-
PDF
- Split View
-
Views
-
Cite
Cite
Enda M Byrne, Manuel A R Ferreira, Angli Xue, Sara Lindström, Xia Jiang, Jian Yang, Douglas F Easton, Naomi R Wray, Georgia Chenevix-Trench, Is Schizophrenia a Risk Factor for Breast Cancer?—Evidence From Genetic Data, Schizophrenia Bulletin, Volume 45, Issue 6, November 2019, Pages 1251–1256, https://doi.org/10.1093/schbul/sby162
Close - Share Icon Share
Abstract
Observational epidemiological studies have found an association between schizophrenia and breast cancer, but it is not known if the relationship is a causal one. We used summary statistics from very large genome-wide association studies of schizophrenia (n = 40675 cases and 64643 controls) and breast cancer (n = 122977 cases and 105974 controls) to investigate whether there is evidence that the association is partly due to shared genetic risk factors and whether there is evidence of a causal relationship. Using LD-score regression, we found that there is a small but significant genetic correlation (rG) between the 2 disorders (rG = 0.14, SE = 0.03, P = 4.75 × 10–8), indicating shared genetic risk factors. Using 142 genetic variants associated with schizophrenia as instrumental variables that are a proxy for having schizophrenia, we estimated a causal effect of schizophrenia on breast cancer on the observed scale as bxy = 0.032 (SE = 0.009, P = 2.3 × 10–4). A 1 SD increase in liability to schizophrenia increases risk of breast cancer 1.09-fold. In contrast, the estimated causal effect of breast cancer on schizophrenia from 191 instruments was not significantly different from zero (bxy = −0.005, SE = 0.012, P = .67). No evidence for pleiotropy was found and adjusting for the effects of smoking or parity did not alter the results. These results provide evidence that the previously observed association is due to schizophrenia causally increasing risk for breast cancer. Genetic variants may provide an avenue to elucidating the mechanism underpinning this relationship.
Introduction
Individuals diagnosed with schizophrenia have increased mortality compared to the general population with a life expectancy of 10–15 years less than those without the disorder.1 Despite having increased levels of risk factors for cancer such as smoking and physical inactivity, epidemiological studies have found evidence that the age-specific incidence rates of most cancers are lower in patients with schizophrenia compared to the general population.2 Breast cancer is an exception to this trend, with several studies showing increased risk in women with schizophrenia. A systematic review of 13 studies found that 6 studies observed an increased risk in schizophrenia patients,3 and the 3 largest studies estimated standardized incidence ratios (SIR) of 1.11 (1.00–1.22),4 1.20 (1.05–1.38)5 and 1.15 (0.98–1.34).6 More recently, a study of insurance claims data in Taiwan found that the risk of breast cancer in female schizophrenia patients after adjustment for confounders was 1.94 (95% CI: 1.43–2.63).7 A meta-analysis including data from 125760 women estimated an SIR of 1.31 (95% CI: 1.14–1.50).8
The mechanism behind the increased rate of breast cancer among schizophrenia patients is unknown. A key question of interest is if the relationship is due to shared genetic and/or lifestyle risk factors or a consequence of the disease itself (eg, related to the disease process or treatment). Here, we use genome-wide association study (GWAS) summary statistics results to investigate the genetic relationship between schizophrenia and breast cancer. First, we investigate if there is a genetic correlation (rG) between the 2 disorders using single nucleotide polymorphisms (SNPs) throughout the genome. Second, we investigate if there is evidence of a causal relationship between the 2 disorders using Mendelian Randomization (MR).9
Methods
Analyses used summary statistics from the Breast Cancer Consortium (BCAC) GWAS10 of 122977 cases and 105974 controls for 11792543 SNPs and from the Schizophrenia Working Group of the Psychiatric Genomics Consortium11 of 40675 cases and 64643 controls for 5471613 SNPs. Merging the 2 data sets, retaining only HapMap 3 SNPs that were genotyped or well imputed in both, gave 937918 SNPs for use in LD-score regression (LDSR)12,13 analyses. LDSR was used to estimate the heritability attributable to common SNPs for both schizophrenia and breast cancer, as well as the rG between the 2 disorders.
Generalized Summary-based MR (GSMR)14 was used to evaluate the hypothesis that having schizophrenia (or the disease process leading to its development) is causally related to breast cancer risk, rather than the relationship being due to pleiotropy (the same genes having independent effects on both disorders) or to shared lifestyle factors. MR is a form of instrumental variable analysis that uses genetic variants associated with the exposure of interest as instrument-based predictors of the exposure which are a proxy of the exposure, to estimate the causal effect (bxy) of the exposure (x) on the outcome (y). In this study, we used genetic variants significantly associated with schizophrenia as instrument-based predictors of schizophrenia. We tested whether these instruments were associated with breast cancer, and conversely whether genetic variants associated with breast cancer risks were associated with schizophrenia. Under a model of causality where schizophrenia causally increases risk of breast cancer, we expect bxy for schizophrenia as exposure, but not bxy for breast cancer as exposure, to be significantly greater than zero. GSMR uses generalized least squares to estimate bxy and is more powerful than other commonly used MR methods because it appropriately accounts for the errors in the effect size estimates of the SNP instruments. Results from other MR methods are shown for comparison (supplementary table 1).
To derive sets of independent SNPs, to use as instruments for schizophrenia and breast cancer respectively, we conducted LD-clumping using PLINK, retaining SNPs with LD r2 < 0.005 and at least 5MB apart. This generated a list of essentially independent genome-wide significant SNPs for schizophrenia, and also for breast cancer. The GSMR analysis model fits a correlation matrix which accounts for any residual correlation between instruments.
A key assumption underlying inference of causality from MR analysis is that there is no pleiotropy—eg, in the case of breast cancer as the outcome trait and schizophrenia as the exposure, the key assumption is that SNPs do not influence breast cancer other than through the pathway that influences schizophrenia risk. To improve robustness of MR estimates, GSMR uses a heterogeneity test (called HEIDI-outlier) to assess the estimate of bxy at each of the instrument SNPs. SNPs that are extreme outliers in bxy estimate may indicate locus-specific pleiotropy and we therefore excluded such SNPs from the analysis.
Sensitivity Analyses—Adjusting for Smoking
One potential mechanism for increased risk of breast cancer in schizophrenia patients is smoking. Schizophrenia patients smoke at higher rate than the general population15 and smoking is a modest risk factor for breast cancer.16 Moreover, one of the genome-wide significant SNPs for schizophrenia (rs3743078-C, bscz = −0.07, SE = 0.01, P = 3.11 × 10–12) is located in a subunit of the nicotinic receptor (CHRNA3), a gene known to be associated with smoking and increased risk for lung cancer.17
We investigated in 2 ways whether there was any evidence that the potential causal association between schizophrenia and breast cancer was driven by smoking. First, we repeated the GSMR analyses after removing SNP rs3743078 as an instrument.
Secondly, we conducted a multi-trait conditional analysis (mtCOJO) to adjust the schizophrenia summary statistics for the effect of heaviness of smoking. To achieve this, a GWAS of self-reported number of cigarettes smoked per day (CPD) was conducted using data from 32510 people within the UK Biobank.18 We adjusted the phenotype by sex and age, and then performed BOLT-LMM19 association analysis with the first 10 principal components fitted as covariates. Three independent genome-wide significant SNPs that did not show evidence of pleiotropy were then used to conduct a GSMR analysis to estimate the causal effect of smoking heaviness on schizophrenia. The schizophrenia summary statistics were then adjusted for the estimated causal effect of CPD according to the method described in Zhu et al.14 The adjusted trait—denoted schizophrenia adjusted for cigarettes per day (SCZ_adj_CPD)—was tested for causal association with breast cancer.
Sensitivity Analyses - Adjusting for Parity
Increased parity is protective for breast cancer20 and patients with schizophrenia have fewer children compared to the general population.21 To investigate if parity may be a confounding factor for the association between schizophrenia and breast cancer, we conducted an mtCOJO analysis to adjust for the causal effect of parity on breast cancer using results from a GWAS analysis of number of children ever born in 193,953 unrelated women of white British descent women from the UK Biobank. Results were downloaded from http://www.nealelab.is/uk-biobank/. A description of the QC steps and analysis are given at http://www.nealelab.is/blog/2017/9/11/details-and-considerations-of-the-uk-biobank-gwas. The phenotype was coded into 3 categories: less than 2 children, 2 children, and more than 2 children and analysis conducted with 10 principal components as covariates. The GWAS effect size estimates were recoded so that they corresponded to the effect on having fewer children. There were not enough SNPs that were genome-wide significant and passed the HEIDI-outlier test, so we included SNPs with a P-value for association with parity less than 1 × 10–7 to estimate the causal effect of reduced parity on breast cancer. We then conducted an mtCOJO analysis to adjust the breast cancer summary statistics for the causal effect of reduced parity (bc_adj_parity) and then tested for a causal effect of schizophrenia on the adjusted trait.
Results
LD-Score Regression
The estimated heritability on the liability scale explained by common SNPs was 0.15 (SE = 0.01) for breast cancer (assuming a population lifetime risk of 0.12 and sample prevalence of 0.54) and 0.23 (SE = 0.008) for schizophrenia (assuming a population lifetime risk of 0.01 and sample prevalence of 0.42). The rG between schizophrenia and breast cancer was 0.14 (S.E = 0.03, P = 4.75 × 10–8), identical to that found in previous analyses using summary statistics from smaller studies of schizophrenia and breast cancer,22 indicating there are shared genetic risk factors between the 2 disorders.
MR Analysis
We used GSMR to investigate if the significant rG was due to a causal relationship between schizophrenia and breast cancer. Results are presented in table 1.
Results from Mendelian Randomization Analysis
| Exposure . | Exposure Sample Size . | Outcome . | Number of Independent Instruments . | Instruments Removed by HEIDI Test . | Number of Instruments Used in GSMR . | bxy . | SE . | P value . |
|---|---|---|---|---|---|---|---|---|
| Schizophrenia | 105318 | Breast Cancer | 150 | 8 | 142 | 0.032 | 0.008 | 2.19E-04 |
| Breast Cancer | 228951 | Schizophrenia | 204 | 13 | 191 | -0.005 | 0.012 | 0.67 |
| Exposure . | Exposure Sample Size . | Outcome . | Number of Independent Instruments . | Instruments Removed by HEIDI Test . | Number of Instruments Used in GSMR . | bxy . | SE . | P value . |
|---|---|---|---|---|---|---|---|---|
| Schizophrenia | 105318 | Breast Cancer | 150 | 8 | 142 | 0.032 | 0.008 | 2.19E-04 |
| Breast Cancer | 228951 | Schizophrenia | 204 | 13 | 191 | -0.005 | 0.012 | 0.67 |
Note: GSMR = Generalized summary-based Mendelian Randomization.
Results from Mendelian Randomization Analysis
| Exposure . | Exposure Sample Size . | Outcome . | Number of Independent Instruments . | Instruments Removed by HEIDI Test . | Number of Instruments Used in GSMR . | bxy . | SE . | P value . |
|---|---|---|---|---|---|---|---|---|
| Schizophrenia | 105318 | Breast Cancer | 150 | 8 | 142 | 0.032 | 0.008 | 2.19E-04 |
| Breast Cancer | 228951 | Schizophrenia | 204 | 13 | 191 | -0.005 | 0.012 | 0.67 |
| Exposure . | Exposure Sample Size . | Outcome . | Number of Independent Instruments . | Instruments Removed by HEIDI Test . | Number of Instruments Used in GSMR . | bxy . | SE . | P value . |
|---|---|---|---|---|---|---|---|---|
| Schizophrenia | 105318 | Breast Cancer | 150 | 8 | 142 | 0.032 | 0.008 | 2.19E-04 |
| Breast Cancer | 228951 | Schizophrenia | 204 | 13 | 191 | -0.005 | 0.012 | 0.67 |
Note: GSMR = Generalized summary-based Mendelian Randomization.
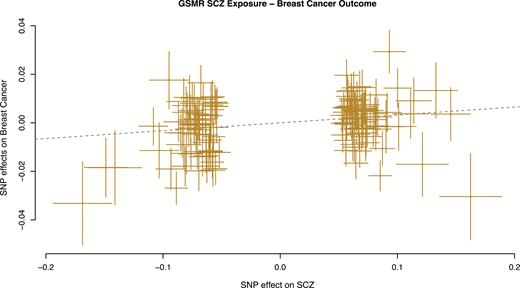
A total of 150 independent SNPs that were associated with schizophrenia at P < 5 × 10−8 were used, of which 8 were filtered by the HEIDI-outlier test, leaving 142 SNPs for analysis. The GSMR estimate of schizophrenia on breast cancer (bxy) was estimated as 0.032 (SE = 0.008, P = 2.2 × 10–4) (figure 1), indicating evidence for a causal effect. Transforming the estimate to the more interpretable scale (ie, fold increase in risk per 1 SD increase in liability to schizophrenia) gives an OR of 1.09 (SE = 0.008).
Generalized summary-based Mendelian Randomization (GSMR) plot for schizophrenia as exposure and breast cancer as outcome. Dotted line shows the GSMR estimate of causal effect.
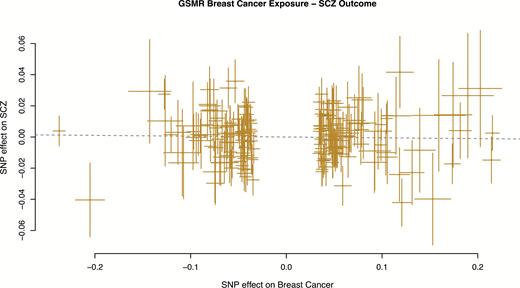
By contrast, there was no evidence of a causal effect of breast cancer on schizophrenia, using 191 SNPs as instruments after HEIDI-outlier filtering removed 13 SNPs (bxy = −0.005, SE = 0.012, P = .67) (figure 2). Other commonly used MR methods such as MR-Egger23 and inverse-variance weighted MR (aka MR-IVW) were consistent with those found using GSMR (supplementary table 1). The intercept from MR-Egger regression, a measure of pleiotropy, was not significantly different from 0 (P = .95) when including all SNPs.
Generalized summary-based Mendelian Randomization (GSMR) plot for breast cancer as exposure and schizophrenia as outcome.
Schizophrenia, Smoking and Breast Cancer
Removing the SNP in CHRNA3 that is associated with smoking and schizophrenia (rs3743078) did not affect the results (bxy = 0.032, S.E. = 0.009, P = 2.34 × 10−4). It is noteworthy that this SNP is not identified as showing significant evidence of pleiotropy by the HEIDI-outlier test (its association with breast cancer is bbreast_cancer = −0.010, P = .44).
The GWAS of CPD in UK Biobank identified 5 independent SNPs associated with heaviness of smoking. Two SNPs showed evidence of pleiotropy. Using the remaining 3 SNPs, the estimated causal effect of heaviness of smoking on schizophrenia was 0.22 (SE = 0.07, P = .001). Adjusting for CPD reduced the number of genome-wide significant SNPs that could be used as instruments from 150 to 123. However, the estimated causal effect of schizophrenia on breast cancer after removing 8 SNPs with evidence of pleiotropy was larger and more significant after adjusting for heaviness of smoking (n instruments = 115; bxy = 0.051; SE = 0.009; P = 6.8 × 10–8).
Adjusting for Parity
The estimated causal effect of reduced parity on breast cancer estimated from 10 SNPs with P < 10−7 was 0.149 (SE = 0.154, P = .330). Despite the causal estimate not being significant, which may reflect low power, we conducted the mtCOJO analysis to adjust the breast cancer summary statistics for the effect of reduced parity.
A total of 137 genome-wide significant SNPs for schizophrenia which overlapped with the parity GWAS in UK Biobank were used as instruments to test for a causal effect on the adjusted breast cancer statistics. After removing 10 SNPs that failed the HEIDI test, the causal effect of schizophrenia on bc_adj_parity was larger and more significant than the estimate without adjustment (bxy = 0.043, SE = 0.009, P = 2.42 × 10−6).
Discussion
Our results provide strong evidence that the association of increased risk of breast cancer in schizophrenia patients reported from epidemiological studies is, at least partly, driven by shared genetic risk factors between the disorders. Epidemiological studies of first-degree relatives have shown inconsistent results with some studies showing that mothers with a child diagnosed with schizophrenia are at decreased risk of breast cancer,5,24 while another study found no evidence of decreased risk.25 The latter study was the only study that compared rates in mothers of patients to those of other mothers, thus controlling for the effect of being a parent. The only study to investigate the rates in sisters of schizophrenia patients found no evidence of a significant difference in risk compared to the general population.24
In conducting MR analyses, several SNPs were excluded as HEIDI-outliers, and these variants may have pleiotropic effects on both disorders. After excluding these outliers, using GSMR we find evidence that SNPs associated with schizophrenia are associated with breast cancer: under the assumption of causality, the predicted OR for breast cancer associated with a 1 SD increase in schizophrenia is 1.09. This risk is modest but consistent with the small increased risk identified by large observational studies. Conversely, the GSMR estimate of the causal effect of breast cancer on schizophrenia was not significantly different from zero. If genetic risk factors were only pleiotropic between disorders, then there would be no expectation that the magnitude of SNP effect sizes of schizophrenia associated SNPs would be correlated with the magnitude of SNP effect sizes in breast cancer. This unidirectional relationship is more consistent with a model in which genetic factors for schizophrenia are on a causal pathway influencing breast cancer risk, rather than purely pleiotropic effects of shared genetic factors.
The MR framework is a powerful paradigm for inferring causality since the cases and controls included in the breast cancer GWAS are unlikely to be enriched for schizophrenia cases due to nongenetic confounding factors, and because genetic variants are assigned at birth, genetic data are free from a number of confounders and biases that are inherent in observational studies. Moreover, it is a cost-effective way to investigate direction of causality because the alternative observational study requires following large cohorts of breast cancer patients or schizophrenia patients over a long period of time.
There are some limitations in interpreting the results. We cannot get insight into the specific mechanism driving the directional relationship between schizophrenia risk and breast cancer risk. We performed 2 sensitivity analyses to investigate the possible effect of smoking, first removing an SNP known to be associated with both schizophrenia and smoking, and second adjusting the schizophrenia summary statistics for the potential causal effects of smoking heaviness. However, the GSMR estimate of causal effect of schizophrenia on breast cancer remained significant. Likewise, we conducted a conditional analysis to adjust breast cancer for the causal effect of parity and found that the estimated causal effect of schizophrenia was not altered. These results suggest that the mechanism is not related to smoking or parity, but it is important to note that only a small number of instruments were available for smoking and parity meaning the causal effects of these traits are estimated with substantial error. Larger studies will allow for further dissection of the relationships between risk factors for breast cancer.
Another possible mechanism underlying this causality is treatment with antipsychotics. Antipsychotics, especially risperidone, are known to increase the levels of prolactin,26 a neuroendocrine hormone that is elevated during pregnancy and lactation. There is some evidence that elevated prolactin levels are a risk factor for breast cancer (Gabrielson et al; in press) and although no association has been found between risperidone use and risk of breast cancer, the average follow-up time for risperidone use was 1.29 years (SD = 1.35) and so longer-term use may still increase breast cancer risk.27 However, it is possible that the causal association is due to another unmeasured confounding variable that is highly genetically associated with both schizophrenia and breast cancer.
Another limitation is that this study has only evaluated breast cancer in women. It is possible that having schizophrenia also increases risk for breast cancer in men, but breast cancer is rare in men, and the breast cancer GWAS included only women.
Our results suggest that investigations of the relationship between schizophrenia and breast cancer at the genetic level may prove fruitful for understanding the biology of breast cancer. Moreover, there needs to be increased awareness in the psychiatry community of the elevated risk of breast cancer in women with schizophrenia and encouragement of patients to seek screening for breast cancer.
Funding
This work was supported by grants from the National Health and Medical Research Council of Australia 1145645, 1078901, 1113400 and 1087889 and the Sylvia & Charles Viertel Charitable Foundation (Senior Medical Research Fellowship). BCAC is funded by Cancer Research UK (C1287/A16563, C1287/A10118), the European Union’s Horizon 2020 Research and Innovation Programme (grant numbers 634935 and 633784 for BRIDGES and B-CAST, respectively), and by the European Community’s Seventh Framework Programme under grant agreement number 223175 (grant number HEALTH-F2-2009-223175) (COGS). The EU Horizon 2020 Research and Innovation Programme funding source had no role in study design, data collection, data analysis, data interpretation or writing of the report. Genotyping of the OncoArray was funded by the National Institutes of Health (NIH) Grant U19 CA148065, and Cancer UK Grant C1287/A16563 and the PERSPECTIVE project supported by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research (grant GPH-129344) and, the Ministère de l’Économie, Science et Innovation du Québec through Genome Québec and the PSRSIIRI-701 grant, and the Quebec Breast Cancer Foundation. Funding for the iCOGS infrastructure came from: the European Community’s Seventh Framework Programme under grant agreement n° 223175 (HEALTH-F2-2009-223175) (COGS), Cancer Research UK (C1287/A10118, C1287/A10710, C12292/A11174, C1281/A12014, C5047/A8384, C5047/A15007, C5047/A10692, C8197/A16565), the National Institutes of Health (CA128978) and Post-Cancer GWAS initiative (1U19 CA148537, 1U19 CA148065 and 1U19 CA148112 - the GAME-ON initiative), the Department of Defence (W81XWH-10-1-0341), the Canadian Institutes of Health Research (CIHR) for the CIHR Team in Familial Risks of Breast Cancer, and Komen Foundation for the Cure, the Breast Cancer Research Foundation, and the Ovarian Cancer Research Fund. The DRIVE Consortium was funded by U19 CA148065.
Acknowledgments
We acknowledge the Schizophrenia Working Group of the Psychiatric Genomics Consortium and the CLOZUK researchers for making data publically available data for use in this project. The PGC-SCZ and CLOZUK studies are the culmination of the work of thousands of people and many funding bodies around the world and we acknowledge their contribution to this study. The authors have declared that there are no conflicts of interest in relation to the subject of this study. This research has been conducted using the UK Biobank Resource, under project 12505.