-
PDF
- Split View
-
Views
-
Cite
Cite
Jaime Puértolas, Marta Pardos, Carlos de Ollas, Alfonso Albacete, Ian C Dodd, Soil moisture heterogeneity regulates water use in Populus nigra L. by altering root and xylem sap phytohormone concentrations, Tree Physiology, Volume 40, Issue 6, June 2020, Pages 762–773, https://doi.org/10.1093/treephys/tpaa037
Close - Share Icon Share
Abstract
Soil moisture heterogeneity in the root zone is common both during the establishment of tree seedlings and in experiments aiming to impose semi-constant soil moisture deficits, but its effects on regulating plant water use compared with homogenous soil drying are not well known in trees. Pronounced vertical soil moisture heterogeneity was imposed on black poplar (Populus nigra L.) grown in soil columns by altering irrigation frequency, to test whether plant water use, hydraulic responses, root phytohormone concentrations and root xylem sap chemical composition differed between wet (well-watered, WW), and homogeneously (infrequent deficit irrigation, IDI) and heterogeneously dry soil (frequent deficit irrigation, FDI). At the same bulk soil water content, FDI plants had greater water use than IDI plants, probably because root abscisic acid (ABA) concentration was low in the upper wetter layer of FDI plants, which maintained root xylem sap ABA concentration at basal levels in contrast with IDI. Soil drying did not increase root xylem concentration of any other hormone. Nevertheless, plant-to-plant variation in xylem jasmonic acid (JA) concentration was negatively related to leaf stomatal conductance within WW and FDI plants. However, feeding detached leaves with high (1200 nM) JA concentrations via the transpiration stream decreased transpiration only marginally. Xylem pH and sulphate concentration decreased in FDI plants compared with well-watered plants. Frequent deficit irrigation increased root accumulation of the cytokinin trans-zeatin (tZ), especially in the dry lower layer, and of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), in the wet upper soil layer. Root hormone accumulation might explain the maintenance of high root hydraulic conductance and water use in FDI plants (similar to well-watered plants) compared with IDI plants. In irrigated tree crops, growers could vary irrigation scheduling to control water use by altering the hormone balance.
Introduction
Under a rapidly changing climate, it is more important than ever to manage future water availability. In this context, it is relevant to understand how tree species can respond to water scarcity and maintain their productivity in drought-prone regions, thus meeting the increasing demand for wood and bioenergy products. However, plant physiological responses to drought are not easy to understand, and the traits controlling those responses can have different impacts depending on the drought scenario (Tardieu 2012). The upper layers of the soil usually dry faster than the deeper layers because of greater evaporation from, and higher root density in, the upper layers, which has a great impact on tree seedling performance during the establishment phase (Padilla and Pugnaire 2007, Liu et al. 2019). Similarly, applying different irrigation regimes to potted plants affects the soil moisture distribution (Turner 2019). Irrigation regimes that aim to maintain a stable suboptimal soil moisture, such as those applied in many phenotyping platforms and drought simulation experiments, can produce large soil moisture gradients between the upper and lower layers. These gradients can alter root-to-shoot hormonal signalling that has been implicated in regulating plant water use (Boyle et al. 2016, Pang et al. 2017, Puértolas et al. 2017).
Leaf water status declines with soil water content, which induces abscisic acid (ABA) accumulation and stomatal closure (Beardsell and Cohen 1975). In tree species, hydraulic features determined by wood anatomical traits (vessel size and density) play an important role in the regulation of water status and stomatal conductance (Cocozza et al., 2011). However, soil drying can induce stomatal closure and changes in xylem sap composition before changes in leaf water potential are recorded (Sobeih et al. 2004) and even when leaf turgor is maintained by pressurizing the root system (Gollan et al. 1986). However, there seems to be strong evidence for both chemical and hydraulic signalling and their coordination in regulating stomatal conductance under specific experimental conditions (Tardieu 2016), including observations that hormonal signals modify leaf and root hydraulic conductance (Pantin et al. 2013, Vandeleur et al. 2014). Thus, mechanisms of stomatal control are complex and might depend on species, plant developmental stage or intensity of water stress.
Soil moisture heterogeneity might have a profound influence on the nature of this long-distance signalling. Early studies with split pots demonstrated that drying only one soil compartment induced partial stomatal closure before any change in leaf water status was detected (Blackman and Davies 1985). Some studies suggested root-sourced xylem-borne abscisic acid (ABA) played a main role in this long-distance signalling mechanism (Zhang and Davies 1990, Tardieu et al. 1991), but experiments under heterogeneous soil moisture conditions often fail to link ABA increase and stomatal closure (Blackman and Davies 1985, Sobeih et al. 2004). In split pot experiments, root ABA accumulation in drying roots is diminished by water redistribution from the wet soil compartment, even more acutely when soil moisture gradients are vertical (Puértolas et al. 2013, 2015). Moreover, sustained soil drying of part of the root zone increasingly limits root ABA export in the xylem sap as the proportion of total water uptake coming from the drying roots diminishes (Dodd et al. 2008, Puértolas et al. 2015). Besides ABA, increased xylem sap pH (Wilkinson 1999), mineral ions like sulphate (Korovetska et al. 2014) and phytohormones like cytokinins, 1-aminocyclopropane-1-carboxylic acid (ACC)/ethylene (Davies et al. 2005) and jasmonic acid (JA) (de Ollas and Dodd 2016) have been identified as root-to-shoot signals involved in stomatal closure in response to drought, often by interacting with ABA. However, these factors have rarely been investigated under heterogeneous soil drying. This drought scenario occurs frequently in rainfed and irrigated plantations, especially during seedling establishment, and can be experimentally induced by manipulating irrigation frequency. The presence of wet layers has similar effects to those reported in split-root experiments, with much lower xylem ABA levels compared with plants subjected to homogeneous soil drying for the same overall soil water content (and similar to those observed in well-watered plants) (Boyle et al. 2016, Puértolas et al. 2017) while inducing partial stomatal closure compared with well-watered plants. Since frequent suboptimal irrigation causes soil moisture heterogeneity inducing partial stomatal closure without triggering massive ABA accumulation (Boyle et al. 2016, Puértolas et al. 2017), it may be especially suitable to investigate the occurrence of other antitranspirants (than ABA) in the xylem sap.
Water use in the plant is mainly regulated by stomatal aperture, but the modulation of the hydraulic resistances along the root-to-leaf pathway can also play an important role. Under heterogeneous soil drying, water uptake increases in wet compared with dry roots (Pérez-Pérez and Dodd 2015, Mackay et al. 2019), which can be explained by an increase of aquaporin expression in the wet roots (McLean et al. 2011). This increase might be related to shoot-sourced JA accumulation in wet roots (Luo et al. 2019). Since ABA, ethylene and salicylic acid (SA) are associated to the regulation of root hydraulic conductivity in response to soil drying (Aroca et al. 2012); hydraulic conductivity might be controlled by differential accumulation of hormones between wet and dry roots.
Black poplar is a fast-growing European native species, and its regeneration is generally limited by soil water before it develops deep roots that can reach the water table (Guilloy et al. 2011). As an isohydric species, its water use is regulated by the interaction of chemical and hydraulic signals (Tardieu and Simonneau 1998). This study aimed to determine the impact of vertical soil moisture gradients on root water potential; plant hydraulic conductivity; root accumulation and xylem sap concentration of phytohormones, as well as xylem sap chemical composition; and the effects of all these changes on stomatal conductance in black poplar. In particular, this study addresses whether:
(i) For the same overall root zone water content, soil moisture gradients alter hormone balance, causing differences in xylem sap hormone concentrations compared with homogeneously dry soil.
(ii) Other root-sourced hormones (than ABA) can explain partial stomatal closure in plants in heterogeneously dry soil.
(iii) Differential root phytohormone accumulation in dry and wet roots can regulate root hydraulic conductivity in plants subjected to heterogeneous soil drying.
Materials and methods
Plant cultivation and treatments
Plant cultivation and treatments followed the same procedure as in Puértolas et al. (2017). Forty-eight hardwood cuttings of Populus nigra (provenance River Ebro basin, Northern Spain) were rooted in perlite for 1 month and then individually transplanted to cylindrical pots (volume: 0.8 l; soil column height: 18 cm; diameter: 6.5 cm) designed to fit in a pressure chamber of the same volume and to allow extraction of roots and soil at different column depths at harvest. Pots were filled with a fertilized organic loam (John Innes no. 2, J. Westland, Dungannon, UK). Before the treatments were assigned, pots were watered to field capacity, left to drain overnight and weighed to obtain weight at field capacity (PWsat). Pots were weighed daily during the experiment and watered according to the pot weight, as plant growth weight was negligible compared with water weight in the pot (<5%). The experiment was set up in a walk-in controlled environment chamber under 400 μmol m−2 s−1 photosynthetically active radiation (PAR) (provided by halogen lamps, HQI-BT 400 W/D Osram GmbH, Munich, Germany), 24/16 °C day/night temperature and 14 h photoperiod.
After watering plants to PWsat daily for 2 weeks, plants were randomly divided into three treatments (16 plants per treatment): for WW, plants were watered daily to PWsat, and for infrequent deficit irrigation (IDI), pots were left to dry until they reached a weight threshold (PWsat—200 g) then rewatered to PWsat. In the first cycle, this threshold was determined to halve stomatal conductance (gs) compared with WW plants. Two or three drying cycles were applied before harvest; for frequent deficit irrigation (FDI), plants were left to dry as in IDI and then re-watered daily to maintain the threshold of (PWsat—200 g) set in IDI.
The different soil drying treatments lasted 4 weeks. Plants were measured over 6 days; six to seven plants were measured per day. Plants from WW and FDI were randomly chosen, while plants from IDI were selected according to pot weight to ensure that they were just below the (PWsat—200 g) threshold at the time of measurement. This ensured that IDI and FDI had comparable pot weight when measured.
Gas exchange, water status and sap extraction
One hour before measurements commenced, both ends of the pot of the selected plant were covered with duct tape to prevent evaporative water losses, and pot weight was recorded. One fully developed leaf from the upper third of the canopy was wrapped in aluminium foil and covered with a plastic bag to stop transpiration. Each pot was measured again 1 h later to calculate the plant water uptake rate. Plant transpiration rate was calculated as water uptake rate divided by leaf area. After the 1 h period, leaf water potential was measured in the covered leaf to measure xylem water potential (Ψxylem). Simultaneously, stomatal conductance (gs) and net photosynthesis (An) were measured in the closest full developed leaf with a portable gas analyser (LI-6400XT, LICOR, Lincoln, NE, USA) ([CO2] = 410 p.p.m.; T = 24 °C; relative humidity = 50%). Leaves were measured at higher radiation than ambient (PAR = 800 μmol m−2 s−1) to guarantee photosynthetic saturation. After the gas exchange measurements, the leaf was excised and immediately frozen in liquid nitrogen, stored at −20 °C and later freeze-dried for ABA determination ([ABAleaf]). Then the pots were weighed to calculate the average water uptake during the previous hour (WU). After weighing, the plant was de-topped, and shoot water potential (ψshoot) was measured with the pressure chamber. After reaching the balancing pressure, an overpressure of up to 0.5 MPa was applied to allow sap collection (~50 μl) for pH determination (pHsap). Next, bulk root water potential (ψroot) was measured by inserting the pot in the pressure chamber of the same volume. After measuring ψroot, pressure was increased at 0.04 MPa intervals. At each step, sap was collected for 20 s in an Eppendorf tube and weighed to calculate sap flow rate. Root hydraulic conductance (Kroot) was determined as the slope between the overpressure applied and sap flow rate. Pressure was increased until sap flow rate matched WU, to obtain a sap sample representative of in vivo transpiration (Else et al. 1994). At this moment, sap was collected (~550 μl), separated into two fractions of ~250 and ~300 μl, frozen in liquid nitrogen and stored at −80 °C for later ion and phytohormone determination, respectively.
Total leaf area for each plant (LA) was measured with a leaf area meter (Li-3100C, LICOR), and fresh shoot and leaf weight were recorded. Whole-plant transpiration rate (Tr) was calculated as WU/LA. Root-to-xylem, xylem-to-shoot and root-to-shoot hydraulic conductances were determined as Ktissue1-tissue2 = Tr/(Ψtissue1 − Ψtissue2), where tissue 1 and tissue 2 are the tissues between each conductance. For each plant, the three conductances were normalized by leaf area to calculate specific hydraulic conductances.
Root sampling and soil water content measurement
Pots were opened individually and soil was divided into two soil sections of equal size (upper and lower). Roots were extracted from each soil section, quickly washed and blotted, and a root sample for phytohormone determination was frozen with liquid nitrogen, stored at −80 °C until freeze-dried. The remaining roots were frozen and stored at −20 °C until root surface area determination. Roots were defrosted and scanned, and root surface area (RSA) was measured using the software WinRHIZO™ (Regents Instrument Inc., Quebec, Canada). After scanning, roots were oven-dried and weighed for specific root area (SRA) calculation. Root samples used for phytohormone analyses were freeze-dried and weighed. RSA of each sample was estimated by multiplying its dry weight by the SRA of the non-sampled roots in that layer. Total RSA in the layer was estimated by summing the RSA of the sampled and non-sampled roots and total RSA per plant (RSAT) as the sum of RSA of both layers. Specific root hydraulic conductance was calculated as Ksroot = Kroot/RSAT.
The soil in each section was weighed, oven-dried at 80 °C until constant weight was reached, and weighed again to calculate soil gravimetric water content. Whole-pot soil gravimetric water content (θg) was calculated as the average of soil moisture in both sections. A soil moisture release curve was constructed by dew point psychrometry to estimate ψsoil from θg. A small volume of saturated soil (0.21 cm3) was placed in each of the three sample holders and packed to match the average apparent density in pots. Soil and holder were weighed and inserted in a psychrometric chamber (C-52, Wescor Inc., Logan UT, USA). Water potential was determined after at least 6 h of equilibrium. To obtain subsequent points of the curve, samples were left to dry on a bench, re-weighed and inserted in the chamber again. This was repeated until the samples dried to 0.18 g g−1 (ψsoil = −1.2 MPa). At the end of the measurements, the sample and the holder were oven-dried to constant weight and holder, and dry weight of each sample was determined to calculate θg at each point. The whole dataset was fitted to an exponential decay curve (see Figure S1 available as Supplementary Data at Tree Physiology Online) with SigmaPlot 12 (Systat Software Inc., San Jose, CA, USA).
Detached leaf transpiration assay
Since both ABA and JA concentrations in the xylem sap were correlated to gs, the interactive effect of these two xylem-borne phytohormones on transpiration was investigated. The combination of three ABA and JA concentrations were assessed, representing the range observed in the experiment (0, 150, 1500 nM for ABA; 0, 300, 1200 nM for JA). Eighteen poplar plants were cultured as described above, and three leaves (seventh, eighth and ninth counting from the most the apical leaf, all fully developed) of each plant were used in the assay. Since ABA was expected to have a greater antitranspirant effect and leaves from a single plant were expected to be less variable than leaves from different plants, each leaf within a plant was assigned to a different JA concentration and a common ABA concentration for the three leaves (different ABA concentrations for each plant). Artificial xylem sap was prepared next (Dodd et al. 2003). The nine different phytohormone combinations were prepared by adding the appropriate JA and ABA amounts to 100 ml of artificial sap.
Leaves were excised from well-watered plants that were kept in the dark overnight; the petiole immersed in its assigned artificial sap and was re-cut (2–3 mm from the initial cut). While immersed, the petiole was tightly inserted in a closed 2 mL Eppendorf centrifuge tube through a narrow puncture in the lid to prevent evaporation. Tubes were randomly placed in a rack and left to equilibrate in darkness for 30 min and then transferred to a transpiration assay cabinet, at 26–27 °C, 40% air humidity and 500 μmol m−2 s−1 PAR provided with a halogen lamp. Tubes were weighed with a four-point analytical balance every 40 min during 4 h. After each weighing, tubes were replenished with artificial sap if necessary and re-weighed. At the end of the assay, leaf area was measured to calculate transpiration rates.
Phytohormone and ion analyses
Freeze-dried leaf and root samples were finely ground. Leaf samples from the soil drying experiment were incubated overnight in distilled water (1:50, w/w) at 4 °C in a shaker. ABA concentration in the aqueous extract was analysed by a radioimmunoassay (Quarrie et al. 1988). Additionally, the main classes of plant hormones, including three phytohormones related to growth (indoleacetic acid (IAA); the cytokinins trans-zeatin (tZ), zeatin riboside (ZR) and isopentenyl adenine (iP); and the gibberellins GA1, GA3 and GA4) and four phytohormones related to stress (the ethylene precursor ACC, ABA, SA and JA, were analysed in freeze-dried root and xylem sap according to the protocol described by Albacete et al. (2008) with some modifications.
Briefly, 50 mg of homogenized freeze-dried material was dropped in 1 ml of cold (−20 °C) extraction mixture of methanol/water (80/20, v/v). Solids were separated by centrifugation (20,000g, 15 min) and re-extracted for 30 min at 4 °C in an additional 1 ml of the same extraction solution. Pooled supernatants were passed through a Sep-Pak Plus †C18 cartridge (Sep-Pak Plus, Waters, Milford, MA USA) to remove interfering lipids and part of the plant pigments and evaporated at 40 °C under vacuum either to near dryness or until the organic solvent was removed. The residue was dissolved in 1 ml methanol/water (20/80, v/v) solution using an ultrasonic bath. The dissolved samples and the xylem sap were filtered through 13 mm diameter Millex filters with 0.22 μm pore size nylon membrane (Millipore, Bedford, MA, USA). Then 10 μl of filtrated extract were injected in a U-HPLC-MS system consisting of an Accela Series U-HPLC (Thermo Fisher Scientific, Waltham, MA, USA) coupled to an Exactive mass spectrometer (Thermo Fisher Scientific) using a heated electrospray ionization (HESI) interface. Mass spectra were obtained using the Xcalibur software version 2.2 (Thermo Fisher Scientific). For quantification of the plant hormones, calibration curves were constructed for each analysed component (1, 10, 50 and 100 μg l−1) and corrected for 10 μg l−1 deuterated internal standards. Recovery percentages ranged between 92 and 95%.
ACC and GA1 in xylem sap and all the gibberellins in the roots were below the detection range in a majority of the samples, so results of these hormones are not reported. ZR was below range in all sap and root samples.
Sulphate concentration in xylem sap was determined in using ion exchange chromatography (LOD = 5 μM; Dionex ICS2500, Thermo Fisher Scientific Inc.,).
Statistical analyses
To detect the effects of irrigation treatment on the different variables analysed, a one-way analysis of variance (ANOVA) was performed. Since hormone accumulation in upper and lower roots substantially differed for some hormones (like ACC or ABA), separate ANOVAs were performed for both column layers. Tukey’s tests were used to compare means. Results from the detached leaf transpiration assay were analysed by repeated measures analysis, with ABA concentration as the between-subject factor and JA as the within-subject factor. A bivariate correlation matrix for hormone concentration and physiological variables was also performed to assess the linear regression parameters (Pearson correlation coefficient and P-value) (see Table S1 available as Supplementary Data at Tree Physiology Online). All these analyses were performed with SPSS Statistics for Windows v. 24 (IBM Corp., Armonk, NY, USA). Linear regression was performed for ABA and JA relationships with SigmaPlot 12 (Systat Software Inc.).
Results
Soil and plant water status and hydraulic conductance
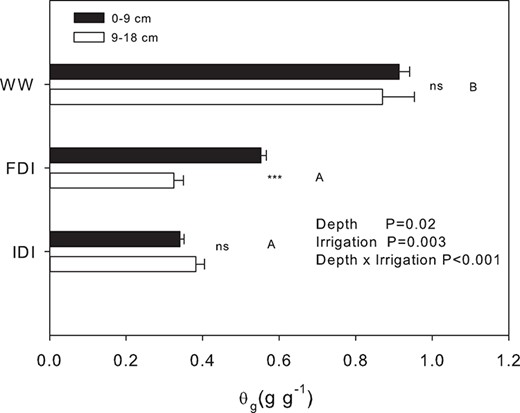
Whole-pot gravimetric soil water content (θg) was twofold higher in WW than FDI and IDI treatments. As intended, θg did not significantly differ between the two DI treatments, although it tended to be higher in FDI (Tukey, P = 0.18; Figure 1). For FDI plants, it was higher in the upper soil layer (0.55 g g−1; ψsoil = −0.02 MPa) than lower layer (0.32 g g−1; ψsoil = −0.18 MPa). Soil water content did not vary between upper and lower sections in WW and IDI treatments (Figure 1). Root distribution was similar across treatments with higher root area and specific root area in the lower layer, but treatment differences were not observed (P = 0.10, P = 0.12 for biomass and area, respectively; Table 1, biomass data not shown). Therefore, soil water content but not root distribution differed across treatments.
Gravimetric soil water content (θ) (mean ± SE) in the upper (grey bars) and bottom (black bars) 9 cm of the soil column for the three irrigation treatments. Different letters denote statistical differences between treatments (Tukey, P < 0.05). Asterisks denote statistical differences between depths within a treatment (P < 0.001). ns, non-significant differences (P > 0.05). P-values for depth, treatment and their interaction are reported following repeated measures ANOVA.
Net photosynthesis rate (An), stomatal conductance (gs), transpiration rate measured gravimetrically (Trg), leaf ABA concentration, root surface area (RSA) and specific root area (SRA) in 0–9 cm and 10–18 of the soil column for the three irrigation regimes (means ± SE; n = 16). Different letters within a row and measurement show statistical differences between irrigation regimes.
| . | WW . | FDI . | IDI . |
|---|---|---|---|
| An (μmol CO2 m−2 s−1) | 10.5 ± 1.4 a | 8.4 ± 1.7 a | 8.4 ± 1.7 a |
| gs (mol H2O m−2 s−1) | 0.29 ± 0.03 a | 0.23 ± 0.02 ab | 0.17 ± 0.04 b |
| Trg (mmol H2O m−2 s−1) | 3.12 ± 0.14 a | 3.00 ± 0.16 a | 1.99 ± 0.21 b |
| [ABA]leaf (ng g−1DW) | 1175 ± 63 b | 1258 ± 71 b | 1973 ± 180 a |
| RSA (0–9 cm) (cm2) | 46.0 ± 10.7 a | 24.2 ± 2.8 a | 38.6 ± 4.0 a |
| RSA (10–18 cm)(cm2) | 87.1 ± 20.6 a | 47.8 ± 7.3 a | 66.9 ± 11.0 a |
| SRA (0–9 cm) (cm2 mg−1) | 0.81 ± 0.15 a | 0.58 ± 0.08 a | 0.68 ± 0.10 a |
| SRA (9–18 cm) (cm2 mg−1) | 1.49 ± 0.25 a | 1.22 ± 0.07 | 1.30 ± 0.17 a |
| . | WW . | FDI . | IDI . |
|---|---|---|---|
| An (μmol CO2 m−2 s−1) | 10.5 ± 1.4 a | 8.4 ± 1.7 a | 8.4 ± 1.7 a |
| gs (mol H2O m−2 s−1) | 0.29 ± 0.03 a | 0.23 ± 0.02 ab | 0.17 ± 0.04 b |
| Trg (mmol H2O m−2 s−1) | 3.12 ± 0.14 a | 3.00 ± 0.16 a | 1.99 ± 0.21 b |
| [ABA]leaf (ng g−1DW) | 1175 ± 63 b | 1258 ± 71 b | 1973 ± 180 a |
| RSA (0–9 cm) (cm2) | 46.0 ± 10.7 a | 24.2 ± 2.8 a | 38.6 ± 4.0 a |
| RSA (10–18 cm)(cm2) | 87.1 ± 20.6 a | 47.8 ± 7.3 a | 66.9 ± 11.0 a |
| SRA (0–9 cm) (cm2 mg−1) | 0.81 ± 0.15 a | 0.58 ± 0.08 a | 0.68 ± 0.10 a |
| SRA (9–18 cm) (cm2 mg−1) | 1.49 ± 0.25 a | 1.22 ± 0.07 | 1.30 ± 0.17 a |
Net photosynthesis rate (An), stomatal conductance (gs), transpiration rate measured gravimetrically (Trg), leaf ABA concentration, root surface area (RSA) and specific root area (SRA) in 0–9 cm and 10–18 of the soil column for the three irrigation regimes (means ± SE; n = 16). Different letters within a row and measurement show statistical differences between irrigation regimes.
| . | WW . | FDI . | IDI . |
|---|---|---|---|
| An (μmol CO2 m−2 s−1) | 10.5 ± 1.4 a | 8.4 ± 1.7 a | 8.4 ± 1.7 a |
| gs (mol H2O m−2 s−1) | 0.29 ± 0.03 a | 0.23 ± 0.02 ab | 0.17 ± 0.04 b |
| Trg (mmol H2O m−2 s−1) | 3.12 ± 0.14 a | 3.00 ± 0.16 a | 1.99 ± 0.21 b |
| [ABA]leaf (ng g−1DW) | 1175 ± 63 b | 1258 ± 71 b | 1973 ± 180 a |
| RSA (0–9 cm) (cm2) | 46.0 ± 10.7 a | 24.2 ± 2.8 a | 38.6 ± 4.0 a |
| RSA (10–18 cm)(cm2) | 87.1 ± 20.6 a | 47.8 ± 7.3 a | 66.9 ± 11.0 a |
| SRA (0–9 cm) (cm2 mg−1) | 0.81 ± 0.15 a | 0.58 ± 0.08 a | 0.68 ± 0.10 a |
| SRA (9–18 cm) (cm2 mg−1) | 1.49 ± 0.25 a | 1.22 ± 0.07 | 1.30 ± 0.17 a |
| . | WW . | FDI . | IDI . |
|---|---|---|---|
| An (μmol CO2 m−2 s−1) | 10.5 ± 1.4 a | 8.4 ± 1.7 a | 8.4 ± 1.7 a |
| gs (mol H2O m−2 s−1) | 0.29 ± 0.03 a | 0.23 ± 0.02 ab | 0.17 ± 0.04 b |
| Trg (mmol H2O m−2 s−1) | 3.12 ± 0.14 a | 3.00 ± 0.16 a | 1.99 ± 0.21 b |
| [ABA]leaf (ng g−1DW) | 1175 ± 63 b | 1258 ± 71 b | 1973 ± 180 a |
| RSA (0–9 cm) (cm2) | 46.0 ± 10.7 a | 24.2 ± 2.8 a | 38.6 ± 4.0 a |
| RSA (10–18 cm)(cm2) | 87.1 ± 20.6 a | 47.8 ± 7.3 a | 66.9 ± 11.0 a |
| SRA (0–9 cm) (cm2 mg−1) | 0.81 ± 0.15 a | 0.58 ± 0.08 a | 0.68 ± 0.10 a |
| SRA (9–18 cm) (cm2 mg−1) | 1.49 ± 0.25 a | 1.22 ± 0.07 | 1.30 ± 0.17 a |
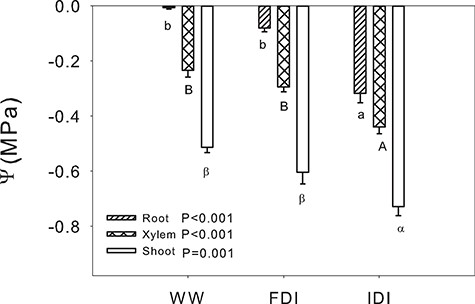
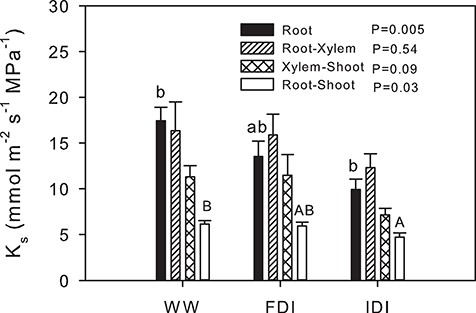
The WW and FDI treatments had equivalent root (ψroot), xylem (ψxylem) and shoot (ψshoot) water potentials, but these were significantly lower in IDI plants (Figure 2). Root-to-shoot Ks in IDI was decreased by 23% compared with WW (Figure 3). Specific root hydraulic conductance measured by the root pressurization method followed a similar pattern as root-to-shoot Ks. It decreased by 43% in IDI compared with WW, and FDI did not significantly differ from the others. Thus, FDI plants had intermediate water relations between IDI and WW plants.
Water potential (Ψ) in different parts of the plant (mean ± SE) for the three irrigation treatments. Striped pattern, root Ψ; cross pattern, xylem Ψ; and white bars, shoot Ψ. P-values for each part of the plant are reported following simple ANOVA. For statistically significant analyses, letters denote statistical differences between treatments within a part of the plant (Tukey, P < 0.05); lowercase letters, root; uppercase letters, xylem; and Greek letters, shoot.
Specific (related to leaf area) hydraulic conductance (Ks) in different parts of the plant. Black, root Ks; striped pattern, root-to-xylem Ks; cross pattern, xylem-to-shoot Ks; and white, root-to-shoot Ks. Letters denote statistical differences between treatments within a part of the plant (Tukey, P < 0.05). Values for each part of the plant are reported following simple ANOVA. For statistically significant analyses, letters denote statistical differences between treatments within a part of the plant (Tukey, P < 0.05); lowercase letters, root; and uppercase letters, root-shoot.
Plant water use and leaf ABA concentration
Leaf stomatal conductance (gs) in FDI and IDI was decreased by 21% (not significantly) and 41%, respectively, compared with WW plants, even though the two deficit irrigation treatments had statistically similar gs (Table 1). However, transpiration rate measured gravimetrically in FDI plants was similar to WW plants and significantly higher than IDI plants, which had two-thirds of their transpiration rate. Foliar ABA concentration [ABA]leaf was significantly higher in IDI plants than in WW and FDI, which showed similar values (Table 1). Again, water use and foliar ABA concentration were similar in WW and FDI plants.
Xylem sap composition and its relation with stomatal conductance
Sap pH was significantly lower in WW plants than in IDI, with FDI presenting intermediate values, with treatments varying by <0.3 pH units (Table 2). ABA was the only phytohormone showing treatment differences in xylem sap concentrations (Table 2). Root xylem ABA concentration ([ABA]root xylem) was significantly higher in IDI plants than WW and FDI plants. Sulphate concentration in root xylem sap ([SO4−]root xylem) was significantly lower in FDI compared with the other treatments (Table 2). Thus, irrigation frequency altered xylem sap composition, at the same soil water content.
Root xylem sap pH and concentration (means ± SE; n = 8) of different hormones (ABA = abscisic acid; tZ = trans-zeatin; iP = isopentenyl adenine; JA = jasmonic acid; GA3, GA4 = gibberellins; IAA = indoleacetic acid; SA = salicylic acid) and the anion SO4. Phytohormone concentration is expressed in nM and SO4 in μM. Different letters within a row and measurement show statistical differences between irrigation regimes.
| . | WW . | FDI . | IDI . |
|---|---|---|---|
| pH | 6.20 ± 0.04 b | 6.34 ± 0.06 ab | 6.44 ± 0.07 a |
| ABA | 15.3 ± 1.5 a | 26.5 ± 7.2 a | 295.1 ± 100.5 b |
| tZ | 123.7 ± 24.5 a | 96.7 ± 15.0 a | 149.6 ± 13.2 a |
| iP | 0.26 ± 0.04 a | 0.23 ± 0.05 a | 0.26 ± 0.03 a |
| JA | 224.3 ± 66.6 a | 299.6 ± 50.2 a | 276.0 ± 51.6 a |
| GA3 | 0.31 ± 0.10 a | 0.15 ± 0.08 a | 0.11 ± 0.04 a |
| GA4 | 0.02 ± 0.00 a | 0.10 ± 0.05 a | 0.08 ± 0.04 a |
| IAA | 2.78 ± 0.33 a | 2.38 ± 0.14 a | 2.87 ± 0.36 a |
| SA | 139.9 ± 29.6 a | 108.1 ± 15.5 a | 98.8 ± 16.6 a |
| SO4 | 446 ± 56 b | 303 ± 2.4 a | 462 ± 4.7 b |
| . | WW . | FDI . | IDI . |
|---|---|---|---|
| pH | 6.20 ± 0.04 b | 6.34 ± 0.06 ab | 6.44 ± 0.07 a |
| ABA | 15.3 ± 1.5 a | 26.5 ± 7.2 a | 295.1 ± 100.5 b |
| tZ | 123.7 ± 24.5 a | 96.7 ± 15.0 a | 149.6 ± 13.2 a |
| iP | 0.26 ± 0.04 a | 0.23 ± 0.05 a | 0.26 ± 0.03 a |
| JA | 224.3 ± 66.6 a | 299.6 ± 50.2 a | 276.0 ± 51.6 a |
| GA3 | 0.31 ± 0.10 a | 0.15 ± 0.08 a | 0.11 ± 0.04 a |
| GA4 | 0.02 ± 0.00 a | 0.10 ± 0.05 a | 0.08 ± 0.04 a |
| IAA | 2.78 ± 0.33 a | 2.38 ± 0.14 a | 2.87 ± 0.36 a |
| SA | 139.9 ± 29.6 a | 108.1 ± 15.5 a | 98.8 ± 16.6 a |
| SO4 | 446 ± 56 b | 303 ± 2.4 a | 462 ± 4.7 b |
Root xylem sap pH and concentration (means ± SE; n = 8) of different hormones (ABA = abscisic acid; tZ = trans-zeatin; iP = isopentenyl adenine; JA = jasmonic acid; GA3, GA4 = gibberellins; IAA = indoleacetic acid; SA = salicylic acid) and the anion SO4. Phytohormone concentration is expressed in nM and SO4 in μM. Different letters within a row and measurement show statistical differences between irrigation regimes.
| . | WW . | FDI . | IDI . |
|---|---|---|---|
| pH | 6.20 ± 0.04 b | 6.34 ± 0.06 ab | 6.44 ± 0.07 a |
| ABA | 15.3 ± 1.5 a | 26.5 ± 7.2 a | 295.1 ± 100.5 b |
| tZ | 123.7 ± 24.5 a | 96.7 ± 15.0 a | 149.6 ± 13.2 a |
| iP | 0.26 ± 0.04 a | 0.23 ± 0.05 a | 0.26 ± 0.03 a |
| JA | 224.3 ± 66.6 a | 299.6 ± 50.2 a | 276.0 ± 51.6 a |
| GA3 | 0.31 ± 0.10 a | 0.15 ± 0.08 a | 0.11 ± 0.04 a |
| GA4 | 0.02 ± 0.00 a | 0.10 ± 0.05 a | 0.08 ± 0.04 a |
| IAA | 2.78 ± 0.33 a | 2.38 ± 0.14 a | 2.87 ± 0.36 a |
| SA | 139.9 ± 29.6 a | 108.1 ± 15.5 a | 98.8 ± 16.6 a |
| SO4 | 446 ± 56 b | 303 ± 2.4 a | 462 ± 4.7 b |
| . | WW . | FDI . | IDI . |
|---|---|---|---|
| pH | 6.20 ± 0.04 b | 6.34 ± 0.06 ab | 6.44 ± 0.07 a |
| ABA | 15.3 ± 1.5 a | 26.5 ± 7.2 a | 295.1 ± 100.5 b |
| tZ | 123.7 ± 24.5 a | 96.7 ± 15.0 a | 149.6 ± 13.2 a |
| iP | 0.26 ± 0.04 a | 0.23 ± 0.05 a | 0.26 ± 0.03 a |
| JA | 224.3 ± 66.6 a | 299.6 ± 50.2 a | 276.0 ± 51.6 a |
| GA3 | 0.31 ± 0.10 a | 0.15 ± 0.08 a | 0.11 ± 0.04 a |
| GA4 | 0.02 ± 0.00 a | 0.10 ± 0.05 a | 0.08 ± 0.04 a |
| IAA | 2.78 ± 0.33 a | 2.38 ± 0.14 a | 2.87 ± 0.36 a |
| SA | 139.9 ± 29.6 a | 108.1 ± 15.5 a | 98.8 ± 16.6 a |
| SO4 | 446 ± 56 b | 303 ± 2.4 a | 462 ± 4.7 b |
Root xylem ABA was negatively correlated with gs for IDI plants, while it remained low in WW and FDI plants irrespective of gs (Figure 4A). Interestingly, JA concentration [JA]root xylem was strongly negatively correlated with gs in WW and FDI plants, but not in IDI (Figure 4B). Sap pH and IAA were weakly but significantly correlated with gs (see Table S1 available as Supplementary Data at Tree Physiology Online). Sap flow rate was not related to either [ABA]root xylem or [JA]root xylem (data not shown). Thus, both ABA and JA were negatively correlated with stomatal conductance, but in different irrigation treatments.
Relationship between stomatal conductance (gs) and (A) abscisic acid [ABA]root xylem and (B) jasmonic acid [JA]root xylem concentration in root xylem sap in the three irrigation treatments (WW = black triangles; FDI = black circles; IDI = white circles). Each point represents an individual plant. Significant (P < 0.05) regression lines and correlation coefficients for pooled WW and FDI (solid line) and IDI (dashed line) are depicted.
Detached leaf transpiration assay
The highest ABA and JA concentrations (1.5 and 1.2 μM, respectively) decreased transpiration rates compared with the other concentrations and the control. However, ABA had a much larger effect than JA (Figure 5), as transpiration rate decreased by 38 and 11%, respectively, compared with the other treatments. No interaction between JA and ABA was observed. Only high xylem ABA concentrations had an effect on transpiration commensurate with the effect of soil drying.
Detached leaf transpiration rate after supplying artificial sap comprising combinations of the three jasmonic acid [JA] and abscisic acid [ABA] sap concentrations via the transpiration stream (white, [ABA] = 0 nM; striped pattern, [ABA] = 150 nM; crossed pattern: [ABA] = 1500 nM). In the insert located in the upper right corner, pooled values for each [JA] across the three [ABA]. P-values for each hormone and their interaction are reported in the main panel following repeated measures ANOVA. Letters denote statistical differences between [JA] (Tukey, P < 0.05).
Root phytohormone concentrations
In general, root hormone concentration was not correlated with local soil moisture. Only tZ was weakly negatively correlated with local θg (P = 0.04, r2 = 0.19 for the root tZ vs soil water content relation in the upper layer; P = 0.03, r2 = 0.21 in the lower layer), while root ABA was correlated with θg but only in the lower layer (P = 0.02, r2 = 0.27). In WW plants, root hormone concentrations were 68, 75 and 32% less in the upper compared with the lower roots for ACC, ABA and SA, respectively (Figure 6). Root ABA concentration differences between lower and upper layers were highest in FDI, with lower roots with [ABA]root as high as in IDI and upper roots as low as in WW (Figure 6A). Trans-zeatin (tZ) was higher in FDI than in the other treatments (Figure 6B). However, lower roots had 29% less tZ than in upper roots for IDI, while they had 16% more for FDI. ACC in the lower layer did not differ between treatments, but in the upper layer, ACC was more than double in FDI than in well-watered plants (Figure 6C). Of these different phytohormones, only root ABA concentration in the lower layers was highly significantly correlated with root xylem concentration (P = 0.001, r2 = 0.43), but this relationship was weaker (P = 0.01, r2 = 0.27) when considering the upper roots (see Table S1 available as Supplementary Data at Tree Physiology Online). Salicylic acid in the upper roots was significantly correlated with SA in the xylem sap but weakly (P = 0.02, r2 = 0.20). Thus, the different irrigation treatments altered the vertical profile of root hormone concentrations according to soil moisture gradients, which sometimes was correlated with changes in root hormone export in the xylem sap.
Abscisic acid [ABA]root (A), trans-zeatin [tZ]root (B), 1-aminocyclopropane-1-carboxylic acid [ACC]root (C) and salicylic acid [SA]root concentrations in the upper (black bars) and lower (white bars) 9 cm of the soil column. Letters denote statistical differences between irrigation treatments within a layer (Tukey, P < 0.05; black, upper layer; white, lower layer) and asterisks denote differences between soil depths (*0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001).
Discussion
Contrary to observations of ABA-induced stomatal closure with homogenous soil drying (Tardieu and Simonneau 1998, Castro et al. 2019), xylem ABA concentration and gs did not correlate in FDI and WW plants (Figure 4A), partially because FDI plants had low ABA concentrations despite similar soil water content to IDI plants. Although xylem-borne ABA has a strong antitranspirant effect (Figure 5), other phytohormones might explain stomatal regulation in heterogeneously dry soil, as ABA transport from dry roots becomes increasingly limited in the absence of any change in leaf water potential (Puértolas et al. 2015). In contrast to ABA, xylem JA concentration was inversely correlated with gs within WW and FDI treatments (Figure 4B). However, neither soil drying treatment (either FDI or IDI) increased JA accumulation in roots or xylem sap compared to WW plants in poplar, in contrast with other studies in tomato (de Ollas et al. 2018) and Eucalyptus (Correia et al. 2014). However, these two studies probably applied more severe water deficits, as leaf water potential reached −0.9 MPa in tomato and soil water content dropped to 18% field capacity in Eucalyptus. Nevertheless, root JA accumulation in response to soil drying can be transient in contrast with the sustained accumulation of ABA (de Ollas et al. 2013).
Xylem-borne JA decreased detached leaf transpiration in poplar (Figure 5), confirming the antitranspirant capacity of JA observed in tomato (de Ollas et al. 2018). However, unlike in tomato, in poplar the effect of the exogenous JA applied via xylem sap was only discerned at a very high concentration (1.2 μM), almost twice the maximum value observed in xylem sap of intact plants (550 nM) (Figure 4). In the absence of root JA accumulation, the slower sap flow rate of plants with lower gs might increase xylem JA concentration, with sustained xylem loading of JA into a slower moving transpiration stream explaining the inverse correlation between xylem JA and gs. Furthermore, other xylem-borne chemicals associated to JA metabolism such as JA-isoleucine or other related oxylipins (Savchenko et al. 2014), and not JA directly, might be responsible for the observed strong correlation between [JA]root xylem and gs in well-watered and FDI intact plants, as both jasmonates tend to increase concomitantly (Andrade et al. 2017).
The deficit irrigation treatments caused (limited) sap alkalisation consistent with other studies, which was inversely correlated with gs (Tables 1 and 2, and see Table S1 available as Supplementary Data at Tree Physiology Online). Sap alkalization increases apoplastic ABA concentration, triggering stomatal closure (Wilkinson 1999). Drought can increase apoplastic pH (Thomas and Eamus 2002), but this effect is not conserved across species (Sharp and Davies 2009). However, small changes in pH, such as those observed in our study (0.2–0.3 units) do not always result in stomatal closure (Else et al. 2006).
Drought-induced increases in xylem sulphate concentration have been suggested as an early drought stress signal that induces stomatal closure (Ernst et al. 2010, Korovetska et al. 2014, Malcheska et al. 2017). Compared with WW plants, homogenous soil drying (IDI plants) did not increase xylem sulphate concentrations (Table 1), but, in contrast, moisture heterogeneity (FDI plants) decreased sulphate concentrations compared with WW plants. Drought elicits long-distance sulphate signalling by altering sulphate transporter expression in the roots, upregulating transporters in the xylem parenchyma that load sulphate into the xylem and downregulating those that transport sulphate in the opposite direction (Malcheska et al. 2017). Therefore, well-watered upper roots in FDI plants, which presumably contribute most to the transpiration stream, might have decreased sulphate uptake compared with roots in IDI. However, this cannot explain the higher sulphate concentration in WW plants, which suggests that the roots in the lower part of the column might have a more active role in sulphate uptake (either because of a higher proportion of roots in that layer or enhanced uptake capacity of younger roots). While sulphate can act as an early signal of drought stress in poplar (Malcheska et al. 2017), it does not seem to explain stomatal responses to heterogeneous soil drying.
Soil drying did not cause root accumulation of most of the measured hormones (Figure 5). Apart from ABA, which accumulated in roots in the dry compartment in split-root plants (Khalil and Grace 1993, Puértolas et al. 2017), only tZ and ACC accumulation in droughted plants was higher than in the control. Interestingly, the tZ accumulation pattern across the root zone was inverted in FDI compared with IDI plants, as root tZ was significantly higher in the upper IDI roots, while the opposite was observed in FDI. Since cytokinin accumulation inhibits root growth (Laplaze et al. 2007, Nehnevajova et al. 2019), the observed tZ patterns might regulate root growth profiles under different soil drying patterns. Thus, lower root tZ concentrations in the lower soil layer of IDI plants could promote root growth to facilitate continued water extraction, while higher root tZ concentrations in the lower roots of FDI plants might restrict root growth (where water uptake is limited).
Root accumulation of ABA, SA and ACC was consistently lower in the upper part of the column across different treatments, perhaps due to higher suberisation of the upper older roots (lower specific root density, Table 1), which might dilute hormone content due to a greater proportion of dry matter. However, the large difference in some hormones, especially ACC, seems to indicate an upregulation of ACC synthesis in lower more active roots. ACC accumulates in response to oxygen depletion in flooded plants (Banga et al. 1996), and it has been suggested that lower roots in potted plants might experience anoxic conditions even in horticultural substrates (Passioura 2006). However, the higher ACC in roots at the bottom half in the drought treatments is unlikely to be attributed to lower oxygen levels as soil water content is rather low. These strong vertical gradients of ACC accumulation in the root zone may affect root-to-shoot ACC signalling, which seems to regulate shoot ethylene evolution (Else and Jackson 1998).
Treatment differences in root and sap hormones and stomatal conductance and transpiration are consistent with the hypothesis of an ABA-based chemical signal regulating plant water use, but plant water status measurements also support the existence of hydraulic signals. Plants grown with IDI had a lower water potential throughout the plant, in parallel with reduced gs and transpiration, whereas FDI and WW did not differ significantly in plant water status and water use. That FDI did not decrease transpiration, in contrast to previous results with Pelargonium (Boyle et al. 2016) and Populus (Puértolas et al. 2017), may be due to higher soil water availability (Ψroot of −0.1 MPa in the current study compared with −0.3 MPa in Puértolas et al. 2017). Regardless, differences in water status and chemical (sap and root ABA) responses between heterogeneous (FDI) and homogeneous (IDI) soil drying at the same overall water content were consistent in both studies with the same poplar genotype. Water potential gradients, hydraulic conductivity and water flow along the root-to-shoot pathway were remarkably similar in FDI and WW plants (Figures 2 and 3), despite large differences in average soil water content (resulting in 0.1 MPa difference in Ψsoil). This indicates that wet soil layers in otherwise dry soil in FDI plants can attenuate root-to-shoot chemical but also hydraulic signals. This necessarily must be achieved by enhanced root hydraulic conductivity and aquaporin expression in the roots in wet soil (McLean et al. 2011), which would keep overall root conductivity similar to well-watered plants.
The mechanisms enhancing root hydraulic conductance in wet soil of FDI plants are more difficult to elucidate. ABA can upregulate aquaporin expression (Parent et al. 2009, Veselov et al. 2018), but the upper roots show marginal differences in root ABA concentration between WW and FDI plants (Figure 4A). Instead, greater ACC accumulation could enhance root hydraulic conductivity of roots in the upper layer of FDI plants. Ethylene can increase root hydraulic conductivity in tree species like Populus (Kamaluddin and Zwiazek 2002) and Larix (Islam et al. 2003) but has the opposite effect in herbaceous crops like Medicago (Li et al. 2009) and tomato (Calvo-Polanco et al. 2017). These contradictory results suggest that root ACC/ethylene synthesis might affect aquaporin expression differently depending on the species. Further research needs to examine whether there is a cause–effect relationship between soil moisture heterogeneity and ethylene-dependent regulation of root water uptake across different species, which could be part of a more complex mechanism regulating root function.
The differential patterns of hormone concentrations in sap and roots in response to contrasting soil moisture distribution in the root zone induced by the different irrigation treatments indicate that plant physiological responses can be profoundly altered by the irrigation timing (Boyle et al. 2016). However, further research should investigate the dynamic responses of these hormonal and physiological responses at different time points during the drying cycles. Thus, complete re-watering of homogenously dry IDI plants could have a different effect than in FDI, where re-watering only enhances soil moisture in the upper layers. Re-watering elicits a series of hormonal and water use regulation responses (Dodd et al. 2015), and its effects in previous cycles could explain the observed responses when the plants were measured.
In conclusion, the strongest phytohormonal response to the moderate soil drying applied to poplar was the increased root and xylem sap abscisic acid levels, associated with decreased root, xylem and leaf water potential. However, moist soil layers in otherwise dry soil (FDI treatment) greatly attenuated this response. Instead, higher ACC accumulation in the upper wet layers in FDI could explain the likely increase in hydraulic conductivity in that part of the root zone necessary to maintain whole root hydraulic conductivity in this treatment. Soil drying caused cytokinin (tZ) accumulation, with opposite responses (higher levels of tZ in upper roots under IDI and higher levels of tZ in lower roots under in FDI) that might regulate possible treatment differences in root growth patterns. Future research should assess the precise role of these two hormones (ACC, tZ) to better understand how wet soil patches in otherwise dry soil regulate physiological functions.
Acknowledgments
The research reported here was conducted in WATBIO (development of improved perennial non-food biomass and bioproduct crops for water stressed environments), which is a collaborative research project funded from the European Union’s Seventh Programme for research, technological development and demonstration under grant agreement no. 311929. The collaboration of Dr Pardos was supported by a Salvador Madariaga scholarship from the Spanish Ministry of Education, Culture and Sports (PR2015-00212). We are very grateful to Ms María del Puerto Sánchez-Iglesias for her technical support on hormonal analysis. We also thank Prof. Gail Taylor and Dr Hazel Smith for providing the poplar cuttings.
Conflict of interest
None declared.
Authors’ contributions
J.P. co-designed and executed the experiment, performed data analyses and led the writing of the manuscript. M.P. co-designed and executed the experiment, performed data analyses and contributed to writing. C.d.O. contributed to the design of the detached leaf transpiration assay and the discussion on the effects of jasmonic acid. A.A. performed the multi-phytohormone determination and helped to discuss its results. I.C.D. contributed to writing.


![Relationship between stomatal conductance (gs) and (A) abscisic acid [ABA]root xylem and (B) jasmonic acid [JA]root xylem concentration in root xylem sap in the three irrigation treatments (WW = black triangles; FDI = black circles; IDI = white circles). Each point represents an individual plant. Significant (P < 0.05) regression lines and correlation coefficients for pooled WW and FDI (solid line) and IDI (dashed line) are depicted.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/treephys/40/6/10.1093_treephys_tpaa037/2/m_tpaa037f4.jpeg?Expires=1716459066&Signature=Jbim6f6ouFcOmrMBHeuK49yjsYHS7c1pm6ywWgydINFxuoX7mOyhlFkOKfC1ygxi3Y9Qq8znqFvUh5ZABlZoaTFqPYtiFHBoWE8IGd9vWfkXU2zBdUdb0GoaAgFWIJMGeF53ndQWNIykc7mWVa4k0j6v~5zJQcwDXovX0ZCB~1pNYpOg6Sskh-PMigkiT9KEpabof1JTaCRmtpS0a02CuPOj95N5ZCkeLSFUjyQnaYo4FWvx-zvgbHuN1Pnggsfu1f-C7pB1W8OXFw5crcCXZj78bsJWgIE-RMYHC3JLqiRYAV~brD18C36vgjrOOhLwThOJjptpW4a6uZ5bFXeoRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Detached leaf transpiration rate after supplying artificial sap comprising combinations of the three jasmonic acid [JA] and abscisic acid [ABA] sap concentrations via the transpiration stream (white, [ABA] = 0 nM; striped pattern, [ABA] = 150 nM; crossed pattern: [ABA] = 1500 nM). In the insert located in the upper right corner, pooled values for each [JA] across the three [ABA]. P-values for each hormone and their interaction are reported in the main panel following repeated measures ANOVA. Letters denote statistical differences between [JA] (Tukey, P < 0.05).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/treephys/40/6/10.1093_treephys_tpaa037/2/m_tpaa037f5.jpeg?Expires=1716459066&Signature=djWnOvBFn9f-h7haEg~JgNc6llmUi5CEtStTbEdWxkYocvw9GsU9iz2qnrlB1wWqTRT~btpQDGLzhq4lSK92pNpirY7FOhL-x9dqs4VtIhEGsg~LhPzKR-soelizGgz4bP~ky5Qso6W-ORM8CFbTJzXngT~kj5LmAs8w8fepnjsjBx9YYpB5GX8NU9YVLqcGnAbkhVE8GaTDIURmlQHTcOGAUFXZkHjZLMWXSTJSu0-87AZKU9lBb-Sqr-x9rVr6ePqkojJn56J8wiwj0ysC9l4H9BqoJpGG19KYqrt9Hlbh6I0dR1KXCy2RazfAj7UuQVHB3QvPafi3aZjs8-uB9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Abscisic acid [ABA]root (A), trans-zeatin [tZ]root (B), 1-aminocyclopropane-1-carboxylic acid [ACC]root (C) and salicylic acid [SA]root concentrations in the upper (black bars) and lower (white bars) 9 cm of the soil column. Letters denote statistical differences between irrigation treatments within a layer (Tukey, P < 0.05; black, upper layer; white, lower layer) and asterisks denote differences between soil depths (*0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/treephys/40/6/10.1093_treephys_tpaa037/2/m_tpaa037f6.jpeg?Expires=1716459066&Signature=XfiGo6EJ7VAgtkd1iDLXyNtDbrGyTrJEWWI2eXzLqEUcgUyaMxiSzZHRKog~3RdPFsf09SBIIuq-zsLETCTtPiIi8ZO7LIdZppo6nGAf0ejToqWVC5yrjekhBfNRLgIoohwbXtFRB1BOpIY0B1gcIgc0dCQJNpGcFxnuck0odv5sX1No8bLYAIcrwmei7CXMsCN9dOzSfw~fPm-IMp7Rag6zUQxGJ6BmhZ4EHdSnJGbj~Kf2-CS5FtgkN2tW1KrDctwq52B~lFoFbw0qr78a-njaRaozbclNOPPXnVI3OOv3ZR9DllFS1fsB7zSO7xR0ztNXE2QpVuMjhZrrNGCfTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
