-
PDF
- Split View
-
Views
-
Cite
Cite
A.A.L. Ajayi, P.V. Halushka, Castration reduces platelet thromboxane A2 receptor density and aggregability, QJM: An International Journal of Medicine, Volume 98, Issue 5, May 2005, Pages 349–356, https://doi.org/10.1093/qjmed/hci054
Close - Share Icon Share
Abstract
Background: Exogenously administered testosterone upregulates platelet thromboxane A2 (TXA2) receptors and increases aggregation response to thromboxane mimetics in healthy male volunteers. However, the biological impact of endogenous testosterone on platelet TXA2 receptor expression, especially in older men at risk of coronary artery disease, is unclear.
Aim: To investigate the impact of reduction in circulating testosterone on platelet TXA2 receptor expression in older men.
Design: Cross-sectional case-control study.
Methods: We studied surgically and/or medically castrated men with prostate cancer (group A, n = 8, aged 71 ± 8 years) and age-matched, uncastrated urology patients (group B, n = 7, aged 67 ± 9 years). Plasma testosterone was measured by radioimmunoassay. Platelet TXA2 receptor expression was assessed by radioligand binding studies using radioactive 125I-BOP. Platelet aggregation responses to TXA2-mimetic I-BOP, and to thrombin, were also studied.
Results: Group A had significantly lower plasma testosterone than group B (16 ± 5 ng/dl vs. 308 ± 47 ng/dl, p<0.001). Platelet TXA2 receptor density (Bmax) but not affinity (Kd) was lower in group A (0.50 ± 0.12 vs. 1.01 ± 0.17 pmol/mg protein, p = 0.03). Maximum platelet aggregation response to I-BOP (Emax), but not sensitivity (EC50) was lower in group A (53 ± 2% vs. 63 ± 2%, p = 0.003 ANOVA). In vitro, high concentrations of hydroxyflutamide (100 μM) competitively inhibited U46619-induced platelet aggregation in washed platelets, without affecting the binding of 125I-BOP to platelet TXA2 receptors.
Discussion: Endogenous testosterone regulates platelet TXA2 receptor Bmax and the Emax aggregation response to thromboxane mimetic I-BOP. Blockade of androgen receptors or inhibition of testosterone production may reduce platelet aggregation responses. Preliminary evidence suggests the presence of functional androgen receptors on human platelets, which may regulate TXA2 receptor expression.
Introduction
There is established evidence of gender differences in the prevalence and severity of vascular thrombosis and/or mortality, with men having a worse prognosis.1–3 The impact of sex hormones4–6 on gender differences in platelet aggregation or endothelial reactivity,7–9 is also increasingly recognized. Exogenously administered testosterone cypionate up-regulated the population of thromboxane A2 (TXA2) receptors on platelets in healthy young male volunteers, concomitant with an enhancement of the aggregation response to TXA2-mimetic I-BOP.10 Human megakaryocytes generated ex vivo expressed RNA for androgen receptors, confirmed by Western immunoblotting to be present in human platelets.11 The same study also demonstrated the regulation of the megakaryocytic androgen receptor by testosterone.11 Testosterone also accelerates vascular plaque development and regulates androgen receptor expression in the arterial wall, thus significantly contributing to atherogenesis.12 These findings provide a potential mechanism by which endogenous testosterone may affect platelet and/or vascular function, and hence the gender differences in thrombotic disorders. However, a direct assessment of how alteration in endogenous testosterone concentration impacts platelet TXA2 receptors, which is the main prothrombotic pathway, is required in order to further elucidate the role of sex hormones and gender in cardiovascular thrombosis. This study aimed to determine the impact of prolonged reduction in testosterone concentration, through castration, on the expression of platelet TXA2 receptors in older men, the age group associated with increased risk of ischaemic heart disease and acute myocardial infarction.
Methods
A cross-sectional study was undertaken in a tertiary referral hospital urology clinic (The Veteran's Administration Hospital, Charleston, SC, USA). The protocol was reviewed by both the Medical University of South Carolina and the VA hospital institutional review board panel. Recruited patients gave informed, written, witnessed consent. The experimental group (group A, n = 8) were patients with prostate cancer, who had undergone surgical orchidectomy (n = 5) or were on treatment with leuprolide, a gonadotrophic agonist and hydroxyflutamide (n = 3) for at least 3 months, and had low plasma testosterone concentration. They were compared with age-matched patients drawn from the same urology clinics. Controls (group B, n = 7) had either prostate cancer which was yet to be treated, or untreated, or had benign prostate hypertrophy awaiting surgery.
Patients with co-morbid states, such as diabetes mellitus or hypertension, who smoked cigarettes or imbibed alcohol, or who were on other medications, were excluded. Group A was aged 71 ± 8 years (mean ± SEM) and group B 67 ± 9 years. The groups were well matched in baseline clinical data, including platelet counts (185 ± 21 and 210 ± 18 × 109/l, respectively).
Blood sampling and platelet isolation
Blood (60 ml) was drawn by venepuncture via a 19-gauge needle into syringes containing EDTA (5 mM) and indomethacin (10 μM) final concentration. Washed platelets were prepared as previously described by us.10,13 Platelet-rich plasma (PRP) was prepared by centrifugation at 175 g for 20 min at room temperature. This was followed by differential centrifugation at 1795 g for another 20 min. The platelet pellet generated was suspended in modified Tyrode's solution buffer, containing NaCl (137 mM), KCL (3 mM), MgCl2 (0.6 mM), NaH2PO4 (12.5 mM), dextrose (5.5 mM) and indomethacin (10 μM) adjusted to a pH of 7.4 for platelet aggregation studies, and pH 6.5 for equilibrium radioligand binding assays with 125I-BOP.14
Equilibrium radioligand binding assay
Radioligand binding studies were as described elsewhere.10,13,14 Briefly, incubations were done in modified Tyrode's buffer at pH 6.5 with a total volume of 200 μl. The optimal assay conditions for the platelet TXA2 receptors occurs at pH 6.5.14 Each reaction mixture contained 100 μl washed platelets (107 platelets/tube). In addition, the reaction mixture contained 20 μl of Tyrode's buffer or unlabelled I-BOP at varying concentrations (10−11 to 10−6 M) and 80 μl of radioactive ‘hot’ 125I-BOP (40 000 cpm). Incubations were performed in sialinized glass tubes (12 × 75 mm) at 37°C for 30 min. Reactions were terminated by the addition of 4 ml ice-cold 50 mM NaCl 100 mM buffer (pH 6.5), followed by rapid filtration under reduced pressure through Whatman GFC filters using a Brandel cell harvester, and washing three times with 4 ml ice-cold buffer within 10 s. Non-specific binding was defined as the amount of radioactivity in the presence of L657925 (10 μM), a stereoselective TXA2 receptor antagonist. Protein concentration was determined using the method of Lowry.
Platelet aggregation studies
Platelet aggregation studies used a Chronolog model 300 aggregometer as previously described.10 Washed platelets suspended in Tyrode's buffer were diluted to 2.5 × 108 platelets/ml, and 450 μl dispensed into sialinized cuvettes, stirred and pre-incubated at 37°C for 1 min. The ascending final concentrations of I-BOP (0.25–100 nM, 50 μl) were added to the washed platelets, and the aggregation at 1 min recorded. Concentration response curves were constructed for I-BOP and thrombin (0.00625–0.1 units/ml) final concentrations. The maximum aggregation response at 1 min (Emax), for each agonist at each experiment was determined. The aggregometer was standardized by arbitrarily setting 100% aggregation as equal to the light transmission with the cuvette containing only Tyrode's buffer. The EC50 values for each agonist were calculated directly from log-logit transformation of the data.
Plasma testosterone assay
Plasma testosterone assay was determined using a radioimmunoassay kit (Diagnostic Products). The inter and intra-assay coefficients of variation were <10%, and the limit of detection was 4 ng/dl.10
In vitro study of hydroxyflutamide on platelet TXA2 receptor density and aggregation
Ascending concentrations of hydroxyflutamide (0.1–100 μM), alone and in combination with testosterone (100 μM), were added to healthy PRP. Platelet TXA2 receptor expression was determined in vitro, using the radioligand binding method described above. Similarly, platelet aggregation response to U46619 (a TXA2 mimetic) (0.05–50 μM) was studied in washed platelets, with and without pre-incubation of the PRP, with hydroxyflutamide (1–100 μM) final concentrations for 10 min.
Data analyses
The radioligand binding dissociation constant (Kd) and receptor density (Bmax) were calculated using Scatchard analysis from the curve fitting program LIGAND.15 The receptor density was corrected for protein, and expressed as pmol/mg protein.
Statistical analyses
Data are expressed as means ± SEM. Unpaired t tests, and two-way repeated measures analysis of variance (ANOVA) were used in data analysis. The null hypothesis was rejected at p<0.05.
Results
Plasma testosterone concentration
The orchidectomized patients (group A, n = 8) had significantly lower plasma testosterone concentrations than did the controls (group B, n = 7) (16.5 ± 5.2 vs. 308 ± 47 ng/dl, p<0.001).
Platelet TXA2 receptor expression
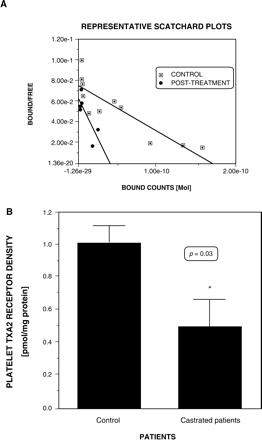
Platelet TXA2 receptor density (Bmax) was significantly lower in group A (0.50 ± 0.12 vs. 1.01 ± 0.17 pmol/mg protein, p = 0.02). The 95%CI for the difference in Bmax was 0.1 ± 0.92 pmol/mg (Figure 1). In one patient, it was possible to measure his plasma testosterone and TXA2 density before and after medical castration. The plasma testosterone was reduced from 934 ng/dl to 12 ng/dl, while Bmax, diminished from 1.1 pmol/mg to 0.25 pmol/mg, after 4 weeks of castration. (Figure 1A). There were no significant differences in the Kd (group A 1.98 ± 0.38 nM, group B 2.8 ± 0.32 nM).
A Representative Scatchard plot of the radioligand binding of 125-I-BOP to platelets of a patient before (squares) and after (diamonds) castration. B Comparison of platelet TXA2 receptor density (Bmax) in uncastrated control (n = 7) and castrated prostate cancer patients (n = 8). Expressed as pmol/mg protein. *p = 0.03 by unpaired t-test.
Platelet aggregation response to thromboxane-mimetic I-BOP and thrombin
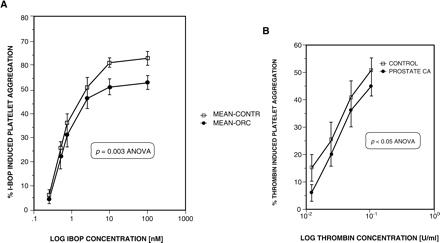
The platelet aggregation responses to I-BOP and to thrombin are shown in Figures 2A and 2B, respectively. The maximum platelet aggregation response to I-BOP was significantly reduced in group A (53 ± 2% vs. 63 ± 2%), with the dose-response relationships being significantly different (p = 0.003, ANOVA) (Figure 2A). The 95%CI for the difference in maximum platelet aggregation was 4.2–15.7%. There was no difference in the EC50 values (group A 0.88 ± 0.24 nM, group B 0.88 ± 0.11 nM). Similarly, thrombin-induced platelet aggregation was slightly, but significantly diminished in the castrated patient group (p = 0.04 ANOVA). The maximum aggregation response was 52 ± 5% in the controls and 43 ± 3% in the castrated patients. The corresponding EC50 values were also not different (0.03 ± 0.005 vs. 0.029 ± 0.004 units/ml). (Figure 2B).
A Platelet aggregation response to I-BOP in uncastrated controls (squares), n = 7, and in castrated prostate cancer patients (diamonds), n = 8. There was a significant difference in the aggregation response between groups (p = 0.003 ANOVA), with the castrated patients having a reduced maximum response. B Platelet aggregation response to thrombin in controls (squares), n = 7, and castrated patients (diamonds), n = 8. p<0.05 ANOVA. Data are mean ± SEM.
In vitro effects of hydroxyflutamide on I-BOP binding to TXA2 receptors and to U46619-induced platelet aggregation response
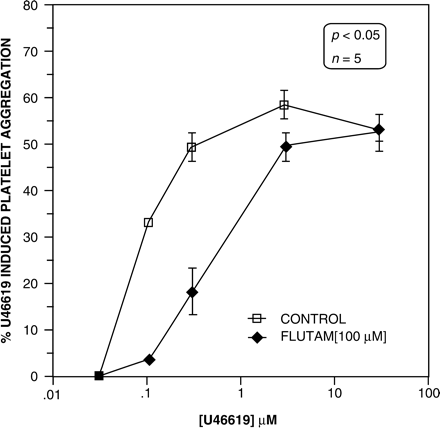
Hydroxyflutamide did not alter the binding of 125-I-BOP to platelet TXA2 receptors. Lower concentrations of hydroxyflutamide (1, 10 μM) incubated with PRP did not alter U46619-induced aggregation in washed platelets. However, very high concentrations (100 μM) caused a significant rightward shift in the dose-response curve for platelet aggregation (Figure 3). The maximum aggregation response was not altered at 54 ± 2%, but there was a significant increase in the EC50 values, in the paired samples (n = 5). The EC50 for U46619-induced platelet aggregation derived from the vehicle-treated PRP (control) was 0.08 ± 0.001 nM, but was 0.60 ± 0.15 nM in the hydroxyflutamide pre-incubated platelets (Figure 3).
U46619-induced platelet aggregation in washed platelets. The control group had physiological saline added to the platelet rich plasma (PRP), whilst the flutamide group was incubated with 100 μM in the PRP. The samples were paired from five different healthy subjects. Incubation of PRP with flutamide resulted in a significant increase in the EC50 to U46619 (decreased sensitivity) induced aggregation in vitro. p<0.05 ANOVA.
Discussion
Our results show that reduction in circulating testosterone concentration following castration is associated with a significant diminution in platelet TXA2 receptor density (Bmax) and maximum aggregation response (Emax) to TXA2-mimetic IBOP. The TXA2 receptor affinity (Kd) and the platelet aggregation sensitivity (EC50) to I-BOP were not altered by castration. These results are consistent with earlier findings, which demonstrated increases in thromboxane receptor density and enhanced aggregation response to mimetics following exogenous testosterone cypionate administration to healthy men.10 It also confirms and extends our observation of a correlation between endogenous testosterone concentration and platelet TXA2 receptor density.10
The present study establishes that the up- or down-regulation of human platelet TXA2 expression, and subsequent aggregation response to TXA2 mimetics, is controlled by endogenous testosterone concentration. A minimum of 4 weeks elapsed between surgical/medical castration and the measurements, which demonstrated altered receptor density and functionality, in a population of elderly men at a greater risk of coronary artery disease. In one patient, measurements of plasma testosterone and the receptor binding and platelet aggregation assays were undertaken before and 4 weeks after castration. This before and after measurement demonstrated a fall in plasma testosterone from 934 to 12 ng/dl, and a reduction in platelet TXA2 receptor density from 1.1 to 0.25 pmol/mg protein (Figure 1A). The aggregation response maximum was also diminished in this individual from 65% before to 56% after castration. Collectively, these findings indicate a definite dynamic regulation of thrombogenicity by testosterone, most likely by a genomic action exerted on bone marrow megakaryocytes.
In vitro and in vivo studies in human erythroleukemia (HEL) cells indicate that testosterone can directly cause an actinomycin D and cycloheximide-reversible increase in transcription and translation of TXA2 receptor, implying a genomic action.16 The difference in testosterone concentration between castrated and control patients (308 vs. 16 ng/dl, 95% reduction) was not linearly related to the difference in TXA2 receptor Bmax (1.01 vs. 0.50 pmol/mg protein, 50% reduction), or the maximum platelet aggregation response difference (53% vs. 63%, 10% difference in effect). These discrepant and non-linear relationships among the changes in the parameters suggest that testosterone modulation of TXA2 receptor expression may entail a change in the population of ‘spare receptors’, which may not be coupled to a functional response in platelets. The thrombin-induced platelet aggregation was slightly but significantly attenuated in the castrated patients, which conforms with the trend to testosterone enhancement of thrombin aggregation (p = 0.07) seen after testosterone injection in healthy young men.10
Age-dependent decline in plasma testosterone levels is well documented in males, although no specific andropause is established. The plasma testosterone concentrations of the elderly men (aged 69 ± 8 years) in this study represent about a 40% decline from the basal values of about 450 ng/dl we previously reported in healthy men aged 29 ± 2 years.10 However despite this 40-year age gap, and 40% plasma testosterone decrease, there were no age-related differences, either in TXA2 receptor density (Bmax) (young vs. elderly, 1.19 ± 023 vs. 1.01 ± 0.17 pmol/mg), affinity (Kd) (2.9 ± 0.4 vs. 2.8 ± 0.32) or platelet aggregation (EC50) to I-BOP (0.70 ± 0.2 vs. 0.88 ± 0.1 nM).
Thus, it appears that the physiological decline in plasma testosterone is not sufficient to alter the platelet TXA2 density and aggregability. This implies that the diminution in endogenous testosterone in elderly men confers no protection in terms of platelet reactivity. It seems that a more profound decrease in plasma testosterone, such as induced by castration (∼95%) is required to alter platelet aggregability to thromboxanes.
In the in vitro study with hydroxyflutamide, we sought to determine if testosterone exerted additional rapid, non-genomic effects on platelet thromboxane receptor expression. We found no effect of hydroxyflutamide on the binding of 125I-BOP to TXA2 receptors on platelets. However, at very high concentrations (100 μM) (unlikely to be attained in vitro), hydroxyflutamide competitively inhibited U46619-induced platelet aggregation, causing an 8-fold increase in the platelet aggregation EC50, from 0.08 ± 0.001 nM to 0.60 ± 0.15 nM (p = 0.008) (Figure 3).
We have previously found that testosterone 1 μM added to PRP lowered the human platelet aggregation threshold to I-BOP, and enhanced aggregation response to I-BOP at low doses of 0.1 and 0.25 nM (unpublished observations). Collectively, these rapid and in vitro opposite effects of testosterone and hydroxyflutamide on platelet aggregation suggest non-genomic actions of these agents on platelets, or the presence of functional androgen receptors on platelets, which modulate the response to TXA2-mimetic-induced platelet aggregation.11 Non-genomic actions have been implicated in testosterone-induced coronary vasodilatation.17,18 Our preliminary observations indicate that androgens may also exert non-genomic actions on human platelets. However, since platelets are anucleate fragments, the mechanism of this non-genomic action is unclear. Several mechanisms have been suggested for the non-genomic action of testosterone, including stimulation of NO-cyclic guanylate cyclase pathway, membrane hyperpolarization, a calcium antagonist action, or through agonist-internalizable, transcription-independent receptors within the cytosol.17–19 A recent study also suggested the presence of androgen receptors within platelets, where it was shown that ADP-induced platelet aggregation was associated with platelet cytosolic binding to radiolabelled testosterone or dihydrotestosterone.20 Hydroxyflutamide action on platelets may reflect its non-genomic action via NO-c-GMP release, independent of binding to AR nuclear receptors.21 This action may underlie its inhibition of platelet aggregation at high doses, which we report here, but it is unclear whether this genomic action has any physiological relevance.
This study clarifies further the dynamic regulation of platelet TXA2 receptors by testosterone, and hence it is of clinical relevance. Earlier studies demonstrated increase in platelet TXA2 receptor density in patients with myocardial infarction 22 and in pregnancy-induced hypertension.23 Thrombotic stroke is associated with changes in circulating testosterone,24 but the change in platelet TXA2 receptor associated with ischaemic stroke is unknown. These clinical research findings are consistent with the efficacy of thromboxane generation inhibition by aspirin, which reduces mortality in these disorders.25 Whether androgen receptor blockade will exert a similar impact on thrombotic cardiovascular mortality, is a concept in need of further intensive and extensive inquiry.
This study has several limitations which warrant highlighting. First, we studied a limited number of patients (n = 16) in a cross-sectional study, albeit the end points were hard and objective. A prospective longitudinal, case–control study of a larger number of castrated patients is needed. Free testosterone (rather than total) may reflect the biological activity of testosterone more accurately, and the role of dihydrotestosterone requires evaluation. Finally, the in vitro study yielded preliminary findings suggestive of a non-genomic action of androgens on human platelets. Definitive studies in androgen receptor knock out mice, using the Cre/lox paradigm and further in vitro studies with radiolabelled androgens and measurement of signal transduction will be needed to establish the non-genomic actions of testosterone and its functionality in platelets.
In conclusion, our present findings indicate that endogenous testosterone regulates human platelet TXA2 receptor density and aggregation responses to TXA2 mimetic I-BOP and to thrombin. The in vitro study suggests a non-genomic action of hydroxyflutamide on platelet aggregation, as well as the presence of androgen receptors within human platelets.
We thank Dr Rajesh Mathur for the measurement of plasma testosterone concentration, and the resident staff of the urology clinics at VA Hospital Charleston, for their help with patient recruitment and screening. We thank the research nurses of the clinical pharmacology unit of the Medical University of South Carolina, Charleston SC, for their assistance. Dr Ajayi was supported by a Merck International Fellowship in clinical pharmacology.
References
National Heart Lung and Blood Institute.
Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary artery disease in women.
Wingard DL. The sex differential in morbidity, mortality and lifestyle.
Malkin CJ, Pugh PJ, Jones TH, Channer KS. Testosterone for secondary prevention in men with ischaemic heart disease?
Ajayi AA, Ogungbade GO, Okorodudu AO. Sex hormone regulation of systemic endothelial and renal microvascular reactivity in type-2 diabetes: studies in gonadectomized and sham operated Zucker rats.
Ajayi AA, Hercule HC, James Cory, Hayes BE, Oyekan AO. Gender differences in vascular and platelet reactivity to TXA2 mimetic U46619 and to endothelial nitric oxide(NO) mediated vasodilation in Zucker fatty (hypertensive, hyperinsulinemic) diabetic rats.
Higashiura K, Mathur RS, Halushka PV. Gender differences in androgen regulation of thromboxane A2 receptors in rat aortic smooth muscle cells.
Johnson M, Ramey E, Ramwell PW. Sex and age differences in human platelet aggregation.
Ajayi AA, Mathur R, Halushka PV. Testosterone increases platelet TXA2 receptor density and aggregation responses.
Khetwat G, Faraday N, Nealen ML, Vijayan VK, Bolton E, Noga SJ, Bray PF. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR) : testosterone regulates AR expression.
Hanke H, Lenz C, Hess B, Spindler KD, Weidemann W. Effect of testosterone on plaque development and androgen receptor expression on the arterial wall.
Okwu AK, Ullian ME, Halushka PV. Homologous desensitization of human platelet thromboxane A2/prostaglandin H2 receptors.
Mayeux PR, Morinelli TA, Williams TC, Hazard ES, Mais DE, Oatis JE, Baron DA, Halushka PV. Differential effects of pH on thromboxane A2/prostaglandin H2 receptor agonist and antagonist binding in human platelets.
Munson PJ, Rodbard D. A versatile computerized approach for characterization of ligand binding systems.
Matsuda K, Mathur RS, Duzic E, Halushka PV. Androgen regulation of thromboxane A2/prostaglandin H2 receptor expression in human erythroleukemia [HEL] cells.
Wynne FC, Khalil RA. Testosterone and coronary vascular tone. Implications for coronary artery disease.
Jones RD, English KM, Jones TH, Channer KS. Testosterone –induced coronary vasodilatation occurs via a non-genomic mechanism : evidence of a direct calcium antagonist action.
Benten WP, Lieberherr M, Stamm O, Wrehlke C, Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages.
Cabeza M, Flores M, Bratoeff E, Pena AL, Mendes-Ceballos G. Intracellular Ca (2+) stimulates binding to androgen receptors on platelets.
Iliescu R, Campus LA, Schlegel WP, Murano I, Baltulatu O, Bader M. Androgen receptor independent cardiovascular action of the anti-androgen flutamide.
Dorn GW 2nd, Liel N, Trask JL, Assey ME, Halushka PV. Increased platelet thromboxane A2/prostaglandin H2 receptors in acute myocardial infarction.
Liel N, Nathan I, Yermiyahu T, Zolotov Z, Lieberman JR, Dvilansky A, Halushka PV. Increased platelet TXA2/PGH2 receptors in patients with pregnancy-induced hypertension.
Jeppessen LL, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K. Decreased serum testosterone in men with acute ischemic stroke.