-
PDF
- Split View
-
Views
-
Cite
Cite
Judith C Masters, Fabien Génin, Sébastien Couette, Colin P Groves, Stephen D Nash, Massimiliano Delpero, Luca Pozzi, A new genus for the eastern dwarf galagos (Primates: Galagidae), Zoological Journal of the Linnean Society, Volume 181, Issue 1, September 2017, Pages 229–241, https://doi.org/10.1093/zoolinnean/zlw028
Close - Share Icon Share
Abstract
The family Galagidae (African galagos or bushbabies) comprises five genera: EuoticusGray, 1872; GalagoGeoffroy Saint-Hilaire, 1796; GalagoidesSmith, 1833; OtolemurCoquerel, 1859; and SciurocheirusGray, 1872, none of which is regarded as monotypic, but some (Euoticus and Otolemur) certainly qualify as oligotypic. We argue for the recognition of a sixth genus, if the taxonomy is to reflect galagid evolution accurately. Genetic evidence has consistently demonstrated that the taxa currently referred to the genus Galagoides are not monophyletic but form two clades (a western and an eastern clade) that do not share an exclusive common ancestor; we review 20 years of genetic studies that corroborate this conclusion. Further, we compare vocalizations emitted by small-bodied galagids with proposed phylogenetic relationships and demonstrate congruence between these data sets. Morphological evidence, however, is not entirely congruent with genetic reconstructions; parallel dwarfing in the two clades has led to convergences in skull size and shape that have complicated the classification of the smaller species. We present a craniodental morphometric analysis of small-bodied galagid genera that identifies distinguishing characters for the genera and supports our proposal that five taxa currently subsumed under Galagoides (Galagoides cocos, Galagoides granti, Galagoides orinus, Galagoides rondoensis and Galagoides zanzibaricus) be placed in their own genus, for which we propose the name Paragalago.
INTRODUCTION
In A Field Guide to the Larger Mammals of Africa (Dorst & Dandelot, 1970), Pierre Dandelot illustrated five species of galagos (or bushbabies) and two species of pottos. Four of the galagid taxa were subsumed under the genus Galago: that is Galago alleni, Galago crassicaudatus, Galago demidovi (sic, now referred to as Galago demidoff in accordance with its initial description: Jenkins, 1987; Groves, 2001) and Galago senegalensis. The needle-clawed galagos were classified in their own genus, Euoticus, in accordance with the taxonomies of Schwarz (1931), Simpson (1945) and Hill (1953). Forty-five years later, all five taxa are regarded as distinct genera, none of which is generally viewed as monotypic, although much of the detailed research has yet to be conducted. In this contribution, we discuss evidence that Galagoides, as construed by Olson (1979) on morphological grounds, does not constitute a clade in molecular phylogenetic reconstructions (DelPero et al., 2000; Masters et al., 2007; Fabre, Rodrigues & Douzery, 2009; Springer et al., 2012; Pozzi, Disotell & Masters, 2014; Pozzi et al., 2015; Pozzi, 2016), and its members are unlikely to have shared an exclusive common ancestor. More specifically, the dwarf galagos confined to the forests of East and southern Africa require the designation of a new genus. In the subsequent text, we abbreviate Galagoides as Gs. to distinguish it from the abbreviation of Galago (G.).
HISTORY OF GALAGID GENERA
The first galagid genus to enter the scientific literature was Galago, described by Etienne Geoffroy Saint-Hilaire (1796) and was based on a lesser galago specimen collected in Senegal, West Africa. The name was taken from the Senegalese vernacular name for these animals. The genus Galagoides was proposed by Sir Andrew Smith (1833) to distinguish the dwarf (G. demidoff) and lesser (G. senegalensis) galagos from what Smith considered to be the ‘true Galagos’ among which he included species now referred to the genera Microcebus and Otolemur. Galagoides is now restricted to the dwarf galago taxa. Otolemur was introduced as the generic epithet to denote the greater galagos by Charles Coquerel (1859) with Otolemur agisymbanus (= garnettii) from Zanzibar as the type species. The genus Euoticus, which distinguishes the needle-clawed species, was introduced initially by John Gray (1863) as a subgenus under the genus Otogale, which also included the greater galagos. A few years later, he elevated Euoticus to the status of a full genus (Gray, 1872). In the same publication, Gray (1872) proposed Sciurocheirus as the generic designation of a squirrel galago specimen (Sciurocheirus alleni, s.l.) deriving from Fernando Po (Bioko Island).
The genus Galagoides was redefined on morphological grounds by Olson (1979) and employed subsequently by many authors (Honess & Bearder, 1996; Kingdon, 1997; Wickings, Ambrose & Bearder, 1998; DelPero et al., 2000; Masters & Bragg, 2000; Masters & Brothers, 2002; Butynski et al., 2006). It comprised the true dwarf galagos (Galagoides demidoff and Galagoides thomasi), the ‘Zanzibar’ galagos (Galagoides cocos, Galagoides granti and Galagoides zanzibaricus) and the squirrel galagos (Galagoides alleni, s.l.). Honess & Bearder (1996) and Kingdon (1997) recognized three new dwarf galago species just before the turn of the 21st century: Galagoides orinus, originally described by Lawrence and Washburn (1936) as a subspecies of Gs. demidoff from the Uluguru Mountains, Galagoides rondoensis from the Rondo plateau and Galagoides udzungwensis from the Udzungwa Mountains. All three localities are in Tanzania. Galagoides udzungwensis has since been downgraded to a subspecies of Gs. zanzibaricus confined to the Tanzanian mainland, while Gs. z. zanzibaricus is reserved for the form on Zanzibar Island. Galagoides orinus and Gs. rondoensis are now recognized as valid species.
The morphological characters uniting Olson’s (1979) genus Galagoides were not made explicit, but from our own observations (Groves, 2001; Masters & Couette, 2015) they include moderate basicranial flexion (i.e. stronger than in most lemuriforms and lorisids, but not as strong as in the genera Galago or Euoticus), anterior upper premolar (P2) not caniniform (sometimes with a hypocone), third upper molar (M3) ≥ posterior upper premolar (P4) and premaxillaries extended into a tube that projects way beyond the lower jaw. The premaxillary tube is longest in dwarf galagos, moderate in ‘Zanzibar’ galagos and least pronounced in squirrel galagos. Euoticus, Galago and Otolemur have no premaxillary tube and the anterior margin of the snout is square in shape; in the absence of a tube, the square-snouted galagos have a relictual nub on the midline beneath the nasal aperture, suggesting that extended premaxillaries may have been the ancestral condition. Among the lorisids, the two small-bodied genera, Arctocebus and Loris, have premaxillary tubes. Galagoides is further characterized by small body size (smaller in the western clade than in the squirrel galagos or most taxa of the eastern clade), a concave nasal profile and dark circumocular rings that range in colour from dark brown to black, separated by a grey to white nose stripe. While the deep russet colouration of squirrel galagos makes them instantly recognizable, a survey of other museum specimens designated as Galagoides revealed variable pelage colouration, both within and between populations. In most cases, the animals were covered in dense, soft hairs with dark grey roots, but brown to bright russet tips on the head, dorsum and outer surfaces of the limbs. The tips of the hairs on the under surfaces are yellow buff to white, and the animals have brown to blackish tails.
OVERVIEW OF MOLECULAR GENETIC EVIDENCE FOR RELATIONSHIPS AMONG GALAGID LINEAGES
Genetic studies – from their earliest days – have consistently indicated that Galagoides is polyphyletic, implying that the grouping based on morphological similarity is probably based on plesiomorphic or homoplastic characters. The first such evidence came from allozymes (Masters et al., 1994), highly repeated DNA sequences (Crovella, Masters & Rumpler, 1994) and 12S ribosomal mitochondrial DNA sequences (rDNA; Bayes, 1998). All of these studies reconstructed the taxon called Gs. alleni (s.l.) as the sister taxon to Otolemur spp., although morphological synapomorphies for this group remain elusive. The only shared character Masters & Brothers (2002) identified from their data set was large, square, bunodont molars, indicative of a predominantly frugivorous diet and potentially homoplastic. The first Zanzibar galago sequences were published by DelPero et al. (2000). The specimen sampled was probably Gs. granti, as it had been collected in northern Mozambique, but was classified as Gs. zanzibaricus on the basis of craniodental morphometrics (Masters & Bragg, 2000). Using partial sequences of three mitochondrial genes (12S and 16S rDNA and cytochrome b), DelPero and colleagues reconstructed Gs. demidoff and the so-called Gs. zanzibaricus as independent lineages that showed higher levels of genetic divergence from one another than either lineage showed from any other galagid taxon in their sample of eight taxa. This result, coupled with the alliance of Gs. alleni with Otolemur, led DelPero et al. (2000) to describe the genus Galagoides as a ‘wastebasket taxon of plesiomorphic species’. This contention has been supported by more recent and more comprehensive studies. The squirrel galagos continue to be recovered as the sister to the Otolemur clade, and Gray’s (1872) genus, Sciurocheirus, has been resuscitated (Grubb et al., 2003; Masters et al., 2007). Despite the paucity of morphological synapomorphies for this grouping, it derives support from the sparse fossil record. Wesselman (1984) described a fossil hypodigm from approximately 3-Myr sediments in Ethiopia that comprises a fragmentary maxilla, an isolated M2 and an edentulous mandible. On the basis of its bunodont teeth and its intermediate size between Otolemur and Sciurocheirus, he interpreted the taxon (now termed Otolemur howelli; Harrison, 2010) as a member of the Sciurocheirus/Otolemur clade, with its closest affinities to Otolemur.
Following the removal of the squirrel galagos from Galagoides, the western and eastern dwarf galagos have continued to be reconstructed as paraphyletic or even polyphyletic in molecular analyses, indicating that the genus still includes two independent clades that did not share an exclusive common ancestor. The western clade comprises the ‘true’ dwarf galagos, Gs. demidoff and Gs. thomasi, and the eastern clade includes Gs. zanzibaricus and its allies. Using complete sequences of the cytochrome b gene, Roos, Schmitz & Zischler (2004) recovered Gs. demidoff as the first galagid lineage to diverge and Gs. zanzibaricus as the sister taxon of Galago, a topology supported by Chatterjee et al. (2009) and Fabre et al. (2009). The tree of Masters et al. (2007) also depicted Galagoides as polyphyletic but did not group Gs. zanzibaricus with the genus Galago. More recently, a more comprehensive phylogenetic study of primates supported a sister taxon relationship between the Zanzibar galagos and the Otolemur/Sciurocheirus clade, with Gs. demidoff and Gs. thomasi again forming an independent clade (Springer et al., 2012).
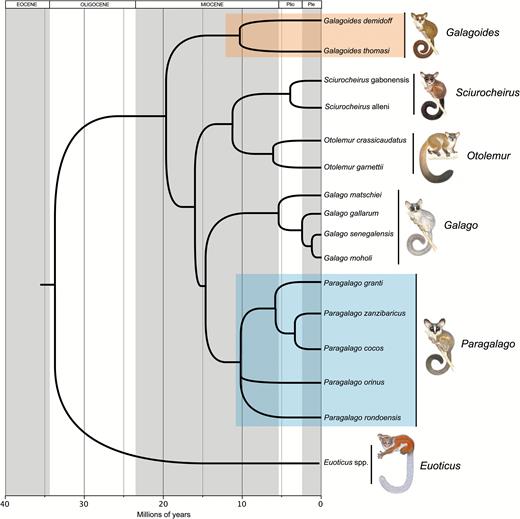
The disagreement among these studies regarding the phylogenetic placement of the eastern and western lineages may be related to incomplete lineage sorting (or the failure of two or more lineages in a population to coalesce, leading one of the lineages to coalesce first with a lineage from a less closely related population) or possibly past introgression events, as they were all based solely on mitochondrial sequences. To address this problem, Pozzi et al. (2014) assembled a molecular data set including 27 independent nuclear loci and inferred phylogenetic relationships using coalescent-based species tree methods to account for incomplete lineage sorting. Their results strongly confirmed the polyphyletic status of Galagoides, as well as a sister taxon relationship between the eastern clade and the lesser galagos (Galago spp.). The largest molecular data set compiled for galagids to date, combining 53 nuclear loci and three mitochondrial genes, confirmed these results (Pozzi, 2016). Figure 1 represents our current understanding of relationships among the lorisoid primates based on both nuclear and mitochondrial sequence data, derived from the studies of Pozzi et al. (2014, 2015) and Pozzi (2016).
Phylogenetic relationships among galagos. The tree represents a summary of our current understanding of relationships among the lorisoid primates based on both nuclear and mitochondrial sequence data, derived from the studies of Pozzi et al. (2014, 2015) and Pozzi (2016). The western dwarf galago clade is identified by a red rectangle, while the eastern clade is enclosed within a blue square.
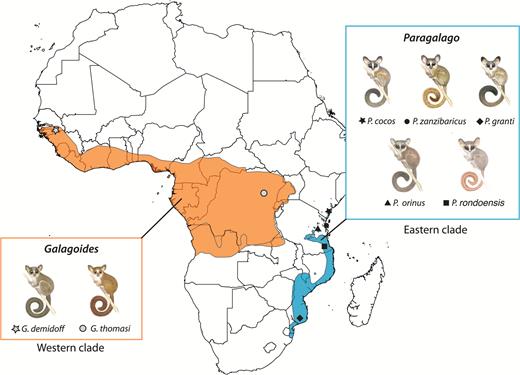
Despite these large nuclear data sets, the sister group relationships of two eastern dwarf galagos, Gs. rondoensis and Gs. orinus, remain unresolved because of limited representative specimens and genetic data; a handful of specimens is distributed across museum collections in North America and Europe. A molecular study based on complete mitochondrial cytochrome b sequences strongly supported an affinity between these species and the Zanzibar galagos to the exclusion of the western Galagoides clade (Pozzi et al., 2015), leading us to include them in the proposed new genus, which hence comprises five species distributed in forests east of the African rift and distinct from the true dwarf galagos, Gs. demidoff and Gs. thomasi, in the west (Fig. 2).
Map showing approximate geographic ranges of the two independent dwarf galago clades, Galagoides (red) and the eastern dwarf galagos (blue). The type localities of the species comprising the genera are indicated by symbols. In the case of Galagoides demidoff, the type locality is estimated from Fischer’s (1806) description.
OVERVIEW OF EVIDENCE FROM VOCAL REPERTOIRES
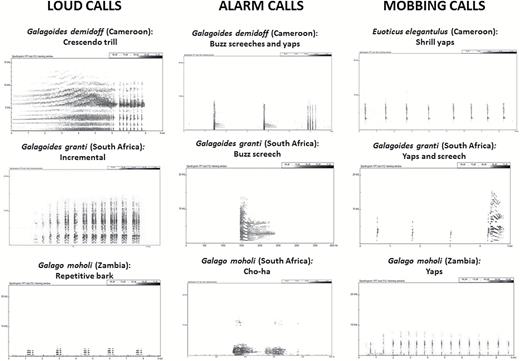
Vocalizations are particularly important indicators of galagid diversity because, as nocturnal animals, galagos do not rely on morphologically encoded visual signals for the location and attraction of conspecific mates. Many galagid species and species groups have been identified on the basis of differences in loud calls (or advertisement calls), which has led to their being grouped according to call structures: crescendo callers, scaling callers, rolling callers, incremental callers and repetitive callers (Bearder et al., 1996; Kingdon, 1997; Butynski, Kingdon & Kalina, 2013). Like all characters that are crucial to specific mate recognition, specific loud calls are qualitatively different between closely related species, and the rapidity of their evolution may obscure their phylogenetic signal at older levels of divergence (Masters, 2007). For instance, rolling and crescendo calls are polyphyletic when compared with species relationships determined by molecular sequence data, indicating a degree of homoplasy: Gs. granti and Gs. cocos both are described as crescendo callers (Bearder et al., 1996; Kingdon, 1997; Butynski et al., 2013), whereas Gs. zanzibaricus, which is reconstructed as the sister taxon to Gs. cocos (see Fig. 1), is a rolling caller, just like Gs. rondoensis. In contrast to advertisement calls, anti-predatory calls tend to be highly conserved phylogenetically, making them more useful as grouping criteria at deeper phylogenetic levels. Vocal homologies among the small-bodied galagos (i.e. excluding Sciurocheirus and Otolemur spp.) can be identified in at least three call types: two anti-predatory calls (mobbing yaps and buzzy alarms; Génin et al., 2016) and the loud socio-territorial calls. These vocal homologies are congruent with phylogenetic relationships among these lineages indicated by molecular analyses and further justify the creation of a new genus for the eastern dwarf galagos.
Mobbing yaps
The mobbing yap is emitted by all small-bodied galagos under similar contexts and is often recorded while an animal is circling around to face the observer (FG, personal observations). It appears to be homologous to the loud call of Euoticus spp. (Fig. 3). It is a high-frequency atonal call repeated at frequent intervals, often after the emission of a few buzzy alarms. The wide frequency range covered by the call that makes it sound atonal to human ears is due to very rapid modulation that is likely to be perceived by the animals.
Sonograms of vocalizations emitted by small-bodied galagid species. Calls of Euoticus (Cameroon) and Galagoides granti (Tshanini, South Africa) were recorded by FG. All other sonograms were downloaded from the East African Primate Diversity and Conservation website (http://www.wildsolutions.nl/vocal-profiles).
Buzzy alarms
Buzzy alarms are homologous in Galagoides, Galago and the eastern dwarf galagos but have very different structures in the three groups (Fig. 3). Buzzy alarms are often the first indicator of the presence of an animal that has not yet been detected visually (FG, personal observations). Animals emit several buzzy alarms that may precede or alternate with yaps. The call is bisyllabic, consisting of an initial high-frequency unit followed by a highly modulated, lower-frequency screech. In western Galagoides spp., the buzz is extremely brief. In Galago spp., it is a brief, noisy, low-frequency cough-like call (cho-ha). In the eastern dwarf galago species, the buzz is a long screech peculiar to the group.
Loud calls
Loud calls are far more variable between taxa than anti-predatory calls. Each of the three small-bodied genera emits a different kind of loud call associated with different contexts, indicating that the various calls evolved different functions associated with different habitats and socio-territorial systems. On the basis of our own observations as well as those of other authors (Bearder et al., 1996; Kingdon, 1997; Butynski et al., 2013), western Galagoides spp. are crescendo callers; the crescendo consists of either a single trill (Gs. demidoff) or a short sequence of trills (Gs. thomasi), starting with an increase in pitch and amplitude (overtone crescendo) followed by repeated, insect-like, high-frequency clicks (Fig. 3). The call is typically used as a gathering call emitted when animals leave or return to their nests. Lesser galagos (Galago spp.) are repetitive callers. They have low-frequency metronomic and tonal calls that are emitted throughout the night, indicating a territorial function. Homology between the loud calls of eastern dwarf galago species and Galagoides crescendo calls is difficult to establish, but such homology with Galago repetitive calls is clear, as they share a basic temporal structure of repeated units forming syllables.
The loud calls emitted by eastern dwarf galagos are so variable that they are difficult to characterize. The group could be called the ‘varied callers’ or ‘modulated callers’, as their loud calls consist of repeated, highly modulated units emitted at higher frequency than Galago repetitive calls. Their function is also less clear, as they are given when animals leave or return to their sleeping sites, as well as throughout the night when animals interact. The calls could hence be categorized as long-distance contact calls adapted to habitats that are generally drier than those of western Galagoides, but wetter and more closed than Galago habitats (Génin et al., 2016). The specific diversity of this group still requires investigation, as only three call structures (i.e. scaling, rolling and incremental) have been described for at least five species.
MORPHOLOGICAL DIFFERENTIATION: NEW ANALYSES
Morphological characterization of the eastern dwarf galagos has been complicated not only by the scarcity of exemplars of some species, but also by their strong convergence with members of the western clade. In a canonical variate morphometric analysis, the skulls of the type specimens of Gs. orinus and Gs. rondoensis clustered with the western clade, contradicting their genetic affinity to Gs. granti and Gs. zanzibaricus (Masters & Couette, 2015). In an attempt to resolve this contradiction, we searched through museum collections in the USA and Europe and identified seven probable Gs. rondoensis specimens in addition to the type specimen held in the Natural History Museum, London. Their identification was based on three factors: the consistent presence of a square M3 with a very small hypocone (a very rare occurrence in other eastern dwarf galagos); collection locality (east of the Rift); and a disjunction between the completion of the eruption of the permanent dentition and skull maturation. In most galagid genera, the attainment of adult body size occurs shortly after the complete eruption of the adult dentition. In the putative Gs. rondoensis specimens we identified, animals with adult (and often worn) dentition had unfused cranial sutures and are likely to have continued to grow had their lives not been prematurely ended. Groves (2001) based his assessment of Gs. rondoensis as the smallest living galagid on the type specimen that had a body weight of 60 g, but animals trapped in the field may be 20–25 g heavier (Andrew Perkin, personal communication). The type specimen has its permanent dentition, but its morphology is juvenile, and fully grown members of this species are likely to be larger than Galagoides orinus adults.
Materials and methods used in the new morphometric analyses
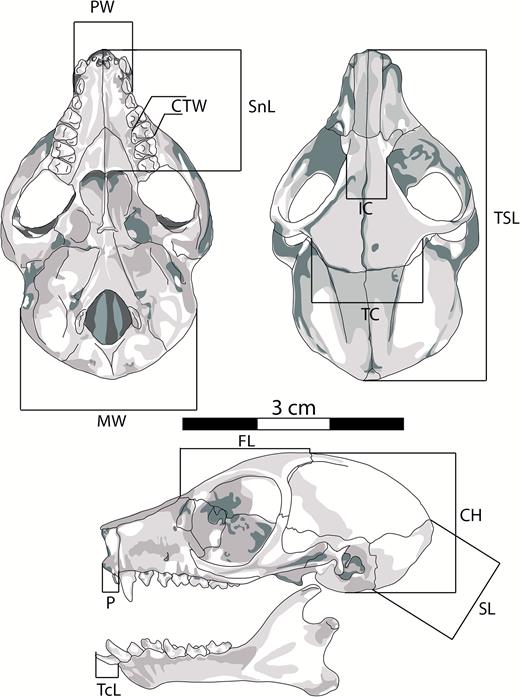
In order to investigate the morphological distinctiveness of the eastern dwarf galagos from other small-bodied galagids, a set of 12 linear craniodental measurements was taken from 610 galago specimens including western dwarf galagos (Gs. demidoff and Gs. thomasi, n = 322; see Masters & Couette, 2015 for specimen list), lesser galagos (Galago moholi, n = 150) and squirrel galagos (Sciurocheirus alleni, s.l., n = 58), plus specimens formerly identified as Gs. cocos, Gs. granti, Gs. orinus, Gs. rondoensis, Gs. udzungwensis and Gs. zanzibaricus (n = 80). Accession details of these specimens are listed in Table 1 of the supplementary data, and the institutions in which mensural data were collected are listed in the ‘Acknowledgements’ section. We followed the methodology of Masters & Couette (2015): measurements (Fig. 4; Table 1) were recorded using digital callipers, and the sample was composed only of specimens with fully erupted dentition.
Cranial measurements used in the study (illustrated in Fig. 1)
| Abbreviation . | Name . | Definition . |
|---|---|---|
| SL | Supraoccipital length | From lambda to opisthion |
| CH | Cranial height | From basioccipital–basisphenoid suture to the highest point of the braincase |
| FL | Frontal length | From bregma to nasion |
| IC | Interorbital constriction | Minimal distance between the inner margins of the orbits |
| CTW | Cheek teeth width | Maximum width of right M1 |
| PW | Palate width | Distance between labial margins of right and left P2 |
| TSL | Total skull length | From prosthion to opisthocranion |
| SnL | Snout length | From prosthion to nasion |
| MW | Mastoid width | Distance between left and right mastoid processes |
| TC | Temporal constriction | Minimum distance between left and right frontotemporals |
| P | Premaxilla | Length of the premaxillary tube |
| TCL | Toothcomb length | From the base to the tip of the incisors |
| Abbreviation . | Name . | Definition . |
|---|---|---|
| SL | Supraoccipital length | From lambda to opisthion |
| CH | Cranial height | From basioccipital–basisphenoid suture to the highest point of the braincase |
| FL | Frontal length | From bregma to nasion |
| IC | Interorbital constriction | Minimal distance between the inner margins of the orbits |
| CTW | Cheek teeth width | Maximum width of right M1 |
| PW | Palate width | Distance between labial margins of right and left P2 |
| TSL | Total skull length | From prosthion to opisthocranion |
| SnL | Snout length | From prosthion to nasion |
| MW | Mastoid width | Distance between left and right mastoid processes |
| TC | Temporal constriction | Minimum distance between left and right frontotemporals |
| P | Premaxilla | Length of the premaxillary tube |
| TCL | Toothcomb length | From the base to the tip of the incisors |
Cranial measurements used in the study (illustrated in Fig. 1)
| Abbreviation . | Name . | Definition . |
|---|---|---|
| SL | Supraoccipital length | From lambda to opisthion |
| CH | Cranial height | From basioccipital–basisphenoid suture to the highest point of the braincase |
| FL | Frontal length | From bregma to nasion |
| IC | Interorbital constriction | Minimal distance between the inner margins of the orbits |
| CTW | Cheek teeth width | Maximum width of right M1 |
| PW | Palate width | Distance between labial margins of right and left P2 |
| TSL | Total skull length | From prosthion to opisthocranion |
| SnL | Snout length | From prosthion to nasion |
| MW | Mastoid width | Distance between left and right mastoid processes |
| TC | Temporal constriction | Minimum distance between left and right frontotemporals |
| P | Premaxilla | Length of the premaxillary tube |
| TCL | Toothcomb length | From the base to the tip of the incisors |
| Abbreviation . | Name . | Definition . |
|---|---|---|
| SL | Supraoccipital length | From lambda to opisthion |
| CH | Cranial height | From basioccipital–basisphenoid suture to the highest point of the braincase |
| FL | Frontal length | From bregma to nasion |
| IC | Interorbital constriction | Minimal distance between the inner margins of the orbits |
| CTW | Cheek teeth width | Maximum width of right M1 |
| PW | Palate width | Distance between labial margins of right and left P2 |
| TSL | Total skull length | From prosthion to opisthocranion |
| SnL | Snout length | From prosthion to nasion |
| MW | Mastoid width | Distance between left and right mastoid processes |
| TC | Temporal constriction | Minimum distance between left and right frontotemporals |
| P | Premaxilla | Length of the premaxillary tube |
| TCL | Toothcomb length | From the base to the tip of the incisors |
Schematic depiction of an eastern dwarf galago skull showing the 12 craniodental measurements included in the multivariate morphometric analyses. Descriptions of the variables are presented in Table 1.
Raw data were size-adjusted using the Burnaby (1966) procedure that consists of extracting an isometric vector from the multivariate data set and back-projecting the values in a multivariate subspace orthogonal to this vector (Klingenberg, 1996). The geometric mean (GM) was computed using the isometric vector and served as a proxy for size. Thus, size (GM) and shape (size-corrected variables = shape variables) are considered independently through analysis of variance (ANOVA; size) or multivariate analysis of variance (MANOVA), principle component analysis (PCA) and canonical variate analysis (log-transformed values of shape). We performed a between-group PCA (BGPCA; Mitteroecker & Bookstein, 2011), which is a classic PCA based on the mean values for each group with no regard for intragroup variation. Specimens are then back-plotted in the morphospace by multiplying the morphological data matrix (log-transformed values of shape) by the coefficient of the BGPCA; the principle components (PCs) are computed only on the intergroup variation rather than on a mix of intra- and intergroup variations. All statistics were performed with R 3.2.3. software (R Core Team, 2015) and the packages ‘candisc’ (Friendly & Fox, 2015), ‘car’ (Fox & Weisberg, 2011), ‘geomorph’ (Adams & Otarola-Castillo, 2013) and ‘smatr’ (Warton et al., 2012).
Results of the morphometric analyses
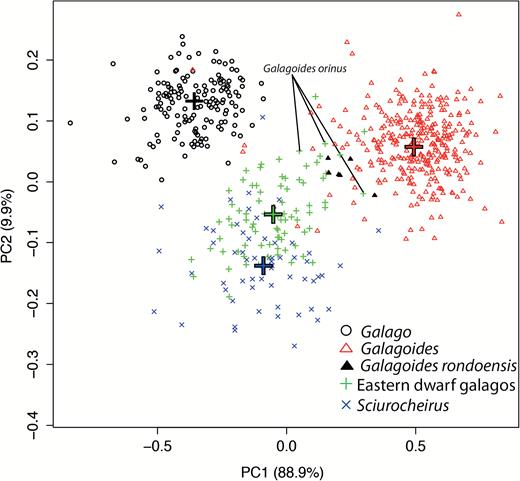
MANOVA results attested to significant differences in cranial morphology among genera (Pillai’s trace = 1.84, F = 86.09, d.f. = 33, P < 0.001). In the BGPCA, three PCs summed up the entire variation in our sample: PC1, PC2 and PC3 accounted for 88.9%, 9.91% and 1.19%, respectively. The genera Galago, Galagoides and Sciurocheirus were well separated in the PC1 × PC2 morphospace, with no overlap except for some outlier specimens (Fig. 5). The presence of outliers and the degree of dispersion evident in Fig. 5 may mean that some subadult skulls were included, along with their correlated allometric shape differences. The cranial morphology of Galagoides individuals was clearly different from that of the Galago specimens, and the differences constituted the major variation along PC1. On this axis, all of the variables had similar loadings (values between −0.24 and 0.07) except for premaxillary length, for which the loading was very high (0.91); hence, the greater part of variation along PC1 was due to differences in the length of the tip of the muzzle. PC2 separated the genus Sciurocheirus (positive values) and the genera Galago and Galagoides (negative values). The variation along PC2 was mainly structured by cheek tooth width (CTW), snout length (SnL) and toothcomb length (TCL), with positive values; and temporal constriction (TC), supraoccipital length (SL) and mastoid width (MW), with negative values. The eastern dwarf galagos showed intermediate cranial morphologies and fell between Galago and Galagoides on PC1 and between Sciurocheirus and the group composed of Galago and Galagoides on PC2. Although the three groups were clearly distinguished on PC1, they overlapped on PC2. The putative Gs. rondoensis specimens were scattered in the space between the eastern dwarf species and Galagoides, and their variation in body size was evident (Fig. 5); despite our best efforts, it is possible that our sample included representatives of more than one species. The Gs. orinus specimens formed part of the main eastern dwarf cluster, although they overlapped with some Gs. rondoensis specimens.
Between-group principle component analysis (BGPCA) calculated on the 12 shape variables. Crosses indicate the mean values of each group that defined the principle component axes to analyse intergroup variation. Specimen data were back-projected in this space. Specimens of the two smallest eastern taxa, orinus and rondoensis, are indicated.
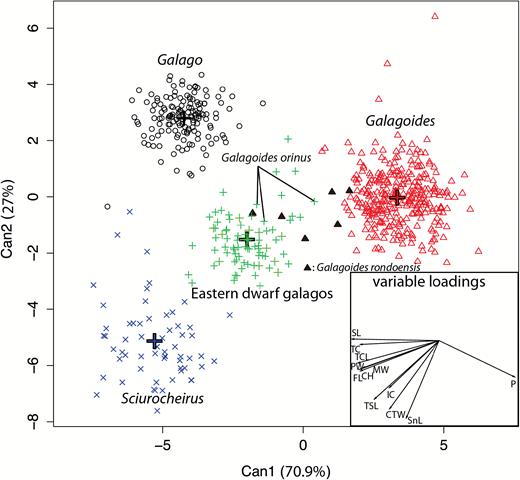
Canonical discriminant analysis defined three significant canonical axes, accounting for 70.98%, 27.06% and 1.96% of interclass variation (Fig. 6). The variable Premaxilla (P) contributed the main discrimination along the first axis. The four genera were well individualized on the first two axes, with high percentages of correct classification: 100% for Galago, 98.14% for Galagoides, 91.3% for the eastern dwarf galagos and 86% for Sciurocheirus. As is evident in visual comparisons of skulls, the elongation of the P is greatest in Galagoides, smaller in the eastern dwarf galagos and smallest in Galago [Tukey’s honest significant difference (HSD) post hoc test P-values < 0.01 among these genera], but the difference in premaxillary length between the eastern dwarf galagos and Sciurocheirus was not significant. The variables with highest loading on the second axis were total skull length (TSL), SnL and CTW. The eastern dwarf galagos differed significantly in SnL from Galago and Sciurocheirus, but not from Galagoides. All of the genera differed significantly in CTW, with values increasing from Galago through Galagoides to the eastern dwarfs and finally Sciurocheirus, the large bunodont molars of which evince its affinity to Otolemur spp. A similar trend is noticeable for TSL measurements, with the smallest values in Galagoides, increasing in Galago and the eastern dwarfs and with Sciurocheirus having the longest skulls. As in the BGPCA, the Gs. rondoensis specimens occupied the morphospace between the eastern dwarf galagos and Galagoides, while Gs. orinus was more closely grouped with the eastern dwarf species. Specimens of Gs. rondoensis and Gs. orinus show intermediate morphology and overlap with eastern dwarf galagos and specimens of the genus Galagoides.
Canonical variate analysis of the 12 shape variables. Crosses indicate the centroid of each group. The first two roots illustrate significant differences in skull shape among genera. Specimens of the two smallest eastern taxa, orinus and rondoensis, are indicated.
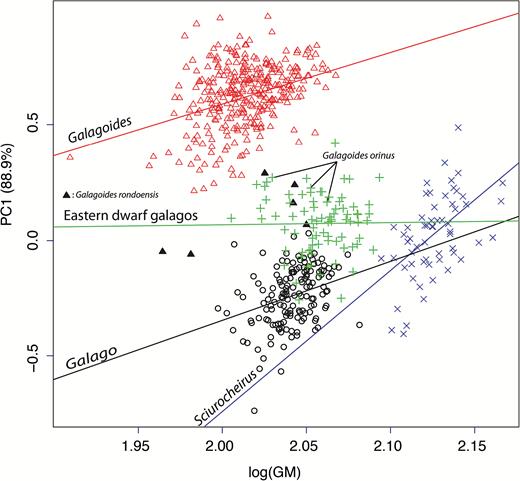
An ANOVA of skull size repeats the pattern shown by TSL (P-value < 0.001 and all Tukey’s HSD multiple comparison of mean P-values below 0.001). In order to test the relationship of size (GM) and shape (logged size-corrected variable), we performed a multivariate regression considering the effect of size on shape, genus and the interaction between size and genus. We used a Type II ANOVA to test each term of the linear model. Size, genus and the interaction had a significant effect on shape with P-values below 0.001, attesting that shape variation is explained by size variation (allometry). The common allometry, that is the proportion of shape explained by size across the entire sample, was 77.7%, but considering the allometric vectors for each genus yielded much lower values: size accounted for 15.1% of shape variation in Galago, 20% in Galagoides, 13.8% in the eastern dwarfs and 8.7% in Sciurocheirus. Pairwise comparison of multivariate allometric patterns demonstrated significant differences between the eastern dwarfs and Galago (P-value of angle between allometric vectors = 0.0428), Galagoides (P = 0.019) and Sciurocheirus (P = 0.014; Fig. 7). With respect to univariate differences in allometries among genera, Sciurocheirus presented a very different pattern from all other genera: the allometric slopes of the variables SL, cranial height, frontal length, CTW, palate width, MW, TC and P were all significantly different. The eastern dwarf galagos differed in slope from Galago for variables SL and CTW and from Galagoides for variables interorbital constriction (IC), CTW, TSL and P.
Allometric trajectories for each genus described by the linear regression of PC1 against the log-transformed centroid size. The allometric patterns are different among genera. Unlike the other genera, the eastern dwarf galago taxa do not show significant allometry. Specimens of the two smallest eastern taxa, orinus and rondoensis, are indicated.
Sciurocheirus is hence clearly differentiated in both size and shape. Comparing the three smaller-sized genera, our multivariate analyses indicated marked differences in cranial shape among them. From a univariate perspective, the eastern dwarf group differed mainly in SL (intermediate between Galago and Galagoides), IC, CTW and TSL (higher values in the eastern dwarf group). These morphometric differences reinforce our proposal to distinguish the eastern dwarf galagos from Galago and Galagoides at the generic level.
SYSTEMATICS
Paragalago gen. nov.
http://zoobank.org/urn:lsid:zoobank.org:act: 4B5DD0A6-BB35-4C9E-B292-AA69A90D6431
Type species:
Galago zanzibaricusMatschie, 1893.
Included species:
Galago grantiThomas & Wroughton, 1907; Galago cocosHeller, 1912; Galago demidovii orinusLawrence & Washburn, 1936; Galagoides udzungwensisHoness, 1996; Galagoides rondoensisHoness, 1996.
Diagnosis:
Medium- to small-sized galagos (60–250 g), overlapping in size with Galago spp. and notably smaller than Sciurocheirus, Euoticus and Otolemur spp. Two species (Paragalago orinus and Paragalago rondoensis) show convergence in shape and size with Galagoides spp. Cranium is ovoid in shape, narrowing posteriorly, so that the pneumatized mastoids protrude. Premaxillaries protracted into a short tube that extends beyond lower jaw, as in Galagoides and Sciurocheirus; the tubular extension in Paragalago is intermediate in length between the premaxillary tubes of the smaller and larger taxa. Anterior upper premolar (P2) double-rooted, slender, but distally trenchant, as in Galagoides, not caniniform as in Euoticus and some Galago spp. Upper posterior premolars (P4s) are slightly larger than upper posterior molars (M3s) in most Paragalago specimens examined. In Galagoides M3 is either larger or equivalent in size to P4, while in Galago M3 is much reduced. In Paragalago spp., the M3 hypocone is absent or minute but commonly observed in Galagoides spp. Coronoid processes delicate and curved, extending almost as far back as glenoid process, as in Sciurocheirus and Galagoides, not flattened and foreshortened, as in Galago and Euoticus.
Distribution:
east of the Great African Rift.
Description:
The snout is longer than in Galago, chiefly because premaxillaries extend well beyond the lower jaw, although not to the degree seen in Galagoides. The facial profile is distinctly concave (Schwarz, 1931) as in Galagoides and Sciurocheirus, not straight or slightly convex as in Galago. Canines are slender. Anterior palatal foramina intrude between medial upper incisors, as in most galagid taxa. P4s fully molarized as in all crown galagids. In most Paragalago specimens examined, the M3 had no hypocone, although a minute hypocone was present in some specimens from the Rondo Forest (probably P. rondoensis). The degree of basicranial flexion is moderate, as seen in Galagoides, Otolemur and Sciurocheirus, not markedly flexed as in Euoticus and in Galago. Cranial shape is oblong as in Galagoides and Sciurocheirus, not globular as in Galago and Euoticus. Postorbital bars are generally slender, lacking the flanges sometimes seen in Euoticus, Galago and even Galagoides, usually in older specimens. Lower anterior premolars (P2) are partially procumbent, but not to the same degree as the toothcomb, and never erect, as usually seen in male Galagoides (Masters & Couette, 2015). Parietal muscle scars/crests on either side of the medial suture outline a broad parietal plate over the orbits that narrows posteriorly.
The colour of the dorsal pelage is drab brown to cinnamon with varying degrees of rufous wash; outer surfaces of limbs are similar to dorsum in colouration. Individual hairs are slate grey near the root, contributing to the overall dark colouration. Hairs on ventrum and inner surfaces of limbs also have grey roots but cream buff to yellow buff tips, and the throat may be yellowish (Groves, 2001). The ears are dark brown to black, depending on the species, and the tail varies from rufous brown to chocolate or even black. Paragalago granti and P. orinus have a darker tail tip. The cream to white nose stripe is emphasized by dark brown to black eye rings. Mature males of all species have unidentate penile spines (Perkin, 2007). Species of Paragalago show behavioural differences that distinguish them in the field from both Galagoides and Galago taxa. Eastern dwarf galagos tend to leap more often than Galagoides, but not as frequently or extensively as Galago spp. Moreover, the three genera can be readily distinguished by vocalizations that differ in structure, in context and probably in function.
Notes:
The new genus embraces several taxa originally allied with lesser or dwarf galagos, depending on body size. Paragalago zanzibaricus was described by Paul Matschie (1893) as a pale cinnamon-coloured lesser galago from western Zanzibar, although the species also occurs on the Tanzanian mainland (see Fig. 2). A recent conservation risk assessment conducted by the Primate Specialist Group of the International Union for the Conservation of Nature considered populations on small islands to be particularly vulnerable and deserving of subspecific recognition for the purpose of conservation monitoring. The Zanzibar population of dwarf galagos was hence designated as the subspecies P. z. zanzibaricus, while the mainland representatives of this species were classified as P. z. udzungwensis. Preliminary genetic studies of mainland and island populations (Pozzi, unpublished data) support their conspecific identity, but a more extensive comparison is necessary to confirm this.
Paragalago cocos, which is morphologically indistinguishable from P. zanzibaricus, was described by Heller (1912) from the Kenyan mainland. Paragalago granti, with a type locality in southern Mozambique, has the largest geographical range among representatives of the genus, extending from the north-east of South Africa throughout Mozambique (and possibly parts of Malawi, where it has been referred to under the rubric Galagoides nyasaeElliot, 1907; Grubb et al., 2003) into southern Tanzania. The type and only known skin of Galago mertensiFrade, 1924, was collected at a locality not far west of the type locality of P. granti and has, rightly or wrongly, been subsumed under this species (Schwarz, 1931).
The two smallest members of the genus, P. orinus and P. rondoensis, are the most recent members of the eastern dwarf clade to have been accorded full species status. Paragalago orinus is a montane endemic and occurs within a restricted habitat at high altitude in the Udzungwa and Uluguru mountains of Tanzania. Paragalago rondoensis has a highly fragmented range in scattered lowland forest patches throughout Tanzania but is no longer considered to be of critical conservation concern (A. Perkin, personal communication). The apparent heterochronic disjunction between the eruption of adult dentition and the cessation of growth in this species may explain why both it and the genus to which it belongs have defied characterization for so long.
Our demonstration that the eastern dwarf galagos constitute a genus entirely distinct from the western dwarf galagos reinforces the conclusions of Groves (in press) that the Eastern Arc Mountains and the Swahilian (Tanzanian/northern Mozambique) coastal forests constitute a separate subregion of the African fauna, the Zanj subregion. The Zanj mammalian fauna is unique and restricted and deserves the highest conservation priority.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Table S1. Eastern dwarf specimens included in the morphometric analysis of skulls.
ACKNOWLEDGEMENTS
Our project was funded primarily by National Research Foundation grants 93924 awarded to F. G. and 92541 and 90772 awarded to J. C. M.; grant number GB-TAF 4120 awarded by the Synthesys Program to S. C.; and an ABIC grant awarded to J. C. M. by the Royal Museum for Central Africa through a Framework Agreement with the Belgian Development Co-operation. Our research was further made possible by the support of the hardworking curators and collection managers of 15 museums who kindly gave us access to their specimens: American Museum of Natural History (New York, USA); Carnegie Museum of Natural History (Pittsburgh, USA); Ditsong Natural History Museum (Transvaal Museum; Pretoria, South Africa); Field Museum of Natural History (Chicago, USA); Museum für Naturkunde (Berlin, Germany); Muséum national d’Histoire naturelle (Paris, France); Museum of Comparative Zoology (Harvard Museum of Natural History, Cambridge, USA); Natural History Museum (London, UK); Royal Museum of Central Africa (Tervuren, Belgium); National Museum of Kenya (Nairobi, Kenya); Powell-Cotton Museum (Birchington, UK); National Museum of Natural History (Smithsonian Institution, Washington DC, USA); National Museum of Scotland (Edinburgh, UK); Naturmuseum Senckenberg (Frankfurt-am-Main, Germany); and Zoologisches Forschungmuseum Alexander Koenig (Bonn, Germany). We express our gratitude to two reviewers whose suggestions improved the final text significantly and to the colleagues who have advised, assisted and encouraged us, particularly Simon Bearder, Iris Dröscher, Paula Jenkins, Nokuthula Kom, John Oates, Andrew Perkin, Hajarimanitra Rambeloarivony, Magdalena Svensson and Ayabulela Yokwana. This is publication No. 5 of the African Primate Initiative for Ecology and Speciation (APIES).
REFERENCES