-
PDF
- Split View
-
Views
-
Cite
Cite
Faith S. Luyster, Patrick J. Strollo, Phyllis C. Zee, James K. Walsh, Sleep: A Health Imperative, Sleep, Volume 35, Issue 6, 1 June 2012, Pages 727–734, https://doi.org/10.5665/sleep.1846
Close - Share Icon Share
Abstract
Chronic sleep deficiency, defined as a state of inadequate or mistimed sleep, is a growing and underappreciated determinant of health status. Sleep deprivation contributes to a number of molecular, immune, and neural changes that play a role in disease development, independent of primary sleep disorders. These changes in biological processes in response to chronic sleep deficiency may serve as etiological factors for the development and exacerbation of cardiovascular and metabolic diseases and, ultimately, a shortened lifespan. Sleep deprivation also results in significant impairments in cognitive and motor performance which increase the risk of motor vehicle crashes and work-related injuries and fatal accidents. The American Academy of Sleep Medicine and the Sleep Research Society have developed this statement to communicate to national health stakeholders the current knowledge which ties sufficient sleep and circadian alignment in adults to health.
The United States' agenda for improving the Nation's health includes Dietary Guidelines for Americans, 20101 and 2008 Physical Activity Guidelines for Americans2. However, significant evidence regarding the duration and timing of sleep as determinants of health remain largely unrecognized by federal agencies, policy makers, scientists, and the public. The American Academy of Sleep Medicine and the Sleep Research Society have developed this document to communicate to national health stakeholders the current knowledge which ties sufficient sleep and circadian alignment in adults (see authors' footnote following acknowledgments) to health as tightly as good nutrition and adequate exercise.
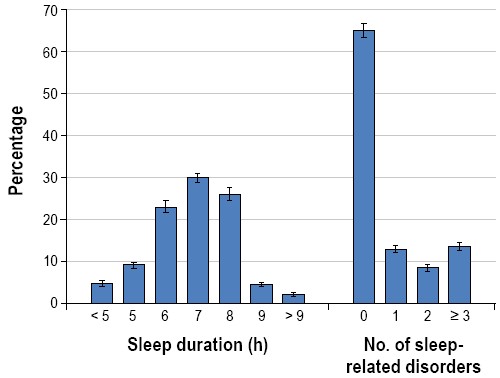
Poor sleep health is largely accounted for by the high prevalence of primary sleep disorders (e.g., sleep apnea, insomnia, narcolepsy, and restless legs syndrome) and the growing number of people living with chronic sleep deficiency, a state of inadequate or mistimed sleep, independent of a primary sleep disorder. The Institute of Medicine estimates that 50 to 70 million adult Americans have a chronic sleep disorder3 that contributes to poor health. This statement focuses upon sleep deficiency and the potential health promotion benefits of addressing this highly prevalent condition. Approximately 1 in 3 adult Americans are sleeping less than 7 hours per night (37.1%),4 an amount at which physiological and neurobehavioral deficits manifest and become progressively worse under chronic conditions (Figure 1)5,6. Broad societal changes, including longer work hours and commute times, shift work, later night life, increased dependence on technology, and a current mindset of “If you snooze, you lose” have contributed to the growing sleep deficiency problem. Further, an estimated 20% of the US workforce is exposed to shift work schedules that contribute to chronic disruption of biological timing as well as increased risk for a number of illnesses.
Percentage of adults in the United States by reported usual sleep durations and by number of sleep-related difficulties. Data taken from the National Health and Nutrition Examination Survey (NHANES), cycles (2005-2006 and 2007-2008).4 A total of 10,896 respondents aged > 20 years completed survey questions on sleep duration and sleep-related difficulties. Sleep duration categories were based on responses to the question “how much sleep do you usually get at night on weekdays or workdays?” Responses to 6 questions from the Functional Outcomes of Sleep Questionnaire92 were used to determine the number of sleep-related difficulties.
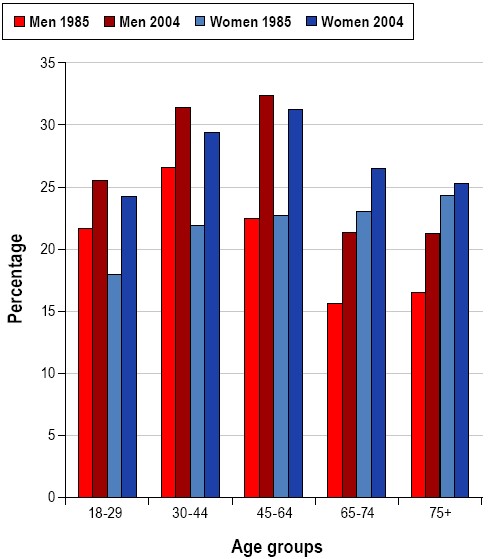
Many people judge their sleep as adequate as long as a minimal level of behavioral functioning can be maintained. With no perceived negative outcome, it is understandable why sleep may be sacrificed for work or pleasure. Indeed, the percentage of men and women reporting sleeping less than 6 hours per night has increased significantly over the last 20 years (Figure 2)7. In contrast to this perception of sleep's limited importance, decades of research clearly indicate that sleep is comprised of complex, active, and highly organized physiological processes, which when disordered or deficient results in ill-health or death.
Sleep is Exquisitely Organized and Controlled
Night after night sleep is remarkably consistent in its organization. Each night is comprised of recurring, complex, active physiologic processes, confirming that sleep is not a biologically inactive time interspersed between periods of wakefulness. Human sleep (and that of most mammals and birds) is composed of two distinct states: non-rapid eye movement (NREM) and rapid eye movement (REM), each with unique characteristics and each actively regulated by distinct neural centers. A typical night involves 4 to 6 repeated cycles of NREM and REM, each lasting approximately 90 to 110 minutes. NREM sleep is divided into 3 stages (N1, N2, N3) characterized by progressively more synchronous cortical neuron activity, stable autonomic function, and increasing arousal thresholds. In contrast, the electroencephalogram (EEG) during REM sleep shows low-voltage, mixed-frequency brain wave activity, more similar to the EEG of relaxed wakefulness than the more synchronous NREM EEG. Periodic bursts of rapid eye movements (hence the name REM), variable autonomic activity, and atonia of skeletal muscles are other features of REM sleep. “Deep” sleep (N3) is considered to be “restorative” in that it is associated with marked reductions in sympathetic activity resulting in decreased heart rate and blood pressure and stable breathing. Low amounts of N3 are associated with increased cardiovascular disease risk8. The evolution of two distinct physiologic states within the behavior of sleep seems extraordinarily unlikely unless critical biological needs are met by each state.
A fundamental homeostatic process is involved in the regulation of sleep. The strength of this process is dependent upon the amount of time elapsed since the last sleep period. The homeostatic pressure to sleep increases as a person stays awake. The longer one stays awake, the stronger the homeostatic drive to sleep becomes. Individuals with sleep deficiency due to short sleep duration accumulate a sleep debt and will exhibit an increased drive to recover the lost sleep leading to shorter sleep latency, greater total sleep time, and enhancement of EEG synchrony in NREM sleep9. During periods of prolonged sleep deprivation, the brain will overwhelm attempts to maintain wakefulness, and sleep will inevitably occur. Even when an individual is active and resisting sleep, if sleep debt is substantial, transitions into brief periods of sleep will occur. These “microsleeps” generally last from 3 to 30 seconds and occur without awareness of the individual, despite clear changes to a sleeping pattern in the EEG10,11. This homeostatic pressure for sleep which can result in the occurrence of sleep even when life is at risk (e.g., when driving), supporting the fact that sleep is indispensable.
In addition to the homeostatic sleep drive, a second process is involved in the control of the timing and organization of sleep. Referred to as the circadian (i.e., “about a day”) process, the timing of sleep is heavily influenced by a circadian timing mechanism located in the suprachiasmatic nucleus in the brain. The circadian timing system incorporates 3 different components: (1) input pathways that transmit light and other signals to the circadian “clock” and thus entrain circadian rhythms to environmental cues, primarily the light-dark cycle; (2) an endogenous circadian pacemaker that generates rhythms having a period of approximately 24 hours; and (3) output pathways controlled by the pacemaker12. In the absence of environmental cues, circadian rhythms are intrinsic and will continue to oscillate with a period of approximately 24 hours. A temporally precise interaction exists between the sleep homeostatic process and the circadian timing of output from wake-promoting centers in the brain. The gradual build-up of sleep debt across the day is opposed by incrementally increasing brain alerting signals, which rather abruptly dissipate around the usual time of sleep onset at night and “permits” sleep to occur. Without the opposition of the alerting system, humans would be unable to sustain wakefulness for the usual 16 hours each day because of the growing homeostatic sleep pressure. Without the prompt dissipation of alerting activity, sleep at night would be difficult to achieve and sustain. The evolution of two temporally and precisely complimentary processes which maximize daily sustained periods of both wakefulness and sleep lends credence to the essential biological need for sleep.
The circadian timing system is also responsible for the daily variations in many physiologic variables, such as core body temperature and the hormones melatonin and cortisol, and properly aligns those rhythms with sleep and with other physiological processes. For example, core body temperature peaks in the late afternoon or evening hours, and reaches its nadir in the latter part of the night. The declining temperature before and during sleep promotes sleep onset and duration. In contrast, levels of melatonin, secreted by the pineal gland, are low during the daytime and rise during the night helping to promote sleep.
The stress hormone cortisol has peak levels in the late morning and reaches a nadir during the night.
Sleep and wake rhythms can be misaligned with daily variations in the physiologic variables noted above through jet lag or shift work. Such misalignment manifesting from altered lighting conditions can also affect circadian oscillations in a host of mammalian peripheral tissues, including the heart, lung, esophagus, and spleen13,14. For example, even a single 6-hour phase-advance in the light-dark cycle, which would mimic travel from the US to Europe, desynchronizes circadian oscillations in peripheral tissues that, in turn, resynchronize at different rates. Over time, whole-body desynchronization may accelerate and lead to metabolic and cardiovascular disease15,16, cancer progression17, and death15,18.
Sleep Occurs Throughout the Animal Kingdom
Sleep or sleep-like states are present in all species studied, although EEG correlates are not demonstrable in all species19. Studies in insects, fish, amphibians, reptiles, turtles, and tortoises have shown sleep-like behavior20. The overall constancy of sleep need across these species is a reflection of a ubiquitous biological need and evolutionary pressures that resulted in the development of the exquisite homeostatic and circadian inputs into consolidated sleep-wake periods in higher order species. Research investigating sleep in multiple species of land mammals clearly shows that these animals have the electrophysiological hallmarks of sleep21, including both NREM and REM sleep. The EEG of birds show both NREM and REM sleep similar to sleep in mammals, except that the REM periods tend to be shorter than those in most mammals22. Even sea mammals (dolphins, whales, fur seals) sleep, despite the need to swim nearly continuously, and EEG recordings demonstrate a capacity for unihemispheric sleep23, allowing the organism to meet two biological requirements simultaneously.
Homeostatic processes are evident in all species studied as is circadian variation in the proportion of sleep and wakefulness. The preservation of most key characteristics of sleep (two states, circadian pattern, homeostasis, common behavioral indicators) throughout the animal kingdom indicates that the survival of the organism is enhanced by the regular occurrence of sleep.
Sleep is Encoded in Our Genes
Human sleep tendencies are heritable. Both twin and family studies suggest that usual sleep duration, excessive sleepiness, and diurnal preference (morning types or “larks” versus evening types or “owls”), are heritable, with heritability estimates (i.e., the proportion of a sleep characteristic that can be explained by genetic variation) ranging from 0.17-0.44, 0.29-0.48, and 0.22-0.47, respectively24–30. Many sleep disorders run in families, and the genetic bases for some of these disorders have been established. Association tests conducted in a family-based sample identified an association between usual bedtime with a non-synonymous coding single-nucleotide polymorphism (SNP) in the NPSRI gene and an association of sleepiness with an SNP located in a non-coding portion of the PDE4D gene28. Familial advance sleep phase syndrome is an inherited disorder characterized by early sleep times and early-morning awakening and was the first human circadian rhythm variant to be well characterized31.
The PER3 gene is involved in sleep regulation. A polymorphism, based on 4-repeat or 5-repeat alleles, results in 3 PER3 genotypes: PER34/4, PER34/5, and PER35/5. The PER35/5 genotype is associated with morning circadian preference and higher homeostatic sleep drive in healthy individuals32. The PER34/4 genotype is associated with the delayed phase syndrome (i.e., evening circadian preference and lower homeostatic sleep drive)32. One manifestation of the delayed phase syndrome is lowered homeostatic sleep drive resulting in prolonged sleep latency (i.e., difficulty falling asleep)33.
A number of genes are essential for the generation of circadian rhythms in mammals31; because of the interaction of circadian processes and sleep, it is not surprising that some clock genes (i.e., genes that affect both the persistence and period of circadian rhythms) appear to have effects on sleep, as well. For example, a mutation in the hDEC2 gene was identified in 2 individuals who had lifelong daily sleep times that were shorter (average 6.25 h) than most normal sleepers (average 8.06 h), and genetically engineered mice carrying this mutation had an increased amount of wakefulness and shortened sleep time.34 Thus, a biological basis for reduced sleep time may be caused by the DEC2 gene mutation. It is important to recognize, however, that reduced amounts of sleep do not necessarily mean less sleep is required for good health.
Sleep is Essential for Survival
The ultimate outcome of prolonged sleep deprivation in animals is death. Rats deprived of sleep die within 2 to 3 weeks.35 Early studies also document the fatal outcome of prolonged sleep deprivation in dogs.36,37 Murine models (e.g., mice and rats) have also demonstrated the detrimental effects of prolonged sleep deprivation on a variety of systems with noticeable changes in endocrine, metabolic, and immune function.38 During the early phase of prolonged sleep deprivation in rats, there is a progressive increase in energy expenditure manifested by weight loss despite increased food intake, decline in thyroid hormones, and increased plasma norepinephrine in response to metabolic demands, and decreased resistance to opportunistic infection as host defense breaks down.35 Following an increase in body temperature in the first few days of prolonged sleep deprivation, the last few days of survival are marked by hypothermia and a progressively debilitated state. Recent work demonstrates that repeated exposure to sleep restriction has persistent physiologic effects even after substantial time for recovery is allowed.39
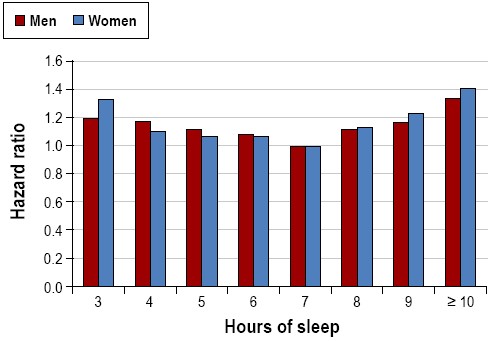
While experimental studies of prolonged sleep deprivation cannot be conducted in humans, growing evidence over the last few decades suggest that habitually shorter or longer sleep duration is associated with greater mortality (Figure 3).40 The mechanisms that underlie these associations are not fully understood. Potential adverse physiologic effects of short sleep duration may contribute to negative health outcomes such as cardiovascular disease, diabetes, and obesity, all of which are associated with increased mortality risk. Healthy young adults subjected to partial sleep restriction demonstrated impaired glucose tolerance, higher evening cortisol levels, alterations in sympathetic nervous system activity, reduced leptin levels (a hormone that regulates satiety), and increased levels of ghrelin (a hormone that regulates hunger).41,42 Increases in inflammatory markers such as C-reactive protein, tumor necrosis factor (TNF)-α, and interleukin (IL)-6 have also been associated with short sleep.43 The mechanisms linking reported long sleep duration with mortality is unknown and may be partly explained by underlying confounders such as depression, low socioeconomic status, undiagnosed medical disease, poor physical health, and low physical activity.44,45From a population perspective, short sleep is much more common than long sleep duration. The Centers for Disease Control and Prevention have reported that 37.1% of US adults slept less than 7 hours, as compared to 2.4% reporting sleep of more than 9 hours.4 In the Nurses' Health Study II, approximately 30% of the participants slept 6 hours or less, whereas only 5% slept 9 hours or more.46 Based upon prevalence, short sleep is likely to substantially impact public health more than long sleep.
Mortality harard ratios for various reported sleep durations for men and women. Data collected from 636,095 men and 480,841 women in the Cancer Prevention Study II (1982-1988). Both men and women with a usual sleep duration of 7 h had the best survival. Participants reporting sleep durations < 6 h and > 8 h had significantly increased mortality hazard. Adapted from Kripke et al.94
Sleep deprivation contributes to a number of molecular, immune, and neural changes that play a role in disease development. Immune-related mechanisms are activated during sleep deprivation in humans and laboratory animals, including increases in inflammatory cytokines, IL-1β, TNF-α, and IL-6, and serum immunoglobins (Ig), IgM, IgG, and IgA,47,48 and a progressive increase and subsequent imbalance in the population of circulating host defense related phagocytic cells, primarily neutrophils.47,49 This imbalance of the host defense cells can promote an inflammatory response and subsequent cell injury. Antioxidant defense responses are decreased as a consequence of sleep deprivation, thus inhibiting the body's ability to remove molecules that promote cell damage, such as free radicals and oxygen reactive species.50 Recovery sleep can restore antioxidant activities and antioxidant capacity in both the liver and the heart.51 Both short-term and long-term sleep deprivation induce a cumulative cell stress response in the brain that leads to adaptive mechanisms involving induction of gene expression (i.e., enhancement of protein folding and protein degradation in order to remove misfolded proteins, and expression of mediators of host defense) to restore normal cellular function and prevent a maladaptive response and cell death (i.e., apoptosis).52 Sleep deprivation also results in increases in the expression of circadian clock-related genes (bmall, clock, cry1, cry2, csnk1ɛ, npas2, per1, and per2) in the cerebral cortex in mice.53–55 These changes in biological processes in response to chronic sleep deprivation may serve as etiological factors for the development and exacerbation of disease and, ultimately, a shortened lifespan.
Sleep Deficiency is Associated with Major Health Risks
Cardiovascular
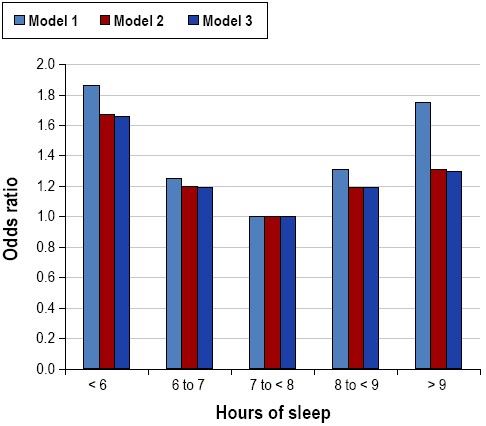
On a population level, 7-8 hours of sleep per night in adults is associated with the lowest risk and incidence of cardiovascular diseases that may ultimately result in death.56–58 Alterations in autonomic nervous system function related to insufficient sleep affect blood pressure59 and have the potential to increase the risk for cardiovascular morbidity and mortality. Associations between self-reported and objectively measured short sleep duration and increased blood pressure and prevalence and incidence of hypertension have been observed in epidemiologic studies (Figure 4).60–62 Epidemiological data linking sleep duration to risk of stroke are limited, but suggest that short (< 6 h) sleep duration may increase the risk for ischemic stroke.63 Short sleep duration is also associated with a greater risk of developing or dying from coronary heart disease and stroke.57 Shift work has been found to be related to elevated risk of cardiovascular morbidity and mortality, including ischemic heart disease, myocardial infarction, and atherosclerosis.64,65 Associations between long sleep duration and cardiovascular outcomes such as hypertension, stroke, and heart disease have been reported.57,66,67
Relationship between sleep duration and prevalence of hypertension from the Sleep Heart Health Study. Data are adapted from Gottlieb et al.61 Odds ratios are for the presence of hypertension, from categorical logistic regression models using 7 to < 8 h sleep per night as the referent category. Model 1 was unadjusted. Model 2 was adjusted for age, sex, race, and apnea-hypopnea index. Model 3 was adjusted for all covariates in Model 2 plus body mass index.
Metabolic
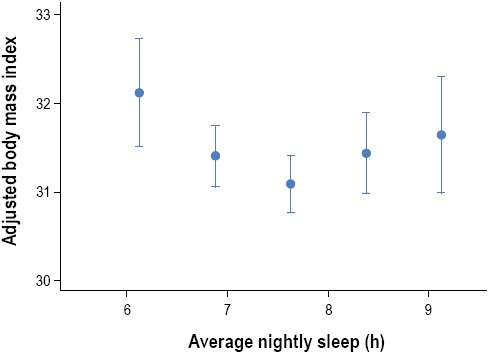
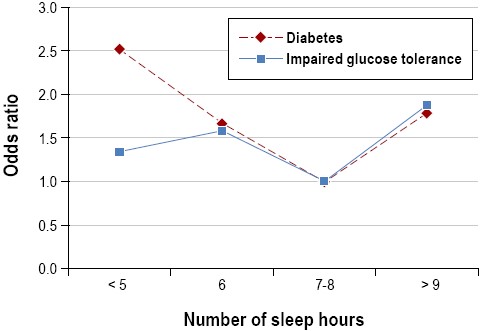
The increasing epidemic of obesity in the U.S. in the last few decades has been paralleled by significant reductions in sleep duration. Accordingly, an increasing number of observational and prospective studies have reported associations between sleep duration and impaired sleep and metabolic risk, including obesity or body mass index (BMI) and type 2 diabetes.58,68-70 In crosssectional studies, short sleep duration (< 5 h per night ) has been found to increase the odds of having obesity 1.5 times, with a 0.35 kg/m2 increase in BMI for every 1-hour reduction of sleep.71 Longitudinal studies of sleep and weight gain have also reported that short sleep duration may increase the risk of becoming obese.69 Short (≤ 5 h or ≤ 6 h per night) sleep durations have been shown to increase the odds of developing type 2 diabetes and impaired glucose tolerance in several large observational studies.70 Some studies have found associations between longer sleep durations (typically > 8 h per night) and obesity or increased BMI.68,69 Both shorter and longer sleep durations were associated with higher BMIs even after accounting for age and sex, with the lowest mean BMI predicted at 7.7 hours (Figure 5).72 An increased prevalence of type 2 diabetes and impaired glucose tolerance has been found in long sleepers (Figure 6).73 Taken together, observational and longitudinal epidemiologic studies suggest that short (and possibly long) sleep duration is a risk factor for obesity and the development of type 2 diabetes.
Curvilinear relationship between body mass index (BMI) and average hours of sleep. “Average nightly sleep” was based on data collected from a 6-day sleep diary and was calculated as the sum of the hours between bedtime and arising, divided by 6. After adjustment for age and sex, those sleeping more and less than 7.7 h/night had an increased BMI. Adapted from Taheri et al.72
Relationships between hours of sleep and prevalence of type 2 diabetes and impaired glucose tolerance. Data adapted from Gottlieb et al.73 Odds ratios are for the presence of diabetes and impaired glucose tolerance, from categorical logistic regression model adjusted for age, race/ethnicity, apnea-hypopnea index, study site, and waist girth, using 7 to 8 h of sleep/night as the referent category. Usual sleep times ≤ 6h or≥ 9h/night were associated with an increased prevalence of type 2 diabetes and impaired glucose tolerance.
Cancer
Emerging evidence suggests that sleep duration may increase risks of several types of cancer. Short sleep duration has been associated with a greater risk of developing breast cancer, colorectal cancer, and prostate cancer.74–77 Women with longer sleep durations (≥ 9 h/night) have been shown to have a decreased risk of breast cancer.78 Epidemiological studies have reported a significantly increased risk of developing a number of malignancies, including breast, colon, prostate, and endometrial cancer in night shift workers.79,80 Nocturnal melatonin suppression due to decreased sleep duration or light exposureat night (in the case of shift workers) may promote cancer cell development and growth.81
Accidents
Sleep deprivation adversely affects neurobehavioral function82–84 and leads to excessive daytime fatigue and sleepiness,85 which increase the risk of human-error related accidents.86,87Sleep deprivation results in impairments in cognitive and motor performance that are comparable to those induced by alcohol consumption at or above the legal limit.88 Drowsy driving is common, with 32% of respondents in the 2008 National Sleep Foundation Sleep in American Poll reporting having driven drowsy, and 36% admitting to having had briefly nodded off while driving in the past year. Drowsy driving is a contributing factor in a significant proportion of traffic accidents, up to 20%.89 In addition to an increased risk for motor vehicle crashes, sleep deprivation and related sleepiness are associated with work-related injuries and fatal accidents.90,91 These serious repercussions of fatigue and sleepiness caused by sleep deprivation emphasize the indispensable need for sleep and the vital importance of sleep for society as a whole.
CONCLUSION
Sleep deficiency (defined as a state of inadequate or mistimed sleep) unrelated to a primary sleep disorder, associated with biological, social, environmental and lifestyle factors, is a growing and underappreciated determinant of health status. The consequences to society are enormous, including disease and accident risk, longevity, and elevated direct medical costs and indirect costs related to work absenteeism and property damage. While primary sleep disorders (i.e., sleep apnea, insomnia, narcolepsy, and restless legs syndrome) largely require the attention of the medical community, sleep deficiency represents an opportunity for individuals, institutions, and communities to promote good health by addressing the factors which lead to short sleep durations and circadian disruption. Sleep deficiency contributes to the risk for several of modern societies' medical epidemics, including cardiovascular disease, diabetes, obesity, and cancer. It is a public health imperative to determine the mechanisms underlying these insidious adverse health effects of sleep deficiency, as well as assessing countermeasures directed at improving sleep and overall health in individuals suffering from chronic sleep deficiency. Indeed, increasing sleep duration may be a more palatable and achievable behavior change than other health-promoting behaviors, such as improved nutrition and increased activity levels. The American Academy of Sleep Medicine and the Sleep Research Society urge healthcare personnel, government agencies, educational institutions, employers, community organizations, industry leaders, individuals, and families to prioritize sleep for the betterment of personal and societal health.
Progress toward three major goals is likely to have significant beneficial effects on health:
Recognize and meet the biological requirement for sleep on a regular basis
Maximize synchrony among the endogenous biological timing systems
Recognize the signs and symptoms of primary sleep disorders and seek medical attention
ABBREVIATIONS
- NREM
non-rapid eye movement
- REM
rapid eye movement
- EEG
electroencephalogram
- SNP
single-nucleotide polymorphism
- TNF
tumor necrosis factor
- IL
interleukin
- BMI
body mass index
ACKNOWLEDGMENT
The contributions and editorial assistance of Allison Brager, Ronald Chervin, David Dinges, Elizabeth Klerman, Jennifer Martin, Janet Mullington, Gina Poe, Susan Redline, Steven Shea, Ronald Syzmusiack, Fred Turek, and Nathaniel Watson are gratefully acknowledged. This paper was reviewed by the Board of Directors of the Sleep Research Society and the American Academy of Sleep Medicine. Sleep Research Society: Phyllis C. Zee, MD, PhD, Ronald S. Szymusiak, PhD, James K. Walsh, PhD, Janet M. Mullignton, PhD, Sean P.A. Drummond, PhD, Elizabeth B. Klerman, MD, PhD, Jennifer L. Martin, PhD, Allan I. Pack, PhD, MBChB, Gina R. Poe, PhD, David B. Rye, MD, PhD, Fred Turek, PhD, and Allison Brager, PhD. American Academy of Sleep Medicine: Nancy Collop, MD, Patrick J. Strollo, Jr., MD, Sam Fleishman, MD, Timothy I. Morgenthaler, MD, Amy Aronsky, DO, M. Safwan Badr, MD, Ronald Chervin, MD, Susan Redline, MD, Ilene Rosen, MD, Steven A. Shea, PhD, Nathaniel F. Watson, MD, Merrill Wise, MD, and Jerome A. Barrett.




Comments