-
PDF
- Split View
-
Views
-
Cite
Cite
James C. Lamsdell, Revised systematics of Palaeozoic ‘horseshoe crabs’ and the myth of monophyletic Xiphosura, Zoological Journal of the Linnean Society, Volume 167, Issue 1, January 2013, Pages 1–27, https://doi.org/10.1111/j.1096-3642.2012.00874.x
Close - Share Icon Share
Abstract
The monophyly of the class Xiphosura is critically re-examined. For the first time a phylogenetic analysis of a number of synziphosurine and xiphosurid taxa is performed together with representatives of the other chelicerate orders also included as ingroup taxa. Xiphosura as currently defined is shown to be paraphyletic, and a revised classification is presented. Previous characteristics used to unite the xiphosurids (possessing a fused thoracetron) and a paraphyletic grade of synziphosurines (retaining freely articulating opisthosomal tergites) include the presence of a cardiac lobe, ophthalmic ridges, an axial region of the opisthosoma, and a reduced first opisthosomal segment. All of these characteristics are, however, here shown to be present in other chelicerate groups, leaving Xiphosura without any defining synapomorphies. A number of other characters, including the form of the chelicerae and appendage VII, indicate that xiphosurans may be paraphyletic with respect to a clade consisting of chasmataspidids, eurypterids, and arachnids. What ramifications this has for the evolution of basal chelicerates is briefly discussed, and it is recognized that most of the currently known ‘synziphosurine’ taxa represent offshoots from the main chelicerate lineage with ghost ranges extending into at least the Middle Ordovician.
Introduction
Few organisms alive today evoke an awareness of the primeval as does the horseshoe crab. Often only seen when shuffling awkwardly onto land to reproduce, yet surprisingly graceful when observed within their usual marine habitat, horseshoe crabs are striking in both their apparent dissimilarity from any of the four familiar extant arthropod groups and their sheer size. Large size among arthropods is something often evoked as a relic of the distant past, from an age when giant arthropods ruled the Carboniferous, and with a maximum length of just over half a metre horseshoe crabs certainly appear to be remnants of grander times. The four surviving species of the class Xiphosura are actually descendants of more diminutive ancestors however, with no currently known fossil species approaching the size of Recent xiphosurans until possible representatives of the extant genus TachypleusLeach, 1819 appear in the Middle Triassic (Diedrich, 2011). Xiphosurans have existed for some 480 Myr, with the earliest unequivocal representatives found from the Lower Ordovician of Morocco (Van Roy et al., 2010). As a group they appear to have had a relatively low species diversity since the late Palaeozoic, although during the Silurian and Devonian there were a plethora of distinct morphologies (see Størmer, 1955). It has been suggested that the post-Palaeozoic limuloids represent a good example of bradytely (Fisher, 1984). Their apparent morphological conservatism has resulted in their being branded as ‘living fossils’, a term which has been rightly criticized as both inaccurate and misleading (Schopf, 1984).
Once considered to be relatives of crustaceans, these creatures have an important place in the history of arthropod research, with the formalization that xiphosurans are chelicerates by Lankester (1881) setting the foundations for the modern interpretation of arthropod relationships. Xiphosurans are now considered to be relatively basal euchelicerates (Dunlop, 2010) with a distinction between those species in which the opisthosoma has become fused into a thoracetron (Xiphosurida) and those that retain freely articulating opisthosomal tergites (‘synziphosurines’), both originally considered monophyletic suborders (Zittel, 1885). A number of other groups have also in the past been considered members of the Xiphosura, namely aglaspidids, chasmataspidids, strabopids, and paleomerids. Aglaspidids (see Raasch, 1939) were included by Størmer (1944) but removed when they were shown not to possess chelicerae (Briggs, Bruton & Whittington, 1979), while chasmataspidids were initially described as aberrant xiphosurans (Caster & Brooks, 1956) and, although they were suggested to have closer affinities to eurypterids by Eldredge (1974), were still retained within Xiphosura until finally being excluded by Anderson & Selden (1997). Strabopids (Beecher, 1901) and paleomerids (Størmer, 1956), shown to be synonyms by Tetlie & Moore (2004), were also originally assigned to Xiphosura by Størmer (1944) as part of an aglaspidid complex, but these were excluded by Bergström (1971). One other problematic taxon, LemoneitesFlower, 1969, was originally considered an aglaspidid before being assigned to Xiphosura incertae sedis by Eldredge (1974) and finally excluded from Xiphosura by Anderson & Selden (1997), eventually to be revealed as a glyptocystitid echinoderm (Moore & Braddy, 2005). Xiphosura therefore at present encompasses solely Xiphosurida and the synziphosurines.
The monophyly of Xiphosura (xiphosurids plus synziphosurines) has not been questioned since synziphosurines were united with xiphosurids by Zittel (1885); although Packard (1886) follows Woodward (1867) in suggesting that synziphosurines showed closer affinities to eurypterids, it is clear that by the turn of the 20th century Zittel's classification had been universally adopted (e.g. Laurie, 1893; Clarke & Ruedemann, 1912; Woodward, 1913). Originally united primarily through general similarity rather then specific synapomorphies, Xiphosura represents a hang-up of pre-phylogenetic thinking that has carried through into early analyses of chelicerate relationships. Supposed synapomorphies were coded as such based solely on their apparent uniqueness, with no attempt to test for homologous structures in non-xiphosuran arthropods. It is important that the characters used to define monophyletic groups are critically evaluated without an a priori bias for or against monophyly, as has been done for trilobites (Ramsköld & Edgecombe, 1991). Assumptions derived from historical classification still influence modern studies, and until such assumptions are critically evaluated the validity of groups based upon them is questionable. The only non-xiphosuran taxa included in previous phylogenetic analyses of Xiphosura was an outgroup (Anderson & Selden, 1997), or the entire group was represented by a single taxon in higher level analyses of the Arthropoda (Briggs & Fortey, 1989; Briggs, Fortey & Wills, 1992; Dunlop & Braddy, 2001; Cotton & Braddy, 2004; Hendricks & Lieberman, 2008). A monophyletic Xiphosura was recovered in the analyses of Dunlop & Selden (1997) and Shultz (2007), but Shultz used Xiphosura as his outgroup and so essentially forced their monophyly. Notably, Wills et al. (1998) included a synziphosurine (WeinberginaRichter & Richter, 1929) and a xiphosurid (Tachypleus) in their analysis and retrieved a paraphyletic Xiphosura, an intriguing result.
Furthermore, in the only comprehensive cladistic treatment of Xiphosura to date synziphosurines were shown to not represent a natural group, instead forming a paraphyletic grade that was interpreted as being the stem lineage to Xiphosurida (Anderson & Selden, 1997). At the time the oldest xiphosurid was known from the Carboniferous with synziphosurines known from the Silurian to Devonian, with the expected stratigraphic distribution from stem lineage to crown group. This congruence between stratigraphic and phylogenetic sequence was upset, however, by the discovery of an unequivocal xiphosurid from the Upper Ordovician of Manitoba, Canada (Rudkin, Young & Nowlan, 2008), followed by further xiphosurid reports from the Lower Ordovician of Morocco (Van Roy et al., 2010). The stratigraphic range of the synziphosurines has also been expanded, with the youngest representative now known from the Carboniferous (Moore, McKenzie & Lieberman, 2007) while the oldest occurs in strata slightly younger than that of the undescribed xiphosurid in Morocco (Van Roy et al., 2010). It is clear that the synziphosurines described to date are at best offshoots of the xiphosurid stem and not the direct ancestors of xiphosurids as inferred by Anderson & Selden (1997) for a few taxa. While the retention of plesiomorphic conditions in taxa is not unusual it should be remembered that these offshoots will have continued following their own evolutionary development, no matter how imperceptible, and autapomorphies, convergences and parallelisms can complicate attempts to resolve their affinities. Hence, character polarity is key to resolving the relationships of groups with a high proportion of ghost ranges and it is because of this that outgroup selection is of utmost importance for any analysis. Unfortunately, as we shall see for Xiphosura, this can be a particularly complex issue.
The monophyly of Xiphosura therefore remains to be fully tested. With the discovery of Ordovician xiphosurids the previous hypotheses of the group's evolutionary history (e.g. Bergström, 1975; Fisher, 1984; Selden & Siveter, 1987; Anderson & Selden, 1997) are all shown to be in need of revision. With the knowledge that there are large gaps in the xiphosuran fossil record it is now opportune to re-evaluate the relationships of the known species without allowing their stratigraphic position to overly influence their hypothesized affinities. Many species have remained largely neglected over the last century, however, and would benefit from restudy. The Mesozoic Limulina, in particular, need revision, so a phylogenetic treatment of all species is not possible, but these species are less critical to working out the origins of the group. Instead, the present work utilizes a number of well-known or recently redescribed taxa to elucidate the exact relationship of the synziphosurines to the xiphosurids within the context of the higher euchelicerates. This includes a critical re-evaluation of the homology statements employed by previous workers and the polarization of these characters.
Terminology
Arthropod segmentation
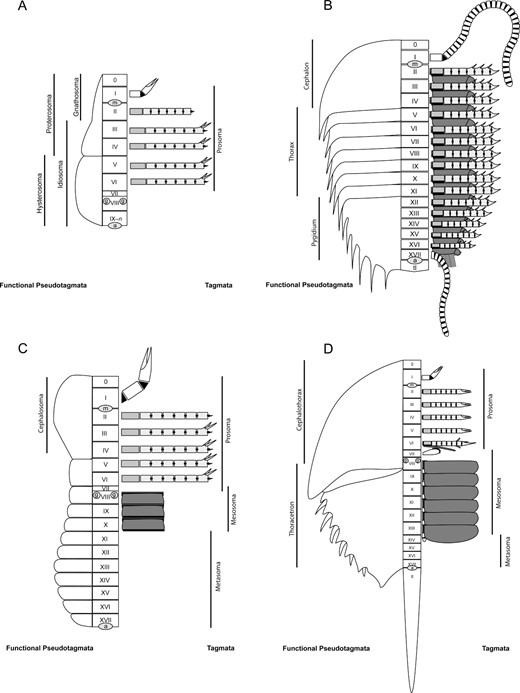
Throughout this discussion a number of aspects of generalized arthropod morphology will be dealt with, and these are briefly defined here. The fundamental division of the arthropod body is the somite, or metamere (Lankester, 1904). The first somite is here termed the ocular/protocerebral region (Scholtz, 1995), which is considered equivalent to the concept of the arthropod acron (see Scholtz & Edgecombe, 2006), and is numbered 0. The subsequent somites are numbered using roman numerals from anterior to posterior; in xiphosurans this ranges from I to XVII. The convention adopted by Selden & Siveter (1987) of separating segments from somites is adopted here; a segment refers to an externally differentiated unit that may comprise multiple somites, and is often used when segmentation is obvious but somite count is uncertain, as can be the case for xiphosurans. Segments are labelled using arabic numerals, with the first post-cephalic segment numbered 1. The dorsal sclerite of each segment is termed the tergite, while the ventral sclerite is the sternite. Somites frequently undergo differentiation as part of specialization of limb form and function. The arthropod body is divided into regions of serial somites exhibiting a characteristic differention and each of these regions are termed tagmata. The term pseudotagma is applied to a division of the body that appears to be a distinct unit but does not represent a true tagma (van der Hammen, 1980).
The definition of what constitutes a tagma has varied throughout its use. Lankester (1904) defined a tagma as a definite region of similar modification of the somites and their appendages, differing in their modification from that observed in regions preceding and succeeding them–modification was defined as a difference in any of the four ‘meromes’ that comprise each somite, in arthropods namely the tergite, sternite, muscular fibres and the appendages. Lankester (1904) noted (and implicitly criticized) a tendency to define tagma based exclusively on the form of the tergites and appendages, and this is a practice that has been largely maintained to this day. Subsequently, some authors have considered tagmosis to be defined by specialization of the appendages (Flessa, Powers & Cisne, 1975), while others view tagmosis to be primarily a property of the dorsal exoskeleton. van der Hammen (1980: 155) described a tagma as a ‘division of the body, composed of a series of more or less similar segments or metameres, and constituting a distinct unity characterised by its own individuality’. In the same publication the term pseudotagma was simply defined as a ‘division of the body, constituting a more or less distinct unity with its own individuality, but not representing a true tagma’ (van der Hammen, 1980: 131). These conflicting (and often vague) definitions of what constitutes a tagma has resulted in a fair degree of subjectivity in defining an arthropod's tagmosis, although in more recent work on trilobites a somewhat more strict definition of a tagma was used: a discrete morphological entity which is distinct in at least the dorsal exoskeleton from its first appearance in ontogeny so that its boundaries with adjacent regions are never crossed by newly recruited or released segments (Minelli, Fusco & Hughes, 2003), a definition already used in many neontological works. Yet this definition does not cover conditions where the dorsal evidence for tagmata does not correspond to the ventral (primarily appendicular) evidence, as appears to be the case in a number of arthropods including trilobites, xiphosurans, Acari, and other chelicerate groups. It also fails to differentiate tagmata from pseudotagmata. The concept of pseudotagmata appears to stem from the work of van der Hammen (1963) on the subdivisions of parasitiform Acari and was then extended for use in the other chelicerate groups (van der Hammen, 1986a, b) but has not found widespread use among other arthropod workers. It seems that there is a great utility in the pseudotagma concept that has gone largely unrecognized but has the potential to aid in providing a clearer definition for arthropod tagmata. Tagmata are most comprehensible as regions of functional specialization, which in arthropods is predominantly mediated through modification or suppression of the appendages. The definition for a tagma adopted herein is therefore a distinct and discrete morphological region that comprises a series of equivalently modified appendages that constitute a unit of specific form (as in the case of the malacostracan abdomen) or sometimes function (as in the hexapod head). The first or last appendages of a tagma may, however, be dramatically differentiated from the others; examples include the chelicera in chelicerates (first appendage of the prosoma), antennula in crustaceans, myriapods, and hexapods (first appendage of the cephalon), the chilaria in xiphosurans (first appendage of the opisthosoma), and the uropods in decapod crustaceans (last appendage of the abdomen). Further to this, the boundaries of a tagma should never be crossed by newly recruited or released segments throughout the organism's ontogeny. Using this definition, the trilobite pygidium would not constitute a tagma as segments of the thorax develop from the anterior end of the pygidium; instead, it would constitute a pseudotagma–see discussion below. While this definition may appear to discount the differentiation produced by the fusion of tergites, Lankester (1904) effectively argued that such fusion was essentially superficial and easily acquired through a disposition of chitinous cuticle of equal thickness across an area instead of the usual thinning at the segment boundary that results in a discrete tergite. Such fusions do, however, fall within the realm of pseudotagma.
Herein the term pseudotagma is used to refer to units defined by differentiation of the tergites or sternites without an associated change in form or function of the appendages. This definition is consistent with its use in Acari for defining the gnathosoma and idiosoma, or proterosoma and hysterosoma, which is based on a dorsal division that is independent of appendage structure (Fig. 1A). For this reason, too, the trilobite pygidium represents a pseudotagma (Fig. 1B). Pseudotagmata can be further subdivided into functional pseudotagmata and non-functional pseudotagmata; functional pseudotagmata impose some type of functional constraint on the organism, and fused tergite series enforce such a constraint. The solifuge cephalosoma (demarcated dorsally by the propeltidium), which like the acariform proterosoma is also differentiated ventrally by the sejugal furrow (see Dunlop, Krüger & Alberti, 2012), is also defined as a functional pseudotagma as there is no differentiation in the form of the appendages of the prosoma (Fig. 1C). Furthermore, if the cephalic appendages in trilobites are in fact undifferentiated from the trunk appendages (Hughes, 2003a; Minelli et al., 2003) then the trilobite cephalon also represents a functional pseudotagma and trilobites therefore possess only a single tagma (Fig. 1B). Non-functional pseudotagmata, meanwhile, are the most subjective type of arthropod body division and are largely limited to changes in tergite or sternite dimensions, most usually width, or are defined by alterations in cuticle ornamentation or the presence or absence of epimera. Examples of non-functional pseudotagmata are the divisions of the olenelline trilobite thorax proposed by Lauterbach (1980, 1983, 1989), demarcated by the third macropleural segment, and the xiphosuran preabdomen and postabdomen, which are discussed further below.
Schematic diagrams of a number of different arthropods showing examples of functional pseudotagmata compared with true tagmata. Somites are labelled 0–XVII; ‘tl’ indicates the telson, ‘m’ the mouth, ‘a’ the anus, and ‘g’ the gonopores. A, the oribatid mite Epilohmannia cylindrica (Berlese, 1904). B, the trilobite Olenoides serratus (Rominger, 1887). C, the solifuge Galeodes armeniacusBirula, 1929. Solifuge arachnids possess opercula on the first three opisthosomal segments associated with the genital ducts and repiratory spiracles. These opercula could be considered homologous to the opercula of the thelyphonid Mastigoproctus giganteus (Lucas, 1835) which Shultz (1993) showed to be opisthosomal appendages that had become completely sutured to the ventral body wall. D, the limulid xiphosurid Limulus polyphemusLinnaeus, 1758.
Xiphosura
The terminology for aspects of xiphosuran morphology largely follows Siveter & Selden (1987) and Selden & Siveter (1987). The prosoma comprises the entirety of the anterior tagma, including the dorsal carapace and the prosomal appendages. The prosomal appendages are denoted by roman numerals I–VI, appendage I being the chelicerae. In limulids the anterior cephalic region is termed the cephalothorax by Shultz (2001) as a number of opisthosomal somites have been at least partially incorporated into the prosoma (Fig. 1D). The carapace/cephalothorax itself bears a number of structures; the cardiac lobe is located at the posterior of the carapace and is axially inflated, while the ophthalmic ridges extend either side of the cardiac lobe and dorsally shade the lateral compound eyes. Anterior to the cardiac lobe the median ocelli, simple eyes, can be identified in a number of taxa. Some species bear extraophthalmic ridges, transverse ridges that are arrayed on the surface of the carapace outside of the ophthalmic ridges. Interophthalmic ridges occur within the area demarcated by the ophthalmic ridges, and may correspond to internal apodemes for the attachment of extrinsic limb musculature, indicating arrangement of the prosomal appendages.
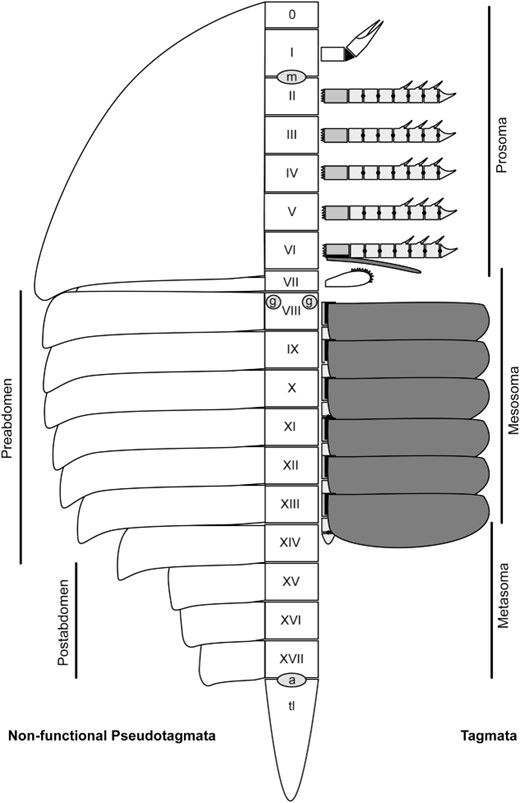
The term opisthosoma refers to the post-prosomal segments, and is equivalent to the trunk region of other arthropods (see Hughes, 2003a, b). The opisthosoma itself is not considered a single tagma, contrary to most recent treatments. Lankester (1904) considered chelicerates to possess three tagmata based on appendage differentiation: the prosoma, mesosoma, and metasoma. The prosoma comprises somites I–VI, the mesosoma somites VII–XIII, and the metasoma somites XIV–XVII (in xiphosurans, eurypterids, scorpions, and probably chasmataspidids the metasoma is formed from somites XIV–XIX). van der Hammen (1986a) termed the mesosoma and metasoma pseudotagmata and this has been followed by subsequent authors including Selden & Siveter (1987) and Rudkin et al. (2008), although van der Hammen considered the mesosoma to consist of somites VII–XIV and the metasoma somites XV–XIX (in scorpions). This in fact corresponds to the preabdomen and postabdomen; these are actually non-functional pseudotagmata defined by a dorsal constriction of the tergites (Fig. 2). In xiphosurans the postabdomen comprises somites XV–XVII, while eurypterids frequently correspond to the condition in scorpions but sometimes have a preabdomen and postabdomen that corresponds to the true tagmata of mesosoma and metasoma. In chasmataspidids the situation is somewhat different, having somites VII–X fused together into a buckler and somites XI–XIX forming a freely articulating postabdomen. These are, however, considered to be functional pseudotagmata, as the fusion of the buckler places a functional constraint on the organism.
Schematic of a generalized synziphosurine arthropod, showing the distinction between the preabdominal and postabdominal non-functional pseudotagmata and the true tagmata of the prosoma, mesosoma, and metasoma.
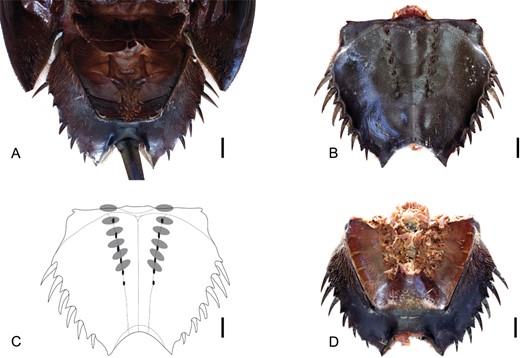
Somite VII is considered to be opisthosomal in origin, as is the conventional view (see Snodgrass, 1952), and not prosomal as suggested by Stürmer & Bergström (1981) (see also Haug et al., 2012a for discussion on this subject). Evidence for this is shown in the dorsal expression of somite VII as a fully sclerotized tergite in synziphosurines (Fig. 3A) and chasmataspidids (Fig. 3B) and in the way the tergite remains attached to the opisthosoma after disarticulation of the carapace (Fig. 4, B). This tergite is termed the microtergite in chasmataspidids, but this refers specifically to the heavily reduced condition seen in these taxa and the term pre-opercular tergite is used herein while microtergite is applied as a condition which the pre-opercular tergite can attain. The tergite of somite VIII may be called the opercular tergite and is hypertrophied in some synziphosurines. Embryological studies have shown that in limulids the tergite of somite VII and part of the tergite of somite VIII are incorporated into the prosoma (Scholl, 1977; Sekiguchi, Yamamichi & Costlow, 1982) with the lateral portions of the opercular tergite distinctly set off from the main body of the tergite, frequently deflected dorsally towards the outer margin, and these regions are termed free lobes. The term thoracetron is used for the fully fused dorsal opisthosomal shield in xiphosurids.
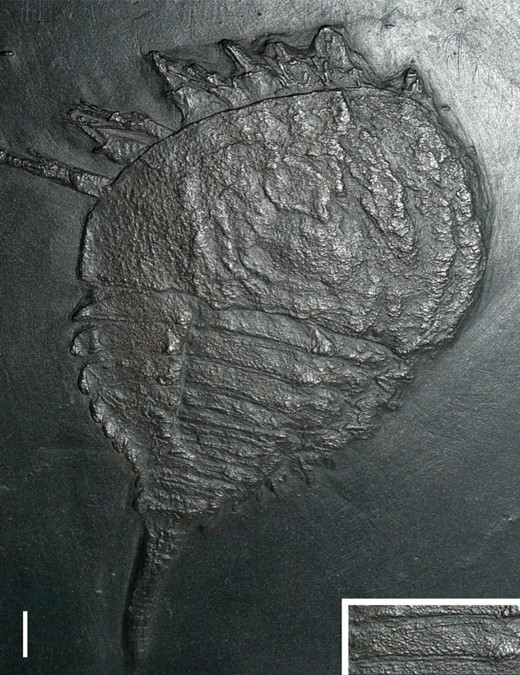
A, Bunodes lunulad'Eichwald, 1854 from the late Silurian (Ludlow) of Oesel, Estonia. Specimen ELM G1:262:2, clearly showing the partially reduced pre-opercula tergite of somite VII (arrowed) between the downturned carapace and the hypertrophied tergite of somite VIII. Scale bar = 10 mm. B, ‘Eurypterus’ stoermeriNovojilov, 1959, a chasmataspidid from the early Devonian (Lochkovian) of Siberia, Russia. Specimen PIN 1138-1, exhibiting the microtergite of somite VII (arrowed) positioned between the carapace and the buckler. The microtergite curves anteriorly towards its lateral edges, and is particularly noticeable on the left-hand side. The occurrence of ridges associated with the lateral eye on the carapace is also labelled. Image courtesy of Dave Marshall. Scale bar = 2 mm.
A, Pasternakevia podolicaSelden & Drygant, 1987 from the late Silurian (Ludlow) of Zalissia, Ukraine. Specimen ISEA I − F/MP/3/1499/08, isolated opsithosoma with articulated microtergite of somite VII (arrowed). Image courtesy of Ewa Krzemińska. Scale bar = 10 mm. B, undescribed chasmataspidid from the Lower Devonian (Emsian) of Siberia, Russia. Specimen PIN 5116-6, disarticulated buckler with microtergite of somite VII (arrowed) still firmly attached to its anterior margin. Image courtesy of Dave Marshall. Scale bar = 2 mm.
The opisthosomal appendages in aquatic chelicerates are modified into flattened opercula that bear the respiratory organs, termed book gills. In xiphosurans the opercula are not medially fused, although in modern xiphosurids they are connected by a thin membrane which would be unlikely to fossilize, and so its presence in extinct taxa is equivocal. The operculum of somite VIII is the genital operculum and bears the paired gonopores; in modern xiphosurids the genital operculum is devoid of respiratory structures, but they are present on the genital operculum of OffacolusOrr et al., 2000 and the condition is unclear in Weinbergina. The appendages of somite VII are functionally incorporated into the prosoma and may be retained as fully pediform walking limbs or reduced to chilaria, which act in a masticatory function.
The institutional abbreviations for xiphosuran specimens figured in this study are as follows: ELM, Estonian Museum of Natural History, Tallinn, Estonia; ISEA, Museum of the Institute of Systematics and Evolution of Animals, Kraków, Poland; MM, Manitoba Museum, Winnipeg, Canada; PIN, Paleontological Institute, Moscow, Russia; PWL, Landessammlung für Naturkunde Rheinland-Pfalz, Mainz, Germany; SMF, Naturmuseum und Forschungsinstitut Senckenberg, Frankfurt am Main, Germany.
Evidence for Xiphosuran Monophyly?
Dunlop & Selden (1997) listed four synapomorphies that united Xiphosura as a monophyletic taxon within Chelicerata: the presence of ophthalmic ridges, a cardiac lobe on the carapace, an axial region of the opisthosoma, and a reduced pre-opercular segment. However, as noted elsewhere (Lamsdell, 2011), at least two of these ‘synapomorphies’ are actually found in several other potentially closely related chelicerate groups: a cardiac lobe is widespread in eurypterids (see Selden, 1981) and present in chasmataspidids (Dunlop, Anderson & Braddy, 2004) while a reduced pre-opercular segment is present as a microtergite in chasmataspidids (Dunlop et al., 2004) and potentially in a heavily reduced form in eurypterids (Dunlop & Webster, 1999). Furthermore, the division of the trunk tergites into an axial and tergopleural region is common in arthropods, being most obvious in the three-lobed bodies of trilobites. The dorsal axial region corresponds to the position of ventral limb insertion, with the appendages arrayed so they attach to the body just inside the lateral margin of the axis (see Whittington & Almond, 1987 for a trilobite example). Initially it would appear that the axis in xiphosurans does not correspond to the opisthosomal limb insertion as the trunk limbs have been modified into laterally expanded opercular flaps that extend across the entirety of the ventrum (Fig. 5A), whereas the axis takes up just one-quarter of the thoracetron's width (Fig. 5B), although after removing the opercula it is apparent that their insertion is limited to the margin of the axial region (Fig. 5C, D). The axis therefore is homologous to the condition found in other arthropods and is not a suitable synapomorphy for Xiphosura. Furthermore, an axial region similar to that of xiphosurans is present in the euchelicerate Offacolus kingiOrr et al., 2000, while a differentiated axial region that may be homologous is observed in Chasmataspis laurenciiCaster & Brooks, 1956. Hints of similar structures (albeit extremely poorly preserved) may exist in Diploaspis muelleriPoschmann, Anderson & Dunlop, 2005 and Diploaspis casteriStørmer, 1972. The presence of these structures in other chelicerates rules out the possibility that the xiphosuran axial region is a re-expression of a characteristic not otherwise present in the chelicerate ground plan.
Limulus polyphemusLinnaeus, 1758 from the Recent of North America. A, ventral view of opisthosoma showing opercula. B, dorsal view of opisthosoma with prosoma and telson removed. C, schematic of opisthosoma in dorsal view with apodemes (shallow pits indicating sites of muscle attachment) marked in black and the insertion points of the opercula shown by grey ovals. D, ventral view of opisthosoma with prosoma, telson, and opercula removed. It can be clearly seen that the opercula are not attached to the lateral regions of the opisthosoma. Scale bars = 10 mm.
Ophthalmic ridges, the final proposed synapomorphy, occupy a similar position to the palpebral lobes of eurypterids and chasmataspidids and may in fact be transformational homologues. Furthermore, in a number of xiphosurans the ophthalmic ridges are only weakly developed or absent altogether. While this does not invalidate ophthalmic ridges as a potential xiphosuran synapomorphy, the presence of ridges associated with the palpebral lobes in some chasmataspidids poses more of a problem. Best known from Octoberaspis ushakoviDunlop, 2002, these structures consist of ridges extending from the palpebral lobe along the length of the carapace and appear almost identical to the ophthalmic ridge, the only difference being the much more prominent nature of the lateral eye (Dunlop, 2002: Fig. 6A). Although poorly preserved, ridges in the same region of the carapace can potentially be discerned on the only known specimen of Forfarella mitchelliDunlop, Anderson & Braddy, 1999 (Dunlop et al., 1999: Fig. 1) and ‘Eurypterus’ stoermeriNovojilov, 1959 (Fig. 3B), a species in need of being redescribed as a chasmataspidid. The presence of ophthalmic ridges in some chasmataspidid species also nullifies this character as a xiphosuran synapomorphy. This leaves them currently with no recognized characters uniting the group to the exclusion of other chelicerates. This is not a unique occurrence; after the discovery that chasmataspidids possessed both a metastoma and a genital appendage (Dunlop, 2002; Tetlie & Braddy, 2004) eurypterids were left without a synapomorphy, until the realization that the fusion of appendages VIII and IX to form the genital operculum was a unique characteristic (Lamsdell, 2011).
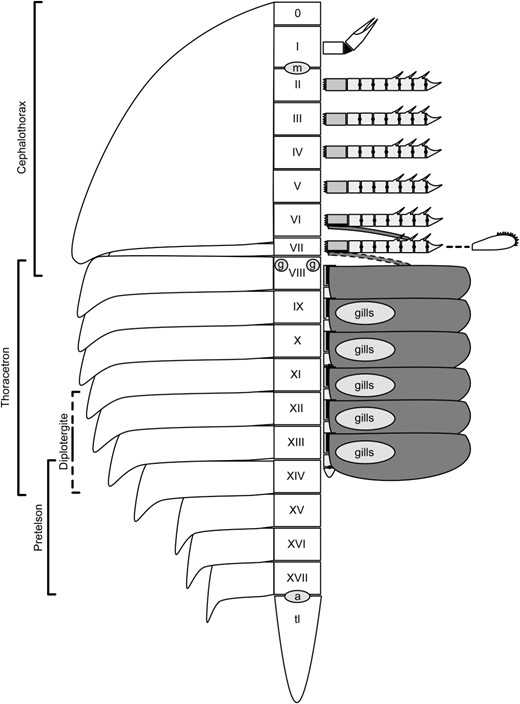
Schematic of the xiphosuran ground pattern. Somites are labelled 0–XVII; ‘tl’ indicates the telson, ‘gills’ the position of gills on the five posterior opisthosomal appendages, ‘m’ the mouth, ‘a’ the anus, and ‘g’ the gonopores. The appendages are shaded with the light ramus representing the endopod, the light grey the basipod, and the dark grey the exopod. The appendage of somite VII is reduced in the majority of taxa into the chilaria. It is possible that appendage VII may also have possessed a reduced exopod as in appendage VI given its intermediate position between appendage VI and the biramous opisthosomal appendages, but there is currently no evidence for this. To the left the somite compositions of the fused xiphosurid cephalothorax, thoracetron, and pretelson are shown, along with the varying combinations of segments that can form the diplotergite in bunodids and pseudoniscids.
The monophyly of chasmataspidids has also been seriously questioned, with Tetlie & Braddy (2004) proposing a scenario in which chasmataspidids form a paraphyletic grade towards eurypterids. This analysis failed to account for a number of characters, however, including the formation of the chasmataspidid buckler, and incorrectly plots the distributions of some characters on the tree (the presence or otherwise of deltoid plates and the anterior opercular plate, specifically) as well as assuming that several characteristics such as the chasmataspidid ventral plate and the chelate prosomal appendage of ChasmataspisCaster & Brooks, 1956 are plesiomorphies. Without a suitable outgroup taxon to polarize the characters there is no way to tell whether these are indeed plesiomorphic (although it is worth noting that ventral plates are at present known solely from chasmataspidids; furthermore, chelate prosomal appendages are known from Offacolus kingi and xiphosurids but are demonstrably absent from synziphosurines when the appendages are preserved and so it appears that this character has evolved more than once within Chelicerata). A metastoma and genital appendage may yet be shown to be present in Chasmataspis, and if undescribed Cambrian resting traces figured in Dunlop et al. (2004) are indeed produced by a Chasmataspis-like animal then it clearly possessed opercula at a minimum. Given the current evidence it seems most likely that chasmataspidids also represent a monophyletic group defined by the possession of a four-segmented buckler with ventral plate (the two characters almost certainly being linked).
Other previously unconsidered characters may serve to define a monophyletic Xiphosura. Opisthosomal segment count is a tempting character, especially given that an opisthosomal segment count of 13 is consistent across chasmataspidids, eurypterids, and scorpions, probably constituting the arachnid ground plan (Dunlop & Webster, 1999). This results in a total post-ocular (all segments except the protocerebral region) somite count of 19. Many arachnids, however, including Pantetrapulmonata, exhibit only 18 somites (Shultz, 2007) but this could potentially be accounted for by the complete suppression of somite VII. The ground pattern for Xiphosura (and potentially Euchelicerata) most likely comprises 11 opisthosomal segments, or 17 somites in total (Fig. 6) as evidenced by a number of fossil taxa and the fact that the neural ganglia for 17 somites are still identifiable in LimulusMüller, 1785 (Scholl, 1977). In terms of unfused segments, LegrandellaEldredge, 1974 clearly shows 11 segments dorsally (Eldredge, 1974: figs 1, 3) while Weinbergina shows ten tergites with a small microtergite assumed to be present but recessed beneath the carapace (Stürmer & Bergström, 1981). However, Anderson, Poschmann & Brauckmann (1998) considered Weinbergina to possess only ten opisthosomal segments, a view adopted by Moore, Briggs & Bartels (2005a). The appendages of Weinbergina show that tergite VII cannot be the first dorsally visible opisthosomal tergite as this segment bears opercula; the pediform walking limbs of somite VII originate from the prosomal region (Stürmer & Bergström, 1981: Fig. 6; Moore, Briggs & Bartels, 2005a: Fig. 2), indicating that they have already been encephalized and their corresponding tergite is either obscured by the carapace fold or has also been incorporated into the prosoma. Thus, both Weinbergina and Legrandella have an 11-segmented opisthosoma. There is a general trend, however, for segments to become fused, the most extreme example being the xiphosurid thoracetron, while certain synziphosurines (some bunodids and pseudoniscids) also exhibit fusion of the two posterior preabdominal segments. Furthermore, the majority of synziphosurine taxa seem to possess only ten opisthosomal segments [two of which are fused in CyamocephalusCurrie, 1927 and PseudoniscusNieszkowski, 1859 (Anderson, 1999)], as do most xiphosurids including Lunataspis auroraRudkin, Young & Nowlan, 2008. LimuloidesWoodward, 1865 and Bunodesd'Eichwald, 1854 also possess fused segments that at first appear to occupy the same position as in Cyamocephalus and Pseudoniscus (Størmer, 1955), but Bunodes and Limuloides possess 11 opisthosomal segments (Bergström, 1975) and so the tergites that have fused are not homologous (XIII and XIV as opposed to XII and XIII). This suggests that tergite fusion has occurred independently in a number of xiphosuran groups. A further complication of using opisthosomal segment count to define Xiphosura is the possibility that the total somite count of 17 represents a plesiomorphic condition. The megacheiran arthropod Yohoia tenuisWalcott, 1912 possesses a 13-segmented trunk and a head bearing the great appendages and three postantennular appendages for a total of 17 somatic segments (Haug et al., 2012b), a somite count potentially shared with Haikoucaris ercaiensisChen, Waloszek & Maas, 2004, although the number of cephalic appendages in this taxon is unclear. Megacheirans are one of the potential sister groups to Chelicerata, as first proposed by Størmer (1944) [not Cotton & Braddy (2004) as suggested by Haug et al. (2012b)] and retrieved through cladistic analysis by Briggs & Fortey (1989), and it is therefore possible that 17 somites is the plesiomorphic state retained by xiphosurans from megacheiran ancestors.
Two other potential synapomorphies remain. Axial nodes are found on a number of xiphosurids and several synziphosurines including Weinbergina (Stürmer & Bergström, 1981), Legrandella (Eldredge, 1974), WillwerathiaStørmer, 1969 (Anderson et al., 1998), and Limuloides (Woodward, 1872). Pseudoniscids, along with the remaining bunodids, lack axial nodes (Anderson, 1999; Krzemiński, Krzemińska & Wojciechowski, 2010). The presence of axial nodes, however, is probably the plesiomorphic condition and therefore not a good clade-defining character, as they are also known from Offacolus kingi, which is considered to be the most basal known euchelicerate (Dunlop, 2006; this study). Finally, a three-segmented postabdomen is present in all synziphosurines (except perhaps Pasternakevia podolicaSelden & Drygant, 1987) with three segments also visible in the fused postabdomen of the xiphosurid Lunataspis aurora, although the postabdomen has become fused into a single segment in all other xiphosurids. The pattern of tagmosis in Offacolus kingi is somewhat unclear; Sutton et al. (2002) describe their specimens as possessing a three-segmented preabdomen (for which they incorrectly use the term mesosoma) and a five-segmented postabdomen (their metasoma). However, the first postabdominal tergite is identical in form to the preabdominal tergites and the terminal segment is little more than a boss that forms the articulating base for the telson; a similar structure is seen in eurypterids, xiphosurids, and ceratiocarids, and is derived from the telson itself rather than the trunk. This would appear to reduce the number of postabdominal segments to three, making this count plesiomorphic with respect to xiphosurans. Accounting for the ventral trunk appendages reveals a different situation, however. Offacolus possesses six pairs of trunk appendages, one pair for each of the first six opisthosomal tergites. This represents the mesosoma and shows that a full, six-segmented mesosoma was part of the euchelicerate ground plan. Sutton et al. (2002) show that the posterior three opercula are reduced and devoid of respiratory structures, although the lack of respiratory structures is not a significant enough difference to warrant the exclusion of these segments from the mesosoma. The erroneous mesosoma/metasoma division employed by Sutton et al. (2002) stems from a failure to appreciate the dichotomous nature of the mesosoma/metasoma and preabdomen/postabdomen tagma (see Terminology), and it is clear that the mesosoma of Offacolus consists of six segments while the metasoma comprises just a single segment.
The division into preabdomen and postabdomen is less clear. Although the postabdomen generally consists of fewer segments than the metasoma (for example three or four metasomal segments in xiphosurans and five or six metasomal segments in eurypterids) it is actually defined solely on dorsal taper and tergite differentiation. The postabdomen of Offacolus is therefore defined as comprising three fused segments, two of which bear opercula. This condition of having opercula-bearing postabdominal segments is not unique among Chelicerata; Upper Cambrian trace fossils that show some morphological affinities to Chasmataspis (Dunlop et al., 2004) clearly possess six pairs of opercula while having a three-segmented preabdomen and a nine-segmented postabdomen. Further uncertainty regarding the utility of a three-segmented postabdomen in defining Xiphosura arises from Yohoia tenuis which also lacks appendages on the three terminal trunk segments. The tergites of these segments are also differentiated from those that precede them, narrowing consecutively and bearing shorter, more acute epimera. This too may indicate that a postabdomen of three segments is the plesiomorphic condition.
In summation, a post-ocular somite count of 17 may in fact serve to unify Xiphosura to the exclusion of all other euchelicerates, as might a postabdomen consisting of three segments, but these could also prove to be plesiomorphic depending on which taxa are eventually shown to constitute the sister group to Chelicerata. Thus all of the characters previously proposed as uniting Xiphosura are either plesiomorphic or also present in other euchelicerates and at present there are no convincing synapomorphies of the group.
Phylogenetic Analysis
While this refutation of the evidence for xiphosuran monophyly may seem compelling, one needs to take into account all the available morphological data. To thoroughly evaluate the signal of the current data a phylogenetic analysis was performed incorporating 12 of the most completely preserved synziphosurines, along with four xiphosurids, Offacolus kingi, and representatives of Eurypterida, Chasmataspidida and Arachnida. In total 27 euchelicerate taxa were included in the analysis, along with a further ten non-euchelicerate arthropods (Table 1), coded based solely on characters either observable in directly studied specimens or in photographs published in the literature. Camera lucida drawings, reconstructions and descriptive text were not used as sources for taxon coding so as to avoid undue interpretation. Pycnogonids are frequently considered the sister group to Euchelicerata, together forming the subphylum Chelicerata; however, it has been proposed that pycnogonids represent the sister group to all other euarthropods, which form a clade termed Cormogonida (see Dunlop & Arango, 2005 for a review). For this analysis pycnogonids were treated as ingroup taxa, contra the Cormogonida hypothesis, as supported by a number of recent molecular analyses (Regier et al., 2010; Rota-Stabelli et al., 2011). Three species were included: the fossil taxa Haliestes dasosSiveter, Sutton, Briggs & Siveter, 2004 and Palaeoisopus problematicusBroili, 1928, and the extant Pycnogonum litorale (Ström, 1762). If pycnogonids do in fact represent the sister group to Euchelicerata, however, then they would appear to be highly apomorphic and it then becomes necessary to resolve the sister group to Chelicerata as a whole, although there is still a degree of controversy surrounding this matter. Previously, trilobites and trilobite-like arthropods had been considered to form the chelicerate stem lineage (Størmer, 1944), a concept termed Arachnomorpha. A number of arachnomorph phylogenies have been carried out (e.g. Cotton & Braddy, 2004) without seriously testing the monophyly of the group, but Scholtz & Edgecombe (2005) explicitly rejected Arachnomorpha as a clade, placing the majority of trilobitomorph taxa in the mandibulate stem lineage. The conclusions of Scholtz & Edgecombe have, however, been ignored by a number of workers who continue to perform phylogenetic analyses solely inclusive of ‘arachnomorph’ taxa (e.g. Hendricks & Lieberman, 2008). Therefore, three trilobitomorphs were included in the analysis: the trilobite Olenoides serratus (Rominger, 1887), Emeraldella brockiWalcott, 1912, and Sidneyia inexpectansWalcott, 1911. Note that no mandibulates were included in the analysis; however, given that trilobites have been shown to resolve as part of a clade including mandibulates when both have been included in an analysis (e.g. Stein & Selden, 2012), Olenoides is here taken to represent Antennata, i.e. a clade including Mandibulata and its stem lineage taxa. Finally, the megacheiran arthropods Alalcomenaeus cambricusSimonetta, 1970, Leanchoilia illecebrosaHou, 1912 and Yohoia tenuis were included in the analysis. Megacheirans are the most recent taxa to have been suggested as a sister group to chelicerates (e.g. Chen et al., 2004; Dunlop, 2006), although whether megacheirans are monophyletic or paraphyletic varies between authors. Two taxa that are not included in the analysis but have previously been considered to be allied in some manner to chelicerates are Aglaspis spiniferRaasch, 1939 and Sanctacaris uncataBriggs & Collins, 1988. The similarities between Aglaspis and chelicerates are purely superficial, however; the supposed chelicerae were shown to be multisegmented and more similar to antenniform appendages by Briggs et al. (1979), while the head incorporates no more than four pairs of postentennula appendages (Hesselbo, 1992). Furthermore it is unclear whether the apparent uniramy of the appendages is preservational or genuine (it is worth noting that the trunk limbs of xiphosurans retain both endopods and exopods, and uniramy would preclude Aglaspis from being a direct chelicerate ancestor). The possession of a telson and the form of the smooth articulating facets on each tergite are also euarthropod plesiomorphies. When aglaspidids have been included in recent phylogenetic analyses their position has proven to be rather inconstant, although they consistently resolve as relatively basal arthropods somewhat removed from chelicerates (Briggs & Fortey, 1989; Dunlop & Selden, 1997; Cotton & Braddy, 2004) and Aglaspis is here considered to show closer affinities to taxa such as Emeraldella as suggested by Van Roy (2006) and retrieved in the recent cladistic analysis by Ortega-Hernández, Legg & Braddy (2012). Sanctacaris meanwhile was originally described as resolving within the subphylum Chelicerata based on its supposed possession of at least six pairs of encephalized appendages, a cardiac lobe, tagmata equivalent to the prosoma and opisthosoma, and the anus being positioned at the rear of the last trunk segment. The presently available material of Sanctacaris, however, bears no clear similarity to chelicerates; numerous phylogenetic analyses have consistently failed to resolve it in any proximity to the chelicerate clade (Briggs & Fortey, 1989; Briggs et al., 1992; Wills, Briggs & Fortey, 1994). The prosomal/opisthosomal tagmosis is essentially a restatement of having more than four appendages in the head and, given that the opisthosoma is here considered equivalent to the trunk of other arthropods, is not a valid character to unite the species with chelicerates. The nature of the head appendages is not clear; it is possible that the rami described represent branches of a single appendage, or that some of the rami are exopods and others endopods, and although Boxshall (2004) has offered an interpretation of the appendages based on Offacolus the published material does not permit easy comparison. The ‘cardiac lobe’ does not resemble a true cardiac lobe, which should be positioned at the very posterior of the carapace, and the position of the anus is known to vary among other fossil arthropods. A number of characters, however, such as the clustered nature of the cephalic appendages and the morphology of the carapace, do invite some comparison with the Burgess Shale arthropods Habelia optataWalcott, 1912 and Habelia (?) brevicaudataSimonetta, 1964.
Taxa included in the phylogenetic analysis including their higher-level taxonomic assignment
| Species . | Assignment . |
|---|---|
| Fuxianhuia protensa | Stem euarthropod |
| Olenoides serratus | Trilobita |
| Emeraldella brocki | Xenopoda |
| Sidneyia inexpectans | |
| Alalcomenaeus cambricus | Megacheira |
| Leanchoilia illecebrosa | |
| Yohoia tenuis | |
| Haliestes dasos | Pycnogonida |
| Palaeoisopus problematicus | |
| Pycnogonum litorale | |
| Offacolus kingi | Euchelicerate |
| Bembicosoma pomphicus | Synziphosurines |
| Bunodes lunula | |
| Camanchia grovensis | |
| Cyamocephalus loganensis | |
| Kasibelinurus amicorum | |
| Legrandella lombardi | |
| Limuloides limuloides | |
| Pasternakevia podolica | |
| Pseudoniscus roosevelti | |
| Venustulus waukeshaensis | |
| Weinbergina opitzi | |
| Willwerathia laticeps | |
| Euproops anthrax | Xiphosurida |
| Limulus polyphemus | |
| Lunataspis aurora | |
| Paleolimulus signatus | |
| Chasmataspis laurencii | chasmataspidids |
| Octoberaspis ushakovi | |
| Eurypterus tetragonophthalmus | Eurypterida |
| Parastylonurus ornatus | |
| Rhenopterus diensti | |
| Stoermeropterus conicus | |
| Centruroides vittatus | Arachnida (Scorpionida) |
| Palaeophonus nuncius | |
| Galeodes armenicus | Arachnida (Solifugae) |
| Mastigoproctus giganteus | Arachnida (Thelyphonida) |
| Species . | Assignment . |
|---|---|
| Fuxianhuia protensa | Stem euarthropod |
| Olenoides serratus | Trilobita |
| Emeraldella brocki | Xenopoda |
| Sidneyia inexpectans | |
| Alalcomenaeus cambricus | Megacheira |
| Leanchoilia illecebrosa | |
| Yohoia tenuis | |
| Haliestes dasos | Pycnogonida |
| Palaeoisopus problematicus | |
| Pycnogonum litorale | |
| Offacolus kingi | Euchelicerate |
| Bembicosoma pomphicus | Synziphosurines |
| Bunodes lunula | |
| Camanchia grovensis | |
| Cyamocephalus loganensis | |
| Kasibelinurus amicorum | |
| Legrandella lombardi | |
| Limuloides limuloides | |
| Pasternakevia podolica | |
| Pseudoniscus roosevelti | |
| Venustulus waukeshaensis | |
| Weinbergina opitzi | |
| Willwerathia laticeps | |
| Euproops anthrax | Xiphosurida |
| Limulus polyphemus | |
| Lunataspis aurora | |
| Paleolimulus signatus | |
| Chasmataspis laurencii | chasmataspidids |
| Octoberaspis ushakovi | |
| Eurypterus tetragonophthalmus | Eurypterida |
| Parastylonurus ornatus | |
| Rhenopterus diensti | |
| Stoermeropterus conicus | |
| Centruroides vittatus | Arachnida (Scorpionida) |
| Palaeophonus nuncius | |
| Galeodes armenicus | Arachnida (Solifugae) |
| Mastigoproctus giganteus | Arachnida (Thelyphonida) |
Taxa shown in bold type are those for which original specimens were investigated for the analysis.
Taxa included in the phylogenetic analysis including their higher-level taxonomic assignment
| Species . | Assignment . |
|---|---|
| Fuxianhuia protensa | Stem euarthropod |
| Olenoides serratus | Trilobita |
| Emeraldella brocki | Xenopoda |
| Sidneyia inexpectans | |
| Alalcomenaeus cambricus | Megacheira |
| Leanchoilia illecebrosa | |
| Yohoia tenuis | |
| Haliestes dasos | Pycnogonida |
| Palaeoisopus problematicus | |
| Pycnogonum litorale | |
| Offacolus kingi | Euchelicerate |
| Bembicosoma pomphicus | Synziphosurines |
| Bunodes lunula | |
| Camanchia grovensis | |
| Cyamocephalus loganensis | |
| Kasibelinurus amicorum | |
| Legrandella lombardi | |
| Limuloides limuloides | |
| Pasternakevia podolica | |
| Pseudoniscus roosevelti | |
| Venustulus waukeshaensis | |
| Weinbergina opitzi | |
| Willwerathia laticeps | |
| Euproops anthrax | Xiphosurida |
| Limulus polyphemus | |
| Lunataspis aurora | |
| Paleolimulus signatus | |
| Chasmataspis laurencii | chasmataspidids |
| Octoberaspis ushakovi | |
| Eurypterus tetragonophthalmus | Eurypterida |
| Parastylonurus ornatus | |
| Rhenopterus diensti | |
| Stoermeropterus conicus | |
| Centruroides vittatus | Arachnida (Scorpionida) |
| Palaeophonus nuncius | |
| Galeodes armenicus | Arachnida (Solifugae) |
| Mastigoproctus giganteus | Arachnida (Thelyphonida) |
| Species . | Assignment . |
|---|---|
| Fuxianhuia protensa | Stem euarthropod |
| Olenoides serratus | Trilobita |
| Emeraldella brocki | Xenopoda |
| Sidneyia inexpectans | |
| Alalcomenaeus cambricus | Megacheira |
| Leanchoilia illecebrosa | |
| Yohoia tenuis | |
| Haliestes dasos | Pycnogonida |
| Palaeoisopus problematicus | |
| Pycnogonum litorale | |
| Offacolus kingi | Euchelicerate |
| Bembicosoma pomphicus | Synziphosurines |
| Bunodes lunula | |
| Camanchia grovensis | |
| Cyamocephalus loganensis | |
| Kasibelinurus amicorum | |
| Legrandella lombardi | |
| Limuloides limuloides | |
| Pasternakevia podolica | |
| Pseudoniscus roosevelti | |
| Venustulus waukeshaensis | |
| Weinbergina opitzi | |
| Willwerathia laticeps | |
| Euproops anthrax | Xiphosurida |
| Limulus polyphemus | |
| Lunataspis aurora | |
| Paleolimulus signatus | |
| Chasmataspis laurencii | chasmataspidids |
| Octoberaspis ushakovi | |
| Eurypterus tetragonophthalmus | Eurypterida |
| Parastylonurus ornatus | |
| Rhenopterus diensti | |
| Stoermeropterus conicus | |
| Centruroides vittatus | Arachnida (Scorpionida) |
| Palaeophonus nuncius | |
| Galeodes armenicus | Arachnida (Solifugae) |
| Mastigoproctus giganteus | Arachnida (Thelyphonida) |
Taxa shown in bold type are those for which original specimens were investigated for the analysis.
Fuxianhuia protensaHou, 1987, considered to be a derivative of the euarthropod stem lineage, was used as outgroup for this analysis. Although phylogenetic analyses have previously placed Fuxianhuia within Euchelicerata (Wills, 1996), or as a component of a paraphyletic Megacheira (Budd, 2002; Kühl, Briggs & Rust, 2009), this is due to misinterpretation of the animal's morphology. These analyses consider a pair of tubular structures located within the head of Fuxianhuia to be subchelate appendages homologous to the great appendages of megacheirans, although these structures have also been interpreted as gut diverticulae (Waloszek et al., 2005). The arguments for these structures being gut diverticulae are supported by the discovery of large gut diverticulae of similar morphology and position in Emeraldella brocki (Stein & Selden, 2012: Fig. 4A) and this, combined with Fuxianhuia having a simple un-segmented exopod lobe and un-differentiated endopod podomeres, suggests that Fuxianhuia is a member of the euarthropod stem lineage with close affinities to crown Euarthropoda, as initially suggested (Chen et al., 1995; Edgecombe & Ramsköld, 1996). Even phylogenetic analyses that maintain an appendicular origin for the tubular structures in the head now resolve Fuxianhuia as the sister taxon to Euarthropoda (Daley et al., 2009). As the most basic division of the Euarthropoda appears to be between Antennata and Chelicerata, both of which are represented as ingroup taxa within the analysis, it is appropriate that Fuxianhuia, as a stem-euarthropod with close affinities to the crown group, be used as the outgroup.
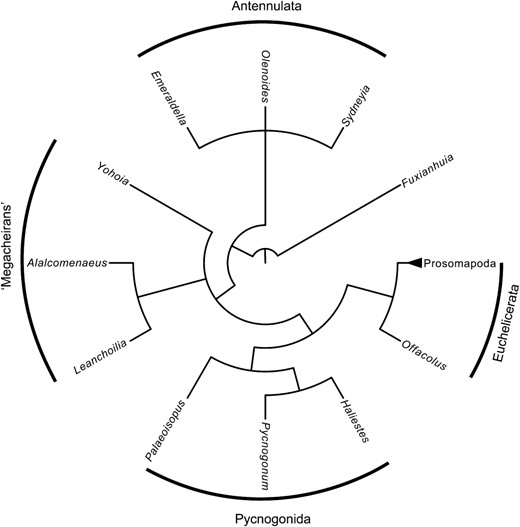
In total 37 taxa were coded for 114 characters (see the Supporting information); the matrix is deposited in morphobank (O'Leary & Kaufman, 2012) with the project code p724 and can be accessed from http://morphobank.org/permalink/?P724. Phylogenetic analysis was performed using random addition sequences followed by branch swapping (the mult command) with 100 000 repetitions with all characters unordered and of equal weight in TNT (Goloboff, Farris & Nixon, 2008; made available with the sponsorship of the Willi Hennig Society). Jackknife (Farris et al., 1996), Bootstrap (Felsenstein, 1985) and Bremer support (Bremer, 1994) values were calculated in TNT and the consistency (CI), retention (RI) and rescaled consistency indices (RCI) were calculated in Mesquite 2.73 (Maddison & Maddison, 2010). The analysis resulted in 12 most-parsimonious trees with a tree length of 283, a strict consensus of which (showing branch support values) is in the Supporting information and is summarized here (Fig. 7, 8). The strict consensus tree has an ensemble CI of 0.583, ensemble RI of 0.770, and an ensemble RCI of 0.449. Pycnogonids are resolved as the monophyletic sister group to Euchelicerata (Fig. 7) which together form Chelicerata, but Pycnogonida are highly autapomorphic and the reduced nature of the dorsal carapace and trunk region in these taxa limit their use in polarizing characters relating to these regions in euchelicerates, although Palaeoisopus does retain a segmented abdomen and telson. Instead the sister group to Chelicerata informs on these characteristics, and in this analysis megacheirans resolve in that position. It is unclear whether they form a clade or a grade, however, as a clade consisting of Alalcomenaeus and Leanchoilia forms a polytomy with Yohoia and Chelicerata. Olenoides, Emeraldella, and Sidneyia form the basalmost clade in the analysis with an unresolved internal topology. While this could be consistent with the arachnomorph hypothesis, following the argumentation of Scholtz & Edgecombe (2005) for trilobites as stem-mandibulates results in this clade being interpreted as representing Antennulata. If the tree is rooted on the pycnogonids, as it would be if the Cormogonida hypothesis were true, then the topology rotates with euchelicerates forming the sister group to a clade with a topology consisting of Fuxianhuia as the sister taxon to Antennulata with megacheirans forming a basal polytomy. Irrespective, the internal topology of Euchelicerata does not change. Offacolus is shown to be the most basal known euchelicerate, resolving as the sister taxon to a clade comprising euchelicerates that have lost the exopods on prosomal appendages II–V. This clade is here termed Prosomapoda nom. nov., and includes xiphosurans (sensu lato), chasmataspidids, eurypterids, and arachnids.
Summary cladogram of higher-level relationships retrieved from the phylogenetic analysis. Chelicerata consists of Pycnogonida and Euchelicerata, with megacheirans forming a polytomy on the node below. Trilobites and xenopods form a basal clade which is here considered to represent Antennata. For the full consensus tree see the Supporting information.
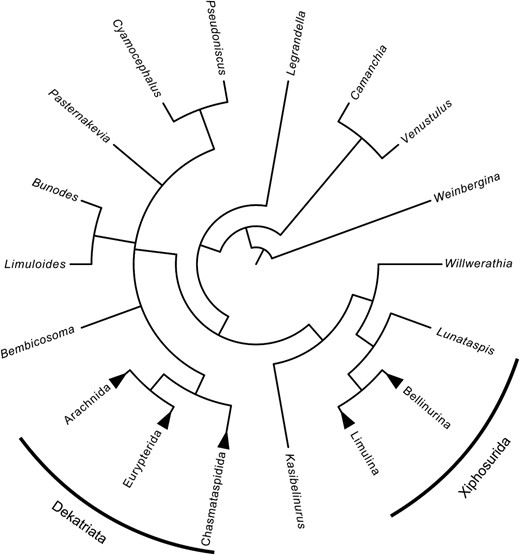
Summary cladogram of the internal relationships of Prosomapoda. Arachnids, eurypterids, and chasmataspidids form a clade, here termed Dekatriata. Xiphosurans are paraphyletic with respect to Dekatriata, with xiphosurids forming a monophyletic clade of their own. All taxa outside the two labels are synziphosurines, which would here be polyphyletic. For the full consensus tree see the Supporting information.
A Revised System–Xiphosuran Paraphyly
Xiphosura, as presently defined, is shown to be paraphyletic (Fig. 8). Xiphosurids do indeed form a monophyletic clade, although synziphosurines represent polyphyletic aquatic chelicerates that resolve at the base of a number of well-supported chelicerate clades. Weinbergina, VenustulusMoore in Moore et al. 2005b, CamanchiaMoore et al., 2011 and LegrandellaEldredge, 1974, taxa that have always been considered to be closely related (either placed within the now defunct Weinberginidae or compared directly with the morphology of Weinbergina), form a grade at the base of Prosomapoda nom. nov. There are a number of characters that support the position of these taxa and the fact they form a grade rather than a clade.
Segment articulations
Lamsdell (2011) proposed two pieces of evidence that suggested synziphosurines formed a paraphyletic grade to a group inclusive of xiphosurids, eurypterids, chasmataspidids, and arachnids, rather than being confined to the xiphosurid stem lineage. One of these lines of reasoning is no longer valid but the other strongly attests to the non-monophyly of Xiphosura. The articulation between the thoracetron and the carapace in xiphosurids is formed by an anterior extension of the articulating facet along the axis into a pseudo half-ring (Fig. 9A), and this type of articulation is vestigial in the tergites of the fused thoracetron of Lunataspis aurora (Fig. 9B). Lamsdell (2011) followed Eldredge (1974) and Eldredge & Plotnick (1974) in suggesting that this type of articulation was common among the synziphosurines, and hypothesized that Weinbergina opitziRichter & Richter, 1929 and Kasibelinurus amicorumPickett, 1993, which possess simple articulating facets without the pseudo half-ring extension, may in fact be ancestral to all the more derived chelicerates as opposed to just Xiphosurida. With the exception of Willwerathia laticeps (Størmer, 1936) (Fig. 9C), however, no synziphosurine demonstrably possesses any form of pseudo half-ring. Instead an articulating facet is present as in Weinbergina opitzi (Fig. 10). Furthermore, possessing an articulating facet is shown to be the plesiomorphic state, being recognizable in megacheirans such as Leanchoilia superlata Walcott, 1912, artiopods such as Emeraldella brocki, and a number of aglaspidids including Aglaspis spinifer, Aglaspis barrandeiHall, 1862, and Chlupacaris dubiaVan Roy, 2006. Therefore, the retention of an articulating facet in synziphosurines provides no information regarding their position on the chelicerate tree.
A, Limulus polyphemusLinnaeus, 1758 from the Recent of North America. Specimen positioned to show the prosoma/opisthosoma joint, with its axially extending articulation. B, Lunataspis auroraRudkin, Young & Nowlan, 2008 from the Upper Ordovician (Katian) of Manitoba, Canada. Rubber mould of specimen MM I-3990, showing the axial extension of the articulating facet on each segment. Image courtesy of Graham Young. C, Willwerathia laticeps (Størmer, 1936) from the Lower Devonian (Emsian) of Willwerath, Germany. Specimen PWL 2011/5690-LS, showing a similar anterior extension of the articulating facets. Image courtesy of Markus Poschmann. Scale bars = 10 mm.
Weinbergina opitziRichter & Richter, 1929 from the Lower Devonian (Emsian) of Bundenbach, Germany. Holotype specimen SMF VIII 7a, showing a flat articulating facet at the anterior of each tergite. Scale bar = 10 mm.
Somite VII
The second line of enquiry focuses on the appendages of somite VII. It has been suggested that this appendage pair is the origin of the xiphosurid chilaria (Dunlop & Webster, 1999), the eurypterid metastoma (Størmer, 1934), and the scorpion sternum (Jeram, 1998), and if so then all of these structures must be treated as transformational homologues. The chilaria have been shown through work on development in Limulus to comprise the appendages of somite VII (Farley, 2010), whilst scorpions possess an embryonic limb pair anterior to the remaining trunk limbs (Brauer, 1895) that is not expressed in adults. Its eventual fate is unknown, but it is conceivable that the pair could proceed to form the sternum. Evidence for the appendicular nature of the eurypterid metastoma stems from the presence of paired muscle scars on many well-preserved specimens and a possible fused median suture in others. If one accepts the homology of these structures, the fully pediform appendage VII of Weinbergina (as shown by Stürmer & Bergström, 1981 and Moore et al., 2005a) needs to be accounted for. There is the possibility that the pediform endopod is an autapomorphy of Weinbergina, especially given the flap-like structures in the same position on Offacolus kingi, although the appendages of Offacolus more closely resemble exopods (bearing some similarity to the exopods of megacheirans) and may represent part of an adaption to a more nektonic mode of life. More likely is that the pediform limb is a plesiomorphic character, irrespective of uncertainty about the euchelicerate outgroup, and this is the case in the new analysis. A fully pediform appendage VII is found in pycnogonids, trilobites, and megacheirans, along with other mooted ancestral taxa such as Sidneyia inexpectans and Emeraldella brocki. Therefore, for Weinbergina to resolve at the base of a monophyletic Xiphosura, the endopod of appendage VII would have to be independently reduced in both the lineage leading to xiphosurids and that leading to eurypterids, chasmataspidids, and arachnids. A more parsimonious model of evolution is for the fully pediform appendages of Weinbergina to be reduced into the chilaria through the synziphosurine lineage (some of which, such as Venustulus waukeshaensisMoore in Moore et al., 2005b, clearly only have five post-cheliceral pediform appendages) that were retained in xiphosurids but fused to form the metastoma in eurypterids and chasmataspidids.
Chelicerae
There are also a number of further characteristics, not explored by Lamsdell (2011), that suggest xiphosuran paraphyly. One of the more conclusive is elongation of the chelicerae, a condition found in Offacolus kingi (Sutton et al., 2002) and in Weinbergina opitzi as shown by the manner in which the chelicerae project beyond the anterior carapace margin (Moore et al., 2005a: Fig. 4). The chelicerae are reduced to their usual, shorter length in the synziphosurines Venustulus waukeshaensis and Camanchia grovensisMoore et al., 2011 as well as the xiphosurids. Short chelicerae are also known in the chasmataspidid Loganamaraspis dunlopiTetlie & Braddy, 2004 and in all eurypterids where preserved except for the pterygotids, the enlarged chelicerae of which represent an independently derived condition. Budd & Legg (2011) have suggested that the elongate chelicerae are derived from multi-annulate antenniform appendages such as those of Sidneyia and Emeraldella, but no chelicerae show any sign of possessing more than four segments [with the possible exception of the chelifores of Palaeoisopus which according to Bergström, Stürmer & Winter (1980) comprise five articles] or being formed from fused annulations. Instead, the increase in length is derived from the peduncle (the first cheliceral segment, which is bipartite in pycnogonids and Offacolus) or the segment bearing the fixed ramus of the cheliceral claw. This state is also shared with the fossil pycnogonids Palaeoisopus problematicus and Haliestes dasos, which have large, robust chelifores similar in width to the walking limbs and of equal length to the palps, while a number of megacheiran taxa also possess great appendages composed of few articles where the peduncle and first armature-bearing segment are elongated (Haug et al., 2012b). Irrespective of whether elongate chelicerae have a great appendage or antenniform origin, it is clear that the condition is plesiomorphic for Euchelicerata and so a similar situation arises as with the form of appendage VII. For Weinbergina to be positioned at the base of a monophyletic Xiphosura, reduction of the chelicerae would be required to have occurred in both the xiphosurid stem and the lineage leading to arachnids, eurypterids, and chasmataspidids. Therefore, it is more likely that the precursors to Weinbergina diverged from the main chelicerate lineage prior to the divergence of the xiphosurid clade.
Marginal rim
Weinbergina, Venustulus, Bunodes, and Camanchia also all lack a carapace marginal rim, something that is present in all other synziphosurines, xiphosurids, and eurypterids, even if it is deflected ventrally as in most xiphosurids. The lack of a carapace marginal rim is likely to be the plesiomorphic condition, it being absent from most other arthropods (including Offacolus) with the notable exception of trilobites and aglaspidids.
Tergopleurae
Of a similar nature is the degree of tergopleural overlap. In Offacolus, along with Weinbergina, Venustulus, and Camanchia, the tergopleurae overlap one another, as is the condition for the majority of artiopods and megacheirans (with the exception of Yohoia) and trilobites (the tergopleurae of which abut each other). Among the remaining synziphosurines, however, the tergopleurae are separated laterally to form a ‘gape’; it is this condition that is also present in Yohoia. Eurypterids, with their reduced epimera, also exhibit a lateral gape, as do xiphosurids that retain the epimera on the lateral margins of the thoracetron despite the fusion of the tergites. This again would be consistent with xiphosurids sharing closer common ancestry with eurypterids than with some of the synziphosurines that were previously meant to comprise part of the xiphosurid stem.
Xiphosura sensu stricto
Kasibelinurus and Willwerathia resolve as a paraphyletic grade in a clade with xiphosurids, and are the only two synziphosurines in the analysis that fall within the revised Xiphosura. Xiphosura sensu stricto is defined by the possession of a cardiac lobe that extends onto the anterior half of the carapace and potentially the possession of opthalmic ridges that curve towards the carapace centre anteriorly and merge with a central cardiac ridge to form a double arch (or ‘m’) shape; although Kasibelinurus amicorum does not appear to possess this ophthalmic ridge morphology the dorsal carapace of the holotype is flattened and these structures may have not been preserved, while other species currently assigned to Kasibelinurus [such as Kasibelinurus (?) randalli (Beecher, 1902)] clearly show the double arch configuration. Willwerathia is placed as the sister taxon to Xiphosurida despite its unusual morphology, united with the order by its possession of an axial anterior extension to the articulating facet on each tergite.
Bunodids and Pseudoniscids
Xiphosura sensu stricto is itself the sister group to a clade named Planaterga nom. nov. consisting of bunodids, pseudoniscids, chasmataspidids, eurypterids, and arachnids, a group defined at its base by the loss of the distinctive axial and subaxial nodes found on other synziphosurines. Chasmataspidids, eurypterids and arachnids are further defined in their ground plan by the possession of a 13-segmented opisthosoma (although this is then reduced in arachnids) and form a monophyletic clade, which is here named Dekatriata nom. nov. The basal node of Planaterga nom. nov. consists of a polytomy of taxa currently assigned to Pseudoniscidae and Bunodidae. Bunodes and Limuloides resolve as sister taxa, as do Pseudoniscus and Cyamocephalus, but neither family is retrieved in its entirety as unequivocally monophyletic. Both are united with Dekatriata nom. nov., however, in lacking axial nodes (with the exception of Limuloides) and in possessing an opisthosoma that has its widest point at the third or fourth tergite. Furthermore, the pseudoniscids and Dekatriata nom. nov. both show a predilection for possessing a carapace that is at least equal in length and width and an increasing lateral gape in the tergopleurae posteriorly. Bunodes and Bembicosoma, meanwhile, possess a distinct tuberculate ornament that is similar to that found in chasmataspidids. At present there is not enough data to indicate whether pseudoniscids and bunodids form a paraphyletic grade leading to Dekatriata nom. nov. or if they are themselves a diverse and previously unrecognized order of chelicerates. Either way, the recognition that they have a closer relationship to chasmataspidids, eurypterids, and arachnids than xiphosurids is important and has ramifications for the evolution of the group; the presence of dorsally visible reduced pre-opercular tergites in chasmataspidids, pseudoniscids, bunodids, and Willwerathia, for example, means that the tergite of somite VII was reduced independently in Xiphosurida and Dekatriata nom. nov. Although it cannot be seen in Weinbergina, Camanchia, or Venustulus, Legrandella clearly shows that while the tergite of somite VII was already partially reduced it still retained its identity as a fully expressed opisthosomal tergite with tergopleurae prior to its reduction in each lineage.
Higher systematics
With phylogenetic analysis supporting the contention that Xiphosura is paraphyletic it is considered sufficient justification to alter the systematics of Euchelicerata to reflect this revised system. Systematics (including taxonomy) should always aim to accurately represent phylogeny whenever possible, and as such formal names should only be applied to monophyletic groups. Xiphosura is therefore restricted to xiphosurids and those synziphosurines that form the stem lineage within the clade exclusive of Planaterga nom. nov.; in this analysis that comprises Willwerathia and Kasibelinurus, although further synziphosurine taxa should be restudied to ascertain whether they fall within or outside of Xiphosura. Rather than muddy the taxonomic waters by introducing a swathe of monotypic families (or orders!), synziphosurines that fall outside of Xiphosura are at present left unranked, although a number of more inclusive clades are named in order to aid discussion of their relationships. This will probably change through time as polytomies such as those of the bunodids and pseudoniscids are resolved and as more taxa are described. Being offshoots of the main chelicerate lineage with a ghost range of at least 45 Myr it is certain that taxa such as Weinbergina and Legrandella are actually representatives of at least marginally successful clades of marine chelicerates that extend as far back as the Ordovician, even if species diversity remained at a minimum throughout their history. It is likely that further studies of Ordovician localities, as well as Silurian and Devonian strata, will reveal both xiphosurids and ‘synziphosurines’.
Taxonomy
Chelicerata Heymons, 1901
Diagnosis
Arthropoda with the preoral appendages of somite I modified into chelate grasping appendages.
Included taxa
EuchelicerataWeygoldt & Paulus, 1979; PycnogonidaLatreille, 1810 [ = ArachnopodaDana, 1853].
Pycnogonida Latreille, 1810 [ = Arachnopoda Dana, 1853]
Diagnosis
Chelicerata with all body segments retaining dorsal differentiation; external proboboscis developed around mouth; appendage III modified into ovigers; abdomen generally reduced.
Euchelicerata Weygoldt & Paulus, 1979
Diagnosis
Chelicerata with the body divided into a prosoma, mesosoma, and metasoma; prosoma consisting of six appendage pairs united dorsally by a carapace; mesosomal appendages modified into flap-like opercula.
Included taxa
OffacolusOrr et al., 2000; Prosomapodanom. nov.
Prosomapoda nom. nov.
Etymology
From the term prosoma, referring to the anterior chelicerate tagma, and the Greek podi (foot).
Diagnosis
Euchelicerata with prosomal appendages II–V lacking exopods in the adult instar.
Included taxa
?AndarellaMoore, McKenzie & Lieberman, 2007; ?BorchgrevinkiumNovojilov, 1959; CamanchiaMoore, Briggs, Braddy & Shultz, 2011; LegrandellaEldredge, 1974; VenustulusMoore in Moore et al. 2005b; WeinberginaRichter & Richter, 1929; Planaterganom. nov.; XiphosuraLatreille, 1802 [ = MerostomataDana, 1852].
Remarks
Prosomapoda incorporates all euchelicerates that have uniramous prosomal appendages in the adult instar apart for the flabellum found on appendage VI. This node-based definition allows for taxa to be incorporated or excluded from Prosomapoda in the future without further altering the taxonomy.
Xiphosura Latreille, 1802 [ = Merostomata Dana, 1852]
Diagnosis
Prosomapoda with a partially reduced tergite of somite VII; appendages of somite VII reduced to chilaria; opisthosoma broadest anteriorly; cardiac lobe extends onto anterior half of carapace; opthalmic ridges merge anteriorly with median ridge to form double arch.
Included taxa
KasibelinurusPickett, 1993; ?MaldybulakiaTesakov & Alekseev, 1998 [ = LophodesmusTesakov & Alekseev, 1992]; WillwerathiaStørmer, 1969; XiphosuridaLatreille, 1802.
Remarks
Merostomata was originally proposed by Dana (1852) as consisting solely of Xiphosura but later expanded to include Eurypterida (Woodward, 1866–1878). Eurypterida being closer related to Arachnida than Xiphosura necessitates their removal from Merostomata and results in Merostomata being a junior synonym of Xiphosura.
ElleriaRaymond, 1944 and KiaeriaStørmer, 1934 probably have a fused thoracetron and are therefore classified as xiphosurids. Maldybulakia has been considered to show similarity to Willwerathia and so is tentatively included within Xiphosura.
Xiphosurida Latreille, 1802
Diagnosis
Xiphosura with the tergites of somites VIII–XIV dorsally fused into a thoracetron.
Planaterga nom. nov.
Etymology
From the Latin plana (level) and terga (back), in reference to the lack of axial nodes that characterizes the clade's ground plan.
Diagnosis
Prosomapoda lacking enlarged axial nodes on opisthosomal tergites; genal spines vestigial; opisthosoma broadest at third or fourth tergite; appendages of somite VII reduced; tergite of somite VII beginning to form microtergite.
Included taxa
BembicosomaLaurie, 1899; ?BunaiaClarke, 1919; Bunodesd'Eichwald, 1854 [ = ExapinurusNieszkowski, 1859]; CyamocephalusCurrie, 1927; LimuloidesWoodward, 1865 [ = HemiaspisWoodward, 1864]; PasternakeviaSelden & Drygant, 1987; PseudoniscusNieszkowski, 1859 [ = NeolimulusWoodward, 1868]; Dekatriatanom. nov.
Remarks
It is currently equivocal as to whether pseudoniscids and bunodids form a clade of their own or are paraphyletic in regard to Dekatriata.
This clade is defined as incorporating all taxa that resolve along the arachnid stem, exclusive of Xiphosurida and those taxa that diverge from the chelicerate lineage prior to the divergence of Xiphosurida.
Dekatriata nom. nov.
Etymology
From the Greek dekatria (thirteen), referring to the 13-segmented opisthosoma that defines the clade's ground plan.
Diagnosis
Planaterga with a total of 19 somites; opisthosoma plesiomorphically consisting of 13 segments; appendages of somite VII fused into plate.
Included taxa
ChasmataspididaCaster & Brooks, 1956 [ = DiploaspidaSimonetta & Delle Cave, 1978]; SclerophorataKamenz, Staude & Dunlop, 2011.
Remarks
The character used to define this clade, the presence of 13 segments in the opisthosoma, is considered to be present in the arachnid groundplan (Dunlop & Webster, 1999). Although the number of segments in other arachnid groups is often fewer, this could be achieved either through suppression of the VII somite or a paedomorphic reduction of segments (see Dunlop, 1998 for a similar concept to this but based on arachnid polyphyly).
Chasmataspidida Caster & Brooks, 1956 [ = Diploaspida Simonetta & Delle Cave, 1978]
Diagnosis
Dekatriata with the tergite of somite VII retained as a dorsally visible microtergite; tergites of somites VII–X dorsally fused into a buckler; buckler underlain by ventral plate.
Sclerophorata Kamenz, Staude & Dunlop, 2011
Diagnosis
Dekatriata possessing sclerophores as part of a spermatophore-mediated sperm transfer system
Included taxa
ArachnidaLamarck, 1801; EurypteridaBurmeister, 1843 [ = GigantostracaHaeckel, 1866; = CyrtoctenidaStørmer & Waterston, 1968].
Remarks
It is possible that Sclerophorata and Dekatriata are synonyms, but until more detail of the genital structures of chasmataspidids is known this clade is considered to comprise solely Arachnida and Eurypterida.
Eurypterida Burmeister, 1843 [ = Gigantostraca Haeckel, 1866] [ = Cyrtoctenida Størmer & Waterston, 1968]
Diagnosis
Sclerophorata with the appendages of somites VIII and IX fused into a genital operculum.
Arachnida Lamarck, 1801
Diagnosis
Sclerophorata with reduced head shield lacking cardiac lobe; anteroventrally directed mouth; proventricular crop lost; adult instars without appendages of the first opisthosomal segment.
Remarks
A monophyletic Arachnida is one of the most strongly supported clades within the analysis, even without characters traditionally considered to unite the group that have been suggested to represent convergences towards a terrestrial mode of life (Dunlop & Webster, 1999). Dunlop et al. (2012) recently reviewed the cephalic tagmosis in solifuges, acariformes, schizomids, and palpigrades, which have the prosoma separated anteriorly into a propeltidium consisting of the first four appendage pairs, that they suggested may be homologous to the ‘euarthropod head’ (see Chen, 2009). Dunlop et al. suggest that a divided prosomal region consisting of a propeltidium and two separate segments bearing walking limbs may be plesiomorphic for arachnids. This would, however, necessitate a separate acquisition of the prosoma in each of the aquatic euchelicerate groups. The solifuge Galeodes armeniacusBirula, 1929 was included in the current analysis with its head composition coded polymorphically as both 1 + 3 and 1 + 5, representing the fact that it possesses a propeltidium as a subdivision of the prosoma. The results, however, show that the plesiomorphic condition for arachnids is a fully consolidated prosoma, and the division into propeltidium is a derived state (or possibly a reversal). Alternatively coding G. armeniacus as only possessing a 1 + 3 head did not change the tree topology, or the arachnid ground plan, in any way.
Conclusions
The redefinition of Xiphosura requires that we rethink the patterns and timing of early chelicerate evolution. With synziphosurines scattered across the chelicerate tree, as either part of the stem lineage to all other euchelicerates except Offacolus, the xiphosurid stem lineage, or even the stem lineage of a clade comprising chasmataspidids, eurypterids, and arachnids, it is clear that the phylogenetic diversity of Palaeozoic aquatic chelicerates as previously defined has been underestimated. Furthermore, the winnowing of Xiphosura sensu stricto also indicates that, except for the proliferation of Bellinurina in the Carboniferous, the clade has always had a relatively low diversity. The new proposed topology of the chelicerate tree also results in large ghost ranges for all of the synziphosurine taxa. While further studies of Ordovician strata in Europe and North America will probably reveal more aquatic chelicerate taxa that may be recognizable as the ancestors of the Silurian and Devonian synziphosurines, attention should also turn to the Gondwanan provinces. Anderson (1996) suggested that the appearance of seemingly primitive synziphosurines during the Devonian was the result of their radiation from Gondwanan refugia, while Legrandella is itself from Bolivia. Lamsdell, Hoşgör & Selden (2012) suggested a Gondwanan origination for eurypterids, the constituent clades of which also all have ghost ranges extending into at least the Katian. Both xiphosurids and synziphosurines have been discovered from the Ordovician of Morocco (Van Roy et al., 2010) and so it is possible that the earliest euchelicerates may be found outside of Laurentia. It is worth noting that megacheirans have a near global distribution, having been recorded from North America (e.g. Briggs & Collins, 1999; Haug et al., 2012b), China (e.g. Chen et al., 2004; Liu, Hou & Bergström, 2007), and Australia (Edgecombe, García-Bellido & Paterson, 2011), and if they are in fact the sister group to Chelicerata then the potential remains for chelicerates to have originated anywhere within this range.
While the balance of evidence presented herein indicates that Xiphosura as has previously been defined is paraphyletic, further discoveries are needed to help elucidate the associations of some of the more problematic taxa (such as Andarella and Borchgrevinkium) and resolve the nature of the relationship between bunodids and pseudoniscids. A restudy of these problematic Palaeozoic ‘synziphosurines’ may provide further information on their relationships, while similar cladistic work should be carried out on the xiphosurids, which have never received a proper phylogenetic treatment. The rearrangement of xiphosurans in a paraphyletic grade also opens up the possibility of the existence of synziphosurines that retain further plesiomorphic characteristics or share derived characters with Dekatriata. While a Weinbergina-like form with biramous prosomal limbs would previously have been difficult to reconcile with the previous model of chelicerate evolution, and a pseudoniscid or bunodid with a genital appendage would have been deeply unsettling, the discovery of either would now be almost expected given the revised relationships set out in this study.
A Note on Dibasterium
Soon after the acceptance of this paper, Briggs et al. (2012) described a new synziphosurine from the Silurian of Herefordshire. This remarkable creature, named Dibasterium durgae, closely resembles the hypothesized ‘Weinbergina-like form with biramous prosomal appendages’ postulated in the conclusion of the present paper. Briggs et al. also include Dibasterium in a phylogenetic analysis that retrieves the traditional Xiphosura (synziphosurines and Xiphosurida) as monophyletic along with Offacolus. Four apparent synapomorphies are cited as supporting this clade, although one of them (character 22–raised axial region) is simply an expression of the arthropod axis as noted here. The status of two of the remaining synapomorphies, character 77 (chelate walking limbs) and character 88 (seventh appendage pair incorporated into the cephalic tagma), is also uncertain as chelate prosomal appendages are known from Chasmataspis (which was not included in the analysis) while the authors did not account for the probability of the eurypterid metastoma being homologous to the seventh appendage pair. This leaves a single synapomorphy, character 38 (an elongate telson), a subjective character that is sensitive to relative changes in both telson and opisthosomal length. Although all of the potential xiphosuran taxa sampled possess elongate telsons, a large number of unsampled synziphosurines do not, and so its suitability as a synapomorphy for the clade is dubious. Synziphosurines and Xiphosurida are united by characters 85 (appendage VI modified into a pusher), 89 (appendage VII reduced and spinose along margin) and 90 (appendage VII as chileria); however, characters 89 and 90 are actually re-expressions of one another, and as such one should be removed. This results in two potential synapomorphies for the clade, both of which were taken into account in the analysis presented in herein. Therefore, while the excellent description and analysis of Dibasterium by Briggs et al. does provide new information about character evolution among basal chelicerates it does not provide any new support for xiphosuran monophyly.
Acknowledgements
Ewa Krzemińska (Museum of the Institute of Systematics and Evolution of Animals), Dave Marshall (Ichron/Palaeocast), Markus Poschmann (Generaldirektion Kulturelles Erbe Rheinland-Pfalz), and Graham Young (Manitoba Museum) are thanked for providing images of specimens. Paul Selden (University of Kansas) provided support and equipment that allowed for the execution of this study. Martin Stein (University of Copenhagen) is thanked for many discussions on arthropod morphology. Bruce Lieberman (University of Kansas) read and commented on an earlier version of the manuscript. Thanks are due to Peter Van Roy (Ghent University), Jason Dunlop (Museum für Naturkunde), and an anonymous reviewer for providing comments that greatly improved the quality of the manuscript.