-
PDF
- Split View
-
Views
-
Cite
Cite
Jennifer L. Vriend, Fiona D. Davidson, Penny V. Corkum, Benjamin Rusak, Christine T. Chambers, Elizabeth N. McLaughlin, Manipulating Sleep Duration Alters Emotional Functioning and Cognitive Performance in Children, Journal of Pediatric Psychology, Volume 38, Issue 10, November/December 2013, Pages 1058–1069, https://doi.org/10.1093/jpepsy/jst033
Close - Share Icon Share
Abstract
Objective To examine the impact of sleep duration on emotional functioning and cognitive performance in children. Methods 32 children (8–12 years) wore actigraphs for 3 weeks. Following a week of typical sleep, each child was randomly assigned to go to bed 1 hr earlier for 4 nights (Long Sleep) or 1 hr later for 4 nights (Short Sleep) relative to their typical bedtime. Each child then completed the opposite condition. After each week, emotional and cognitive functioning were assessed using objective and subjective measures. Results Results revealed impaired functioning in the Short- relative to the Long-Sleep condition on measures of positive affective response, emotion regulation, short-term memory, working memory, and aspects of attention. Conclusions Results suggest that even modest differences in sleep duration over just a few nights can have significant consequences for children’s daytime functioning. These findings demonstrate the important impact of sleep duration on children’s daytime functioning.
Sleep problems are common in children, affecting ∼25% of the population (Owens, 2007). Furthermore, a number of reports suggest that children’s total sleep duration has shown a steady decline over the past several decades (Dollman, Ridley, Olds, & Lowe, 2007; Iglowstein, Jenni, Molinari, & Largo, 2003; Matricciani, Olds, Blunden, Rigney, & Williams, 2012). A poll of >600 American parents of school-aged children found that although children are estimated to need 10–11 hr of sleep nightly (Meltzer & Mindell, 2006), they averaged only 9.0–9.5 hr (National Sleep Foundation, 2004). However, it is also important to acknowledge the lack of empirical foundation for recommendations for sleep duration (Matricciani, Olds, & Williams, 2011; Matricciani et al., 2012).
Given the high prevalence of sleep problems and the suggestion that many children sleep less than is recommended, it is important to understand the impact of sleep duration on their daytime functioning. The daytime consequences of sleep loss have been well documented in adults (e.g., Walker & Stickgold, 2006), and studies examining adolescent populations are accumulating (see Beebe, 2011 for review). There is, however, only limited pediatric literature examining the impact of sleep duration on daytime functioning in pre-adolescents (for review, see Sadeh, 2007).
A number of correlational studies have associated poor sleep or the presence of sleep disorders with symptoms of emotional dysregulation in children (Fredriksen, Rhodes, Reddy, & Way, 2004; Gregory, Rijsdijk, & Eley, 2006; Kurnatowski, Putyński, Łapienis, & Kowalska, 2008), but these cannot establish causality. Longitudinal studies suggest that poor sleep precedes the emergence of emotional problems in children (e.g., depression and low self-esteem ratings, Fredriksen et al., 2004; anxiety, depression, and aggressive behavior, Gregory, Van der Ende, Willis, & Verhulst, 2008). Studies involving experimental manipulations of sleep are much more limited. Talbot, McGlinchey, Kaplan, Dahl, and Harvey (2010) found that adolescents who were restricted to 6.5 hr sleep for one night and 2 hr the following night reported less positive affect, but no change in negative affect. Using an affective response task, Leotta, Carskadon, Acebo, Seifer, and Quinn (1997) found that 8–12-year-old children reported higher levels of negative emotions following sleep restriction.
Similarly, correlational studies relating sleep and cognitive functioning in children found that early school start times (which result in shortened sleep durations; Wahlstrom, 2002) are linked to poor academic performance (e.g., Wolfson & Carskadon, 1998), and poor sleep is linked to impaired attention (Gruber et al., 2007; Sadeh, Gruber, & Raviv, 2002; Vriend et al., 2012) and memory (Steenari et al., 2003). The effects of experimental sleep restriction in children on cognition have also been studied, and most reports describe impaired functioning in some domains: Arithmetic and word memory tasks (Carskadon, Harvey, & Dement, 1981a); verbal creativity, learning new abstract concepts, and abstract thinking (Randazzo, Muehlbach, Schweitzer, & Walsh, 1998); inattention, as assessed by a research assistant (Fallone, Acebo, Arnedt, Seifer, & Carskadon, 2001); simple reaction time (Sadeh, Gruber, & Raviv, 2003); teacher-reported academic problems and severity of attention problems (Fallone, Acebo, Seifer, & Carskadon, 2005; Gruber, Cassoff, Frenette, Wiebe, & Carrier, 2012); and vigilance and sustained attention (Gruber et al., 2011; Peters et al., 2009).
Studies of memory and attention have shown that performance correlates with sleep duration (Gruber et al., 2009; Steenari et al., 2003) and that these domains are particularly vulnerable to experimentally shortened sleep (Carskadon, Harvey, & Dement, 1981b; Fallone et al., 2001, 2005; Gruber et al., 2011; Sadeh et al., 2003). A few studies, however, have reported that sleep loss does not impair performance on some tests of memory and attention (Carskadon et al., 1981a; Fallone et al., 2001; Randazzo et al., 1998). Most studies have used acute overnight sleep restriction. It is important, however, to understand the impact of more chronic, and limited sleep loss, which many children experience.
We enlisted parents and children to lengthen sleep for four consecutive nights (1 hr earlier than their habitual bedtime: Long-Sleep condition) and shorten sleep for four consecutive nights (1 hr later than their habitual bedtime: Short-Sleep condition) while children slept at home and otherwise maintained their usual activities. These manipulations resulted in sleep duration differences during the 2 weeks that might realistically occur spontaneously among children. Based on previous research, we predicted that shorter sleep would increase negative emotions, decrease positive emotions, and impair emotion regulation, mood, and cognitive performance (e.g., memory, attention).
Methods
Participants
Participants were typically developing school-aged children between 8 and 12 years old. Potential participants were excluded if they had a chronic illness, a history of neurological impairments (e.g., epilepsy), a history of psychiatric illness, or a known learning disability. With regard to sleep history, exclusion criteria included major sleep complaints (e.g., frequent night-time awakenings), use of medication during the month prior to the study that would likely affect sleep, crossing more than two time zones in the last month, sleeping regularly <8 hr or >12 hr nightly, or napping more than once per week.
Of 36 participants enrolled initially, 32 completed the entire protocol, including weeks of habitual sleep, Short Sleep, and Long Sleep. Four participants withdrew following the baseline period (three had erratic sleep schedules with bedtimes shifting by >3 hr from night to night and one withdrew following the death of a family member). The 32 participants who completed the study had an average age of f 9.8 years (SD = 1.4), and included 14 boys (M = 9.1 years, SD = 1.3) and 18 girls (M = 10.3 years, SD = 1.3). Half completed the Short Sleep protocol and half the Long Sleep protocol first. To be included in analyses, participants’ sleep duration was required to be at least 30 min longer in the Long-Sleep relative to the Short-Sleep condition. All 32 participants met this criterion.
Four parents identified their children as multi-racial, while 28 were identified as Caucasian. Mothers’ average age was 39.5 years (SD = 5.6) and fathers’ average age was 42.2 years (SD = 6.6). All 32 mothers indicated that they had completed high school, 8 had completed courses in community college, and 22 had attended university. The highest level of education reported for the fathers was high school (n = 5), community college (n = 4), and university (n = 22). Two sets of parents were divorced; one mother was widowed; and the rest were married.
Procedure
This project was reviewed and approved by the Research Ethics Board of the IWK Health Centre, Halifax, Nova Scotia, in conformity with the Canadian Tricouncil Policy Statement: Ethical Conduct for Research Involving Humans. Participants were recruited using a variety of methods, including word-of-mouth and public advertisements at community centers, universities, hospitals, and on internet sites directed at parents. Individuals who were interested in the study contacted the principal investigator who provided the study details and administered a brief screening questionnaire over the phone. Participants and their parent(s) who met inclusion/exclusion criteria were asked to come to our laboratory to complete the consent process and the Sleep Evaluation Questionnaire (Mindell & Owens, 2003).
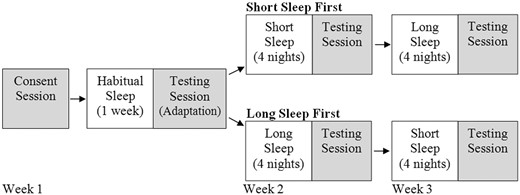
A 3-week protocol was used (1 week to measure habitual sleep patterns and 2 weeks of modified sleep schedules), which required participants to come to the laboratory on four separate occasions (see Figure 1). Following the consent process, participants were given an actigraph (see below) and a daily sleep diary. For the 3-week duration of the study, parents were instructed to complete the sleep diary each day, and participants were instructed to wear the actigraphs at all times, except when they bathed, swam, or engaged in sports where it might get damaged. Participants were told to follow their typical sleep schedules for the first week. The sleep diary and actigraphy data from the first week were used to calculate each participant’s typical daily bedtime and wake time during the week. Using these data, bedtimes were adjusted to create two different sleep schedules: One with bedtimes that were 1 hr later than usual (Short Sleep), and one with bedtimes that were 1 hr earlier than usual (Long Sleep). Participants were randomly assigned to have either the Short-Sleep condition first followed by the Long-Sleep condition, or vice versa. Participants maintained their usual sleep schedules Saturday through Monday nights, and sleep schedule manipulations were imposed for four nights (Tuesday through Friday nights) during the second and third weeks of the study.
Study design. Shaded boxes represent when the participants came to the laboratory.
At the end of each week, participants returned to the laboratory on Saturday morning for testing sessions, which took 90–120 min and involved completing various measures of emotional functioning, memory, attention, and math fluency including objective tasks and self-report questionnaires. Meanwhile, parents completed questionnaires to assess their child’s attention and emotion regulation. Once the child left the laboratory, a research assistant (RA), who administered the tests to the participants and was blinded with respect to treatment condition, completed a scale to provide observations of the child’s sleepiness. Participants were provided with compensation of $10 in movie/store gift cards each week, and the parent received $10 each week. The relation of habitual sleep duration to daytime performance was reported previously (Vriend et al., 2012). The schedule of events during the testing session was as follows: Child-reported sleepiness scale; short-term and working memory tasks; math fluency task; affective response task; computerized alerting, orienting, and executive attention task (Block 1); break; computerized alerting, orienting, and executive attention task (Block 2); Children’s Colour Trails Test (CCTT, Williams et al., 1995) attention tasks; child-reported emotion regulation questionnaire.
Measures
Screening
After phone screening, parents completed the Sleep Evaluation Questionnaire (Mindell & Owens, 2003) at the laboratory following the consent session. This questionnaire requires parents to answer questions about their child’s sleep history, current sleep problems, and medical and psychiatric history, as well as demographic questions.
Sleep
To estimate sleep durations, Octagonal Basic Motionlogger Actigraphs (Ambulatory Monitoring, Inc. Ardsley, New York) were used. An actigraph resembles a wristwatch and is worn on the non-dominant wrist; it uses an accelerometer to measure activity as an indirect measure of sleep and waking. The detected movements are translated into digital counts, which accumulate over a predetermined period (1-min epochs for this study) and are stored within the device. Data are downloaded to a computer, which uses algorithms to score wake and sleep (Sadeh & Acebo, 2002). Actigraphy has been shown to provide valid and reliable estimates of children’s sleep and wake periods that are highly correlated with the “gold-standard” polysomnography (PSG) measures (Sadeh & Acebo, 2002). In addition, participants’ parents were asked to complete the daily Sleep Diary (Corkum, 1996).
Sleepiness
One question in the Sleep Diary asked parents to assess their child’s sleepiness on awakening using a 5-point Likert scale with anchors of 1 = alert to 5 = lethargic. Child self-report of sleepiness was measured using the Child’s Pictorial Sleepiness Scale (Maldonado, Bentley, & Mitchell, 2004). This scale displays five cartoon faces representing degrees of sleepiness. The child was asked to circle the face that best matched how he/she felt prior to each testing session (1 = picture of an alert face; 5 = picture of a very sleepy face). RAs completed one question to assess the child’s sleepiness during the session, using a scale of 1 = alert to 5 = tired.
Emotion Regulation
Parent-reported emotion regulation was measured using the Emotion Questionnaire (Rydell, Thorell, & Bohlin, 2007; EQ-P), a 40-item questionnaire that assesses parents’ views of children’s emotional reactions with regard to anger, fear, positive emotions/exuberance, and sadness in response to statements depicting everyday situations. Items are ranked on a 5-point scale where 1 = Does not apply at all and 5 = Applies very well. For this study, only the 24-item Emotion Regulation subscale was used. Child-reported emotion regulation was assessed using the Emotion Questionnaire for Children (Rydell et al., 2007; EQ-C). The 29 items pertaining to emotion regulation (e.g., “When I am angry I calm down pretty quickly”) were used for this study. Items are rated on a 4-point scale where 1 = Does not apply to me at all and 4 = Applies to me very well. This measure has been shown to have adequate reliability and validity (Rydell et al., 2007). The instructions for both parent and child questionnaires were modified to ensure ratings were based on the past few days (e.g., “Please read each statement carefully and mark your response based on your child’s behavior the past three days.”). This measure has been shown to have adequate reliability and validity (Rydell et al., 2007) using the standard instructions, but has not been specifically validated for the 3-day interval.
Using methods from Leotta et al. (1997, 1999), an Affective Response Task (ART) was designed to examine emotional responses. This task has been validated for use with pediatric populations and has been found to be sensitive to sleep restriction (Leotta, 1999). It includes 33 pictures from the International Affective Picture System (IAPS), a set of visual stimuli used in investigations of emotions (Lang, Bradley, & Cuthbert, 2008). These stimuli include a variety of images such as pictures of a baby, a fierce dog, and garbage. Three practice pictures were presented followed by 30 test pictures. Following the presentation of each picture, children were asked to report their affective responses along six 100 mm lines (visual analog scales) representing the feelings happy, sad, angry, disgusted, scared, and interested. Endpoints of each line were represented by a neutral face and the word “neutral” on the left side and a face representing the emotion and the word “very” on the right side. The measure of positive affective response was a composite mean score of “happy” and “interested,” while negative affective response was assessed using a composite score of “sad,” “angry,” “disgusted,” and “scared.” The range of possible scores for both positive and negative affective responses was 0–100.
Short-Term and Working Memory
The Digit Span (modified from the Wechsler Intelligence Scale for Children, Fourth Edition; Wechsler, 2003) and Finger Windows (Wide Range Assessment of Memory and Learning 2; Sheslow & Adams, 2003) tasks were used to measure short-term and working memory. In the Digit Span task, participants were asked to remember sequences of numbers, while in the Finger Windows task, they were required to point out patterns of visual sequences. Short-term memory scores were based on children’s abilities to repeat the sequences in the same order as presented by the RA, whereas working memory tasks required participants to repeat the sequences in reverse order. Scores were based on percentage of sequences recalled correctly, resulting in a possible range of 0–100. The Digit Span task has been found to have good reliability (0.78–0.91) for children (Wechsler, 2003). The Finger Windows task has been found to have good internal consistency (0.81–0.83) for children (Sheslow & Adams, 2003).
Attention
The Conners’ Parent Rating Scale-Revised (Long Form; CRS-R:L; Conners, Sitarenios, Parker, & Epstein, 1998) is a standardized behavior rating system that is completed by parents to assess problem behaviors in children. It has adequate psychometric properties as demonstrated by good internal reliability coefficients, high test–retest reliability, and effective discriminatory power (Conners et al., 1998). It consists of 80 items that comprise a number of subscales. For this study, the 9-item DSM-IV Criteria for Inattentive Type Attention-Deficit/Hyperactivity Disorder (ADHD) subscale was used to measure parent-reported attention. Parents were asked to focus on the preceding 4 days when rating children’s attention each week.
The Attention Network Test-Interaction (ANT-I; Callejas, Lupianez, & Tudela, 2004; Fan, McCandliss, Sommer, Raz, & Posner, 2002), a 20-min computerized task, was used to measure alerting, orienting, and executive attention. The participant is instructed to press the “c” key if the target stimulus (an arrow) points left and the “m” key if the arrow points right.
Functioning of the alerting network is measured by subtracting the reaction times on trials with a tone preceding the target from those without a tone. The orienting network is assessed by subtracting reaction times on trials with a valid asterisk cue from those with an invalid asterisk cue. The executive network is assessed by examining the difference in reaction times between congruent trials and incongruent trials. For review see Callejas et al. (2004) and Fan et al. (2002). Reaction time was calculated for correct responses only. The task includes one block of 24 practice trials and two blocks of 96 experimental trials. The experimental trial blocks were separated by a 5-min break.
An earlier version of the ANT-I, the ANT, has good test–retest reliability and has been validated for measuring attention in children (Fan et al., 2002). Reliability estimates for the modified version used in this study, the ANT-I, are only beginning to emerge. However, a recent study found that the reliability of the network scores is generally greater with the ANT-I than with the ANT and that scores are robust against practice effects (Ishigami & Klein, 2010).
The Children’s Colour Trails Test (CCTT; Williams et al., 1995), a standardized, two-part, paper-and-pencil task, was also used to assess attention as measured by number of seconds required to complete each task. The CCTT-1 requires participants to rapidly draw a line from numbers 1 through 15 in consecutive order. For the CCTT-2, each of the 15 numbers is printed twice, once in a pink colored circle and once in a yellow colored circle. Participants are instructed to alternate between yellow and pink circles while drawing the line from number 1 to 15. The CCTT-1 measures perceptual tracking, sustained attention, and graphomotor skills, while the CCTT-2 requires these skills as well as divided attention, sequencing skills, and inhibition–disinhibition. The CCTT has been shown to have good test–retest reliability (moderate range) and adequate validity (Llorente, Voigt, Williams, Frailey, Satz, & D’Elia, 2009).
Math Fluency
A Math Fluency Task (MFT) based on the Woodcock Johnson Tests of Achievement-III (Woodcock, McGrew, & Mather, 2001) measures speed and accuracy of mathematical computations using a series of addition problems. Scores were based on the number of questions answered correctly within a 3-min time limit. Reliability coefficients for this subtest range from .90 to .98 for children between 7 and 19 years of age (McGrew, Schrank, & Woodcock, 2007).
Results
Average habitual sleep duration during the first week was 530.7 (SD = 32.7) min or (M = 8.8 hr). Analysis of variance (ANOVA) revealed that the sleep manipulation had the desired effect: Mean sleep duration in the Short-Sleep condition (M = 484.6 min, SD = 38.9) was significantly shorter (F(1,30) = 145.34, p < .001) than in the Long-Sleep condition (M = 558.0 min, SD = 36.8). Individual participants slept 30.2–140.2 min longer (M = 73.4 min; SD = 33.9) in the Long-Sleep condition. ANOVA revealed no significant effect of condition order on sleep duration. Paired samples t-tests indicated that children were sleepier in the Short-Sleep condition according to parental report (t(31) = 3.41, p = .002), child report (t(31) = 3.39, p = .002), and RA report (t(31) = 3.43, p = .008).
Effects of Sleep Duration on Daytime Functioning Variables
To examine the effects of sleep duration on emotional and cognitive functioning, we conducted repeated-measures multivariate analyses of variance (MANOVAs). For the primary analyses, one MANOVA was performed to examine emotional functioning and a separate MANOVA was performed to examine cognition, including attention, memory, and math fluency. For both analyses, condition (Short Sleep vs. Long Sleep) was the within-participant factor and order (Short Sleep First vs. Long Sleep First) was the between-participant factor. The dependent variables in the analysis of emotional functioning included positive affective response (ART), negative affective response (ART), parent-reported emotion regulation (EQ-P), and child-reported emotion regulation (EQ-C).
For the second MANOVA, the following dependent variables were analyzed: Short-term memory (Digit Span Forward and Finger Windows Forward), working memory (Digit Span Backward and Finger Windows Backward), alerting attention (ANT-I), orienting attention (ANT-I), executive attention (ANT-I), CCTT-1, CCTT-2, math fluency (MFT), and parent-reported inattention (CRS-R:L).The mean and standard deviation of the raw scores as well as effect sizes for each outcome variable are presented in Table I for the emotional functioning variables and Table II for the cognitive functioning variables. Although MANOVA tests controlled for experiment-wise (Type 1) error at the multivariate level, given the relatively small sample size, no correction was made for multiple univariate tests. This decision was in keeping with other studies in this area (e.g., Randazzo et al., 1998; Sadeh et al., 2003). An alpha level of .05 was used to determine statistical significance.
Means and Standard Deviations for Emotional Outcome Measures in Long Versus Short Sleep Conditions
| Measure . | Long sleep . | Short sleep . | F . | P . | η2 . |
|---|---|---|---|---|---|
| Positive affective response (ART) | 33.38 (18.13) | 31.26 (17.68) | 5.05 | .022 | .16 |
| Negative affective response (ART) | 14.33 (13.53) | 14.86 (13.71) | 0.61 | .442 | .02 |
| Parent-reported emotion regulation (EQ-P) | 4.11 (0.53) | 3.80 (0.66) | 7.62 | .006 | .23 |
| Child-reported emotion regulation (EQ-C) | 2.80 (0.49) | 2.77 (0.56) | 0.31 | .586 | .01 |
| Measure . | Long sleep . | Short sleep . | F . | P . | η2 . |
|---|---|---|---|---|---|
| Positive affective response (ART) | 33.38 (18.13) | 31.26 (17.68) | 5.05 | .022 | .16 |
| Negative affective response (ART) | 14.33 (13.53) | 14.86 (13.71) | 0.61 | .442 | .02 |
| Parent-reported emotion regulation (EQ-P) | 4.11 (0.53) | 3.80 (0.66) | 7.62 | .006 | .23 |
| Child-reported emotion regulation (EQ-C) | 2.80 (0.49) | 2.77 (0.56) | 0.31 | .586 | .01 |
Note. Values shown are means (standard deviations). F values represent results from the univariate ANOVAs. η2 = partial eta squared.
ART scores are the averaged sum of the mean score on a 100-mm visual analog scale. EQ-P values are scores on the Emotion Regulation Scale (range: 1–5). EQ-C values are scores on the Emotion Regulation Scale (range: 1–4).
ART = Affective Response Task; EQ-P = Emotion Questionnaire (Parent); EQ-C = Emotion Questionnaire (Child).
Means and Standard Deviations for Emotional Outcome Measures in Long Versus Short Sleep Conditions
| Measure . | Long sleep . | Short sleep . | F . | P . | η2 . |
|---|---|---|---|---|---|
| Positive affective response (ART) | 33.38 (18.13) | 31.26 (17.68) | 5.05 | .022 | .16 |
| Negative affective response (ART) | 14.33 (13.53) | 14.86 (13.71) | 0.61 | .442 | .02 |
| Parent-reported emotion regulation (EQ-P) | 4.11 (0.53) | 3.80 (0.66) | 7.62 | .006 | .23 |
| Child-reported emotion regulation (EQ-C) | 2.80 (0.49) | 2.77 (0.56) | 0.31 | .586 | .01 |
| Measure . | Long sleep . | Short sleep . | F . | P . | η2 . |
|---|---|---|---|---|---|
| Positive affective response (ART) | 33.38 (18.13) | 31.26 (17.68) | 5.05 | .022 | .16 |
| Negative affective response (ART) | 14.33 (13.53) | 14.86 (13.71) | 0.61 | .442 | .02 |
| Parent-reported emotion regulation (EQ-P) | 4.11 (0.53) | 3.80 (0.66) | 7.62 | .006 | .23 |
| Child-reported emotion regulation (EQ-C) | 2.80 (0.49) | 2.77 (0.56) | 0.31 | .586 | .01 |
Note. Values shown are means (standard deviations). F values represent results from the univariate ANOVAs. η2 = partial eta squared.
ART scores are the averaged sum of the mean score on a 100-mm visual analog scale. EQ-P values are scores on the Emotion Regulation Scale (range: 1–5). EQ-C values are scores on the Emotion Regulation Scale (range: 1–4).
ART = Affective Response Task; EQ-P = Emotion Questionnaire (Parent); EQ-C = Emotion Questionnaire (Child).
Means and Standard Deviations for Cognitive Outcome Measures in Long Sleep Versus Short Sleep Conditions
| Measure . | Long sleep . | Short sleep . | F . | P . | η2 . |
|---|---|---|---|---|---|
| Short-term memory (Digit Span and Finger Windows) | 56.73 (9.75) | 53.43 (10.07) | 5.27 | .030 | .17 |
| Working memory (Digit Span and Finger Windows) | 44.32 (10.41) | 39.50 (11.40) | 7.52 | .011 | .22 |
| Alerting attention (ANT-I) | 90.35 (74.17) | 116.48 (125.09) | 0.71 | .406 | .03 |
| Orienting attention (ANT-I) | 35.63 (86.48) | 24.64 (106.49) | 0.89 | .356 | .03 |
| Executive attention (ANT-I) | 104.81 (91.06) | 87.96 (72.68) | 0.51 | .48 | .02 |
| CCTT-1 | 42.11 (26.22) | 47.96 (29.44) | 1.57 | .221 | .06 |
| CCTT-2 | 71.61 (36.93) | 80.89 (41.34) | 4.61 | .041 | .151 |
| Math fluency (MFT) | 54.07 (19.51) | 50.85 (19.36) | 5.87 | .023 | .18 |
| Parent-reported inattention (CRS-R:L) | 3.93 (4.54) | 7.55 (6.76) | 15.26 | .001 | .36 |
| Measure . | Long sleep . | Short sleep . | F . | P . | η2 . |
|---|---|---|---|---|---|
| Short-term memory (Digit Span and Finger Windows) | 56.73 (9.75) | 53.43 (10.07) | 5.27 | .030 | .17 |
| Working memory (Digit Span and Finger Windows) | 44.32 (10.41) | 39.50 (11.40) | 7.52 | .011 | .22 |
| Alerting attention (ANT-I) | 90.35 (74.17) | 116.48 (125.09) | 0.71 | .406 | .03 |
| Orienting attention (ANT-I) | 35.63 (86.48) | 24.64 (106.49) | 0.89 | .356 | .03 |
| Executive attention (ANT-I) | 104.81 (91.06) | 87.96 (72.68) | 0.51 | .48 | .02 |
| CCTT-1 | 42.11 (26.22) | 47.96 (29.44) | 1.57 | .221 | .06 |
| CCTT-2 | 71.61 (36.93) | 80.89 (41.34) | 4.61 | .041 | .151 |
| Math fluency (MFT) | 54.07 (19.51) | 50.85 (19.36) | 5.87 | .023 | .18 |
| Parent-reported inattention (CRS-R:L) | 3.93 (4.54) | 7.55 (6.76) | 15.26 | .001 | .36 |
Note. Values shown are means (standard deviations). F values represent results from the univariate ANOVAs. η2 = partial eta squared.
Digit Span and Finger Windows scores are the percentage of sequences correctly recalled. ANT-I scores are difference scores measured in ms. CCTT scores are raw scores measured in milliseconds. MFT scores are raw scores based on number of questions correctly answered. CRS-R:L values represent scores on the Inattentive Type Attention-Deficit/Hyperactivity Disorder subscale (range: 1–9).
ANT-I = Attention Network Test-Interaction; CCTT = Children’s Color Trails Test; MFT = Math Fluency Task.
Means and Standard Deviations for Cognitive Outcome Measures in Long Sleep Versus Short Sleep Conditions
| Measure . | Long sleep . | Short sleep . | F . | P . | η2 . |
|---|---|---|---|---|---|
| Short-term memory (Digit Span and Finger Windows) | 56.73 (9.75) | 53.43 (10.07) | 5.27 | .030 | .17 |
| Working memory (Digit Span and Finger Windows) | 44.32 (10.41) | 39.50 (11.40) | 7.52 | .011 | .22 |
| Alerting attention (ANT-I) | 90.35 (74.17) | 116.48 (125.09) | 0.71 | .406 | .03 |
| Orienting attention (ANT-I) | 35.63 (86.48) | 24.64 (106.49) | 0.89 | .356 | .03 |
| Executive attention (ANT-I) | 104.81 (91.06) | 87.96 (72.68) | 0.51 | .48 | .02 |
| CCTT-1 | 42.11 (26.22) | 47.96 (29.44) | 1.57 | .221 | .06 |
| CCTT-2 | 71.61 (36.93) | 80.89 (41.34) | 4.61 | .041 | .151 |
| Math fluency (MFT) | 54.07 (19.51) | 50.85 (19.36) | 5.87 | .023 | .18 |
| Parent-reported inattention (CRS-R:L) | 3.93 (4.54) | 7.55 (6.76) | 15.26 | .001 | .36 |
| Measure . | Long sleep . | Short sleep . | F . | P . | η2 . |
|---|---|---|---|---|---|
| Short-term memory (Digit Span and Finger Windows) | 56.73 (9.75) | 53.43 (10.07) | 5.27 | .030 | .17 |
| Working memory (Digit Span and Finger Windows) | 44.32 (10.41) | 39.50 (11.40) | 7.52 | .011 | .22 |
| Alerting attention (ANT-I) | 90.35 (74.17) | 116.48 (125.09) | 0.71 | .406 | .03 |
| Orienting attention (ANT-I) | 35.63 (86.48) | 24.64 (106.49) | 0.89 | .356 | .03 |
| Executive attention (ANT-I) | 104.81 (91.06) | 87.96 (72.68) | 0.51 | .48 | .02 |
| CCTT-1 | 42.11 (26.22) | 47.96 (29.44) | 1.57 | .221 | .06 |
| CCTT-2 | 71.61 (36.93) | 80.89 (41.34) | 4.61 | .041 | .151 |
| Math fluency (MFT) | 54.07 (19.51) | 50.85 (19.36) | 5.87 | .023 | .18 |
| Parent-reported inattention (CRS-R:L) | 3.93 (4.54) | 7.55 (6.76) | 15.26 | .001 | .36 |
Note. Values shown are means (standard deviations). F values represent results from the univariate ANOVAs. η2 = partial eta squared.
Digit Span and Finger Windows scores are the percentage of sequences correctly recalled. ANT-I scores are difference scores measured in ms. CCTT scores are raw scores measured in milliseconds. MFT scores are raw scores based on number of questions correctly answered. CRS-R:L values represent scores on the Inattentive Type Attention-Deficit/Hyperactivity Disorder subscale (range: 1–9).
ANT-I = Attention Network Test-Interaction; CCTT = Children’s Color Trails Test; MFT = Math Fluency Task.
Effects of Sleep Duration on Emotional Functioning
The first MANOVA revealed significant differences (F(1,27) = 3.09, p = .014, partial η2 = .37) in emotional functioning. This result indicates that 37% of the variation in emotional functioning was attributable to the influence of sleep duration on the outcome measures. This significant effect prompted examination of four separate repeated-measures ANOVAs, of which two were significant. Participants showed less positive affective response (F(1,30) = 5.80, p = .022) and poorer parent-reported emotion regulation (F(1,30) = 8.87, p = .006) in the Short-Sleep compared with Long-Sleep condition. The other two ANOVAs revealed no significant differences in negative affective response or child-reported emotion regulation.
There was no main effect of order, but there were significant interactions between condition and order for both positive affective response (F(1,30) = 5.58, p = .025) and parent-reported emotion regulation (F(1,30) = 6.07, p = .020). Post-hoc paired samples t-tests revealed that those who experienced the Long-Sleep condition first reported higher levels of positive affective response in the Long Sleep (M = 35.44, SD = 15.85) compared with the Short Sleep (M = 31.24, SD = 17.37) condition (t(1,15) = 3.14, p = .007). However, there was no significant effect of sleep condition on positive affective response for those who were in the Short-Sleep condition first (Long Sleep: M = 31.32, SD = 20.47; Short Sleep: M = 31.28, SD = 18.56). In terms of parent-reported emotion regulation, those participants who experienced the Short-Sleep condition first had a decreased ability to control their emotions in the Short-Sleep condition (M = 3.52, SD = .53) compared with when they were in the Long-Sleep (M = 4.09, SD = .48) condition (t(1,15) = 3.06, p = .008). However, there was no significant effect of sleep condition on parent-reported emotion regulation for those who were in the Long-Sleep condition first (Short Sleep: M = 4.08, SD = .66; Long Sleep: M = 4.13, SD = .60).
Effects of Sleep Manipulation on Cognitive Functioning
The MANOVA examining the effects of sleep duration on attention, memory, and math fluency also revealed significant differences (F(1,18) = 3.65, p = .011, partial η2 = .64), indicating that 64% of the variance in cognitive functioning was attributable to sleep duration. Of the nine univariate ANOVAs, five were significant. There was a significant difference in short-term memory (Digit Span and Finger Windows; F(1,26) = 5.27, p = .030), working memory (Digit Span and Finger Windows; F(1,26) = 7.52, p = .011), the CCTT-2 (F(1,26) = 4.61, p = .041), math fluency (Math Fluency Test; F(1,26) = 5.87, p = .023), and parent-reported inattention (F(1,26) = 15.26, p = .001), with lower scores on memory and math fluency tasks, and decreased attention in the Short-Sleep compared with Long-Sleep condition. No significant differences were found for reaction time on alerting, orienting or executive attention tasks (ANT-I) or on the CCTT-1.
There were significant main effects of order for working memory (F(1,26) = 6.78, p = .015) and the CCTT-1 (F(1,26) = 5.13, p = .032). Those who were in the Short-Sleep condition first (M = 46.04, SD = 8.29) performed better on the working memory and CCTT-1 (M = 35.54, SD = 23.23) tasks overall, compared with those who were in the Long-Sleep condition first (M = 37.15, SD = 11.63; M = 55.88, SD = 28.16, respectively). There was also a significant interaction (F(1,26) = 4.31, p = .048) between Condition and Order for orienting attention (ANT). Those who were in the Short-Sleep condition first performed better in the Short-Sleep condition (M = 6.43, SD = 121.47) relative to the Long-Sleep condition (M = 62.29, SD = 77.14). However, this difference only approached significance (t(1,15) = 1.88, p = .080). There was no significant effect of Sleep condition on orienting attention for those who were in the Long-Sleep condition first (Long Sleep: M = 5.16, SD = 89.12; Short Sleep: M = 45.45, SD = 86.00; t(1,15) = 1.13, p = .278). There were no other effects of order.
Exploratory Analyses
Exploratory bivariate correlations were performed to examine whether there were correlations between the difference scores (i.e., sleep duration in Long Sleep minus sleep duration in Short Sleep relative to performance on outcomes variables in Long Sleep minus performance on outcome variables in Short Sleep). Sleep duration difference scores were negatively correlated with CCTT-2 difference scores (r = −.433, p = .021) and child reported emotion regulation difference scores (r = −.391, p = .027), indicating that those who had greater reductions in sleep had greater decreases in CCTT-2 performance and ability to regulate their emotions. Individual differences in the impact of sleep loss on performance and sleepiness in children may explain the weak correlations between degree of difference in sleep duration and outcome measures. This phenomenon has been studied explicitly in adults. There is clear evidence that individual responses to the same degree of sleep loss can vary widely both with respect to subjective and objective measures of sleepiness, and that these differences are stable individual characteristics (Van Dongen et al., 2004).
Discussion
This study examined the effects of modest cumulative differences in sleep duration over several days on emotional functioning, memory, attention, and math fluency in children. Our sleep manipulation was successful in achieving an average of >1-hr difference in sleep duration during the two experimental weeks. In addition, reports from the parent, child, and RA were consistent in indicating that children were sleepier in the Short-Sleep condition. Our results confirmed our hypothesis that imposing different sleep durations would affect aspects of emotional functioning, memory, attention, and math fluency; however, this finding was not consistent across all measures.
Our results are consistent with earlier findings that either acute sleep loss or moderate, cumulative sleep restriction in children increases daytime sleepiness (Carskadon et al., 1981b; Fallone et al., 2001; Sadeh et al., 2003). Parent and child reports could have been confounded by their knowledge of the current sleep condition, but their consistency with the blinded evaluations made by RAs demonstrates that this sleep manipulation was adequate to generate overt, readily detectable changes in children’s sleepiness. Thus, even as few as four nights of modest differences in sleep duration (∼1 hr per night) can increase daytime sleepiness in children.
Participants expressed less positive affective responses to stimuli presented, and parents reported children had more difficulty regulating their emotions when they were in the Short-Sleep compared with Long-Sleep condition. These results demonstrate a causal relation between differences in sleep duration and differences in emotion regulation, and are consistent with the only other experimental study in this area (Talbot et al., 2010). Additionally, results are consistent with a number of correlational studies showing that sleep problems and child emotional problems occur together (El-Sheikh, Buckhalt, Cummings, & Keller, 2007; Gregory et al., 2006). For example, higher levels of depressed mood have been reported in short-sleeping children (Smaldone, Honig, & Byrne, 2009).
Although there were no order effects for the majority of the dependent variables, there were unanticipated significant interactions between condition and order for both positive affective response and parent-reported emotion regulation. The manipulation of sleep duration altered positive affective response, but only when participants were in the Long-Sleep condition first. On the other hand, the manipulation significantly affected parent-reported emotion regulation only when participants were in the Short-Sleep condition first. These results suggest that there may be carry-over effects on emotional functioning if a child is sleep restricted prior to a sleep extension protocol or vice versa. For example, it is possible that having the Long-Sleep condition first buffered the effects of the Short-Sleep condition on children’s ability to regulate their emotion; similarly, there may have been carry-over effects related to the Short-Sleep condition occurring first, which dampened children’s positive affective response in the Long-Sleep condition. Confirmation of these hypotheses in future studies would demonstrate that even modest differences in sleep duration can have impacts that extend beyond the duration of a planned manipulation. These results also suggest that manipulating sleep duration may affect aspects of emotional functioning differentially. For example, one of our measures asked how the child felt about an image while the other asked parents how well their child behaves in emotionally challenging situations.
Our findings are also consistent with the adult literature, which strongly suggests a role for sleep in emotional functioning. Reduced sleep has been reported to have numerous effects including lowered positive mood (Franzen, Siegle, & Buysse, 2008), reduced positive affective response (Van der Helm & Walker, 2009), emotional blunting (Zohar, Tzischinsky, Epstein, & Lavie, 2005), and heightened negative mood and emotion (Franzen et al., 2008; Zohar et al., 2005), although one study of the effects of acute total sleep deprivation reported an increase in positive evaluations of neutral stimuli (Gujar, Yook, Hu, & Walker, 2011). Our findings are consistent with most of the adult literature in pointing to a role for sleep in emotional regulation; however, more research is needed to understand whether this relationship is the same in children and adults.
Longitudinal studies have found that poor sleep precedes the emergence of depressive symptoms (Fredriksen et al., 2004; Gregory et al., 2008), and that infant sleep problems are linked to later emergence of mood-related (internalizing) symptoms during childhood (Hemmi, Wolke, & Schneider, 2011). Others have reported improvements in emotional problems following treatment for sleep difficulties (Goldstein, Post, Rosenfeld, & Campbell, 2000; Walters et al., 2000). In these studies, however, it is unclear whether effects are on negative emotions, positive emotions or both. The current investigation did not examine clinical populations; however, it is important to consider our results in the context of such studies. In our study, modest changes in sleep duration over four days affected positive, but not negative, emotions. It should be noted that we used the same set of stimulus pictures for both conditions, and the negative pictures were not very disturbing. Whether more extreme or sustained changes in sleep, or the use of more strongly negative images, would demonstrate broader effects of sleep loss on emotional regulation needs to be evaluated.
Sleep condition did not produce significant changes in children’s self-report of emotion regulation. Since parents reported much greater impairment of emotion regulation in the Short-Sleep condition, the lack of self-reported impairment in children may reflect an inability to adequately reflect on and assess their own ability to regulate emotional state. Similarly, Beebe et al. (2008) found that adolescent self-report following sleep restriction was a less robust measure than parental report. In addition, we administered these questionnaires at the end of a lengthy tiring testing session. Perhaps children would be more able to attend to and reflect on emotional regulation if assessed earlier in the testing protocol.
Short-term and working memory, some aspects of attention, and math fluency were impaired during the Short-Sleep relative to Long-Sleep condition. The differences in short-term and working memory performance are consistent with a previous finding that sleep extension for 3 days led to improved performance on a short-term memory task (Sadeh et al., 2003). Correlational studies have also suggested that sleep disturbances are associated with impaired working memory performance (Steenari et al., 2003). In contrast, Carskadon et al. (1981a) and Randazzo et al. (1998) found that performance on verbal memory tasks was unaffected by a single night of sleep restriction. However, significant impairments were detected on a word memory task after 38 h of sleep deprivation (Carskadon et al., 1981b).
Parents in this study reported problems with children’s attention during the Short Sleep week, and performance on a task of attention (CCTT-2) showed significant impairments when sleep was shortened. These results are consistent with those from teacher reports of children undergoing moderate cumulative sleep restriction, which indicated that they had increased academic problems and impaired attention (Fallone et al., 2005), and with evidence that sleep-restricted children show more inattentive behavior in the laboratory (Fallone et al., 2001) and in school (Gruber et al., 2012). Similarly, children with poor sleep quality were reported to show poorer attention (Gruber et al., 2009, Vriend et al., 2012), and those undergoing sleep restriction showed impairments in sustained attention and vigilance (Gruber et al., 2011; Peters et al., 2009). It is noteworthy, however, that Fallone et al. (2001) did not find deficits in computerized tasks that measure sustained attention and response inhibition, despite evidence for more inattention in the laboratory.
Although our results suggest that short sleep impairs attention on some measures, this finding was not true for the attention networks of the ANT-I or the CCTT-1. It is possible that our modest manipulation of sleep duration did not affect some aspects of attention, or, that the measures used (ANT-I and CCTT-1) are not sensitive to modest changes in sleep duration. Some cognitive tasks are known to be more resistant to sleep loss than others (Randazzo et al., 1998).
Finally, math fluency was also significantly worse in the Short-Sleep condition. This result is consistent with the findings of Carskadon et al. (1981a) that performance on an addition task was impaired following a full night of sleep deprivation.
The main strengths of this study include the following: (1) it is one of few studies to assess how cumulative moderate changes in sleep duration affect daytime functioning in children; (2) manipulation of sleep duration was moderate to mimic sleep changes that occur spontaneously in everyday life; (3) a within-participant design was used to avoid between-group differences in both sleep and performance on the outcome measures; (4) both subjective and objective measures of daytime functioning were used; and (5) use of actigraphy provided objective measures of sleep in children’s usual home environment.
Future research could address some of the limitations of this study. First, we used subjective measures to assess sleepiness and emotional functioning. This aspect could be strengthened by adding objective measures such as Multiple Sleep Latency Tests to assess sleepiness, and blinded systematic coding of facial expression to assess emotional status (e.g., Ekman & Friesen, 1975). Secondly, the sample was drawn from families with relatively high levels of education and income. There is evidence that inadequate sleep has the greatest effects on children from low-income homes (El-Sheikh, Kelly, Buckhalt, & Hinnant, 2010), so studies of children from a broader range of socioeconomic backgrounds would be informative. Also, future studies would benefit from larger sample sizes and use of measures with a large range of variability. It is important to note that this study compared the effects of two experimental sleep manipulation conditions, rather than examining the effects of a decrease in sleep duration relative to a child’s habitual sleep pattern, as has been done in previous studies.
This study indicates that even modest differences in sleep duration, accumulated over a few days, can affect critical cognitive and emotional functions in children. One can assume that more chronic sleep loss would result in much greater impairments. Our results suggest that problems with emotional functioning, short-term memory, working memory, attention, and math fluency may sometimes be symptoms of inadequate sleep; thus, children experiencing difficulties in these areas should be screened for sleep problems. Furthermore, this study highlights the need to educate health care professionals, educators, parents, and children about the importance of sleep and healthy sleep habits, and the potential negative consequences of inadequate sleep.
Acknowledgments
The authors would like to thank all of the children and parents who participated in this study. The authors would also like to thank Sunny Shaffner, Alyssa Beaudette, Jessica Waldon, Ashton Parker, Sarah Melkert, Abby Poirier, Jill Tonet, and Kait Sullivan for their help in data collection and administrative support.
Funding
Dalhousie Psychiatry Research Fund Grant, Nova Scotia Health Research Foundation Student Research Awards, an IWK Summer Studentship Award, and an IWK Graduate Student Research Award.
Conflicts of interest: None declared.