-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew J Kittelson, Thomas J Hoogeboom, Margaret Schenkman, Jennifer E Stevens-Lapsley, Nico L U van Meeteren, Person-Centered Care and Physical Therapy: A “People-Like-Me” Approach, Physical Therapy, Volume 100, Issue 1, January 2020, Pages 99–106, https://doi.org/10.1093/ptj/pzz139
Close - Share Icon Share
Abstract
In health care, “person centeredness” is a valued (though nebulous) concept. In physical therapy, clinical interactions often strive to be person-centered, for example, by focusing on participation and valuing patient empowerment. However, the available evidence has mostly been constructed around populations (or study samples) rather than individuals. In this perspective, an alternative evidence framework is described, constructed around measurements in routine practice. Specifically, the authors propose developing “people-like-me” reference charts, generated with historical outcomes data, to provide real-time information on an individual’s status relative to similar people. The authors present an example of how this could work using their experience with people rehabilitating after total knee arthroplasty. They also describe several challenges that must be addressed to bring this innovation into practice. First, the most important outcome measures for stakeholders (eg, patients, clinicians) need to be identified and monitored longitudinally to ensure that “people-like-me” estimates are useful and support the goals of person-centered care. Statistical methods for selecting “people-like-me” need to be examined and refined. Finally, the “people-like-me” information needs to be packaged in such a way that it is accessible, intuitive, and helpful at the point of care. Ideally, the entire process should recognize from the outset that practice patterns evolve, so databases, statistical models, and decision tools should be dynamic by design. Ultimately, the authors propose this framework as a practical mechanism to advance person-centered decisions in physical therapy according to the ideals of evidence-based practice.
According to the Sicily Statement on Evidence-Based Practice (EBP), “healthcare decisions should be made by those receiving care, informed by the tacit and explicit knowledge of those providing care, within the context of available resources.”1 In this definition, the patient is in the lead, reflecting a cultural shift away from paternalism and toward person-centered health care. Person-centered health care has been referred to in a number of contexts and has taken on a variety of specific meanings: an emphasis on individualized approaches to care, a focus on patient participation rather than pathology, a recognition of the patient as an expert in her or his care, and an overall goal of patient empowerment.2 , 3 Recently, the American Geriatrics Society defined “person-centered care” as follows: “individuals’ values and preferences are elicited and, once expressed, guide all aspects of their healthcare, supporting their realistic health and life goals.” Thus, “person-centered” care and EBP are somewhat overlapping, visionary ideals, which are likely born out of (and potential solutions for) the major health challenges facing society (eg, increasing rates of chronic disease, aging population, rising health care costs).4 , 5 However, to ensure these ideals persist as more than just platitudes, practical, actionable strategies are needed.6
In particular, an evolution in research and clinical practice may be required.7 Traditionally, research and practice guidelines have been designed around study samples, with the researcher or health care provider (rather than the patient) in the lead. The most highly regarded experiments report group-level comparisons (eg, clinical trials, meta-analyses),8–10 and treatment recommendations are typically diagnosis-centered, relying on the recommendations of expert panels.11–13 These traditional sources of evidence have certain advantages; controlled studies are scientifically rigorous,14 and the simple act of standardizing treatments according to guidelines often results in improvements in the aggregate. Yet this reliance on group-level, expert-generated evidence arguably runs counter to the ideals of person-centeredness and EBP.15 , 16
In this perspective, we propose a possible route forward: a renewed emphasis in research and practice on the interpretation of outcome measures in clinical practice, with the goal of building an evidence base that utilizes an individual patient’s outcomes measurements to inform clinical decisions. It is our perspective that when an outcome measurement is made, it should be imbued with meaning; it should relate to the person’s goals, and it should enable judgments of how the person is progressing in care. Implicit in our proposal is the notion that patients and clinicians have a meaningful understanding of the clinical course. That is to say, prognosis for an outcome measure should be known as precisely as possible for individual patients to serve as a reference for measurements over time. Here, a “people-like-me” approach is conceptually appealing.17 The central idea is to use historical outcomes data from similar (past) patients as a template of what to expect for a new patient.18 This template can then be used in practice to inform patient expectations, augment clinical decisions, and provide an evidence-based framework for anchoring judgments of treatment success or failure over time. In the following sections, we describe this “people-like-me” approach and some associated challenges. We also present an example of how this could work in practice, using our experience with people undertaking rehabilitation following total knee arthroplasty (TKA).
The Importance of Monitoring Important Outcome Measures
Physical therapy draws on a rich tradition of outcomes research and epistemological work in the areas of disablement,19 functioning,20 , 21 and clinical decision-making.22 , 23 Still, there are opportunities to continue to advance the usefulness of outcome measures in practice. The alignment of outcome measures with the ideals of person-centeredness and EBP should be constantly scrutinized. How well does a particular outcome measure relate to a patient’s goals or participation in society? Does the act of performing this measurement help to inform the patient with the best available evidence? These questions are undoubtedly complicated by the heterogeneity of patient populations and the variability in peoples’ reasons for seeking care, and a single outcome measure cannot realistically serve all purposes.24 For example, outcome measures such as the global rating of change or the Patient Specific Functional Scale are desirable, because they allow people to report status according to personal goals and perceptions.25 , 26 However, measures such as these have also been criticized for being subjective, poorly responsive, or regarded as not credible.27 , 28 Measures of physical performance may be more sensitive to change (thus, potentially more useful for monitoring progress) and provide a different picture of patient functioning than is captured with patient-reported measures.29 , 30 Yet these measures are also surrogates rather than direct measures of participation and should be recognized as such. Ultimately, the discussion surrounding outcome measures and “person-centeredness” will be most productive if outcome measures are regularly used in practice to inform clinical decisions.
The routine adoption of outcome measures in busy clinical settings is itself a challenge. Although therapists are open to using outcome measures in practice, surveys suggest they are not frequently used. Moreover, the act of documenting outcome measures is often seen as a necessary chore to satisfy payers or to provide a defensible record of treatment decisions.31–36 Thus, measurements are sometimes used as bookends to an episode of care (to justify the need for therapy or to support a discharge decision) rather than to inform decisions at more frequent intervals.28 Additionally, the act of documentation is often made unnecessarily burdensome, with difficult-to-navigate electronic health records. With some creativity, user-centered design approaches could be harnessed to create health records that facilitate the documentation of outcome measures via easy interfaces, where the act of recording this measurement during a clinical interaction additionally generates information to guide decisions. Whereas Alan Jette called for therapists to “become interested in data” in his 2012 McMillan Lecture,37 perhaps innovations in psychometrics research, technology, and analytics could be used in turn to make data more interesting and useful to patients and therapists.
People-Like-Me Reference Charts: a Novel Framework for Monitoring
To support person-centeredness and individualized care, clinical measurements should have context; how does a given measurement compare with what is expected for a particular person? In health care, one of the most well-known, empirically derived decision-making frameworks is the reference chart used to monitor infant growth. With this framework, the individual growth of an infant is plotted against the growth of a representative sample of similarly aged infants. Using the reference chart, both parents and providers can monitor the growth progression and—if necessary—adjust management strategies (eg, feeding schedule) when the growth trajectory deviates substantially from the expected curve. Importantly, the role of the provider in this scenario is not to apply an intervention per se (the pediatrician does not feed the infant) but to guide management strategies for the infants’ parents. We envision possibilities for a similar approach in physical therapy, where the therapist serves as a guide to the patients’ self-management (eg, through advice regarding exercise content and intensity) utilizing reference charts derived from clinically collected outcomes data.
How would this look in physical therapy practice? Consider what is learned from a Timed Up and Go (TUG) test performed at an initial clinical evaluation. TUG measurements may be used to establish the baseline status of the patient or perhaps to determine fall risk according to published cut-offs. However, since the published data are largely represented in the aggregate, it could also be argued that the single baseline measurement adds quite little to the understanding of the patient’s actual status or potential to change over time. We propose to put this observation into a people-like-me context, for example, by plotting on a reference chart of TUG times from similar patients. With this approach, both the therapist and patient get a better understanding of the current status and likely clinical course. Over time, patients and therapists might also be able to make informed judgements on the success and failure of treatment by monitoring the patient’s TUG time relative to the reference derived from people-like-me.
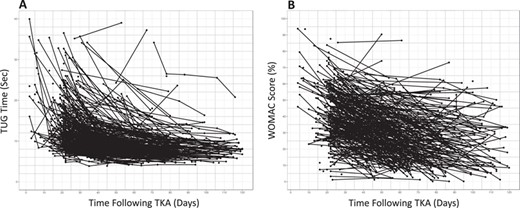
Clinically collected outcomes data from people following total knee arthroplasty (TKA) surgery for the (A) Timed Up and Go (TUG) test (n = 244) and (B) Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index (n = 203). The heterogeneity in outcomes is apparent, illustrating the limitations of diagnosis-based protocols for decisions with individual patients. For the purposes of the example in this Perspective, deidentified outcomes data (collected in routine practice at ATI Therapy, Greenville, SC, USA) were accessed retroactively for the TUG test and the WOMAC Osteoarthritis Index.
Identifying people-like-me and modeling measurements over time presents some methodological challenges. This is an area worthy of continued research, although a few compelling templates already exist. In psychotherapy, so-called Estimated Treatment Response (ETR) projections have been developed using nonlinear models (mixed effects models).38 , 39 These equations allow for calculation of an individual’s predicted outcome measurement at a given future time point based on the measurement at initial evaluation.39 The expected rate of change can also be adjusted at the individual level given certain patient characteristics. During therapy, the ETR models can be used to calculate the centile position of the patient (relative to his or her peers) at any particular treatment session, which may inform the discussion of therapy success and failure over time. These ETR models are built at a population level and adjusted for the individual. An alternative would be to select a patient’s peers (ie, people-like-me) from a historical databank using statistical approaches (eg, k-nearest neighbors analysis) and then utilize the recorded outcome measures from only these peers to model the expected trajectory for a patient.17 , 18 Various modelling techniques (eg, quantile regression, generalized additive models) are widely available in statistical packages.18 One possible advantage of this approach compared with the ETR approach is that the modelling would be built around empirical data of people-like-me, whereas population-level models—even with adjustments for covariates—might not be flexible enough to produce trajectory estimates that accurately reflect what is observed in reality.17 Regardless of the specific approach selected, the information needs to be packaged in such a way that it can be seamlessly accessed in practice. The statistical modelling should simply live in the background of the clinical tool and any complexity in terms of calculations should be automated for the user.
Example: Rehabilitation for People Following TKA
To illustrate how this people-like-me approach might work, we have accessed clinical data from patients rehabilitating after TKA surgery, as it is the most commonly performed inpatient elective procedure and is also a common indicator for physical therapy.40 There are no agreed-upon practice standards for postoperative care or rehabilitation following TKA, although treatments are traditionally protocol based. These treatment protocols are diagnosis based and contrast with the ideals of person-centeredness. They fail to accommodate the heterogeneity in patients’ initial presentations, recovery trajectories, and reasons that people elect to have surgery. Postoperative protocols also struggle to provide meaningful benchmarks for functional recovery. Since functional goals are critically dependent on the characteristics of the person, it is problematic to set objective functional goals in a diagnosis-based protocol.
For this example, we have retrospectively accessed de-identified outcomes data for the TUG test and the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index. These data were collected in routine practice at ATI Therapy (Greenville, SC, USA). The WOMAC is a 24-item questionnaire that assesses patient reports of pain, stiffness, and level of difficulty with daily activities via a 5-point (0–4) Likert scale.41 The total WOMAC score is calculated by summing an individual’s responses to all the questions and dividing by 96 possible points to generate a percentage between 0 (no functional limitation) and 100 (maximum limitation). The TUG test assesses the speed with which a patient can rise from a chair, walk a distance of 3 m, pivot, walk back to the chair, and return to a seated position. A total of 266 patient records for the TUG and 268 patient records for the WOMAC were available for this illustration. Whether these 2 outcome measures adequately capture what is most important to patients and therapists is debatable (and should be debated), but we can use them to illustrate how people-like-me references might be helpful in practice.
The people-like-me approach
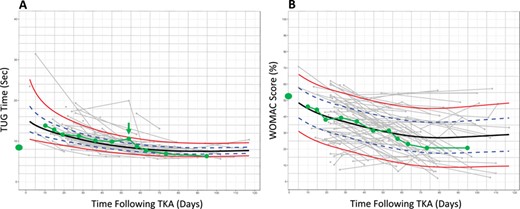
Suppose a person considering TKA surgery sees a physical therapist for a preoperative consultation and asks his or her therapist what to expect following surgery. The therapist administers a TUG test and WOMAC questionnaire to assess the patient’s preoperative status. Historical TUG and WOMAC data suggest substantial heterogeneity in functional recovery following surgery (Fig. 1), which itself could be informative and stimulate a discussion. However, the people-like-me reference chart, built using outcomes data from 100 similar patients (Fig. 2), provides more context. Suppose these charts could be generated automatically during the clinical interaction. The therapist could share this information with the patient, orienting him or her to the reference charts and pointing out, for example, the extent of immediate postoperative worsening, the timing of a return to preoperative status, and at what point functional recovery is expected to plateau.
People-like-me reference charts for a single patient for the (A) Timed Up and Go (TUG) test and (B) Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index. Similar previous patients were identified on the basis of preoperative status. The green line illustrates outcomes monitoring for a hypothetical patient. A deviation in the TUG trajectory is noted at the 8-week visit (arrow). For the purposes of the example in this Perspective, deidentified outcomes data (collected in routine practice at ATI Therapy, Greenville, SC) were accessed retroactively for the TUG test and the WOMAC Osteoarthritis Index.
After surgery, the charts could be used during therapy to monitor recovery and detect deviations in the expected course. For example, in our illustration, the 8-week TUG time is slightly slower than the previous visit (Fig. 2). Additionally, whereas the patient’s TUG times had been at (or near) the median of people-like-me from weeks 3 to 6, the 8-week TUG observation is at the 85th percentile (among the slowest 15% of his or her peers). This could inform a conversation between the patient and the therapist regarding possible changes to the plan of care. For example, the therapist might recommend increasing the frequency of visits in the short term. Finally, the recovery plateau is clearly visible on the chart, potentially informing difficult decisions such as when to stop physical therapy care.
Limitations to this example
This case is hypothetical; the details were manufactured for the purposes of illustration. However, the decision-making framework is entirely possible in practice. The people-like-me reference charts were built from clinically collected data using open-source statistical modeling techniques (GAMLSS package, R Foundation for Statistical Computing, Vienna, Austria, 2018).42 The people-like-me selection was done using only preoperative status; the 100 patient records in the dataset with preoperative TUG times or WOMAC scores most similar to the hypothetical patient were selected, and postoperative data from those records were used to construct the charts. It would be interesting to investigate alternative selection strategies (eg, k-nearest neighbors) capable of incorporating additional patient characteristics (eg, age, sex, health status) to see if more precise estimates can be achieved. The number of people-like-me matches required to produce useful estimates is also an open question. Here we used 100 matches, but it is possible that more precise estimates could be achieved with fewer matches or with a larger dataset.
This example utilizes only patient records containing both pre- and postoperative data. Therefore, patients who did not attend postoperative rehabilitation (eg, because of a postoperative complication or because they decided not to have surgery) are not reflected in the estimates. Thus, there are limitations to the use of this approach for informed surgical decision-making, as the alternative clinical course (ie, what would be the estimated trajectory if a patient were to elect not to have surgery) cannot be illustrated, and the risk of experiencing postoperative complications may not be adequately captured with the current data. These limitations could inform the type of data that are collected in clinical settings moving forward (eg, incidence of postoperative complications, hospital discharge status, etc.). There is also the question of whether postoperative data are biased (eg, whether low- or high-performing patients are more likely to have a greater number of data points documented). It would be possible to probe the dataset to illuminate these biases and communicate any limitations to therapists and patients. Importantly, the advantage of this approach is that end-users (patients and providers) and researchers can work from the same dataset with the same goals in mind. This is likely to encourage the overall person-centeredness of the evidence and contrasts with traditional research approaches where end-users of the evidence are not involved in its derivation and are rarely privy to all the potential biases and pitfalls.
The validity of the people-like-me estimates might also be questioned. Do these estimates adequately match the realized observations that occur prospectively? It seems possible (perhaps likely) that the mere presence of the people-like-me framework could affect measurements, through changes in treatment plans, goal setting, or for a variety of reasons. Therefore, it would be important to build dynamic validation procedures, where the performance of the charts could be regularly adjudicated and the relevance of the modelling approach and underlying dataset to the end-users could be periodically maintained (eg, on a quarterly basis). This would require the integration of research and data management expertise within the clinical practice setting.
Use of this approach in practice
There are several challenges to adopting this approach in practice. One important consideration is how the charts would be interpreted by patients and providers. For example, what if a patient is presented with an illustration of a poor prognosis? Would the patient become motivated to prove the prognosis wrong, or would it be demoralizing? It could be argued that the mere opportunity to encounter this situation is an advance in person-centered care.43 However, there is also evidence from the pediatric literature that difficulties with the implementation might be expected. Interpretation of childhood growth charts varies across parents and health care professionals, and low parental numeracy is related to less accurate understanding of growth charts.44–47 These problems have prompted a number of studies aimed at improving the use of growth charts in pediatric care. Separate growth charts have been created for screening versus monitoring purposes. Innovations in design have been examined (eg, de-emphasizing the 50th centile to better illustrate the wide range of normal growth), and targeted educational modules have been developed.48 , 49 It would be wise to learn from these experiences, to anticipate potential difficulties with numeracy and literacy, and draw inspiration from past successes in other health care disciplines.
Ultimately, we envision the people-like-me approach as a bridge to empowering patient self-management and monitoring. For example, imagine that a patient is provided with a reference chart, a home-based therapy program, and the ability to monitor his or her own recovery. Both the therapist (remotely) and the patient could observe measurements relative to the expected recovery over time (perhaps via uploads to the electronic health record), and the patient or therapist would have the opportunity to initiate a conversation in the event of an unanticipated deviation. This could reduce the burden and cost of unnecessary follow-up visits and might also allow providers and payers to reserve resources for longer-term monitoring or treatment. It is important to keep in mind that people are only administratively labelled as patients upon entering the clinic. The rest of the time they are people, managing their own health and attempting to adapt to new challenges. The structure of evidence and practice should inform health decisions where and when they are required, regardless of the physical location of the patient.
Conclusion
The difficulty in adopting person-centered practices may be partly a function of habit or tradition, as clinical reasoning in rehabilitation has historically been driven by the research community and enacted with little input from patients.50 Although some have advocated for a shift in the power structure of health care (where the patient is entirely in the lead), actionable strategies for achieving person-centered care remain challenging to envision.51 We have presented a framework for clinical research and practice where the evidence is designed to inform the interpretation of outcome measurements with individual patients. To realize this approach, we have outlined several necessary steps. First, the most important outcomes must be identified and monitored. Electronic health records should be constructed to facilitate documentation of these outcomes, and the act of monitoring these outcomes should be useful. Relevant information should be clearly and intuitively presented to the clinician and patient in real time. People-like-me reference charts are 1 possible vehicle for person-centered information regarding prognosis and progress. We have described the methods for generating people-like-me estimates as well as possible limitations and pitfalls.
Author Contributions and Acknowledgments
Concept/idea/research design: A.J. Kittelson, T.J. Hoogeboom, M. Schenkman, J.E. Stevens-Lapsley, N.L.U. van Meeteren
Writing: A.J. Kittelson, T.J. Hoogeboom, M. Schenkman, J.E. Stevens-Lapsley, N.L.U. van Meeteren
Data analysis: A.J. Kittelson, N.L.U. van Meeteren
Project management: A.J. Kittelson, T.J. Hoogeboom, J.E. Stevens-Lapsley, N.L.U. van Meeteren
Fund procurement: A.J. Kittelson, J.E. Stevens-Lapsley
Providing facilities/equipment: M. Schenkman
Consultation (including review of manuscript before submitting): T.J. Hoogeboom
The authors wish to acknowledge Dawn Waugh, Jacqueline Davenport, Charles Thigpen, and the physical therapists at ATI therapy in Greenville, SC, for their collaboration on this work.
Funding
A. Kittelson and J. Stevens-Lapsley received grants from the National Institutes of Health (T32 AG000279), the Agency for Health-Related Quality (R03 HS024316, R01 HS025692), and the National Institutes of Health (K12 HD055931) related to this Perspective.
Role of Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
Author notes
A.J. Kittelson and T.J. Hoogeboom contributed equally to the writing of this perspective manuscript.




Comments