-
PDF
- Split View
-
Views
-
Cite
Cite
Chad Cook, Jean-Michel Brismée, Robert Fleming, Phillip S Sizer, Identifiers Suggestive of Clinical Cervical Spine Instability: A Delphi Study of Physical Therapists, Physical Therapy, Volume 85, Issue 9, 1 September 2005, Pages 895–906, https://doi.org/10.1093/ptj/85.9.895
Close - Share Icon Share
Abstract
Background and Purpose Clinical cervical spine instability (CCSI) is controversial and difficult to diagnose. Within the literature, no clinical or diagnostic tests that yield valid and reliable results have been described to differentially diagnose this condition. The purpose of this study was to attempt to obtain consensus on symptoms and physical examination findings that are associated with CCSI. Subjects One hundred seventy-two physical therapists who were Orthopaedic Certified Specialists (OCS) or Fellows of the American Academy of Orthopaedic Manual Physical Therapists (FAAOMPT) participated in the survey. Methods This study was a 3-round Delphi survey designed to obtain consensual symptoms and physical examination findings for CCSI. Results The symptoms that reached the highest consensus among respondents were “intolerance to prolonged static postures,” “fatigue and inability to hold head up,” “better with external support, including hands or collar,” “frequent need for self-manipulation,” “feeling of instability, shaking, or lack of control,” “frequent episodes of acute attacks,” and “sharp pain, possibly with sudden movements.” The physical examination findings related to cervical instability that reached the highest consensus among respondents included “poor coordination/neuromuscular control, including poor recruitment and dissociation of cervical segments with movement,” “abnormal joint play,” “motion that is not smooth throughout range (of motion), including segmental hinging, pivoting, or fulcruming,” and “aberrant movement.” Discussion and Conclusion The Delphi method is useful in situations where clinical judgments are encountered but empirical evidence to provide evidence-based decision making does not exist. Findings of this study may provide beneficial clinical information, specifically when the identifiers are clustered together, because no set of clinical examination and symptom standards for CCSI currently exists. Diagnosis of CCSI is challenging; therefore, appropriate clinical reasoning is required for distinctive physical therapy assessment using pertinent symptoms and physical examination findings.
Cervical spine pain is a common musculoskeletal condition reportedly affecting 70% of people within their lifetime.1 Instability is one element of cervical pain and may contribute to the clinical presentation of various conditions, including cervicogenic headaches,2,3 chronic whiplash dysfunction,4,5 rheumatoid arthritis,6 osteoarthritis,7 and segmental degeneration.8 Situations involving trauma,9,10 genetic predisposition,11 disk degeneration,12 and surgery13,14 may compromise the stabilizing mechanisms of the cervical spine.
It has been suggested that different categories of cervical instability exist.15,16 Radiographically appreciable cervical spine instability (RACSI) may lead to compression of neural or vascular structures,17 pain,18 and neurological signs and symptoms.19 In most cases, RACSI reflects marked disruption of passive osseoligamentous anatomical constraints and hypermobility.20–23
Panjabi13,14 proposed that spinal stability is a component of 3 interactive subsystems: passive, active, and neural. The 3 systems work in concert to provide dynamic stability during the application of external forces. Instability may occur when the active and neural subsystems fail to maintain control within the intervertebral neutral zone of the cervical spine.15 Unlike RACSI, dysfunction of the active and neural subsystems is more appropriately described as an abnormality of movement rather than hypermobility22,24 and can present indicators of instability in the absence of passive system (osseoligamentous) pathology. These indicators may include cervical pain,25 aberrant cervical movements,26 referred shoulder pain,26,27 radiculopathy or myelopathy,28 paraspinal muscle spasms, decreased cervical lordosis,26 tinnitus,29 pain during sustained postures,26 complaints of “catching” or “locking,”16,25,30,31 and altered range of motion.16,25,30,31 In addition, a history of major trauma or repetitive microtrauma may predate report of symptoms.25
Within the literature, instability associated with active and neural cervical subsystem failure is identified as clinical cervical spine instability (CCSI), but it also has been characterized as nonradiographic or minor cervical instability.25,32 Clinical cervical spine instability may demonstrate only subtle symptoms and clinical examination features25,32 and frequently normal radiographic findings.33–35 At present, although numerous diagnostic identifiers are suggested for CCSI, a valid and effective criterion standard does not exist. Consequently, the condition is speciously associated with degeneration,4 kinematic measurements of anterior to posterior shear,4 abnormal or excessive coupling of the cervical spine,36 and unquantifiable physical examination findings.36,37
The purpose of our study was to obtain consensus of symptoms and physical examination findings associated with CCSI. Using a Delphi method survey, expert practitioners consensually outlined common symptoms and physical examination findings of CCSI. The consensus agreement could be used to enhance the knowledge base required in clinical reasoning during differential diagnosis.
Method
Study Design
Our study used a Delphi survey instrument that incorporated both a work group and a respondent group.
Subjects
Respondent group
The population selected for the study consisted of volunteers from 2 “expert” categories. The first group was all board-certified Orthopaedic Certified Specialists (OCS) from the American Physical Therapy Association (APTA) who identified cervical and lumbar dysfunction as their primary practice specialty. The second group targeted was all Fellows of the American Academy of Orthopaedic Manual Physical Therapists (FAAOMPT). This group was targeted because of their clinical expertise obtained through residency or fellowship preparation and because members of the group are acknowledged by the American Academy of Orthopaedic Manual Physical Therapists (AAOMPT) for recognized competence and expertise in the practice of orthopedic manual physical therapy.38 All targeted participants were contacted using traditional direct mail and e-mail (when possible) and then were pooled into a single group upon their agreement to participate.
Work group
The work group comprised those investigators who summarized the returned data from round 1 and redesigned the follow-up instruments. This group contained 3 investigators, including the primary investigator (CC) and 2 investigators (JMB and PSS) who were experienced in qualitative research. All principal work group members were board-certified orthopedic physical therapists with a minimum of 14 years and an aggregate of 51 years of research and clinical experience in orthopedic manual therapy. The primary investigator was a certified manual physical therapist with an emphasis on the Maitland/Australian approach to manual therapy, and the other 2 investigators were certified within the International Academy of Orthopaedic Medicine. All investigators had various levels of training in other orthopedic manual therapy models, including McKenzie, Cyriax, Kaltenborn, Paris, Grimsby, and the osteopathic model. The 2 coinvestigators were Fellows of the AAOMPT.
Procedure
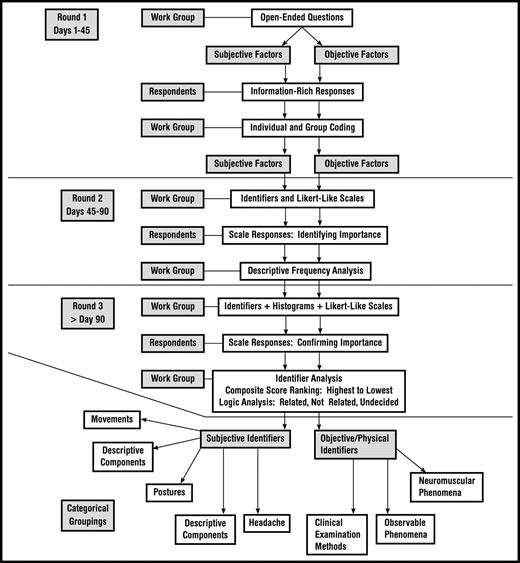
This Delphi survey consisted of 3 rounds of questionnaires that respondents consecutively answered as illustrated in Figure 1.39,40 Invitations to round 1 of the study were distributed through e-mail for the OCS group and direct mail for the FAAOMPT group. Each invitation, e-mail, and direct mail provided a Web address link to the Web-based consent form and survey. Potential respondents who did not answer the request for participation were sent a reminder notice to encourage participation using a method suggested by Dillman.41 Two consecutive follow-up reminders were delivered at 10 and 20 days after the initial invitation was sent.42–44 Invitations to rounds 2 and 3 of the instrument were automatically distributed through e-mail to all respondents from round 1, providing the respondents with a Web link to the appropriate survey.
Instrument
The instrument used in round 1 of the survey included questions regarding basic demographic information and open-ended questions related to symptoms and physical examination findings for patients with CCSI. After defining CCSI, the first open-ended question in round 1 queried respondents to distinguish the symptoms they deemed to be associated with CCSI. The second open-ended question queried respondents to distinguish physical examination findings they believed to be associated with CCSI. The responses to the open-ended questions provided the multiple identifiers used for rounds 2 and 3. The symptoms and physical examination findings used throughout the 3 rounds were selected solely by the Delphi survey participants and were not generated by the work group.
The invitation to round 1 included specific directions and an operational definition of CCSI: “painful hypermobility, inappropriate dynamic control, and/or nonradiographic instability.” For the sake of classification, we directed the respondents to consider symptoms as “activities that result in pain and the nature of that pain: Examples include the immediate onset of headaches during extension or pain that occurs through range of motion.” Physical examination findings were defined as “activities, motions, and movement patterns that are uniquely identifiable for cervical spine instability: Examples include reduced willingness to volitionally move the head, or forward head posture.”
The instrument used in round 2 of the survey was a list of the symptoms and physical examination findings constructed from the work group's qualitative analysis of the responses from round 1. The purposes of round 2 were to allow respondents to (1) review the categories of responses from round 1 for clarification and correction of terminology and (2) identify the most important identifiers related to the diagnosis of clinical instability of the cervical spine. Respondents were instructed to use a 5-point Likert scale to score each of these identifiers in terms of their level of agreement that the identifier was related to CCSI. Demographics were not collected during round 2, because much of the information was redundant to that from round 1.
The instrument used in round 3 of the survey contained the same identifier list and rating scale used in round 2, with additional tables and graphs demonstrating the descriptive statistical score outcomes for each identifier statement. Figure 2 depicts an example of a graphic representation similar to those used during round 3. The graphic information identified the percentage of total respondents who selected each possible score for the given item in round 2. The respondents were instructed to re-score each identifier with the scale after viewing the scoring results from round 2. Consequently, round 3's list of CCSI identifiers included a re-score of the same identifiers from round 2, only after each respondent reviewed the round 2 scores of the other respondents.
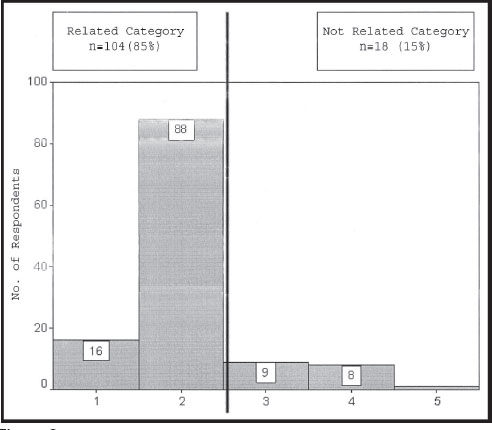
Example of a consensus-scoring tally indicating consensus or no consensus. Identifier displayed is “catching, clicking, clunking, and popping sensation.” 1=strongly agree, 2=agree, 3=not applicable, 4=disagree, and 5=strongly disagree.
Data Analysis
After respondents completed each round, the WebSurveyor program* automatically downloaded response data onto a spreadsheet for work group analyses. The tally of scores for “strongly disagree” and “disagree” represented the total percentage of scores in the “Not Related” category, meaning that the symptoms or physical examination findings were not important for the diagnosis of cervical spine instability. Conversely, the tally of scores for “strongly agree” and “agree” were placed in the “Related” category, meaning that the particular identifiers for symptoms or physical examination findings were important for that diagnosis. Consensus was established if 75% or more of the respondents39 scored the symptoms or physical examination findings as “Consensus, Not Related” or “Consensus, Related.” Figure 2 provides an example of a consensus-scoring tally.
If the tally for “Not Related” or “Related” was between 50% and 74.9%, consensus was not established and a decision was forced among “Near-Consensus, Not-Related,” “Near-Consensus, Related,” and “Undecided.”45 A logic analysis was conducted in order to derive a decision among “Near-Consensus, Related,” “Near-Consensus, Not Related,” and “Undecided.”45 If the tally for “strongly agree” and “agree” was greater than the tally for “strongly disagree” and “disagree,” the identifier was labeled as “Near-Consensus, Related.” Similarly, if the tally for “strongly disagree” and “disagree” was greater than the tally for “agree” and “strongly agree,” the identifier was labeled as “Near-Consensus, Not Related.” However, if the tally for “agree” and “disagree” was greater than the tally for “strongly agree” and agree” or the tally for “strongly disagree” and “disagree,” the identifier was labeled as “Undecided.”
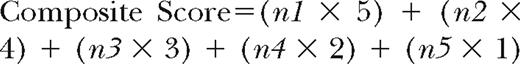
The identifiers for symptoms or physical examination findings were tallied as:
n1=number of respondents who scored the identifier as “strongly agree”
n2=number of respondents who scored the identifier as “agree”
n3=number of respondents who scored the identifier as “undecided”
n4=number of respondents who scored the identifier as “disagree”
n5=number of respondents who scored the identifier as “strongly disagree”
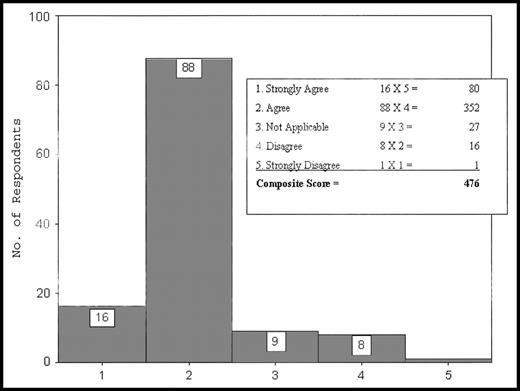
For clarification purposes, a graphic example of this composite score tally is presented in Figure 3. The composite score value for each identifier was derived from the tally of scores. For example, the identifier in Figure 3 was assigned a composite score of 476. This composite score then was compared with the composite scores of the other symptoms or physical examination findings to establish rank or priority for each heading. The highest score represented the identifer that the respondent group outlined as most explicit for CCSI.
Composite score tally sheet. The text bar represents the calculations associated with composite score ranking. The total composite score then is compared with the scores of other identifiers.
The respondents assigned scores both without (round 2) and with (round 3) graphic feedback from the other respondents; therefore, it was expected that changes might occur between rounds. We used Megastat, version 9.0,† and a Mann-Whitney U test (α=.05) to compare ranked scores between rounds 2 and 3 for both symptoms and physical examination findings.
Results
Round 1
We solicited 1,111 Orthopedic Certified Specialists from APTA and 334 Fellows of the AAOMPT (1,445 total) for participation in the study. Microsoft Outlook Express, version 6.1,‡ identified 92 potential respondents who were inaccessible because of incorrect e-mail address, server difficulties, or relocation without a new address. One hundred seventy-two clinicians (11.9%; mean age=42.3 years, range=27–61 years) responded to round 1. These respondents reported 3 to 39 years of physical therapist practice (X̄=17.5 years). Ninety-six respondents were male and 72 were female; 4 respondents failed to answer this question. One hundred seven respondents (64%) indicated that 50% or more of their clinical practice time was spent in a non-hospital-based outpatient clinical practice. Table 1 outlines pertinent respondent data.
Respondent Characteristicsa
| Age | X̅=42.3 y Range=27–61 y Missing values=3 | |
| Sex | Male=96 Female=72 Missing values=4 | |
| Credentials | FAAOMPT=66 OCS=78 Both=49 Missing values=28 | |
| Experience | X̅=17.5 y Range=3–39 y Missing values=3 | |
| Work setting | >50% of clinical time in nonhospital-based outpatient setting=107 >50% of clinical time in hospital- based outpatient setting=38 Missing values=27 | |
| Reported background | Grimsby | 4.12% |
| Kaltenborn | 8.24% | |
| Maitland | 24.12% | |
| McKenzie | 14.71% | |
| NA | 0% | |
| NAIOMPT | 7.65% | |
| Osteopathic | 19.41% | |
| Other | 8.24% | |
| Paris | 12.35% | |
| Winkel | 1.18% | |
| Age | X̅=42.3 y Range=27–61 y Missing values=3 | |
| Sex | Male=96 Female=72 Missing values=4 | |
| Credentials | FAAOMPT=66 OCS=78 Both=49 Missing values=28 | |
| Experience | X̅=17.5 y Range=3–39 y Missing values=3 | |
| Work setting | >50% of clinical time in nonhospital-based outpatient setting=107 >50% of clinical time in hospital- based outpatient setting=38 Missing values=27 | |
| Reported background | Grimsby | 4.12% |
| Kaltenborn | 8.24% | |
| Maitland | 24.12% | |
| McKenzie | 14.71% | |
| NA | 0% | |
| NAIOMPT | 7.65% | |
| Osteopathic | 19.41% | |
| Other | 8.24% | |
| Paris | 12.35% | |
| Winkel | 1.18% | |
FAAOMPT=Fellow of the American Academy of Orthopaedic Manual Physical Therapists, OCS=Orthopaedic Certified Specialist, NAIOMPT=North American Institute of Orthopaedic Manual Therapy, NA=not applicable.
Respondent Characteristicsa
| Age | X̅=42.3 y Range=27–61 y Missing values=3 | |
| Sex | Male=96 Female=72 Missing values=4 | |
| Credentials | FAAOMPT=66 OCS=78 Both=49 Missing values=28 | |
| Experience | X̅=17.5 y Range=3–39 y Missing values=3 | |
| Work setting | >50% of clinical time in nonhospital-based outpatient setting=107 >50% of clinical time in hospital- based outpatient setting=38 Missing values=27 | |
| Reported background | Grimsby | 4.12% |
| Kaltenborn | 8.24% | |
| Maitland | 24.12% | |
| McKenzie | 14.71% | |
| NA | 0% | |
| NAIOMPT | 7.65% | |
| Osteopathic | 19.41% | |
| Other | 8.24% | |
| Paris | 12.35% | |
| Winkel | 1.18% | |
| Age | X̅=42.3 y Range=27–61 y Missing values=3 | |
| Sex | Male=96 Female=72 Missing values=4 | |
| Credentials | FAAOMPT=66 OCS=78 Both=49 Missing values=28 | |
| Experience | X̅=17.5 y Range=3–39 y Missing values=3 | |
| Work setting | >50% of clinical time in nonhospital-based outpatient setting=107 >50% of clinical time in hospital- based outpatient setting=38 Missing values=27 | |
| Reported background | Grimsby | 4.12% |
| Kaltenborn | 8.24% | |
| Maitland | 24.12% | |
| McKenzie | 14.71% | |
| NA | 0% | |
| NAIOMPT | 7.65% | |
| Osteopathic | 19.41% | |
| Other | 8.24% | |
| Paris | 12.35% | |
| Winkel | 1.18% | |
FAAOMPT=Fellow of the American Academy of Orthopaedic Manual Physical Therapists, OCS=Orthopaedic Certified Specialist, NAIOMPT=North American Institute of Orthopaedic Manual Therapy, NA=not applicable.
Rounds 2 and 3
Twenty-eight subjects did not leave e-mail contact information during round 1; therefore, only 140 of the 172 respondents were contacted for participation in round 2. One hundred thirty-three respondents (81.4% retention rate between rounds 1 and 2; 9.7% overall response rate) completed round 2, and 122 respondents (70.9%) completed round 3, producing a 92% retention rate between rounds 2 and 3 and an overall response rate of 8.4%. The total composite score tallies for rounds 2 and 3 are reported in Table 2 for symptom identifiers and Table 3 for physical examination finding identifiers.
Symptoms of Consensus and Rank Outcomes for Clinical Cervical Spine Instability (CCSI), Listed in Descending Rank
| Identifier . | Round 3 Consensus Statusa . | Round 2 Composite Score . | Round 3 Composite Score . |
|---|---|---|---|
| Intolerance to prolonged static postures | CR | 481 | 502 |
| Fatigue and inability to hold head up | CR | 464 | 499 |
| Better with external support, including hands or collar | CR | 487 | 493 |
| Frequent need for self-manipulation | CR | 466 | 488 |
| Feeling of instability, shaking, or lack of control | CR | 464 | 485 |
| Frequent episodes of acute attacks | CR | 466 | 483 |
| Sharp pain, possibly with sudden movements | CR | 470 | 481 |
| Head feels heavy | CR | 473 | 480 |
| Neck gets stuck, or locks, with movement | CR | 462 | 479 |
| Better in unloaded position such as lying down | CR | 449 | 476 |
| Catching, clicking, clunking, and popping sensation | CR | 462 | 476 |
| Past history of neck dysfunction or trauma | CR | 480 | 476 |
| Trivial movements provoke symptoms | CR | 456 | 469 |
| Muscles feel tight or stiff | CR | 464 | 467 |
| Unwillingness, apprehension, or fear of movement | CR | 435 | 462 |
| Temporary improvement with clinical manipulation | CR | 442 | 464 |
| Increased pain as day progresses | NCR | 445 | 453 |
| Complaints of dull ache | U | 438 | 443 |
| Reports of sleep disturbances | U | 438 | 439 |
| Inconsistency of symptoms, including pain that shifts from side to side | U | 425 | 435 |
| Feeling that head is disconnected from neck | U | 416 | 433 |
| Complaints of headache | U | 436 | 430 |
| History of disorder or syndrome, such as Ehlers-Danlos syndrome, Marfan syndrome, or Down syndrome | U | 401 | 395 |
| Pain with the initiation of motion | U | 363 | 385 |
| Pain through the range of motion | U | 372 | 355 |
| Vertebrobasilar insufficiency symptoms that include dizziness, diplopia, drop attacks, and nausea | U | 371 | 352 |
| Spinal cord symptoms with neck movement | U | 361 | 325 |
| Temporomandibular joint symptoms | U | 343 | 323 |
| Cervical instability does not exist | CNR | 190 | 157 |
| Identifier . | Round 3 Consensus Statusa . | Round 2 Composite Score . | Round 3 Composite Score . |
|---|---|---|---|
| Intolerance to prolonged static postures | CR | 481 | 502 |
| Fatigue and inability to hold head up | CR | 464 | 499 |
| Better with external support, including hands or collar | CR | 487 | 493 |
| Frequent need for self-manipulation | CR | 466 | 488 |
| Feeling of instability, shaking, or lack of control | CR | 464 | 485 |
| Frequent episodes of acute attacks | CR | 466 | 483 |
| Sharp pain, possibly with sudden movements | CR | 470 | 481 |
| Head feels heavy | CR | 473 | 480 |
| Neck gets stuck, or locks, with movement | CR | 462 | 479 |
| Better in unloaded position such as lying down | CR | 449 | 476 |
| Catching, clicking, clunking, and popping sensation | CR | 462 | 476 |
| Past history of neck dysfunction or trauma | CR | 480 | 476 |
| Trivial movements provoke symptoms | CR | 456 | 469 |
| Muscles feel tight or stiff | CR | 464 | 467 |
| Unwillingness, apprehension, or fear of movement | CR | 435 | 462 |
| Temporary improvement with clinical manipulation | CR | 442 | 464 |
| Increased pain as day progresses | NCR | 445 | 453 |
| Complaints of dull ache | U | 438 | 443 |
| Reports of sleep disturbances | U | 438 | 439 |
| Inconsistency of symptoms, including pain that shifts from side to side | U | 425 | 435 |
| Feeling that head is disconnected from neck | U | 416 | 433 |
| Complaints of headache | U | 436 | 430 |
| History of disorder or syndrome, such as Ehlers-Danlos syndrome, Marfan syndrome, or Down syndrome | U | 401 | 395 |
| Pain with the initiation of motion | U | 363 | 385 |
| Pain through the range of motion | U | 372 | 355 |
| Vertebrobasilar insufficiency symptoms that include dizziness, diplopia, drop attacks, and nausea | U | 371 | 352 |
| Spinal cord symptoms with neck movement | U | 361 | 325 |
| Temporomandibular joint symptoms | U | 343 | 323 |
| Cervical instability does not exist | CNR | 190 | 157 |
CR=Consensus, Related; NCR=Near-Consensus, Related; CNR=Consensus, Not Related; U=Undecided.
Symptoms of Consensus and Rank Outcomes for Clinical Cervical Spine Instability (CCSI), Listed in Descending Rank
| Identifier . | Round 3 Consensus Statusa . | Round 2 Composite Score . | Round 3 Composite Score . |
|---|---|---|---|
| Intolerance to prolonged static postures | CR | 481 | 502 |
| Fatigue and inability to hold head up | CR | 464 | 499 |
| Better with external support, including hands or collar | CR | 487 | 493 |
| Frequent need for self-manipulation | CR | 466 | 488 |
| Feeling of instability, shaking, or lack of control | CR | 464 | 485 |
| Frequent episodes of acute attacks | CR | 466 | 483 |
| Sharp pain, possibly with sudden movements | CR | 470 | 481 |
| Head feels heavy | CR | 473 | 480 |
| Neck gets stuck, or locks, with movement | CR | 462 | 479 |
| Better in unloaded position such as lying down | CR | 449 | 476 |
| Catching, clicking, clunking, and popping sensation | CR | 462 | 476 |
| Past history of neck dysfunction or trauma | CR | 480 | 476 |
| Trivial movements provoke symptoms | CR | 456 | 469 |
| Muscles feel tight or stiff | CR | 464 | 467 |
| Unwillingness, apprehension, or fear of movement | CR | 435 | 462 |
| Temporary improvement with clinical manipulation | CR | 442 | 464 |
| Increased pain as day progresses | NCR | 445 | 453 |
| Complaints of dull ache | U | 438 | 443 |
| Reports of sleep disturbances | U | 438 | 439 |
| Inconsistency of symptoms, including pain that shifts from side to side | U | 425 | 435 |
| Feeling that head is disconnected from neck | U | 416 | 433 |
| Complaints of headache | U | 436 | 430 |
| History of disorder or syndrome, such as Ehlers-Danlos syndrome, Marfan syndrome, or Down syndrome | U | 401 | 395 |
| Pain with the initiation of motion | U | 363 | 385 |
| Pain through the range of motion | U | 372 | 355 |
| Vertebrobasilar insufficiency symptoms that include dizziness, diplopia, drop attacks, and nausea | U | 371 | 352 |
| Spinal cord symptoms with neck movement | U | 361 | 325 |
| Temporomandibular joint symptoms | U | 343 | 323 |
| Cervical instability does not exist | CNR | 190 | 157 |
| Identifier . | Round 3 Consensus Statusa . | Round 2 Composite Score . | Round 3 Composite Score . |
|---|---|---|---|
| Intolerance to prolonged static postures | CR | 481 | 502 |
| Fatigue and inability to hold head up | CR | 464 | 499 |
| Better with external support, including hands or collar | CR | 487 | 493 |
| Frequent need for self-manipulation | CR | 466 | 488 |
| Feeling of instability, shaking, or lack of control | CR | 464 | 485 |
| Frequent episodes of acute attacks | CR | 466 | 483 |
| Sharp pain, possibly with sudden movements | CR | 470 | 481 |
| Head feels heavy | CR | 473 | 480 |
| Neck gets stuck, or locks, with movement | CR | 462 | 479 |
| Better in unloaded position such as lying down | CR | 449 | 476 |
| Catching, clicking, clunking, and popping sensation | CR | 462 | 476 |
| Past history of neck dysfunction or trauma | CR | 480 | 476 |
| Trivial movements provoke symptoms | CR | 456 | 469 |
| Muscles feel tight or stiff | CR | 464 | 467 |
| Unwillingness, apprehension, or fear of movement | CR | 435 | 462 |
| Temporary improvement with clinical manipulation | CR | 442 | 464 |
| Increased pain as day progresses | NCR | 445 | 453 |
| Complaints of dull ache | U | 438 | 443 |
| Reports of sleep disturbances | U | 438 | 439 |
| Inconsistency of symptoms, including pain that shifts from side to side | U | 425 | 435 |
| Feeling that head is disconnected from neck | U | 416 | 433 |
| Complaints of headache | U | 436 | 430 |
| History of disorder or syndrome, such as Ehlers-Danlos syndrome, Marfan syndrome, or Down syndrome | U | 401 | 395 |
| Pain with the initiation of motion | U | 363 | 385 |
| Pain through the range of motion | U | 372 | 355 |
| Vertebrobasilar insufficiency symptoms that include dizziness, diplopia, drop attacks, and nausea | U | 371 | 352 |
| Spinal cord symptoms with neck movement | U | 361 | 325 |
| Temporomandibular joint symptoms | U | 343 | 323 |
| Cervical instability does not exist | CNR | 190 | 157 |
CR=Consensus, Related; NCR=Near-Consensus, Related; CNR=Consensus, Not Related; U=Undecided.
Physical Examination Findings of Consensus and Rank Outcomes for Clinical Cervical Spine Instability (CCSI), Listed in Descending Rank
| Identifiera . | Round 3 Consensus Statusb . | Round 2 Composite Score . | Round 3 Composite Score . |
|---|---|---|---|
| Poor coordination/neuromuscular control, including poor recruitment and dissociation of cervical segments with movement | CR | 481 | 508 |
| Abnormal joint play | CR | 492 | 508 |
| Motion that is not smooth throughout range (of motion), including segmental hinging, pivoting, or fulcruming | CR | 491 | 499 |
| Aberrant movement | CR | 459 | 486 |
| Hypomobility of upper thoracic spine | CR | 467 | 478 |
| Increased muscle guarding, tone, or spasms with test movements | CR | 474 | 477 |
| Palpable instability during test movements | CR | 469 | 475 |
| Jerkiness or juddering of motion during cervical movement | CR | 450 | 472 |
| Decreased cervical muscle strength | CR | 428 | 468 |
| Catching, clicking, clunking, popping sensation heard during movement assessment | CR | 454 | 467 |
| Fear, apprehension, or decreased willingness to move during examination | CR | 457 | 465 |
| Pain provocation with joint-play testing | CR | 451 | 456 |
| Motion disparity between AROM and PROM | NCR | 434 | 455 |
| Poor posture; postural deviations | U | 443 | 448 |
| Decreased AROM in weight bearing | NCR | 419 | 446 |
| Need to support head during examination movements | U | 425 | 441 |
| Positive radiographic evidence | U | 425 | 439 |
| Palpable segmental changes, such as step-off at C5-C6 | U | 426 | 429 |
| Positive ligament shear test | U | 423 | 424 |
| Painful arc, including through range of pain | U | 423 | 422 |
| Forward head posture | U | 369 | 412 |
| Positive test for transverse ligament of atlas | U | 414 | 396 |
| Hypomobility of upper cervical spine | U | 387 | 391 |
| Positive Alar Ligament Stress Test | u | 406 | 389 |
| Positive Sharp-Purser Test | u | 412 | 352 |
| Pain at end range of movement | u | 395 | 374 |
| Positive VBI tests | u | 348 | 321 |
| Segmental instability does not exist | CNR | 249 | 152 |
| Identifiera . | Round 3 Consensus Statusb . | Round 2 Composite Score . | Round 3 Composite Score . |
|---|---|---|---|
| Poor coordination/neuromuscular control, including poor recruitment and dissociation of cervical segments with movement | CR | 481 | 508 |
| Abnormal joint play | CR | 492 | 508 |
| Motion that is not smooth throughout range (of motion), including segmental hinging, pivoting, or fulcruming | CR | 491 | 499 |
| Aberrant movement | CR | 459 | 486 |
| Hypomobility of upper thoracic spine | CR | 467 | 478 |
| Increased muscle guarding, tone, or spasms with test movements | CR | 474 | 477 |
| Palpable instability during test movements | CR | 469 | 475 |
| Jerkiness or juddering of motion during cervical movement | CR | 450 | 472 |
| Decreased cervical muscle strength | CR | 428 | 468 |
| Catching, clicking, clunking, popping sensation heard during movement assessment | CR | 454 | 467 |
| Fear, apprehension, or decreased willingness to move during examination | CR | 457 | 465 |
| Pain provocation with joint-play testing | CR | 451 | 456 |
| Motion disparity between AROM and PROM | NCR | 434 | 455 |
| Poor posture; postural deviations | U | 443 | 448 |
| Decreased AROM in weight bearing | NCR | 419 | 446 |
| Need to support head during examination movements | U | 425 | 441 |
| Positive radiographic evidence | U | 425 | 439 |
| Palpable segmental changes, such as step-off at C5-C6 | U | 426 | 429 |
| Positive ligament shear test | U | 423 | 424 |
| Painful arc, including through range of pain | U | 423 | 422 |
| Forward head posture | U | 369 | 412 |
| Positive test for transverse ligament of atlas | U | 414 | 396 |
| Hypomobility of upper cervical spine | U | 387 | 391 |
| Positive Alar Ligament Stress Test | u | 406 | 389 |
| Positive Sharp-Purser Test | u | 412 | 352 |
| Pain at end range of movement | u | 395 | 374 |
| Positive VBI tests | u | 348 | 321 |
| Segmental instability does not exist | CNR | 249 | 152 |
AROM=active range of motion, PROM=passive range of motion, VBI=vertebrobasilar insufficiency.
CR=Consensus, Related; NCR=Near-Consensus, Related; CNR=Consensus, Not Related; U=Undecided.
Physical Examination Findings of Consensus and Rank Outcomes for Clinical Cervical Spine Instability (CCSI), Listed in Descending Rank
| Identifiera . | Round 3 Consensus Statusb . | Round 2 Composite Score . | Round 3 Composite Score . |
|---|---|---|---|
| Poor coordination/neuromuscular control, including poor recruitment and dissociation of cervical segments with movement | CR | 481 | 508 |
| Abnormal joint play | CR | 492 | 508 |
| Motion that is not smooth throughout range (of motion), including segmental hinging, pivoting, or fulcruming | CR | 491 | 499 |
| Aberrant movement | CR | 459 | 486 |
| Hypomobility of upper thoracic spine | CR | 467 | 478 |
| Increased muscle guarding, tone, or spasms with test movements | CR | 474 | 477 |
| Palpable instability during test movements | CR | 469 | 475 |
| Jerkiness or juddering of motion during cervical movement | CR | 450 | 472 |
| Decreased cervical muscle strength | CR | 428 | 468 |
| Catching, clicking, clunking, popping sensation heard during movement assessment | CR | 454 | 467 |
| Fear, apprehension, or decreased willingness to move during examination | CR | 457 | 465 |
| Pain provocation with joint-play testing | CR | 451 | 456 |
| Motion disparity between AROM and PROM | NCR | 434 | 455 |
| Poor posture; postural deviations | U | 443 | 448 |
| Decreased AROM in weight bearing | NCR | 419 | 446 |
| Need to support head during examination movements | U | 425 | 441 |
| Positive radiographic evidence | U | 425 | 439 |
| Palpable segmental changes, such as step-off at C5-C6 | U | 426 | 429 |
| Positive ligament shear test | U | 423 | 424 |
| Painful arc, including through range of pain | U | 423 | 422 |
| Forward head posture | U | 369 | 412 |
| Positive test for transverse ligament of atlas | U | 414 | 396 |
| Hypomobility of upper cervical spine | U | 387 | 391 |
| Positive Alar Ligament Stress Test | u | 406 | 389 |
| Positive Sharp-Purser Test | u | 412 | 352 |
| Pain at end range of movement | u | 395 | 374 |
| Positive VBI tests | u | 348 | 321 |
| Segmental instability does not exist | CNR | 249 | 152 |
| Identifiera . | Round 3 Consensus Statusb . | Round 2 Composite Score . | Round 3 Composite Score . |
|---|---|---|---|
| Poor coordination/neuromuscular control, including poor recruitment and dissociation of cervical segments with movement | CR | 481 | 508 |
| Abnormal joint play | CR | 492 | 508 |
| Motion that is not smooth throughout range (of motion), including segmental hinging, pivoting, or fulcruming | CR | 491 | 499 |
| Aberrant movement | CR | 459 | 486 |
| Hypomobility of upper thoracic spine | CR | 467 | 478 |
| Increased muscle guarding, tone, or spasms with test movements | CR | 474 | 477 |
| Palpable instability during test movements | CR | 469 | 475 |
| Jerkiness or juddering of motion during cervical movement | CR | 450 | 472 |
| Decreased cervical muscle strength | CR | 428 | 468 |
| Catching, clicking, clunking, popping sensation heard during movement assessment | CR | 454 | 467 |
| Fear, apprehension, or decreased willingness to move during examination | CR | 457 | 465 |
| Pain provocation with joint-play testing | CR | 451 | 456 |
| Motion disparity between AROM and PROM | NCR | 434 | 455 |
| Poor posture; postural deviations | U | 443 | 448 |
| Decreased AROM in weight bearing | NCR | 419 | 446 |
| Need to support head during examination movements | U | 425 | 441 |
| Positive radiographic evidence | U | 425 | 439 |
| Palpable segmental changes, such as step-off at C5-C6 | U | 426 | 429 |
| Positive ligament shear test | U | 423 | 424 |
| Painful arc, including through range of pain | U | 423 | 422 |
| Forward head posture | U | 369 | 412 |
| Positive test for transverse ligament of atlas | U | 414 | 396 |
| Hypomobility of upper cervical spine | U | 387 | 391 |
| Positive Alar Ligament Stress Test | u | 406 | 389 |
| Positive Sharp-Purser Test | u | 412 | 352 |
| Pain at end range of movement | u | 395 | 374 |
| Positive VBI tests | u | 348 | 321 |
| Segmental instability does not exist | CNR | 249 | 152 |
AROM=active range of motion, PROM=passive range of motion, VBI=vertebrobasilar insufficiency.
CR=Consensus, Related; NCR=Near-Consensus, Related; CNR=Consensus, Not Related; U=Undecided.
Sixteen symptom identifiers were ranked as “Consensus, Related” with CCSI and 1 was ranked as “Near-Consensus, Related” in round 3. In addition, 1 symptom identifier was ranked as “Consensus, Not Related” and 11 were ranked as “Undecided.” Twelve physical examination finding identifiers were ranked as “Consensus, Related” with CCSI whereas 2 were ranked as “Near-Consensus, Related,” 1 was ranked as “Consensus, Not Related,” and 13 were ranked as “Undecided.”
Each identifier's ranked outcomes are reported by composite rank in Tables 2 and 3. “Intolerance to prolonged static postures” was the symptom identifier that was most related to CCSI. “Fatigue and inability to hold head up” ranked second, followed by “better with external support, including hands or collar.” “Spinal cord symptoms with neck movement,” “temporomandibular (TMJ) symptoms,” and “cervical instability does not exist” ranked as the 3 symptom identifiers that were least related to CCSI.
Overall, “poor coordination/neuromuscular control, including poor recruitment and disassociation of cervical segments with movement” ranked as the physical examination finding that was most related to CCSI, followed by “abnormal joint play.” The third most related physical examination finding was “motion that is not smooth throughout range (of motion), including segmental hinging, pivoting, or fulcruming.” In addition, the 3 physical examination findings that were determined to be least related to CCSI included “pain at end range of movement,” “positive VBI (vertebrobasilar insufficiency) tests,” and “segmental instability does not exist.” Finally, no differences in composite score rankings were detected through data analysis for rounds 2 and 3 in the symptom identifiers (P=.19) or physical examination finding identifiers (P=.41).
Discussion
The Delphi method is useful in situations where frequent clinical or practical judgments are encountered but where empirical evidence to provide evidence-based decision making does not exist.45–47 Past studies have used the Delphi method to create standards in quality assessment, components of diagnosis, and refinement of treatment.48–52 At present, clinical detection of CCSI using pathoanatomical, radiological, and selected clinical assessment methods has inherent limitations.22,53,54 Subsequently, the use of a Delphi method may provide beneficial clinical information because no set of clinical examination and symptom standards for CCSI currently exists.
The success of a Delphi study rests explicitly on the expertise of the participants who make up the respondent group. Two group characteristics may influence the success of the Delphi method: panel size and qualifications. Some authors47,55,56 have suggested that appropriate panel sizes range from 10 to more than 1,000. Murphy et al57 argued that the more expert participants, the better, although little empirical evidence exists on whether more participants affect the reliability or validity of data for a consensus process.57,58 The Delphi method does not require expert panels to be representative samples for statistical purposes, nor is a specific volume required for appropriate sampling validity.47 Nonetheless, to lend credibility to the findings, it is essential that the panel consist of heterogeneous members who work in the appropriate targeted area.47 It is our assumption that the OCS and FAAOMPT have the expertise to identify CCSI.
In this study, the panel members selected were Fellows of the AAOMPT and board-certified Orthopedic Clinical Specialists of APTA. Fellows of the AAOMPT were targeted as experts based on their previous residency or fellowship training, which is designed to advance the physical therapist fellow's preparation as a provider of patient care services in a defined area of clinical practice. In addition, APTA proposes that the designation of orthopedic specialist certification depicts a clinician with “knowledge, skill, and experience exceeding that of the physical therapist at entry to the profession and unique to the specialized area of practice.”59
Proposed Identifiers for Symptoms
The Delphi survey participants consensually selected symptoms that were qualitatively grouped by the work group members into 5 conceptually similar areas: (1) movements, (2) descriptive components, (3) postures, (4) neurological phenomena, and (5) headaches. Movement-related identifiers included “sharp pain, possibly with sudden movements,” “neck gets stuck, or locks, with movement,” and “trivial movements provoke symptoms.” In addition, “unwillingness, apprehension, or fear of movement” was identified, a finding supported by Klein et al,31 who reported an unwillingness of patients with whiplash-associated disorders to move their neck beyond comfort zones into ranges where higher muscle activity is engaged.
Descriptive components included identifiers that describe the type of pain or an action that modulates the pain. Within this category, the Delphi survey participants selected “past history of neck dysfunction or trauma,” “better with external support, including hands or collar,” “frequent need for self-manipulation,” “feeling of instability, shaking, or lack of control,” “frequent episodes of acute attacks,” “head feels heavy,” “catching, clicking, clunking, and popping sensation,” “muscles feel tight or stiff,” “temporary improvement with clinical manipulation,” and “increased pain as day progresses.” Several authors11,60,61 have identified the coexistence of trauma and cervical spine instability. Other authors62 have related cervical spine instability with comorbidities, such as spondylosis or spine degeneration, although these relationships appear less definitive. These studies did not determine whether the instability condition was radiographically appreciable.
Postural identifiers included “intolerance to prolonged static postures” and “better in unloaded position such as lying down”—2 findings supported by other authors.26,63 Lying down may reduce intolerance to segmental physiological loading, as reported by Oxland and Panjabi10 Mid-postural position of the cervical spine displayed the highest area of load sensitivity. Hypothetically, mid-position is the posture that requires the most dynamic control of the neutral zone and is the position most prone to instability problems.11 Subjects with long-term rheumatologic-related instability show changes in muscle fibers, which can lead to losses of postural stability and decreased control of the neutral zone.63
The Delphi survey respondents were undecided about “spinal cord symptoms with neck movement” or “complaints of headache” as specific identifiers of CCSI in our study. Past studies11,62,64 have suggested that cervical myelopathy and radiculopathy are associated with cervical spine instability. Most authors who have evaluated cord-related and radicular symptoms related to cervical spine instability have done so following severe trauma or dislocation of the cervical spine. Still, some symptomatic complaints may be related to repeated episodes of severe 2 neck pain with minor provocation65–67 and may be less obviously deduced. Moreover, several authors have suggested the relationship between headaches and instability, most notably secondary to instability within the upper cervical spine3,27,68 as well as the C5–6 intervertebral disk.69
Proposed Identifiers for Physical Examination Findings
The composite scores for neuromuscular-related phenomena were scored high as identifiers of CCSI. “Poor coordination/neuromuscular control, including poor recruitment and dissociation of cervical segments with movement” was ranked first, “increased muscle guarding, tone, or spasms with test movements” was ranked sixth, and “decreased cervical muscle strength” was ranked ninth. Jull and colleagues27,68 found dysfunction of the deep neck flexors (longus colli and longus capitus muscles) in people with cervicogenic headache and whiplash, accompanied by their inability to generate tension and sustain this tension under a low load. They hypothesized that the coexistence of poor coordination and strength of the deep neck flexors and cervical spine instability may be a contributor to cervicogenic symptoms such as headaches. Other researchers26,70,71 have observed overactivity of the upper trapezius muscle in people with long-term, chronic instability-related conditions such as whiplash, further suggesting a distortion of motor control strategies.
Phenomena that involve observation during the physical examination dominated the identifiers selected by the Delphi survey participants. The participants selected “motion that is not smooth throughout range (of motion), including segmental hinging, pivoting, or fulcruming,” “aberrant movement,” “jerkiness or juddering of motion during cervical movement,” “catching, clicking, clunking, popping sensation heard during movement assessment,” “fear, apprehension, or decreased willingness to move during examination,” “motion disparity between AROM (active range of motion) and PROM (passive range of motion),” and “decreased AROM in weight bearing” as consensual or near-consensual identifiers. Other authors have associated catching or locking32 and abnormalities in range of motion of the cervical spine15 with CCSI.
Clinical examination methods to determine the integrity of ligaments or the active stabilization capabilities of the cervical spine often offer little conclusive evidence and are fraught with poor reliability.39,60,72 Despite this, numerous clinical tests for cervical spine instability exist. Most methods examine the integrity of the alar and transverse ligaments, with varied reported levels of reliability.73 Nearly all manual instability assessment methods are finite, require very skilled assessment, and have not been corroborated by simultaneous diagnostic measurement.5 Notable exclusions from the Delphi list of consensus identifiers were the special tests associated with CCSI. The Delphi survey participants did not reach consensus for a “positive ligament shear test,” a “positive test for transverse ligament of atlas,” a “positive Alar Ligament Stress Test,” “positive (vertebrobasilar insufficiency) VBI tests,” and a “positive Sharp-Purser Test.” Although the Sharp-Purser test has been found to be a valid indicator73,74 for detection of radiographic instability, this method was not consensually chosen as an identifier for CCSI.
Historically, hypermobility, or “greater range of motion,” has been erroneously confused with spine instability.54,75 However, the Delphi survey participants aligned well with literature-based findings and did not recognize hypermobility or greater range of motion as forms of CCSI. The group did identify “abnormal joint play” and “palpable instability during test movements,” suggesting the assumption that abnormal segmental movements are clinically discernible from normal movements.76 Past studies72,77 have suggested that most passive joint assessment or palpatory tests traditionally have poor interrater reliability. Further investigation is necessary to determine whether physical therapists are able to make such diminutive joint assessment or palpatory judgments.
Clinical Application
Jensen et al78 reported that expert clinicians were comfortable with ambiguity and had the capacity to self-monitor their data collection and thinking patterns. They are able to do this by combining clusters of information together into workable sets, based on past experience and cooperative decision making. A growing body of expertise literature suggests that orthopedic clinical experts have the capacity to recognize inconsistencies or links between data variables collected and have the capability to distill appropriate information for diagnostic and treatment purposes.79 Clinical cervical spine instability is multidimensional, fraught with ambiguity, and may involve various convoluted identifiers.
Adler and Ziglio58 stated that, in the absence of complete information, the health care provider has 2 options. First, they may wait until they have enough information to create an adequate theory. Second, they may make the most of the available information and use this knowledge for the best possible consequence. This investigation suggests that judicious use of the Delphi survey findings may contribute to a growing pool of data for identification of CCSI. Thus, by using the clusters of identifiers proposed within the Delphi survey consensus, practitioners may glean additional information for successful assessment of CCSI.
Limitations
It has been proposed that the Delphi method builds on the Lockean notion of agreement, a notion that learning is a collective action process and is the basis of truth.80 Although some authors47,51,80 have stated that, if the shared members demonstrate expertise and consensus, an empirical generalization is judged to be objective, true, or factual, other authors45,81 have countered that Delphi survey findings are relegated to experience, sharing, and wisdom of the panel members. Opponents to the Delphi method argue that findings should not be judged by the same validation criteria as hard science derived by scientific method45,81 but rather that the findings should be considered to be a process for making best use of available information in the absence of a criterion standard and in the presence of ambiguity.
The Delphi method is a qualitative analysis and does not have the sampling requirements of a randomized design.58 However, it is worth noting that fewer than 12% of the targeted population responded to initial recruitment. There may be several reasons for the low response rate. First, e-mail annual response rates for surveys dropped consistently from 1992 to 2000.82 On average, response rates dropped nearly 10% per year during that time.83 Second, it is estimated that the average e-mail user receives 39 unsolicited e-mails each day.82 Bradley84 stated that this phenomenon has prompted many users to create several e-mail addresses, thus maintaining an address for “bulk,” unsolicited mail. Third, this study used the Microsoft Outlook mass e-mail function to distribute to participants. The Microsoft Outlook distribution reports when e-mail addresses are no longer in service but does not automatically report when an e-mail blocking program is limiting access to the targeted user or when e-mail users “churn” addresses, such as switching to a different provider but not closing an old account.84 Therefore, chance exists that the introductory e-mails did not arrive at all of the potential 1,015 eligible OCS respondents who Microsoft Outlook did not recognize as having a bad e-mail address. Another potential limitation is that the findings may not be representative of the group of therapists we sampled because a large majority may not have been reached via e-mail.
A documented weakness of a Delphi method is the stand-alone principle.58 The stand-alone principle allows the respondent to evaluate only one variable at a time. Within this study, respondents were asked if one single variable was associated with spine instability, a process that was repeated throughout the study. This process is analogous to asking if A (one identifier)=Z (spine instability), B (a different identifier)=Z, and C (a different identifier)=Z, and so on. In reality, some of the identifiers may be associated with spine instability only when combined with other identifiers (A + B + C may=Z). Subsequently, using a cluster of identifiers is likely a more pertinent application of this information for clinical practice. Expert clinicians may be able to integrate the proposed evidence provided and improve their clinical decision making.
Conclusion
Clinical cervical spine instability is difficult to diagnose and may involve subtle clinical features. Our Delphi investigation was designed to identify common symptoms and physical examination findings for cervical spine instability used by expert physical therapists in daily practice. Most identifiers involved assessment methods that encompass intricate palpation and visual assessment skills, poor tolerance to certain postures, and movement-related similarities. Although selected identifiers within each of these categories met consensus, it does not suggest that these variables are individual predictors of CCSI. Diagnosis and prediction of CCSI are marred by the failure to determine a criterion standard for this pathology, and appropriate clinical reasoning is required for distinctive assessment.
Future studies should prospectively cluster the Delphi method identifiers using a cross-impact analysis. A cross-impact analysis minimizes this drawback of the Delphi process and can predict the probability of 2 or more individual components detecting if a conclusive finding is present, allowing for better analytical depth in assessment. In addition, identification of the confidence of expert physical therapists in detecting CCSI may lead to further beneficial findings.
Dr Cook, Dr Sizer, and Mr Fleming provided concept/idea/research design. All authors contributed writing, data collection and analysis, and consultation (including review of manuscript before submission). Dr Cook provided project management, subjects, facilities/equipment, and clerical/secretarial support. Dr Cook and Mr Fleming provided fund procurement. Dr Cook and Dr Sizer provided institutional liaisons.
This study was approved by the Texas Tech University Health Sciences Center Institutional Ethics Review Board.
This study was supported by the 2003 Steens/USA Grant.
WebSurveyor Corp, 505 Huntmar Park Dr, Ste 225, Herndon, VA 20170.
JB Orris, Butler University, College of Business Administration, 4600 Sunset Ave, Indianapolis, IN 46208.
Microsoft Corp, One Microsoft Way, Redmond, WA 98052.
References
Who Is a Fellow of the American Academy of Orthopaedic Manual Physical Therapists (FAAOMPT)? Available at: http://www.aaompt.org. Accessed October 28,
Overview of the Specialist Certification Program.




Comments