-
PDF
- Split View
-
Views
-
Cite
Cite
Christine E Spadola, Na Guo, Dayna A Johnson, Tamar Sofer, Suzanne M Bertisch, Chandra L Jackson, Michael Rueschman, Murray A Mittleman, James G Wilson, Susan Redline, Evening intake of alcohol, caffeine, and nicotine: night-to-night associations with sleep duration and continuity among African Americans in the Jackson Heart Sleep Study, Sleep, Volume 42, Issue 11, November 2019, zsz136, https://doi.org/10.1093/sleep/zsz136
Close - Share Icon Share
Abstract
We examined the night-to-night associations of evening use of alcohol, caffeine, and nicotine with actigraphically estimated sleep duration, sleep efficiency, and wake after sleep onset (WASO) among a large cohort of African American adults.
Participants in the Jackson Heart Sleep Study underwent wrist actigraphy for an average of 6.7 nights and completed concurrent daily sleep diary assessments to record any consumption of alcohol, caffeine, and nicotine within 4 hours of bedtime. Linear mixed-effect models were fit and adjusted for age, sex, educational attainment, body mass index, depression, anxiety, stress, and having work/school the next day.
Eligible participants (n = 785) were an average of 63.7 years (SD: 10.6), and were predominantly female (67.9%). There were 5164 days of concurrent actigraphy and sleep diary data. Evening alcohol use was associated with that night’s lower sleep efficiency (−0.98% [95% CI: −1.67% to −0.29%], p = 0.005), but not with WASO or sleep duration. Evening nicotine use was associated with that night’s lower sleep efficiency [1.74% (95% CI: −2.79 to −0.68), p = 0.001] and 6.09 minutes higher WASO ([95% CI: 0.82 to 11.35], p = 0.02), but was not associated with sleep duration. Evening caffeine use was not associated with any of the sleep parameters.
Nicotine and alcohol use within 4 hours of bedtime were associated with increased sleep fragmentation in the associated night, even after controlling for multiple potential confounders. These findings support the importance of sleep health recommendations that promote the restriction of evening alcohol and nicotine use to improve sleep continuity.
Avoiding the use of alcohol, caffeine, and nicotine close to bedtime represents modifiable behaviors that can improve sleep. Nonetheless, among community-dwelling adults and specifically African Americans, there is a lack of longitudinal research investigating the use of these substances and the associations with objective measures of sleep. In one of the largest longitudinal investigations to date, we found that among an African American cohort, alcohol and nicotine consumption within 4 hours of bedtime is associated with higher sleep fragmentation (nicotine) and lower sleep efficiency (nicotine and alcohol). These new data support the value of sleep hygiene efforts to reduce evening alcohol and nicotine use. Future work should examine the dose-response relationships of these substances and the impact on sleep.
Introduction
Short sleep duration and sleep fragmentation are associated with adverse health outcomes, including cardiovascular disease, diabetes, hypertension, and certain cancers [1, 2]. Behavioral sleep health interventions commonly target evening consumption of nicotine, alcohol, and caffeine [3]. However, the impact of these substances on objectively measured sleep parameters within community, non-laboratory settings have not been thoroughly examined. Examination of the impact of use and nonuse patterns (within persons) on sleep parameters is also missing from the literature [3].
African Americans have been underrepresented in investigations examining the associations of nicotine, alcohol, and caffeine use on sleep. This is of particular salience as African Americans are more likely to experience: (1) short sleep duration and fragmented sleep compared to non-Hispanic Whites, and (2) more deleterious health consequences associated with inadequate sleep than other racial/ethnic groups [4–6].
Here, we will briefly review the literature examining the association of alcohol, caffeine, and nicotine use on sleep parameters, as well as the racial/ethnic representation of the studies to date. Alcohol is considered a common over-the-counter sleep aid and may be frequently used to promote sleep initiation [7]. However, experimental research conducted with predominantly non-Hispanic white populations indicates that alcohol may promote sleep in the short-term, but results in fragmented and lighter sleep later in the sleep period (see review: Ebrahim et al.[8]). A recent cross-sectional study indicates the relationship between alcohol and sleep might differ between race, with black male and female infrequent heavy drinkers more likely to report short sleep duration than their white male and female counterparts [9]. Overall, research to date is mainly based on cross-sectional data using subjective assessments of sleep, or laboratory studies that administer alcohol within 60 minutes of bedtime. Thus, there is a paucity of investigations conducted in naturalistic settings that address the impact of alcohol ingestion within several hours of bedtime, which could more commonly represent timing of alcohol use in the general population (i.e., at dinner, happy hour, etc.) [3].
Experimental and observational studies indicate caffeine use close to bedtime is associated with increases in sleep onset latency and WASO as well as reduced sleep efficiency and total sleep time (see review: Clark and Landolt [10]). However, the literature is not conclusive and some studies report no effect of caffeine on sleep parameters [11, 12]. Moreover, studies to date are limited by small sample sizes not representative of racial/ethnic diversity, objective measures of sleep, and investigations conducted in naturalistic, non-laboratory, settings [3].
Nicotine use is also associated with poor sleep outcomes, including subjective reports of decreased total sleep time, increased sleep onset latency, and higher sleep fragmentation [13]. Of the limited literature utilizing objective assessments of sleep, a large, single night polysomnography study among a predominantly non-Hispanic white sample reveals nicotine use is associated with higher sleep onset latency and lower total sleep duration [14].
Overall, considering the public health importance of obtaining quality sleep and the widespread use of alcohol, nicotine, and caffeine, relatively few studies have thoroughly investigated the association between evening substance use and sleep parameters. The literature particularly lacks investigations among community-dwelling, racial/ethnic minority populations that utilize objective assessments of sleep across several nights to capture individuals’ natural patterns in their home environment [13, 15].
To address this noteworthy gap in the literature and to further elucidate modifiable behaviors that can impact sleep, we studied the night-to-night associations between evening use of alcohol, caffeine, or nicotine and concurrent actigraphically-measured sleep parameters (i.e., total sleep time, sleep efficiency, and wake after sleep onset [WASO]) among a cohort of community-dwelling African American men and women enrolled in the Jackson Heart Sleep Study. This study represents one of the largest longitudinal examinations of the associations of evening use of alcohol, caffeine, and nicotine with objectively measured sleep outcomes. This investigation also advances the literature by accounting for within-person variation of substance use, thereby examining the night-to-night association of substance use with sleep duration and continuity [3]. We hypothesized that evening use of alcohol, caffeine, and nicotine are associated with lower sleep duration, increased WASO, and lower sleep efficiency.
Methods
Study participants and data collection
Participants were enrolled in the sleep ancillary study of the Jackson Heart Study (JHS). The JHS is an observational, prospective cohort study conducted from 2000 to 2013, that enrolled 5301 African American adults from the Jackson, Mississippi metropolitan statistical area. Follow-up visits occurred approximately every 4 years [16]. JHS participants were eligible for the Jackson Heart Sleep Study if they participated in the third follow-up visit of the JHS or participated in other JHS ancillary studies, were not a first-degree relative of already consented participants, and did not report regular use of continuous positive airway pressure. Details of recruitment and study methods for sleep ancillary study of the JHS were previously described [17]. In brief, the JHS sleep ancillary study was conducted between 2012 and 2016 and had a total sample of 913 participants. The current analyses focus on individuals who had ≥4 nights of concurrent actigraphy and sleep diary data, as well as complete data for potential confounders that were also measured as a part of the ancillary study. Of the 913 participants included in the Jackson Heart Sleep Ancillary Study, 824 participants had continuous day-to-day sleep diary and actigraphy data for at least four nights. Of those 824 participants, there were less than 5% of participants with missing covariate data, so we conducted complete-case analysis (n = 785).
Participants’ evening use of alcohol, tobacco, and caffeine within 4 hours of bedtime was assessed using the following questions from the sleep diary, with binary/yes–no response options: “Did you have any alcoholic drinks in the 4 hours before bedtime (beer, wine, hard liquor)?”; “Did you have any drinks with caffeine in the 4 hours before bedtime (coffee, tea, soda)?”; “Did you smoke in the 4 hours before you went to sleep last night?” Overall smoking status was defined using the question “Do you smoke cigarettes?”
Participants were asked to wear a wrist actigraph (GT3X + Activity Monitor) for seven nights and complete a daily sleep diary for every night. ActiLife version 6.13 analysis software (ActiGraph Corp, Pensicola, FL) was used to score 60-second epoch data as sleep or wake using the well-established Cole–Kripke algorithm [18]. Actigraphically measured sleep parameters include sleep duration, WASO, and sleep efficiency. Sleep duration is defined as total time asleep during a main sleep, not including time spent awake after sleep onset and any time spent napping; WASO is defined as total time awake after initial sleep onset; and sleep efficiency is defined as percent sleep time of total time in bed.
Potential confounders included in our statistical models were: age, sex, body mass index (BMI), educational attainment (less than high school, high school or GED, some college/training, and college degree or above), depressive symptomatology (measured by the 20-item Center for Epidemiologic Studies Depression Scale [CESD-20], without the item “my sleep was restless”); anxiety (trait anxiety inventory of the State Trait Anxiety Inventory [STAI]) [19]; perceived stress (Perceived Stress Scale [PSS]) [20]; and having work/school the next day which could vary within a person. Participants also completed the Women’s Health Initiative Insomnia Rating Scale (WHIIRS) a self-report measure of severity of insomnia symptoms [21]. The scale ranged from 0 to 20, with scores of ≥10 indicative of insomnia.
Statistical Analyses
Night-to-night associations between evening substance intake and sleep parameters
To examine the night-to-night relationships between evening use of alcohol, caffeine, and nicotine and the associated night’s sleep while accounting for the correlation within persons, we fit adjusted linear mixed-effect models to estimate the average difference in each sleep measure (continuous variables of sleep duration, WASO, and sleep efficiency) between a night with and without each exposure (binary variables) 4 hours before bedtime. We also conducted an exploratory analysis testing whether baseline sleep symptoms of insomnia, as measured by a WHIIRS score ≥ 10, moderated the effect of substance use on sleep outcomes. We did this by fitting an interaction term between evening substance use and actigraphically assessed sleep duration, WASO, and sleep efficiency.
We used restricted maximum likelihood method to estimate parameters and compared models using maximum likelihood statistics. First, we fit null random-intercept (unadjusted and without sleep traits) models to estimate within-subject and between-subject variance components for each sleep outcome. Linearity assumptions were assessed separately for continuous covariates (e.g., age, BMI, anxiety) in the unadjusted random-intercept model by applying likelihood ratio tests on the improvement in model fit after adding quadratic terms. To assess the independent associations between evening consumption behaviors and each sleep outcome, we adjusted for all potential confounders as previously indicated. In the sensitivity analyses, we excluded the first-night sleep measure for each participant and additionally adjusted for the sleep outcome measure at previous night as a within-person level covariate in the mixed-effect models (n = 4408 days). Analyses were conducted in Stata v 14 [22] and SAS 9.4 [23]; two-sided p-values were reported for statistical testing.
Associations between smoking status and average sleep parameters
Finally, to examine the mean difference in each sleep outcome averaged over all nights between current smokers and never smokers or former smokers, we fit multivariable linear regression models for each sleep outcome while adjusting for potential within- and between-person level confounders as described earlier (Table 3).
Results
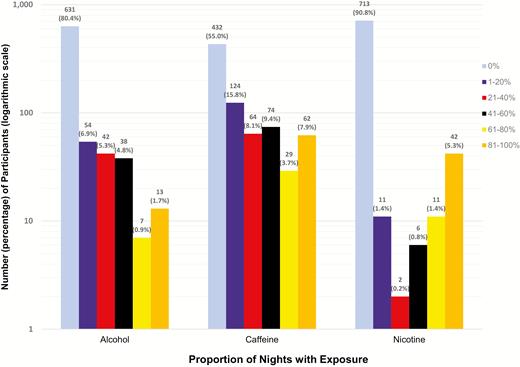
There were a total of 5164 days of concurrent actigraphy and sleep diary data. Participants were predominantly female (67.9%), an average age of 63.7 years (SD: 10.6), and 53.8% had at least some college or a college degree (Table 1). One or more nights of caffeine, alcohol, and nicotine consumption within 4 hours of bedtime was reported by 45.0%, 19.6%, and 9.2% of the analytic sample, respectively (Figure 1). Nineteen percent of the sample had symptoms indicative of insomnia, as measured by a WHIIRS score ≥ 10; 35% of the sample reported high-moderate levels of stress, and 14% had elevated scores for depressed mood (Table 1). Evening nicotine use was higher in individuals reporting increased symptoms of insomnia, depressed mood, and anxiety; evening caffeine use was higher among those reporting increased stress; and evening alcohol use was higher among those with increased stress (Supplementary Table 1). We adjusted for these factors as confounders but chose not to test for interactions due to concerns about multiple testing.
Sample demographics, sleep hygiene, and sleep characteristics (n = 785)
| Demographics | |
| Female, N (%) | 533 (67.9%) |
| Age, mean ± SD | 63.7 ± 10.6 |
| Education, N (%) | 77 (9.8%) |
| <High school | 119 (15.2%) |
| High school or GED | 167 (21.3%) |
| Some college/training | 422 (53.8%) |
| College degree | |
| Percent days with work or school the next day (%), mean ± SD | 27.9 ± (34.2 %) |
| BMI (kg/m2), mean ± SD | 31.7 ± 6.8 |
| Current smoker, N (%) | 62 (7.9%) |
| Psychosocial factors | |
| CESD-20 score (without restless sleep item), mean ± SD | 7.7 ± 7.2 |
| High depressive symptoms (CESD-20 score without restless sleep item ≥ 16), N (%) | 108 (13.8%) |
| Trait anxiety inventory (TAI-20) score, mean ± SD | 30.4 ± 8.1 |
| Perceived stress scale (PSS-10) score, mean ± SD | 11.1 ± 6.8 |
| Moderate or high perceived stress (PSS-10 score ≥ 14), N (%) | 273 (34.8%) |
| Substance intake within 4 hours of bedtime* | |
| Percent nights had any alcoholic beverages(%), mean ± SD | 7.0 ± 17.5 |
| Percent nights had any caffeinated beverages (%), mean ± SD | 19.5 ± 29.0 |
| Percent nights smoked cigarettes (nicotine) (%), mean ± SD | 7.0 ± 23.9 |
| Sleep characteristics | |
| Actigraphy-measured sleep (daily average over all actigraphy days) | |
| Sleep duration (hours), mean ± SD | 6.7 ± 1.1 |
| WASO (minutes), mean ± SD | 53.9 ± 23.4 |
| Sleep efficiency (%), mean ± SD | 86.9 ± 4.9 |
| Insomnia Rating Scale (WHIIRS) score (n = 780), mean ± SD | 6.0 ± 5.0 |
| Demographics | |
| Female, N (%) | 533 (67.9%) |
| Age, mean ± SD | 63.7 ± 10.6 |
| Education, N (%) | 77 (9.8%) |
| <High school | 119 (15.2%) |
| High school or GED | 167 (21.3%) |
| Some college/training | 422 (53.8%) |
| College degree | |
| Percent days with work or school the next day (%), mean ± SD | 27.9 ± (34.2 %) |
| BMI (kg/m2), mean ± SD | 31.7 ± 6.8 |
| Current smoker, N (%) | 62 (7.9%) |
| Psychosocial factors | |
| CESD-20 score (without restless sleep item), mean ± SD | 7.7 ± 7.2 |
| High depressive symptoms (CESD-20 score without restless sleep item ≥ 16), N (%) | 108 (13.8%) |
| Trait anxiety inventory (TAI-20) score, mean ± SD | 30.4 ± 8.1 |
| Perceived stress scale (PSS-10) score, mean ± SD | 11.1 ± 6.8 |
| Moderate or high perceived stress (PSS-10 score ≥ 14), N (%) | 273 (34.8%) |
| Substance intake within 4 hours of bedtime* | |
| Percent nights had any alcoholic beverages(%), mean ± SD | 7.0 ± 17.5 |
| Percent nights had any caffeinated beverages (%), mean ± SD | 19.5 ± 29.0 |
| Percent nights smoked cigarettes (nicotine) (%), mean ± SD | 7.0 ± 23.9 |
| Sleep characteristics | |
| Actigraphy-measured sleep (daily average over all actigraphy days) | |
| Sleep duration (hours), mean ± SD | 6.7 ± 1.1 |
| WASO (minutes), mean ± SD | 53.9 ± 23.4 |
| Sleep efficiency (%), mean ± SD | 86.9 ± 4.9 |
| Insomnia Rating Scale (WHIIRS) score (n = 780), mean ± SD | 6.0 ± 5.0 |
*The distribution of substance intake is provided in Figure 1.
Sample demographics, sleep hygiene, and sleep characteristics (n = 785)
| Demographics | |
| Female, N (%) | 533 (67.9%) |
| Age, mean ± SD | 63.7 ± 10.6 |
| Education, N (%) | 77 (9.8%) |
| <High school | 119 (15.2%) |
| High school or GED | 167 (21.3%) |
| Some college/training | 422 (53.8%) |
| College degree | |
| Percent days with work or school the next day (%), mean ± SD | 27.9 ± (34.2 %) |
| BMI (kg/m2), mean ± SD | 31.7 ± 6.8 |
| Current smoker, N (%) | 62 (7.9%) |
| Psychosocial factors | |
| CESD-20 score (without restless sleep item), mean ± SD | 7.7 ± 7.2 |
| High depressive symptoms (CESD-20 score without restless sleep item ≥ 16), N (%) | 108 (13.8%) |
| Trait anxiety inventory (TAI-20) score, mean ± SD | 30.4 ± 8.1 |
| Perceived stress scale (PSS-10) score, mean ± SD | 11.1 ± 6.8 |
| Moderate or high perceived stress (PSS-10 score ≥ 14), N (%) | 273 (34.8%) |
| Substance intake within 4 hours of bedtime* | |
| Percent nights had any alcoholic beverages(%), mean ± SD | 7.0 ± 17.5 |
| Percent nights had any caffeinated beverages (%), mean ± SD | 19.5 ± 29.0 |
| Percent nights smoked cigarettes (nicotine) (%), mean ± SD | 7.0 ± 23.9 |
| Sleep characteristics | |
| Actigraphy-measured sleep (daily average over all actigraphy days) | |
| Sleep duration (hours), mean ± SD | 6.7 ± 1.1 |
| WASO (minutes), mean ± SD | 53.9 ± 23.4 |
| Sleep efficiency (%), mean ± SD | 86.9 ± 4.9 |
| Insomnia Rating Scale (WHIIRS) score (n = 780), mean ± SD | 6.0 ± 5.0 |
| Demographics | |
| Female, N (%) | 533 (67.9%) |
| Age, mean ± SD | 63.7 ± 10.6 |
| Education, N (%) | 77 (9.8%) |
| <High school | 119 (15.2%) |
| High school or GED | 167 (21.3%) |
| Some college/training | 422 (53.8%) |
| College degree | |
| Percent days with work or school the next day (%), mean ± SD | 27.9 ± (34.2 %) |
| BMI (kg/m2), mean ± SD | 31.7 ± 6.8 |
| Current smoker, N (%) | 62 (7.9%) |
| Psychosocial factors | |
| CESD-20 score (without restless sleep item), mean ± SD | 7.7 ± 7.2 |
| High depressive symptoms (CESD-20 score without restless sleep item ≥ 16), N (%) | 108 (13.8%) |
| Trait anxiety inventory (TAI-20) score, mean ± SD | 30.4 ± 8.1 |
| Perceived stress scale (PSS-10) score, mean ± SD | 11.1 ± 6.8 |
| Moderate or high perceived stress (PSS-10 score ≥ 14), N (%) | 273 (34.8%) |
| Substance intake within 4 hours of bedtime* | |
| Percent nights had any alcoholic beverages(%), mean ± SD | 7.0 ± 17.5 |
| Percent nights had any caffeinated beverages (%), mean ± SD | 19.5 ± 29.0 |
| Percent nights smoked cigarettes (nicotine) (%), mean ± SD | 7.0 ± 23.9 |
| Sleep characteristics | |
| Actigraphy-measured sleep (daily average over all actigraphy days) | |
| Sleep duration (hours), mean ± SD | 6.7 ± 1.1 |
| WASO (minutes), mean ± SD | 53.9 ± 23.4 |
| Sleep efficiency (%), mean ± SD | 86.9 ± 4.9 |
| Insomnia Rating Scale (WHIIRS) score (n = 780), mean ± SD | 6.0 ± 5.0 |
*The distribution of substance intake is provided in Figure 1.
Frequency of nights with substance use over actigraphy period.
Night-to-night associations between evening alcohol, caffeine, and nicotine intake and sleep parameters
Table 2 shows the adjusted night-to-night associations among evening intake of alcohol, caffeine, and nicotine and actigraphically estimated sleep duration, WASO, and sleep efficiency. Comparing to a night without alcohol use before 4 hours of bedtime, a night with evening use of alcohol had 0.98% lower sleep efficiency on average (95% CI: −1.67% to −0.29%, p = 0.005), but did not have statistically significant differences in WASO or sleep duration. A night with evening nicotine use, compared to a night without, had 6.09 minutes longer WASO (95% CI: 0.82 to 11.35, p = 0.02), 1.74% lower sleep efficiency (95% CI: −2.79% to −0.68%, p = 0.001), and no statistically significant difference in sleep duration. No associations were observed between caffeine use and any sleep parameters.
Night-to-night associations of alcohol, caffeine, and nicotine intake and actigraphically measured sleep (n = 5164 days nested within 785 participants)
| Behavioral predictors . | Sleep duration (minutes) (95% CI) . | WASO (minutes) (95% CI) . | Sleep efficiency (%) (95% CI) . |
|---|---|---|---|
| Alcohol use within 4 hours of bedtime | −3.62 (−14.37 to 7.14) p = 0.51 | 3.02 (−0.52 to 6.55) p = 0.09 | −0.98 (−1.67 to −0.29) p = 0.005 |
| Caffeine use within 4 hours of bedtime | −1.15 (−8.39 to 6.10) p = 0.76 | 1.13 (−1.25 to 3.52) p = 0.35 | −0.15 (−0.62 to 0.31) p = 0.53 |
| Nicotine use within 4 hours of bedtime | −12.97 (−28.53 to 2.59) p = 0.10 | 6.09 (0.82 to 11.35) p = 0.02 | −1.74 (−2.79 to −0.68) p = 0.001 |
| Behavioral predictors . | Sleep duration (minutes) (95% CI) . | WASO (minutes) (95% CI) . | Sleep efficiency (%) (95% CI) . |
|---|---|---|---|
| Alcohol use within 4 hours of bedtime | −3.62 (−14.37 to 7.14) p = 0.51 | 3.02 (−0.52 to 6.55) p = 0.09 | −0.98 (−1.67 to −0.29) p = 0.005 |
| Caffeine use within 4 hours of bedtime | −1.15 (−8.39 to 6.10) p = 0.76 | 1.13 (−1.25 to 3.52) p = 0.35 | −0.15 (−0.62 to 0.31) p = 0.53 |
| Nicotine use within 4 hours of bedtime | −12.97 (−28.53 to 2.59) p = 0.10 | 6.09 (0.82 to 11.35) p = 0.02 | −1.74 (−2.79 to −0.68) p = 0.001 |
Significant p values are bolded. Adjusted for age, gender, BMI, education, work/school next day, depressive symptoms, anxiety, stress.
Night-to-night associations of alcohol, caffeine, and nicotine intake and actigraphically measured sleep (n = 5164 days nested within 785 participants)
| Behavioral predictors . | Sleep duration (minutes) (95% CI) . | WASO (minutes) (95% CI) . | Sleep efficiency (%) (95% CI) . |
|---|---|---|---|
| Alcohol use within 4 hours of bedtime | −3.62 (−14.37 to 7.14) p = 0.51 | 3.02 (−0.52 to 6.55) p = 0.09 | −0.98 (−1.67 to −0.29) p = 0.005 |
| Caffeine use within 4 hours of bedtime | −1.15 (−8.39 to 6.10) p = 0.76 | 1.13 (−1.25 to 3.52) p = 0.35 | −0.15 (−0.62 to 0.31) p = 0.53 |
| Nicotine use within 4 hours of bedtime | −12.97 (−28.53 to 2.59) p = 0.10 | 6.09 (0.82 to 11.35) p = 0.02 | −1.74 (−2.79 to −0.68) p = 0.001 |
| Behavioral predictors . | Sleep duration (minutes) (95% CI) . | WASO (minutes) (95% CI) . | Sleep efficiency (%) (95% CI) . |
|---|---|---|---|
| Alcohol use within 4 hours of bedtime | −3.62 (−14.37 to 7.14) p = 0.51 | 3.02 (−0.52 to 6.55) p = 0.09 | −0.98 (−1.67 to −0.29) p = 0.005 |
| Caffeine use within 4 hours of bedtime | −1.15 (−8.39 to 6.10) p = 0.76 | 1.13 (−1.25 to 3.52) p = 0.35 | −0.15 (−0.62 to 0.31) p = 0.53 |
| Nicotine use within 4 hours of bedtime | −12.97 (−28.53 to 2.59) p = 0.10 | 6.09 (0.82 to 11.35) p = 0.02 | −1.74 (−2.79 to −0.68) p = 0.001 |
Significant p values are bolded. Adjusted for age, gender, BMI, education, work/school next day, depressive symptoms, anxiety, stress.
Associations between smoking status and indices of average sleep
Given that nicotine was the substance most strongly associated with sleep disruption, we conducted a post hoc analysis examining the differences in average sleep parameters comparing current smokers to non-current smokers (Table 3). Participants who reported as current smokers had an average of 8.01 minutes higher WASO (95% CI: 1.94 to 14.07, p = 0.01) and 1.74% lower sleep efficiency (95% CI: −3.00% to −0.48%, p = 0.007) than nonsmokers or former smokers after adjusting for covariates.
Adjusted mean differences in sleep parameters by smoking status
| Sleep parameter . | Adjusted mean difference comparing smokers vs. nonsmokers (%95 CI) . | p-value . |
|---|---|---|
| Sleep duration (minutes) | 1.51 (−15.88 to 18.89) | 0.865 |
| WASO (minutes) | 8.01 (1.94 to 14.07) | 0.010 |
| Sleep efficiency (%) | −1.74 (−3.00 to −0.48) | 0.007 |
| Sleep parameter . | Adjusted mean difference comparing smokers vs. nonsmokers (%95 CI) . | p-value . |
|---|---|---|
| Sleep duration (minutes) | 1.51 (−15.88 to 18.89) | 0.865 |
| WASO (minutes) | 8.01 (1.94 to 14.07) | 0.010 |
| Sleep efficiency (%) | −1.74 (−3.00 to −0.48) | 0.007 |
Significant p values are bolded. Adjusted for age, sex, BMI, education, work/school next day, depressive symptoms, anxiety, stress.
Adjusted mean differences in sleep parameters by smoking status
| Sleep parameter . | Adjusted mean difference comparing smokers vs. nonsmokers (%95 CI) . | p-value . |
|---|---|---|
| Sleep duration (minutes) | 1.51 (−15.88 to 18.89) | 0.865 |
| WASO (minutes) | 8.01 (1.94 to 14.07) | 0.010 |
| Sleep efficiency (%) | −1.74 (−3.00 to −0.48) | 0.007 |
| Sleep parameter . | Adjusted mean difference comparing smokers vs. nonsmokers (%95 CI) . | p-value . |
|---|---|---|
| Sleep duration (minutes) | 1.51 (−15.88 to 18.89) | 0.865 |
| WASO (minutes) | 8.01 (1.94 to 14.07) | 0.010 |
| Sleep efficiency (%) | −1.74 (−3.00 to −0.48) | 0.007 |
Significant p values are bolded. Adjusted for age, sex, BMI, education, work/school next day, depressive symptoms, anxiety, stress.
Interactions between self-report insomnia symptoms, evening substance use, and sleep parameters
Baseline self-report of insomnia (as indicated by a WHIIRS score ≥ 10), was associated with evening nicotine use (p = 0.02); 4.33 minutes of higher WASO (95% CI: 0.07 to 8.60, p = 0.047); and 0.95% lower sleep efficiency (95% CI: −1.83% to −0.06%, p = 0.007). There were no interactions between baseline report of insomnia and evening alcohol or caffeine use in the associations with sleep parameters. However, there was a statistically significant interaction between evening nicotine use and insomnia in relation to sleep duration. Specifically, among participants with a baseline report of insomnia (WHIIRS score ≥ 10), nightly nicotine use was associated with an average 42.47-minute reduction in sleep duration (95% CI: −73.37 to −11.56, p = 0.007). Among those without a baseline report of insomnia (WHIIRS score < 10), nicotine use was not associated with sleep duration. Similarly, interactions with insomnia and nicotine were not observed for sleep efficiency or WASO.
Discussion
This study represents one of the largest examinations of the night-to-night associations of alcohol, caffeine, and nicotine with objectively measured sleep duration and continuity in the general population and among African Americans. In this sample, we did not observe an association between ingestion of caffeine within 4 hours of bedtime with any of the sleep parameters. However, a night with use of nicotine and/or alcohol within 4 hours of bedtime demonstrated worse sleep continuity than a night without these substances, even after controlling for age, BMI, educational attainment, having work/school the next day, and mental health symptomatology. These results are especially meaningful as they were observed in individuals unselected for sleep problems and who generally had high sleep efficiency. Moreover, they were based on longitudinal data so that the associations can take account of not only between-person differences but also within-person variations in exposures and covariates.
Our findings that alcohol consumed within 4 hours of bedtime was associated with a small but statistically significant reduction in sleep efficiency is consistent with other studies demonstrating that alcohol can enhance sleep onset but decrease sleep continuity during the second half of the sleep period. Our multilevel modeling of repeated measures of substance use and objective sleep outcomes in a naturalistic setting allowed us to extend these findings, estimating the average effect of alcohol consumption on objectively assessed sleep while modeling variances at within- and between-person levels, an approach lacking in the sleep hygiene literature [3]. Experimental and cross-sectional research has revealed that the impact of alcohol on sleep may vary according to dosing and drinking patterns [8, 9, 24]. Thus, we might have observed larger effect size of alcohol use on sleep, including associations with duration or WASO, if we had more detailed information on quantity of alcohol consumed before bedtime. With nearly 60% of adults in the United States consuming at least one alcoholic beverage per month, further research investigating the impact of dosing and timing of alcohol ingestion on sleep parameters is salient [3, 25]. “Happy hours,” which generally occur during early evening hours, are popular times for individuals to consume alcohol [26, 27]. While limited research has indicated that alcohol can negatively impact sleep even after alcohol metabolizes (indicating early evening use), the investigations to date are small and conducted among clinical populations [28, 29]. Thus, elucidating the impact of early evening alcohol use on objectively measured sleep parameters among larger, community-dwelling populations is needed. Moreover, for adults who choose to consume alcohol, these investigations could perhaps inform a “harm-reduction” approach surrounding alcohol dosing and timing to negate the impact of alcohol on sleep.
Caffeine use before bedtime was relatively common in this sample: 40% of participants endorsed at least one evening of caffeine use over the actigraphy period, which reflects the popularity of caffeine in the general population [30]. Physiologically, caffeine promotes wakefulness by acting as an adenosine antagonist, and blocking sleep-promoting neurochemicals [31]. However, similar to other studies, we did not find an association between evening caffeine consumption and any sleep parameters in our longitudinal assessment [11, 32]. When interpreting our null findings, it is important to consider that we were unable to consider caffeine dosing, caffeine sensitivity, and caffeine tolerance, which are potentially important moderating factors to be studied in future investigations on examining the effects of caffeine on sleep disruption [3, 10].
Nicotine was the substance used least frequently. However, it was also the substance associated with more disturbed sleep (lower sleep efficiency and higher WASO) even after controlling for covariates that commonly affect sleep (i.e., age, gender, BMI, education, work/school the following day, depressive symptoms, anxiety, and stress). Nicotine is a stimulant and can disturb sleep through the disruption of sleep-involved neurotransmitters, as well as through nicotine withdrawal that occurs while sleeping [33]. The estimates we observed (1.74% reduction in sleep efficiency) were larger than in a study by Zhang et al.[33] who reported a 0.7% reduction in sleep efficiency (84.1% vs. 83.4%) in smokers versus nonsmokers using at home polysomnography. We add to this important work by objectively assessing sleep among a large sample of African Americans across multiple nights, therefore accounting for more natural physiological responses to nicotine compared to a single night of polysomnography. It is important to note that single night polysomnography may not provide a representative assessment of usual sleep and the higher estimates we observed may reflect phenomena observed in more natural sleep settings, with enhanced reliability due to multiple days of measurement. Moreover, in exploratory analyses, we found a significant interaction between self-reported insomnia symptoms and evening nicotine use such that nicotine use prior to bedtime was associated with over 40 minutes of reduced sleep duration in those with insomnia symptoms. This suggests that the effects of nicotine may be particularly significant among individuals with insomnia. These findings underscore the importance of addressing substance use in clinical settings and in individuals with insomnia symptoms.
Unlike other studies that used questionnaires or polysomnography, we did not find any significant changes in sleep duration associated with evening nicotine use. This may reflect differences in the underlying samples, and the potential for numerous other factors influencing sleep in this African American population. Alternatively, the sample had relatively few individuals who smoked frequently before bedtime, and thus we may have been under-powered.
Sleep quality is influenced by multiple behavioral, environmental, and biological factors. The magnitude of the individual effects of alcohol and nicotine use on sleep continuity in our study were relatively small. However, these effect estimates are consistent with other studies that have used actigraphy to estimate the effects of various social and behavioral exposures on sleep fragmentation or duration. For example, in a recent investigation of neighborhood disadvantage and associations with actigraphy-estimated sleep among predominantly African American adults, Troxel et al.[34] found that perceived neighborhood safety was significantly associated with 1.14% higher sleep efficiency, and 5.64 minutes lower WASO. The literature that shows small effects of social, behavioral, and environmental factors on sleep suggests that multi-pronged and multilevel approaches may be needed for improving sleep in the community. Specifically, avoidance of alcohol and nicotine before bedtime is one modifiable target that not only has general health benefits but also helps to improve sleep continuity.
Limitations and Strengths
Results from our study should be interpreted within the context of study limitations. The primary limitation was the lack of dosing information for alcohol, caffeine, and nicotine. In addition, our sample of African Americans was recruited from a well-established cohort from a single but large geographic area and had relatively high education levels; thus, the findings may not generalize to other samples. Our study also has a number of strengths, particularly our longitudinal design (7 days) that included over 5000 nights of data, and the use of objective assessment of sleep parameters via wrist actigraphy, among a large epidemiologic cohort of African Americans. The study design allowed us to model the nightly association of each exposure with sleep—a strategy that accounts for within—and between-person variances. We also had extensive data on stress and mental health and were able to address confounding by these factors. Objective assessment of sleep duration and quality among African Americans is especially important as subjectively measured sleep duration has been found to be especially misreported among this demographic group [35].
Conclusion
This study comprises one of the largest night-to-night examinations of the association of alcohol, caffeine, and nicotine on sleep duration and continuity utilizing objective assessments of sleep. Among a large African American cohort, evening consumption of alcohol and nicotine were associated with reductions in sleep continuity during the associated night. These associations were independent of factors such as age, gender, BMI, level of education, having work/school the next day, depressive symptoms, anxiety, and stress. While these estimated effect sizes were small, they were within the range of effects commonly reported in the sleep health field for other behavioral as well as social and environmental exposures [33, 34]. Moreover, nicotine was associated with large decrements in nightly sleep duration among individuals with insomnia, suggesting that individuals with sleep problems may be particularly sensitive to nightly use. Our findings support sleep health interventions that promote cessation of alcohol and nicotine and highlight the need for additional longitudinal investigations that examine dose-response effects and timing on sleep parameters.
Funding
C.E.S. was supported in part by Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. D.A.J. was supported in part by K01HL138211. S.R. was supported in part by 5R35HL135818. J.G.W. is supported by U54GM115428 from the National Institute of General Medical Sciences. This work was funded, in part, by the Intramural Program at the NIH, National Institute of Environmental Health Sciences (Z1AES103325-01). The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I, and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD).
Conflict of interest statement. None declared.
Acknowledgments
The authors thank the participants and data collection staff of the Jackson Heart Study. The lead author also thanks Dr. Jarvis T. Chen for his helpful review of the manuscript, and Dr. Brian Healy for his statistical instruction. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the U.S. Department of Health and Human Services; Harvard Catalyst; and Harvard University and its affiliated academic healthcare centers.
References
Cole RJ, et al. Automatic sleep/wake identification from wrist activity. Sleep 1992;15(5):461–469.
Rosenthal L, et al. Alerting effects of caffeine after normal and restricted sleep. Neuropsychopharmacol 1991b;4:103–108





Comments