-
PDF
- Split View
-
Views
-
Cite
Cite
V. C. Moser, P. M. Phillips, K. L. McDaniel, R. S. Marshall, D. L. Hunter, S. Padilla, Neurobehavioral Effects of Chronic Dietary and Repeated High-Level Spike Exposure to Chlorpyrifos in Rats, Toxicological Sciences, Volume 86, Issue 2, August 2005, Pages 375–386, https://doi.org/10.1093/toxsci/kfi199
Close - Share Icon Share
Abstract
This study aimed to model long-term subtoxic human exposure to an organophosphorus pesticide, chlorpyrifos, and to examine the influence of that exposure on the response to intermittent high-dose acute challenges. Adult Long-Evans male rats were maintained at 350 g body weight by limited access to a chlorpyrifos-containing diet to produce an intake of 0, 1, or 5 mg/kg/day chlorpyrifos. During the year-long exposure, half of the rats in each dose group received bi-monthly challenges (spikes) of chlorpyrifos, and the other half received vehicle. Rats were periodically tested using a neurological battery of evaluations and motor activity to evaluate the magnitude of the acute response (spike days) as well as recovery and ongoing chronic effects (non-spike days). Effects of the spikes differed as a function of dietary level for several endpoints (e.g., tremor, lacrimation), and in general, the high-dose feed groups showed greater effects of the spike doses. Animals receiving the spikes also showed some neurobehavioral differences among treatment groups (e.g., hypothermia, sensory and neuromotor differences) in the intervening months. During the eleventh month, rats were tested in a Morris water maze. There were some cognitive deficits observed, demonstrated by slightly longer latency during spatial training, and decreased preference for the correct quadrant on probe trials. A consistent finding in the water maze was one of altered swim patterning, or search strategy. The high-dose feed groups showed more tendency to swim in the outer annulus or to swim very close to the walls of the tank (thigmotaxic behavior). Overall, dietary exposure to chlorpyrifos produced long-lasting neurobehavioral changes and also altered the response to acute challenges.
Inhibition of acetylcholinesterase (AChE) has been associated with clinical signs of acute toxicity of organophosphorus (OP) and carbamate pesticides (Ecobichon, 1991; Marrs, 1993). While there are numerous reports in the literature of behavioral, neurological, and physiological effects due to acute OP exposure with relatively high AChE inhibition, considerably less research has been directed towards determining the precise relationship between various levels of lower ChE inhibition and alterations in behavior following repeated exposures. AChE-inhibiting pesticides have been among the most widely used pesticides in the environment, leading to high potential for human long-term exposures. Indeed, monitoring studies (Hill et al., 1995; Saieva et al., 2004) revealed that as much as 82% of the population had measurable levels of urinary 3,5,6-trichloro-2-pyridinol (TCP), a primary metabolite of chlorpyrifos, indicating recent exposure. Despite decades of epidemiological and clinical research on these pesticides, there is still much concern and controversy over the adverse health outcomes of long-term subclinical human exposure. Recent reviews of the human exposure literature conclude that most studies report neurological symptoms along with changes in cognitive and psychomotor function (Albers et al., 2004; Colosio et al., 2003; Jamal et al., 2002; Kamel and Hoppin, 2004). The clinical syndrome, however, is vague and not represented well using standard quantitative examinations.
Despite the wealth of literature on acute effects of OP pesticides in laboratory animals, there are relatively few reports of long-term exposure, and even fewer involving exposure greater than three to four months. Few neurobehavioral effects have been reported in studies of long-term exposures. Decreased motor activity was seen transiently with subchronic dietary exposure to chlorpyrifos and tebupirimphos (Mattsson et al., 1996; Sheets et al., 1997), whereas four-month exposure to dietary triphenyl phosphate produced no measurable behavioral effects (Sobotka et al., 1986). A subchronic study of dietary exposure to parathion produced no adverse behavioral changes, only a transient improvement in learning at the low dose (Ivens et al., 1998). Shorter-term exposures, however, appear to produce various neuromotor and cognitive effects. For example, six-week exposure to methyl parathion by oral gavage lowered activity and arousal (Schulz et al., 1990), and 38-day exposure to subcutaneous chlorpyrifos decreased hindlimb grip strength at the end of exposure (Terry et al., 2003). Fourteen-day subcutaneous exposure to chlorpyrifos (Terry et al., 2003) or to diisopropylfluorophosphate (DFP; Prendergast et al., 1997) altered acquisition in the Morris water maze; these effects were seen after AChE had recovered in the one study that measured it (Prendergast et al., 1997). A 30-day study of intraperitoneally administered disulfoton produced overt toxicity early on, followed by tolerance to some, but not all, of the behavioral effects (Llorens et al., 1993). Overall, the comparability of these findings to human exposures is limited due to the dose, length, and route of exposures.
This study was initiated to examine the health effects of long-term, low-level exposure to organophosphate (OP) pesticides, as well as the influence of that exposure on intermittent high-dose challenges. Chlorpyrifos (CPF) was chosen as a model OP which is still used extensively world-wide, despite recent limitations of its use in the U.S. Many poisoning episodes, e.g., during crop spraying, occur in farm workers/applicators, who probably also have ongoing low-level exposure. These ongoing exposures may make the individual more sensitive to acute poisoning episodes.
There were two basic questions addressed in this study: (1) Does long-term, low-level OP exposure produce neurotoxicity? And, (2) does ongoing OP exposure influence the subsequent response to higher-level acute exposure? One hypothesis of this study was that the low levels used in this study would not produce acute effects, but would produce tolerance in the form of muscarinic receptor down-regulation. If that occurred, treated rats would show fewer neurobehavioral effects following an acute, high dose exposure. An alternate hypothesis was that the already-compromised rats would show a greater response to acute exposure. A large, multi-investigator project was undertaken to address these hypotheses using endpoints reported to be sensitive to acute or repeated chlorpyrifos exposures. Other papers report biochemical endpoints (Padilla et al., submitted) and measures of operant responding (Samsam, T.E., Bushnell, P.J., Marshall, R.S., and Hunter, D.L., unpublished data, 2005). The present article presents data collected using a neurobehavioral screening battery and a Morris water maze test of cognitive function during the year-long exposure.
MATERIALS AND METHODS
Animals.
Adult Long-Evans hooded male rats were obtained from Charles River Laboratories (Raleigh, NC). Rats were maintained at 350 g body weight by restricting food access to about 15 g/day. They were housed on heat-treated pine shavings. The animal facility was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and was maintained at 70 ± 2°F, 50 ± 10% humidity, with a 12-h light:dark cycle.
Chemicals.
CPF feed was provided in 5-g wafers (Bio-Serv, Frenchtown, NJ), and was color-coded (using vegetable food dyes) to prevent feeding errors. The wafer concentrations were approximately 117 and 23 mg/kg, which would deliver daily doses of 5 and 1 mg/kg, respectively, when 15 g/day of feed was given to rats weighing 350 g. Shipments were monitored for CPF levels, and were rejected if the nominal values were outside 10% of target (see Padilla et al. for details of analysis).
For CPF challenges, CPF (Chem-Serve, West Chester, PA) was dissolved in corn oil and administered via po gavage at 1 ml/kg.
Experimental design.
There were six treatment groups: three levels of dietary exposure (control, low, high) and for each diet group, half of the rats received bimonthly CPF challenges (spikes) by po gavage and the other half received vehicle (corn oil). This was a large multi-investigator project, and the entire study was run in two cohorts. Twenty rats/treatment group were designated for these behavioral evaluations, divided into two equal cohorts (n = 10/treatment/cohort; total, 120 rats). Feed exposure lasted one year.
The first CPF spike was 60 mg/kg; however, several rats died at this dose (this occurred in rats allocated to other investigators, and did not affect the sample size in these behavioral studies), and therefore the subsequent spikes were lowered to 45 mg/kg. The unanticipated lethality occurred in the first cohort, but the second cohort was dosed the same way to keep them comparable; in other words, the second cohort also received 60 mg/kg for the first challenge, and 45 mg/kg for subsequent doses.
Neurobehavioral testing.
Rats were tested periodically throughout the year-long exposure period. Testing for neurobehavioral changes, using a functional observational battery (FOB) and an evaluation of motor activity, took place before exposure began and approximately monthly thereafter, except in month 11. On spike days, rats were tested at 3.5 h (time of peak effect; see Moser and Padilla, 1998) after the dose. During the 11th month of exposure, cognitive testing, using a Morris water maze, started two weeks after the 5th spike and continued daily for a month. Table 1 presents the test times by week.
Testing by Week during the Year-Long CPF Feed Exposure
Week . | Test . |
|---|---|
| 0 | FOB, MA before feed exposure began |
| 4 | FOB, MA |
| 8 | FOB, MA 3½ h after 1st spike—60 mg/kg |
| 13 | FOB, MA |
| 17 | FOB, MA 3½ h after 2nd spike—45 mg/kg |
| 21 | FOB, MA |
| 27 | FOB, MA 3½ h after 3rd spike—45 mg/kg |
| 31 | FOB, MA |
| 36 | FOB, MA 3½ h after 4th spike—45 mg/kg |
| 40 | FOB, MA |
| 44 | FOB, MA 3½ h after 5th spike—45 mg/kg |
| 46–50 | MWM—cohort 1 |
| 46–48 | MWM—cohort 2 |
| 52 | FOB, MA 3½ h after 6th spike—45 mg/kg |
Week . | Test . |
|---|---|
| 0 | FOB, MA before feed exposure began |
| 4 | FOB, MA |
| 8 | FOB, MA 3½ h after 1st spike—60 mg/kg |
| 13 | FOB, MA |
| 17 | FOB, MA 3½ h after 2nd spike—45 mg/kg |
| 21 | FOB, MA |
| 27 | FOB, MA 3½ h after 3rd spike—45 mg/kg |
| 31 | FOB, MA |
| 36 | FOB, MA 3½ h after 4th spike—45 mg/kg |
| 40 | FOB, MA |
| 44 | FOB, MA 3½ h after 5th spike—45 mg/kg |
| 46–50 | MWM—cohort 1 |
| 46–48 | MWM—cohort 2 |
| 52 | FOB, MA 3½ h after 6th spike—45 mg/kg |
Note. FOB, functional observational battery; MA, motor activity; MWM, Morris water maze.
Testing by Week during the Year-Long CPF Feed Exposure
Week . | Test . |
|---|---|
| 0 | FOB, MA before feed exposure began |
| 4 | FOB, MA |
| 8 | FOB, MA 3½ h after 1st spike—60 mg/kg |
| 13 | FOB, MA |
| 17 | FOB, MA 3½ h after 2nd spike—45 mg/kg |
| 21 | FOB, MA |
| 27 | FOB, MA 3½ h after 3rd spike—45 mg/kg |
| 31 | FOB, MA |
| 36 | FOB, MA 3½ h after 4th spike—45 mg/kg |
| 40 | FOB, MA |
| 44 | FOB, MA 3½ h after 5th spike—45 mg/kg |
| 46–50 | MWM—cohort 1 |
| 46–48 | MWM—cohort 2 |
| 52 | FOB, MA 3½ h after 6th spike—45 mg/kg |
Week . | Test . |
|---|---|
| 0 | FOB, MA before feed exposure began |
| 4 | FOB, MA |
| 8 | FOB, MA 3½ h after 1st spike—60 mg/kg |
| 13 | FOB, MA |
| 17 | FOB, MA 3½ h after 2nd spike—45 mg/kg |
| 21 | FOB, MA |
| 27 | FOB, MA 3½ h after 3rd spike—45 mg/kg |
| 31 | FOB, MA |
| 36 | FOB, MA 3½ h after 4th spike—45 mg/kg |
| 40 | FOB, MA |
| 44 | FOB, MA 3½ h after 5th spike—45 mg/kg |
| 46–50 | MWM—cohort 1 |
| 46–48 | MWM—cohort 2 |
| 52 | FOB, MA 3½ h after 6th spike—45 mg/kg |
Note. FOB, functional observational battery; MA, motor activity; MWM, Morris water maze.
Both cohorts were treated equally with only two exceptions, as follows. The same observer was used for all observations over the two years except that a second observer had to fill in for the week-13 testing (non-spike day) in the first cohort. Comparison of the two cohorts at that time point indicated no differences in the observations, therefore the data were combined. The first cohort was tested at week 2, but the second cohort could not be tested during that week due to a weather emergency. Comparison of the week-4 data for both cohorts indicated there were no differences at that time point, implying that the week-2 test did not have an influence on subsequent tests. The week-2 test was therefore removed for data analysis purposes.
The FOB and motor activity evaluation are widely used as a screening battery for general neurobehavioral toxicity. Procedural details and scoring criteria for the FOB protocol are provided elsewhere (McDaniel and Moser, 1993). Each rat was evaluated for changes in general appearance, lacrimation, and salivation, in addition to ranking of the animal's reactivity. Open field measurements included ranking the rat's arousal and activity levels, counting the number of times the animal reared, and noting any tremorigenic activity. The rat's reaction to a click stimulus, tail pinch, and penlight were also ranked. Forelimb and hindlimb grip strength, landing foot splay, and rectal temperature were quantified. Motor activity data were collected shortly after FOB testing, using a photocell-based chamber shaped like a figure-eight (Reiter 1983). Activity counts were recorded in 12 five-min intervals to evaluate habituation of activity during the session. For all testing, the observer was blind with respect to the treatment levels.
Cognitive testing took place in a water maze using procedures described by Morris (1981). The round tank (140-cm diameter) was filled with water made opaque with white tempera paint, and the plexiglass escape platform (9 cm in diameter) was placed 2 cm beneath the surface of the water. Water temperature was maintained at 25–26°C and water was changed and the tank cleaned daily. Electronic tracking equipment (HVS Imaging, Ormond Crescent, Hampton, U.K.) was used to measure behavior, including latency to find the platform, pathlength, swim speed, and distribution of swimming throughout the quadrants and concentric zones of the tank. For place training, the platform was located in the same quadrant, and the starting position alternated across trials in a semi-random order.
For cohort 1, place training consisted of one trial per day (Monday through Friday) for three weeks, whereas for cohort 2, place training involved two trials per day for two weeks. Rats had 60 s in which to find the platform, and were guided there by the observer if they did not succeed, followed by 15 s on the platform. Memory was tested in 60-s probe trials, with the platform removed, on day 8 and 15 for cohort 1, and day 5 and 10 for cohort 2. Working memory was evaluated for cohort 1 only. For this, the platform moved each day (five possible positions) for five days, and rats were given two trials per day. The last test for both groups was a visual task using a platform with a black strip along edge, raised 2.5 cm above the water. Dependent variables included latency to find the platform, pathlength, swim speed, percent time in cocentric zones as well as the outermost ring of the tank, and, for probe trials only, percent time in quadrants (dwell time) and proximity score (Gallagher et al., 1993).
Statistical approach.
For analysis of these data, multivariate ANOVAs would need to include multiple factors: CPF feed (three levels), spike (two levels), cohort (two), and repeated testing over 12 months (for FOB/activity data) or over daily training (for water maze). To focus and decrease the number of overall statistical analyses, only data that targeted three specific questions were analyzed. The first question was, did the feed alone have an effect, and was there a difference in effect across time? For these analyses, data from the non-spike groups (i.e., received oil for the spike) were analyzed across all 12 time points. The second question was, was the response to the spikes different depending on the level of CPF in the feed, and did this change over time? For this, data from the spike groups (i.e., received chlopyrifos for the spike) were analyzed across all spike days. However, since the first spike day used a higher dose (which could erroneously appear as a spike-by-time interaction), that one day was analyzed separately, and the subsequent five spikes were analyzed together (as repeated measures). Finally, were there differences in spike rats compared to non-spike rats, did this depend on the level of CPF in the feed, and did this change over time? For the FOB and motor activity data, these analyses included all groups, analyzed across non-spike days only. Focusing the analyses in this way eliminated the high incidence of overall interactions due to combining spike and non-spike data across all time points. We also felt this approach strengthened the interpretation of this large dataset.
Using this modified approach, overall analyses using factors of feed, spike, cohort, and repeated testing were conducted as described above. Where overall interactions were significant, step-down analyses were conducted to clarify the specific interaction. If there were no interactions between cohort and other factors, the data were combined for subsequent analyses. For analysis of the Morris water maze data, the cohorts were analyzed separately since the experimental testing was different. Analysis procedures for these behavioral test measures have been previously described (Creason, 1989; Moser et al., 1988). Continuous data (e.g., motor activity, habituation, continuous endpoints of the FOB, and water maze measures) were analyzed by a general linear model (GLM; SAS, 1990). Rank-order and categorical data of the FOB were analyzed using a categorical modeling procedure (CATMOD; SAS, 1990). In all cases, probability values p < 0.05 were considered significant.
RESULTS
To simplify the treatment levels for each group, the treatments are indicated with the first number being the dose level in the feed, and the second being whether the rats received vehicle or CPF for the spike. For example, “1-CPF” represents the group that was given 1 mg/kg CPF in the feed and also received CPF with each spike dose.
None of the rats in the first cohort died during the year-long study, but in the second cohort, one rat in the 1-0 group and two rats in the 5-CPF group were euthanized for health reasons unrelated to treatment. Otherwise, rats showed relatively good health. The rats were about 15 months old at the end of the study. However, since rats that are weight-maintained in our facility live at least two years (P. Bushnell, personal communication), they cannot be considered aged. Body weight was consistent across treatment groups, since the amount of feed provided each day was adjusted so as to maintain a 350 g body weight. The only exception was that, after each spike, the rats who received CPF spike tended to lose weight overnight and therefore received a bit more food the next day.
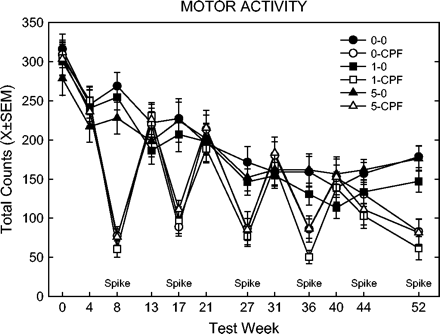
As expected, the bimonthly (every two months) spikes produced overt toxicity, which recovered in each intervening month. A typical pattern of behavior is shown in Figure 1, which shows motor activity counts over the year. There was a sharp drop in activity in the groups receiving each CPF spike. The first spike, being a higher dose, produced greater effects (relative to the controls for that time point) than the subsequent doses. Note that there was also a significant decline in activity across testing for all non-spike groups, and for the spike groups on the non-spike testing days.
Motor activity counts during 60-min sessions over one-year study. Key: the first number refers to the CPF dietary dose (0, 1, or 5 mg/kg/day), second entry indicates spike (CPF) or no spike (0).
The significant interactions and/or overall main effects across all the FOB and motor activity analyses are summarized in Table 2. The total number of multivariate analyses considered, including FOB, motor activity, and water maze data, was 139, of which 43 showed significance overall. Additional analyses were conducted only where there were significant overall effects. The results below are described in terms of the specific questions of the study.
Summary of Significant Overall Interactions for Each Endpoint of the FOB and Motor Activity Evaluations
Effects in non-spike groups across all test days | ||
| Activity habituation | interval*cohort*feed F(22,539) = 2.22, p = 0.0013 | |
| Open field activity | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}38.45,\) p = 0.0163 | |
| Rearing | feed*cohort*time F(22,583) = 1.89, p = 0.0087 | |
| Arousal | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}73.59,\) p = 0.0000 | |
| Handling reactivity | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}62.84,\) p < 0.0000 | |
| Click response | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}37.66,\) p = 0.0200 | |
| Spike groups on spike days | ||
| Open field activity | 1st spike: feed*cohort \(\mathrm{{\chi}}_{(2)}^{2}{=}6.57,\) p = 0.0375 | |
| Arousal | 2nd-6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}32.76,\) p = 0.0001 | |
| Click response | 2nd–6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}38.05,\) p < 0.0000 | |
| Lacrimation | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}8.26,\) p = 0.0161 2nd-6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}15.73,\) p = 0.0464 | |
| Pupil response | 2nd–6th spikes: feed*cohort*time \(\mathrm{{\chi}}_{(8)}^{2}{=}28.27,\) p = 0.0004 | |
| Tremors | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}9.94,\) p = 0.0069 | |
| Landing foot splay | 1st spike: feed F(2,59) = 4.29, p = 0.0187 | |
| Righting response | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}7.82,\) p = 0.0200 | |
| Effects in all groups across non-spike days | ||
| Activity habituation | time*interval*cohort*spike F(55,5885) = 1.37, p = 0.0350 | |
| Open field activity | cohort*spike \(\mathrm{{\chi}}_{(1)}^{2}{=}5.45,\) p = 0.0196 | |
| Rearing | time*feed*cohort*spike F(10,525) = 1.99, p = 0.0323 | |
| Arousal | feed*spike*time \(\mathrm{{\chi}}_{(10)}^{2}{=}23.96,\) p = 0.0077 | |
| Handling reactivity | spike*time \(\mathrm{{\chi}}_{(5)}^{2}{=}19.71,\) p = 0.0014 | |
| Tail pinch response | spike*time \(\mathrm{{\chi}}_{(5)}^{2}{=}11.26,\) p = 0.0464 | |
| Forelimb grip strength | feed*time F(10,525) = 2.05, p = 0.0272; spike*time F(5,525) = 3.06, p = 0.0098 | |
| Hindlimb grip strength | spike*time F(5,525) = 2.24, p = 0.0488 | |
| Landing foot splay | spike F(1,105) = 5.09, p = 0.0262 | |
| Body temperature | spike F(1,105) = 6.94, p = 0.0097 | |
Effects in non-spike groups across all test days | ||
| Activity habituation | interval*cohort*feed F(22,539) = 2.22, p = 0.0013 | |
| Open field activity | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}38.45,\) p = 0.0163 | |
| Rearing | feed*cohort*time F(22,583) = 1.89, p = 0.0087 | |
| Arousal | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}73.59,\) p = 0.0000 | |
| Handling reactivity | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}62.84,\) p < 0.0000 | |
| Click response | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}37.66,\) p = 0.0200 | |
| Spike groups on spike days | ||
| Open field activity | 1st spike: feed*cohort \(\mathrm{{\chi}}_{(2)}^{2}{=}6.57,\) p = 0.0375 | |
| Arousal | 2nd-6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}32.76,\) p = 0.0001 | |
| Click response | 2nd–6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}38.05,\) p < 0.0000 | |
| Lacrimation | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}8.26,\) p = 0.0161 2nd-6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}15.73,\) p = 0.0464 | |
| Pupil response | 2nd–6th spikes: feed*cohort*time \(\mathrm{{\chi}}_{(8)}^{2}{=}28.27,\) p = 0.0004 | |
| Tremors | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}9.94,\) p = 0.0069 | |
| Landing foot splay | 1st spike: feed F(2,59) = 4.29, p = 0.0187 | |
| Righting response | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}7.82,\) p = 0.0200 | |
| Effects in all groups across non-spike days | ||
| Activity habituation | time*interval*cohort*spike F(55,5885) = 1.37, p = 0.0350 | |
| Open field activity | cohort*spike \(\mathrm{{\chi}}_{(1)}^{2}{=}5.45,\) p = 0.0196 | |
| Rearing | time*feed*cohort*spike F(10,525) = 1.99, p = 0.0323 | |
| Arousal | feed*spike*time \(\mathrm{{\chi}}_{(10)}^{2}{=}23.96,\) p = 0.0077 | |
| Handling reactivity | spike*time \(\mathrm{{\chi}}_{(5)}^{2}{=}19.71,\) p = 0.0014 | |
| Tail pinch response | spike*time \(\mathrm{{\chi}}_{(5)}^{2}{=}11.26,\) p = 0.0464 | |
| Forelimb grip strength | feed*time F(10,525) = 2.05, p = 0.0272; spike*time F(5,525) = 3.06, p = 0.0098 | |
| Hindlimb grip strength | spike*time F(5,525) = 2.24, p = 0.0488 | |
| Landing foot splay | spike F(1,105) = 5.09, p = 0.0262 | |
| Body temperature | spike F(1,105) = 6.94, p = 0.0097 | |
Note. F-values are given for continuous variables, chi-square values given for ordinal variables.
Summary of Significant Overall Interactions for Each Endpoint of the FOB and Motor Activity Evaluations
Effects in non-spike groups across all test days | ||
| Activity habituation | interval*cohort*feed F(22,539) = 2.22, p = 0.0013 | |
| Open field activity | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}38.45,\) p = 0.0163 | |
| Rearing | feed*cohort*time F(22,583) = 1.89, p = 0.0087 | |
| Arousal | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}73.59,\) p = 0.0000 | |
| Handling reactivity | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}62.84,\) p < 0.0000 | |
| Click response | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}37.66,\) p = 0.0200 | |
| Spike groups on spike days | ||
| Open field activity | 1st spike: feed*cohort \(\mathrm{{\chi}}_{(2)}^{2}{=}6.57,\) p = 0.0375 | |
| Arousal | 2nd-6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}32.76,\) p = 0.0001 | |
| Click response | 2nd–6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}38.05,\) p < 0.0000 | |
| Lacrimation | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}8.26,\) p = 0.0161 2nd-6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}15.73,\) p = 0.0464 | |
| Pupil response | 2nd–6th spikes: feed*cohort*time \(\mathrm{{\chi}}_{(8)}^{2}{=}28.27,\) p = 0.0004 | |
| Tremors | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}9.94,\) p = 0.0069 | |
| Landing foot splay | 1st spike: feed F(2,59) = 4.29, p = 0.0187 | |
| Righting response | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}7.82,\) p = 0.0200 | |
| Effects in all groups across non-spike days | ||
| Activity habituation | time*interval*cohort*spike F(55,5885) = 1.37, p = 0.0350 | |
| Open field activity | cohort*spike \(\mathrm{{\chi}}_{(1)}^{2}{=}5.45,\) p = 0.0196 | |
| Rearing | time*feed*cohort*spike F(10,525) = 1.99, p = 0.0323 | |
| Arousal | feed*spike*time \(\mathrm{{\chi}}_{(10)}^{2}{=}23.96,\) p = 0.0077 | |
| Handling reactivity | spike*time \(\mathrm{{\chi}}_{(5)}^{2}{=}19.71,\) p = 0.0014 | |
| Tail pinch response | spike*time \(\mathrm{{\chi}}_{(5)}^{2}{=}11.26,\) p = 0.0464 | |
| Forelimb grip strength | feed*time F(10,525) = 2.05, p = 0.0272; spike*time F(5,525) = 3.06, p = 0.0098 | |
| Hindlimb grip strength | spike*time F(5,525) = 2.24, p = 0.0488 | |
| Landing foot splay | spike F(1,105) = 5.09, p = 0.0262 | |
| Body temperature | spike F(1,105) = 6.94, p = 0.0097 | |
Effects in non-spike groups across all test days | ||
| Activity habituation | interval*cohort*feed F(22,539) = 2.22, p = 0.0013 | |
| Open field activity | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}38.45,\) p = 0.0163 | |
| Rearing | feed*cohort*time F(22,583) = 1.89, p = 0.0087 | |
| Arousal | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}73.59,\) p = 0.0000 | |
| Handling reactivity | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}62.84,\) p < 0.0000 | |
| Click response | feed*cohort*time \(\mathrm{{\chi}}_{(22)}^{2}{=}37.66,\) p = 0.0200 | |
| Spike groups on spike days | ||
| Open field activity | 1st spike: feed*cohort \(\mathrm{{\chi}}_{(2)}^{2}{=}6.57,\) p = 0.0375 | |
| Arousal | 2nd-6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}32.76,\) p = 0.0001 | |
| Click response | 2nd–6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}38.05,\) p < 0.0000 | |
| Lacrimation | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}8.26,\) p = 0.0161 2nd-6th spikes: feed*time \(\mathrm{{\chi}}_{(8)}^{2}{=}15.73,\) p = 0.0464 | |
| Pupil response | 2nd–6th spikes: feed*cohort*time \(\mathrm{{\chi}}_{(8)}^{2}{=}28.27,\) p = 0.0004 | |
| Tremors | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}9.94,\) p = 0.0069 | |
| Landing foot splay | 1st spike: feed F(2,59) = 4.29, p = 0.0187 | |
| Righting response | 1st spike: feed \(\mathrm{{\chi}}_{(2)}^{2}{=}7.82,\) p = 0.0200 | |
| Effects in all groups across non-spike days | ||
| Activity habituation | time*interval*cohort*spike F(55,5885) = 1.37, p = 0.0350 | |
| Open field activity | cohort*spike \(\mathrm{{\chi}}_{(1)}^{2}{=}5.45,\) p = 0.0196 | |
| Rearing | time*feed*cohort*spike F(10,525) = 1.99, p = 0.0323 | |
| Arousal | feed*spike*time \(\mathrm{{\chi}}_{(10)}^{2}{=}23.96,\) p = 0.0077 | |
| Handling reactivity | spike*time \(\mathrm{{\chi}}_{(5)}^{2}{=}19.71,\) p = 0.0014 | |
| Tail pinch response | spike*time \(\mathrm{{\chi}}_{(5)}^{2}{=}11.26,\) p = 0.0464 | |
| Forelimb grip strength | feed*time F(10,525) = 2.05, p = 0.0272; spike*time F(5,525) = 3.06, p = 0.0098 | |
| Hindlimb grip strength | spike*time F(5,525) = 2.24, p = 0.0488 | |
| Landing foot splay | spike F(1,105) = 5.09, p = 0.0262 | |
| Body temperature | spike F(1,105) = 6.94, p = 0.0097 | |
Note. F-values are given for continuous variables, chi-square values given for ordinal variables.
Question 1: Did the feed alone have an effect, and was there a difference in effect across time?
FOB/Motor Activity
Rats receiving CPF feed but only the vehicle spikes showed significant interactions on most of the endpoints evaluating activity and reactivity. There were, however, no consistent or dose-related changes. In addition, there were significant cohort interactions with every endpoint, indicating differences in the cohorts.
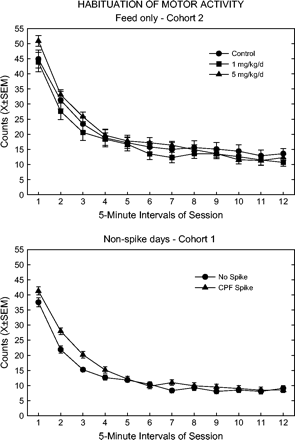
In cohort 1, the high feed group showed increased open field activity, but only at the 27-week test, and decreased handling reactivity, only at 52 weeks. However, Cohort 2 rats showed a few more effects. Habituation differences were suggested by the significant influence of cohort and feed, but not time, which was shown by further analyses to be due to the high-dose feed (presented in Fig. 2). The high-dose feed group showed slightly higher activity early in the session, but asymptotic activity later in the session was similar to the other feed groups. Arousal was transiently altered, with decreases in the low feed group at 44 weeks and in the high feed group at 52 weeks. The magnitude of these changes was quite small. Step-down analyses of rearing and click response did not produce any significant differences.
Motor activity counts in intervals of the session. Top panel: data for rats receiving CPF feed only (i.e., vehicle spike), collapsed across test times. Bottom panel: data for spike and no-spike (vehicle) rats, all feed groups combined, collapsed across non-spike days.
Morris Water Maze
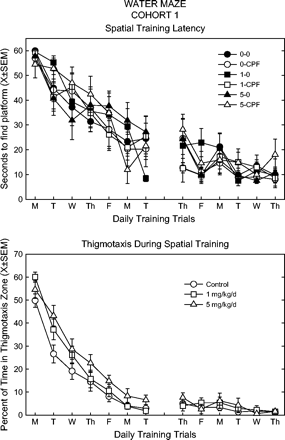
CPF dietary exposure did not alter acquisition of place learning (platform in the same location) in the cohort 1 rats. Figure 3 shows that the latency to find the platform decreased with daily training, but there were no significant differences or interactions across treatment groups. During the last week of training, asymptotic performance was evident. There were also no differences among treatment groups in swim speed or pathlength at any time.
Latency to find platform (top panel) and time spent very close to tank wall (bottom panel) during spatial training (one trial/day) in the Morris water maze for cohort 1. Latency key: the first number refers to the CPF dietary dose (0, 1, or 5 mg/kg/day), second entry indicates spike (CPF) or no spike (0). Data for thigmotaxis as a function of CPF dietary dose, i.e., spike and no-spike rats combined.
While latency was similar across treatment groups, the spatial distribution of swimming was not. There was a significant overall effect of feed (F(2,54) = 4.5, p = 0.0156) on the percent time spent next to the tank wall (hugtime), which could be interpreted as thigmotaxis. When the spiked groups were combined, the high-dose feed group had higher hugtime than controls in the first week of training, shown in Figure 3. These data indicate that while the high-dose feed group was able to find the platform in a similar period of time, they stayed closer to the tank wall while searching.
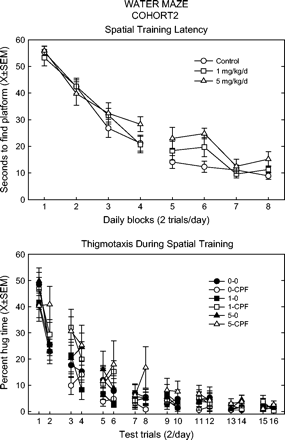
The paradigm used for cohort 2 training involved two trials each day, and for the statistical analyses trial was nested within daily blocks. Unlike in cohort 1, there was an overall effect of feed (F(2,54) = 3.53, p = 0.0366) on the latency to find the platform in cohort 2 during the second week of training. As shown in Figure 4, the high-dose feed group latency was significantly higher. There were no differences among treatment groups in swim speed or pathlength at any time.
Latency to find platform (top panel) and time spent very close to tank wall (bottom panel) during spatial training (two trials/day) in the Morris water maze for cohort 2. Latency presented in daily blocks (two trials averaged), as a function of CPF dietary dose, i.e., spike and no-spike rats combined. Data for thigmotaxis are shown for each trial. Key: the first number refers to the CPF dietary dose (0, 1, or 5 mg/kg/day), second entry indicates spike (CPF) or no spike (0).
On the first probe trial in cohort 1 rats, there was a significant overall influence of feed (F(2,54) = 4.07, p = 0.0225) on the proximity measure, in that the high feed group showed a slightly higher (worse) proximity score than the control feed groups. This was not reflected in the percent time spent in the correct quadrant, and was only significant in the first 30 s of the trial. Analysis of the spatial distribution of swimming indicated that the high-dose feed group spent significantly more time in the outer zone (feed F(2,54) = 5.18, p = 0.0088), and less time in the middle zone (feed F(2,54) = 4.33, p = 0.0181) compared to controls, as was observed during the spatial training. Thus, the rats showed preference for swimming in the correct quadrant, but during the first half of the trial they stayed in the outer zone and therefore were further away from the platform (reflected in the higher proximity measure).
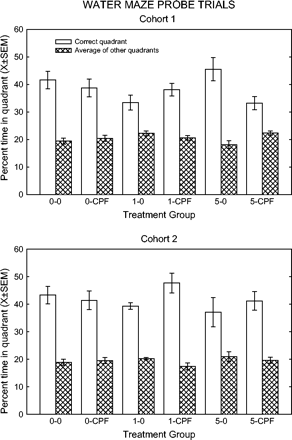
Unlike the first cohort, neither probe trial showed any significant effects on any dependent variables in the second cohort. The time in quadrants data for the second probe trial are shown in Figure 5.
Quadrant preferences during 60-s probe trials in the Morris water maze for each cohort; the second probe trial for each group. Key: the first number refers to the CPF dietary dose (0, 1, or 5 mg/kg/day), second entry indicates spike (CPF) or no spike (0).
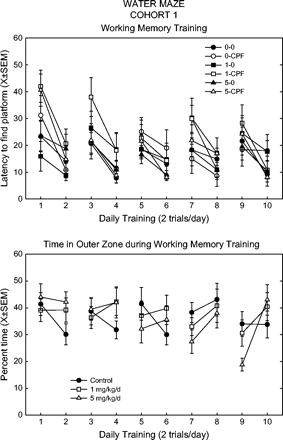
Working memory was evaluated by moving the platform each day. Using this paradigm, the latency in the first trial was typically high while the latency in the second trial that day was lower, as shown by a significant effect of trial across daily blocks. This pattern was observed in all treatment groups (see Fig. 6). There was an overall effect of feed (F(2,54) = 4.42, p = 0.0167) with the low-dose feed group showing higher latencies, and an overall effect of spike (F(1,54) = 4.68, p = 0.0350) with the spike groups showing higher latencies. There were, however, no interactions between feed and spike. Pathlength also showed a significant feed effect (F(2,54) = 6.05, p = 0.0043), again with the low-dose feed having greater pathlengths. The spatial pattern of swimming was again altered. The feed groups significantly varied across trials in the middle (trial*feed F(2,54) = 3.39, p = 0.0410) and outer zones (trial*feed F(2,54) = 5.69, p = 0.0057). As shown in Figure 6, control rats typically spent less time in the outer zone on the second trial, whereas treated rats showed either no spatial difference between the two trials, or else spent significantly more time in the outer zone in the second trial of the day compared to the first trial.
Working memory training in the Morris water maze. Top panel: latency to find the new platform position each day (two trials/day). Key: the first number refers to the CPF dietary dose (0, 1, or 5 mg/kg/day), second entry indicates spike (CPF) or no spike (0). Bottom panel: time in outer zone each day (two trials/day). Data presented as a function of CPF dietary dose, i.e., spike and no-spike rats combined.
In both cohorts, there were no treatment-related differences in the latency to find a raised platform (visual probe). There were, however, differences between the two cohorts. For cohort 1, the average latency was 6.0 ± 0.3 s, and for cohort 2, it was 14.9 ± 1.9 s. The reason for this difference is unclear, although the latencies for cohort 2 are more similar to the historical control in our laboratory.
Question 2: Was the response to the spikes different depending on the level of CPF in the feed, and did this change over time?
FOB/Motor Activity
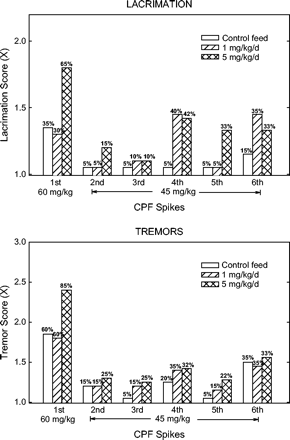
Although the feed itself had relatively little effect, CPF dietary exposure resulted in greater response on some endpoints in the rats receiving the CPF challenges. This was generally true for both feed levels, but was not consistent across spikes nor always dose-responsive. A summary of the influences of feed exposure on the response to CPF challenge is presented in Table 3. Dietary exposure made the most difference following the first spike (60 mg/kg). For lacrimation, tremor, righting reflex, foot splay, and open field activity, there was a main effect of feed; only open field activity showed an interaction with cohort (see Table 2). The data for lacrimation and tremor severity scores are shown in Figure 7. After the first spike, the high-dose feed group showed a greater response in terms of lacrimation, tremor, abnormal righting ability, and increased landing foot splay in both cohorts. Open field activity was more depressed in the high-dose feed group in cohort 1, but the opposite finding (less depression) was seen in the low-dose feed group in cohort 2.
Lacrimation (top panel) and tremor (bottom panel) data following bi-monthly spikes as a function of CPF dietary dose. For each measure, mean score is plotted with incidence of rats showing each sign above each bar.
Summary of the Significant Influences of Feed Exposure on the Response to CPF Challenges
. | 1 mg/kg feed . | 5 mg/kg feed . |
|---|---|---|
| 1st spike | LESS effect on open-field activity depressionb | MORE effect on altered righting response, increased foot splay, tremors, lacrimation, open-field activity depressiona |
| 2nd spike | ND | ND |
| 3rd spike | MORE effect on pupil responsea, decreased click response | MORE effect on pupil responsea |
| 4th spike | MORE effect on lacrimation, decreased arousal | MORE effect on lacrimation |
| 5th spike | ND | MORE effect on lacrimation, decreased click response |
| 6th spike | ND | LESS effect on decreased click response |
. | 1 mg/kg feed . | 5 mg/kg feed . |
|---|---|---|
| 1st spike | LESS effect on open-field activity depressionb | MORE effect on altered righting response, increased foot splay, tremors, lacrimation, open-field activity depressiona |
| 2nd spike | ND | ND |
| 3rd spike | MORE effect on pupil responsea, decreased click response | MORE effect on pupil responsea |
| 4th spike | MORE effect on lacrimation, decreased arousal | MORE effect on lacrimation |
| 5th spike | ND | MORE effect on lacrimation, decreased click response |
| 6th spike | ND | LESS effect on decreased click response |
Note. The first spike was 60 mg/kg, subsequent spikes were 45 mg/kg. There was no effect of feed exposure on CPF-induced hypothermia, decreased rearing, motor activity, tail pinch response, or gait changes at any of the spikes. ND, no significant differences.
Cohort 1 only.
Cohort 2 only.
Summary of the Significant Influences of Feed Exposure on the Response to CPF Challenges
. | 1 mg/kg feed . | 5 mg/kg feed . |
|---|---|---|
| 1st spike | LESS effect on open-field activity depressionb | MORE effect on altered righting response, increased foot splay, tremors, lacrimation, open-field activity depressiona |
| 2nd spike | ND | ND |
| 3rd spike | MORE effect on pupil responsea, decreased click response | MORE effect on pupil responsea |
| 4th spike | MORE effect on lacrimation, decreased arousal | MORE effect on lacrimation |
| 5th spike | ND | MORE effect on lacrimation, decreased click response |
| 6th spike | ND | LESS effect on decreased click response |
. | 1 mg/kg feed . | 5 mg/kg feed . |
|---|---|---|
| 1st spike | LESS effect on open-field activity depressionb | MORE effect on altered righting response, increased foot splay, tremors, lacrimation, open-field activity depressiona |
| 2nd spike | ND | ND |
| 3rd spike | MORE effect on pupil responsea, decreased click response | MORE effect on pupil responsea |
| 4th spike | MORE effect on lacrimation, decreased arousal | MORE effect on lacrimation |
| 5th spike | ND | MORE effect on lacrimation, decreased click response |
| 6th spike | ND | LESS effect on decreased click response |
Note. The first spike was 60 mg/kg, subsequent spikes were 45 mg/kg. There was no effect of feed exposure on CPF-induced hypothermia, decreased rearing, motor activity, tail pinch response, or gait changes at any of the spikes. ND, no significant differences.
Cohort 1 only.
Cohort 2 only.
The subsequent spikes were a lower dose (45 mg/kg), and the overall magnitude of response was accordingly lower across most endpoints. As with the first spike, there was an influence of feed, but this was more variable; there were generally fewer differences in feed groups after the second and third spikes. The high-dose feed group showed significantly more pupil effects, i.e., miosis, (cohort 1, third spike only), more lacrimation (fourth and fifth spikes; evident in Fig. 7), and altered the click response (greater response, fifth spike; less response, sixth spike). The low-dose feed group also showed more pupil effects (cohort 1, third spike), more lacrimation (fourth spike, Fig. 7), greater decreased arousal (fourth spike) and more click response depression (third spike). Effects on several endpoints (specifically, decreased rearing and motor activity, gait changes, salivation, hypothermia, and depressed response to handling and the tail pinch), were always observed after each CPF spikes, but the magnitude of response was similar across feed groups.
The water maze testing took place between the spikes, not during, so those data cannot be used to address the second question.
Question 3: Were there differences in spike rats compared to non-spike rats, did this depend on the level of CPF in the feed, and did this change over time?
FOB/Motor Activity
On non-spike days, groups receiving the CPF spikes were different on some measures from the vehicle control groups. Statistically, the spike factor had the greatest influence; only a few of these measures depended on the level of feed or differed between the cohorts (Table 2).
There were significant overall effects of spike on several FOB measures on some, but not all, non-spike days, regardless of the level of CPF in the feed. These differences in the spike group included lowered tail-pinch response (week 21), increased handling reactivity (week 13), and slightly (6–7%) higher forelimb grip strength (weeks 21 and 32). The effect on hindlimb grip strength was not due to spike exposures, since it was significant at four weeks (before the first spike) as well as later time points.
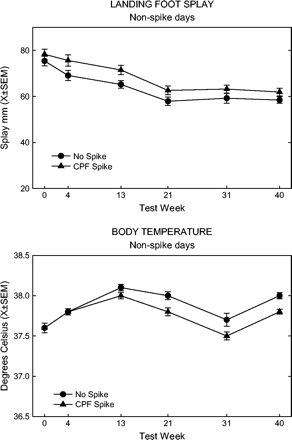
Landing foot splay and body temperature (presented in Fig. 8) showed significant overall effects of spike. Foot splay values were higher in the spike group even before the first spike, and the magnitude of difference did not change throughout the study. Even though there was no significant influence of time, body temperature was equal between the groups until after the first spike, and the spike group remained about 0.2°C lower thereafter.
Landing foot splay (top panel) and body temperature (bottom panel) for spike and no-spike rats, all feed groups combined, collapsed across non-spike days.
Arousal was the only endpoint that indicated an interaction between feed and spike on certain non-spike days. Specifically, at week 32 the 5-CPF group showed lower arousal than 0-CPF, and at week 40 the high feed groups (both spike and non-spike) showed lower arousal.
For several endpoints, the influence of spike and/or feed depended on the cohort. Open field activity was lower in spiked groups across all times in cohort 1. Habituation was also altered in this cohort (Fig. 2). As was observed in the feed-only groups, the spike group had higher activity levels early in the session, but the asymptotic levels were the same between the spike and non-spike groups. In cohort 2, the high feed group had less rearing activity than controls only at week 21, regardless of whether or not they received the CPF spike.
Morris Water Maze
During spatial training of cohort 1, the time in each annulus (zone) was altered by CPF exposure; however, the pattern of effect was not clear. In the first week of training, the spike groups (F(1,54) = 6.58, p = 0.0132) spent less time in the innermost zone. There were significant interactions of trial, spike, and feed (trial*feed F(12,324) = 2.24, p = 0.0102; trial*spike F(6,324) = 2.21, p = 0.0419) in the middle zone data with effects only in certain trials and in opposite directions. Time in the outer zone showed an interaction of feed and spike (trial*feed*spike F(10,270) = 1.89, p = 0.0467) during the second week, but step-down analyses did not reach significance.
With cohort 2, there were again significant interactions of feed, spike, block, and trial for the measure of thigmotaxis during spatial training (trial*block*feed*spike F(6,153) = 2.18, p = 0.0475). As shown in Figure 4, this complex interaction appeared to be due to the increase in percent hugtime in the feed groups in the second trial of each day. However, step-down analyses showed only a significant overall feed effect in trial 3, and a feed-by-spike interaction in trial 8. In the first week of training, the feed groups showed an interaction between trials and the time spent in the innermost zone (trial*feed F(2,51) = 5.90, p = 0.0050), but no trial showed a difference in feed groups. During the second week, the interactions were among the spike groups in the inner zone (time*trial*spike F(3,153) = 2.91, p = 0.0364) and the middle zone (trial*spike F(1,51) = 6.67, p = 0.0127). The spike groups spent less time in these zones in certain trials only, specifically trial 9 and 14 for the inner zone, the middle zone showed only a trend (p's < 0.07) in trials 12 and 13.
In the second probe test for cohort 1, there was a significant interaction of feed and spike in the percent time in quadrant variable, for both the first 30 s (quadrant*feed*spike F(2,54) = 3.69, p = 0.0316) as well as the full 60-s measure (quadrant*feed*spike F(2,54) = 3.93, p = 0.0255). Figure 5 shows that the 5-CPF group spent less time in the correct quadrant compared to the 0-0 control group. The proximity measure for the first 30 s showed a significant interaction with spike (quadrant*spike F(1,54) = 6.65, p = 0.0127), in that the spike groups had lower proximity values in the incorrect quadrants. This finding was not evident in the 60-s analysis. There were no differences among treatment groups on the time in the different areas of the tank in the second probe. There was a significant effect on swim speed in the second probe (feed*spike F(2,54) = 3.55, p = 0.0355). Post-hoc comparisons revealed that the 1–0 group swam faster than 1-CPF, 0-CPF, and 5-0 groups.
As mentioned above, the working memory paradigm revealed an overall effect of spike, with the spike groups showing higher latencies. In the analyses of spatial distribution, there was an interaction with spike on the hugtime measure (trial*time*spike F(4,216) = 2.81, p = 0.0266), but step-down analyses did not identify significant contrasts.
DISCUSSION
This study provides data regarding neurotoxicological effects of CPF during dietary exposure to relatively low doses and intermittent high-dose challenges, as well as the interactions between these exposure conditions. One goal of this study was to evaluate potential behavioral effects of exposure to the CPF feed alone. The behavioral effects can be compared to the changes in ChE activity and muscarinic receptor levels measured in other animals from this study, as described by Padilla and coworkers (submitted). The low-dose feed produced 78% inhibition of whole blood ChE and no inhibition in brain, whereas the high-dose feed produced 93% blood inhibition and 63% brain ChE inhibition. The ChE inhibition had reached steady-state by six months. This level of dietary exposure produced long-lasting neurobehavioral changes and altered the response to acute challenges.
We have shown that acute doses of CPF which produce 60–70% brain inhibition will produce hypothermia, altered gait, and decreased motor activity (Nostrandt et al., 1997). Even though the high-dose feed group had similar levels of brain ChE inhibition on a chronic basis, such signs were not evident. While the FOB and motor activity measures indicated increased activity and decreased excitability, the dose-response and effective times were not consistent between cohorts and the data are not convincing. Other reports of subchronic dietary OP exposures (Ivens et al., 1998; Mattsson et al., 1996; Sheets et al., 1997) also found relatively few behavioral effects using similar observational batteries and activity measures.
A possible explanation for the lack of behavioral response to dietary exposure (despite clear ChE inhibition) could be tolerance. Repeated exposure to OPs leads to the development of tolerance, presumably due to down-regulation of the muscarinic receptors mediating the toxicity (reviewed by Costa, 1982). While tolerance is the accepted sequelae of repeated anticholinesterase exposure, studies have often used doses which produce high ChE inhibition and/or clear toxicity. In this study, muscarinic receptor assays, using QNB as the ligand, showed decreased binding in the whole brain (but not the pons or retina; Padilla et al., submitted). The high-dose feed group showed 12% decrease in binding, and the spiked groups showed ∼6% lower binding across all levels of feed. As with ChE inhibition, this parameter had reached steady-state by six months. This muscarinic receptor down-regulation could have contributed to a form of tolerance, and attenuated the effects of ChE inhibition following the dietary exposure.
In rats receiving the spike, both brain and blood ChE were greatly inhibited at 24 h, and the inhibition in the high-dose feed group was greater than that of the control feed group receiving the same spike (brain, 92% vs. 79% inhibition, and blood, 98% vs. 93% inhibition for the 5-CPF vs 0-CPF groups, respectively; Padilla, S., Marshall, R.S., Hunter, D.L., Oxendine, S., Moser, V., Southerland, S.B., and Mailman, R.B., unpublished data, 2005). Likewise, the level of dietary CPF clearly impacted the behavioral effects of the spikes. Generally, exposure to CPF in the feed potentiated the behavioral responses, and this was significant with both feed levels for some endpoints. This phenomenon was clearer following the first spike, which produced greater effects than the lower dose used thereafter. Overall, the CPF feed potentiated some autonomic, neuromuscular, and convulsive endpoints, while less consistent effects were observed on the activity and excitability endpoints.
We had hypothesized that tolerance would develop during the long-term exposure, which was indeed evident in the receptor down-regulation. We anticipated that this would have been measurable as an attenuated response to the CPF spikes, but we observed the opposite. Potentiation of effect was evident with measures of ChE inhibition and behavior, with the rats receiving CPF feed showing greater inhibition following the spikes. The magnitude of muscarinic receptor down-regulation was not great (6–12%), and this may not be sufficient to attenuate the greater ChE inhibition experienced in the CPF-feed rats. Higher dietary levels, which would produce greater persistent ChE inhibition, and greater receptor down-regulation, may show a completely different pattern of response.
Another goal of this study was to evaluate the persistent effects of the spikes. Generally, there were more effects in the spike groups with little influence of feed, indicating that, for some measures, recovery was not complete in the intervening months. Some of the differences correlated with the acute effects of CPF, specifically, decreased tail pinch and hypothermia (Moser, 1995). However, increased reactivity, grip strength, and foot splay were also observed, and these are responses that are opposite to the acute effects. Since ChE was not measured in the intervening months between spikes, we do not know if the inhibition had recovered by a month. Behavioral changes like this have not been reported in the literature. Indeed, Gordon and Padnos (2002) reported that, using a telemetry system, increased temperature was measured in the high-dose feed rats, in contrast to the persistent decreased temperature that we measured. They did, however, report a similar potentiation of the hypothermic response to the spikes. In another study, Terry et al. (2003) reported decreased hindlimb grip strength at the end of 38-day CPF exposure. While we observed an increase in hindlimb grip strength, this was most likely a pre-existing difference since it emerged before the first spike.
There were significant differences between the cohorts on some endpoints, despite our extreme efforts to keep everything the same. Furthermore, there was variation between responses to the six spikes administered over the year. Every batch of feed, and all the dosing solutions were analyzed for CPF concentration and found to be within 10% of the nominal concentration. The source of the rats, down to the breeding area at the supplier, was the same. Gaines and Linder (1986) reported some seasonal differences in the lethality of one OP (parathion), and it is apparent that this confounding factor is not well understood. Seasonal effects could at least partially explain the differences across spikes, which took place every two months, and between cohorts, since the cohorts did not start at the same time of year.
There is general agreement in human studies that cognitive deficits occur during episodic or prolonged pesticide exposure, although the cognitive construct altered varies depending on the report. We evaluated spatial learning using the Morris water maze, which has been used in other studies of OPs as well (Prendergast et al., 1997; Sanchez-Santed et al., 2004; Terry et al., 2003). Using the typical measures of learning and memory, we observed several changes that we interpret as evidence of learning deficits. As with the FOB, there were cohort differences in the outcomes, but since a different training paradigm was used in the second cohort, there is no way to conduct formal comparisons. In the first cohort, learning (acquisition during place learning) was not altered. The second probe trial provided evidence of impaired memory in the high-dose feed/spike group, in that they showed less preference for the correct quadrant compared to controls. Working memory training showed no evidence of cognitive differences. In the second cohort, the high-dose feed group showed slower latencies during acquisition (second week of training), but there were no differences on the probe trials, indicating that they had learned and remembered the platform location. The effects were mostly a function of dietary exposure (high-dose feed group), although there were a few interactions with the spike.
The first cohort was tested with one trial per day, a paradigm that may be more difficult and therefore more sensitive to disruption by chemical treatment. This was not the case in this study, but the general effects were somewhat different—memory but no learning differences in cohort 1, learning but no memory differences in cohort 2. The magnitude of these cognitive differences was not as great as reported elsewhere. In the Morris water maze, repeated exposures of varying length to CPF or other OP pesticides has been reported to alter both acquisition and memory (e.g., Llorens et al., 1993; Prendergast et al., 1997; Terry et al., 2003). Likewise, other cognitive tasks are altered as well (e.g., Bushnell et al., 1991; Cohn and MacPhail, 1997; McDonald et al., 1988). In another project from the present study, Samsam et al. (submitted) reported that this exposure paradigm altered acquisition of an autoshaped operant response. After exposure (when ChE activity had returned to normal) response accuracy was reduced in the high-dose-feed/spike group.
Alterations in the search strategy used by the CPF-treated rats were the most reproducible finding in this study. A detailed analysis of swimming patterns revealed a consistently altered spatial distribution in the high-dose feed group, which consisted of either thigmotaxis (hugging closely to the side of the tank) or higher percentages of time in the outer zone of the tank. This was sometimes accompanied by a decrease of time in the middle zone as well, which is where the platform is located. Since the latency or quadrant preference was often not altered, this implies that the rats were swimming along the outside of the tank until the platform area was reached, at which time they swam directly to the platform.
Such tendencies to not venture into the middle of an open field is interpreted as anxiety, which is often measured as thigmotaxis in rodents (Simon et al., 1994; Treit and Fundytus, 1988). Sanchez-Amate and coworkers (2001) reported an anxiolytic effect of CPF using the elevated plus-maze, although much higher, acute doses with the subcutaneous route were used. In another study (Sanchez-Amate et al., 2002), CPF substituted for pentylenetetrazol, a GABA antagonist, in a drug discrimination paradigm. The pattern of spatial behavior in an open field was altered (i.e., thigmotaxis) a pyrethroid pesticide (Righi and Palermo-Neto, 2003). Given the number of human complaints of anxiety (Levin et al., 1976; Mearns et al., 1994), these findings are even more interesting. Such finding indicate similarities in the actions of pesticides, and this common action could underlie the thigmotaxic effects of CPF that we observed in this study. On the other hand, studies of rats with dorsomedial caudate-putamen (Devan et al., 1999) or prefrontal cortical (Goss et al., 2003) lesions reported a dissociation between thigmotaxis in the water maze compared to a dry-land open field. In mice overexpressing the alpha2C-adrenoceptor, thigmotaxis in the water maze was not released by treatment with the classic antianxiolytic, diazepam (Björklund et al., 1999). Thus, the relationship between water maze thigmotaxis and anxiety is not clear. A potential follow-up would be to repeat these studies using commonly accepted measures of anxiety.
Based on the overall pattern of behavioral effects evaluated in this study, one could predict that humans similarly exposed would exhibit mild cognitive dysfunction and subtle neuromotor changes. If exposed to a high dose (poisoning, accidental exposure), those with ongoing exposure would show greater effects. It should be noted, however, that both doses used in this study produced ChE inhibition in blood, and we therefore did not identify a no-effect dose. These levels of intake are about 30-fold higher than the dose used to base determination of the chlorpyrifos chronic reference dose, and many orders of magnitude higher than estimates for chronic dietary exposure in the general population (U.S. EPA, 2000). While occupational exposures may be higher, few epidemiological studies have solid measures of exposure other than blood ChE inhibition and/or urinary TCP. In a review of human studies, Colosio et al. (2003) included increased reaction time, fatigue, and non-specific emotional and intellectual changes. Other studies have reported changes in sustained attention and information processing, general malaise, and poor performance on motor and vestibular tasks, to list a few (Dick et al., 2001; Kamel and Hoppin, 2004; Steenland et al., 2000; Stephens et al., 1996). The data we present here may be reconciled with at least some of these human reports, but no clear pattern or syndrome of effects has emerged from either the present study or the human literature. Thus, the available data are insufficient to assess fully the predictability of chronic animal studies to outcomes in the human population.
The information in this document has been funded wholly by the U.S. Environmental Protection Agency. It has been reviewed by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents necessarily reflect the views of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Portions of this research were presented at the International Neurotoxicology Association meeting, June 2001, and at the 41st annual meeting of the Society of Toxicology, March 2002, Nashville, TN.
Conflict of interest: none declared.
References
Albers, J. W., Berent, S., Garabrant, D. H., Giordani, B., Schweitzer, S. J., Garrison, R. P., and Richardson, R. J. (
Björklund, M., Sirviö, J., Sallinen, J., Scheinin, M., Kobilka, B. K., and Riekkinen, Jr., P. (
Bushnell, P. J., Padilla, S. S., Ward, T., Pope, C. N., and Olszyk, V. B. (
Cohn, J., and MacPhail, R. C. (
Colosio, C., Tiramani, M., and Maroni, M. (
Costa, L. G., Schwab, B. W., and Murphy, S. D. (
Creason, J. P. (
Devan, B. D., McDonald, R. J., and White, N. M. (
Dick, R. B., Steenland, K., Krieg, E. F., and Hines, C. J. (
Ecobichon, D. J. (
Gaines, T. B., and Linder, R. E. (
Gallagher, M., Burwell, R., and Burchinal, M. (
Gordon, C. J., and Padnos, B. K. (
Goss, C. W., Hoffman, S. W., and Stein, D. G. (
Hill, R. H., Jr., Head, S. L., Baker, S., Gregg, M., Shealy, D. B., Bailey, S. L., Williams, C. C., Sampson, E. J., and Needham, L. L. (
Ivens, I. A., Schmuck, G., and Machemer, L. (
Jamal, G. A., Hansen, S., Julu, P. O. O. (
Kamel, F., and Hoppin, J. A. (
Levin, H. S., Rodnitzky, R. L., and Mick, D. L. (
Llorens, J., Crofton, K. M., Tilson, H. A., Ali, S. F., and Mundy, W. R. (
Mattsson, J. L., Wilmer, J. W., Shankar, M. R., Berdasco, N. M., Crissman, J. W., Maurissen, J. P., and Bond, D. M. (
McDaniel, K. L., and Moser, V. C. (
McDonald, B. E., Costa, L. G., and Murphy, S. D. (
Mearns, J., Dunn, J., and Lees-Haley, P. R. (
Morris, R. G. M. (
Moser, V. C. (
Moser, V. C., McCormick, J. P., Creason, J. P., and MacPhail, R. C. (
Moser, V. C., and Padilla, S. (
Nostrandt, A. C., Padilla, S., and Moser, V. C. (
Prendergast, M. A., Terry, A. V., Jr., and Buccafusco, J. J. (
Reiter, L. W. (
Righi, D. A., and Palermo-Neto, J. (
Saieva, C., Aprea, C., Tumino, R., Masala, G., Salvini, S., Frasca, G., Giurdanella, M. C., Zanna, I., Decarli, A., Sciarra, G., and Palli, D. (
Sanchez-Amate, M. C., Davila, E., Canadas, F., Flores, P., and Sanchez-Santed, F. (
Sanchez-Amate, M. C., Flores, P., and Sanchez-Santed, F. (
Sanchez-Santed, F., Canadas, F., Flores, P., Lopez-Grancha, M., and Cardona, D. (
Schulz, H., Desi, I., and Nagymajtenyi, L. (
Sheets, L. P., Hamilton, B. F., Sangha, G. K., and Thyssen, J. H. (
Simon, P., Dupuis, R., and Costentin, J. (
Sobotka, T. J., Brodie, R. E., Arnold, A., West, G. L., and O'Donnell, M. W. (
Steenland, K., Dick, R. B., Howell, R. J., Chrislip, D. W., Hines, C. J., Reid, T. M., Lehman, E., Laber, P., Krieg, E. F., Jr., and Knott, C. (
Stephens, R., Spurgeon, A., and Berry, H. (
Terry, A. V., Jr., Stone, J. D., Buccafusco, J. J., Sickles, D. W., Sood, A., and Prendergast, M. A. (
Treit, D., and Fundytus, M. (
US Environmental Protection Agency. (




Comments