-
PDF
- Split View
-
Views
-
Cite
Cite
Alexis E Whitton, Alan I Green, Diego A Pizzagalli, Robert M Roth, Jill M Williams, Mary F Brunette, Potent Dopamine D2 Antagonists Block the Reward-Enhancing Effects of Nicotine in Smokers With Schizophrenia, Schizophrenia Bulletin, Volume 45, Issue 6, November 2019, Pages 1300–1308, https://doi.org/10.1093/schbul/sby185
Close - Share Icon Share
Abstract
Antipsychotics that are potent dopamine (DA) D2 receptor antagonists have been linked to elevated levels of nicotine dependence in smokers with schizophrenia. Because activation of D2 receptors mediates motivation for nicotine, we examined whether potent D2 antagonists would diminish nicotine’s ability to stimulate reward processing—a mechanism that may drive compensatory increases in smoking. Smokers with schizophrenia (n = 184) were recruited and stratified into medication groups based on D2 receptor antagonist potency. The effects of smoking on reward function were assessed using a probabilistic reward task (PRT), administered pre- and post-smoking. The PRT used an asymmetrical reinforcement schedule to produce a behavioral response bias, previously found to increase under conditions (including smoking) that enhance mesolimbic DA signaling. Among the 98 participants with valid PRT data and pharmacotherapy that could be stratified into D2 receptor antagonism potency, a medication × smoking × block interaction emerged (P = .005). Post-hoc tests revealed a smoking × block interaction only for those not taking potent D2 antagonists (P = .007). This group exhibited smoking-related increases in response bias (P < .001) that were absent in those taking potent D2 antagonists (P > .05). Our findings suggest that potent D2 antagonists diminish the reward-enhancing effects of nicotine in smokers with schizophrenia. This may be a mechanism implicated in the increased rate of smoking often observed in patients prescribed these medications. These findings have important clinical implications for the treatment of nicotine dependence in schizophrenia.
Introduction
Nicotine use in individuals with schizophrenia is extremely common, with recent estimates indicating that 80% of individuals with this condition smoke.1 Greater smoking in this population has been linked to worse symptoms,2 susceptibility to respiratory and cardiovascular diseases, and early mortality;3 hence, reducing smoking in these patients is a key priority. Evidence suggests that patients taking first-generation antipsychotics, many of which produce potent dopamine (DA) D2 receptor antagonism, experience greater difficulty quitting with evidence-based cessation treatment.4,5 Because these medications are foundational to the management of schizophrenia, an important question is whether DA D2 receptor antagonists modulate nicotine’s reinforcing effects.
Nicotine’s addictive properties are driven in large part by activity within the mesolimbic DA reward system.6,7 Nicotine stimulates DA neurons via nicotinic acetylcholine receptors (nAChRs) in the ventral tegmental area, causing the release of DA into striatal synapses.8,9 Human positron emission tomography studies have shown evidence of acute increases in DA signaling in the ventral striatum following nicotine intake.10 Nicotine has been found to enhance reward processing on several paradigms in both animals11 and humans,12 and is associated with increased neural activation in key reward regions.13 Conversely, nicotine withdrawal has been linked to decreases in reward processing.14
Mesolimbic DA system dysfunction is central to the pathophysiology of schizophrenia,15,16 and may be a pathway through which comorbid schizophrenia and nicotine dependence develops.17 Supporting this notion, deficits in reward learning—a process reliant on phasic striatal DA signaling18—is correlated with increased nicotine dependence in schizophrenia.19 Furthermore, the nAChR partial agonist varenicline has been shown to restore nicotine withdrawal-related deficits in reversal learning (a process that is also reliant on phasic striatal DA signaling)20 in rodents,21 and has been found to improve cessation and prolong abstinence,22 and boost reward processing in patients with schizophrenia.23
Central to reducing smoking rates in schizophrenia is understanding how antipsychotic medications moderate nicotine’s rewarding effects. Evidence indicates that treatment with potent DA D2 receptor antagonists increases smoking in individuals with schizophrenia.24–26 Although the precise mechanisms are unknown, potent DA D2 receptor antagonists may diminish nicotine’s ability to stimulate mesolimbic DA release, which may drive a compensatory increase in smoking to achieve the same level of stimulation. Supporting this, studies in psychiatrically healthy smokers show that pretreatment with the potent DA D2 antagonist haloperidol resulted in increased blood plasma nicotine (due to greater smoking during a free smoking period) compared to placebo.27 Pretreatment with haloperidol has also been linked to a faster rate of smoking and greater total puffing time than treatment with the DA agonist, bromocriptine.28 Similarly, 8 weeks of haloperidol treatment has been linked to significant increases in nicotine dependence in individuals with schizophrenia.26
Conversely, smoking reductions have been observed in patients treated with lower DA D2 receptor affinity medications.26,29 Specifically, clozapine—a potent blocker of the serotonin 5-HT2A receptor and the norepinephrine α2 receptor, as well as a weak DA D2 receptor antagonist30—has been associated with reduced daily cigarette use.29 Similarly, aripiprazole—a partial agonist at both pre- and post-synaptic DA D2 and 5-HT1A receptors—has been associated with reduced nicotine dependence compared to haloperidol.26 Quetiapine is also of interest, given its broad spectrum of action, including weak DA D2 receptor antagonism.31
Together, these findings suggest that potent DA D2 receptor antagonists may reduce the effects of nicotine on reward processing in smokers with schizophrenia; however, no study to date has tested this hypothesis. We aimed to address this gap by examining the effects of smoking on reward learning, a key aspect of reward processing that is defined as the process by which behavior is modified as a function of prior reinforcement. Reward learning was assessed using a behavioral probabilistic reward task (PRT)32 that uses an asymmetrical reinforcement schedule to induce a response bias toward a more frequently rewarded (rich) stimulus. In healthy individuals, stimulants that increase phasic DA signaling—including nicotine—potentiated response bias.12 Individual differences in response bias have also been linked to DA release,33 DA transporter binding,34 and functional connectivity between key reward system nodes in nonsmokers.34 Furthermore, response bias has been found to be inversely related to cigarette craving intensity in smokers.35 Consistent with prior studies,12 we predicted that individuals with schizophrenia would show greater response bias after smoking compared to following abstinence, but that this increase would be attenuated in individuals taking potent DA D2 receptor antagonists.
Methods
Participants
Participants were 184 chronic tobacco smokers with schizophrenia (122 male) enrolled in a multisite smoking cessation study (Randomized Controlled Trial of a Motivational Decision Support System [RCTEDSS]; ClinicalTrials.gov #NCT02086162). The aim of this multisite study was to evaluate whether the use of a web-based decision support system resulted in higher rates of initiation of smoking cessation treatment compared to use of a computerized educational pamphlet. The primary outcomes of this study will be reported separately. To be eligible, participants had to be English speaking, daily smokers, with a diagnosis of schizophrenia or schizoaffective disorder according to the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV)36 and stable in mental health treatment. Exclusion criteria were psychiatric instability (a score >75 on the Brief Psychiatric Rating Scale [BPRS]37); current moderate/severe alcohol/drug dependence; pregnancy/breastfeeding.
Measures
Demographic and Clinical Assessments.
Demographics and psychiatric history were assessed with a structured interview at baseline. Diagnosis of schizophrenia, schizoaffective disorder, and current drug/alcohol dependence was determined using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV). Psychotic symptom severity was assessed using the BPRS. All assessments were administered by trained interviewers.
Classification of Antipsychotic Medication.
Current antipsychotic medications were obtained by self-report and confirmed by record review. Medications were classified into groups based on the potency with which they blocked the DA D2 receptor. This classification was carried out based on a theoretical hypothesis that the relative potency of DA D2 receptor antagonism might contribute to the reward-enhancing effects of nicotine in patients with schizophrenia. Medications known to be potent DA D2 receptor antagonists included haloperidol, fluphenazine, chlorpromazine, risperidone, olanzapine, and paliperidone.38 Participants who were taking a combination of antipsychotics that included a potent D2 receptor antagonist and other types of antipsychotics were included in this class. Medications were considered “non-potent DA D2 antagonists” if they were known to have lower DA D2 antagonism (clozapine and quetiapine)38–40 or DA D2 partial agonism, resulting in a lack of “functional antagonism” at DA D2 receptors (aripiprazole).41–44 Patients who were not taking antipsychotic medication were excluded, as were those taking lurasidone, due to its complex pharmacologic profile.
Measures of Nicotine Dependence.
Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND),45 and smoking urges were assessed using the Tiffany Questionnaire of Smoking Urges (QSU).46 Both measures have been found to have adequate psychometric properties among smokers with schizophrenia.47 Expired carbon monoxide (CO)—associated with greater levels of smoking48—was obtained using a Smokerlyzer monitor (Bedfont Scientific).
Probabilistic Reward Task.
To probe reward responsiveness, all participants completed the PRT.32 Rooted within signal detection theory, the PRT allows for an objective assessment of the propensity to modulate behavior based on prior reinforcement. The PRT consisted of schematic faces presented on a monitor, each with 2 eyes, a nose, and a horizontal line for a mouth. On each trial, a fixation cross appeared for 500 ms, followed by a mouthless face. After 500 ms, either a “short mouth” (10 mm) or “long mouth” (11 mm) was presented for 100 ms. Participants indicated via keypress whether the short or long mouth appeared. There were 2 blocks of 100 trials, and 40 correct trials in each block were followed by a monetary reward (Correct!! You won 20 cents). Long mouths and short mouths were presented at equal frequencies; however, unbeknownst to participants, one of the mouth lengths (the “rich stimulus”) was rewarded 3 times more frequently than the other mouth length (the “lean stimulus”). Participants were told to try and win as much money as possible, as they would be given the money that they earned. To avoid practice effects from pre- to post-smoking, 2 versions of the PRT were used—a version where the length of the mouth varied, and a version where the length of the nose varied, with different versions administered in a counterbalanced order across participants.
Procedure
Participants were recruited through flyers and clinician referral. After providing informed consent, demographic, smoking, diagnostic, and symptom information was collected. Eligible participants returned to do the PRT and other cognitive assessments. They were instructed to smoke prior to the study visit. At the beginning of the visit, participants indicated that they had smoked within the past hour, which was confirmed with a breath CO sample ≥10 ppm. They then completed cognitive assessments, and after being abstinent for at least 1 hour, completed the PRT for the first time. They then smoked one of their own cigarettes over a period of 15 minutes. After this smoking period, a second breath CO sample was taken, and participants repeated a counterbalanced version of the PRT performed within 10 minutes of finishing smoking.
Data Reduction and Statistical Analyses
PRT Quality Assurance and Data Reduction
In line with prior PRT studies,32,49 data were subjected to quality control analyses that involved excluding trials with reaction times of less than 150 ms or greater than 2500 ms, and data from participants who had accuracy scores below 50% (indicative of below-chance level performance), or from participants who had more than 25% reaction time outlier trials. Next, signal detection analysis50 was used to calculate response bias (the tendency to bias responding to the rich stimulus) and discriminability (the ability to accurately distinguish between the 2 mouth sizes), according to the following formulas:
In accordance with recommendations,51 0.5 was added to every cell of the detection matrix to allow for log-transformation of cells containing a zero.
Statistical Analysis
A repeated measures analysis of variance (ANOVA) with medication (D2 antagonism+, D2 antagonism−) as the between-subject factor, and smoking (pre-smoke, post-smoke) and block (block 1, block 2) as within-subject factors, was used to examine the degree to which the presence of potent D2 receptor antagonists moderated the effects of smoking on response bias. Significant interaction effects were followed up with tests of simple effects. Furthermore, bivariate Pearson correlations were used to examine the degree to which smoking-induced changes in response bias correlated with nicotine dependence on the FTND and smoking urges on the QSU.
Results
Sample
Sample characteristics are shown in table 1. Half of the sample were African American (n = 93, 50.5%), most were non-Hispanic, (n = 158, 85.9%) and unemployed (n = 166, 90.2%). The mean (±SEM) age was 45.73 ± 11.35 and range = 20–70, and the sample was two-thirds male (n = 122, 66.3%). Symptoms were moderate (BPRS score = 41.32 ± 11.45, mean lifetime hospitalizations = 11.65 ± 15.0). They reported smoking 15.57 ± 13.34 cigarettes/day in the past 3 months.
Demographic, Clinical and Smoking Characteristics of the Entire Sample and Medication Subgroups
| . | D2 antagonism + (n = 71) . | D2 antagonism − (n = 27) . | Group Comparisons . | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, M (SD) | 45.0 | (10.8) | 47.4 | (9.1) | t = 1.04, P = .30 |
| Years of education, M (SD) | 11.9 | (2.1) | 12.2 | (2.7) | t = 0.58, P = .56 |
| Male, N (%) | 50 | (70.4) | 13 | (48.1) | χ2 = 0.43, P = .04 |
| Race | χ2 = 3.03, P = .39 | ||||
| White, N (%) | 21 | (29.6) | 10 | (37.0) | — |
| Black, N (%) | 34 | (47.9) | 14 | (51.9) | — |
| Other, N (%) | 16 | (22.5) | 3 | (11.1) | — |
| Hispanic, N (%) | 15 | (21.1) | 0 | (0.0) | χ2 = 6.74, P = .01 |
| Employed, N (%) | 8 | (11.3) | 2 | (7.4) | χ2 = 1.63, P = .80 |
| Clinical characteristics | |||||
| Hosp., M (SD) | 13.4 | (18.6) | 9.2 | (7.4) | t = 1.15, P = .25 |
| BPRS Total, M (SD) | 39.6 | (11.3) | 43.2 | (12.2) | t = 1.36, P = .18 |
| BPRS Pos Sx, M (SD) | 11.6 | (5.0) | 13.1 | (5.1) | t = 1.31, P = .19 |
| BPRS Neg Sx, M (SD) | 9.5 | (3.7) | 9.6 | (3.3) | t = 0.04, P = .97 |
| BPRS Agit Sx, M (SD) | 8.3 | (2.7) | 9.3 | (4.0) | t = 1.40, P = .17 |
| BPRS Dep Sx, M (SD) | 10.2 | (4.7) | 11.3 | (4.6) | t = 0.97, P = .34 |
| Smoking characteristics | |||||
| Cigs./day, M (SD) | 16.1 | (13.4) | 15.5 | (17.3) | t = 0.19, P = .85 |
| FTND Total, M (SD) | 5.2 | (2.0) | 5.4 | (2.0) | t = 0.42, P = .67 |
| Baseline CO, M (SD) | 28.4 | (22.0) | 30.2 | (19.4) | t = 0.37, P = .71 |
| Post-smoke CO, M (SD) | 29.2 | (20.4) | 30.4 | (19.0) | t = 0.23, P = .82 |
| QSU Total, M (SD) | 142.8 | (33.0) | 146.7 | (48.4) | t = 0.46, P = .65 |
| . | D2 antagonism + (n = 71) . | D2 antagonism − (n = 27) . | Group Comparisons . | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, M (SD) | 45.0 | (10.8) | 47.4 | (9.1) | t = 1.04, P = .30 |
| Years of education, M (SD) | 11.9 | (2.1) | 12.2 | (2.7) | t = 0.58, P = .56 |
| Male, N (%) | 50 | (70.4) | 13 | (48.1) | χ2 = 0.43, P = .04 |
| Race | χ2 = 3.03, P = .39 | ||||
| White, N (%) | 21 | (29.6) | 10 | (37.0) | — |
| Black, N (%) | 34 | (47.9) | 14 | (51.9) | — |
| Other, N (%) | 16 | (22.5) | 3 | (11.1) | — |
| Hispanic, N (%) | 15 | (21.1) | 0 | (0.0) | χ2 = 6.74, P = .01 |
| Employed, N (%) | 8 | (11.3) | 2 | (7.4) | χ2 = 1.63, P = .80 |
| Clinical characteristics | |||||
| Hosp., M (SD) | 13.4 | (18.6) | 9.2 | (7.4) | t = 1.15, P = .25 |
| BPRS Total, M (SD) | 39.6 | (11.3) | 43.2 | (12.2) | t = 1.36, P = .18 |
| BPRS Pos Sx, M (SD) | 11.6 | (5.0) | 13.1 | (5.1) | t = 1.31, P = .19 |
| BPRS Neg Sx, M (SD) | 9.5 | (3.7) | 9.6 | (3.3) | t = 0.04, P = .97 |
| BPRS Agit Sx, M (SD) | 8.3 | (2.7) | 9.3 | (4.0) | t = 1.40, P = .17 |
| BPRS Dep Sx, M (SD) | 10.2 | (4.7) | 11.3 | (4.6) | t = 0.97, P = .34 |
| Smoking characteristics | |||||
| Cigs./day, M (SD) | 16.1 | (13.4) | 15.5 | (17.3) | t = 0.19, P = .85 |
| FTND Total, M (SD) | 5.2 | (2.0) | 5.4 | (2.0) | t = 0.42, P = .67 |
| Baseline CO, M (SD) | 28.4 | (22.0) | 30.2 | (19.4) | t = 0.37, P = .71 |
| Post-smoke CO, M (SD) | 29.2 | (20.4) | 30.4 | (19.0) | t = 0.23, P = .82 |
| QSU Total, M (SD) | 142.8 | (33.0) | 146.7 | (48.4) | t = 0.46, P = .65 |
Note: M, Mean; SD, standard deviation, Hosp., hospitalizations; BPRS, Brief Psychiatric Rating Scale; Pos Sx, Positive Symptom subscale; Neg Sx, Negative Symptom subscale; Agit Sx, Agitation Symptom subscale; Dep Sx, Depressive Symptom subscale; FTND, Fagerstrom Test of Nicotine Dependence; CO, carbon monoxide; QSU, Questionnaire of Smoking Urges.
Demographic, Clinical and Smoking Characteristics of the Entire Sample and Medication Subgroups
| . | D2 antagonism + (n = 71) . | D2 antagonism − (n = 27) . | Group Comparisons . | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, M (SD) | 45.0 | (10.8) | 47.4 | (9.1) | t = 1.04, P = .30 |
| Years of education, M (SD) | 11.9 | (2.1) | 12.2 | (2.7) | t = 0.58, P = .56 |
| Male, N (%) | 50 | (70.4) | 13 | (48.1) | χ2 = 0.43, P = .04 |
| Race | χ2 = 3.03, P = .39 | ||||
| White, N (%) | 21 | (29.6) | 10 | (37.0) | — |
| Black, N (%) | 34 | (47.9) | 14 | (51.9) | — |
| Other, N (%) | 16 | (22.5) | 3 | (11.1) | — |
| Hispanic, N (%) | 15 | (21.1) | 0 | (0.0) | χ2 = 6.74, P = .01 |
| Employed, N (%) | 8 | (11.3) | 2 | (7.4) | χ2 = 1.63, P = .80 |
| Clinical characteristics | |||||
| Hosp., M (SD) | 13.4 | (18.6) | 9.2 | (7.4) | t = 1.15, P = .25 |
| BPRS Total, M (SD) | 39.6 | (11.3) | 43.2 | (12.2) | t = 1.36, P = .18 |
| BPRS Pos Sx, M (SD) | 11.6 | (5.0) | 13.1 | (5.1) | t = 1.31, P = .19 |
| BPRS Neg Sx, M (SD) | 9.5 | (3.7) | 9.6 | (3.3) | t = 0.04, P = .97 |
| BPRS Agit Sx, M (SD) | 8.3 | (2.7) | 9.3 | (4.0) | t = 1.40, P = .17 |
| BPRS Dep Sx, M (SD) | 10.2 | (4.7) | 11.3 | (4.6) | t = 0.97, P = .34 |
| Smoking characteristics | |||||
| Cigs./day, M (SD) | 16.1 | (13.4) | 15.5 | (17.3) | t = 0.19, P = .85 |
| FTND Total, M (SD) | 5.2 | (2.0) | 5.4 | (2.0) | t = 0.42, P = .67 |
| Baseline CO, M (SD) | 28.4 | (22.0) | 30.2 | (19.4) | t = 0.37, P = .71 |
| Post-smoke CO, M (SD) | 29.2 | (20.4) | 30.4 | (19.0) | t = 0.23, P = .82 |
| QSU Total, M (SD) | 142.8 | (33.0) | 146.7 | (48.4) | t = 0.46, P = .65 |
| . | D2 antagonism + (n = 71) . | D2 antagonism − (n = 27) . | Group Comparisons . | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, M (SD) | 45.0 | (10.8) | 47.4 | (9.1) | t = 1.04, P = .30 |
| Years of education, M (SD) | 11.9 | (2.1) | 12.2 | (2.7) | t = 0.58, P = .56 |
| Male, N (%) | 50 | (70.4) | 13 | (48.1) | χ2 = 0.43, P = .04 |
| Race | χ2 = 3.03, P = .39 | ||||
| White, N (%) | 21 | (29.6) | 10 | (37.0) | — |
| Black, N (%) | 34 | (47.9) | 14 | (51.9) | — |
| Other, N (%) | 16 | (22.5) | 3 | (11.1) | — |
| Hispanic, N (%) | 15 | (21.1) | 0 | (0.0) | χ2 = 6.74, P = .01 |
| Employed, N (%) | 8 | (11.3) | 2 | (7.4) | χ2 = 1.63, P = .80 |
| Clinical characteristics | |||||
| Hosp., M (SD) | 13.4 | (18.6) | 9.2 | (7.4) | t = 1.15, P = .25 |
| BPRS Total, M (SD) | 39.6 | (11.3) | 43.2 | (12.2) | t = 1.36, P = .18 |
| BPRS Pos Sx, M (SD) | 11.6 | (5.0) | 13.1 | (5.1) | t = 1.31, P = .19 |
| BPRS Neg Sx, M (SD) | 9.5 | (3.7) | 9.6 | (3.3) | t = 0.04, P = .97 |
| BPRS Agit Sx, M (SD) | 8.3 | (2.7) | 9.3 | (4.0) | t = 1.40, P = .17 |
| BPRS Dep Sx, M (SD) | 10.2 | (4.7) | 11.3 | (4.6) | t = 0.97, P = .34 |
| Smoking characteristics | |||||
| Cigs./day, M (SD) | 16.1 | (13.4) | 15.5 | (17.3) | t = 0.19, P = .85 |
| FTND Total, M (SD) | 5.2 | (2.0) | 5.4 | (2.0) | t = 0.42, P = .67 |
| Baseline CO, M (SD) | 28.4 | (22.0) | 30.2 | (19.4) | t = 0.37, P = .71 |
| Post-smoke CO, M (SD) | 29.2 | (20.4) | 30.4 | (19.0) | t = 0.23, P = .82 |
| QSU Total, M (SD) | 142.8 | (33.0) | 146.7 | (48.4) | t = 0.46, P = .65 |
Note: M, Mean; SD, standard deviation, Hosp., hospitalizations; BPRS, Brief Psychiatric Rating Scale; Pos Sx, Positive Symptom subscale; Neg Sx, Negative Symptom subscale; Agit Sx, Agitation Symptom subscale; Dep Sx, Depressive Symptom subscale; FTND, Fagerstrom Test of Nicotine Dependence; CO, carbon monoxide; QSU, Questionnaire of Smoking Urges.
Of those who completed the PRT (n = 174), 114 (65.5%) had valid data both prior to and post-smoking. Those who were excluded (81.7% male; mean age = 46.7, SD = 12.8) did not differ from those who were retained in terms of symptom severity (BPRS) or nicotine dependence and craving (FTND and QSU; all Ps > .20).
Smoking and Clinical Characteristics of Antipsychotic Medication Groups
Of those with valid PRT data, 98 could be classified as taking or not taking potent DA D2 receptor antagonists (taking potent D2 antagonists [D2 antagonism+], n = 71; not taking potent D2 antagonists [D2 antagonism−], n = 27). Table 2 shows how the medication profiles were grouped. Independent samples t tests revealed no group differences in the average number of cigarettes smoked daily (P = .85), pre-assessment expired CO (P = .71), post-smoking expired CO (P = .82), self-reported nicotine dependence on the FTND (P = .67), craving on the QSU (P = .65), or symptom severity on the BPRS (total score and subscales, all Ps > .15). However, the groups did differ with respect to gender and ethnicity, where the D2 antagonism + group contained a higher proportion of males, χ2 = 0.43, P = .04, and Hispanic individuals, χ2 = 6.74, P = .01.
Medication Profiles Classified as Having Potent DA D2 Receptor Antagonism (D2 antagonism +) and Non-potent DA D2 Receptor Antagonism (D2 Antagonism −)
| Medication . | D2 antagonism + (n = 71) . | D2 antagonism − (n = 27) . | n per Subcategory . |
|---|---|---|---|
| Aripiprazole | X | 11 | |
| Aripiprazole + quetiapine | X | 1 | |
| Chlorpromazine | X | 2 | |
| Chlorpromazine + quetiapine | X | 1 | |
| Clozapine | X | 7 | |
| Clozapine + haloperidol | X | 1 | |
| Clozapine + risperidone | X | 1 | |
| Fluphenazine | X | 12 | |
| Haloperidol | X | 13 | |
| Haloperidol + risperidone | X | 1 | |
| Olanzapine | X | 12 | |
| Paliperidone | X | 14 | |
| Quetiapine | X | 8 | |
| Risperidone | X | 14 | |
| Risperidone + aripiprazole | X | 1 | |
| Risperidone + olanzapine | X | 1 | |
| Ziprasidone | X | 2 |
| Medication . | D2 antagonism + (n = 71) . | D2 antagonism − (n = 27) . | n per Subcategory . |
|---|---|---|---|
| Aripiprazole | X | 11 | |
| Aripiprazole + quetiapine | X | 1 | |
| Chlorpromazine | X | 2 | |
| Chlorpromazine + quetiapine | X | 1 | |
| Clozapine | X | 7 | |
| Clozapine + haloperidol | X | 1 | |
| Clozapine + risperidone | X | 1 | |
| Fluphenazine | X | 12 | |
| Haloperidol | X | 13 | |
| Haloperidol + risperidone | X | 1 | |
| Olanzapine | X | 12 | |
| Paliperidone | X | 14 | |
| Quetiapine | X | 8 | |
| Risperidone | X | 14 | |
| Risperidone + aripiprazole | X | 1 | |
| Risperidone + olanzapine | X | 1 | |
| Ziprasidone | X | 2 |
Note: “X” shows how the different medication profiles (left-most column) within the sample were grouped.
Medication Profiles Classified as Having Potent DA D2 Receptor Antagonism (D2 antagonism +) and Non-potent DA D2 Receptor Antagonism (D2 Antagonism −)
| Medication . | D2 antagonism + (n = 71) . | D2 antagonism − (n = 27) . | n per Subcategory . |
|---|---|---|---|
| Aripiprazole | X | 11 | |
| Aripiprazole + quetiapine | X | 1 | |
| Chlorpromazine | X | 2 | |
| Chlorpromazine + quetiapine | X | 1 | |
| Clozapine | X | 7 | |
| Clozapine + haloperidol | X | 1 | |
| Clozapine + risperidone | X | 1 | |
| Fluphenazine | X | 12 | |
| Haloperidol | X | 13 | |
| Haloperidol + risperidone | X | 1 | |
| Olanzapine | X | 12 | |
| Paliperidone | X | 14 | |
| Quetiapine | X | 8 | |
| Risperidone | X | 14 | |
| Risperidone + aripiprazole | X | 1 | |
| Risperidone + olanzapine | X | 1 | |
| Ziprasidone | X | 2 |
| Medication . | D2 antagonism + (n = 71) . | D2 antagonism − (n = 27) . | n per Subcategory . |
|---|---|---|---|
| Aripiprazole | X | 11 | |
| Aripiprazole + quetiapine | X | 1 | |
| Chlorpromazine | X | 2 | |
| Chlorpromazine + quetiapine | X | 1 | |
| Clozapine | X | 7 | |
| Clozapine + haloperidol | X | 1 | |
| Clozapine + risperidone | X | 1 | |
| Fluphenazine | X | 12 | |
| Haloperidol | X | 13 | |
| Haloperidol + risperidone | X | 1 | |
| Olanzapine | X | 12 | |
| Paliperidone | X | 14 | |
| Quetiapine | X | 8 | |
| Risperidone | X | 14 | |
| Risperidone + aripiprazole | X | 1 | |
| Risperidone + olanzapine | X | 1 | |
| Ziprasidone | X | 2 |
Note: “X” shows how the different medication profiles (left-most column) within the sample were grouped.
Interactive Effects of Antipsychotic Type and Smoking on Reward Processing
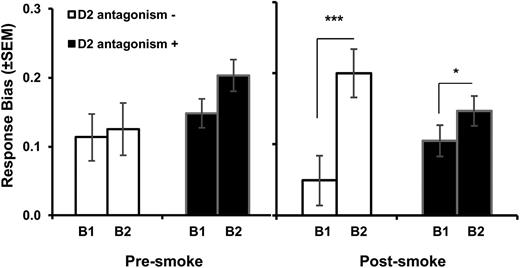
A significant 3-way interaction emerged from the medication (D2 antagonism+, D2 antagonism−) × smoking × block ANOVA, F(1,96) = 8.17, P = .005 ηp2 = .08 (figure 1). This interaction was followed up by considering all 2-way interactions, and Bonferroni-corrected post hoc tests of simple effects were conducted in cases where the 2-way interaction was significant. For brevity, only main effects and interactions involving smoking or medication are reported.
Response bias in block 1 (B1) and block 2 (B2), prior to smoking and post-smoking, for the D2 antagonism+ and D2 antagonism− groups. Bars show mean (±SEM). *P < .05; ***P < .001.
When examining the smoking × block interaction separately within each medication group, we observed a significant smoking × block interaction for the D2 antagonism− group, F(1,26) = 8.56, P = .007, ηp2 = 0.25, but not for the D2 antagonism+ group (P = .64). In the D2 antagonism− group, an increase in response bias from block 1 to block 2 of the task was observed following smoking (P < .001) but not prior to smoking (P = .72), indicating that smoking enhanced reward learning in this group.
When examining the medication × block interaction separately for each smoking condition, at pre-smoking, the medication × block interaction was not significant (P = .28) nor was there a main effect of medication (P = .14). For the post-smoking condition, however, a significant medication × block interaction emerged, F(1,96) = 9.07, P = .003, ηp2 = 0.09. Specifically, response bias increased from block 1 to block 2 in both the D2 antagonism− (block 1 = 0.05, block 2 = 0.20, P < .001) and the D2 antagonism+ group (block 1 = 0.11, block 2 = 0.15, P = 0.03); however, this increase was greater in the D2 antagonism− group.
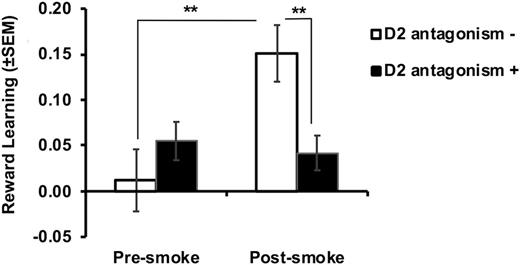
Figure 2 summarizes the interactive effects of medication and smoking on reward learning, conceptualized as the increase in response bias from block 1 to block 2. The increase in response bias was greater in the D2 antagonism− group, indicating greater reward learning in these patients.
Interactive effects of potent D2 antagonist and smoking on reward learning (defined as response bias in block 2 − response bias in block 1). Bars show mean (±SEM). **P < .01.
Independent samples t tests confirmed that the D2 antagonism+ and the D2 antagonism− groups did not differ significantly in terms of the ratio of rich to lean rewards that they received (summed across blocks) at pre- or post-smoking (both Ps > 0.10), indicating that effects were not due to differential rates of exposure to the asymmetrical reinforcement schedule.
Because the 2 medication groups differed in terms of gender and ethnic makeup, further analyses were conducted to examine these variables as potential confounds. To maximize statistical power, we performed 2 medication × smoking ANCOVAs containing reward learning as the dependent variable and gender/ethnicity as the covariate. For the model containing gender as a covariate, a medication × smoking interaction emerged, F(1,95) = 7.23, P = .008, ηp2 = 0.07. Bonferroni-corrected post hoc tests showed that after controlling for gender, reward learning increased from pre- to post-smoking in the D2 antagonism− group (P = .004), but not in the D2 antagonism+ group (P = .69). Furthermore, in this model, the main effect of gender was not significant, F(1,95) = 0.06, P = .80, ηp2 = 0.001, nor was the gender × smoking interaction, F(1,95) = 0.01, P = .64, ηp2 = 0.002. For the model containing ethnicity as a covariate, the medication × smoking interaction emerged, F(1,95) = 8.29, P = .005, ηp2 = 0.08. Post hoc tests showed that after controlling for ethnicity, reward learning increased from pre- to post-smoking in the D2 antagonism− group (P = .006), but not in the D2 antagonism+ group (P = .19). Similarly, the main effect of ethnicity was not significant in this model, F(1,95) = 1.11, P = .30, ηp2 = 0.01, and ethnicity did not interact with smoking, F(1,95) = 0.25, P = .62, ηp2 = 0.003. These results suggest that differences in the gender and ethnic makeup of the medication groups did not drive our primary findings.
Effects of Potent D2 Antagonists and Smoking on Discriminability
Mean discriminability at block 1 and block 2, pre- and post-smoking, across the 2 medication groups is shown in supplementary figure S1. No main effects or interactions involving medication or smoking emerged from the medication × smoking × block ANOVA on discriminability scores (all Ps > .10). This indicates that our primary findings were specific to reward processing, and not due to the effect of D2 antagonists or smoking on more general task performance.
Associations Between Smoking-Related Increases in Reward Processing and Smoking Severity
Reward learning was not correlated with scores on the FTND or QSU (both Ps > .05).
Discussion
The use of potent DA D2 receptor antagonists has been linked to increased smoking and difficulty quitting in smokers with schizophrenia.24–26 The aim of our study was to evaluate a putative mechanism for these effects by examining whether taking potent DA D2 receptor antagonists was associated with a reduction in nicotine’s ability to enhance reward learning. Consistent with predictions, smoking increased reward learning, but only in individuals who were not taking potent DA D2 antagonists. This effect was specific to reward learning, as effects were not observed on a measure of general task performance (ie, discriminability). Effects were also not attributable to demographic or clinical differences between the medication groups, or differences in nicotine dependence (FTND scores, expired CO, cigarettes per day), urges to smoke (QSU scores), or to post-smoking breath CO readings. Taken together, these findings point to a potential mechanistic explanation for increased smoking in individuals with schizophrenia who are treated with potent DA D2 receptor antagonists. Specifically, these antagonists appear to diminish the effects of tobacco smoking on reward processing. Although not directly assessed in this study, we suggest that this may be a factor that drives compensatory increases in smoking behavior in order to achieve the same level of reward/stimulation.
Our findings align with prior work linking greater tobacco smoking in patients with schizophrenia to the use of first-generation antipsychotics,52 and research showing reductions in dependence and craving severity in individuals prescribed second-generation antipsychotics, such as aripiprazole and clozapine.26,29 Although we are the first to show that increased smoking in those treated with potent DA D2 receptor antagonists may be related to a blunting of nicotine’s effects on reward processing, our interpretation is consistent with findings from an earlier study, which examined links between antipsychotic-related DA D2 receptor occupancy and smoking in schizophrenia. Specifically, in patients who were randomized to olanzapine or risperidone, de Hann and colleagues53 found that striatal D2 receptor occupancy following antipsychotic treatment (stable dose for at least 6 weeks) was associated with future smoking, where greater D2 receptor blockade predicted greater smoking frequency in the following 3 years. Recent studies suggest that nicotine may enhance associative conditioning.54,55 Therefore, a possible alternative explanation for our findings is that rather than blocking the effects of nicotine on reward sensitivity, potent DA D2 receptor antagonists may diminish nicotine’s ability to facilitate the formation of an association between the rich stimulus and reward receipt. This, and other studies linking use of typical antipsychotics to blunted striatal activation in response to monetary reward cues,56 supports the notion that potent DA D2 receptor antagonists induce changes in the brain reward pathways, which may confer increased risk for nicotine dependence in individuals with schizophrenia.
Our initial hypothesis was based around evidence pointing to increased smoking in schizophrenia patients taking potent DA D2 receptor antagonists;4,5 however, the 2 medication groups did not differ in smoking urges, dependence, or average number of cigarettes smoked per day. This may be because medication grouping occurred on a naturalistic basis, which meant that many participants were receiving several medications. It is possible that the polypharmacological profile of our sample may have reduced the magnitude of group differences in overt smoking behavior by comparison to other studies that have, for example, compared the effects of haloperidol monotherapy to no medication.24 Additionally, other measures of smoking behavior may be more sensitive to group differences in nicotine dependence. For example, although de Haan and colleagues53 found a relationship between antipsychotic-induced DA D2 receptor occupancy and future smoking, they failed to find an association between D2 receptor occupancy and smoking frequency at the time of scanning. As such, longitudinal measures of smoking may be more sensitive to medication-related differences in smoking behavior.
Our findings have important clinical implications for treating individuals with schizophrenia and co-occurring nicotine dependence. All patients with schizophrenia should be advised to quit and offered pharmacologic assistance with quitting. If a patient is unable to quit with evidence-based cessation treatment and is taking a potent DA D2 receptor antagonist, switching to a different antipsychotic with lower affinity DA D2 antagonism (eg, clozapine) or partial DA D2 agonism (eg, aripiprazole) may be appropriate prior to a second trial of evidence-based cessation treatment. This recommendation aligns with recent findings suggesting that clozapine may be an effective treatment for comorbid schizophrenia and nicotine dependence.57 In addition, when considering the use of a potent DA D2 receptor antagonist for a patient with schizophrenia who smokes, it may be beneficial to implement concurrent efforts aimed at minimizing increases in smoking behavior.
Some limitations must be kept in mind when interpreting our findings. In our study, medication groupings were naturalistic rather than randomly assigned. Although the groups did not differ in terms of major demographic, clinical, or smoking characteristics, future research is needed to determine whether the blunted effect of smoking on reward processing observed in the D2 antagonism+ group is specifically caused by potent DA D2 receptor antagonism. It is possible that separate factors may cause a weaker effect of smoking on reward processing in certain individuals who are also more likely to be prescribed potent DA D2 receptor antagonists. Future studies could also use direct administration of nicotine, rather than tobacco smoking, to ensure that effects are not confounded by ingestion of other substances in tobacco. Furthermore, in the pre-smoking condition, participants were tested after a 60-minute period of smoking abstinence. Because this corresponds approximately with the half-life of nicotine (1–2 hours), it is possible that the baseline response bias would have been influenced by some residual levels of nicotine. Additional studies should incorporate a longer abstinence period, while avoiding withdrawal, which worsens reward function.58 Finally, we did not examine smoking-induced changes in response bias in a non-psychiatric control sample. Future studies using a comparison sample are needed to determine whether the effects of smoking on response bias in patients not taking potent DA D2 receptor antagonists is normative or blunted in comparison with control smokers.
In sum, our findings indicate that the use of potent DA D2 receptor antagonists may reduce the ability of tobacco smoking to enhance reward-related processes in smokers with schizophrenia. Future longitudinal research is needed to examine the role of specific antipsychotic pharmacotherapies in the pharmacological management of schizophrenia and co-occurring tobacco smoking.
Funding
National Cancer Institute (R01CA168778).
Conflict of Interest
The funding body had no role in the preparation of this manuscript, interpretation of the findings, or the decision to publish this research. These findings were previously presented as a poster at the 73rd Annual Meeting of the Society of Biological Psychiatry in New York, May 10–12, 2018. Over the past 3 years, Dr Green has received funding for research studies from Novartis and Alkermes; he has served on a Data Safety Board for Eli Lilly, and he has served as an (unpaid) consultant to Otsuka and Alkermes. He is the coinventor of a patent, as well as a patent application, regarding development of a treatment of substance abuse. Over the past 3 years, Dr Brunette received funding from Alkermes to conduct research on medication treatment for co-occurring schizophrenia and alcohol use disorder. Drs Green and Brunette’s interests were reviewed and are managed by the Geisel School of Medicine at Dartmouth in accordance with their conflict of interest policies. Over the past 3 years, Dr Pizzagalli received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Posit Science, and Takeda for activities unrelated to this study. Dr Pizzagalli has a financial interest in BlackThorn Therapeutics, which has licensed the copyright to the Probabilistic Reward Task through Harvard University. Dr Pizzagalli’s interests were reviewed and are managed by McLean Hospital and Partners HealthCare in accordance with their conflict of interest policies. Drs. Whitton, Roth, and Williams report no biomedical financial interests or potential conflicts of interest. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.