-
PDF
- Split View
-
Views
-
Cite
Cite
Dominik Mischkowski, Jennifer Crocker, Baldwin M. Way, From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain, Social Cognitive and Affective Neuroscience, Volume 11, Issue 9, September 2016, Pages 1345–1353, https://doi.org/10.1093/scan/nsw057
Close - Share Icon Share
Abstract
Simulation theories of empathy hypothesize that empathizing with others’ pain shares some common psychological computations with the processing of one’s own pain. Support for this perspective has largely relied on functional neuroimaging evidence of an overlap between activations during the experience of physical pain and empathy for other people’s pain. Here, we extend the functional overlap perspective to the neurochemical level and test whether a common physical painkiller, acetaminophen (paracetamol), can reduce empathy for another’s pain. In two double-blind placebo-controlled experiments, participants rated perceived pain, personal distress and empathic concern in response to reading scenarios about another's physical or social pain, witnessing ostracism in the lab, or visualizing another study participant receiving painful noise blasts. As hypothesized, acetaminophen reduced empathy in response to others’ pain. Acetaminophen also reduced the unpleasantness of noise blasts delivered to the participant, which mediated acetaminophen's effects on empathy. Together, these findings suggest that the physical painkiller acetaminophen reduces empathy for pain and provide a new perspective on the neurochemical bases of empathy. Because empathy regulates prosocial and antisocial behavior, these drug-induced reductions in empathy raise concerns about the broader social side effects of acetaminophen, which is taken by almost a quarter of adults in the United States each week.
“I feel your pain.” - President William J. Clinton.
Bill Clinton’s memorable line during the 1992 presidential campaign (New York Times, 1992) became emblematic of his ability to connect with the American populace. This empathic ability to ‘put oneself in other people’s shoes’ and feel their pain is important not only in leadership, but also in daily social interactions with friends, family members, coworkers and strangers. Among its many forms, empathy for other people’s pain is particularly vital for societally important processes. For example, empathizing with another’s suffering is considered an important trigger of prosocial actions (Batson, 1998; see Eisenberg and Miller, 1987, for a meta-analysis). Similarly, empathy for another’s potential pain can act as a brake on aggressive behavior (Miller and Eisenberg, 1988; but see Vachon et al., 2014, for an updated meta-analysis).
A substantial body of functional magnetic resonance imaging (fMRI) research suggests that observing others experiencing pain (e.g. observing a person receiving a hot probe placed on the hand), activates brain regions that are also activated during one’s own experience of pain—the anterior cingulate cortex (ACC) and the anterior insular (AI) cortex (see Lamm et al., 2011, for a meta-analysis). Evidence of this functional overlap coincided with the development of simulation theories of empathy, which suggest that empathy for pain relies on similar psychological and neural representations as the experience of physical pain (for reviews, see Gallese and Goldman, 1998; Preston and De Waal, 2002; Decety and Jackson, 2004; Singer, 2009; Lamm et al., 2011; but see Decety, 2010; Lamm and Majdandžić, 2015; Zaki et al., 2016). However, fMRI studies of the neural overlap between pain and empathy for pain have inherent methodological and analytical limitations. Specifically, correlating changes in neural activity with changes in the psychological task precludes testing whether neural networks traditionally assigned to the physical pain system are causally involved in the experience of empathy. Furthermore, overlapping neural activation may disguise underlying functional separation between pain and empathy for pain because of the limited spatial resolution currently possible in fMRI. Recent fMRI studies used new analytical methods to address the problem of low spatial resolution, but results are still mixed on whether neural activation patterns in the AI and ACC represent a process specific to physical pain (Corradi-Dell’Acqua et al., 2011; Iannetti et al., 2013; Wager et al., 2013; Rütgen et al., 2015a,b). These limitations highlight the need for additional evidence to determine if there is a common psychological mechanism underlying the experience of both physical pain and empathy for pain.
Pharmacological intervention constitutes an alternative approach for addressing the psychological commonality between empathy for pain and personal pain experience. If similar neurochemical and psychological computations of one’s own pain are also involved in processing another’s pain, pharmacologically inhibiting the neural circuits for experiencing one’s own pain should also inhibit experiences of another’s pain. Alternatively, if pain experience and empathy for pain are fundamentally different psychological processes, pharmacologically inhibiting pain should not affect empathy for pain.
We tested these alternative predictions using the analgesic acetaminophen (or paracetamol, under its international denotation). Acetaminophen, the active ingredient in Tylenol, is the most popular painkiller in the USA. An estimated 23% of all US adults consume a drug containing acetaminophen during an average week (Kaufman et al., 2002). Multiple randomized controlled trials document acetaminophen’s analgesic proprieties, including clinically significant effects on dental, arthritic and postoperative pain (for reviews, see Hyllested et al., 2002; Perrott et al., 2004; Zhang et al., 2004; Toms et al., 2008; McNicol et al., 2011; De Oliveira et al., 2015). Acetaminophen also has analgesic effects in studies using experimental pain inductions, such as cold pressor, nasal dry air or thermal laser stimulation (e.g. Nielsen et al., 1991; Bromm et al., 1992; Yuan et al., 1998; Renner et al., 2007; but Olesen et al., 2007). Because acetaminophen reduces neural activity in the ACC and AI during social pain (DeWall et al., 2010), we hypothesized that this analgesic would also impair empathy when witnessing another person in physical or social pain.
Research on the effect of acetaminophen on empathy for pain also adds to the emerging literature on the neurochemical basis of empathy. Though accumulating evidence suggests a role of oxytocin (for a review, see Barraza and Zak, 2013; but see Singer et al., 2008), the endogenous opioid system (Rütgen et al., 2015a,b), and serotonin (e.g. Kuypers et al., 2014) in modulating empathy, the degree to which the neurochemical modulation of empathy is related to the physical pain experience is not well understood. Testing the effect of a well-established analgesic such as acetaminophen on reducing empathy for pain thus provides an important step toward establishing a neurochemical link between the experience of physical pain and empathy for pain.
Research overview
In two randomized, double-blind, parallel-group, placebo-controlled trials, we tested the effect of acetaminophen on empathy while participants read vignettes describing hypothetical people in physical pain (e.g. cutting a finger) or social pain (e.g. death of father). Participants in the second experiment also met other study participants, two of whom ostensibly ostracized a third participant during a virtual ball-tossing game, an actual event of social pain. To test whether personal pain mediated the effects of acetaminophen on empathy for others’ pain, participants in Experiment 2 also rated the unpleasantness of white noise blasts and completed measures of empathy while visualizing another study participant receiving the same blasts. In both experiments, we also tested the effect of acetaminophen on empathic affect and cognition (for reviews, see e.g. Davis, 1994; Preston and De Waal, 2002; Decety and Jackson, 2004). We operationalized cognitive empathy as perceiving another person’s pain and affective empathy as the distress in response to another’s pain and the empathic concern for another’s well-being.
Materials and methods
Participants
Eighty undergraduate students in Experiment 1 (26 females; Mage = 19.4, SD = 1.44; 59 Whites, 7 Asian-Americans, 3 African-Americans, 11 mixed race/others) and 114 undergraduate students in Experiment 2 (48 females; Mage = 18.8, SD = 1.31; 83 Whites, 12 Asian-Americans, 7 African-Americans, 12 mixed race/others) participated for partial course credit toward their introductory psychology requirement. Four participants in Experiment 1 and seven participants in Experiment 2 dropped out at various stages during the study. We retained these participants when they had provided sufficient data for a particular set of analyses. The Institutional Review Board at the Ohio State University approved all experimental procedures.
Statistical power
In Experiment 1, we determined sample size based on previous research which indicated that a sample size of about 40 participants per cell provides sufficient power to detect a behavioral effect of acetaminophen (Durso et al., 2015). For Experiment 2, a power-analysis based on a power criterion of (1−β) = 0.80 and effect sizes obtained in Experiment 1 indicated that a mean cell size of n = 54 was sufficient to replicate significant findings. In addition to this power analysis, we took sample attrition into account when predetermining the sample size of Experiment 2.
Pharmacological procedures
In Experiments 1 and 2, the pharmacological procedures were identical. After signing up for the experiment, participants received an email about the risk factors associated with acetaminophen (e.g. currently taking a drug containing acetaminophen, a history of liver disorder, an allergic reaction to acetaminophen or a history of alcohol abuse) and asked them to refrain from participation if they had any of these risk factors. To facilitate drug absorption, we also asked participants to refrain from consuming food for three hours before the experiment.
Upon arrival, participants gave informed consent and were randomly assigned to consume a liquid containing 1000 mg acetaminophen (Experiment 1: n = 40; Experiment 2: n = 59) or a placebo (Experiment 1: n = 40; Experiment 2: n = 55). Acetaminophen and placebo solutions were prepared by Pharmacy Specialists Compounding Pharmacy (Altamonte Springs, Florida; http://www.makerx.com/). The drug solution consisted of acetaminophen (100 mg/ml) dissolved in Ora-Plus suspension liquid and flavored with Ora-Sweet Syrup. The placebo solution consisted of Avicel Microcrystalline powder (100 mg/ml) dissolved in the same vehicle. Participants and the experimenter were blind to drug condition. Participants were only told that they would consume a liquid containing either acetaminophen or placebo. The experimenter assigned drug condition using a random number generator and did not know whether she administered drug or placebo.
Next, the experimenter led participants to individual cubicles. We waited 60 minutes for the drug to be absorbed (Møller et al., 2000; Randles et al., 2013; Durso et al., 2015) before administering measures of general affect and empathy. During the initial portion of this time, participants completed questionnaires not analyzed for this study. After completing all the tasks, participants guessed whether they had received acetaminophen or placebo. Before participants left, the experimenter reminded them to refrain from taking acetaminophen or drinking more than two alcoholic beverages in the upcoming 15 h.
Experiment 1
‘General affect’ was measured with the Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988). Participants rated their current affect (i.e. ‘right now’) on 10 positive (e.g. ‘excited’) and 10 negative (e.g. ‘irritable’) items on a scale from 1 (‘Very slightly or not at all’) to 5 (‘Extremely’). We averaged items to create measures of positive (α = 0.85) and negative affect (α = 0.82).
Empathy scenarios
Participants rated eight short scenarios (Bruneau et al., 2012) describing various protagonists experiencing physical pain (cutting a finger, catching fingers in a slammed door, scraping a shin and stepping barefoot on a thumb tack) or social pain (father passing away, getting rejected from college, disapproval after a bad sports performance, overhearing being disliked). Half of the protagonists had female names. Scenario order was randomized for each participant. For each scenario, we measured ‘perceived pain’ with two measures. First, participants rated the pain of each protagonist using a scale from 1 (‘No pain at all’) to 5 (‘Worst possible pain’). Second, participants rated on three items how much each protagonist felt ‘hurt’, ‘wounded’ and ‘pained’ (Buckley et al., 2004) on scales ranging from 1 (‘Not at all’) to 5 (‘Extremely’). We averaged items to create perceived hurt feeling measures across physical (0.89 ≤ α ≤ 0.94) and social pain scenarios (0.82 ≤ α ≤ 0.83). Within each scenario type, both perceived pain ratings correlated highly, rs(76) ≥ 0.61, Ps > 0.001. Therefore, we standardized and averaged these measures into indices of perceived physical and social pain. Participants also rated their ‘personal distress’ when reading each scenario. On a scale from 1 (‘Not at all’) to 5 (‘Extremely’), participants rated the extent to which they felt ‘uncomfortable’, ‘pained’, ‘bothered’, ‘unpleasant’, ‘distress’, as well as ‘wanted to cringe’ while imagining the feelings of each scenario protagonist. We averaged items to create separate personal distress measures for physical (0.95 ≤ α ≤ 0.96) and social pain scenarios (0.90 ≤ α ≤ 0.94).
Experiment 2
About 45 min after drug administration, participants gathered in groups of four to eight in a large room where they engaged for 15 min in a relationship closeness induction task (Twenge et al., 2001, Experiment 4). The experimenter asked participants to get to know each other, using a list of provided questions (e.g. ‘Where are you from?’). Participants chose which questions to answer and in which order. This task was intended to make subsequent tasks involving other study participants relevant. As in Experiment 1, we administered all critical measures at least 60 min after drug administration. Participants completed the PANAS (Watson et al., 1988) as a measure of ‘general affect’. We averaged items to create positive (α = 0.89) and negative (α = 0.74) affect measures. In this experiment, we used three different paradigms to test for the effect of acetaminophen on empathy. First, participants completed a similar version of the hypothetical scenario measures used in Experiment 1. Second, we measured participants’ sensitivity to noise pain and empathy to other’s noise pain. Third, we measured empathic responses when witnessing an actual incident of social pain. Participants completed all empathy measures within less than two hours after drug administration.
Empathy scenarios
Participants read the same eight physical and social pain empathy scenarios as in Experiment 1. After reading each scenario, participants rated ‘perceived pain’ of the protagonist, using a scale from −4 (‘Worst possible pain’) to +4 (‘Most possible pleasure’). We reverse-coded participants’ ratings, so higher ratings indicated higher empathy for pain. Using the same measure as in Experiment 1, participants rated their ‘personal distress’ while reading each of the physical and social pain scenarios. We averaged items to create separate personal distress measures for physical (αs = 0.93) and social pain scenarios (0.91 ≤ α ≤ 0.93). Extending the measurement of empathy in Experiment 1, participants also rated their ‘empathic concern’ while reading each pain scenario, using an established scale (Batson et al., 1995). On six items, participants indicated the extent to which they felt empathic concern (e.g. ‘sympathetic’, ‘compassionate’), using a scale from 1 (‘Not at all’) to 5 (‘Extremely’). We averaged items to create separate empathic concern scales for physical (0.82 ≤ α ≤ 0.87) and social pain scenarios (0.83 ≤ α ≤ 0.86).
Noise pain
After playing a competitive game with another (anonymous) ostensible study participant (Bushman and Baumeister, 1998, Study 3; data to be reported elsewhere), participants received four blasts of 2 s white noise (75–105 dB) in random order through headphones. To capture a shared affective mechanism for pain and empathy for pain (Singer et al., 2004), we specifically measured unpleasantness of the noise blasts (Price et al., 1983). Consequently, participants rated each noise blast on a scale from 1 (‘Not unpleasant at all’) to 10 (‘Extremely unpleasant’). We averaged these ratings across noise blasts into a measure of ‘affective noise pain’. Next, participants imagined another (anonymous) study participant receiving the same noise blasts. Completing the same measures of perceived pain as in Experiment 1, participants rated the extent to which the other participant was bound to experience pain and hurt feelings (0.91 ≤ α ≤ 0.93). Pain and hurt feelings ratings correlated highly for each noise blast, r(106)s ≥ 0.74, Ps < 0.001. We averaged pain and hurt feelings ratings across each noise blast, standardized the two measures, and averaged them to create a measure of ‘perceived noise pain’.
Participants rated their ‘personal distress’ and ‘empathic concern’ while picturing their partner receiving each noise blast using the items from the empathy scenarios. We averaged items across noise blasts to create ‘personal distress’ (0.85 ≤ α ≤ 0.95) and ‘empathic ‘concern (0.82 ≤ α ≤ 0.90) scales for noise pain.
Cyberball
After completing the noise pain paradigm, participants watched two other study participants ostracize a third participant during a virtual ball-tossing game, ‘Cyberball’ (Williams and Jarvis, 2006; Wesselmann et al., 2009). In actuality, the computer simulated the players, who tossed the ball to each other for 60 rounds. After the third round, two players ostracized the third player for the rest of the game, not tossing the ball to this player anymore. After the game, participants completed measures of empathy for each of the three players.
We used the same measures as in Experiment 1 to measure perceived pain in each of the three players. Participants rated the extent to which each player experienced pain and hurt feelings during the game. We averaged hurt feelings items to create a perceived hurt feelings measure for each player (0.82 ≤ α ≤ 0.91). Pain and hurt feelings ratings correlated, r(112)s ≥ 0.36, Ps < 0.001. We standardized and averaged these ratings separately for each player, to create measures of perceived social pain. Using the same items as in response to the empathy scenarios, participants rated the extent to which they felt ‘personal distress’ and ‘empathic concern’ while imagining how each of the three players must have felt during the game. For each player rated, we averaged items to create personal distress (0.89 ≤ α ≤ 0.94) and empathic concern scales (0.89 ≤ α ≤ 0.92).
In addition to the empathy measures, participants completed an established measure of ‘perceived negativity’ (Berntson et al., 2011) after each empathy scenario and after watching the Cyberball game. On a scale from −5 (‘Extremely negative’) to +5 (‘Extremely positive’), participants rated the extent to which each scenario as well as the events during the game were positive or negative. We averaged ratings across physical and across social pain scenarios. To reduce burden, participants did not complete this measure after receiving the noise stimuli.
Results
Preliminary analyses
Blinding
Participants in Experiment 1 were not able to identify above chance whether they had taken acetaminophen or placebo, Pearson’s χ2(1, n = 76) = 0.00, P = 1.00, ϕ = 0.00. Unexpectedly, some participants in Experiment 2 were able to accurately identify whether they had taken acetaminophen or placebo, Pearson’s χ2 (1, n = 113) = 6.49, P = 0.011, ϕ = 0.24. When the data from the two experiments were combined, participants identified above chance whether they had taken acetaminophen or placebo, Pearson’s χ2(1, n = 189) = 3.90, P = 0.048, ϕ = 0.14. However, adjusting for perceived drug consumption did not affect results, except one: the effect of acetaminophen on perceived pain of the ostracized Cyberball player in Experiment 2, which went from P = 0.043 to P = 0.091. We did not control for perceived drug consumption in subsequent analyses reported here. For these same analyses including perceived drug condition as a covariate see Supplementary Tables S1–S4.
Effects by scenario and noise level
To determine whether the effect of acetaminophen on empathy differed between scenarios, we conducted repeated measurement Analyses of Variances (ANOVAs) with Drug Condition as the between-subjects factor and Scenario as the within-subjects factor. In both Experiments 1 and 2, the Drug Condition × Scenario interaction did not significantly predict perceived pain or personal distress in response to physical pain scenarios, Fs ≤ 1.86, Ps ≥ 0.135, , or social pain scenarios, Fs ≤ 2.41, Ps ≥ 0.068, . In Experiment 1, The Drug Condition × Scenario interaction approached significance when predicting personal distress while witnessing social pain (P = 0.068), indicating that the effect of acetaminophen on personal distress depended on scenario. However, this effect was in the same direction for each scenario. We therefore averaged perceived pain, personal distress, and empathic concern ratings within physical and social pain scenarios in both Experiments 1 and 2, and tested the effect of acetaminophen on these aggregated measures.
Similarly, in Experiment 2 we conducted repeated measurement ANOVAs with Drug Condition as the between-subjects factor and noise level as the within-subjects factor to determine whether the effect of acetaminophen on noise unpleasantness and empathy measures differed across noise levels. The Drug Condition × Noise Level interaction did not significantly predict noise unpleasantness, nor did it predict perceived pain, personal distress or empathic concern when imagining noise blasts inflicted on another study participant, F(3,315)s ≤ 2.45, Ps ≥ 0.064, . The Drug Condition × Noise Level interaction approached significance when predicting noise unpleasantness (P = 0.064). However, acetaminophen relative to placebo affected experienced noise unpleasantness in the same direction at all noise levels. We thus averaged noise unpleasantness and empathy ratings across all noise blast levels in Experiment 2.
Main results
Experiment 1
Experiment 1 provided a first test of the hypothesis that acetaminophen reduces empathy for another’s pain. Table 1 displays the effects of acetaminophen relative to the placebo condition on measures of perceived pain, personal distress and general affect. As predicted, acetaminophen reduced perceived pain and personal distress when reading scenarios about people in both physical and social pain; the effect of acetaminophen on perceived social pain was marginally significant. Furthermore, acetaminophen did not significantly affect general positive or negative affect.
General affect and empathy scenario measures by drug condition (Experiment 1)
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| General affecta | |||||||
| Positive affect | 2.14 | 0.65 | 2.21 | 0.70 | 0.19 | 0.662 | 0.003 |
| Negative affect | 1.62 | 0.62 | 1.42 | 0.44 | 2.55 | 0.115 | 0.034 |
| Physical pain scenarios | |||||||
| Perceived painb | −0.22 | 1.00 | 0.22 | 0.82 | 4.66 | 0.034 | 0.058 |
| Personal distress | 2.15 | 0.89 | 2.75 | 1.01 | 7.68 | 0.007 | 0.092 |
| Social pain scenarios | |||||||
| Perceived painb | −0.19 | 1.01 | 0.19 | 0.74 | 3.49 | 0.066 | 0.044 |
| Personal distress | 2.00 | 0.78 | 2.45 | 0.85 | 5.92 | 0.017 | 0.072 |
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| General affecta | |||||||
| Positive affect | 2.14 | 0.65 | 2.21 | 0.70 | 0.19 | 0.662 | 0.003 |
| Negative affect | 1.62 | 0.62 | 1.42 | 0.44 | 2.55 | 0.115 | 0.034 |
| Physical pain scenarios | |||||||
| Perceived painb | −0.22 | 1.00 | 0.22 | 0.82 | 4.66 | 0.034 | 0.058 |
| Personal distress | 2.15 | 0.89 | 2.75 | 1.01 | 7.68 | 0.007 | 0.092 |
| Social pain scenarios | |||||||
| Perceived painb | −0.19 | 1.01 | 0.19 | 0.74 | 3.49 | 0.066 | 0.044 |
| Personal distress | 2.00 | 0.78 | 2.45 | 0.85 | 5.92 | 0.017 | 0.072 |
Notes. dfs = 1,76; two participants failed to complete the scenario measures, reducing sample size in these analyses to n = 78.
adfs = 1,72; due to a programming oversight, we did not collect the PANAS data from our first four participants.
bAveraged composite of standardized perceived pain subscales.
General affect and empathy scenario measures by drug condition (Experiment 1)
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| General affecta | |||||||
| Positive affect | 2.14 | 0.65 | 2.21 | 0.70 | 0.19 | 0.662 | 0.003 |
| Negative affect | 1.62 | 0.62 | 1.42 | 0.44 | 2.55 | 0.115 | 0.034 |
| Physical pain scenarios | |||||||
| Perceived painb | −0.22 | 1.00 | 0.22 | 0.82 | 4.66 | 0.034 | 0.058 |
| Personal distress | 2.15 | 0.89 | 2.75 | 1.01 | 7.68 | 0.007 | 0.092 |
| Social pain scenarios | |||||||
| Perceived painb | −0.19 | 1.01 | 0.19 | 0.74 | 3.49 | 0.066 | 0.044 |
| Personal distress | 2.00 | 0.78 | 2.45 | 0.85 | 5.92 | 0.017 | 0.072 |
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| General affecta | |||||||
| Positive affect | 2.14 | 0.65 | 2.21 | 0.70 | 0.19 | 0.662 | 0.003 |
| Negative affect | 1.62 | 0.62 | 1.42 | 0.44 | 2.55 | 0.115 | 0.034 |
| Physical pain scenarios | |||||||
| Perceived painb | −0.22 | 1.00 | 0.22 | 0.82 | 4.66 | 0.034 | 0.058 |
| Personal distress | 2.15 | 0.89 | 2.75 | 1.01 | 7.68 | 0.007 | 0.092 |
| Social pain scenarios | |||||||
| Perceived painb | −0.19 | 1.01 | 0.19 | 0.74 | 3.49 | 0.066 | 0.044 |
| Personal distress | 2.00 | 0.78 | 2.45 | 0.85 | 5.92 | 0.017 | 0.072 |
Notes. dfs = 1,76; two participants failed to complete the scenario measures, reducing sample size in these analyses to n = 78.
adfs = 1,72; due to a programming oversight, we did not collect the PANAS data from our first four participants.
bAveraged composite of standardized perceived pain subscales.
Experiment 2
We replicated and extended our findings from Experiment 1. Table 2 displays effects of acetaminophen relative to the placebo condition on general affect and empathic responses to the scenario measures. Replicating Experiment 1, acetaminophen reduced personal distress when reading about physical and social pain. Unlike Experiment 1, acetaminophen did not change perceived physical or social pain. In addition, we extended findings of Experiment 1 to other-focused empathic affect; acetaminophen reduced empathic concern for others depicted in physical or social pain.
General affect and empathy scenario measures by drug condition (Experiment 2)
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| General affect | |||||||
| Positive affect | 2.54 | 0.71 | 2.62 | 0.85 | 0.32 | 0.322 | 0.003 |
| Negative affect | 1.27 | 0.33 | 1.27 | 0.36 | 0.00 | 0.997 | 0.000 |
| Physical pain scenarios | |||||||
| Perceived pain | 2.70 | 0.67 | 2.55 | 1.05 | 0.83 | 0.364 | 0.007 |
| Personal distress | 2.42 | 0.85 | 2.94 | 0.90 | 9.82 | 0.002 | 0.081 |
| Empathic concern | 1.68 | 0.55 | 2.00 | 0.74 | 6.95 | 0.010 | 0.058 |
| Social pain scenarios | |||||||
| Perceived pain | 2.63 | 0.75 | 2.58 | 0.90 | 0.09 | 0.770 | 0.001 |
| Personal distress | 2.26 | 0.77 | 2.57 | 0.86 | 3.95 | 0.049 | 0.034 |
| Empathic concern | 2.04 | 0.66 | 2.31 | 0.76 | 4.07 | 0.046 | 0.035 |
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| General affect | |||||||
| Positive affect | 2.54 | 0.71 | 2.62 | 0.85 | 0.32 | 0.322 | 0.003 |
| Negative affect | 1.27 | 0.33 | 1.27 | 0.36 | 0.00 | 0.997 | 0.000 |
| Physical pain scenarios | |||||||
| Perceived pain | 2.70 | 0.67 | 2.55 | 1.05 | 0.83 | 0.364 | 0.007 |
| Personal distress | 2.42 | 0.85 | 2.94 | 0.90 | 9.82 | 0.002 | 0.081 |
| Empathic concern | 1.68 | 0.55 | 2.00 | 0.74 | 6.95 | 0.010 | 0.058 |
| Social pain scenarios | |||||||
| Perceived pain | 2.63 | 0.75 | 2.58 | 0.90 | 0.09 | 0.770 | 0.001 |
| Personal distress | 2.26 | 0.77 | 2.57 | 0.86 | 3.95 | 0.049 | 0.034 |
| Empathic concern | 2.04 | 0.66 | 2.31 | 0.76 | 4.07 | 0.046 | 0.035 |
Note. dfs = 1,112.
General affect and empathy scenario measures by drug condition (Experiment 2)
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| General affect | |||||||
| Positive affect | 2.54 | 0.71 | 2.62 | 0.85 | 0.32 | 0.322 | 0.003 |
| Negative affect | 1.27 | 0.33 | 1.27 | 0.36 | 0.00 | 0.997 | 0.000 |
| Physical pain scenarios | |||||||
| Perceived pain | 2.70 | 0.67 | 2.55 | 1.05 | 0.83 | 0.364 | 0.007 |
| Personal distress | 2.42 | 0.85 | 2.94 | 0.90 | 9.82 | 0.002 | 0.081 |
| Empathic concern | 1.68 | 0.55 | 2.00 | 0.74 | 6.95 | 0.010 | 0.058 |
| Social pain scenarios | |||||||
| Perceived pain | 2.63 | 0.75 | 2.58 | 0.90 | 0.09 | 0.770 | 0.001 |
| Personal distress | 2.26 | 0.77 | 2.57 | 0.86 | 3.95 | 0.049 | 0.034 |
| Empathic concern | 2.04 | 0.66 | 2.31 | 0.76 | 4.07 | 0.046 | 0.035 |
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| General affect | |||||||
| Positive affect | 2.54 | 0.71 | 2.62 | 0.85 | 0.32 | 0.322 | 0.003 |
| Negative affect | 1.27 | 0.33 | 1.27 | 0.36 | 0.00 | 0.997 | 0.000 |
| Physical pain scenarios | |||||||
| Perceived pain | 2.70 | 0.67 | 2.55 | 1.05 | 0.83 | 0.364 | 0.007 |
| Personal distress | 2.42 | 0.85 | 2.94 | 0.90 | 9.82 | 0.002 | 0.081 |
| Empathic concern | 1.68 | 0.55 | 2.00 | 0.74 | 6.95 | 0.010 | 0.058 |
| Social pain scenarios | |||||||
| Perceived pain | 2.63 | 0.75 | 2.58 | 0.90 | 0.09 | 0.770 | 0.001 |
| Personal distress | 2.26 | 0.77 | 2.57 | 0.86 | 3.95 | 0.049 | 0.034 |
| Empathic concern | 2.04 | 0.66 | 2.31 | 0.76 | 4.07 | 0.046 | 0.035 |
Note. dfs = 1,112.
Furthermore, Experiment 2 showed that acetaminophen not only reduces empathy to hypothetical scenarios, but also to an actual incident of social pain. Table 3 depicts effects of acetaminophen relative to placebo on the Cyberball empathy measures separately for each Cyberball player. As expected, acetaminophen reduced perceived pain, empathic concern, and personal distress (marginally) when witnessing ostracism. As predicted, this effect was restricted to the ostracized victim; acetaminophen did not significantly reduce empathy for the ostracism perpetrators.
Cyberball empathy measures by drug condition (Experiment 2)
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| Ostracized player | |||||||
| Perceived paina | −0.17 | 0.99 | 0.19 | 0.87 | 4.20 | 0.043 | 0.036 |
| Personal distress | 2.25 | 0.98 | 2.61 | 1.01 | 3.70 | 0.057 | 0.032 |
| Empathic Concern | 1.68 | 0.74 | 2.05 | 0.92 | 5.73 | 0.018 | 0.049 |
| Included first player | |||||||
| Perceived paina | −0.06 | 0.61 | 0.06 | 1.00 | 0.63 | 0.430 | 0.006 |
| Personal distress | 1.24 | 0.47 | 1.34 | 0.61 | 1.01 | 0.318 | 0.009 |
| Empathic concern | 1.08 | 0.29 | 1.14 | 0.37 | 0.77 | 0.383 | 0.007 |
| Included second player | |||||||
| Perceived paina | −0.04 | 0.80 | 0.04 | 1.06 | 0.23 | 0.631 | 0.002 |
| Personal distress | 1.22 | 0.34 | 1.38 | 0.56 | 3.34 | 0.070 | 0.029 |
| Empathic concern | 1.08 | 0.23 | 1.13 | 0.31 | 0.78 | 0.381 | 0.007 |
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| Ostracized player | |||||||
| Perceived paina | −0.17 | 0.99 | 0.19 | 0.87 | 4.20 | 0.043 | 0.036 |
| Personal distress | 2.25 | 0.98 | 2.61 | 1.01 | 3.70 | 0.057 | 0.032 |
| Empathic Concern | 1.68 | 0.74 | 2.05 | 0.92 | 5.73 | 0.018 | 0.049 |
| Included first player | |||||||
| Perceived paina | −0.06 | 0.61 | 0.06 | 1.00 | 0.63 | 0.430 | 0.006 |
| Personal distress | 1.24 | 0.47 | 1.34 | 0.61 | 1.01 | 0.318 | 0.009 |
| Empathic concern | 1.08 | 0.29 | 1.14 | 0.37 | 0.77 | 0.383 | 0.007 |
| Included second player | |||||||
| Perceived paina | −0.04 | 0.80 | 0.04 | 1.06 | 0.23 | 0.631 | 0.002 |
| Personal distress | 1.22 | 0.34 | 1.38 | 0.56 | 3.34 | 0.070 | 0.029 |
| Empathic concern | 1.08 | 0.23 | 1.13 | 0.31 | 0.78 | 0.381 | 0.007 |
Notes. dfs = 1,112.
aAveraged composite of standardized perceived pain subscales.
Cyberball empathy measures by drug condition (Experiment 2)
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| Ostracized player | |||||||
| Perceived paina | −0.17 | 0.99 | 0.19 | 0.87 | 4.20 | 0.043 | 0.036 |
| Personal distress | 2.25 | 0.98 | 2.61 | 1.01 | 3.70 | 0.057 | 0.032 |
| Empathic Concern | 1.68 | 0.74 | 2.05 | 0.92 | 5.73 | 0.018 | 0.049 |
| Included first player | |||||||
| Perceived paina | −0.06 | 0.61 | 0.06 | 1.00 | 0.63 | 0.430 | 0.006 |
| Personal distress | 1.24 | 0.47 | 1.34 | 0.61 | 1.01 | 0.318 | 0.009 |
| Empathic concern | 1.08 | 0.29 | 1.14 | 0.37 | 0.77 | 0.383 | 0.007 |
| Included second player | |||||||
| Perceived paina | −0.04 | 0.80 | 0.04 | 1.06 | 0.23 | 0.631 | 0.002 |
| Personal distress | 1.22 | 0.34 | 1.38 | 0.56 | 3.34 | 0.070 | 0.029 |
| Empathic concern | 1.08 | 0.23 | 1.13 | 0.31 | 0.78 | 0.381 | 0.007 |
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| Ostracized player | |||||||
| Perceived paina | −0.17 | 0.99 | 0.19 | 0.87 | 4.20 | 0.043 | 0.036 |
| Personal distress | 2.25 | 0.98 | 2.61 | 1.01 | 3.70 | 0.057 | 0.032 |
| Empathic Concern | 1.68 | 0.74 | 2.05 | 0.92 | 5.73 | 0.018 | 0.049 |
| Included first player | |||||||
| Perceived paina | −0.06 | 0.61 | 0.06 | 1.00 | 0.63 | 0.430 | 0.006 |
| Personal distress | 1.24 | 0.47 | 1.34 | 0.61 | 1.01 | 0.318 | 0.009 |
| Empathic concern | 1.08 | 0.29 | 1.14 | 0.37 | 0.77 | 0.383 | 0.007 |
| Included second player | |||||||
| Perceived paina | −0.04 | 0.80 | 0.04 | 1.06 | 0.23 | 0.631 | 0.002 |
| Personal distress | 1.22 | 0.34 | 1.38 | 0.56 | 3.34 | 0.070 | 0.029 |
| Empathic concern | 1.08 | 0.23 | 1.13 | 0.31 | 0.78 | 0.381 | 0.007 |
Notes. dfs = 1,112.
aAveraged composite of standardized perceived pain subscales.
Experiment 2 also showed that acetaminophen reduces empathy for noise pain. Table 4 displays effects of acetaminophen on empathic responses when imagining another participant receiving the noise blasts. As expected, acetaminophen reduced perceived pain, personal distress and empathic concern in response to picturing noise pain.
Noise pain measures by drug condition (Experiment 2)
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| Noise unpleasantness | 5.86 | 1.35 | 6.48 | 1.27 | 6.05 | 0.016 | 0.055 |
| Perceived noise paina | −0.21 | 0.79 | 0.24 | 1.08 | 6.08 | 0.015 | 0.055 |
| Personal distressb | 1.75 | 0.61 | 2.06 | 0.92 | 4.51 | 0.036 | 0.041 |
| Empathic concern | 1.42 | 0.45 | 1.68 | 0.63 | 6.12 | 0.015 | 0.055 |
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| Noise unpleasantness | 5.86 | 1.35 | 6.48 | 1.27 | 6.05 | 0.016 | 0.055 |
| Perceived noise paina | −0.21 | 0.79 | 0.24 | 1.08 | 6.08 | 0.015 | 0.055 |
| Personal distressb | 1.75 | 0.61 | 2.06 | 0.92 | 4.51 | 0.036 | 0.041 |
| Empathic concern | 1.42 | 0.45 | 1.68 | 0.63 | 6.12 | 0.015 | 0.055 |
Notes. dfs = 1,105; seven participants did not complete the noise pain measures, reducing sample size in these analyses to n = 107.
aAveraged composite of standardized perceived pain subscales.
bdf = 1,106; compared with the rest of these analyses, one more participant completed this measure.
Noise pain measures by drug condition (Experiment 2)
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| Noise unpleasantness | 5.86 | 1.35 | 6.48 | 1.27 | 6.05 | 0.016 | 0.055 |
| Perceived noise paina | −0.21 | 0.79 | 0.24 | 1.08 | 6.08 | 0.015 | 0.055 |
| Personal distressb | 1.75 | 0.61 | 2.06 | 0.92 | 4.51 | 0.036 | 0.041 |
| Empathic concern | 1.42 | 0.45 | 1.68 | 0.63 | 6.12 | 0.015 | 0.055 |
| . | Acetaminophen . | Placebo . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Dependent variable . | M . | SD . | M . | SD . | F . | P . | . |
| Noise unpleasantness | 5.86 | 1.35 | 6.48 | 1.27 | 6.05 | 0.016 | 0.055 |
| Perceived noise paina | −0.21 | 0.79 | 0.24 | 1.08 | 6.08 | 0.015 | 0.055 |
| Personal distressb | 1.75 | 0.61 | 2.06 | 0.92 | 4.51 | 0.036 | 0.041 |
| Empathic concern | 1.42 | 0.45 | 1.68 | 0.63 | 6.12 | 0.015 | 0.055 |
Notes. dfs = 1,105; seven participants did not complete the noise pain measures, reducing sample size in these analyses to n = 107.
aAveraged composite of standardized perceived pain subscales.
bdf = 1,106; compared with the rest of these analyses, one more participant completed this measure.
The noise pain paradigm also allowed us to test whether acetaminophen reduced responsiveness to the noise pain of others as well as the self. As predicted, acetaminophen reduced the unpleasantness of the noise blasts for participants (see Table 4), in addition to effects on empathy.
Gender did not consistently moderate the effect of acetaminophen on empathy for the hypothetical protagonists in the empathy scenarios, the ostracized Cyberball player or the other participant receiving noise blasts, Fs ≤ 2.80, Ps ≥ 0.098, . These results suggest that acetaminophen reduces empathy for pain to a similar extent in both men and women.
Replicating Experiment 1, acetaminophen did not change positive or negative affect. Furthermore, controlling for perceived negativity of the empathy scenarios or the events during the Cyberball game did not diminish effects of acetaminophen on empathy, indicating that perceived negativity of the painful events does not account for acetaminophen’s effects on empathy (see Supplementary Tables S5 and S6). Finally, omitting participants who indicated they were not at all motivated or who failed to indicate their motivation did not weaken results; if anything, it strengthened our findings (see Supplementary Tables S7–10).
Mediation
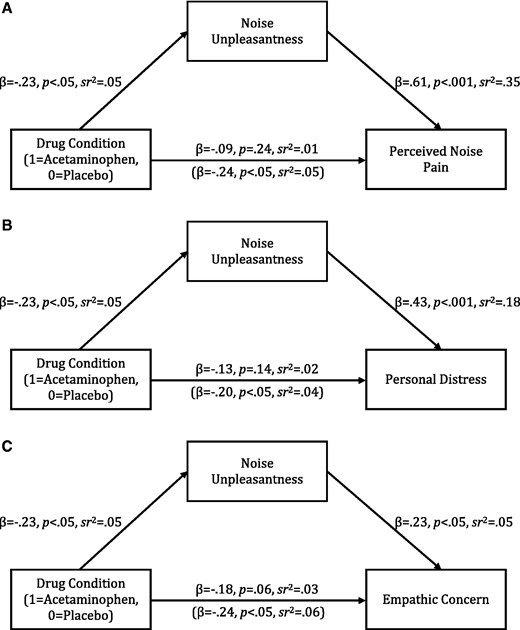
Experiment 2 allowed us to test whether acetaminophen predicted perceived noise pain of the partner (see Figure 1, upper panel), personal distress of the partner (see Figure 1, middle panel) and empathic concern for the partner (see Figure 1, lower panel) through personal noise unpleasantness. We drew 5000 bootstrapping samples to construct bias-corrected 95% confidence intervals around these indirect effects using the PROCESS macro for SPSS (Hayes, 2012). As expected, the indirect effect of drug condition through personal noise unpleasantness was significant for perceived noise pain of the partner [−0.272, −0.034], personal distress of the partner [−0.207, −0.024] and empathic concern for the partner [−0.143, −0.011]. Noise unpleasantness did not account for the effects of acetaminophen on perceived pain, personal distress, or empathic concern in response to the scenario measures or the ostracized Cyberball player; all 95% confidence intervals constructed around the indirect effects through noise unpleasantness included zero. These findings suggest that acetaminophen has overlapping effects on personal pain and empathy for the pain of others, but only for pain in the same stimulus domain.
Model of the effect of drug condition on (A) perceived noise pain, (B) personal distress and (C) empathic concern through noise unpleasantness (Experiment 2). Statistics in parentheses indicate the effect of drug condition on empathy measures while controlling for noise unpleasantness.
Meta-analytic integration
Because effects of acetaminophen on empathic affect and cognition were not always significant, we conducted a fixed-effects meta-analysis, adjusting effect sizes for multiple measurements of empathic affect and cognition within a sample (Borenstein et al., 2009). We tested whether acetaminophen reduced perceived pain, personal distress, and empathic concern for others’ pain, independent from pain modality and across experiments. As hypothesized, acetaminophen relative to placebo reduced perceived pain in others, Hedge’s g = 0.23, z = 2.45, P = 0.014, 95% CI [0.047, 0.41], personal distress in response to another’s pain, Hedge’s g = 0.47, z = 3.95, P < 0.001, 95% CI [0.24, 0.72], and empathic concern for others in pain, Hedge’s g = 0.45, z = 2.79, P = 0.005, 95% CI [0.13, 0.76].
Discussion
As predicted, acetaminophen reduced empathy for other people’s pain across two experiments. In Experiment 1, acetaminophen reduced perceived pain and personal distress when reading hypothetical scenarios depicting people in physical or social pain. Experiment 2 partially replicated and extended the findings of Experiment 1, showing that acetaminophen also decreased empathic concern when reading about the pains of others. Experiment 2 also showed that acetaminophen reduced perceived pain, personal distress and empathic concern in response to an actual incident of social pain: ostracism during Cyberball (Williams and Jarvis, 2006; Wesselmann et al., 2009). This effect was limited to the ostracism victim and did not extend to the ostracism perpetrators, suggesting that these effects are not due to a general tendency for less extreme social judgments under the influence of acetaminophen. These reductions in empathic concern are particularly noteworthy because empathic concern is thought to be a key driver of prosocial behavior (Batson, 1998). It is thus conceivable that acetaminophen may also reduce willingness to help others in physical or emotional distress, though this prediction has to be tested in future studies.
Furthermore, we explored two explanations for the effect of acetaminophen on empathy. In both experiments, acetaminophen failed to affect general positive or negative affect, suggesting that flattened mood did not account for our findings. Also, if reduced perceived negativity underlay effects of acetaminophen on empathy, blunted perceived negativity of empathy arousing-events should account for acetaminophen’s effects on empathic affect and cognition. However, as Experiment 2 showed, this was clearly not the case. These findings thus suggest that acetaminophen decreases empathy over-and-above an effect on general negative evaluation (Durso et al., 2015) and therefore may be due to a different mechanism. It remains an open question, however, whether acetaminophen affects other specific types of emotions (e.g. anger, sadness) in response to empathy-arousing events, and whether such a reduction in affectivity could account for the effects of acetaminophen on decreased empathy.
Experiment 2 also showed that acetaminophen had correlated effects on both personal pain and empathy for the pain of others. Experiment 2 showed that acetaminophen reduced affective noise pain, specifically noise unpleasantness, while also reducing perceived pain, personal distress, and empathic concern in response to noise pain in others. Noise pain accounted for the effect of acetaminophen on reduced empathy for others’ noise pain, but not empathy for pain in other domains. These findings suggest that empathy for pain relies on a closely matching, domain-specific affective simulation of pain, consistent with the simulation account of empathy (Preston and De Waal, 2002).
We also tested whether manipulating pain responsiveness affects different aspects of empathy. In contrast to the effects of acetaminophen on personal distress and empathic concern, the effect on perceived pain was inconsistent across experiments. However, a meta-analysis of our data across samples and pain modalities showed that acetaminophen significantly reduced perceived pain when witnessing others in pain. Inspecting effect sizes suggested moderate effects of acetaminophen on empathic affect measures, and a small effect of acetaminophen on empathic cognition (Cohen, 1988).
Unexpectedly, participants in Experiment 2 guessed above chance whether they had consumed acetaminophen or the placebo. In contrast, participants in Experiment 1 did not, consistent with previous research Durso et al. (2015) and Randles et al. (2013). We did not collect systematic data to further probe this finding. However, controlling for perceived drug consumption across both experiments did not substantially alter results (see Supplementary Tables S1–S4), giving us confidence that participants’ awareness of their drug status did not affect their responses on the empathy measures in a systematic way.
Implications and future directions
The finding that the physical painkiller acetaminophen reduces empathy for pain has several theoretical and practical implications. First, these findings underscore the need for further research on the neurochemical bases of empathy for pain. Although some research implicates a role of oxytocin in empathy (Barraza and Zak, 2013), there are no documented effects of acetaminophen on oxytocin. Thus, it remains unclear which neurotransmitter system is involved in the effects of acetaminophen, but evidence supports roles for the serotonin, opioid, cannabinoid and prostanoid systems, among others (Toussaint et al., 2010; Graham et al., 2013). Each of these neurochemical systems is poised to affect anterior cingulate and insula activity and thus could be a candidate for explaining the effect of acetaminophen on reduced empathy.
A single drug, acetaminophen, affected both the experience of pain and empathy for pain suggesting a common neurochemical process underpinning these experiences. These findings provide pharmacological support for simulation theories of empathy (e.g. Gallese and Goldman, 1998; Preston and De Waal, 2002; Decety and Jackson, 2004; Singer, 2009). However, acetaminophen affects multiple neurochemical and psychological processes (DeWall et al., 2010; Randles et al., 2013; Durso et al., 2015; for reviews on the neurochemistry of acetaminophen, see e.g. Toussaint et al., 2010; Graham et al., 2013) and it is therefore possible that acetaminophen could act via one mechanism to reduce physical pain and another to reduce empathy for pain. Thus, although the current findings are consistent with a shared mechanism hypothesis, more research is needed to conclusively demonstrate this commonality.
Second, a pharmacological approach may be useful in research on the functional neuroanatomy of empathy, for which there are multiple theoretical frameworks (Premack and Woodruff, 1978; Baron-Cohen, 1997; Preston and De Waal, 2002; Frith and Frith, 2003; Decety and Jackson, 2004; Iacoboni, 2009; Saxe, 2009; Dvash and Shamay-Tsoory, 2014; Zaki, 2014). Pharmacologically manipulating the psychological experience of empathy should lead to changes in the neural regions involved in generating empathic responses. Analogous to a lesion approach, pharmacologically reducing empathy provides an underused methodology for testing causal hypotheses about the neural underpinnings of the psychological experience of empathy. A variant of this approach was recently used to show that placebo analgesia reduces empathy for others, an effect mediated by the opioid system (Rütgen et al., 2015a,b).
Finally, our findings raise important questions about the societal impact of acetaminophen. Empathy for another’s pain and suffering is an important motivator of compassionate actions (Eisenberg and Miller, 1987; Batson, 1998). Empathic affect and cognition can also serve as brakes on aggressive and hurtful impulses (Miller and Eisenberg, 1988; but see Vachon et al., 2014). Based on the drug-induced reductions in empathy seen here, acetaminophen, and potentially other analgesics, might interfere with social processes that are critical for the promotion of social bonds and social order. Given the millions of people who consume acetaminophen on a regular basis (Kaufman et al., 2002), the social consequences of acetaminophen could be far more costly than previously assumed. However, little is known about how clinically approved drug therapies such as analgesics influence social cognition, affect or behavior. Clearly, more research on the social side effects of these medications is needed.
Conclusion
In summary, our research is the first to show that the popular physical painkiller acetaminophen can reduce empathy to the pain of others. Physical pain is an aversive experience, and undoubtedly many people can attest to acetaminophen’s beneficial ability to suppress physical pain. However, acetaminophen can also have unappreciated psychosocial side effects by interrupting the fundamental capacity to empathically connect with other people’s painful experiences. Quite literally, acetaminophen reduces one’s ability to feel another’s pain.
Acknowledgements
We are grateful to Caitlyn Erra, Linda Fleming, Katie Lewis, Megan Mohler, Paige Schlagbaum and Diandra Showe for their invaluable assistance with data collection.
Funding
This project was supported in part by funds from the Ohio State University as well as from the National Center for Advancing Translational Sciences (Award Number 8KL2TR000112-05). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. Data are available upon request.
References