-
PDF
- Split View
-
Views
-
Cite
Cite
Willian M Ohara, Murilo Pastana, Priscila Camelier, The monophyly of Crenuchinae and description of two new species of Poecilocharax (Teleostei: Crenuchidae) based on phenotypic and genotypic evidence, Zoological Journal of the Linnean Society, Volume 197, Issue 2, February 2023, Pages 442–473, https://doi.org/10.1093/zoolinnean/zlac026
Close - Share Icon Share
Abstract
Crenuchinae is a subfamily of the fish family Crenuchidae distributed in the Amazon Basin with pronounced sexual dimorphism and exuberant colour patterns. Recent fieldwork in the tributaries of the Rio Aripuanã drainage, a large tributary of the Rio Madeira (Amazon Basin), resulted in the discovery of two distinctive, undescribed species of the crenuchin genus Poecilocharax, which are formally described herein, combining morphological and molecular data. These are the first representatives of Crenuchinae discovered after a gap of 57 years and the first records of Poecilocharax from the tributaries of the right bank of the Rio Amazonas draining the Brazilian crystalline shield. Based on a taxonomic review including all species of the subfamily, we provide an expanded morphological diagnosis for Crenuchinae. This now includes characteristics related to the lateral-line canals of head and body, the number of dorsal-fin rays and sexually dimorphic traits. In addition, we review previous characteristics used to diagnose Crenuchus and Poecilocharax, providing comments on their polarity and distribution across the subfamily. A dichotomous key is provided for the first time for species of Crenuchinae.
INTRODUCTION
Crenuchidae is a family of Neotropical fishes with 112 valid species (Fricke et al., 2021a) inhabiting most freshwater drainages from eastern Panama to Argentina (Buckup, 1998, 2003). All crenuchid genera can be found in the Rio Amazonas and Orinoco basins: AmmocryptocharaxWeitzman & Kanazawa, 1976 (four species), Characidium Reinhardt, 1867 (82 species), CrenuchusGünther, 1863 (one species), Elachocharax Myers, 1927 (four species), KlausewitziaGéry, 1965 (one species), Leptocharacidium Buckup, 1993 (one species), Melanocharacidium Buckup, 1993 (nine species), Microcharacidium Buckup, 1993 (five species), Odontocharacidium Buckup, 1993 (two species), PoecilocharaxEigenmann, 1909 (two species) and SkiotocharaxPresswell et al., 2000 (one species). From these, almost two-thirds of the crenuchids are allocated in Characidium, and over 60% of the known diversity of Characidium is found outside the Rio Amazonas and Orinoco basins (Buckup, 1993a, 2003; Fricke et al., 2021b). Crenuchid fish are usually small-sized, rarely surpassing more than 8 cm of standard length and include several miniature species (Weitzman & Vari, 1988; Buckup, 1993b; Toledo-Piza et al., 2014). These fish occur in an array of freshwater environments, from small streams to large rivers, lakes or floodplains, but with most taxa preferring fast-flowing small streams, where they hover around pebbles, rocks and aquatic vegetation (Buckup, 2003).
Phylogenetic hypotheses based on both morphological and molecular data are discordant regarding the positioning of Crenuchidae in Characiformes (e.g. Buckup, 1993a, 1998; Ortí & Meyer, 1997; Calcagnotto et al., 2005; Mirande, 2009; Oliveira et al., 2011; Arcila et al., 2017; Betancur-R et al., 2019). Nevertheless, after Buckup’s (1998) revisionary study, the monophyly of the family has never been questioned and Crenuchidae are still diagnosed by six synapomorphies. The family also has a stable composition and internal classification (see: Buckup, 1993b,c, 1998, 2003; Betancur-R et al., 2019), with crenuchid species allocated in two subfamilies: Characidiinae, which harbour most of the richness of the family, with seven genera and 109 valid species, and Crenuchinae currently known from two genera and three species (Fricke et al., 2021a).
Crenuchinae are composed of Crenuchus spilurusGünther, 1863 (type species of the family Crenuchidae), Poecilocharax bovaliorumEigenmann, 1909 and Poecilocharax weitzmaniGéry, 1965. Poecilocharax is currently distinguished from Crenuchus based on four morphological characteristics: (1) absence of an adipose fin; (2) maxillar short; (3) presence of maxillary teeth; and (4) absence of an asymmetrical caudal-peduncle spot (Eigenmann, 1909; Géry, 1965; Buckup, 1998). In the aquarium trade, C. spilurus is known as ‘sailfin tetra’ and the species of Poecilocharax, in special P. weitzmani, as ‘black morpho tetra’, ‘colibri tetra’ or ‘black darter tetra’ (Froese & Pauly, 2000). Both genera inhabit either black- or clear-water rivers of the Amazon Basin, occurring preferentially in slow-flowing body waters or marginal lakes. Whereas C. spilurus is abundant and broadly distributed in the Amazon drainage (Pires et al., 2016), Poecilocharax weitzmani is restricted to the upper Rio Negro, Solimões and Orinoco Basins (Brazil, Peru, Colombia and Venezuela) and P. bovaliorum is known only from the Potaro River Basin in Guyana (Buckup, 2003; Fricke et al., 2021b).
From a phylogenetic perspective, the subfamily Crenuchinae is widely accepted as monophyletic and supported by 12 morphological synapomorphies (Buckup, 1998). Among the diagnostic characters supporting this subfamily, the most remarkable is the presence of a frontal organ formed by a subcutaneous space filled with a thick collagenous pad fitting a depression of the frontal bone, innervated by trunks of the ophthalmicus superficialis branch of the trigeminal nerve (Alexander, 1963; Géry, 1963). Up to the present date, no morphological or molecular phylogenetic studies have verified the interrelationships in Crenuchinae, and both the monophyly of the subfamily and of its genera still rely on Buckup (1998: 136).
Ichthyological expeditions in 2015 and 2016 in the Rio Aripuanã drainage – a major tributary of the Rio Madeira Basin in the state of Amazonas, Brazil – resulted in the discovery of two additional and distinctive crenuchin species belonging to the genus Poecilocharax, representing the first new species of the subfamily discovered in the last 57 years.
In this contribution, the two new species are formally described using a combined approach based on morphological and molecular data. In addition, we conduct a detailed, comparative, morphological study that discusses current synapomorphies for the subfamily. This adds five putative new synapomorphies for Crenuchinae and provides an updated diagnosis for Poecilocharax accounting for the description of the two new species. Moreover, we report a considerable expansion on the geographical distribution of Poecilocharax, because these are the first records of the genus from the right bank tributaries of the Rio Amazonas Basin that drains the Brazilian crystalline shield.
MATERIAL AND METHODS
Measurements follow Buckup (1993c) and Melo & Oyakawa (2015), except for fin lengths, which are measured from fin origin to the distal tip of the longest ray. Standard length (SL) is expressed in millimetres and all other measurements are expressed as percentages of SL, except subunits of the head, which are expressed as percentages of head length (HL). Measurements related to sexual dimorphism are provided independently. Counts follow Buckup (1993c), except for the number of horizontal scale rows above and below the lateral line, which are not particularly irregular or variable in size in Crenuchinae and thus follow Fink & Weitzman (1974). The posteriormost double dorsal and anal-fin rays, which are associated with a single pterygiophore, are counted as a single element. The circuli and radii patterns are examined on scales sampled from the scale-row located immediately dorsal to the longitudinal scale row containing lateral-line pores and taken from the vertical that cuts through the dorsal-fin origin. Lateral-line canal and pore terminology follow Pastana et al. (2019).
Frequency of each count is provided in parentheses after the respective count; asterisks indicate holotype values. Counts of teeth, vertebrae, supraneurals, procurrent caudal-fin rays, gill rakers of the first branchial arch, cephalic lateral-line branches and pores of both species are taken from cleared and stained specimens (C&S), prepared according to Taylor & Van Dyke (1985). Because of its small size, counts of pectoral- and anal-fin rays of the second species described herein are based on C&S specimens only. Vertebrae of the Weberian apparatus are counted as four elements and the fused PU1 + U1 of the caudal region as a single one. Pre-caudal vertebrae counts include the Weberian apparatus and the vertebrae associated with ribs or haemal arches lacking a haemal spine. Caudal-vertebrae counts consider only vertebrae having a haemal spine. Sex of specimens was confirmed by direct observation of gonads. Colour in life was described based on freshly collected material photographed alive. Cleared and stained specimens and specimens fixed for molecular studies (MOL) are listed following the number of formalin-preserved specimens for each lot and listing their SL range. Institutional abbreviations follow Sabaj (2020).
Phylogenetic interpretations of morphological characters relevant for the monophyly of Crenuchinae are based on maximum parsimony optimizations performed on TNT v.1.5 for Windows (Goloboff & Catalano, 2016). These include only non-ambiguous characters and use the phylogenies of Buckup (1998) and Buckup (1993b), which are trees that synthesize the currently accepted hypotheses of relationships based on phenotypic data for Crenuchidae and Characidiinae, respectively.
Molecular analyses
A total of 85 individuals of the family Crenuchidae were used in the molecular analyses, representing 16 specimens of all species of Crenuchinae (including those described here) and 69 individuals of Characidiinae (sampling Ammocryptocharax and Characidium). Additionally, sequences of two non-crenuchid species were also included: Hoplias malabaricus (Bloch, 1794) and Hoplerythrinus unitaeniatus (Spix & Agassiz, 1829) (Erythrinidae). Tissue samples from species of Crenuchinae were obtained from fish collections [Instituto Nacional de Pesquisas da Amazônia (INPA), Laboratório de Genética de Peixes (LBP) and Auburn University Museum of Natural History (AUM)] and field expeditions conducted between 2015 and 2016 (in the case of the new species). All vouchers of the new species were deposited in the Museu de Zoologia da Universidade de São Paulo (MZUSP), São Paulo, Brazil. Sequences of all non-crenuchin species were obtained from the GenBank database, deposited by Pereira et al. (2013), Pansonato-Alves et al. (2014) and Scacchetti et al. (2015). Species of Characidiinae and Erythrinidae were used as outgroups in the phylogenetic analyses, and their identification codes, specimen vouchers and GenBank accession numbers are given in the Supporting Information, Table S1.
Total genomic DNA was extracted from muscle and fin tissues preserved in 96% ethanol with a DNeasy Blood and Tissue Kit (Qiagen), according to the manufacturer’s protocol. Partial sequences of the mitochondrial gene cytochrome c oxidase subunit I (COI) were amplified by polymerase chain reaction (PCR) with the primers described by Ward et al. (2005) and Melo et al. (2011). Amplifications were performed in a total volume of 20 μL, with 2.0 μL of 10 × buffer (10 mmol/L Tris-HCl + 15 mmol/L MgCl2), 0.6 μL MgCl2 (50 nmol/L), 0.4 μL dNTPs (200 nmol/L of each), 0.4 μL each 5 mmol/L primer, 0.05 μL Platinum Taq Polymerase (Invitrogen), 15.65 μL of double-distilled water and 0.5 μL template DNA (12 ng). The thermocycler profile consisted of an initial denaturation step at 95 °C for 5 min; followed by 35 cycles of chain denaturation (30 s at 94 °C), annealing (35 s at 50 °C) and nucleotide extension (1:10 min at 72 °C), plus a final extension step at 72 °C for 7 min. The PCR products were first visually identified on a 1% agarose gel and then purified using ExoSap-IT1 (USB Corporation) following the instructions of the manufacturer. The purified PCR products were sequenced in the Instituto de Biociências, Universidade Estadual Paulista, Botucatu, Brazil, and Centro de Pesquisas sobre o Genoma Humano e Células-Tronco Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil. All sequences were read twice (forward and reverse). All sequences produced in this study were deposited in the GenBank.
Electropherograms were inspected and assembled in contigs from forward and reverse strands using GENEIOUS v.4.8.5 (http://www.geneious.com, Kearse et al., 2012). Sequences were aligned using the MUSCLE algorithm under default parameters (http://www.ebi.ac.uk/Tools/msa/muscle/; Edgar, 2004). After alignment, the matrix was checked visually for obvious misalignments and to detect potential cases of sequencing error due to contamination, paralogy or pseudogenes using BioEdit v.7.0.9.0 (Hall, 1999). Nucleotide variation and substitution patterns were examined using MEGA X (Kumar et al., 2018). To evaluate the occurrence of substitution saturation in the sequences, the index of substitution saturation (Iss), described by Xia et al. (2003) and Xia & Lemey (2009) in DAMBE 5.3.48 (Xia, 2013), was estimated.
Phylogenetic relationships among species of Crenuchinae and between this subfamily and outgroups were inferred by Bayesian inference (BI) and maximum likelihood (ML) methods. Sequences of species of the Erythrinidae were used to root the phylogenetic analyses.
The best-fit nucleotide evolution model was estimated using MrModelTest v.2.2 (Nylander, 2014) based on the Akaike information criterion (AIC), in conjunction with PAUP* (Swofford, 1998). BI analysis was conducted in MrBayes v.3.2.6 (Ronquist et al., 2012). Two independent Bayesian runs of 20 million generations with four Monte Carlo Markov chains (MCMC) each were performed, saving trees each 500 generations. Chain convergence (effective sample size – ESS values > 200) was checked using the likelihood plots for each run using TRACER v.1.5.1 (Rambaut & Drummond, 2009). The potential scale reduction factor (PSRF) was also used to check chain convergence and burn-in; values close to 1 indicate good convergence between runs (Gelman & Donal, 1992). After a graphical analysis of the evolution of the likelihood scores, and checking for the stationarity of all model parameters, the first four thousand generations (10%) were discarded as burn-in. The remaining trees were used to calculate the consensus tree and posterior probability values were calculated to determine the level of support to the Bayesian topology. The ML phylogenetic reconstructions were performed using RAxML v.8.0.24 (Stamatakis, 2014), random starting trees and a GTRGAMMA model of nucleotide substitution. One-thousand bootstrap pseudoreplicates were used to investigate the support of each node in the most likely topology. In general, bootstrap values above 75% in the ML analyses were interpreted as well supported, and in the BI analyses a posterior probability value of 0.99 was taken as a threshold. MrBayes and RAxML analyses were performed remotely at the CIPRES Science Gateway portal (Miller et al., 2010).
The automatic barcode gap discovery (ABGD; Puillandre et al., 2012), a computationally efficient distance-based method of species delimitation, which seeks to quantify the location of the barcode gap that separates intra from interspecific distances (Puillandre et al., 2012; Blair & Bryson, 2017), was used to delimit lineages in the subfamily Crenuchinae. Thus, ABGD analyses were run through the software command line. Default settings were used for the prior range for maximum intraspecific divergence (0.001, 0.1). Results were compared using both JC69 and K80 corrected distances and minimum slope increase (X) of 1.0.
RESULTS
Molecular results
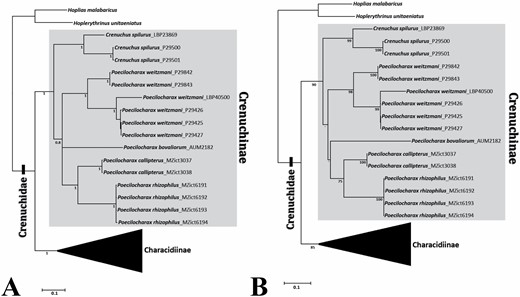
The sequences from 87 specimens (Table 1; Supporting Information, Table S1) resulted in a matrix for the mitochondrial gene COI with 666 base pairs (bp) from which 386 positions were conserved, 266 were variable and 250 positions were informative. The sequences did not have insertions, deletions, stop-codons or sequencing errors due to contamination. The Iss index was significantly lower than the Iss.c (critical substitution saturation index), indicating no saturation in both transitions and transversions, and in both asymmetrical (Iss.cAsym) and symmetrical (Iss.cSym) topologies. The best-fit model of evolution estimated by MrModelTest was GTR+I+G. Both phylogenetic methods (BI and ML) produced gene trees with similar topologies (Fig. 1A, B, respectively), in which Crenuchinae are recovered as a strongly supported monophyletic group, including two genera (Crenuchus and Poecilocharax) and five species (C. spilurus, P. bovaliorum, P. weitzmani, and the two new species described in this study, P. callipterus and P. rhizophilus) (Fig. 1). Both the BI and ML analyses recovered Poecilocharax as monophyletic but with low support. Overall, the relationships among species of Poecilocharax were not clearly resolved except for the clade (P. callipterus, P. rhizophilus), which had high statistical support in both hypotheses (1 of posterior probability and 75% of bootstrap).
Species, lot number, voucher and GenBank accession numbers for the representatives of Crenuchinae used in this study.
| Species . | Lot number . | Voucher . | GenBank number (COI) . |
|---|---|---|---|
| Crenuchus spilurus | LBP 4275 | LBP 23869 | KF914693 |
| Crenuchus spilurus | INPA-ICT 049574 | P 29500 | ON059686 |
| Crenuchus spilurus | INPA-ICT 049574 | P 29501 | ON062375 |
| Poecilocharax bovaliorum | AUM 2182 | AUM 2182 | ON042212 |
| Poecilocharax callipterus | MZUSP 117568 | MZict 3037 | ON067495 |
| Poecilocharax callipterus | MZUSP 117568 | MZict 3038 | ON067498 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6191 | ON076904 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6192 | ON067501 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6193 | ON067502 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6194 | ON067500 |
| Poecilocharax weitzmani | LBP 7078 | LBP 40500 | HQ289701 |
| Poecilocharax weitzmani | INPA-ICT 049036 | P 29842 | ON063015 |
| Poecilocharax weitzmani | INPA-ICT 049036 | P 29843 | ON063044 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29425 | ON062533 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29426 | ON063012 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29427 | ON063016 |
| Species . | Lot number . | Voucher . | GenBank number (COI) . |
|---|---|---|---|
| Crenuchus spilurus | LBP 4275 | LBP 23869 | KF914693 |
| Crenuchus spilurus | INPA-ICT 049574 | P 29500 | ON059686 |
| Crenuchus spilurus | INPA-ICT 049574 | P 29501 | ON062375 |
| Poecilocharax bovaliorum | AUM 2182 | AUM 2182 | ON042212 |
| Poecilocharax callipterus | MZUSP 117568 | MZict 3037 | ON067495 |
| Poecilocharax callipterus | MZUSP 117568 | MZict 3038 | ON067498 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6191 | ON076904 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6192 | ON067501 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6193 | ON067502 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6194 | ON067500 |
| Poecilocharax weitzmani | LBP 7078 | LBP 40500 | HQ289701 |
| Poecilocharax weitzmani | INPA-ICT 049036 | P 29842 | ON063015 |
| Poecilocharax weitzmani | INPA-ICT 049036 | P 29843 | ON063044 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29425 | ON062533 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29426 | ON063012 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29427 | ON063016 |
Species, lot number, voucher and GenBank accession numbers for the representatives of Crenuchinae used in this study.
| Species . | Lot number . | Voucher . | GenBank number (COI) . |
|---|---|---|---|
| Crenuchus spilurus | LBP 4275 | LBP 23869 | KF914693 |
| Crenuchus spilurus | INPA-ICT 049574 | P 29500 | ON059686 |
| Crenuchus spilurus | INPA-ICT 049574 | P 29501 | ON062375 |
| Poecilocharax bovaliorum | AUM 2182 | AUM 2182 | ON042212 |
| Poecilocharax callipterus | MZUSP 117568 | MZict 3037 | ON067495 |
| Poecilocharax callipterus | MZUSP 117568 | MZict 3038 | ON067498 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6191 | ON076904 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6192 | ON067501 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6193 | ON067502 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6194 | ON067500 |
| Poecilocharax weitzmani | LBP 7078 | LBP 40500 | HQ289701 |
| Poecilocharax weitzmani | INPA-ICT 049036 | P 29842 | ON063015 |
| Poecilocharax weitzmani | INPA-ICT 049036 | P 29843 | ON063044 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29425 | ON062533 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29426 | ON063012 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29427 | ON063016 |
| Species . | Lot number . | Voucher . | GenBank number (COI) . |
|---|---|---|---|
| Crenuchus spilurus | LBP 4275 | LBP 23869 | KF914693 |
| Crenuchus spilurus | INPA-ICT 049574 | P 29500 | ON059686 |
| Crenuchus spilurus | INPA-ICT 049574 | P 29501 | ON062375 |
| Poecilocharax bovaliorum | AUM 2182 | AUM 2182 | ON042212 |
| Poecilocharax callipterus | MZUSP 117568 | MZict 3037 | ON067495 |
| Poecilocharax callipterus | MZUSP 117568 | MZict 3038 | ON067498 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6191 | ON076904 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6192 | ON067501 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6193 | ON067502 |
| Poecilocharax rhizophilus | MZUSP 121651 | MZict 6194 | ON067500 |
| Poecilocharax weitzmani | LBP 7078 | LBP 40500 | HQ289701 |
| Poecilocharax weitzmani | INPA-ICT 049036 | P 29842 | ON063015 |
| Poecilocharax weitzmani | INPA-ICT 049036 | P 29843 | ON063044 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29425 | ON062533 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29426 | ON063012 |
| Poecilocharax weitzmani | INPA-ICT 042889 | P 29427 | ON063016 |
Abbreviated phylogenetic trees of Crenuchidae obtained in this study based on mitochondrial gene cytochrome c oxidase subunit I (COI, 666 bp), indicating the monophyly of Crenuchinae (highlighted) and the relationships among its representatives. A, Bayesian tree, numbers at branches are posterior probabilities; B, maximum likelihood tree, numbers at branches are bootstrap values. Values below 75% (-) are not shown.
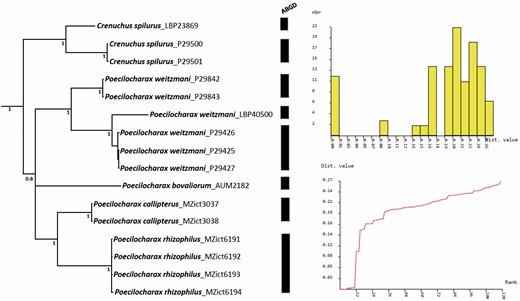
The ABGD analyses suggested a total of nine species based on initial partitioning over a range of prior values for maximum intraspecific divergence. Results based on JC69 and K80 distances were identical, indicating that there is no doubt about the species boundaries based on the analysed specimens, although there is a split within both Crenuchus spilurus and Poecilocharax weitzmani (Fig. 2). Similar results were obtained by using the 2% standard barcode threshold of genetic distance calculated based on the COI matrix. They indicate a high genetic divergence among specimens of both Crenuchus spilurus and Poecilocharax weitzmani, 10.3 and 11.1%, respectively. The rate of genetic variation ranges from 16.9 to 25.2% among species of Poecilocharax (Supporting Information, Table S2).
Results from ABGD analysis on Crenuchinae data shown in the IB topology. Analyses were run with a minimum slope increase (X) of 1.0. Distances were calculated based on the Kimura 2-parameter (K80) model. Graphs indicate the histogram of distances (right, above) and ranked distances (right, below).
Taxonomy
Genus: PoecilocharaxEigenmann, 1909
Type species:
Poecilocharax bovaliiEigenmann, 1909 (by original description); now Poecilocharax bovaliorum (mandatory name correction: Fricke et al., 2021b).
Diagnosis:
Poecilocharax can be distinguished from the only other crenuchin genus, Crenuchus, by the following morphological characteristics: (1) adipose fin absent (vs. present); (2) pelvic-fin rays eight, with the following formula: i,seven or i,six,i (vs. pelvic-fin rays nine or ten, with formula: i,eight or i,nine); and (3) posterior terminus of maxilla not surpassing vertical through anterior margin of the orbit (vs. posterior terminus of maxilla extending posterior to orbital margin, reaching vertical through pupils).
Poecilocharax callipterus sp. nov.
(Figs 3–9; Table 2)
Zoobank registration:
urn:lsid:zoobank.org:act:A55ADF69-B919-415B-8BAB-38F7516AB9AD.
Holotype:
MZUSP 121650, male, 30.9 mm SL, BRAZIL, Amazonas State, Apuí, tributary of Rio Canadá, Rio Juma, Rio Aripuanã drainage, Rio Madeira Basin, road AM 174 between Apui and Novo Aripuanã towns, 6º51′36′′S 59º58′36′′W, 140 m above sea level (a.s.l.), 23 June 2015, W. Ohara and V. Abrahão (collectors).
Paratypes:
BRAZIL, Amazonas State, Apuí. MZUSP 117568 (43, 19.1–31.2 mm SL; 2 C&S, 23.3–28.1 mm SL; 3 MOL, 23.9–29.0 mm SL); INPA 59405 (15, 23.0–29.6 mm SL); MCP 54291 (15, 21.7–28.1 mm SL), MNRJ 51747 (10, 22.7–28.4 mm SL), same data as the holotype. MZUSP 121653 (17, 24.6–30.6 mm SL; 3 C&S, 29.1–31.6 mm SL), same locality as the holotype, 9 October 2016, O. Oyakawa, W. Ohara, M. Pastana and T. Teixeira (collectors).
Diagnosis:
Poecilocharax callipterus can be promptly distinguished from its congeners by the presence of a single, conspicuous, dark spot ventrally positioned on caudal peduncle (vs. absence); by the absence of a dark midlateral stripe on the body (vs. presence); and by the presence of dark blotches on the flank (vs. absence). Additionally, Poecilocharax callipterus can be distinguished from its congeners, except P. bovaliorum, by having the anterior and posterior nares separated only by a narrow skin fold (vs. the anterior and posterior nares separated from each other by a distance equal or greater than anterior nostril diameter; see Géry, 1965: fig. 11); and by the presence of a nasal flap (vs. absence). Poecilocharax callipterus can be further distinguished from P. bovaliorum by having a long dorsal-fin filament in mature males (vs. dorsal fin not particularly elongated in mature males).
Description:
Morphometric data are presented in Table 2. Largest specimen reaching 31.2 mm SL. Body moderately compressed and deep (Fig. 3). Greatest body depth approximately at vertical through tip of pectoral fin. Dorsal profile convex between tip of snout and posterior region of frontal bone; slightly concave or straight from that point to tip of supraoccipital spine; convex from supraoccipital spine to base of last dorsal-fin ray; straight or slightly concave between that point to origin of anterior most dorsal procurrent caudal-fin ray. Ventral profile convex between anterior tip of dentary and anal-fin origin; slightly convex at anal-fin base; almost straight between terminus of anal-fin base to origin of anteriormost ventral procurrent caudal-fin ray. Body elliptical in cross-section at pectoral-fin origin, broader ventrally and gradually more compressed toward caudal-fin base.
Morphometric data for holotype and paratypes (range) of Poecilocharax callipterus sp. nov. (N = 25). SD = standard deviation
| Characters . | Holotype . | Range . | Median ± SD . |
|---|---|---|---|
| Total length (mm) | 39.83 | 30.6–39.9 | 36.9 ± 2.1 |
| Standard length (mm) | 30.91 | 23.5–31.2 | 28.4 ± 1.8 |
| Percentage of standard length | |||
| Head length | 26.4 | 25.9–30.2 | 27.6 ± 1.1 |
| Prepectoral distance | 30.3 | 27.8–32.1 | 30.4 ± 0.9 |
| Pectoral-fin height | 17.1 | 15.5–19.5 | 17.5 ± 0.9 |
| Predorsal distance | 51.4 | 47.5–51.8 | 49.7 ± 1.1 |
| Dorsal-fin height females | - | 38.4–49.3 | 44.2 ± 6.7 |
| Dorsal-fin height males | 63.7 | 51.3–67.5 | 60.1 ± 4.3 |
| Dorsal-fin base | 26.0 | 23.9–27.2 | 25.2 ± 0.9 |
| Prepelvic distance | 54.4 | 50.8–55.4 | 53.2 ± 1.2 |
| Pelvic-fin height | 19.5 | 17.1–22.3 | 19.2 ± 1.3 |
| Pre-anal distance | 72.4 | 71.0–76.2 | 73.7 ± 1.6 |
| Anal–apex distance females | - | 98.4–104.0 | 102.7 ± 3.3 |
| Anal–apex distance males | 111.7 | 104.0–112.4 | 109.7 ± 2.4 |
| Anus to anal-fin distance | 3.3 | 1.9–3.9 | 3.1 ± 0.5 |
| Anal-fin height females | - | 24.7–31.7 | 28.6 ± 2.2 |
| Anal-fin height males | 38.7 | 31.9–39.8 | 36.8 ± 2.7 |
| Anal-fin base | 13.1 | 11.1–14.1 | 12.5 ± 0.8 |
| Peduncle length | 17.7 | 15.7–18.5 | 17.0 ± 0.7 |
| Body depth at dorsal-fin origin | 33.2 | 30.4–33.4 | 31.9 ± 0.9 |
| Body depth at anal-fin origin | 24.5 | 22.3–24.8 | 23.8 ± 0.6 |
| Body depth at caudal peduncle | 16.9 | 14.7–16.9 | 15.6 ± 0.6 |
| Body width | 13.6 | 12.1–14.6 | 13.4 ± 0.6 |
| Percentage of head length | |||
| Snout length | 28.8 | 24.6–29.8 | 27.3 ± 1.3 |
| Snout–maxillary length | 35.4 | 31.8–37.8 | 34.7 ± 1.8 |
| Anterior naris-orbit | 13.8 | 10.7–15.0 | 13.2 ± 1.2 |
| Posterior naris-orbit | 9.5 | 9.4–12.1 | 10.2 ± 0.7 |
| Cheek | 11.9 | 9.3–13.4 | 11.2 ± 0.9 |
| Orbital diameter | 33.4 | 30.0–35.0 | 31.5 ± 1.2 |
| Interorbital diameter | 28.8 | 24.3–30.5 | 28.2 ± 1.4 |
| Characters . | Holotype . | Range . | Median ± SD . |
|---|---|---|---|
| Total length (mm) | 39.83 | 30.6–39.9 | 36.9 ± 2.1 |
| Standard length (mm) | 30.91 | 23.5–31.2 | 28.4 ± 1.8 |
| Percentage of standard length | |||
| Head length | 26.4 | 25.9–30.2 | 27.6 ± 1.1 |
| Prepectoral distance | 30.3 | 27.8–32.1 | 30.4 ± 0.9 |
| Pectoral-fin height | 17.1 | 15.5–19.5 | 17.5 ± 0.9 |
| Predorsal distance | 51.4 | 47.5–51.8 | 49.7 ± 1.1 |
| Dorsal-fin height females | - | 38.4–49.3 | 44.2 ± 6.7 |
| Dorsal-fin height males | 63.7 | 51.3–67.5 | 60.1 ± 4.3 |
| Dorsal-fin base | 26.0 | 23.9–27.2 | 25.2 ± 0.9 |
| Prepelvic distance | 54.4 | 50.8–55.4 | 53.2 ± 1.2 |
| Pelvic-fin height | 19.5 | 17.1–22.3 | 19.2 ± 1.3 |
| Pre-anal distance | 72.4 | 71.0–76.2 | 73.7 ± 1.6 |
| Anal–apex distance females | - | 98.4–104.0 | 102.7 ± 3.3 |
| Anal–apex distance males | 111.7 | 104.0–112.4 | 109.7 ± 2.4 |
| Anus to anal-fin distance | 3.3 | 1.9–3.9 | 3.1 ± 0.5 |
| Anal-fin height females | - | 24.7–31.7 | 28.6 ± 2.2 |
| Anal-fin height males | 38.7 | 31.9–39.8 | 36.8 ± 2.7 |
| Anal-fin base | 13.1 | 11.1–14.1 | 12.5 ± 0.8 |
| Peduncle length | 17.7 | 15.7–18.5 | 17.0 ± 0.7 |
| Body depth at dorsal-fin origin | 33.2 | 30.4–33.4 | 31.9 ± 0.9 |
| Body depth at anal-fin origin | 24.5 | 22.3–24.8 | 23.8 ± 0.6 |
| Body depth at caudal peduncle | 16.9 | 14.7–16.9 | 15.6 ± 0.6 |
| Body width | 13.6 | 12.1–14.6 | 13.4 ± 0.6 |
| Percentage of head length | |||
| Snout length | 28.8 | 24.6–29.8 | 27.3 ± 1.3 |
| Snout–maxillary length | 35.4 | 31.8–37.8 | 34.7 ± 1.8 |
| Anterior naris-orbit | 13.8 | 10.7–15.0 | 13.2 ± 1.2 |
| Posterior naris-orbit | 9.5 | 9.4–12.1 | 10.2 ± 0.7 |
| Cheek | 11.9 | 9.3–13.4 | 11.2 ± 0.9 |
| Orbital diameter | 33.4 | 30.0–35.0 | 31.5 ± 1.2 |
| Interorbital diameter | 28.8 | 24.3–30.5 | 28.2 ± 1.4 |
Morphometric data for holotype and paratypes (range) of Poecilocharax callipterus sp. nov. (N = 25). SD = standard deviation
| Characters . | Holotype . | Range . | Median ± SD . |
|---|---|---|---|
| Total length (mm) | 39.83 | 30.6–39.9 | 36.9 ± 2.1 |
| Standard length (mm) | 30.91 | 23.5–31.2 | 28.4 ± 1.8 |
| Percentage of standard length | |||
| Head length | 26.4 | 25.9–30.2 | 27.6 ± 1.1 |
| Prepectoral distance | 30.3 | 27.8–32.1 | 30.4 ± 0.9 |
| Pectoral-fin height | 17.1 | 15.5–19.5 | 17.5 ± 0.9 |
| Predorsal distance | 51.4 | 47.5–51.8 | 49.7 ± 1.1 |
| Dorsal-fin height females | - | 38.4–49.3 | 44.2 ± 6.7 |
| Dorsal-fin height males | 63.7 | 51.3–67.5 | 60.1 ± 4.3 |
| Dorsal-fin base | 26.0 | 23.9–27.2 | 25.2 ± 0.9 |
| Prepelvic distance | 54.4 | 50.8–55.4 | 53.2 ± 1.2 |
| Pelvic-fin height | 19.5 | 17.1–22.3 | 19.2 ± 1.3 |
| Pre-anal distance | 72.4 | 71.0–76.2 | 73.7 ± 1.6 |
| Anal–apex distance females | - | 98.4–104.0 | 102.7 ± 3.3 |
| Anal–apex distance males | 111.7 | 104.0–112.4 | 109.7 ± 2.4 |
| Anus to anal-fin distance | 3.3 | 1.9–3.9 | 3.1 ± 0.5 |
| Anal-fin height females | - | 24.7–31.7 | 28.6 ± 2.2 |
| Anal-fin height males | 38.7 | 31.9–39.8 | 36.8 ± 2.7 |
| Anal-fin base | 13.1 | 11.1–14.1 | 12.5 ± 0.8 |
| Peduncle length | 17.7 | 15.7–18.5 | 17.0 ± 0.7 |
| Body depth at dorsal-fin origin | 33.2 | 30.4–33.4 | 31.9 ± 0.9 |
| Body depth at anal-fin origin | 24.5 | 22.3–24.8 | 23.8 ± 0.6 |
| Body depth at caudal peduncle | 16.9 | 14.7–16.9 | 15.6 ± 0.6 |
| Body width | 13.6 | 12.1–14.6 | 13.4 ± 0.6 |
| Percentage of head length | |||
| Snout length | 28.8 | 24.6–29.8 | 27.3 ± 1.3 |
| Snout–maxillary length | 35.4 | 31.8–37.8 | 34.7 ± 1.8 |
| Anterior naris-orbit | 13.8 | 10.7–15.0 | 13.2 ± 1.2 |
| Posterior naris-orbit | 9.5 | 9.4–12.1 | 10.2 ± 0.7 |
| Cheek | 11.9 | 9.3–13.4 | 11.2 ± 0.9 |
| Orbital diameter | 33.4 | 30.0–35.0 | 31.5 ± 1.2 |
| Interorbital diameter | 28.8 | 24.3–30.5 | 28.2 ± 1.4 |
| Characters . | Holotype . | Range . | Median ± SD . |
|---|---|---|---|
| Total length (mm) | 39.83 | 30.6–39.9 | 36.9 ± 2.1 |
| Standard length (mm) | 30.91 | 23.5–31.2 | 28.4 ± 1.8 |
| Percentage of standard length | |||
| Head length | 26.4 | 25.9–30.2 | 27.6 ± 1.1 |
| Prepectoral distance | 30.3 | 27.8–32.1 | 30.4 ± 0.9 |
| Pectoral-fin height | 17.1 | 15.5–19.5 | 17.5 ± 0.9 |
| Predorsal distance | 51.4 | 47.5–51.8 | 49.7 ± 1.1 |
| Dorsal-fin height females | - | 38.4–49.3 | 44.2 ± 6.7 |
| Dorsal-fin height males | 63.7 | 51.3–67.5 | 60.1 ± 4.3 |
| Dorsal-fin base | 26.0 | 23.9–27.2 | 25.2 ± 0.9 |
| Prepelvic distance | 54.4 | 50.8–55.4 | 53.2 ± 1.2 |
| Pelvic-fin height | 19.5 | 17.1–22.3 | 19.2 ± 1.3 |
| Pre-anal distance | 72.4 | 71.0–76.2 | 73.7 ± 1.6 |
| Anal–apex distance females | - | 98.4–104.0 | 102.7 ± 3.3 |
| Anal–apex distance males | 111.7 | 104.0–112.4 | 109.7 ± 2.4 |
| Anus to anal-fin distance | 3.3 | 1.9–3.9 | 3.1 ± 0.5 |
| Anal-fin height females | - | 24.7–31.7 | 28.6 ± 2.2 |
| Anal-fin height males | 38.7 | 31.9–39.8 | 36.8 ± 2.7 |
| Anal-fin base | 13.1 | 11.1–14.1 | 12.5 ± 0.8 |
| Peduncle length | 17.7 | 15.7–18.5 | 17.0 ± 0.7 |
| Body depth at dorsal-fin origin | 33.2 | 30.4–33.4 | 31.9 ± 0.9 |
| Body depth at anal-fin origin | 24.5 | 22.3–24.8 | 23.8 ± 0.6 |
| Body depth at caudal peduncle | 16.9 | 14.7–16.9 | 15.6 ± 0.6 |
| Body width | 13.6 | 12.1–14.6 | 13.4 ± 0.6 |
| Percentage of head length | |||
| Snout length | 28.8 | 24.6–29.8 | 27.3 ± 1.3 |
| Snout–maxillary length | 35.4 | 31.8–37.8 | 34.7 ± 1.8 |
| Anterior naris-orbit | 13.8 | 10.7–15.0 | 13.2 ± 1.2 |
| Posterior naris-orbit | 9.5 | 9.4–12.1 | 10.2 ± 0.7 |
| Cheek | 11.9 | 9.3–13.4 | 11.2 ± 0.9 |
| Orbital diameter | 33.4 | 30.0–35.0 | 31.5 ± 1.2 |
| Interorbital diameter | 28.8 | 24.3–30.5 | 28.2 ± 1.4 |
Poecilocharax callipterus sp. nov.: A, holotype, male, 30.9 mm SL, MZUSP 121653; B, paratype, female, 27.7 mm SL, MZUSP 117568.
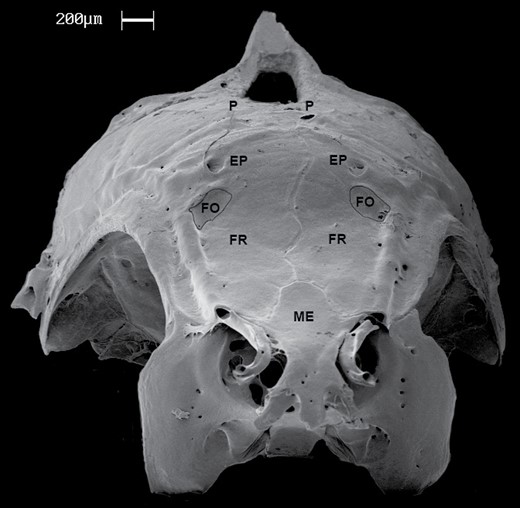
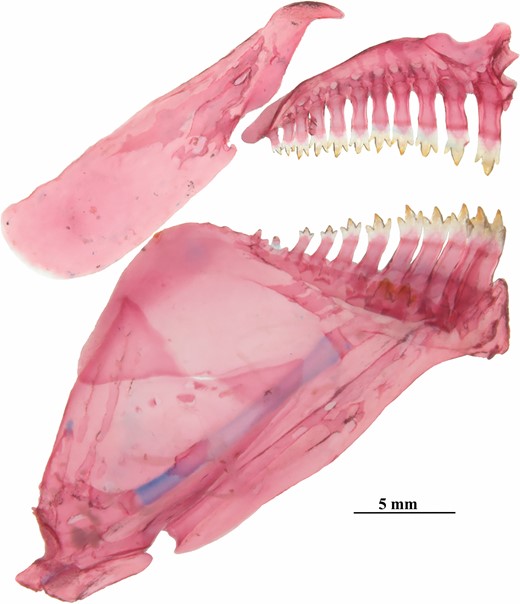
Mouth terminal, jaws vertically aligned anteriorly. Snout gently rounded, shorter than eye diameter. Distal tip of maxilla barely reaching anterior margin of orbit. Orbital margin free. Cheek depth about half of eye diameter. Anterior and posterior nares close to each other, separated by skin flap. Anterior naris tubular and posterior crescent-shaped. Frontals concave anteriorly, with wide depression between orbits extending from posterior portion of mesethmoid bone to anterior portion of epiphyseal branch of supraorbital canal (Fig. 4). Concavity allocating frontal organ. Each frontal containing one relatively large foramen for passage of nerve branch ophthalmicus superficialis that innervates the organ. Foramen located medial to supraorbital canal of cephalic lateral line, anterior to epiphyseal branch of supraorbital canal (Fig. 4). Supraorbital bone absent. Infraorbitals one to six present (Fig. 5), with infraorbitals three and four occasionally fused to each other; antorbital bone located anterodorsally to first infraorbital. Pseudotympanum present, underneath third to fifth scales of longitudinal line.
SEM photograph of the of neurocranium of Poecilocharax callipterus sp. nov. in frontal view, paratype, male, 28.1 mm SL. Epiphyseal branch of the supraorbital laterosensory canal (EP), foramina (FO), frontal bone (FR), mesethmoid (ME) and parietal branch of supraorbital laterosensory canal (P).
Infraorbital series of Poecilocharax callipterus sp. nov., depicting antorbital bone and infraorbitals 1–6, MZUSP 117568, paratype, male, 28.1 mm SL, left lateral view.
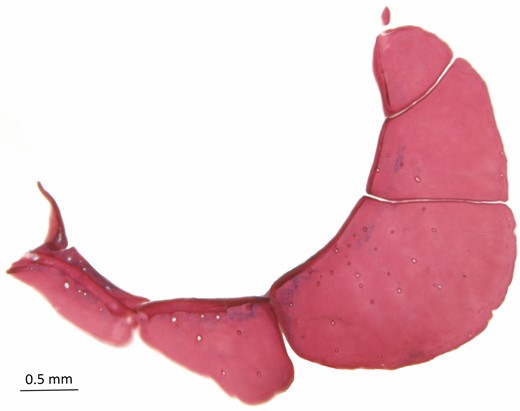
Dentary teeth in one row with seven to 13 (5) conical or tricuspid teeth. Premaxillary teeth in single row with ten or 11 (5) conical to tricuspid teeth. Premaxillary and dentary teeth increasing in size toward symphysis. Maxillary teeth absent (Fig. 6). Ectopterygoid with small teeth. Branchiostegals five (5); four attached to anterior ceratohyal and one to posterior ceratohyal. First arch with three (5) gill rakers on hypobranchial, seven (1), eight (1), nine (2) or ten (1) on ceratobranchial, one (5) on cartilage between ceratobranchial and epibranchial, and six (1) or eight (4) on epibranchial. Gill rakers slightly shorter than brachial filaments. Supraneurals three (5), small and rod-like dorsally positioned and anterior to first neural spine (5).
Poecilocharax callipterus sp. nov., left medial view of premaxilla, maxilla and dentary, MZUSP 117568, paratype, male, 28.1 mm SL.
Laterosensory system developmentally truncated, tubules opening in individual pores (i.e. never forming subpores). Supraorbital canal associated with nasal, frontal and parietal bones. Epiphyseal and parietal branch of supraorbital canal present (Fig. 4). Supraorbital lacking connection to otic canal, falling short near posterolateral margin of frontal bone. Infraorbital canal developmentally truncated, restricted to infraorbital plates 1, 2 (Fig. 5) and variably associated with infraorbital plates 5, 6. Otic canal present, associated with anterior portion of pterotic. Post-otic canal running along pterotic, extrascapular, post-temporal and supracleithrum. Supratemporal canal associated with extrascapula and posterior margin of parietal bone, running dorsomedially and opening in a pore near cranial fontanel. Preopercular canal extending along entire extension of pre-opercular bone, continuous with mandibular canal. Mandibular canal associated with the anguloarticular and dentary bones.
Scales cycloid, circuli distributed over entire scale surface. Two to four parallel and well-defined radii present on posterior scale surface. Trunk lateral-line perforating a single scale, either fifth (3) or sixth* (19) scale of the longitudinal series (Fig. 7). Perforated scale inconspicuous or covered by skin in some specimens. Longitudinal scale series, including perforated scale, 25 (1), 26 (3), 27 (8), 28* (13) or 29 (1). Horizontal scale rows above lateral line four* (26). Horizontal scale rows below lateral line three* (26). Scale rows around caudal peduncle 14* (26). Predorsal scales series six (8), seven* (14) or eight (2). Isthmus scaled. Scales between anus and anal fin, none (1), one* (18) or two (6). Superficial neuromasts arranged vertically on basal portion of each scale, more conspicuous on anterior half of body (Fig. 8).
Poecilocharax callipterus sp. nov., lateral line with a single perforated scale, MZUSP 117568, paratype, 27.5 mm SL.
Superficial neuromasts (white arrows) on lateral body scales of Poecilocharax callipterus sp. nov., MZUSP 117568, paratype, 29.4 mm SL.
Pectoral-fin rays ii, nine (2) or 10* (22). Tip of longest pectoral-fin ray slightly anterior to vertical through dorsal-fin origin. Pelvic-fin rays i,seven* (24). Tip of longest pelvic-fin rays slightly anterior to vertical through insertion of last dorsal-fin ray. Dorsal-fin rays iii,13 (1), iii,14 (2), iv,12 (1), iv,13* (13) or iv,14 (7). Dorsal-fin ray length variable (Figs 3, 9; see ‘sexual dimorphism’). Dorsal-fin origin nearer snout than caudal-fin base, slightly anterior to vertical through pelvic-fin origin. First dorsal-fin pterygiophore located behind neural spine of 18th (5) vertebra. Anal-fin rays ii,seven (5), ii,eight (9), iii,seven* (8), iii,eight (3) with variable length (Figs 3, 9; see ‘sexual dimorphism’). Anal-fin origin posterior to vertical through base of last dorsal-fin ray. Single row of one to three scales covering base of anteriormost anal-fin rays. First anal-fin pterygiophore located behind neural spine of 18th (5) vertebra. Principal caudal-fin rays i8,8i (1), i9,8i* (25), i9,7i (1) or i10,8i (2). Caudal fin with one to three large scales covering basal portion of each lobe. Dorsal procurrent caudal-fin rays five (1) or six (4), and ventral procurrent caudal-fin rays four (1) or five (4). Hypurals one and two fused to each other; hypurals five and six fused to each other in some specimens (2); hypurals three and four always autogenous. Adipose fin absent. Total vertebrae 29 (1) or 30 (4); precaudal vertebrae 19 (5), caudal vertebrae ten (1) or 11 (4).
Poecilocharax callipterus sp. nov., immediately after capture, paratypes: A, male, 30.6 mm SL, MZUSP 121653; B, female, 27.2 mm SL, MZUSP 117568.
Colour in alcohol:
Overall background coloration of head and body beige to brown, darker dorsally (Fig. 3). Snout, premaxilla, tip of dentary, maxilla, antorbital and infraorbitals one and two densely covered by small, dark chromatophores. Ventral portion of head, cheeks, gular and opercular regions with scattered chromatophores. Eyes mostly dark, with brown pigments surrounding pupils. Suborbital stripe absent. Guanine deposits on head not visible in alcohol preserved specimens. Dark chromatophores densely distributed along dorsal and dorsolateral scale rows. Scales of anterior-dorsal half of body with chromatophores disposed vertically, mostly on anterior margin of scales, forming small, dark blotches on each scale. Scale borders forming a conspicuous reticulate pattern on anterior-dorsal half of body. Ventral portion of the body lighter, abdominal region lacking pigment, except for few sparse dark chromatophores. Body somewhat mottled, having nine to 12 conspicuous dark blotches. First blotch somewhat round, variably present immediately posterior to the opercle, followed by two other blotches. Midline of body often having blotches irregular in shape with undefined borders. A series of two to five vertically elongated, dark blotches encompassing one scale horizontally and three to four scales vertically, positioned below the dorsal-fin base. Midbody with two to three dark blotches: one located at the abdominal region, one or two at the anal-fin base. Midbody blotches highly variable in number, position and conspicuity among specimens, with blotches occasionally forming a discontinuous dark stripe; none to four dark spots or blotches positioned above anal-fin base. Caudal peduncle with one conspicuous dark spot, located on ventral half of peduncle, near the base of ventral caudal-fin rays (referred hereafter as asymmetrical caudal-peduncle spot), encompassing two or two and a half scales horizontally and one to one and a half vertically (Fig. 3). Spot remarkably similar to that present in Crenuchus spilurus (see Discussion).
Dorsal-fin coloration sexually dimorphic. Dorsal fin mostly dusky in adult males, with dark pigment more concentrated distally on rays and interradial membranes; dorsal-fin filament densely pigmented. Remaining dorsal-fin area with melanophores sparsely distributed, more concentrated in the distal portions of the fin. Posteriormost dorsal-fin rays hyaline. Dorsal fin of females and immature individuals has less pigmentation, with melanophores sparsely distributed on border of rays, more concentrated on distal half of anteriormost rays; dorsal-fin filament, whenever present, short and dark. Pectoral fins predominantly hyaline in both sexes, with few scattered dark chromatophores present along margins of rays. Pelvic fin varying from hyaline in females and juveniles or dusky in adult males, with scattered chromatophores along fin-rays and membranes. Anal-fin coloration sexually dimorphic; adult males with dusky anal-fin, and dark chromatophores along border of rays and membranes. One horizontal dark stripe present distally on the anal-fin, encompassing the first five anal-fin rays. Dark stripe contrasting with an unpigmented anal-fin margin. Anal fin of females and immature specimens considerably less pigmented. Caudal fin with concentration of dark chromatophores on upper and lower caudal-fin base, and a series of small and elongated spots along interradial membrane of middle caudal-fin rays. Caudal-fin spots more numerous in adult males.
Colour in life:
Overall body coloration yellow to red, darker dorsally (Fig. 9). Abdominal region yellow with silvery hue. Adult males with a more vivid coloration. Dark blotches on body as described in ‘Colour in alcohol’, usually visible in live specimens. Guanine deposits on cheeks, ventral portion opercular bone and along sub- and interopercle. Lower half of opercle and infraorbitals two to four silvery, golden or red. Upper half of opercle transparent, red gill filaments visible through bone in some individuals. Upper and lower jaw mostly yellow; orange hue present in large adult males.
Dorsal-fin coloration sexually dimorphic (Fig. 9). Adult males with dorsal fin orange to red along first 12 to 14 dorsal-fin rays, chromatophores more concentrated on interradial membranes. Tip of dorsal-fin filament strongly red pigmented. Females and immature specimens of both sexes with dorsal fin mostly yellow, with chromatophores concentrated on fin-rays. Pectoral fins predominantly pale orange to yellow in both sexes. Pelvic fins yellow to orange, more intensely coloured in adult males. Anal-fin coloration sexually dimorphic. Adult males with orange to red fin, chromatophores concentrated on interradial membranes of first nine anal-fin rays; and with an evident horizontal red stripe located on the leading margin of first five to six anal-fin rays. Females and immature specimens of both sexes with anal fin yellow to orange, less vivid than adult males, with red stripe absent or inconspicuous. Caudal fin pale red to yellowish in both sexes.
Sexual dimorphism:
Dorsal-fin filament length sexually dimorphic. Dorsal-fin height of adult males ranging from 51.3 to 67.5% of SL with a long dorsal-fin filament projecting from the eighth to 11th dorsal-fin rays. In some specimens, depressed filament reaching the proximal half of the upper caudal-fin lobe (Figs 3, 9). Adult females with much shorter dorsal-fin filaments, projecting from the eighth to tenth dorsal-fin rays, with tip of filament never reaching base of caudal-fin rays. Adult males with longer anal-fin, tip of longest ray reaching to distal half of ventralmost principal caudal-fin ray. Adult females with shorter anal-fin rays, tip of longest ray never reaching principal caudal-fin rays (Figs 3, 9).
Adult males more vividly coloured than females (Fig. 9). Coloration in live orange to red, with dorsal and anal fins more intensely pigmented. Adult males with inconspicuous red stripe on leading margin of dorsal-fin filaments and conspicuous band along first six anal-fin rays (Fig. 9A). Females and immature specimens of both sexes paler, with overall yellow to gold coloration, and dorsal, pelvic and anal fins yellow to orange. Dorsal and anal fins lacking red stripe on leading margin (see ‘Colour in life’ section for further details) (Fig. 9B). Bony processes were not observed on fin-rays of either sex.
Etymology:
The specific name callipterus is Latinized from the Greek κάλλη [kalli], beauty, and πτερων [pteron], feather or wing, in reference to the vivid coloration of the dorsal-fin of adult males. It is a declinable adjective.
Geographic distribution:
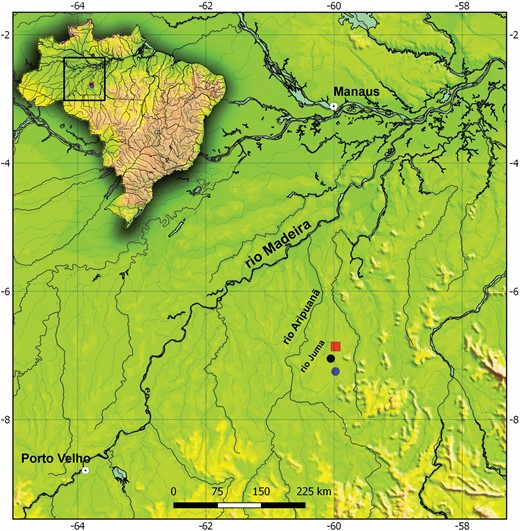
Poecilocharax callipterus is known from the type locality, a black water tributary of the Rio Juma, in the Rio Aripuanã drainage, Rio Madeira Basin, Apuí Municipality, Amazonas State, Brazil (Fig. 10).
Type-locality (red square) of Poecilocharax callipterus sp. nov. and P. rhizophilus sp. nov. (blue dot), tributary of the Rio Juma, Rio Aripuanã drainage, Rio Madeira Basin, Apuí, Amazonas, Brazil.
Ecological notes:
The type locality of Poecilocharax callipterus is a small, black-water stream (Fig. 11). The stream is located at an elevation of 140 m, is 1–3 m wide, 0.3–1.0 m deep, with slow-flowing water and a predominantly rocky substrate. The stream runs across a small Cerrado (= savannah) enclave into the Amazon Forest. Specimens were collected during daytime and were in low abundance. Different environments and habitats were explored, but the new species was captured specifically among aquatic grasses or roots near the margins. Schools were not observed. Individuals of P. callipterus were observed during the night in deeper portions of the stream (1 m) and near the marginal vegetation or between subaquatic roots of riparian vegetation (Fig. 11). Several small streams were sampled in two field expeditions carried out in 2015 and 2016, but only the type locality had aquatic vegetation and a rocky bottom. Thus, we infer that P. callipterus may have specific habitat requirements. At the type locality of P. callipterus, characids are absent and this is apparently the only species to forage the medium-upper water column. Fish species sampled syntopically in the type locality were Callichthys callichthys (Linnaeus, 1758), Erythrinus erythrinus (Bloch & Schneider, 1801) and three unidentified species of Aequidens Eigenmann & Bray, 1894, Brachyhypopomus Mago-Leccia, 1994 and Lepthoplosternum Reis, 1997. The stomach content analysis of five paratypes revealed the presence of filamentous algae, nematodes, chironomids, Cladocera, sediment and sand. One male and two female specimens collected in October had gonads moderately developed.
Tributary of Rio Canadá, Rio Juma drainage, Rio Aripuanã Basin, Apuí, Amazonas, Brazil, type-locality of Poecilocharax callipterus sp. nov. In detail the microenvironment where P. callipterus sp. nov. was captured.
Conservation assessment:
The risk of extinction for this species is preliminarily assessed as critical. Poecilocharax callipterus is a species of restricted geographical range, known only from one small, black-water tributary of the Rio Juma (Rio Aripuanã drainage), near the city of Apuí. The new species was discovered in a stream that crosses the highway AM-174 (linking Apuí and Novo Aripuanã), a region surrounded by a small and relatively degraded forest area currently being occupied by pasture. There are three Conservation Units surrounding the type locality (Floresta Nacional do Jatuarana to the south, Parque Nacional do Acari to the east and Floresta Nacional do Aripuanã to the west), and some still preserved, unprotected areas near the type locality. However, the new species was not found elsewhere other than the type locality, despite intense collecting efforts, and the presence of P. callipterus within these Conservation Units or in surrounding areas remains uncertain but seems unlikely. Sampling activities took place in 2015 and 2016, and at that time, the type locality did not present signs of silting, water turbidity and erosion on the banks (Fig. 11). However, the territory of Apuí has been intensely impacted by deforestation ever since the discovery of the new species, and it is currently ranked in second place in a list of Brazilian municipalities with greatest loss of native forest (Fonseca et al., 2021). The Area of Occupation of the new species is estimated as 4 km2 (B2). A continuous decline in the habitat quality is expected and inferred based on erosion, silting and increased turbidity, as results of continued high deforestation in the region. The number of locations is one. There are no estimates of population size or population decline. For these reasons, P. callipterus is tentatively assessed as Near Threatened (NT) approaching Critically Endangered (CR) by criterion B2ab(iii) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Sub-Committee, 2019).
Poecilocharax rhizophilus sp. nov.
(Figs 12–14; Table 3)
Zoobank registration:
urn:lsid:zoobank.org:act:1B74686B-BA11-4F5F-829F-28ECD9A13C1E.
Holotype:
MZUSP 121652, male, 20.3 mm SL, BRAZIL, Amazonas State, Apuí, Igarapé Mutum, tributary of the Rio Juma, Rio Aripuanã drainage, Rio Madeira Basin, 7º14′58′′S 59º58′40′′W, 123 m a.s.l., 10 October 2016, O. Oyakawa, W. Ohara, M. Pastana and T. Teixeira (collectors).
Paratypes:
BRAZIL, Amazonas State, Apuí. MZUSP 121651 (30, 15.9–23.4 mm SL; 3 C&S, 14.9–17.9 mm SL; 4 MOL, 16.7–19.5 mm SL); INPA 59405 (5, 16.6–17.5 mm SL); MNRJ 51748 (5, 16.1–19.1 mm SL), same data as the holotype. MZUSP 117653 (1, 16.2 mm SL), Rio Madeira Basin, Rio Aripuanã drainage, Rio Juma upstream from Paredão waterfall, 7º02′58′′S 60º03′04′′W, 23 June 2015, W. M. Ohara and V. Abrahão (collectors).
Diagnosis:
Poecilocharax rhizophilus can be promptly distinguished from its congeners, except P. weitzmani, by having the anterior and posterior nares separated from each other by a distance equal to or greater than anterior nostril diameter (see Géry, 1965: fig. 11; vs. anterior and posterior nares separated only by a narrow skin fold); and by the absence of a nasal flap (vs. presence). The new species can be distinguished from P. weitzmani by the absence of a dark suborbital bar (vs. presence); absence of maxillary teeth (vs. presence); presence of a small unbranched ray preceding the two long unbranched anal-fin rays (vs. absence); presence of a dark humeral spot (vs. absence); absence of dark pigmentation on the median margin of the branchiostegal membrane in dimorphic males (vs. presence) and by its overall yellowish colour in life (vs. overall colour in life reddish).
Description:
Morphometric data presented in Table 3. Miniature species, largest specimen analysed reaching 23.3 mm SL (Fig. 12). Body moderately compressed and elongated. Greatest body depth approximately at vertical through tip of pectoral fin. Dorsal profile convex between tip of snout and base of last dorsal-fin ray; straight or slightly concave between that point to origin of anteriormost dorsal procurrent caudal-fin ray. Ventral profile of head convex; ventral profile of the body straight or slightly convex from posterior portion of the head to anal-fin origin; slightly convex along anal-fin base; straight or slightly concave between terminus of anal fin to origin of anteriormost ventral procurrent caudal-fin ray. Body elliptical in cross-section at pectoral-fin origin, broader ventrally, gradually becoming more compressed toward caudal-fin base.
Morphometric data for holotype and paratypes (range) of Poecilocharax rhizophilus sp. nov. (N = 30) SD = standard deviaton
| Characters . | Holotype . | Range . | Median ± SD . |
|---|---|---|---|
| Total length (mm) | 26.0 | 20.6–29.6 | 22.1 |
| Standard length (mm) | 20.3 | 16.0–23.3 | 17.4 ± 1.8 |
| Percentage of standard length | |||
| Head length | 30.6 | 27.8–31.9 | 30.3 ± 1.1 |
| Prepectoral distance | 30.9 | 30.1–33.7 | 32.0 ± 1.0 |
| Pectoral-fin height | 15.0 | 12.5–16.1 | 14.2 ± 1.0 |
| Predorsal distance | 48.8 | 47.2–51.4 | 49.2 ± 1.1 |
| Dorsal-fin height | 19.5 | 19.0–24.0 | 21.9 ± 1.6 |
| Dorsal-fin base | 24.0 | 21.1–25.7 | 23.5 ± 1.1 |
| Prepelvic distance | 49.1 | 49.0–53.1 | 50.6 ± 1.1 |
| Pelvic-fin height | 18.3 | 15.0–19.3 | 17.9 ± 1.0 |
| Pre-anal distance | 68.0 | 68.0–73.6 | 70.3 ± 1.3 |
| Anal–apex distance | 88.8 | 86.1–92.6 | 88.8 ± 1.5 |
| Anus to anal-fin distance | 1.9 | 1.4–2.7 | 2.1 ± 0.3 |
| Anal fin height | 19.9 | 16.4–21.1 | 18.2 ± 1.1 |
| Anal fin base | 12.7 | 10.8–13.8 | 12.6 ± 0.9 |
| Peduncle length | 20.2 | 17.4–21.9 | 19.3 ± 1.2 |
| Body depth at dorsal-fin origin | 27.2 | 23.5–28.4 | 26.6 ± 1.1 |
| Body depth at anal-fin origin | 19.9 | 16.0–20.1 | 18.0 ± 1.0 |
| Body depth at caudal peduncle | 12.6 | 9.4–12.6 | 11.6 ± 0.7 |
| Body width | 9.8 | 7.8–11.1 | 9.5 ± 0.8 |
| Percentage of head length | |||
| Snout length | 17.6 | 15.8–21.1 | 17.9 ± 1.2 |
| Maxila length | 33.4 | 29.2–34.1 | 32.3 ± 1.4 |
| Anterior naris-orbit | 12.3 | 12.3–16.7 | 13.8 ± 1.6 |
| Posterior naris-orbit | 15.8 | 11.8–16.7 | 14.4 ± 1.5 |
| Cheek | 13.2 | 10.9–15.8 | 13.3 ± 1.2 |
| Orbital diameter | 37.8 | 37.0–42.1 | 39.5 ± 1.3 |
| Interorbital diameter | 30.5 | 27.9–32.5 | 31.2 ± 1.3 |
| Characters . | Holotype . | Range . | Median ± SD . |
|---|---|---|---|
| Total length (mm) | 26.0 | 20.6–29.6 | 22.1 |
| Standard length (mm) | 20.3 | 16.0–23.3 | 17.4 ± 1.8 |
| Percentage of standard length | |||
| Head length | 30.6 | 27.8–31.9 | 30.3 ± 1.1 |
| Prepectoral distance | 30.9 | 30.1–33.7 | 32.0 ± 1.0 |
| Pectoral-fin height | 15.0 | 12.5–16.1 | 14.2 ± 1.0 |
| Predorsal distance | 48.8 | 47.2–51.4 | 49.2 ± 1.1 |
| Dorsal-fin height | 19.5 | 19.0–24.0 | 21.9 ± 1.6 |
| Dorsal-fin base | 24.0 | 21.1–25.7 | 23.5 ± 1.1 |
| Prepelvic distance | 49.1 | 49.0–53.1 | 50.6 ± 1.1 |
| Pelvic-fin height | 18.3 | 15.0–19.3 | 17.9 ± 1.0 |
| Pre-anal distance | 68.0 | 68.0–73.6 | 70.3 ± 1.3 |
| Anal–apex distance | 88.8 | 86.1–92.6 | 88.8 ± 1.5 |
| Anus to anal-fin distance | 1.9 | 1.4–2.7 | 2.1 ± 0.3 |
| Anal fin height | 19.9 | 16.4–21.1 | 18.2 ± 1.1 |
| Anal fin base | 12.7 | 10.8–13.8 | 12.6 ± 0.9 |
| Peduncle length | 20.2 | 17.4–21.9 | 19.3 ± 1.2 |
| Body depth at dorsal-fin origin | 27.2 | 23.5–28.4 | 26.6 ± 1.1 |
| Body depth at anal-fin origin | 19.9 | 16.0–20.1 | 18.0 ± 1.0 |
| Body depth at caudal peduncle | 12.6 | 9.4–12.6 | 11.6 ± 0.7 |
| Body width | 9.8 | 7.8–11.1 | 9.5 ± 0.8 |
| Percentage of head length | |||
| Snout length | 17.6 | 15.8–21.1 | 17.9 ± 1.2 |
| Maxila length | 33.4 | 29.2–34.1 | 32.3 ± 1.4 |
| Anterior naris-orbit | 12.3 | 12.3–16.7 | 13.8 ± 1.6 |
| Posterior naris-orbit | 15.8 | 11.8–16.7 | 14.4 ± 1.5 |
| Cheek | 13.2 | 10.9–15.8 | 13.3 ± 1.2 |
| Orbital diameter | 37.8 | 37.0–42.1 | 39.5 ± 1.3 |
| Interorbital diameter | 30.5 | 27.9–32.5 | 31.2 ± 1.3 |
Morphometric data for holotype and paratypes (range) of Poecilocharax rhizophilus sp. nov. (N = 30) SD = standard deviaton
| Characters . | Holotype . | Range . | Median ± SD . |
|---|---|---|---|
| Total length (mm) | 26.0 | 20.6–29.6 | 22.1 |
| Standard length (mm) | 20.3 | 16.0–23.3 | 17.4 ± 1.8 |
| Percentage of standard length | |||
| Head length | 30.6 | 27.8–31.9 | 30.3 ± 1.1 |
| Prepectoral distance | 30.9 | 30.1–33.7 | 32.0 ± 1.0 |
| Pectoral-fin height | 15.0 | 12.5–16.1 | 14.2 ± 1.0 |
| Predorsal distance | 48.8 | 47.2–51.4 | 49.2 ± 1.1 |
| Dorsal-fin height | 19.5 | 19.0–24.0 | 21.9 ± 1.6 |
| Dorsal-fin base | 24.0 | 21.1–25.7 | 23.5 ± 1.1 |
| Prepelvic distance | 49.1 | 49.0–53.1 | 50.6 ± 1.1 |
| Pelvic-fin height | 18.3 | 15.0–19.3 | 17.9 ± 1.0 |
| Pre-anal distance | 68.0 | 68.0–73.6 | 70.3 ± 1.3 |
| Anal–apex distance | 88.8 | 86.1–92.6 | 88.8 ± 1.5 |
| Anus to anal-fin distance | 1.9 | 1.4–2.7 | 2.1 ± 0.3 |
| Anal fin height | 19.9 | 16.4–21.1 | 18.2 ± 1.1 |
| Anal fin base | 12.7 | 10.8–13.8 | 12.6 ± 0.9 |
| Peduncle length | 20.2 | 17.4–21.9 | 19.3 ± 1.2 |
| Body depth at dorsal-fin origin | 27.2 | 23.5–28.4 | 26.6 ± 1.1 |
| Body depth at anal-fin origin | 19.9 | 16.0–20.1 | 18.0 ± 1.0 |
| Body depth at caudal peduncle | 12.6 | 9.4–12.6 | 11.6 ± 0.7 |
| Body width | 9.8 | 7.8–11.1 | 9.5 ± 0.8 |
| Percentage of head length | |||
| Snout length | 17.6 | 15.8–21.1 | 17.9 ± 1.2 |
| Maxila length | 33.4 | 29.2–34.1 | 32.3 ± 1.4 |
| Anterior naris-orbit | 12.3 | 12.3–16.7 | 13.8 ± 1.6 |
| Posterior naris-orbit | 15.8 | 11.8–16.7 | 14.4 ± 1.5 |
| Cheek | 13.2 | 10.9–15.8 | 13.3 ± 1.2 |
| Orbital diameter | 37.8 | 37.0–42.1 | 39.5 ± 1.3 |
| Interorbital diameter | 30.5 | 27.9–32.5 | 31.2 ± 1.3 |
| Characters . | Holotype . | Range . | Median ± SD . |
|---|---|---|---|
| Total length (mm) | 26.0 | 20.6–29.6 | 22.1 |
| Standard length (mm) | 20.3 | 16.0–23.3 | 17.4 ± 1.8 |
| Percentage of standard length | |||
| Head length | 30.6 | 27.8–31.9 | 30.3 ± 1.1 |
| Prepectoral distance | 30.9 | 30.1–33.7 | 32.0 ± 1.0 |
| Pectoral-fin height | 15.0 | 12.5–16.1 | 14.2 ± 1.0 |
| Predorsal distance | 48.8 | 47.2–51.4 | 49.2 ± 1.1 |
| Dorsal-fin height | 19.5 | 19.0–24.0 | 21.9 ± 1.6 |
| Dorsal-fin base | 24.0 | 21.1–25.7 | 23.5 ± 1.1 |
| Prepelvic distance | 49.1 | 49.0–53.1 | 50.6 ± 1.1 |
| Pelvic-fin height | 18.3 | 15.0–19.3 | 17.9 ± 1.0 |
| Pre-anal distance | 68.0 | 68.0–73.6 | 70.3 ± 1.3 |
| Anal–apex distance | 88.8 | 86.1–92.6 | 88.8 ± 1.5 |
| Anus to anal-fin distance | 1.9 | 1.4–2.7 | 2.1 ± 0.3 |
| Anal fin height | 19.9 | 16.4–21.1 | 18.2 ± 1.1 |
| Anal fin base | 12.7 | 10.8–13.8 | 12.6 ± 0.9 |
| Peduncle length | 20.2 | 17.4–21.9 | 19.3 ± 1.2 |
| Body depth at dorsal-fin origin | 27.2 | 23.5–28.4 | 26.6 ± 1.1 |
| Body depth at anal-fin origin | 19.9 | 16.0–20.1 | 18.0 ± 1.0 |
| Body depth at caudal peduncle | 12.6 | 9.4–12.6 | 11.6 ± 0.7 |
| Body width | 9.8 | 7.8–11.1 | 9.5 ± 0.8 |
| Percentage of head length | |||
| Snout length | 17.6 | 15.8–21.1 | 17.9 ± 1.2 |
| Maxila length | 33.4 | 29.2–34.1 | 32.3 ± 1.4 |
| Anterior naris-orbit | 12.3 | 12.3–16.7 | 13.8 ± 1.6 |
| Posterior naris-orbit | 15.8 | 11.8–16.7 | 14.4 ± 1.5 |
| Cheek | 13.2 | 10.9–15.8 | 13.3 ± 1.2 |
| Orbital diameter | 37.8 | 37.0–42.1 | 39.5 ± 1.3 |
| Interorbital diameter | 30.5 | 27.9–32.5 | 31.2 ± 1.3 |
Poecilocharax rhizophilus sp. nov.: A, holotype, male, 20.3 mm SL, MZUSP 121652; B, paratype, male, 23.1 mm SL, MZUSP 121651; C, paratype, female, 23.3 mm SL, MZUSP 121651.
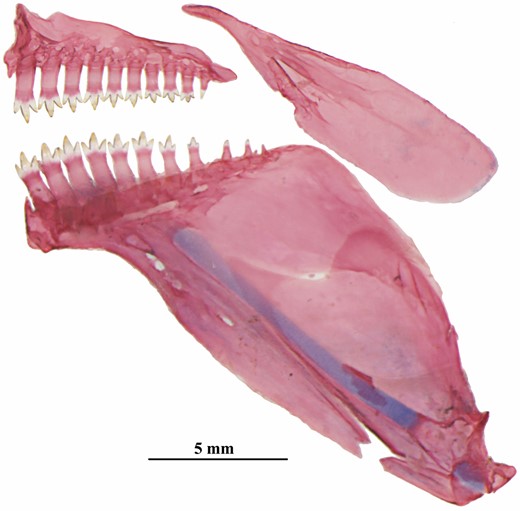
Mouth small, terminal, jaws vertically aligned anteriorly. Snout shorter than eye diameter, gently rounded. Maxilla short, reaching level of anterior margin of orbit. Orbital margin free. Cheek narrow, less than half of eye diameter. Anterior and posterior nares conspicuously separated from each other; lacking a skin flap. Anterior naris circular, posterior naris crescent-shaped. Dorsal profile of head concave, concavity extending anteriorly from mesethmoid to midlength of frontal bones portion, allocating a frontal organ. Each frontal containing one relatively large foramen for passage of the trigeminal branch ophthalmicus superficialis that innervates the organ. Supraorbital absent. Antorbital positioned dorso-anteriorly to infraorbital 1; infraorbitals 2, 3 and 4 present (3); infraorbitals 5 and 6 absent (3). Pseudotympanum present, underneath third to fifth scales of longitudinal line.
Dentary in one row with 11–13 (3) conical to tricuspid teeth. Premaxillary teeth in a single row with ten to 11 (3) conical to tricuspid teeth. Premaxillary and dentary teeth increasing in size toward symphysis. Maxillary teeth absent (Fig. 13). Ectopterygoid with minute teeth. Branchiostegals five (2); four attached to anterior ceratohyal and one to posterior ceratohyal. First arch with two gill rakers on hypobranchial (3), six on ceratobranchial (3), one on cartilage between ceratobranchial and epibranchial (3) and five (2) or six (1) on epibranchial. Supraneurals four (3), cartilaginous.
Poecilocharax rhizophilus sp. nov., medial view of premaxilla, maxilla and dentary, right side, MZUSP 121651, paratype, 16.5 mm SL.
Laterosensory system developmentally truncated, tubules opening in individual pores (i.e. never forming subpores). Supraorbital canal present, posteriorly truncated, associated with the nasal and frontal bones only. Parietal branch of supraorbital canal absent. Pre-opercular canal present, restricted to the horizontal axis of the pre-opercular bone. Infraorbital, mandibular, otic, postotic, supratemporal canals absent.
Scales cycloid; circuli distributed over entire area of scale. Two to five parallel and well-defined radii present on posterior portion of scale. Trunk lateral-line poorly developed, short, visible along first five to seven scales from longitudinal row. No visible pores on scales (except in two specimens, with pores on fifth and seventh scales, respectively). Longitudinal series with 28 (7), 29* (8), 30 (10) or 31 (2) scales. Horizontal scale rows from dorsal-fin origin to lateral line four* (29). Horizontal scale rows from pelvic-fin origin to lateral line four* (26). Scales around caudal peduncle ten (1), 11(3), 12* (21) or 13 (1). Predorsal scales series seven (1), eight* (8), nine (12), ten (4) or 12 (1), covered by skin. Isthmus with scales reaching anteroventral margin of cleithrum anteriorly. Scales between anus and anal fin none (6) or one* (24). Anterior portions of scales with vertically oriented superficial neuromasts. Neuromast rows arched anteriorly and more conspicuous on anterior half of body.
Pectoral-fin rays i, ten (2) or 11 (1). Tip of longest pectoral-fin rays anterior to pelvic-fin origin and nearly at vertical through dorsal-fin origin. Pelvic-fin rays i,six,i* (30). Tip of longest pelvic-fin rays slightly anterior to anal-fin origin, close to vertical through last ray of dorsal-fin base. Dorsal-fin rays iii,12,i (3), iii,13,i* (25) or iii,14,i (2); in the three C&S specimens there is a fourth additional anteriormost unbranched ray below the skin, not visible in alcohol-preserved specimens. Distal margin of dorsal fin straight to rounded. Dorsal-fin origin slightly anterior to vertical through pelvic-fin origin. First dorsal-fin pterygiophore located behind neural spine of eighth (1) or ninth (2) vertebrae. Externally visible anal-fin rays ii,six (12) or ii,seven* (18) with distal margin straight to rounded; in three C&S specimens there is a third, small anteriormost unbranched anal-fin ray below skin, not visible in alcohol-preserved specimens. Anal-fin origin slightly posterior to vertical through last dorsal-fin ray. Scales covering base of anteriormost anal-fin rays none (5), one* (22) or two (3). First dorsal anal-fin pterygiophore located behind neural spine of 18th (2) or 19th (1) vertebrae. Principal caudal-fin rays i,9,8,i* (2). Caudal fin naked. Five (1) or six (2) dorsal procurrent caudal-fin rays and five (3) ventral procurrent caudal-fin rays. Adipose fin absent. Hypurals one and two fused to each other; hypurals three to six autogenous. Total vertebrae 31 (3); precaudal vertebrae 18 (2) or 19 (1), caudal vertebrae 12 (1) or 13 (2).
Colour in alcohol:
Overall background coloration of head and body beige, darker dorsally, with chromatophores densely distributed along two or three dorsalmost series of scales (Fig. 12). Small, dark chromatophores densely concentrated on premaxilla, tip of dentary and maxilla. Head with a dark diffuse stripe extending from tip of snout to dorsal half of opercle, crossing eyes. Ventral portion of head and gular region with silvery tinge and few, scattered, dark chromatophores. Eyes predominantly dark with scattered pigmentation on ventral portion. Suborbital stripe absent. Guanine on ventral portion of opercle, pre-opercle, subopercle and interopercle. Body with one dark, irregularly shaped blotch present immediately posterior to opercle, continuous with a dark midlateral stripe, which extends to tip of median caudal-fin rays. Midlateral stripe encompasses two series of scales vertically, overlapping with dark blotch on its anterior portion and extending to tip of middle caudal-fin rays.
Dorsal-fin coloration sexually dimorphic (Fig. 12). Adult males having dark chromatophores on rays and interradial membranes; chromatophores roughly forming two to five descending dark stripes. Stripes restricted to anterior two-thirds of the dorsal fin, encompassing the first ten to12 dorsal-fin rays. Remaining dorsal-fin area hyaline. Females and immature specimens of both sexes with dorsal fin mostly hyaline, except for few, dark, scattered chromatophores restricted to anterior edge of fin. Pectoral and pelvic fins predominantly hyaline, with few, scattered chromatophores present along margins of rays. Anal-fin coloration sexually dimorphic. Adult males having three to four ascending, dark, irregular stripes on anal fin formed by chromatophores along rays and interradial membranes. Stripes present on first eight to nine anal-fin rays. Remaining anal-fin area hyaline. Anal fin of females and immature specimens of both sexes with chromatophores restricted to margin of rays and interradial membrane, lacking stripes. Caudal fin mostly hyaline, except of middle caudal-fin rays; dorsal and ventral edge with chromatophores along rays and interradial membranes. Upper and lower caudal-fin base with concentration of brown pigmentation.
Colour in life:
Overall body coloration yellowish, darker dorsally (Fig. 14). Abdominal region yellow to silvery. Humeral spot and midlateral stripe usually visible in live specimens. Males and females similar in life coloration. Head yellow dorsally, silver ventrally. Guanine deposits on infraorbitals 2 and 3, cheeks, ventral portion opercular bone, along sub- and interopercle. Upper half of opercle golden. Dentary, premaxillary, and maxillary yellowish.
Poecilocharax rhizophilus sp. nov., immediately after capture. A, holotype, male, 20.3 mm SL, MZUSP 121652. Paratypes: B, male, 23.1 mm SL; C, female, 23.3 mm SL, MZUSP 121651.
Dorsal-fin coloration sexually dimorphic. Adult males lacking yellow pigment on the base of the fin and with dark pigments arranged in descending stripes (see ‘Colour in alcohol’). Females and immature specimens of both sexes with proximal portion of interradial membranes yellow from first 11–14 anteriormost dorsal-fin rays and lacking dark pigmentation. Pectoral fin predominantly hyaline and pelvic fin yellowish in both sexes. Yellow pigmentation more concentrated on base of fin rays. Anal-fin coloration sexually dimorphic; adult females with more conspicuous yellow coloration, and adult males with dark blotches forming irregular ascending dark stripes. Pigmentation on females and juveniles of both sexes stronger on base of first five branched anal-fin rays. Caudal fin mostly yellowish, except from middlemost caudal fin rays, with black pigmentation following from dark midlateral stripe.
Sexual dimorphism:
Dorsal fin of adult males with two to five conspicuous dark descending stripes, restricted to anterior two-thirds of the fin; anal fin with three or four ascending dark stripes on rays, present along the first eight or nine anal-fin rays (Fig. 14A). Females and immature specimens of both sexes with dorsal and anal fins mostly hyaline (Fig. 14B). Further details of dimorphic coloration in ‘Colour in alcohol’ section. Bony hooks were not observed on fins of either sex.
Etymology:
The specific name rhizophilus is Latinized from the Greek words ρίζα [rhiza], root, and φίλος [philos], friend. The name refers to habitat where the species was collected, in between subaquatic roots of riparian vegetation. It is a declinable adjective.
Geographic distribution:
Poecilocharax rhizophilus is known from two localities, both upstream from Paredão Falls, in the Rio Juma, middle Rio Aripuanã drainage, Rio Madeira Basin, Apuí Municipality, Amazonas State, Brazil (Fig. 10).
Ecological notes:
Poecilocharax rhizophilus occurs in black (Rio Juma) and clear water (Igarapé Mutum). The type locality is a tributary of the Rio Juma (Igarapé Mutum), that runs through impacted pasture area, at an elevation of 123 m, with swift current, 2–4 m wide, 1–2 m deep and a substrate composed mainly of mud and sand. One specimen was captured in the Rio Juma, which is a black-water river. Different micro-environments were exhaustively sampled during collecting activities, but P. rhizophilus was only captured in the medium-upper water column between subaquatic roots of riparian vegetation (Fig. 15). Other species sampled syntopically with P. rhizophilus were Acestrorhynchus falcatus (Bloch, 1794), Bario steindachneri (Eigenmann, 1893), Characidium sp., Corydoras gracilis Nijssen & Isbrücker, 1976, Gymnotus coropinae Hoedeman, 1962, Hemigrammus ocellifer (Steindachner, 1882), Hoplias malabaricus (Bloch, 1794), Hyphessobrycon platyodus Ohara, Abrahão & Espíndola, 2017, H. procyonPastana & Ohara, 2016, Moenkhausia comma Eigenmann, 1908, Otocinclus mura Schaefer, 1997, Satanoperca jurupari (Heckel, 1840) and Tatia dunni (Fowler, 1945). The analysis of the stomach contents of three paratypes of P. rhizophilus revealed the presence of Cladocera (Chydoridae), Chironomidae, unidentified insect fragments and inorganic sediments.
Igarapé Mutum, tributary of Rio Juma, Rio Aripuanã Basin, Apuí, Amazonas, Brazil, type-locality of Poecilocharax rhizophilus sp. nov. Inset details the microenvironment where P. rhizophilus sp. nov. was captured.
Conservation assessment:
The extinction risk for this species is preliminarily assessed as high. Poecilocharax rhizophilus is a species of restricted geographical range, with an Extent of Occurrence (EOO) of 50 km2 and known from two localities (Fig. 10). The type locality has moderate riparian forest surrounded by pasture, and the other site is a touristic area (Paredão Falls), intensely impacted by agriculture. There are three Conservation Units surrounding the type locality (Floresta Nacional do Jatuarana to the south, Parque Nacional do Acari to the east and Floresta Nacional do Aripuanã to the west) and there are some preserved, but unprotected areas nearby the type locality. However, the new species was not found elsewhere other than the type locality, despite intense collecting efforts, and it is uncertain if P. rhizophilus is present in these Conservation Units or in other areas. Sampling activities took place in 2015 and 2016, and at that time, the two localities where the species was found (i.e. the Igarapé Mutum and Rio Juma) did not present signs of silting, water turbidity and erosion on the banks (Fig. 15). However, the territory of Apuí has been heavily impacted by deforestation since the discovery of the new species, and it is currently ranked in second place in a list of Brazilian municipalities with greatest loss of native forest (Fonseca et al., 2021). A continuous decline in the habitat quality is expected and inferred based on erosion, silting and increased turbidity, as results of continued deforestation in the region. There are no estimates of population size or population decline. For these reasons, P. rhizophilus is tentatively assessed Near Threatened (NT) by criterion B2ab(iii) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Sub-Committee, 2019).
1a. Asymmetrical dark spot on caudal peduncle present; dark midlateral stripe on flanks absent....................2
1b. Asymmetrical dark spot on caudal peduncle absent; dark midlateral stripe on flanks present (sometimes diffuse)…..………................................................................................................................................................3
2a. Adipose fin present; dark blotches on flank absent; posterior terminus of maxilla surpassing vertical through anterior margin of orbit.……………..................................................................…Crenuchus spilurus
2b. Adipose fin absent; dark blotches on flank present; posterior terminus of maxilla never surpassing vertical through anterior margin of the orbit...…...................................................................Poecilocharax callipterus
3a. Two dark, longitudinal stripes positioned dorsally and ventrally along the head and body; anterior and posterior nasal openings separated by a narrow skin fold; presence of nasal flap between anterior and posterior nares………….….……................................................................................Poecilocharax bovaliorum
3b. One dark, longitudinal stripe positioned on middle of flank; anterior and posterior nasal openings widely separated from each other; absence of a nasal flap….……...............……........................................................4
4a. Suborbital bar present; humeral blotch absent; dimorphic males with dark pigmentation on medial margin of branchiostegal membrane....................................................................................…Poecilocharax weitzmani
4b. Suborbital bar absent; humeral blotch present; dimorphic males lacking dark pigmentation on medial margin of branchiostegal membrane.....................................................................….Poecilocharax rhizophilus
DISCUSSION
Genetic structure and monophyly of Crenuchinae based on molecular and morphological data
Currently, molecular analyses are used as an additional methodology to help with species delimitation in Neotropical fishes and to support new species descriptions (e.g. Barreto et al., 2018; Ota et al., 2020). In this study, the results of genetic analysis (DNA barcodes and species delimitation method ABGD) confirmed Poecilocharax callipterus and P. rhizophilus as distinct taxa in Crenuchinae, which have been formally described as species herein based on morphological characters. The values of genetic divergence between the analysed species were high, indicating genetic structuring. Several authors have suggested that inferences regarding species boundaries, based on genetic data alone, are probably inadequate, and that species delimitation should be conducted with consideration of other factors, such as morphology, geographical distribution and behaviour (Carstens et al., 2013; Camelier et al., 2018), as is done in this study. Because some studies involving different orders, families and genera of fishes have indicated that high intraspecific values (as found in both Crenuchus spilurus and Poecilocharax weitzmani) could signal cryptic species and/or possible new species within the analysed group (e.g. Ward et al., 2009; April et al., 2011; Pereira et al., 2013), this variation plus the ABGD results should be investigated in more detail in future studies, but it is not within the scope of our study.
The monophyly of Crenuchinae has never been contested on the grounds of cladistic analyses. Previous molecular phylogenetic studies have indicated a robust sister-group relationship between Crenuchus spilurus and Poecilocharax weitzmani (Oliveira et al., 2011: fig. 6), a clade that is recovered as sister-group to Characidiinae. Accordingly, our phylogenetic analysis indicated a monophyletic Crenuchinae that is strongly supported by COI sequences (Fig. 1). The results obtained herein are the same as obtained by Oliveira et al. (2011) in retrieving Crenuchus and Poecilocharax as sister-lineages, a topology that remains stable even after expanding the analysis to encompass all five representatives of this subfamily (i.e. two genera and five species, including the two described herein).
Morphological studies, on the other hand, have never tested the monophyly of Crenuchinae, including all species and using a cladistic framework. Currently, the synapomorphies listed for the subfamily follows Buckup (1998), a study that provided a list of 12 morphological characters to support the monophyly of Crenuchinae. The analysis included only one representative of the subfamily (Crenuchus spilurus; Buckup, 1998: table 1). Poecilocharax weitzmani and P. bovaliorum were not included as terminals in Buckup’s cladistic analysis, although they were analysed as comparative material and confirmed for the synapomorphies retrieved for Crenuchinae.
From the 12 synapomorphies listed for the monophyly of Crenuchinae, three (Buckup, 1998: 136, chars 1–3) are related to modifications involving the frontal organ (Fig. 4): (1) the enlargement of the frontal foramina; (2) the presence of the frontal organ; and (3) the depression of the frontal bone that serves to allocate the frontal organ and that contains the frontal foramina. Our examination confirms the existence of a frontal organ and the modification of the frontal bones in all species of Crenuchinae, and thus corroborates it as a synapomorphy for the subfamily. Little has been published concerning the morphology and function of the frontal organ, and the available descriptions of this structure dates back to Alexander (1963), Géry (1963) and Bossy et al. (1965). According to these authors, the frontal organ runs across the paired frontal foramina and provides a direct contact between the central nervous system (via ophthalmic branching from the trigeminal nerve and a dedicated ophthalmic ganglion) and the superficial epithelium. Although probably a sense organ (Alexander, 1963), the frontal organ is not related to the cephalic laterosensory system (Bossy et al., 1965). The similarities between the frontal organ and the cephalic lateral-lines are too distant, both anatomically and structurally, to trace a relation between both systems (Bossy et al., 1965).
Buckup (1998: 136, chars 4–12) provided nine additional synapomorphies for Crenuchinae: (4) lateral ethmoidal wing well developed; (5) lack of connection between supraorbital and otic canals; (6) absence of supraorbital bones; (7) presence of mesopterygoid (= endopterygoid) teeth; (8) dentary bones with hinge-like joint; (9) lamellar portion of large supraneural without contact with neural spine; (10) coracoid small; (11) fusion of postcleithra 1 and 2; and (12) fusion of hypurals 1 and 2. Although these characters are not exclusive to Crenuchinae, they were considered phylogenetically informative due to their absence in Characidiinae (the immediate outgroup of Crenuchinae) and the restricted occurrence in Characiformes. Our detailed morphological analysis of Crenuchus spilurus and the four species of Poecilocharax corroborates the occurrence of all nine character-states in members of the subfamily. Complementary to Buckup’s (1998) list of synapomorphies, we provide five morphological characteristics with restricted occurrence in Crenuchidae and Characiformes that further strengthens the monophyly of Crenuchinae. Phylogenetic interpretations offered below are based on maximum parsimony optimizations of non-ambiguous characters on to Buckup’s (1993b, 1998) trees. They are provisionally listed as putative synapomorphies awaiting a formal cladistic study of the subfamily Crenuchinae to confirm their synapomorphic status.
Infraorbital canals truncated
All Crenuchinae examined herein have some degree of truncation in the infraorbital canal of the cephalic lateral-line system. In Characiformes, the infraorbital canal is usually disposed along the inner portion of the infraorbital bones 1 to 6, where it contacts the supraorbital and otic canals posterodorsally (Pastana et al., 2019). Within Crenuchidae, the integrity of the infraorbital canal is retained only in Characidiinae. Although these fishes lack true infraorbital bones (Buckup, 1993a; Pastana et al., 2019), the infraorbital canal is still continuous along the ventral margin of the orbit, and its posterodorsal end anastomoses with both the supraorbital and otic canals (Pastana et al., 2019: figs 4, 9). Representatives of Crenuchinae instead have a modification of this general pattern: in Crenuchus spilurus, Poecilocharax bovaliorum and P. callipterus, the infraorbital canal is absent along infraorbital plates 3 to 5, and in the two other species of Poecilocharax (i.e. P. rhizophilus and P. weitzmani), the entire infraorbital canal is missing.
Truncation of the infraorbital canal is common among Characiformes, although rarely used as phylogenetic information. Similar truncation of the infraorbital canal is observed in Gasteropelecidae (Pastana et al., 2019), some small-sized lebiasinids (e.g. Pyrrhulina Valenciennes, 1846 and Nannostomus Günther, 1872) (Weitzman, 1964), alestids (e.g. Ladigesia Géry, 1968 and Lepidarchus Roberts, 1966) (Zanata & Vari, 2005), distichodontids (e.g. some species of Neolebias Steindachner, 1894) (Vari, 1979) and several characids (e.g. species of Paracheirodon Géry, 1960, Erythrocharax altipinnisNetto-Ferreira et al., 2013a and Hyphessobrycon myrmexPastana et al., 2017) (Weitzman & Fink, 1983; Netto-Ferreira et al., 2013a; Pastana et al., 2017). According to Pastana et al. (2019), losses of lateral-line canal segments result from developmental truncation events, whereas canal segments associated with infraorbital bones 3 to 5 are among the first to be lost. Losses restricted to portions of the infraorbital canals (as occurs in Crenuchus spilurus, Poecilocharax bovaliorum and P. callipterus) are likely to be a result of truncations on the last steps of the development of the lateral-line system. Truncations present on P. rhizophilus and P. weitzmani are instead much more severe and probably occur in earlier steps of the lateral-line ontogeny (Pastana et al., 2019). Either way, all crenuchin species have some level of lateral-line truncation affecting the infraorbital canal.
Given the widespread occurrence of a complete infraorbital canal in Characidiinae, as well as in most Characiformes, the infraorbital-canal truncation of Crenuchinae (affecting segments associated with infraorbital plates 3 to 5 in Crenuchus spilurus, Poecilocharax callipterus and P. bovaliorum; and the entire infraorbital canal in P. rhizophilus and P. weitzmani) is herein listed as an additional synapomorphy for the subfamily.
Trunk lateral-line canal truncated
All Crenuchinae have the trunk lateral-line truncated posteriorly. In these fishes, the trunk canal is either restricted to the anterior half of the body associated with few longitudinal scales or entirely absent. In Crenuchus spilurus, the longitudinal series of scales is pored from the first five to eight scales. Among species of Poecilocharax, the number of pored scales varies from none (P. weitzmani and P. rhizophilus) up to five or six (P. bovaliorum and P. callipterus, respectively).
In Characidiinae, the immediate outgroup of Crenuchinae, lateral-line truncation occurs in several taxa, i.e. Characidium, Elachocharax, Microcharacidium, Odontocharacidium and Skiotocharax (e.g. Weitzman & Kanazawa, 1977; Weitzman, 1986; Buckup, 1993c; Zarske, 1997; Netto-Ferreira et al., 2013b; Mendonça & Netto-Ferreira, 2015; Rodrigues & Netto-Ferreira, 2020), although these events are often concentrated in phylogenetically restricted clades (Buckup, 1993b: char. 44, clade 6, C4; Netto-Ferreira et al., 2013b; Mendonça & Netto-Ferreira, 2015). Character optimization shows that a complete trunk lateral-line is the plesiomorphic condition among the subfamily Characidiinae (Buckup, 1993a). Similarly, a complete trunk lateral-line is widespread among Characiformes (Pastana et al., 2019), including the characoid families possibly closely related to Crenuchidae (Buckup, 1998). Therefore, the truncated lateral-line present in Crenuchinae is most parsimoniously interpreted as an apomorphic condition present in the subfamily and is listed herein as an additional putative synapomorphy for this group of fishes.
Interestingly, all examined crenuchin species share another modification of their laterosensory system, i.e. an abundance of superficial neuromasts distributed along the dorsal and lateral portions of the head, and on the proximal portion of the scales (Fig. 8). The superficial neuromasts (hereafter treated as SNs) of crenuchins are similar in size, orientation and position to SNs reported for other ostariophysans (e.g. Schmitz et al., 2008: 753, fig. 1A; Beckmann et al., 2010: 275, fig.1H; Menezes & Lucena, 2014: 196, figs 1, 2). Abundance of SNs are found in fish species that inhabit calm water habitats and that usually have a sedentary life-style (e.g. Dijkgraaf, 1963; Merrilees & Crossman, 1973a, b; Vischer, 1990), although some exceptions have been documented (i.e. Beckmann et al., 2010; Schmitz et al., 2014). In contrast, species that prefer running waters often have well-developed lateral-line canals and relatively fewer SNs (e.g. Engelmann et al., 2002, 2003; Bassett et al., 2006). Both the habitat and life style described for species with abundant SNs match our observations for the species of Poecilocharax described herein and the remaining of the crenuchin species, which all inhabit calm water and have a sedentary life-style.
Truncation of lateral-line canals has been documented to occur together with proliferation of superficial neuromasts (Poulson, 1963; Rizzato & Bichuette, 2016), and this process appears to be correlated with life in still waters or confined spaces. Since crenuchins display both a truncated lateral-line and densely distributed SNs along the dorsal and dorsolateral portions of the head and body, we opt for a cautious interpretation and treat both variables provisorily as co-dependent of each other. We thus list the lateral-line truncation as an additional putative synapomorphy for the Crenuchinae, interpreting the SN distribution as a possible morphological adaptation that is correlated to the trunk canal truncation.
Dorsal-fin rays numerous
Species of Crenuchinae have a high number of dorsal-fin rays when compared to other Characiformes. Both Crenuchus and Poecilocharax have three or four unbranched dorsal-fin rays, which are followed by 13 to 17 branched rays in the former, or 11 to 14 branched rays in the latter, thus ranging from 14 to 21 total elements.
Among the crenuchin subfamily Characidiinae, the dorsal-fin ray number varies from 11 to 14 (including both branched and unbranched rays), a count considered plesiomorphic for the subfamily and among Characiformes (Géry, 1971; Weitzman & Géry, 1981; Buckup, 1993b). Exceptions are found only among members of Elachocharax that may exhibit up to 22 dorsal-fin rays, including both branched and unbranched elements (Géry, 1965, 1971; Weitzman & Géry, 1981; Buckup, 1993c), a feature considered derived for the genus (Weitzman & Géry, 1981: fig. 7, char. 1). In Characiformes in general, the number of dorsal fin rays ranges from 11 to 14 elements (in total) and a high dorsal-fin ray count that matches those observed among Crenuchinae is known only in citharinids (e.g. Citharinus Cuvier, 1817: 20–23 elements), distichodontids (e.g. Xenocharax Günther, 1867: 19–24 elements) and serrasalmids (16–32 elements; Machado-Allison, 1983: 158, char. 2).
Current relationship hypotheses of Characiformes do not corroborate a sister-group relationship of Crenuchidae with Citharinoidei or Serrasalmidae, and the monophyly of both Citharinoidei (positioning Citharinidae as sister-group of Distichodontidae, sensu Vari, 1979; Buckup, 1998) and Serrasalmidae (sensuMachado-Allison, 1983) has remained unquestioned. Therefore, the elevated number of dorsal-fin rays seems to be an independently derived feature of Crenuchinae and is listed herein as an additional putative synapomorphy for the subfamily.
Presence of sexual dichromatism
All species of Crenuchinae have sexual dichromatism in some degree. Documentation of this dichromatism is available for Poecilocharax weitzmani (Beltrão et al., 2018), P. bovaliorum (Pastana et al., 2017) and for Crenuchus spilurus (Borghezan et al., 2019), all of which have males with more intense coloration in their dorsal and anal fins than females (e.g. Borghezan et al., 2019: fig. 1). In addition to these taxa, we expand the presence of sexual dichromatism to encompass both new species described herein, Poecilocharax callipterus and P. rhizophilus, based on observation of live and alcohol preserved specimens. In these, sexual dichromatism is expressed as differential presence of both black (melanophores) and red to orange (carotenoids) pigmentation. Adult males have an overall more intense coloration and a darker pigmentation on body and fins than females (Figs 9, 14).
Within Crenuchidae, dichromatic coloration can also be found in Characidiinae. However, the distribution of this trait among Characidiinae is restricted to a small number of species when considering the richness of the subfamily, all belonging to the genus Characidium (e.g. Buckup, 1993b; Buckup & Hahn, 2000; Melo & Espíndola, 2016; Pastana et al., 2017: supporting information; Teixeira & Melo, 2021). Within Characidium, sexual dichromatism is most parsimoniously interpreted as an apomorphic condition of a small group of species (Buckup, 1993b: clade C4) and possibly homoplastically present in several other taxa (Pastana et al., 2017: supporting information; Teixeira & Melo, 2021). When optimized in Buckup’s (1993b) tree, sexual dichromatism is retrieved as a synapomorphy for clade C4 and indicates that the lack of differential coloration among males and females is the plesiomorphic condition among Characidiinae.
Dichromatic coloration among sexes is of limited occurrence among Characiformes. Pastana et al. (2017) reviewed and summarized the occurrence of this type of sexual dimorphism in Characiformes, indicating that dichromatism occurs in 108 species of six characiform families: Alestidae, Characidae, Crenuchidae, Curimatidae, Lebiasinidae and Serrasalmidae. Available morphology-based phylogenies of Characiformes (i.e. Buckup, 1998) do not support a close relationship of Crenuchidae with any of these characiform families, thus indicating that the dichromatism is a derived feature of crenuchids. When optimized on to the phylogenies of Buckup (1993b, 1998), sexual dichromatism is homoplastically present in both subfamilies. Thus, we list it as a putative synapomorphy for Crenuchinae with independent occurrence in a subset of Characidium species (Characidiinae).
Sexually dimorphic elongation of the seventh to 11th dorsal-fin rays
Sexually dimorphic elongation of dorsal-fin rays in males is present in all known crenuchins with the exception of Poecilocharax rhizophilus. In some crenuchins, fin elongation is so pronounced that the elongated rays form a long dorsal-fin filament that can reach the upper caudal-fin lobe (e.g. Poecilocharax callipterus: Figs 3A, 9A). Sexually dimorphic males have a dorsal-fin elongation of the median dorsal-fin rays, more specifically from the seventh–eighth to the eighth–11th fin rays (except in P. rhizophilus that lacks dorsal-fin elongation).
Aside from crenuchins, fin elongation related to sexual dimorphism is herein reported to occur in only six of the 23 families currently recognized in Characiformes (i.e. Alestidae, Characidae, Erythrinidae, Hemiodontidae, Lebiasinidae and Serrasalmidae). However, a detailed literature review indicates that the pattern of dorsal-fin elongation present in Crenuchinae, i.e. seventh–eighth to the eighth–11th dorsal-fin rays, contrasts from those observed in most other Characiformes. In sexually dimorphic Characidae, for example, the elongation usually affects the second unbranched dorsal-fin ray, as in Odontostilbe Cope, 1870 (Bührnheim & Malabarba, 2006, 2007) and Serrapinnus Malabarba, 1998 (Malabarba & Jerep, 2014); or the last unbranched and first three to five branched dorsal-fin rays, such as in Carlana Strand, 1928 (Cardoso, 2003), Erythrocharax (Netto-Ferreira et al., 2013a), Hemigrammus spp. (e.g. Benine & Lopes, 2007; Zarske, 2011), Hyphessobrycon spp. (e.g. Moreira et al., 2002; Ohara et al., 2017a; Pastana & Ohara, 2016), Moenkhausia spp. (e.g. Eigenmann, 1920), NematocharaxWeitzman et al., 1986 (Weitzman et al., 1986; Menezes et al., 2015; Barreto et al., 2018), Parastremma Eigenmann, 1912 (cf. Dahl, 1960), Rhoadsia Fowler, 1911 (cf. Aguirre et al., 2016) and TrochilocharaxZarske, 2010 (Zarske, 2010). Likewise, representatives of the Alestidae (i.e. Alestopetersius Hoedeman, 1951, Bryconaethiops Günther, 1873, Bryconalestes Hoedeman, 1951, Nannopetersius Hoedeman, 1956 and Phenacogrammus Eigenmann, 1907; Zanata & Vari, 2005) and Lebiasinidae (i.e. Pyrrhulina spp. and Copella spp.; Zarske, 2011; Marinho & Menezes, 2017) have the dorsal-fin elongation affecting generally the anteriormost dorsal-fin rays. Fin elongation is also present in Serrasalmidae (e.g. Mylesinus Valenciennes, 1850, Myleus Müller & Troschel, 1844, Myloplus Gill, 1896, Ossubtus Jégu, 1992 and Tometes Valenciennes, 1850; Andrade et al., 2016a, 2017; Jégu et al., 1989: Ohara et al., 2017b) and Hemiodontidae [Hemiodus atranalis (Fowler, 1940); WMO, pers. obs.]. Yet, dorsal-fin filaments in Serrasalmidae and Hemiodontidae are unique among Characiformes in not forming a single lobe or filament, but several filaments continuing from different elongated fin rays.
Among Characiformes, the only dorsal-fin elongation matching the condition described for Crenuchinae occurs in the erythrinid Erythrinus erythrinus. Dimorphic males of this species have the seventh to tenth dorsal-fin rays elongated, a morphology remarkably similar to that observed in crenuchins. However, given the restrict occurrence of this character-state within Erythrinidae (present only in Erythrinus), and the latest phylogenetic hypotheses rejecting a sister-group relationship between crenuchids and Erythrinidae (Oliveira et al., 2011; Arcila et al., 2017; Mirande, 2019), the dorsal-fin elongation present in Crenuchinae and Erythrinus is most parsimoniously interpreted as homoplastic. The crenuchin fin elongation is listed herein as an additional putative synapomorphy for the subfamily.
Further modification of the dorsal-fin elongation occurs in Crenuchinae. While in Crenuchus spilurus, Poeciliocharax bovaliorum and P. callipterus the dimorphism affects the seventh or eighth to the eighth or 12th rays, this condition is further modified in P. weitzmani. Dimorphic males of this species have an autapomorphic elongation of all dorsal-fin rays. Poecilocharax rhizophilus, on the other hand, is the sole crenuchin species that lacks dorsal-fin elongation, a morphology that is herein interpreted as a reversal to the plesiomorphic characiform condition.
Comments on the diagnosis of the genus Poecilocharax
Poecilocharax has historically been distinguished from Crenuchus based on four morphological characters, namely the short maxilla (not surpassing the vertical through anterior margin of the orbit), absence of an adipose fin, presence of maxillary teeth and absence of an asymmetrical caudal-peduncle spot (Eigenmann, 1909, Géry, 1965; Buckup, 1998). Although the long maxillar is a character that still awaits a formal test based on phylogenetic grounds and encompassing a larger sample of characiform lineages as outgroup, within Crenuchidae this feature is only present in Crenuchus. Both Characidiinae and the crenuchin genus Poecilocharax exhibit a short maxillary bone that never surpasses the vertical through the anterior margin of the orbit. Thus, within Crenuchinae, a maxilla not surpassing the anterior orbital margin is a valid diagnostic character of Poecilocharax.
Although the adipose fin was initially reported as absent in the original description of Crenuchus (Günther, 1863: 443), this information was incorrect and later amended by Günther (1864: 365). No species of Poecilocharax has an adipose fin, while it is always present in C. spilurus and plesiomorphically present in Crenuchidae (Buckup, 1993b: char. 45). Among characidiins, adipose-fin loss is observed in Elachocharax (Weitzman, 1986), in some Characidium species (e.g. C. sterbai, C. nana, C. nupelia, C. xavante, C. mirim and C. stigmosum) (Zarske, 1997; Melo & Buckup, 2002; Graça et al., 2008, 2019; Netto-Ferreira et al., 2013b; Mendonça & Netto-Ferreira, 2015) and variably in Odontocharacidium species (Weitzman & Kanazawa, 1977; Rodrigues & Netto-Ferreira, 2020). Phylogenetically, adipose-fin loss is apomorphic for the crenuchin clade E1 in Buckup’s (1993b) analysis, while according to Mendonça & Netto-Ferreira (2015) this is synapomorphic for a Characidium clade containing C. nupelia, C. xavante, C. mirim, C. stigmosum, C. rachovii, C. occidentale, C. orientale and C. vestigipinne (although the four latter species exhibit a reversal in this characteristic). Similarly, the absence of an adipose fin in Poecilocharax is probably a modification of the plesiomorphic condition present in Chrenuchidae and could be interpreted as a putative synapomorphy diagnostic for the genus.
While the absence of an adipose fin and the presence of a short maxilla still stand as valid diagnostic features for Poecilocharax, the description of Poecilocharax callipterus and P. rhizophilus directly affects other diagnostic characteristics traditionally used to separate this genus from Crenuchus (i.e. maxillary teeth and asymmetrical caudal-peduncle spot). Maxillary teeth are indeed present in P. bovaliorum and P. weitzmani (Eigenmann, 1909; Géry, 1965), but are lacking in P. callipterus and P. rhizophilus (and in C. spilurus). Within characidiins, maxillary teeth are variously present in some lineages, i.e. Ammocryptocharax, Odontocharacidium and Klausewitzia ritae (Géry, 1965; Weitzman & Kanazawa, 1976; Buckup, 1993c; Rodrigues & Netto-Ferreira, 2020), but plesiomorphically absent in this subfamily (Buckup, 1993b). In addition, the peculiar caudal-peduncle spot of Crenuchus spilurus, often used to differentiate this species from other crenuchins (e.g. Buckup & Van der Sleen, 2017), is also present in P. callipterus. The spot is located on the ventral portion of the caudal peduncle (vs. on the middle portion of the peduncle in most Characiformes) and only present in these two species (Figs 3, 9).
In addition to the maxillary teeth and the asymmetrical caudal-peduncle spot, our study also yielded three additional characteristics with an unresolved optimization in crenuchins: (1) the fusion between fifth and sixth hypurals; (2) the number of branched pelvic-fin rays; and (3) the number of unbranched anal-fin rays. Crenuchins are characterized by having hypural plates 1 and 2 fused to each other (Buckup, 1998). Among species of Poecilocharax, further hypural fusion between plates 5 and 6 characterizes P. bovaliorum, P. weitzmani and most specimens of P. callipterus (five of seven C&S specimens). However, all three C&S specimens of P. rhizophilus have autogenous hypurals 5 and 6. Given that fusions between hypural plates are variable interspecifically among Poecilocharax, and intraspecifically within P. callipterus, we refrain from listing this characteristic as an additional diagnosis for the genus.
Among Crenuchinae, the highest count of pelvic-fin rays occurs in C. spilurus, which has one unbranched and eight or nine branched pelvic-fin rays. In Poecilocharax, this number is stable with one unbranched and seven branched rays (being the medialmost pelvic-fin ray variably unbranched in smaller specimens), which is similar to species of the subfamily Characidiinae (Buckup, 1993c). Yet, given that the relationships of the family Crenuchidae with other Characiformes remain controversial (e.g. Oliveira et al., 2011; Arcila et al., 2017; Mirande, 2019), it is impossible to establish if the seven branched rays of Poecilocharax is an apomorphic reduction within crenuchins, or if it is a retained characiform plesiomorphy (and the increase in pelvic-fin ray count is autapomorphic in Crenuchus spilurus). Although still a diagnostic character separating Poecilocharax from Crenuchus, a proper polarization of this character awaits a comprehensive phylogenetic analysis of the family with a larger sampling of Crenuchidae.
A third and uncommon characteristic present in most species of Poecilocharax and shared with Characidiinae (Buckup, 1993c) is the presence of only two unbranched anal-fin rays. This condition contrasts with P. rhizophilus and C. spilurus, which have three unbranched anal-fin rays, being the anteriormost a small unbranched ray often covered by tissue (not visible in alcohol-preserved specimens). Given that this characteristic is not exclusive to Poecilocharax, it is not listed as a diagnosis for the genus. The lack of knowledge regarding Crenuchidae relationships in Characiformes hampers a proper polarization of this character. It is currently not possible to attest if two unbranched rays is a synapomorphy for Crenuchidae (making the extra unbranched anal-fin ray a reversal of P. rhizophilus and C. spilurus), or if the reduction of anal-fin rays occur homoplastically in both subfamilies.
Many of the above-mentioned characters that vary between Poecilocharax and Crenuchus are reductive features present in the former. For instance, miniaturization traits are present in two species of Poecilocharax: P. weitzmani and P. rhizophilus. Both species have a skeleton with noticeable reductive characteristics, with losses of the infraorbital plates 5 and 6 and of the extrascapula, and lack of the ossification of the supraneurals and the dorsal- and anal-fin distal radials (which are predominantly cartilaginous even in adults). Poecilocharax weitzmani and P. rhizophilus also have a severely truncated cephalic lateral-line system (sensuPastana et al., 2019), lacking the infraorbital, mandibular, otic, postotic and supratemporal canals. The lateral-line canals that are left still show reductive traits, noticeable as a posteriorly shortened supraorbital canal (lacking a parietal branch), a pre-opercular canal restricted to the horizontal axis of the pre-opercle and a trunk lateral-line lacking pored scales. Yet, P. rhizophilus is the only taxon in the genus that can be categorized as a miniature species (sensuWeitzman & Vari, 1988), as it is the only that does not exceed 26 mm SL. The largest specimen recorded was 23.3 mm SL (among 25 examined specimens) and sexual dimorphism was observed in specimens with less than 20 mm SL, indicating sexual maturity. According to Toledo-Piza et al. (2014), Crenuchidae includes 15 miniature species, with all of them belonging to Characidiinae. We list herein P. rhizophilus as the 16th miniature crenuchid species, and the first belonging to subfamily Crenuchinae.
Distribution and endemism of Poecilocharax
The restricted distribution of the two species described herein may be the result of an allopatric or peripatric speciation of phylogenetically close species occurring in Poecilocharax. Like P. callipterus and P. rhizophilus, P. bovaliorum occurs in highland drainages, but is restricted to the Guiana Shield, being known only from the Potaro River in the Essequibo Basin, Guyana, in a distribution pattern similar to the disjunctive distribution already reported for other fish species (e.g. Lima & Ribeiro, 2011; Collins et al., 2018; Ohara et al., 2019).
Poecilocharax bovaliorum is found mainly in small ‘terra firme’ streams of black water (like P. callipterus) in the Essequibo Basin, although they can also occur with less frequency in others small drainages of clear water (Teixeira, pers. comm.). Poecilocharax weitzmani occurs in the black-water system of the Rio Negro, Solimões and Orinoco Basins. The right bank of the lower Rio Madeira has several black-water tributaries (e.g. Marmelos, Manicoré, Acarí, Sucunduri and some tributaries of the Rio Aripuanã). The fish fauna of these rivers includes several species shared with others black-water rivers like the Rio Negro, Orinoco and Essequibo [e.g. Acestrocephalus sardina (Fowler, 1913), Acestrorhynchus grandoculisMenezes & Géry, 1983, Biotoecus opercularis (Steindachner, 1875), Creagrutus maxillaris (Myers, 1927), Dekeyseria scaphirhynchus (Kner, 1854), Hemigrammus coeruleus Durbin, 1908, H. stictus (Durbin, 1909), Heterocharax spp., Hoplarchus psittacus (Heckel, 1840), Hoplocharax goethei Géry, 1966, Hyphessobrycon rheophilusOhara et al., 2019, Metynnis melanogrammus Ota et al., 2016, Pseudolithoxus kinja Bifi et al., 2018, Pygidianops spp., Rhinobrycon negrensis Myers, 1944, Satanoperca lilith Kullander & Ferreira, 1988, Symphysodon discus Heckel, 1840 and Thrissobrycon pectinifer Böhlke, 1953]. Currently, these black-water systems are isolated from each other by the Rio Madeira, a large white-water river. Yet, the species distribution observed nowadays indicates a previous common history between the black-water tributaries of the Rio Madeira Basin and other black-water river systems of the region. The apparent disjunct distributions of species between black-water tributaries of the Rio Madeira and other black-water rivers may be explained by proximity between them, before the capture by the Rio Madeira at around 2 Mya (cf. Collins et al., 2018).
The two species of Poecilocharax described herein were captured in the drainages of Juma Plateau, a strand of the Brazilian Shield. They constitute the first records of the genus from the right bank tributaries of the Rio Amazonas Basin that drains the Brazilian Shield. Despite several studies conducted in the Rio Madeira (i.e. Queiroz et al., 2013a, b; Vieira et al., 2016; Costa et al., 2017), including the Rio Aripuanã drainage (e.g. Camargo & Giarrizzo, 2007; Rapp Py-Daniel et al., 2007; Pedroza et al., 2012; Anjos et al., 2019), none reported the occurrence of Poecilocharax species, which is the reason why we list Poecilocharax callipterus and P. rhizophilus as endemic with a restricted distribution in the Brazilian highland. A similar distribution was reported for the rheophilic suckermouth catfish Pseudolithoxus kinja Bifi et al., 2018 (Siluriformes: Loricariidae), described from Rio Guariba (another Brazilian Shield tributary of the Rio Aripuanã) and representing the first record of a species of this genus from a right bank tributary of the Amazon River (Collins et al., 2018).
The Rio Aripuanã can be considered as a distinct tributary of the Rio Madeira Basin in Brazilian territory regarding its geomorphology, because this river has a series of great waterfalls along its length. Several species have been considered endemic from this drainage. Kullander (1995) and later Deprá et al. (2014) provided lists including 11 species of fishes occurring exclusively in the upper Rio Aripuanã. We expand this list to include 15 species described from the same drainage during the last few years (cf. Andrade et al., 2016b; Tencatt & Ohara, 2016a, b; Esguícero & Castro, 2017; Ohara et al., 2017a, b; Oliveira et al., 2017; Calegari et al., 2018; Dias et al., 2018; Garcia-Ayala & Benine, 2019; Shibatta, 2019; Kim et al., 2020; Menezes et al., 2020). Shibatta (2019) recently listed 26 endemic species for the Rio Aripuanã. We update Shibatta’s list to add the new species described herein (Poecilocharax callipterus and P. rhizophilus), plus Corydoras gracilis, Curculionichthys jumaorum (Dias et al., 2018), Curculionichthys scaiusCalegari et al., 2018, Gymnotus aripuanaKim et al., 2020, Knodus angustusMenezes et al., 2020 and Poptella actenolepisGarcia-Ayala & Benine, 2019. We also modify this list to remove Potamoglanis anhanga (Dutra et al., 2012), since it also occurs in the Amazonas and Tapajós Basins, and Hyphessobrycon procyon (MZUSP 122408) and Inpaichthys kerri Géry & Junk, 1977 (MZUSP 115795), both recently captured in the Tapajós Basin. The updated list contains 31 endemic species of fish known from the Rio Aripuanã.
Comparative material
Erythrinidae:
BRAZIL. Erythrinus erythrinus: MZUSP 64702 (2, 76.3–112.9 mm SL) Amazonas State, Buriti river, Rio Tiquié Basin; MZUSP 43127 (3, 111.9–124.4 mm SL) São Paulo State, Rio Tietê Basin; MZUSP 81484 (4, 54.1–103.8 mm SL) Amazonas state, Açaí river, Rio Tiquié Basin.
Characidiinae:
BRAZIL. Melanocharacidium dispiloma MZUSP 115699 (26, 22.7–31.9 mm SL) Amazonas State, Rio Mutum, Rio Tapajós Basin; Leptocharacidium osmopilus MZUSP 108323 (1, 57.5 mm SL) Amazonas state, tributary of Rio Urubu, Rio Amazonas Basin; Ammocryptocharax elegans MZUSP 122241 (2, 40.3–40.7 mm SL) Amazonas state, tributary from Rio Roosevelt, Rio Aripuanã Basin; Microcharacidium sp. MZUSP 122569 (3, 14.1–16.9 mm SL) Amazonas state, Rio dos Couros, Rio Acari drainage; Characidium lauroi MZUSP 110359 (5, 39.2–46.4 mm SL) São Paulo state, Rio das Cobras, Paraíba do Sul drainage; Elachocharax pulcher MZUSP 122229 (6, 14.8–23.1 mm SL) Roraima State, Caicubi river, Rio Negro Basin; Odontocharacidium aphanes MZUSP 113226 (3, 12.1–13.5 mm SL) Roraima State, Caicubi river, Rio Negro Basin.
Crenuchinae:
Crenuchus spilurus: BRAZIL: MZUSP 101986 (13, 18.6–32.9 mm SL), Amapá State, Ting Ling River, Rio Jari Basin. MZUSP 55115 (4, 20.9–35.2 mm SL), Amazonas State, river near Tapurucuara. GUYANA: MZUSP 62773 (30, 1 C&S, 26.4–46.3 mm SL), Demerara, black-water creek, Essequibo River Basin. Poecilocharax bovaliorum: GUYANA: Essequibo River Basin: USNM 120430 (1 cotype, 23.9 mm SL), Potaro River; USNM 344495 (4, 15.89–19.46 mm SL), Dakara Creek; USNM 204386 (10, 17.26–25.97 mm SL), Savannah Landing; USNM 343443 (9, 20.80–36.57 mm SL), Kaieteur National Park, tributary of upper Potaro River, just above Kaieteur Falls; USNM 327197 (4, 19.84–35.97 mm SL), black-water creek at Kaieteur National Park, tributary of upper Potaro River, just above Kaieteur Falls; MZUSP 109092 (6, 19.6–38.4 mm SL), Koribrong River; MZUSP 109160 (9, 12.9–31.7 mm SL), Amaila River; MZUSP 109026 (17, 15.7–31.9 mm SL), Koribrong River; MZUSP 109083 (12, 16.6-34.2 mm SL), Koribrong River; MZUSP 109010 (2, 22.8–23.6 mm SL), Koribrong River; MZUSP 109024 (11, 1C&S, 19.3–34.1 mm SL), Koribrong River; MZUSP108845 (2, 22.1–30.2 mm SL), Koribrong River; MZUSP 99749 (5, 19.8–27.7 mm SL), Potaro River; MZUSP 108881(39, 10.4–35.4 mm SL), Kuribrong River. Poecilocharax weitzmani: BRAZIL: MZUSP 113449 (1, 22.1 mm SL), Roraima State, temporary pound on the side banks of Rio Pupunha, Rio Negro Basin. MZUSP 113390 (49, 15.1–22.9 mm SL), Roraima State, temporary pound on the side banks of Pupunha River, Rio Negro Basin. ZUEC PIS 15082 (4, 20.1–21.2 mm SL), Amazonas State, Tabatinga, small river at Urumutum road. INPA-ICT 9186 (12, 15.7–23.6 mm SL), Amazonas State, São Gabriel da Cachoeira, Rio Negro Basin. INPA-ICT 32819 (242, 14.9–20.2 mm SL), Amazonas State, first order creek near Novo Airão, Parque Nacional do Jaú, Rio Jaú Basin. INPA-ICT 36377 (24, 15.2–16.3 mm SL), Roraima State, Rio Anauá, Rio Branco Basin. INPA-ICT 38890 (34, 12.5–24.2 mm SL), Amazonas State, Santa Isabel do Rio Negro, Rio Negro Basin. INPA-ICT 49036 (35, 12.5–24.2 mm SL), small creek on the left bank of Rio Japurá, Rio Japurá Basin. USNM 391811 (1, 50.8 mm SL – aquarium specimen), original locality data unknown; GUYANA: USNM 228091 (16, 17.5–24.1 mm SL), aquarium specimens out of Georgetown; VENEZUELA: USNM 270196 (25, 12.32–20.7 mm SL), Rio Platanillal on road from Puerto Ayacucho to Samariapo; USNM 270197 (133, 5 C&S, 14.6–20.6 mm SL), Rio Negro, Cano Tremblador, on road from San Carlos to Solano; USNM 270191 (3, 12.6–13.9 mm SL), Platanillal River on road from Puerto Ayacucho to Samariapo; USNM 270192 (8, 12.2–16.4 mm SL), small stream down road from Rio Paraiba Grande, c. 25 km from Puerto Ayacucho; USNM 270193 (8, 14.7–23.1 mm SL), flooded grassland, 2 km c. from San Carlos; USNM 270194 (24, 12.4–17.3 mm SL), Small cano crossed by road, 2 km south of Mirabel; USNM 270195 (6, 12.7–15.8 mm SL), Cano provincial, c. 20 km north of Puerto Ayacucho; USNM 270189 (14, 12.8–15.4 mm SL), Cano Chola, on road from San Carlos to Solano; USNM 222029 (22, 12. 81–19.91 mm SL), small stream about 32 km from Puerto Ayacucho to Samariapo; BRAZIL: USNM 302071 (10, 15.1–17.4 mm SL), Amazonas State, rocks near Ilha Paraíso, Rio Negro Basin; USNM 302089 (1, 20.7 mm SL), Amazonas State, rocks near Ilha Paraíso, Rio Negro Basin; USNM 302094 (1, 15.6 mm SL), Amazonas State, Irere River, Caatinga lake, Rio Negro Basin; USNM 302102 (2, 15.1–18.2 mm SL), Amazonas State, Rio Negro Basin; USNM 302111 (34, 14.6–19.1 mm SL), Amazonas State, Rio Negro Basin; USNM 302121 (11, 12.19–17.68 mm SL) Amazonas State, Rio Irere, Caatinga lake, Rio Negro Basin. Poecilocharax sp.: BRAZIL: all from Amazonas state, São Gabriel da Cachoeira: ZUEC PIS 16201 (14, 17.5–24.3 mm SL) Uacatuna River; ZUEC PIS 16172 (49, 11.2–20.1 mm SL) small river near dirt road.
[Version of record, published online 16 May 2022; http://zoobank.org/urn:lsid:zoobank.org:pub:3F65DD5D-3C73-49A8-88F5-E047CFDEB68B]
ACKNOWLEDGEMENTS
The authors are grateful to L. Rapp Py-Daniel, C. Ribas, S. Hashimoto (INPA) and C. Oliveira (UNESP) for curatorial assistance, loan of specimens and donation of tissue samples. Sequence data for the species Poecilocharax bovaliorum and P. weitzmani (LBP 40500) were provided by C. Oliveira and E. Rodrigues (UNESP/Mamirauá Institute). The authors are grateful to V. Abrahão, O. Oyakawa (MZUSP) and T. Teixeira (PUC-MG) for help and assistance during fieldwork. M. Gianeti (MZUSP) helped in curatorial assistance and T. Teixeira provided thoughtful comments on the description of sexual dimorphism of Crenuchinae. SEM images were taken by L. Guimarães (MZUSP). Part of the type series was collected during an expedition funded by the South American Characiformes Inventory project (FAPESP Grant # 2011/502827). Authors received financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (WMO: grant number 2013/22473-8; PC: grant numbers 2012/00840-6, 2016/03966-1, 2016/19075-9 and 2017/09321-5), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, Sara E. and Bruce B. Collette Postdoctoral Fellowship in Systematic Ichthyology (MNLP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (PC: grant number 423760/2018-1).
DATA AVAILABILITY
DNA sequence data that support the findings of this study have been deposited in the GenBank database with the following accession codes ON041209, ON042212, ON059686, ON062375, ON063012, ON063015, ON063016, ON063044, ON067464, ON067495, ON067498, ON067500, ON067501, ON067502 and ON076904.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Table S1. List of lot number, voucher and GenBank accession number for each taxon used in this study (with exception of Crenuchinae). Collection abbreviations follow Sabaj (2020). Gray highlight indicates sequences generated in this study.
Table S2. Distance genetic between species of Crenuchinae based on the mitochondrial marker cytochrome c oxidase subunit I.