-
PDF
- Split View
-
Views
-
Cite
Cite
Simone Harmsen, Carina A C M Pittens, Eva Vroonland, Annemiek J M L van Rensen, Jacqueline E W Broerse, Supporting health researchers to realize meaningful patient involvement in research: Exploring researchers’ experiences and needs, Science and Public Policy, Volume 49, Issue 5, October 2022, Pages 751–764, https://doi.org/10.1093/scipol/scac024
Close - Share Icon Share
Abstract
Involving patients in health research requires a new way of working for all stakeholders involved, including researchers. This research aimed (1) to gain deeper insight into the experiences and needs of researchers regarding meaningful patient involvement and (2) to incorporate these insights into an online tool. This was done in a transdisciplinary research process, including three focus group discussions and three test sessions. We used the Social Cognitive Theory in the analysis process to reflect on how the tool addresses the complex personal, behavioural, and environmental factors that shape researchers’ experiences and needs. Identified factors were categorized into three themes: added value, perceived difficulty and patient-researcher role patterns. A tool was developed that addresses these factors, aiming to stimulate meaningful involvement by encouraging (self)reflection, experimentation, and learning-by-doing. It provides one element in a bigger systems approach to further stimulate patient involvement.
1. Introduction
Patient involvement in health research has gained much ground in the last 20 years and can be defined as: ‘The involvement of patients (or their representatives) in health research decision-making processes on the basis of their experiential knowledge’ (Schölvinck 2018: 25). Patients, people with any type of experiential knowledge of disabilities or chronic diseases, who are involved in health research, can complement the expert knowledge of researchers and health professionals (Caron-Flinterman et al. 2005). Integrating this knowledge into the health research cycle could improve its outcomes, such as its enhanced relevance, feasibility, quality, and appropriateness (Baxter et al. 2016; Brett et al. 2014; Ennis and Wykes 2013).
Worldwide, patient involvement is increasingly included in health research policy and programmes. For instance, the Netherlands Organization for Health Research and Development (ZonMw) and the UK’s National Institute for Health Research have made it a criterion for some of their funding programmes (Den Oudendammer et al. 2019). Patients have successfully participated throughout the health research cycle. They have aided in agenda-setting and prioritizing research topics (Manafò et al. 2018; Pittens et al. 2014) and have been involved in grant writing and research design (Baxter et al. 2016), as well as proposal appraisal (de Wit et al. 2018b). Patient co-researchers join research teams and sometimes also participate in data interpretation (de Wit et al. 2018b; Frost et al. 2018; Gillard et al. 2012; Jennings et al. 2018; Nierse et al. 2012). Furthermore, their involvement becomes visible in the publishing stage, as The British Medical Journal (BMJ) now demands BMJ Open article submissions to include a Patient and Public Involvement statement, ‘hoping that requiring the reporting will encourage the practice’ (Aldcroft 2018).
As patient involvement becomes more widespread, many have tried to set standards for patient involvement (Greenhalgh et al. 2019). We argue that patient involvement should be ‘meaningful’, for which the following principles have been formulated:
Inclusion and diversity: create a welcoming attitude and environment towards (a diversity of) patients (Black et al. 2018) and appreciate the value of their knowledge (Kirwan et al. 2017).
Mutual learning: learning of researchers and patients as a result of the interaction between them (Schölvinck 2018; Staley and Barron 2019).
Responsiveness: make the effort to act upon patient knowledge (Bellows et al. 2015).
Various studies have, however, shown that putting these principles into practice is neither straightforward nor easy (Domecq et al. 2014; Schölvinck et al. 2018). A review by Domecq et al. (2014) found that many studies reported challenges in patient involvement, such as time constraints on the part of both researchers and patients and the risk of tokenism. Schölvinck et al. (2018) reported in a case study how a lack of ownership by both researchers and patients made it hard to create sustainable collaboration, with researchers reportedly doubting whether they have the required skills and finding it hard to allocate enough time. Faulkner et al. (2021) identified several gaps in the engagement of patients in medicines development, such as paying little attention to the diversity of and support for patients regarding their roles and responsibilities.
Conducting research in which patients have an active role requires a new way of working for all parties involved. Both researchers and policymakers have aimed to support patients to fulfil their role in patient involvement processes by establishing organizations like INVOLVE (Elberse et al. 2011; Staniszewska et al. 2018), EUPATI (Pushparajah et al. 2015), and PFMD (Boutin et al. 2017), and by setting out patients’ needs and perceptions (Elberse et al. 2011; Leese et al. 2018). Nevertheless, studies like those by Schölvinck et al. (2018) and Domecq et al. (2014) show that researchers also need to be supported to fulfil their role in patient–researcher collaborations. Some initiatives to support researchers have become available in recent years, such as the online Patient Engagement Management Suite (Patient Engagement for Medicines Development 2021) with practical tools to plan, assess, and execute patient involvement in medicines development (de Wit et al. 2018a; Turk et al. 2017). As the health foundations that funded our research receive frequent questions from researchers on patient involvement, it seems that the existing tools do not meet their specific needs.
Scholars have recently gained increasing insights into the challenges researchers experience. These include doubts about who to involve and how to recruit patients (Carroll et al. 2017; Lander et al. 2019), the value of the investment of time (Maccarthy et al. 2019; Schölvinck 2018; Vroonland et al. 2019), questions about the quality and influence of patients’ input (Carroll et al. 2017; Gillard et al. 2012; Maccarthy et al. 2019), doubts about the added value of patients’ knowledge for scientific research (Gibson et al. 2019), and a lack of competence regarding patient involvement on the part of (pre-clinical) researchers (Carroll et al. 2017; Gibson et al. 2019; Maccarthy et al. 2019). Also, more external challenges, like lack of time due to commissioner-imposed timelines or lack of funding, have been reported (Pittens et al. 2014). In order to understand how to best support researchers in employing meaningful patient involvement, it is important to take into account this complexity of factors that shape researcher’s experiences and needs. The Social Cognitive Theory (SCT) might be helpful to study this complexity, as it poses that learning and behaviour, such as involving patients, are shaped by personal, behavioural, and environmental factors that all affect each other (Bandura 1986).
In this study, we aimed (Schölvinck 2018) to gain deeper insight into the experiences and needs of researchers regarding meaningful patient involvement and (Caron-Flinterman et al. 2005) to incorporate these insights into an online tool that supports researchers to involve patients in a meaningful way. In this paper, we describe the experiences and needs of researchers, guided by the SCT, and we report on the tool’s design process.
2. Method
This study was commissioned by ZonMw, three Dutch health foundations, and one patient organization with the ultimate goal of developing an online tool to support researchers in employing meaningful patient involvement in health research. Data collection and design activities were carried out between September 2018 and September 2019. Our research and design method was based on the Interactive Learning and Action (ILA) approach (Broerse and Bunders 2000), a transdisciplinary research approach that involves various (societal) stakeholders in innovation development, in order to address persistent complex problems (Schuitmaker 2012). We chose this approach as we believe the development of an effective tool to stimulate meaningful patient involvement requires the critical input of various stakeholders.
Following ILA, our research comprised four phases:
Preparation - an overview of relevant stakeholders and a preliminary overview of their issues and views are made;
Exploration and design - issues and views are deepended and the first outlines of atool design are drawn;
Reflection and improvement - through testing and reflection, the innovation is further refined; and
Implementation - the resulting innovation is implemented and explanded.
Phases 1–3 were conducted by the research team comprising two academic researchers (first two authors) and two advisors of an independent organization supporting patient involvement (third and fourth authors).1 The subsequent promotion and dissemination of the online tool were led by the organizations that funded this research, supported by the organization of the third and fourth authors. The first three phases are reported in this article.
2.1 Phase 1: preparation (September–October 2018)
2.1.1 Scoping search
A non-systematic scoping search of grey literature was conducted to gain some insights into (Schölvinck 2018) existing online tools and (Caron-Flinterman et al. 2005) the prerequisites for patient involvement set by funding organizations in the Netherlands. We did so using Google, visiting websites of relevant institutions, and inquiring within our networks.
2.1.2 Steering committee
A steering committee of six members was formed to advise the research team throughout the project. The committee represented three important stakeholder groups in patient involvement in research: funders, researchers, and patient organizations. Members were invited because of their key positions in networks within the health research system and patient involvement in the Netherlands and complementary expertise. The steering committee met at three moments throughout the project and had an advisory role (i.e. participant selection, the interpretation of data, and the consequences of outcomes for the online tool).
2.1.3 Preliminary exploration
Semi-structured interviews were held with all six members of the steering committee (see Table 1), in which they provided advice on the set-up of the focus group discussions (FGDs; Phase 2) and expressed their initial ideas regarding an online tool. Additionally, we aimed to gain a preliminary understanding of the experiences and needs that health researchers have with respect to patient involvement. Interviewees were asked to draw on their experiences as a funder, researcher, and/or patient organization. They were asked about (their and/or other) researcher’s general experiences with patient involvement, the challenges they encountered, and the questions they have. All interviews were audio-recorded. They were transcribed verbatim and read thoroughly and analysed by the first author (S.H.). We aimed to create a preliminary overview of things that researchers might need support with. Therefore, the interviews were transcribed verbatim, and the challenges and questions that researchers have were inductively coded, through reflexive thematic coding and then categorized in an emerging chronological order: from deciding to involve patients, designing involvement, to implementing involvement and post-involvement. The results were formulated in a list as either questions or challenge statements (i.e. ‘who should I involve in my study?’ and ‘younger scientists are sometimes hindered by their supervisors’). The analysis results were discussed with the other authors and used as a starting point for the subsequent FGDs.
Characteristics of participants throughout the project. Numbers indicate the number of participants with certain characteristics.
| . | . | Phase 1 . | Phase 2 . | Phase 3 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aspect . | Category . | Interviews . | FGD 1 . | FGD 2 . | FGD 3 . | Test Session 1 . | Test Session 2 . | Patient check . | Total . |
| Number of participants | 6 | 7 | 8 | 8 | 9 | 3 | 4 | 45 | |
| Sex | Male | 2 | 3 | 1 | 4 | 1 | 1 | – | 12 |
| Female | 4 | 4 | 7 | 4 | 8 | 2 | – | 29 | |
| Role/research experience | Junior researcher | – | 4 | 3 | 1 | 4 | 2 | – | 14 |
| Senior researcher | 2 | 2 | 4 | 5 | 1 | 1 | – | 15 | |
| Other role in research (coordinator, nursing specialist, team manager) | 1 | 1 | 1 | 2 | 4 | – | – | 9 | |
| Policymaker from patient organization | 2 | – | – | – | – | – | – | 2 | |
| Patient representative | 1 | – | – | – | – | – | 4 | 4 | |
| Background of researchers in FGDs and test sessions | |||||||||
| Area of expertise | Oncology | – | – | 1 | 6 | – | – | – | 7 |
| Psychology | – | 1 | – | 1 | – | 1 | – | 3 | |
| Pulmonology | – | – | – | – | 2 | – | 2 | ||
| Paediatrics | – | 6 | – | – | – | 1 | – | 6 | |
| Epidemiology | – | – | 2 | – | 1 | – | 3 | ||
| Other | – | – | 5 | 2 | 6 | 1 | – | 14 | |
| Type of research | Participatory | – | 2 | 2 | – | – | 2 | – | 4 |
| Pre-clinical | – | 1 | 1 | – | – | – | – | 2 | |
| Clinical | – | 3 | 5 | 6 | 7 | 1 | – | 14 | |
| Applied care research | – | – | – | 2 | 2 | – | – | 4 | |
| Organization | Academic hospital | – | 6 | 6 | 8 | 8 | 3 | – | 30 |
| Applied sciences | – | 1 | – | – | 1 | – | – | 2 | |
| Research institute | – | – | 2 | – | – | – | – | 2 | |
| . | . | Phase 1 . | Phase 2 . | Phase 3 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aspect . | Category . | Interviews . | FGD 1 . | FGD 2 . | FGD 3 . | Test Session 1 . | Test Session 2 . | Patient check . | Total . |
| Number of participants | 6 | 7 | 8 | 8 | 9 | 3 | 4 | 45 | |
| Sex | Male | 2 | 3 | 1 | 4 | 1 | 1 | – | 12 |
| Female | 4 | 4 | 7 | 4 | 8 | 2 | – | 29 | |
| Role/research experience | Junior researcher | – | 4 | 3 | 1 | 4 | 2 | – | 14 |
| Senior researcher | 2 | 2 | 4 | 5 | 1 | 1 | – | 15 | |
| Other role in research (coordinator, nursing specialist, team manager) | 1 | 1 | 1 | 2 | 4 | – | – | 9 | |
| Policymaker from patient organization | 2 | – | – | – | – | – | – | 2 | |
| Patient representative | 1 | – | – | – | – | – | 4 | 4 | |
| Background of researchers in FGDs and test sessions | |||||||||
| Area of expertise | Oncology | – | – | 1 | 6 | – | – | – | 7 |
| Psychology | – | 1 | – | 1 | – | 1 | – | 3 | |
| Pulmonology | – | – | – | – | 2 | – | 2 | ||
| Paediatrics | – | 6 | – | – | – | 1 | – | 6 | |
| Epidemiology | – | – | 2 | – | 1 | – | 3 | ||
| Other | – | – | 5 | 2 | 6 | 1 | – | 14 | |
| Type of research | Participatory | – | 2 | 2 | – | – | 2 | – | 4 |
| Pre-clinical | – | 1 | 1 | – | – | – | – | 2 | |
| Clinical | – | 3 | 5 | 6 | 7 | 1 | – | 14 | |
| Applied care research | – | – | – | 2 | 2 | – | – | 4 | |
| Organization | Academic hospital | – | 6 | 6 | 8 | 8 | 3 | – | 30 |
| Applied sciences | – | 1 | – | – | 1 | – | – | 2 | |
| Research institute | – | – | 2 | – | – | – | – | 2 | |
Characteristics of participants throughout the project. Numbers indicate the number of participants with certain characteristics.
| . | . | Phase 1 . | Phase 2 . | Phase 3 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aspect . | Category . | Interviews . | FGD 1 . | FGD 2 . | FGD 3 . | Test Session 1 . | Test Session 2 . | Patient check . | Total . |
| Number of participants | 6 | 7 | 8 | 8 | 9 | 3 | 4 | 45 | |
| Sex | Male | 2 | 3 | 1 | 4 | 1 | 1 | – | 12 |
| Female | 4 | 4 | 7 | 4 | 8 | 2 | – | 29 | |
| Role/research experience | Junior researcher | – | 4 | 3 | 1 | 4 | 2 | – | 14 |
| Senior researcher | 2 | 2 | 4 | 5 | 1 | 1 | – | 15 | |
| Other role in research (coordinator, nursing specialist, team manager) | 1 | 1 | 1 | 2 | 4 | – | – | 9 | |
| Policymaker from patient organization | 2 | – | – | – | – | – | – | 2 | |
| Patient representative | 1 | – | – | – | – | – | 4 | 4 | |
| Background of researchers in FGDs and test sessions | |||||||||
| Area of expertise | Oncology | – | – | 1 | 6 | – | – | – | 7 |
| Psychology | – | 1 | – | 1 | – | 1 | – | 3 | |
| Pulmonology | – | – | – | – | 2 | – | 2 | ||
| Paediatrics | – | 6 | – | – | – | 1 | – | 6 | |
| Epidemiology | – | – | 2 | – | 1 | – | 3 | ||
| Other | – | – | 5 | 2 | 6 | 1 | – | 14 | |
| Type of research | Participatory | – | 2 | 2 | – | – | 2 | – | 4 |
| Pre-clinical | – | 1 | 1 | – | – | – | – | 2 | |
| Clinical | – | 3 | 5 | 6 | 7 | 1 | – | 14 | |
| Applied care research | – | – | – | 2 | 2 | – | – | 4 | |
| Organization | Academic hospital | – | 6 | 6 | 8 | 8 | 3 | – | 30 |
| Applied sciences | – | 1 | – | – | 1 | – | – | 2 | |
| Research institute | – | – | 2 | – | – | – | – | 2 | |
| . | . | Phase 1 . | Phase 2 . | Phase 3 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aspect . | Category . | Interviews . | FGD 1 . | FGD 2 . | FGD 3 . | Test Session 1 . | Test Session 2 . | Patient check . | Total . |
| Number of participants | 6 | 7 | 8 | 8 | 9 | 3 | 4 | 45 | |
| Sex | Male | 2 | 3 | 1 | 4 | 1 | 1 | – | 12 |
| Female | 4 | 4 | 7 | 4 | 8 | 2 | – | 29 | |
| Role/research experience | Junior researcher | – | 4 | 3 | 1 | 4 | 2 | – | 14 |
| Senior researcher | 2 | 2 | 4 | 5 | 1 | 1 | – | 15 | |
| Other role in research (coordinator, nursing specialist, team manager) | 1 | 1 | 1 | 2 | 4 | – | – | 9 | |
| Policymaker from patient organization | 2 | – | – | – | – | – | – | 2 | |
| Patient representative | 1 | – | – | – | – | – | 4 | 4 | |
| Background of researchers in FGDs and test sessions | |||||||||
| Area of expertise | Oncology | – | – | 1 | 6 | – | – | – | 7 |
| Psychology | – | 1 | – | 1 | – | 1 | – | 3 | |
| Pulmonology | – | – | – | – | 2 | – | 2 | ||
| Paediatrics | – | 6 | – | – | – | 1 | – | 6 | |
| Epidemiology | – | – | 2 | – | 1 | – | 3 | ||
| Other | – | – | 5 | 2 | 6 | 1 | – | 14 | |
| Type of research | Participatory | – | 2 | 2 | – | – | 2 | – | 4 |
| Pre-clinical | – | 1 | 1 | – | – | – | – | 2 | |
| Clinical | – | 3 | 5 | 6 | 7 | 1 | – | 14 | |
| Applied care research | – | – | – | 2 | 2 | – | – | 4 | |
| Organization | Academic hospital | – | 6 | 6 | 8 | 8 | 3 | – | 30 |
| Applied sciences | – | 1 | – | – | 1 | – | – | 2 | |
| Research institute | – | – | 2 | – | – | – | – | 2 | |
2.2 Phase 2: exploration of experiences and needs, and design of tool (December 2018–February 2019)
To gain further insights into the experiences health researchers have with patient involvement, as well as their needs we conducted three FGDs. Based on the gathered insights, we iteratively designed an outline for the online tool, through continuous consultation and reflection within the steering committee and in FGDs.
2.2.1 Participant recruitment
Participants for FGDs were recruited through the steering committee and the network of the research team. They were affiliated with three academic hospitals, a research institute, and a university for applied sciences in the Netherlands. We invited health researchers that had some experience with or interest in patient involvement. We believed that they would have more in-depth questions on patient involvement than those with no prior experience with or interest in patient involvement. Furthermore, we aimed for heterogeneity in terms of discipline, research experience, and type of research. FGD1 involved people from different specialities within paediatrics, FGD2 consisted of researchers from a broad mix of backgrounds, and FGD3 engaged researchers from one clinical department. Many participants were both a researcher and a clinician, and the first two FGDs included two researchers from the field of participatory action research (PAR).
2.2.2 FGDs and tool design
The three FGDs, comprising a total of 23 health researchers (Table 1), explored their experiences and needs with respect to patient involvement. The focus throughout the FGDs gradually shifted from the inventory of experiences and needs to the design and content of the online tool. Each FGD builds on the insights of the previous FGD, deepening and validating the experiences and needs. All FGDs were facilitated by either C.A.C.M.P. or A.J.M.L.vR., assisted by E.V. or S.H., who also made research notes. All FGDs were audio-recorded.
The overall set-up of the FGDs is shown in Table 2. At the start of each FGD, participants were asked about experiences with patient involvement (Table 2, Elements 1 and 2). The middle part of the three FGDs differed. During FGD1, participants reflected on, added to, and prioritized the list of challenges and questions that had been drafted at the end of Phase 1. The list that resulted after FGD1 was used as a starting point of FGD2, following the same focus group design. During FGD1, a member of the steering committee was present observing as a non-participant. Both FGDs ended with an exercise in which participants sketched the desired functionalities and structure of the tool.
Design of the three FGDs.
| Element . | . | FGD 1 . | FGD 2 . | FGD 3 . |
|---|---|---|---|---|
| 1 | Inventory of experiences with patient involvement (perceived added value of patient involvement, encountered challenges and subsequent questions) | x | x | x |
| 2 | Plenary discussion of Element 1 | x | x | x |
| 3 | Validation of previously collected needs and questions (participants were asked to reflect, comment, and add to the results of from previous interviews or FGDs. In FGD3 these had been incorporated into the tool’s outline) | x | x | x |
| 4 | Prioritization of needs and questions (participants prioritized the three most important needs and questions they wanted to be addressed in the online tool) | x | x | |
| 5 | Reflection on tool outline (participants reflected on how the content was structured in the outline of the tool) | x | ||
| 6 | Prioritization of tool content (participants indicated which topic they would click first) | x | ||
| 7 | Wishes for functionalities and structural design of the tool (participants brainstormed on the desired functionalities and structure of the tool) | x | x | x |
| Element . | . | FGD 1 . | FGD 2 . | FGD 3 . |
|---|---|---|---|---|
| 1 | Inventory of experiences with patient involvement (perceived added value of patient involvement, encountered challenges and subsequent questions) | x | x | x |
| 2 | Plenary discussion of Element 1 | x | x | x |
| 3 | Validation of previously collected needs and questions (participants were asked to reflect, comment, and add to the results of from previous interviews or FGDs. In FGD3 these had been incorporated into the tool’s outline) | x | x | x |
| 4 | Prioritization of needs and questions (participants prioritized the three most important needs and questions they wanted to be addressed in the online tool) | x | x | |
| 5 | Reflection on tool outline (participants reflected on how the content was structured in the outline of the tool) | x | ||
| 6 | Prioritization of tool content (participants indicated which topic they would click first) | x | ||
| 7 | Wishes for functionalities and structural design of the tool (participants brainstormed on the desired functionalities and structure of the tool) | x | x | x |
Design of the three FGDs.
| Element . | . | FGD 1 . | FGD 2 . | FGD 3 . |
|---|---|---|---|---|
| 1 | Inventory of experiences with patient involvement (perceived added value of patient involvement, encountered challenges and subsequent questions) | x | x | x |
| 2 | Plenary discussion of Element 1 | x | x | x |
| 3 | Validation of previously collected needs and questions (participants were asked to reflect, comment, and add to the results of from previous interviews or FGDs. In FGD3 these had been incorporated into the tool’s outline) | x | x | x |
| 4 | Prioritization of needs and questions (participants prioritized the three most important needs and questions they wanted to be addressed in the online tool) | x | x | |
| 5 | Reflection on tool outline (participants reflected on how the content was structured in the outline of the tool) | x | ||
| 6 | Prioritization of tool content (participants indicated which topic they would click first) | x | ||
| 7 | Wishes for functionalities and structural design of the tool (participants brainstormed on the desired functionalities and structure of the tool) | x | x | x |
| Element . | . | FGD 1 . | FGD 2 . | FGD 3 . |
|---|---|---|---|---|
| 1 | Inventory of experiences with patient involvement (perceived added value of patient involvement, encountered challenges and subsequent questions) | x | x | x |
| 2 | Plenary discussion of Element 1 | x | x | x |
| 3 | Validation of previously collected needs and questions (participants were asked to reflect, comment, and add to the results of from previous interviews or FGDs. In FGD3 these had been incorporated into the tool’s outline) | x | x | x |
| 4 | Prioritization of needs and questions (participants prioritized the three most important needs and questions they wanted to be addressed in the online tool) | x | x | |
| 5 | Reflection on tool outline (participants reflected on how the content was structured in the outline of the tool) | x | ||
| 6 | Prioritization of tool content (participants indicated which topic they would click first) | x | ||
| 7 | Wishes for functionalities and structural design of the tool (participants brainstormed on the desired functionalities and structure of the tool) | x | x | x |
After FGD2, the resulting overview of challenges and questions and summaries of both FGDs were discussed with the steering committee. Together with the research team, they critically reflected on the FGD results, and the support researchers need to employ meaningful involvement. The implications for the content and structure of the tool were discussed, and the steering committee did a brainstorming session, sketching design ideas for the online tool.
Subsequently, the research team designed an outline for the tool. This outline showed the main headings and structure of the homepage and four topic-specific pages and indicated what type of information would be discussed there. The outline was presented in FGD3 on five posters, on which participants could reflect, add topics, discuss, and annotate content (Table 2, Elements 3 and 5). They also prioritized topics and sketched the tool’s desired functionalities and structure (Table 2, Element 6).
2.3 Phase 3: reflection and improvement (March–September 2019)
Based on the insights gathered during Phase 2, the research team built and wrote a first prototype of the online tool in cooperation with a web designer. This, along with FGD3 results, was discussed with the steering committee. Additionally, it was presented and discussed in a session with the commissioners of this project.
2.3.1 Test sessions
The prototype was further developed and tested among 12 researchers in two test sessions (Table 1). Participants of Test Session 1 had not previously participated in the FGDs, while two out of three participants in Session 2 had participated in FGD2. All test session participants were interested in patient involvement, although some in Session 1 not yet had experience. Participants in both test sessions were presented with three scenarios and asked about the information they deemed necessary in this situation on the tool (Box 1). A facilitator from the research team observed how participants used the tool (i.e. what pages were instantly clicked, if they explored various pages) and discussed their questions and/or issues.
Finally, to ensure the tool did not contain inappropriate or insensitive language, we performed a patient check. Four patient representatives reflected on three draft texts for the tool. They provided valuable feedback on the language and identified words they felt were inappropriate. The tool was adapted accordingly.
You are considering submitting a proposal for funding with a commissioning health organization. Patients are involved in a review of the proposal. As a researcher, you have not previously worked together with patients and are wondering whether and how you can improve your chances of getting the grant.
Previous research of a colleague at your department did not go as expected. You consider trying something different. Your colleague suggests involving representatives from the relevant patient group this time. This immediately leaves you with many practical questions…
What other questions do you have about patient involvement?
2.4 Data analysis
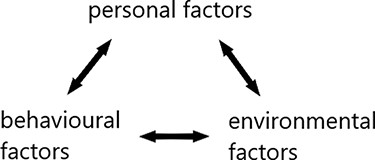
We aimed to do a critical analysis of the rich data collected during the project. Transcripts of all interviews and FGDs were analysed, using the SCT. The SCT explains learning and behaviour in a dynamic and reciprocal three-component model with personal, behavioural, and environmental factors (Fig. 1), also called ‘reciprocal determinism’ (Bandura 1986). Using reciprocal determinism as a framework for the analysis of experiences and needs allowed us to identify various personal, behavioural, and environmental factors that shaped their experiences and explicitly voiced needs. This gave us insight into the (non-explicitly voiced) support researchers need to be stimulated to employ meaningful patient involvement in their complex reality wherein they operate which helped us to develop the tool (see results).
Drawing on various scholars, we articulated a broad definition of each component to guide the analysis but left space for the contextuality and complexity of the subject matter.
Personal factors are specific to the individual, such as age, education, knowledge, skills, self-confidence, values, beliefs, and attitudes towards the behaviour (Schunk and DiBenedetto 2020; Selemani et al. 2018).
In environment, we include the social, economic, and legal standards and dynamics in which people operate, including for example the organization in which they work and others’ behaviours and beliefs, also called (role) models by Bandura (1986).
Behavioural factors include their practice and the experienced behavioural outcome (Selemani et al. 2018).
This means that factors like department culture (environmental), education on patient involvement (personal), and experiences with involving patients (behavioural) might shape if and how researchers involve patients and which challenges they experience when doing so.
Using the SCT, we performed a reflexive thematic analysis to allow us to use theory as a starting point and broad guideline, providing us with ‘rich and detailed, yet complex account’ of our data (Braun and Clarke 2006). We approached this as a two-step process (Maguire and Delahunt 2017). First, content analysis was performed, guided by the components of reciprocal determinism. For this, all interviews and FGD transcripts were read multiple times by the first author, to get familiar with their content. Then, possible factors in each of the three components (individual, behavioural, and environmental) were coded by the first author, using ATLAS.ti. These were continuously refined and discussed with authors C.A.C.M.P and J.E.W.B. Then a thematic analysis was performed, for which the first author explored and mapped relationships between different factors, using the ‘network’ function in ATLAS.ti to create a data display. From these relationships, themes were constructed.
2.5 Ethical considerations
Permission from a Dutch ethics committee was not needed for this research. We adhered to the national Code of Ethics for Research in the Social and Behavioural Sciences involving Human Participants (VCWE 2016). All participants gave permission to use their anonymized data, and FGD participants agreed with a summary report of their FGD. To stimulate a critical approach to the views of researchers, we included patient(s) (representatives) in both the steering committee and Phase 3 (testing of the online tool).
3. Results
This study aimed to develop an online tool that meets the needs of researchers to employ meaningful patient involvement. In this section, we first describe the results of the analysis of researchers’ experiences and needs, guided by the SCT. Second, we describe the development of the online tool, and how the tool addresses the identified factors that shape researchers’ experiences and needs in order to stimulate the principles of meaningful involvement.
3.1 Researchers’ experiences and needs
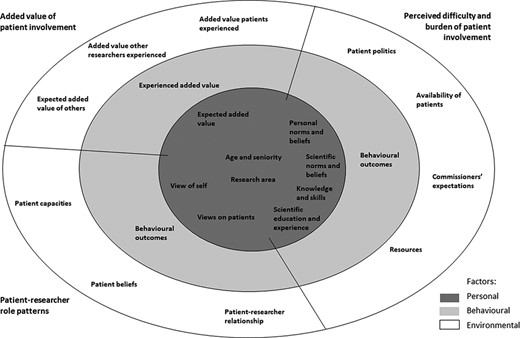
The factors that we found to shape researchers’ experiences and needs with respect to patient involvement are visualized as a nested model in Fig. 2. It is important to note that personal, behavioural, and environmental factors are reciprocal and therefore attribution of a factor to one of the three components can be arbitrary. We categorized the factors into three themes: (Schölvinck 2018) added value of patient involvement; (Caron-Flinterman et al. 2005) patient–researcher role patterns; and (Brett et al. 2014) perceived difficulty and burden of patient involvement.
Different personal, behavioural, and environmental factors that shape researchers’ experiences and needs with respect to patient involvement shown in a nested model.
3.1.1 Added value of patient involvement
Our results indicate that both the expected and experienced added value of patient involvement shape researchers’ experiences and needs. This includes not only their own expectations and experiences but also those of other researchers and patients. Interestingly, the added values that researchers expected tended to be instrumental (improves the research), while the added value that researchers had experienced were more value-driven (being fun, motivating, and broadening their own views).
3.1.1.1 Personal factors
Expected added value.
Almost all participants expressed at the beginning of the FGDs that they (had) expected added value from patient involvement. In all FGDs, various participants mentioned they (had) expected direct improvements to their research, such as improved research quality, inclusion rates, and support from patient organizations. Additionally, many participants expected the involvement of patients to broaden their view, and a number of senior researchers expected it to stimulate critical reflection on their research. This participant illustrates this:
‘I think it is very important that when you are working on a specific area, with a sort of tunnel vision, that you get stimulated in that way to substantiate the societal importance or the importance for that patient group. And therefore also for yourself. (…) I think that because you are forced to do that, you will also look at your own research question more critically’. [Participant A]
However, a few participants did not expect an added value. For example, junior researchers in FGD3 were quite vocal about involvement being ‘a check box’ that they have to complete in order to get a grant. In addition, many FGD participants expressed the wish for ‘quick fix’ instructions on how to involve patients. While this may be caused by multiple factors, (discussed in the third theme), this indicates that they do not yet see the true added value. Additionally, participants expressed doubts about the added value in relation to certain (vulnerable) groups, such as children or people with dementia, and specific types of research, particularly more fundamental/basic research.
Age, seniority, and research area.
Our data indicate that age and seniority in the research field may influence the added value researchers expect. For example, interviewees heard how some (junior) researchers were discouraged from employing patient involvement, because their superiors saw no added value. Also, a person’s background might influence their expectations, as the participants involved in PAR and paediatrics in FGD1 and FGD2 saw more potential positive effects of patient involvement than did the participants from oncology and surgery in FGD3.
3.1.1.2 Environmental factors
Added value other researchers experienced and expected.
Being exposed to the added value that peers have experienced or expect, may have large impact on participants’ own expectations, as during all FGDs participants described how they were inspired to involve patients by anecdotes from other researchers they talked to, heard speaking at a conference, or read about.
Added value patients experienced.
Many participants of both the FGDs mentioned they were interested in the added value patients experience from participating in research, as it motivated them to involve patients and resolved any hesitation about whether patients wanted to be involved.
‘It [the information document she once received] contained very simple things, such as that patients enjoy cooperating on research. That, I already found very helpful’. [Participant C]
3.1.1.3 Behavioural factors
Experienced added value.
The experienced added value appeared to influence the attitude towards patient involvement. Some had not yet experienced added value, either because they never involved patients or because doing so had not yet resulted in added value. While they were generally sympathetic towards the idea of patient involvement, they also wanted more ‘proof’ of the added value, for example in peer-reviewed literature.
However, many participants said that they had experienced positive effects from involving patients. For some, it had improved their research, such as increased inclusion rates, or generated more patient-relevant outcome measures. Particularly junior participants in the first two FGDs said that it was often fun and motivating to be in contact with those for whom you undertake research. Two participants in FGD2 had experience with patient co-interviewers, who had provided them with new insights into the disease and improved data collection by asking different questions. Several senior participants also expressed strategical advantages, such as patients stressing the relevance and importance of a topic with politics or commissioners and increased patient support for a research topic. Finally, the most frequently cited added value of patient involvement was that it exposed researchers’ ‘blind spots’ and differences in focus:
‘What I mostly noticed was that indeed, the ‘hard outcomes’ that we find really fantastic, like “do you have a chance of rejection on a cellular level, yes or no?”, that they really found these ways less interesting. They found more emotional outcomes [important]: “we have rejection, my body is letting me down, what are we going to do about this?”. That was interesting. For me patients became no longer numbers, but people. That is also a bit of a switch as a researcher’. [Participant C]
Interestingly, while many experienced added value as ‘being an eye opener’, FGD3 indicated that it does not automatically create an overall positive attitude towards involving patients in research. Two young participants in this FGD started by explaining how they had changed their entire research set-up after a poll among patients about their research design, indicating they took their input very seriously. However, they maintained a very positivistic view, criticizing patients’ rights and ability to be involved in research. It appeared they viewed patient involvement in an instrumental way—mainly as a means to improve research and only doing it when it made practical sense to them.
3.1.2 Patient–researcher role patterns
Some participants were very open to the idea of patients being involved in research, while others were more sceptical. During all FGDs, participants expressed doubts and raised questions about the division of power and roles when patients are involved in research. However, the tone of the discussions on this topic varied, as did the beliefs that seem to underpin their questions: from being sceptical or even frustrated about patients having power in research, to questioning on what patients can and cannot do, to self-reflective discussions on their own role as researcher. Here, we describe what personal factors underpin these ideas on the divisions of roles and power, how experiences can reinforce or challenge these ideas, and what environmental factors play a role.
3.1.2.1 Personal factors
Self-view and views on patient.
Two personal factors seem to be at the heart of the discussions on the roles and power patients (should) have: the way researchers view themselves and their view of patients.
Their self-view includes beliefs about authority, knowledgeability, and their ability to speak on behalf of patients. One participant gave an outspoken example of when these beliefs create a very negative attitude towards patient involvement. She recalled a very defensive reaction from international consortium partners when her team suggested patients looked at some of these partners’ work:
‘What were we thinking, running this by patients? Really, such outrage. That was a really heated gathering. And arguments, I did not really hear. I think it was mostly emotional. The idea that patients could possibly have something to say about the amazing scientific work they had done. That was impossible. Imagine they [patients] could find fault with it […]. They [researchers] were the ones who understood it’. [Participant D]
These researchers seemed to feel patients were trespassing on their territory when patients got a say in the research. During the FGDs, these beliefs were less explicitly voiced, but all FGD participants mentioned struggles with the balance of power. One researcher summarized:
‘I think it is important that patients give their input, because they are the ones that it’s about. But I also think that you can think a decision is better, from your clinicians’ perspective. […] I think that is something difficult to find a balance in’. [Participant E]
An interviewee [Participant F], who had worked with numerous researchers on patient involvement, recognized this attitude and attributed it to researchers’ feeling of authority and knowledgeability, stating researchers felt ‘I am the one who studied for this’, and that ‘they are sometimes afraid of losing control of their study’. On the other side of the spectrum, some participants, especially those involved in PAR, reflected on their authority over patients, ability to speak on their behalf, and their own knowledgeability compared to that of patients.
Some participants viewed patient involvement as also requiring that researchers adopt a new way of working and thinking. This was mostly expressed in FGD1, where some participants asked for information on how to evaluate patient involvement and their own role in it, and how to be more open to patient input. Much discussed was how the patients involved in research should be able to represent a broader group and go beyond their own experience—something participants felt not everyone could do. Many mentioned they thought patients needed a certain level of scientific knowledge. These results indicate that many participants particularly focus on how the patient can get more up to their level and fulfil their needs as researchers.
Research area, age, and seniority.
Several other personal factors might influence the beliefs described above. Our study seems to indicate that the field of health research makes a difference. The participants who were more open towards the influence of patients, and reflecting on their own role, were from paediatrics and/or PAR. A participant from FGD1 mentioned that their paediatrics background might give them a different view to begin with, although he did not explain what that meant to him exactly. It also appears that the more research directly affects patients (like in clinical research), the more participants appeared to see the added value and the more they seemed to value the input of patients.
3.1.2.2 Environmental factors
Capacities and beliefs of patients and patient–researcher relationship.
Some participants expressed patients struggling with differences in power and knowledge. A few participants had experienced that patients did not provide the critical feedback they had hoped for, which was attributed to a lack of knowledge or that they are being ‘too polite’. The personal relationship that sometimes exists between a researcher–clinician and patients and resulting co-dependency were mentioned as possible hurdles. A few participants noted that patients may also view the researcher as an ‘expert’ and do not see what they could add.
3.1.2.3 Behavioural factors
Behavioural outcomes.
Experiencing added value from patient involvement appeared to have opened up some participants making them reflect on their own knowledgeability and their capacity to speak on behalf of patients. Some highlighted how patients’ participation had opened their eyes to the fact that patients had a perspective that they themselves could not provide:
‘But what we have really noticed is that we assumed, they will find this or that important. And we should have just asked. So, you have to get a kind of openness that you do not naturally have, you really have to learn that’. [Participant D]
In this way, experience may create a snowball effect on attitudes. In our FGDs, those with more positive experiences of involving patients tended to be more self-reflective and open to patient input. They were also more likely to ask for information on how to evaluate their patient involvement or support in being more open to patient input. This may create more positive (learning) experiences and thus a more positive attitude towards patients getting a say in research.
On the other hand, some experiences exposed more traditional and more closed ideas on the division of power and value of patient knowledge. While most researchers expressed finding it important to involve patients in research, in practice many struggled to value and incorporate patients’ input in research. Several researchers said they sometimes got input they could not use, either because they deemed the patients’ input to be scientifically wrong or irrelevant. Some found this difficult because they wanted to take patients’ input seriously, and make them feel heard. A researcher who did a qualitative analysis with patient partners on a study on cerebral palsy illustrated this:
‘Then we did an analysis and scientifically viewed, the analysis was totally wrong. So, you could not use the analysis. But you still want to show them in some way: “your input is valuable, we are doing something with it”’. [Participant G]
3.1.3 Perceived difficulty and burden of patient involvement
Many researchers appeared to perceive patient involvement as difficult and sometimes a burden, mostly due to lack of clarity and scarcity of resources. Many lack the know-how for involvement and are not sure what commissioners expect of them or of the rules of involvement. In addition, patients are not always available, researchers cannot easily find information to help them design involvement, and the time and money to employ involvement are scarce owing to the many other demands on their research.
3.1.3.1 Personal factors
Knowledge and skills
The perception of patient involvement as complicated, time-consuming, and expensive seems partly caused by a lack of knowledge and skills, resulting in reluctance. Researchers mentioned many questions regarding patient involvement, of which the most important were the following:
What goals can I pursue with patient involvement?
What involvement methods can I use to achieve these goals?
At which phases or points in my research can I involve patients?
Who do I involve in my research and how do I find these patients?
What about the representativeness of the involved patients?
Will involving patients lead to bias in my research?
What can I ask from patients and patient organizations without overburdening them?
Do I reimburse patients and how much?
Is my research suitable for patient involvement?
Their reluctance to act is further strengthened by the common idea that there is a correct way to involve patients, as they are used to science being bound to strict regulations. In line with this, many researchers asked for clear guidelines and rules on how to set up patient involvement. A researcher noted:
‘Patient involvement is actually in its infancy and the rules are still developing. So, it is important that people can also clearly read that it is not set in concrete. Like all other parts of your research proposal are’. [Participant H]
3.1.3.2 Environmental factors
Resources.
Researchers had difficulty finding accessible and usable information. For ‘beginners’, existing information is often too lengthy and too vague, while researchers more committed to patient involvement were looking for more in-depth information, and examples and experiences from other researchers, which they could not easily find. During FGD1 and several interviews, it was mentioned that currently there is a lack of reporting on patient involvement experiences and outcomes. This was seen as leading to the lack of clear information, and some hoped that more reporting about patient involvement in (non)academic sources would increase knowledge and the availability of examples.
Commissioners’ expectations
Many researchers recognized that as patient involvement was increasingly a prerequisite from commissioners, this would also increase its practice. However, they voiced that when it is obligatory, there need to be clear criteria, rules, and standards by which it can be assessed. FGD1 and FGD3 discussed the difficulty of lacking clear criteria, and in FGD3, some expressed irritation with commissioners’ lack of clarity in this regard. When were their efforts sufficient? How did the commissioner evaluate them? A few participants had just lost a major application, which they assumed was due to a negative verdict by patient reviewers. These experiences had created frustration that appeared to have influenced their perceptions of patient involvement:
‘…and we also know that patient participation should be added, and rightfully so. But what are the rules? Can patients, out of this whole application, of which they actually maybe do not know the ins and outs, can they have their say in that?’ [Participant I]
In FGD2 and FGD3, researchers suggested that commissioners need to re-think their ideas on patient involvement, and clarify their standards and procedures. An interviewee [Participant J] compared research to a Christmas tree that has to be adorned with ever more baubles on a limited budget and timeframe.
Availability of patients
The (limited) capacity of patients and patient organizations was often mentioned to pose constraints. Interviewees from patient organizations, as well as researchers in interviews and FGDs, acknowledged that it is difficult to bring enough motivated patients together at the same place and time. They see that there is a lack of good ‘patient infrastructure’; currently only a small group of motivated, highly educated patients is involved. Both researchers and patient organizations worried that this limited group of patients will be too much in demand. It was mentioned that reimbursing patients to compensate for their time and efforts would address this concern, but it was also pointed out that this could result in higher expectations of the efforts and quality of these patients’ input.
Patient politics
Several senior researchers worried about the potential impact of ‘patient politics’ emerging as patients gain influence in the research arena, influencing funding outcomes. One researcher saw a potential conflict of interest between patients versus potential patients:
‘We see this a lot in cancer. If you have cancer patient organizations, they all have cancer. And often they are afraid it will return. And this group of patients, if somewhere there is an amount of money to be divided, then they are biased to purpose that to new treatment options. Often they will not put a cent towards prevention – now I’m exaggerating a little’. [Participant K]
Other possible perceived issues included certain patient groups being disproportionately powerful, or exclusion of researchers who could not find patients to support them.
3.1.3.3 Behavioural factors
Behavioural outcomes.
Our findings suggest that the experience of patient involvement is an important factor in reducing the perceived difficulty by increasing knowledge and skills and establishing a network to find patient participants. For example, some researchers said they had learnt that they should have included patients in their research at an earlier stage and would do that next time—a learning process several interviewees recognized. For others, experiences had created a better network to find future patients.
3.2 Designing an online tool
We aimed to develop an online tool that supports researchers to employ meaningful patient involvement by gaining insights into their experiences and needs with respect to employing meaningful patient involvement. To translate the identified experiences and needs into a useful online tool, the data gathered in the FGDs were discussed in subsequent FGDs and with the steering committee throughout the research project. What did the results tell us about what researchers need to employ meaningful patient involvement? How should the online tool address their needs, and what should its content, functionalities, and structure be?
In this section, we first describe how the insights from the FGDs relate to the principles of meaningful involvement. Then, we describe three design dilemmas that emerged in discussion with the steering committee and with participants of FGDs. Finally, we will discuss tools’ design and explain how it aims to navigate the dilemmas, and the factors identified with the SCT in order to stimulate meaningful involvement.
3.3 Reflection on the meaningfulness of involvement
When reflecting with the steering committee on researchers’ experiences and needs, in relation to the three principles of meaningful involvement (inclusion and diversity, mutual learning, and responsiveness) we observed the following.
Inclusion and diversity require a welcoming attitude and environment towards patients and appreciation of the value of patient knowledge. Many researchers wanted to hear the patients’ views on participation and often found it important they feel welcomed and heard. However, they did not always truly appreciate the value of patient knowledge, focusing on proof of its usefulness and the (scientific) appropriateness of the input. We also found researchers might not see the value of including certain (vulnerable) groups.
The second principle is mutual learning between researchers and patients as a result of their interaction. Our results show that the involvement of patients can lead to experiencing their unique insights and knowledge. However, this does not necessarily lead to reflexivity on the division of power and knowledge, as researchers were focused on the training of knowledge and skills that patients need to develop before they can be involved in research. In addition, instrumental questions prevailed, together with a demand for clear guidelines and rules, with limited interest in how to learn from the experience itself. The steering committee stressed that a mindset change and increased reflexivity are essential to improve mutual learning.
Finally, researchers’ responsiveness was found to be limited, as researchers were often unsure how to act upon patient knowledge and found it hard to pinpoint how they had used it. Both their experiences with and questions on patient involvement show a profound fear that the patient knowledge will collide with scientific norms.
3.4 Emerging design dilemmas
We identified three design dilemma’s for the translation of the researchers’ needs and experiences into a useful online tool.
The first dilemma concerns the extent to which a tool can indicate (uniform) norms and standards. We wanted to provide information specific enough to be practical and answer to the expressed need for guidelines on ‘good’ involvement. Meanwhile, the information also had to be applicable to many different research types and contexts and align with different commissioners’ expectations of involvement.
The second dilemma concerns addressing researchers’ need both for accessible, practical guidance and also teach them about the contextual nature of patient involvement, and stimulate reflection. We could end up with a highly user-friendly, but checklist-like product that would encourage instrumentalism and easily lead to tokenism, or a tool that focuses on creating awareness and reflexivity but that is too lengthy and vague to be useful. We wanted to appeal to researchers’ needs and language, but also stimulate reflection and learning.
The third dilemma concerns the tension between norms and values of science and those of patient involvement. Researchers have to adhere to scientific rules and standards that teachers, colleagues, and policymakers demand. Health research traditionally entails quantitative research that strongly values concepts like replicability and objectivity, while patient involvement is often qualitative, context-dependent, and sometimes more subjective in nature. It can be challenging to merge scientific knowledge with patients’ sometimes anecdotal experiential knowledge. This can give rise to tension as researchers are increasingly expected to create impact with patient involvement while still being held to scientific standards in decision-making, execution, and analysis.
3.5 The online tool
The final tool was written in Dutch and is freely available online.2 The tool is elaborate and aims to cater to various health researchers with different backgrounds, experience levels, and needs. It is not specifically intended (or likely) that users read the entire tool (at once), but that they navigate easily to parts of the tool where their questions and needs regarding patient involvement lie.
Here we explain how we designed the tool, in order to stimulate the three principles of meaningfulness, by addressing the factors shaping their experiences and needs and navigating the dilemmas. Table 3 provides an overview of the tool’s content, and which factors each chapter addresses.
Content of the main page of the online tool (translated from Dutch).
| Main chapters . | Content . | The following factors that shape experiences and needs are addressed in all chapters . | The following factors that shape experiences and needs are addressed in the indicated chapter . |
|---|---|---|---|
| 1. Involving patients, also when less self-evident | Demonstrates how participation is possible in situations where researchers often said that patients’ involvement was ‘unlikely’ or ‘not useful’ and provides examples | Knowledge Resources Behavioural outcomes Added value other researchers experienced | Expected added value Behavioural outcomes |
| 2. Designing patient involvement | Provides a step-by-step guide to design patient involvement, with practical information on implementation and how to get started | Added value patient experienced | |
| 3. Finding the right experience expert | Provides multiple steps and suggestions on finding ‘experience experts’ for the intended roles and creating sustainable infrastructure for patient involvement | Availability of patients | |
| 4. Addressing commissioners’ requirements | Provides general information on how to gain more insights into commissioners’ requirements and procedures of (patient) reviews | Commissioners’ expectations | |
| 5. Deepening patient involvement | A guide into helpful directions for increasing, evaluating, and improving involvement for those with more experience | View of self | |
| Shortcuts to general information | |||
| Definitions | |||
| Pitfalls | Behavioural outcomes | ||
| Why patient involvement? | Expected added value | ||
| Methods and instruments | |||
| Main chapters . | Content . | The following factors that shape experiences and needs are addressed in all chapters . | The following factors that shape experiences and needs are addressed in the indicated chapter . |
|---|---|---|---|
| 1. Involving patients, also when less self-evident | Demonstrates how participation is possible in situations where researchers often said that patients’ involvement was ‘unlikely’ or ‘not useful’ and provides examples | Knowledge Resources Behavioural outcomes Added value other researchers experienced | Expected added value Behavioural outcomes |
| 2. Designing patient involvement | Provides a step-by-step guide to design patient involvement, with practical information on implementation and how to get started | Added value patient experienced | |
| 3. Finding the right experience expert | Provides multiple steps and suggestions on finding ‘experience experts’ for the intended roles and creating sustainable infrastructure for patient involvement | Availability of patients | |
| 4. Addressing commissioners’ requirements | Provides general information on how to gain more insights into commissioners’ requirements and procedures of (patient) reviews | Commissioners’ expectations | |
| 5. Deepening patient involvement | A guide into helpful directions for increasing, evaluating, and improving involvement for those with more experience | View of self | |
| Shortcuts to general information | |||
| Definitions | |||
| Pitfalls | Behavioural outcomes | ||
| Why patient involvement? | Expected added value | ||
| Methods and instruments | |||
Content of the main page of the online tool (translated from Dutch).
| Main chapters . | Content . | The following factors that shape experiences and needs are addressed in all chapters . | The following factors that shape experiences and needs are addressed in the indicated chapter . |
|---|---|---|---|
| 1. Involving patients, also when less self-evident | Demonstrates how participation is possible in situations where researchers often said that patients’ involvement was ‘unlikely’ or ‘not useful’ and provides examples | Knowledge Resources Behavioural outcomes Added value other researchers experienced | Expected added value Behavioural outcomes |
| 2. Designing patient involvement | Provides a step-by-step guide to design patient involvement, with practical information on implementation and how to get started | Added value patient experienced | |
| 3. Finding the right experience expert | Provides multiple steps and suggestions on finding ‘experience experts’ for the intended roles and creating sustainable infrastructure for patient involvement | Availability of patients | |
| 4. Addressing commissioners’ requirements | Provides general information on how to gain more insights into commissioners’ requirements and procedures of (patient) reviews | Commissioners’ expectations | |
| 5. Deepening patient involvement | A guide into helpful directions for increasing, evaluating, and improving involvement for those with more experience | View of self | |
| Shortcuts to general information | |||
| Definitions | |||
| Pitfalls | Behavioural outcomes | ||
| Why patient involvement? | Expected added value | ||
| Methods and instruments | |||
| Main chapters . | Content . | The following factors that shape experiences and needs are addressed in all chapters . | The following factors that shape experiences and needs are addressed in the indicated chapter . |
|---|---|---|---|
| 1. Involving patients, also when less self-evident | Demonstrates how participation is possible in situations where researchers often said that patients’ involvement was ‘unlikely’ or ‘not useful’ and provides examples | Knowledge Resources Behavioural outcomes Added value other researchers experienced | Expected added value Behavioural outcomes |
| 2. Designing patient involvement | Provides a step-by-step guide to design patient involvement, with practical information on implementation and how to get started | Added value patient experienced | |
| 3. Finding the right experience expert | Provides multiple steps and suggestions on finding ‘experience experts’ for the intended roles and creating sustainable infrastructure for patient involvement | Availability of patients | |
| 4. Addressing commissioners’ requirements | Provides general information on how to gain more insights into commissioners’ requirements and procedures of (patient) reviews | Commissioners’ expectations | |
| 5. Deepening patient involvement | A guide into helpful directions for increasing, evaluating, and improving involvement for those with more experience | View of self | |
| Shortcuts to general information | |||
| Definitions | |||
| Pitfalls | Behavioural outcomes | ||
| Why patient involvement? | Expected added value | ||
| Methods and instruments | |||
We aimed to deal with the first dilemma, regarding (uniform) norms and standards, by urging researchers to gain insights into commissioners’ specific requirements (chapter 4). In addition, we used many examples from different research fields aiming to appeal to their research type and background. The design choices that we made to address the second dilemma, and stimulate the principles of meaningful involvement by addressing the factors that shape experiences and needs, can be divided into (1) transformation of instrumental ‘quick and dirty’ questions into reflection and learning, (2) stimulating action, and (3) exemplifying and referencing. They are elaborated on below. The third dilemma could not be addressed in an online tool.
3.5.1 Transforming instrumental ‘quick and dirty’ questions into reflection and learning
Some FGD participants expected instrumental added values of involvement and looked at patient knowledge with conventional scientific norms, education, and experiences in mind: asking questions on what is right or wrong involvement, useful or useless input, and a suitable patient to involve. The steering committee agreed that we needed to appeal to the language and needs of the user while being careful not to legitimize or reinforce scepticism and doubts (as described in the second dilemma). To do so, we used the main page to appeal to the large variety of experiences, questions, and attitudes that researchers have while encouraging meaningful involvement. We therefore structured the main page into five main sections on researchers’ most common needs, as expressed during the FGDs. We used more instrumental language for the questions and other headings, because we wanted researchers to recognize their own questions. The answers and explanations, however, provided nuance. In addition, researchers expressed a wish for a tool that is ‘to the point’. We decided to include various step-by-step guides that included open questions they had to ask themselves, with suggested answers. For example, ‘what is your goal?’, followed by several possible goals, with the aim to stimulate more reflection and learning. Drop-down menus and different links provide follow-up and background information. To make researchers reflect on inclusion and diversity, we added questions on who they wanted to involve and provided information on how to reach and involve groups that are seen as more ‘difficult’.
3.5.2 Stimulating action
Our findings indicate that experiencing (the added value of) patient involvement is an important factor in creating positive attitudes and building knowledge and skills to further improve meaningful involvement. We aimed to ‘kick-start’ users and stimulate quick action: to be open and start participation in conversation with patients at an early stage. We hoped this would stimulate mutual learning and responsiveness. Many participants in FGDs expressed the lack of clarity on rules and standards for patient involvement, but both the steering committee and the commissioners recognized that patient organizations and commissioners are also unsure of the exact rules of involvement. They felt the online tool could not take the place of policymakers, in setting strict norms and rules, so the tool emphasizes that rules are not set in stone. We avoided presenting a utopian vision of involvement, with clear regulations to follow, but encouraged getting started and learning-by-doing.
3.8.3 Exemplifying and referencing
Experiences of other researchers and patients were included so that researchers could see what added value other researchers had experienced, and what they had learnt along the way, thereby improving attitude, knowledge, and skills. Examples also provided more concrete information on ways in which involvement can be done. Some were described and written as ‘pitfalls’ and given a prominent place. To add to the tool’s legitimacy, we included academic references on effectiveness and examples of case studies involving patients.
4. Discussion
This research aimed to gain insights into researchers’ experiences and needs with respect to patient involvement. These insights were used to develop an online tool that stimulates the meaningful involvement of patients.
Experiencing the added value of patient involvement and learning-by-doing appear to be important kick-starters to more meaningful involvement. Experiencing the added value is associated with increased motivation for patient involvement, but also builds the necessary competences and networks. Currently, many researchers view involvement as difficult while also struggling with unclear rules and expectations of commissioners. We found that researchers particularly have difficulty determining what roles patients can have, what patient profile fits with this, and how to integrate patient input into research.
Our findings show that the scientific background, education, and resulting scientific norms and values play a role in how researchers view patient involvement. Even though some want to value the input of patients, they struggle with incorporating patients’ knowledge into the decision-making process. Schölvinck et al. (2018) also observed that the scientific research paradigm prevented ‘integration of the subjective experiential knowledge brought in by patients’ (p. 260). While decisions are rarely made based on evidence alone, and ‘human’ factors (e.g. power) and context play an important role (Oliver et al. 2012), researchers hardly acknowledged this. While some scholars argue that actors increasingly understand that the evidence-based medicine paradigm also implies including other evidence types such as patient knowledge (Oliver and Pearce 2017), our findings indicate that in the research decision-making process, scientific evidence is still viewed as superior, frustrating the integration of patient knowledge.
Given their focus on evidence-based practices, it is no surprise that researchers wished for clearer rules and standards of patient involvement (Staley and Barron 2019). This focus might not be limited to researchers, as Schölvinck et al. describe a similar focus on rules by policymakers (Schölvinck et al. 2018). However, as we argued in the second design dilemma, standardizing methods may not be very useful in improving the process and outcomes of a contextual practice such as patient involvement. Previously, McCoy et al. (2018) pointed out the potential of standards for involvement missing the essence of its underlying goals. In an evaluation of standards for biomedical research, they found that these ‘fail to address fundamental questions about when, why and with whom public involvement should be undertaken in the first place’ (p. 803), eventually leading researchers to use it inappropriately. They conclude that standards therefore should take account of the underlying goals and rationales for involvement. Staley and Barron (2019) suggest a different way of thinking about involvement to address this issue, conceptualizing it as ‘conversations that support two-way learning’ (p. 3). We applied a similar approach to the online tool, hoping that the stimulation of early open conversations between researchers and patients will stimulate more meaningful patient involvement. This is a different way of thinking than what researchers are used to, usually having to pre-determine research designs according to strict criteria, and this will not be easy to address using an online support tool only.
4.1 So what else is needed to stimulate meaningful patient involvement?
To successfully foster more meaningful patient involvement, a broader change in the research system will be needed. Realizing such a change is notoriously complex due to the resilience of social systems and involves not only the practice of doing research but also the culture (thinking, such as scientific paradigms) and structure (rules and regulations, incentives, and infrastructures for reaching experiential knowledge experts) of the system (De Haan 2010; Rotmans et al. 2001). Our tool provides one element in a system change by increasing researcher competences (knowledge, attitude, and skills) to apply patient involvement in the practice of their research.
However, many factors cannot be fully addressed by an online tool, or even lie beyond researchers’ control. To address these, different characteristics of the system should be considered (Van Mierlo et al. 2010). Knowledge infrastructure should be improved, increasing the exchange of knowledge and experiences with patient involvement between peers. This could be done through formal and informal actions, ranging from accessible, local social networking events intended for knowledge and experience sharing, to specialized courses organized by funders. Additionally, reporting on patient involvement efforts and experiences should be increased in scientific literature, but perhaps more importantly, also less formal formats (e.g. organizations’ websites, professional journals, or conference speeches). Legislation and regulations should consider the context of patient involvement and leave room for experimentation, learning-by-doing, and flexibility in changing the course of the research in response to patient input. Funders could provide more learning and experimentation space, either by specific calls for experimental, innovative patient involvement initiatives, but also by stimulating applicants to not rigidly pre-plan their research, but actively build-in patient involvement, leaving room for adjustments. Values and norms of science and the place of patient knowledge within these norms should be reconsidered through dialogue among all stakeholders. Interactions between stakeholders, such as patient organizations, funders, and researchers should be embedded in a stimulating and friendly research environment that is welcoming to all. Finally, an improved (physical) infrastructure to match researchers and patients could be beneficial.
4.2 Strengths and limitations
One of the strengths of this research is the use of behavioural determinism as a broad programme theory, allowing us to look beyond researchers’ initially voiced needs and gain a deeper understanding of the many factors that shape their experiences and needs with respect to meaningful patient involvement. The continuous discussion and evaluation of results in both the FGDs and the steering committee further deepened and validated our findings. The experiences of all our participants with patient involvement provided concrete issues and exposed deeper problems than those without such experience might have done. A potential drawback of this approach was that it could draw a distorted picture of the average researcher’s experiences and needs, as our research population might have a stronger intrinsic motivation to involve patients. However, attitudes expressed by researchers varied throughout different FGDs. Most importantly, in FGD3, researchers were overall more sceptical of involvement and patients’ capacities, and more focused on the instrumental added values patient involvement could provide than in the other two FGDs. Given the generally limited experience with patient involvement of Dutch researchers, we hypothesize that most of the online tool’s target population will have limited knowledge of patient involvement, and possibly less positive and open attitudes than most of our FGD participants. We adjusted the tool to this likelihood through wording and titles that would appeal to these researchers’ questions while aiming to create a positive attitude towards patient involvement.
Although we hypothesize that many Dutch researchers have limited experience, the country is one of the patient involvement frontrunners. Our results are likely generalizable to other Western ‘frontrunners’, such as Canada, Denmark, and the UK, where a similar basic understanding of patient involvement is present in most researchers. However, as funders may have different prerequisites, and different regulations may exist, those looking to develop innovations to stimulate meaningful involvement in these countries will likely have to make adaptations. The description of our approach could inform such future efforts.
5. Conclusion
This study found that when employing patient involvement, researchers often struggle with patient–researcher role patterns. While they often find it difficult to involve patients, hearing or experiencing the added value of patient involvement is an important motivational factor. The online support tool, developed with behavioural determinism to understand the multiple factors that shape researchers’ needs and experiences with respect to patient involvement, can be helpful for researchers looking to start, improve, or innovate their research together with patients. As meaningful patient involvement is difficult to achieve, we however argue the need for a wider system change to further stimulate meaningful patient participation in research.
Funding
This research was supported by the following organizations: ZonMw, KWF Kankerbestrijding, Alzheimer Nederland, De Hartstichting, and Harteraad.
Conflict of interest statement.
None declared.
Acknowledgements
We would like to thank the members of the steering committee for their critical reflections throughout the research process and the development of the online tool. We would also like to thank the participants of the focus groups and test sessions for sharing their experiences with us.
Endnotes
The academic researchers are specialized in transdisciplinary research. The advisors have expertise in training and consultancy on patient involvement in health research, healthcare, and health policy for both patient representatives and stakeholders (including researchers) who collaborate with them.