified using calibration curves prepared with appropriate standards. Control assays were reactions lacking CsSGRa (labelled as “No enzyme added”) or reactions containing CsSGRa that was heat-denatured by treatment at 95°C for 5 min (labelled as “CsSGRa (heat-inactivated)”).
Prokaryotic expression
Functional assays were performed with E. coli BL21 (DE3) strains (TransGen Biotech). In the first set of experiments, bacterial genes, including bacterial PSY (crtB), were used to construct minicarotenoid pathways for testing with CsSGR constructs. The experiment was conducted with two plasmids containing Pantoea ananas (formerly known as Erwinia uredovora) genes that encode carotenoid biosynthetic enzymes. pACCRT-E harbors crtE encoding bacterial GGPP synthase. Escherichia coli strains harboring this plasmid produce GGPP. pACCRT-EB harbors crtE and crtB, encoding bacterial GGPP synthase and bacterial PSY. Esherichia coli strains harboring this plasmid produce phytoene. pACCRT-EIB harbors crtE, crtI and crtB encoding bacterial GGPP synthase, bacterial PSY, and bacterial phytoene desaturase. E. coli strains harboring this plasmid produce lycopene. pACCAR16ΔcrtX harbors crtE, crtI, crtB, and crtY encoding bacterial GGPP synthase, bacterial PSY, bacterial phytoene desaturase, and bacterial lycopene β-cyclase. Escherichia coli strains harboring pACCAR16ΔcrtX produce β-carotene (Misawa et al., 1995). Escherichia coli cells were co-transformed with one plasmid encoding carotenoid biosynthetic enzymes (pACCRT-EIB or pACCAR16ΔcrtX) and with either pGEX-6p-1-CsSGRa, pGEX-6p-1-CsSGRb, pGEX-6p-1-CsSGRaSTOP, or the EV pGEX-6p-1 (negative control). In the second set of experiments, we also included a plant PSY gene and compared with bacterial PSY constructs as follows. Escherichia coli cells were transformed with pACCRT-E, pACCRT-EB, pACCRT-E+EV, pACCRT-E+CsPSY1, pACCRT-E+CsPSY1+CsSGRa, pACCRT-E+CsPSY1+CsSGRb, pACCRT-E+CsPSY1+CsSGRaSTOP. The detailed information on these plasmid combinations is listed in Supplemental Table S16 and detailed information about constructs is listed in Supplemental Table S15. The detailed methods were described previously (Lu et al., 2016).
Citrus callus transformation
The overexpression vector pH7WG2D with full-length cDNAs derived from CsSGRaSTOP, CsSGRa, and CsSGRb were also used in transgenic callus experiments (see detailed information about constructs in Supplemental Table S15). Agrobacterium tumefaciens strain (EHA105) was transformed with these constructs or an EV (as a control). The citrus callus, derived from Valencia sweet orange (C. sinensis cv Valencia) was used because it accumulates abundant carotenoids. Explant preparation and transformation were performed as described previously (Zheng et al., 2019). As a result, at least 20 independent transgenic callus lines for each target gene were obtained. Three lines overexpressing CsSGRa (OX3, OX12, and OX16), CsSGRb (OX2, OX5, and OX6), and CsSGRaSTOP (OX5, OX6, and OX8) were chosen for further investigation. The transgenic callus harboring EV was used as the negative control.
Subcellular localization
The full-length cDNAs from the CsSGR alleles and CsPSY1 without the stop codon were amplified and inserted into pM999-35 (see detailed information about the constructs in Supplemental Table S15). The relative plasmids were extracted and purified using the Plasmid Midi Kit (QIAGEN). The isolation of the citrus leaf and the co-transformation of pairwise plasmids into citrus protoplasts were performed as described previously (Lu et al., 2018). GFP fluorescence was detected using a Leica TCS SP8 (Leica Microsystems) confocal laser scanning microscope with the following properties: laser, 488 nm; detector, PMT; intensity, 1.0005%; collection bandwidth, 505 to 545 nm; gain, 688.4. Chloroplasts were detected with the following properties: laser, 488 nm; detector, PMT; intensity, 0.4999%; collection bandwidth, 680–700 nm; gain, 613.9.
BiFC analysis and Y2H assay
In the Arabidopsis mesophyll protoplast system, full-length cDNAs from the CsSGR alleles and CsPSY1 genes without the stop codon were amplified using PCR with gene-specific primers (Supplemental Table S14) and cloned into pSPYCE-Bar containing the C-terminal half of YFPn, YFPc, and the pSPYNE-Kan vector containing the N-terminal half of YFPn), respectively (Walter et al., 2004; see detailed information about the constructs in Supplemental Table S15). Detailed methods for the isolation and transfection of Arabidopsis mesophyll protoplasts were described previously (Wu et al., 2009). In the N. benthamiana leaf system, full-length cDNAs from the CsSGR alleles and CsPSY1 genes without the stop codon were cloned into the Gateway vectors pCL112 containing the N-terminal half of YFPn and pCL113 containing the C-terminal half of YFPn, YFPc, respectively (Maloney et al., 2015; See detailed information about the constructs in Supplemental Table S15). Nicotiana benthamiana leaves were transformed with these plasmids. YFP was detected using a Leica TCS SP8 (Leica Microsystems) confocal laser scanning microscope with the following properties: laser, 514 nm; detector, HyD; intensity, 4.9998%; collection bandwidth, 516– 610 nm; gain, 75.9. Chloroplasts were detected with the following properties: laser, 514 nm; detector, PMT; intensity, 4.9998%; collection bandwidth, 650–750 nm; gain, 549.6. In the Y2H assay, the cDNAs sequences from the CsSGR alleles and CsPSY1 genes without the sequences encoding their transit peptides were amplified using PCR with gene-specific primers (Supplemental Table S14) and cloned into Nub and Cub plasmids, respectively (Zhou et al., 2015; see detailed information about the constructs in Supplemental Table S15). Plasmids were transformed into yeast strain THY.AP4 (for Nub constructs) or THY.AP5 (for Cub constructs) and mated with each other. The resulting diploid cells were grown and subcultured in a synthetic complete medium lacking both leucine and tryptophan (−LW). To detect interactions between Nub and Cub fusions, the diploid yeast cultures were serially diluted and grown for 2 d at 29°C on a selective medium lacking −leucine, tryptophan, adenine, and histidine but supplemented with 150-µM methionine. The detailed Y2H assay and the split ubiquitin system were used as described previously (Zhou et al., 2015).
Statistical analysis
The data were analyzed using the SAS statistical software to test for significant differences. Analysis of variance was used to compare the statistical difference based on Student’s t test at the significance levels of *P < 0.05 and **P < 0.01, respectively.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers listed in Supplemental Tables S13 and S14.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Assessment of color values (L*, a*, and b*) in developing fruit of WT and MT at various DAF.
Supplemental Figure S2. Chromatographic profiles of chlorophylls and carotenoids in WT and MT during fruit ripening.
Supplemental Figure S3. Transcriptome comparison of developing flavedos from MT and WT fruits.
Supplemental Figure S4. Chimeras on MT plants.
Supplemental Figure S5. Expression profiles of CsSGR.
Supplemental Figure S6. PCR-based sequence analysis of MT CsSGR alleles compared to WT.
Supplemental Figure S7. Nucleic acid sequence alignment of CsSGR alleles.
Supplemental Figure S8. Phylogenetic analysis of the SGR and SGRL amino acid sequences.
Supplemental Figure S9. Expression profiles of CsSGRL, CsSGRa, CsSGRb, CsPSY1 in different citrus fruit tissues, developmental stages, and species.
Supplemental Figure S10. Citrus CsSGRa transcript and protein levels.
Supplemental Figure S11. Mg-dechelating activity of CsSGRa.
Supplemental Figure S12. Phenotype of withered leaves in WT and MT.
Supplemental Figure S13. Functional analysis of plant CsPSY1 and CsSGR alleles in E. coli.
Supplemental Figure S14. Localization of CsSGR-GFP proteins and CsPSY1-GFP.
Supplemental Figure S15. Testing interaction between CsSGRa, CsSGRb, or CsSGRaSTOP with PSY from citrus and Arabidopsis.
Supplemental Figure S16. The construction of anti-SGR, the peptide used for raising polyclonal antibodies against SGR.
Supplemental Figure S17. Overexpression of anti-SGR.
Supplemental Figure S18. The antibody specificity of anti-SGR.
Supplemental Table S1. Chlorophylls and their concentrations in the flavedo of WT and MT during development and maturation.
Supplemental Table S2. Carotenoids and their concentrations in the flavedo of WT and MT during development and maturation.
Supplemental Table S3. DEGs from comparison of WT and MT.
Supplemental Table S4. Gene set 1A.
Supplemental Table S5. Gene set 1B.
Supplemental Table S6. DEGs from comparison of flavedo sectors in chimeric MT fruit.
Supplemental Table S7. Gene set 2.
Supplemental Table S8. All of the mutations identified using Whole Genome Sequence (WGS) analysis.
Supplemental Table S9. Gene set 3.
Supplemental Table S10. Sequencing confirmation of mutations in the eight candidate genes.
Supplemental Table S11. Citrus genes encoding enzymes required for chlorophyll degradation.
Supplemental Table S12. Citrus genes encoding enzymes required for carotenoid biosynthesis.
Supplemental Table S13. Protein accession numbers of SGR from various species.
Supplemental Table S14. Primers used in this study.
Supplemental Table S15. Constructs used in this study.
Supplemental Table S16. Plasmid combinations used for prokaryotic expression.
X.D. conceived and coordinated this project; K.Z., X.D., Q.X., J.Y., and X.Z. designed the experiments; K.Z. performed most of the experiments with contributions from X.Z., Y.H., H.C., and X.M.; L.C. and Z.X. collected citrus tissue samples and assisted with field management; K.Z. and E.T.W. analyzed the data; Y.Z., R.M.L., Q.X., J.Y., E.P.-R., M.T. and C.Z.-C. contributed to improvement of the manuscript. K.Z., X.D. and E.T.W. wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Xiuxin Deng (xxdeng@mail.hzau.edu.cn).
Acknowledgments
We thank Dr Misawa for providing the plasmid pACCRT-EIB and pACCAR16ΔcrtX, Dr Li Li for providing pXNgate21-3HA, pNXgate33-3HA, pMetYCgate, and pMetYCgate-AtPSY vectors.
Funding
The research in China was supported by the National Key Research and Development Program of China (2018YFD1000200) and National Natural Science Foundation of China (No. 31930095, 31630065). The research at Centro de Genomica in Spain was supported by the Ministerio de Ciencia, Innovación y Universidades (grant # RTI2018-097790-R-100); the Instituto Valenciano de Investigaciones Agrarias (grants 51915 and 52002), and also the Lehman College and The Graduate Center of the City University of New York and the New York Botanical Garden.
Conflict of interest statement. None declared.
References
Author notes
Senior authors.