-
PDF
- Split View
-
Views
-
Cite
Cite
Janet K Sluggett, Gillian E Caughey, Tracy Air, Max Moldovan, Catherine Lang, Grant Martin, Stephen R Carter, Shane Jackson, Andrew C Stafford, Steve L Wesselingh, Maria C Inacio, Provision of a comprehensive medicines review is associated with lower mortality risk for residents of aged care facilities: a retrospective cohort study, Age and Ageing, Volume 51, Issue 7, July 2022, afac149, https://doi.org/10.1093/ageing/afac149
Close - Share Icon Share
Abstract
no studies have examined the impact of residential medication management review (RMMR, a 24-year government subsidised comprehensive medicines review program) in Australian residential aged care facilities (RACFs) on hospitalisation or mortality.
to examine associations between RMMR provision in the 6–12 months after RACF entry and the 12-month risk of hospitalisation and mortality among older Australians in RACFs.
retrospective cohort study.
individuals aged 65–105 years taking at least one medicine, who entered an RACF in three Australian states between 1 January 2012 and 31 December 2015 and spent at least 6 months in the RACF (n = 57,719).
Cox regression models estimated adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for associations between RMMR provision and mortality. Adjusted subdistribution hazard ratios were estimated for associations between RMMR provision and next (i) emergency department (ED) presentation or unplanned hospitalisation or (ii) fall-related ED presentation or hospitalisation.
there were 12,603 (21.8%) individuals who received an RMMR within 6–12 months of RACF entry, of whom 22.2% (95%CI 21.4–22.9) died during follow-up, compared with 23.3% (95%CI 22.9–23.7) of unexposed individuals. RMMR provision was associated with a lower risk of death due to any cause over 12-months (aHR 0.96, 95%CI 0.91–0.99), but was not associated with ED presentations or hospitalisations for unplanned events or falls.
provision of an RMMR in the 6–12 months after RACF entry is associated with a 4.4% lower mortality risk over 12-months but was not associated with changes in hospitalisations for unplanned events or falls.
Key Points
Few studies have examined the impact and outcomes of medicines reviews among residents of aged care facilities or care homes.
We examined associations between medicines review provision for long-term residents and hospitalisation or mortality.
Medicines review provision was associated with a 4.4% lower mortality risk.
No association between medicines review provision and emergency department presentations or hospitalisations was observed.
Introduction
Multiple safety and quality issues are encountered across all aspects of the medicines management pathway [1] for individuals in residential aged care facilities (RACFs; similar to care homes), including prescribing, dispensing and administration errors [2, 3]. Overall, half of all residents are exposed to at least one potentially inappropriate medicine, defined as where harms typically outweigh any anticipated benefits [4–7]. Polypharmacy is common, with up to three quarters of residents taking nine or more medicines [8]. In addition, residents frequently experience care transitions, with 37% of residents of Australian RACFs presenting to an emergency department (ED) at least once over a 12-month period in 2018–2019 [9]. Consequently, their risk of medicines-related harm (i.e. arising from preventable adverse drug events such as medicines errors, and non-preventable events such as adverse drug reactions) due to medicines discrepancies and information transfer problems is exacerbated [10, 11].
Comprehensive medicines reviews involving pharmacist, general medical practitioner (GP) and resident/family collaboration can resolve medicines-related problems in RACFs [12–14]. On average, between 2.7 and 3.9 medicines-related problems are identified during a Residential Medication Management Review (RMMR) [13], a longstanding Australian Government-funded program that aims to identify, resolve and prevent medicines-related problems in Australian RACFs [15]. To provide RMMRs, pharmacists must complete a two-stage accreditation process, and a GP referral is required for a resident to access the service. RMMRs involve the accredited pharmacist visiting the RACF to obtain consent and interview the resident, review clinical documentation and speak with staff to identify medicines-related problems. Strategies to address medicines-related problems are communicated to the GP in a written report, which the GP uses to prepare a medicines management plan. Best practice guidelines for RMMR provision are available [15] although pharmacists are encouraged to tailor their activities to the resident’s goals and reason for RMMR referral.
Studies examining the impact of comprehensive medicines reviews on clinical outcomes have largely focused on community-dwelling individuals [16], with few studies specific to RACFs. Previous systematic reviews of medicines review interventions in RACFs have reported uncertain or nil impact on hospitalisations or mortality, with the small number of heterogeneous studies included often underpowered to detect outcome differences between groups [14, 17–19]. No Australian studies have examined associations between RMMR provision and hospitalisation or mortality despite the 25-year history of this national program [13]. Hence, this study examined associations between RMMR provision and the risk of hospitalisation or mortality among older Australians in RACFs. Although RMMRs are generally recommended on RACF entry and when clinical circumstances change [15, 20–22], RMMRs provided at RACF entry likely have a different focus to RMMRs provided to long-term residents. Medicines reconciliation is typically prioritised at RACF entry because there are often hospitalisations before RACF entry and/or changes to a resident’s usual GP [23–24]. This is in line with current RMMR guidelines that emphasise the importance of medicines reconciliation during care transitions [15]. Other activities (i.e. identifying deprescribing opportunities) likely receive greater focus during RMMRs for residents who are more settled in the RACF [23]. Therefore, we focused on individuals living in the RACF for at least 6 months.
Methods
Study design and data sources
This retrospective cohort study utilised data from the National Historical cohort of the Registry of Senior Australians (ROSA) [20, 25]. ROSA contains deidentified data for all individuals aged ≥65 years who access government-subsidised aged care services linked to their subsidised health care services. Briefly, we utilised datasets containing information from aged care eligibility assessments [26] and entry into RACF assessments [27], aged care service records, the Australian Institute of Health and Welfare (AIHW) National Death Index, the Australian Government Pharmaceutical Benefits Scheme (PBS, Australia’s national medicines subsidy scheme), Medicare Benefits Schedule (MBS) and state-based hospitalisation records. Prescription claims are captured via the PBS dataset, and GP and other health services accessed through the MBS are linked to ROSA. Hospitalisation and ED presentations from New South Wales (NSW), Victoria (VIC) and South Australia (SA) are included.
Study cohort
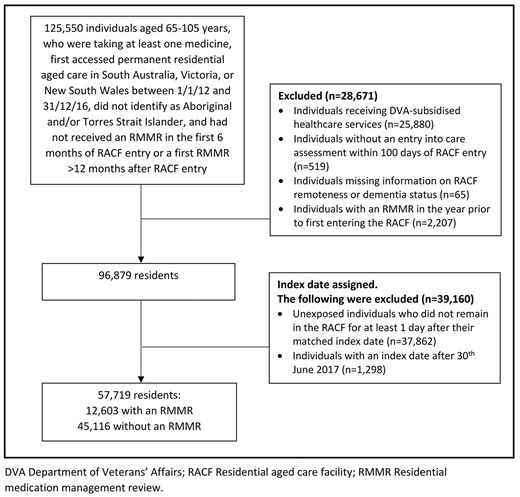
Eligible individuals were those aged 65–105 years with an aged care eligibility assessment who first entered an RACF between 1 January 2012 and 31 December 2016 in SA, VIC or NSW, received at least one PBS medicine in the 6 months before RACF entry, did not identify as Aboriginal or Torres Strait Islander and had not received an RMMR in the first 6 months of RACF entry or > 12 months after RACF entry (n = 125,550, Figure 1). Because RMMRs were not subsidised during respite, the RACF entry date was the date an individual first entered permanent care and did not include prior time in respite. Residents who received a first RMMR >12 months post-RACF entry were not eligible for inclusion as many individuals with RMMRs are provided with the service within 12-months of RACF entry [28].
Study flow chart. DVA: Department of Veterans’ Affairs; RACF: residential aged care facility; RMMR: residential medication management review.
Consistent with previous studies [20, 23, 28], the following residents were excluded (Figure 1): individuals receiving Australian Government Department of Veterans’ Affairs subsidised medicines, individuals without an entry into care assessment within 100 days of RACF entry, individuals missing information on RACF remoteness or dementia status (required for index date assignment in the unexposed group) and those with an RMMR in the year before RACF entry (as RMMRs can be provided during transition care). Of the n = 96,879 individuals eligible for index date assignment, n = 57,719 spent >6 months in the RACF and were included.
Exposure of interest
The exposure of interest was the first GP MBS claim for an RMMR (MBS item code 903) within 6–12 months of RACF entry. In keeping with previous studies [20, 23, 28], claims for Home Medicines Reviews (HMRs; MBS item code 900) post-RACF entry were also considered to represent RMMRs (see Appendix 1a).
Index date assignment
For residents with an RMMR, the date of RMMR provision was the index date. Index date assignment for unexposed individuals (i.e. those never receiving an RMMR) was undertaken in accordance with a previous study [23] (see Appendix 1b). For an individual to be considered unexposed, they needed to be alive at the index date and not receive an RMMR during follow-up.
The index date had to occur before 30 June 2017. The index date was Day 1 of follow-up and individuals needed to survive the index date to be included in the study.
Outcomes of interest
Outcomes were examined over 12-months and included: (a) next ED presentation or unplanned hospitalisation, (b) next fall-related ED presentation or hospitalisation and (c) death (all-cause). Unplanned hospitalisations were those where the urgency status was recorded as ‘emergency’ and not ‘scheduled’. Fall-related ED presentations and hospitalisations were determined using International Classification of Diseases, 10th revision, Australian modification (ICD-10-AM) codes (i.e. W00*–W19*, R29.6), as previously defined in this cohort [29].
Covariates
Covariates for all models are comprehensively described in Appendix 1c and included: age and year of RACF entry, sex, main language spoken, country of birth, RACF location, remoteness of residence [30], RACF provider type, number of unique PBS prescriptions dispensed in the year before RACF entry, comorbidity score [31] derived from prescription claims in the 6 months before RACF entry, dementia, number of standard GP visits and unplanned hospitalisations in the year before RACF entry, provision of a multidisciplinary care plan or a case conference in the 6 months prior to the index date, and care needs with respect to palliative or end of life care, assistance with medicines administration, activities of daily living, behavioural daily living and complex care needs. For models where the outcome was a fall-related hospitalisation, covariates included: (1) number of unique medicines associated with increased falls risk [32] dispensed in the previous 6 months and (2) fall-related hospitalisations in the previous year.
Statistical analysis
The survival probability was determined from Kaplan–Meier analyses for the outcome of mortality. The cumulative incidence function was used to determine time to next ED visit/hospitalisation of interest, adjusted for the competing risk of death using the Fine–Gray method [33]. Individuals were censored on 1 September 2017, on receipt of a subsequent HMR/RMMR (among exposed individuals), RACF transfer or return to the community, whichever occurred first.
Cox regression models estimated unadjusted and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for associations between RMMR provision and mortality, adjusting for covariates described above. For outcomes other than mortality, subdistribution hazard ratios (sHRs) were estimated using Fine–Gray subdistribution hazard regression models, accounting for the competing risk of death [33]. Proportional hazards assumptions were verified by formal statistical tests and visually by plotting Schoenfeld residuals against the time-dependent coefficient for the linear predictor. Complete case analysis was conducted; 0.6% of the cohort with missing data were excluded.
Analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, NC) and R statistical package v3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Sensitivity analyses
Sensitivity analyses were conducted among individuals experiencing polypharmacy (i.e. dispensed ≥9 unique medicines in the 4 months before the index date). Taking ≥9 medicines is a commonly used polypharmacy definition in RACF studies [8] and is similarly defined in Australia’s national aged care mandatory quality indicator for polypharmacy [34]. Additional sensitivity analyses examined time to (i) next unplanned hospitalisation or (ii) fall-related hospitalisation only (i.e. excluding ED presentations).
Ethical approval
Ethical approvals were provided by the University of South Australia Human Research Ethics Committee (HREC) (Ref: 200489), AIHW HREC (EO2018/1/418), SA Department for Health and Wellbeing HREC (HREC/18/SAH/90) and NSW Population and Health Services Research HREC (2019/ETH12028).
Results
There were 57,719 residents from 1945 RACFs included (Figure 1). Of these, 12,603 (21.8%) received an RMMR within 6–12 months of RACF entry and 45,116 (78.2%) did not receive an RMMR. Individuals with and without RMMRs were similar in terms of median age (84 (interquartile range [IQR] 79–89) vs. 85 (IQR 80–89) years), sex (34.2% vs. 34.1% males) and median comorbidity score (5 (IQR 3–7) vs. 5 (IQR 3–7)) (Table 1). Residents with an RMMR had fewer standard GP visits in the previous year than those without an RMMR (82.8% vs. 87.6% with ≥1 visit) but were more likely to have received a multidisciplinary care plan (28.0% vs. 17.6%) or case conference (5.5% vs. 2.6%). Individuals with an RMMR had fewer unplanned hospitalisations in the preceding year than those without RMMRs (57.2% vs. 61.9% with ≥1 unplanned hospitalisation).
Baseline characteristics of the n = 57,719 residents included in the study
| Characteristic . | Received RMMR (n = 12,603, 21.8%) . | Unexposed (no RMMR) (n = 45,116, 78.2%) . |
|---|---|---|
| Number of RACFs | 1,776 | 1,922 |
| Age (years) at RACF entry, median (IQR) | 84.0 (79.0–89.0) | 85.0 (80.0–89.0) |
| Male, n (%) | 4,313 (34.2) | 15,367 (34.1) |
| Born in Australia, n (%)a | 8,333 (66.5) | 29,417 (65.5) |
| Primary language other than English, n (%)b | 1,471 (11.7) | 5,833 (13.0) |
| RACF provider type, n (%) For profit Government Not for profit | 5,244 (41.6) 740 (5.9) 6,619 (52.5) | 18,507 (41.0) 2,390 (5.3) 24,219 (53.7) |
| Remoteness of residence, n (%) Major cities Outside major cities | 8,482 (67.3) 4,121 (32.7) | 31,329 (69.4) 13,787 (30.6) |
| State of residence, n (%) New South Wales South Australia Victoria | 5,524 (43.8) 1,687 (13.4) 5,392 (42.8) | 21,188 (47.0) 7,449 (16.5) 16,479 (36.5) |
| Standard GP visits in the year before the index date, n (%) No visits 1–5 visits 6–15 visits 16 or more visits | 2,164 (17.2) 6,413 (50.9) 3,165 (25.1) 861 (6.8) | 5,608 (12.4) 24,632 (54.6) 11,772 (26.1) 3,104 (6.9) |
| Multidisciplinary care plan in the 6 months before the index date, n (%) | 3,522 (28.0) | 7,945 (17.6) |
| Case conference in the 6 months before the index date, n (%) | 694 (5.5) | 1,184 (2.6) |
| Flagged as requiring palliative care/end of life care on the entry into permanent residential aged care assessment, n (%) | 67 (0.5) | 236 (0.5) |
| Rx-risk comorbidity score, n (%) 0–1 2–3 4–5 6–8 9 or more | 784 (6.2) 2,396 (19.0) 3,553 (28.2) 4,414 (35.0) 1,456 (11.6) | 3,114 (6.9) 8,835 (19.6) 12,979 (28.8) 15,123 (33.5) 5,065 (11.2) |
| Dementia, n (%) | 6,138 (49.8) | 22,461 (48.7) |
| No. of unique prescription medicines dispensed in the year before RACF entry, n (%) 1–5 6–10 11–15 16–20 21 or more | 1,848 (14.7) 4,069 (32.3) 3,606 (28.6) 1,940 (15.4) 1,140 (9.1) | 7,001 (15.5) 14,869 (33.0) 12,646 (28.0) 6,627 (14.7) 3,973 (8.8) |
| Polypharmacy, n (%)c | 6,005 (47.7) | 21,097 (46.8) |
| Number of unique falls risk medicines dispensed in the 6 months prior to the index date, median (IQR) | 3 (2–5) | 3 (2–5) |
| Need for assistance with medicines, n (%) Resident takes no medicines/self-manages Requires assistance for <6 min/day and/or patches every 2–7 days Requires assistance for 6–11 min/day Requires assistance >11 min/day and/or daily parenteral medicines | 369 (2.9) 4,205 (33.4) 4,480 (35.6) 3,549 (28.2) | 1,361 (3.0) 15,393 (34.1) 15,625 (34.6) 12,737 (28.2) |
| Assisted daily living level, n (%) Nil or low Medium High | 4,397 (34.9) 4,241 (33.7) 3,965 (31.5) | 15,002 (33.3) 15,565 (34.5) 14,549 (32.3) |
| Behavioural daily living level, n (%) Nil or Low Medium High | 4,602 (36.5) 3,271 (26.0) 4,730 (37.5) | 16,116 (35.7) 11,673 (25.9) 17,327 (38.4) |
| Complex health care level, n (%) Nil or low Medium High | 5,275 (41.9) 3,383 (26.8) 3,945 (31.3) | 18,579 (41.2) 12,261 (27.2) 14,276 (31.6) |
| ED presentation or unplanned hospitalisation in the year prior to index date, n (%) No hospitalisations 1 hospitalisation 2–4 hospitalisations 5 or more hospitalisations | 5,401 (42.9) 4,061 (32.2) 2,898 (23.0) 243 (1.9) | 17,189 (38.1) 15,626 (34.6) 11,461 (25.4) 840 (1.9) |
| History of a fall in the 12 months prior to the index date, n (%) | 3,397 (27.0) | 12,950 (28.7) |
| Characteristic . | Received RMMR (n = 12,603, 21.8%) . | Unexposed (no RMMR) (n = 45,116, 78.2%) . |
|---|---|---|
| Number of RACFs | 1,776 | 1,922 |
| Age (years) at RACF entry, median (IQR) | 84.0 (79.0–89.0) | 85.0 (80.0–89.0) |
| Male, n (%) | 4,313 (34.2) | 15,367 (34.1) |
| Born in Australia, n (%)a | 8,333 (66.5) | 29,417 (65.5) |
| Primary language other than English, n (%)b | 1,471 (11.7) | 5,833 (13.0) |
| RACF provider type, n (%) For profit Government Not for profit | 5,244 (41.6) 740 (5.9) 6,619 (52.5) | 18,507 (41.0) 2,390 (5.3) 24,219 (53.7) |
| Remoteness of residence, n (%) Major cities Outside major cities | 8,482 (67.3) 4,121 (32.7) | 31,329 (69.4) 13,787 (30.6) |
| State of residence, n (%) New South Wales South Australia Victoria | 5,524 (43.8) 1,687 (13.4) 5,392 (42.8) | 21,188 (47.0) 7,449 (16.5) 16,479 (36.5) |
| Standard GP visits in the year before the index date, n (%) No visits 1–5 visits 6–15 visits 16 or more visits | 2,164 (17.2) 6,413 (50.9) 3,165 (25.1) 861 (6.8) | 5,608 (12.4) 24,632 (54.6) 11,772 (26.1) 3,104 (6.9) |
| Multidisciplinary care plan in the 6 months before the index date, n (%) | 3,522 (28.0) | 7,945 (17.6) |
| Case conference in the 6 months before the index date, n (%) | 694 (5.5) | 1,184 (2.6) |
| Flagged as requiring palliative care/end of life care on the entry into permanent residential aged care assessment, n (%) | 67 (0.5) | 236 (0.5) |
| Rx-risk comorbidity score, n (%) 0–1 2–3 4–5 6–8 9 or more | 784 (6.2) 2,396 (19.0) 3,553 (28.2) 4,414 (35.0) 1,456 (11.6) | 3,114 (6.9) 8,835 (19.6) 12,979 (28.8) 15,123 (33.5) 5,065 (11.2) |
| Dementia, n (%) | 6,138 (49.8) | 22,461 (48.7) |
| No. of unique prescription medicines dispensed in the year before RACF entry, n (%) 1–5 6–10 11–15 16–20 21 or more | 1,848 (14.7) 4,069 (32.3) 3,606 (28.6) 1,940 (15.4) 1,140 (9.1) | 7,001 (15.5) 14,869 (33.0) 12,646 (28.0) 6,627 (14.7) 3,973 (8.8) |
| Polypharmacy, n (%)c | 6,005 (47.7) | 21,097 (46.8) |
| Number of unique falls risk medicines dispensed in the 6 months prior to the index date, median (IQR) | 3 (2–5) | 3 (2–5) |
| Need for assistance with medicines, n (%) Resident takes no medicines/self-manages Requires assistance for <6 min/day and/or patches every 2–7 days Requires assistance for 6–11 min/day Requires assistance >11 min/day and/or daily parenteral medicines | 369 (2.9) 4,205 (33.4) 4,480 (35.6) 3,549 (28.2) | 1,361 (3.0) 15,393 (34.1) 15,625 (34.6) 12,737 (28.2) |
| Assisted daily living level, n (%) Nil or low Medium High | 4,397 (34.9) 4,241 (33.7) 3,965 (31.5) | 15,002 (33.3) 15,565 (34.5) 14,549 (32.3) |
| Behavioural daily living level, n (%) Nil or Low Medium High | 4,602 (36.5) 3,271 (26.0) 4,730 (37.5) | 16,116 (35.7) 11,673 (25.9) 17,327 (38.4) |
| Complex health care level, n (%) Nil or low Medium High | 5,275 (41.9) 3,383 (26.8) 3,945 (31.3) | 18,579 (41.2) 12,261 (27.2) 14,276 (31.6) |
| ED presentation or unplanned hospitalisation in the year prior to index date, n (%) No hospitalisations 1 hospitalisation 2–4 hospitalisations 5 or more hospitalisations | 5,401 (42.9) 4,061 (32.2) 2,898 (23.0) 243 (1.9) | 17,189 (38.1) 15,626 (34.6) 11,461 (25.4) 840 (1.9) |
| History of a fall in the 12 months prior to the index date, n (%) | 3,397 (27.0) | 12,950 (28.7) |
ED: emergency department; GP: general medical practitioner; IQR: interquartile range; RACF: residential aged care facility; RMMR: residential medication management review.
Data missing for 259 individuals
Data missing for 95 individuals
Defined as ≥9 unique PBS prescriptions dispensed in the 4 months prior to the index date
Baseline characteristics of the n = 57,719 residents included in the study
| Characteristic . | Received RMMR (n = 12,603, 21.8%) . | Unexposed (no RMMR) (n = 45,116, 78.2%) . |
|---|---|---|
| Number of RACFs | 1,776 | 1,922 |
| Age (years) at RACF entry, median (IQR) | 84.0 (79.0–89.0) | 85.0 (80.0–89.0) |
| Male, n (%) | 4,313 (34.2) | 15,367 (34.1) |
| Born in Australia, n (%)a | 8,333 (66.5) | 29,417 (65.5) |
| Primary language other than English, n (%)b | 1,471 (11.7) | 5,833 (13.0) |
| RACF provider type, n (%) For profit Government Not for profit | 5,244 (41.6) 740 (5.9) 6,619 (52.5) | 18,507 (41.0) 2,390 (5.3) 24,219 (53.7) |
| Remoteness of residence, n (%) Major cities Outside major cities | 8,482 (67.3) 4,121 (32.7) | 31,329 (69.4) 13,787 (30.6) |
| State of residence, n (%) New South Wales South Australia Victoria | 5,524 (43.8) 1,687 (13.4) 5,392 (42.8) | 21,188 (47.0) 7,449 (16.5) 16,479 (36.5) |
| Standard GP visits in the year before the index date, n (%) No visits 1–5 visits 6–15 visits 16 or more visits | 2,164 (17.2) 6,413 (50.9) 3,165 (25.1) 861 (6.8) | 5,608 (12.4) 24,632 (54.6) 11,772 (26.1) 3,104 (6.9) |
| Multidisciplinary care plan in the 6 months before the index date, n (%) | 3,522 (28.0) | 7,945 (17.6) |
| Case conference in the 6 months before the index date, n (%) | 694 (5.5) | 1,184 (2.6) |
| Flagged as requiring palliative care/end of life care on the entry into permanent residential aged care assessment, n (%) | 67 (0.5) | 236 (0.5) |
| Rx-risk comorbidity score, n (%) 0–1 2–3 4–5 6–8 9 or more | 784 (6.2) 2,396 (19.0) 3,553 (28.2) 4,414 (35.0) 1,456 (11.6) | 3,114 (6.9) 8,835 (19.6) 12,979 (28.8) 15,123 (33.5) 5,065 (11.2) |
| Dementia, n (%) | 6,138 (49.8) | 22,461 (48.7) |
| No. of unique prescription medicines dispensed in the year before RACF entry, n (%) 1–5 6–10 11–15 16–20 21 or more | 1,848 (14.7) 4,069 (32.3) 3,606 (28.6) 1,940 (15.4) 1,140 (9.1) | 7,001 (15.5) 14,869 (33.0) 12,646 (28.0) 6,627 (14.7) 3,973 (8.8) |
| Polypharmacy, n (%)c | 6,005 (47.7) | 21,097 (46.8) |
| Number of unique falls risk medicines dispensed in the 6 months prior to the index date, median (IQR) | 3 (2–5) | 3 (2–5) |
| Need for assistance with medicines, n (%) Resident takes no medicines/self-manages Requires assistance for <6 min/day and/or patches every 2–7 days Requires assistance for 6–11 min/day Requires assistance >11 min/day and/or daily parenteral medicines | 369 (2.9) 4,205 (33.4) 4,480 (35.6) 3,549 (28.2) | 1,361 (3.0) 15,393 (34.1) 15,625 (34.6) 12,737 (28.2) |
| Assisted daily living level, n (%) Nil or low Medium High | 4,397 (34.9) 4,241 (33.7) 3,965 (31.5) | 15,002 (33.3) 15,565 (34.5) 14,549 (32.3) |
| Behavioural daily living level, n (%) Nil or Low Medium High | 4,602 (36.5) 3,271 (26.0) 4,730 (37.5) | 16,116 (35.7) 11,673 (25.9) 17,327 (38.4) |
| Complex health care level, n (%) Nil or low Medium High | 5,275 (41.9) 3,383 (26.8) 3,945 (31.3) | 18,579 (41.2) 12,261 (27.2) 14,276 (31.6) |
| ED presentation or unplanned hospitalisation in the year prior to index date, n (%) No hospitalisations 1 hospitalisation 2–4 hospitalisations 5 or more hospitalisations | 5,401 (42.9) 4,061 (32.2) 2,898 (23.0) 243 (1.9) | 17,189 (38.1) 15,626 (34.6) 11,461 (25.4) 840 (1.9) |
| History of a fall in the 12 months prior to the index date, n (%) | 3,397 (27.0) | 12,950 (28.7) |
| Characteristic . | Received RMMR (n = 12,603, 21.8%) . | Unexposed (no RMMR) (n = 45,116, 78.2%) . |
|---|---|---|
| Number of RACFs | 1,776 | 1,922 |
| Age (years) at RACF entry, median (IQR) | 84.0 (79.0–89.0) | 85.0 (80.0–89.0) |
| Male, n (%) | 4,313 (34.2) | 15,367 (34.1) |
| Born in Australia, n (%)a | 8,333 (66.5) | 29,417 (65.5) |
| Primary language other than English, n (%)b | 1,471 (11.7) | 5,833 (13.0) |
| RACF provider type, n (%) For profit Government Not for profit | 5,244 (41.6) 740 (5.9) 6,619 (52.5) | 18,507 (41.0) 2,390 (5.3) 24,219 (53.7) |
| Remoteness of residence, n (%) Major cities Outside major cities | 8,482 (67.3) 4,121 (32.7) | 31,329 (69.4) 13,787 (30.6) |
| State of residence, n (%) New South Wales South Australia Victoria | 5,524 (43.8) 1,687 (13.4) 5,392 (42.8) | 21,188 (47.0) 7,449 (16.5) 16,479 (36.5) |
| Standard GP visits in the year before the index date, n (%) No visits 1–5 visits 6–15 visits 16 or more visits | 2,164 (17.2) 6,413 (50.9) 3,165 (25.1) 861 (6.8) | 5,608 (12.4) 24,632 (54.6) 11,772 (26.1) 3,104 (6.9) |
| Multidisciplinary care plan in the 6 months before the index date, n (%) | 3,522 (28.0) | 7,945 (17.6) |
| Case conference in the 6 months before the index date, n (%) | 694 (5.5) | 1,184 (2.6) |
| Flagged as requiring palliative care/end of life care on the entry into permanent residential aged care assessment, n (%) | 67 (0.5) | 236 (0.5) |
| Rx-risk comorbidity score, n (%) 0–1 2–3 4–5 6–8 9 or more | 784 (6.2) 2,396 (19.0) 3,553 (28.2) 4,414 (35.0) 1,456 (11.6) | 3,114 (6.9) 8,835 (19.6) 12,979 (28.8) 15,123 (33.5) 5,065 (11.2) |
| Dementia, n (%) | 6,138 (49.8) | 22,461 (48.7) |
| No. of unique prescription medicines dispensed in the year before RACF entry, n (%) 1–5 6–10 11–15 16–20 21 or more | 1,848 (14.7) 4,069 (32.3) 3,606 (28.6) 1,940 (15.4) 1,140 (9.1) | 7,001 (15.5) 14,869 (33.0) 12,646 (28.0) 6,627 (14.7) 3,973 (8.8) |
| Polypharmacy, n (%)c | 6,005 (47.7) | 21,097 (46.8) |
| Number of unique falls risk medicines dispensed in the 6 months prior to the index date, median (IQR) | 3 (2–5) | 3 (2–5) |
| Need for assistance with medicines, n (%) Resident takes no medicines/self-manages Requires assistance for <6 min/day and/or patches every 2–7 days Requires assistance for 6–11 min/day Requires assistance >11 min/day and/or daily parenteral medicines | 369 (2.9) 4,205 (33.4) 4,480 (35.6) 3,549 (28.2) | 1,361 (3.0) 15,393 (34.1) 15,625 (34.6) 12,737 (28.2) |
| Assisted daily living level, n (%) Nil or low Medium High | 4,397 (34.9) 4,241 (33.7) 3,965 (31.5) | 15,002 (33.3) 15,565 (34.5) 14,549 (32.3) |
| Behavioural daily living level, n (%) Nil or Low Medium High | 4,602 (36.5) 3,271 (26.0) 4,730 (37.5) | 16,116 (35.7) 11,673 (25.9) 17,327 (38.4) |
| Complex health care level, n (%) Nil or low Medium High | 5,275 (41.9) 3,383 (26.8) 3,945 (31.3) | 18,579 (41.2) 12,261 (27.2) 14,276 (31.6) |
| ED presentation or unplanned hospitalisation in the year prior to index date, n (%) No hospitalisations 1 hospitalisation 2–4 hospitalisations 5 or more hospitalisations | 5,401 (42.9) 4,061 (32.2) 2,898 (23.0) 243 (1.9) | 17,189 (38.1) 15,626 (34.6) 11,461 (25.4) 840 (1.9) |
| History of a fall in the 12 months prior to the index date, n (%) | 3,397 (27.0) | 12,950 (28.7) |
ED: emergency department; GP: general medical practitioner; IQR: interquartile range; RACF: residential aged care facility; RMMR: residential medication management review.
Data missing for 259 individuals
Data missing for 95 individuals
Defined as ≥9 unique PBS prescriptions dispensed in the 4 months prior to the index date
Risk of ED presentation or hospitalisation
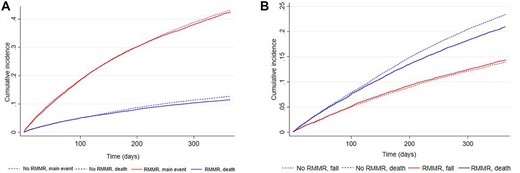
Figure 2 presents the cumulative incidence of ED presentation or hospitalisation over 12-months and the competing risk of death. Of those with an RMMR in the 6–12 months after RACF entry, 42.5% (95%CI 41.6–43.4) had at least one ED presentation or unplanned hospitalisation at 12-month follow-up, compared with 43.1% (95%CI 42.6–43.6) without an RMMR (Figure 2a,Appendix 2). Fall-related ED presentations or hospitalisations were experienced by 14.3% (95%CI 13.7–15.0) in the RMMR group and 13.9% (95%CI 13.6–14.2) without an RMMR at 12-month follow-up (Figure 2b). Adjusted multivariable analyses showed no statistically significant difference in the risk of (a) ED presentation or unplanned hospitalisation or (b) fall-related ED presentation or hospitalisation during follow-up among those who did and did not receive an RMMR in the 6–12 months after RACF entry (Table 2). Consistent findings were observed in sensitivity analyses among individuals experiencing polypharmacy (47% of total cohort) or that only included hospitalisation outcomes (i.e. no ED presentations) (Appendix 3).
Cumulative incidence of (A) ED presentation or unplanned hospitalisation and (B) fall-related ED presentation or hospitalisation and the competing risk of death.
Unadjusted and adjusted associations between RMMR provision and outcomes of interest
| Outcome of interesta . | Unadjusted sHR (95% CI)b . | P-value . | Adjusted sHR (95% CI)c . | P-value . | Unadjusted HR (95% CI)d . | P-value . | Adjusted HR (95% CI)e . | P-value . |
|---|---|---|---|---|---|---|---|---|
| ED presentation or unplanned hospitalisation | 0.99 (0.96–1.02) | 0.38 | 1.01 (0.98–1.03) | 0.63 | ||||
| Fall-related ED presentation or hospitalisation | 1.04 (0.98–1.10) | 0.17 | 1.05 (0.99–1.12) | 0.07 | ||||
| Death (all-cause) | 0.95 (0.91–0.99) | 0.02 | 0.96 (0.91–0.99) | 0.048 |
| Outcome of interesta . | Unadjusted sHR (95% CI)b . | P-value . | Adjusted sHR (95% CI)c . | P-value . | Unadjusted HR (95% CI)d . | P-value . | Adjusted HR (95% CI)e . | P-value . |
|---|---|---|---|---|---|---|---|---|
| ED presentation or unplanned hospitalisation | 0.99 (0.96–1.02) | 0.38 | 1.01 (0.98–1.03) | 0.63 | ||||
| Fall-related ED presentation or hospitalisation | 1.04 (0.98–1.10) | 0.17 | 1.05 (0.99–1.12) | 0.07 | ||||
| Death (all-cause) | 0.95 (0.91–0.99) | 0.02 | 0.96 (0.91–0.99) | 0.048 |
CI: confidence interval; HR: hazard ratio; RMMR: residential medication management review; sHR: subdistribution hazard ratio
Complete case analysis conducted; sample size = 57,378 for all models
Estimates from unadjusted competing risk regression model
Estimates from competing risk regression model, adjusted for all covariates described in methods; competing event was death
Estimates from unadjusted Cox regression model
Estimates from Cox regression model, adjusted for all covariates described in methods
Unadjusted and adjusted associations between RMMR provision and outcomes of interest
| Outcome of interesta . | Unadjusted sHR (95% CI)b . | P-value . | Adjusted sHR (95% CI)c . | P-value . | Unadjusted HR (95% CI)d . | P-value . | Adjusted HR (95% CI)e . | P-value . |
|---|---|---|---|---|---|---|---|---|
| ED presentation or unplanned hospitalisation | 0.99 (0.96–1.02) | 0.38 | 1.01 (0.98–1.03) | 0.63 | ||||
| Fall-related ED presentation or hospitalisation | 1.04 (0.98–1.10) | 0.17 | 1.05 (0.99–1.12) | 0.07 | ||||
| Death (all-cause) | 0.95 (0.91–0.99) | 0.02 | 0.96 (0.91–0.99) | 0.048 |
| Outcome of interesta . | Unadjusted sHR (95% CI)b . | P-value . | Adjusted sHR (95% CI)c . | P-value . | Unadjusted HR (95% CI)d . | P-value . | Adjusted HR (95% CI)e . | P-value . |
|---|---|---|---|---|---|---|---|---|
| ED presentation or unplanned hospitalisation | 0.99 (0.96–1.02) | 0.38 | 1.01 (0.98–1.03) | 0.63 | ||||
| Fall-related ED presentation or hospitalisation | 1.04 (0.98–1.10) | 0.17 | 1.05 (0.99–1.12) | 0.07 | ||||
| Death (all-cause) | 0.95 (0.91–0.99) | 0.02 | 0.96 (0.91–0.99) | 0.048 |
CI: confidence interval; HR: hazard ratio; RMMR: residential medication management review; sHR: subdistribution hazard ratio
Complete case analysis conducted; sample size = 57,378 for all models
Estimates from unadjusted competing risk regression model
Estimates from competing risk regression model, adjusted for all covariates described in methods; competing event was death
Estimates from unadjusted Cox regression model
Estimates from Cox regression model, adjusted for all covariates described in methods
Mortality
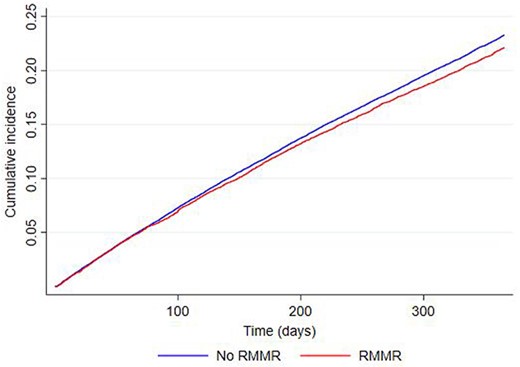
At 12-months, 22.2% (95%CI 21.4–22.9) of residents provided an RMMR in the 6–12 months after RACF entry had died, compared with 23.3% (95%CI 22.9–23.7) without an RMMR (Figure 3,Appendix 2). Adjusted multivariable analyses showed RMMR provision in the 6–12 months after RACF entry was associated with a 4.4% (95%CI 0.02–8.6, P = 0.048) lower risk of death due to any cause over 12-months (Table 2). Similar findings were observed among individuals experiencing polypharmacy (6.6% lower all-cause mortality risk, 95%CI 0.8–12.0, P = 0.025) (Appendix 3).
Kaplan–Meier graph showing time to death in the RMMR group compared with those without an RMMR over 12-month follow-up.
Discussion
This is the first evaluation of long-term health outcomes linked with RMMR provision in the 25-year history of this national government-funded program. In this population-based study of 57,719 individuals, RMMR provision in the 6–12 months after RACF entry was associated with a 4.4% lower risk of death due to any cause over 12 months. In addition, the cumulative incidence of death was consistently lower among those with an RMMR for the other endpoints where death was a competing event. However, no differences in the risk of ED presentations or hospitalisations (neither unplanned nor fall-related) were observed for those who received an RMMR during the period examined compared with those who did not. Importantly, consistent findings were observed in our sensitivity analyses. Our finding that RMMR provision in the 6–12 months after RACF entry was associated with a 6.6% lower risk of death among residents receiving ≥9 unique medicines suggests these impacts are particularly important for individuals exposed to polypharmacy.
Of the few studies that have examined links between comprehensive medicines review provision and mortality in RACFs, this is the first to demonstrate a lower risk of death. Roberts et al., who examined a clinical pharmacy model of care that is different to the RMMR program in a cluster randomised controlled trial (RCT) of 52 Australian RACFs, found no difference in mortality between the intervention and comparison groups [35]. In four other RCTs, there was either no difference in mortality between those with and without a medicines review [36, 37], fewer deaths observed during the intervention period but not throughout the overall study period [38] or no formal analysis of mortality differences between groups [39]. The lower risk of mortality observed in our study is encouraging, as it is a definitive and important outcome for aged care recipients [40]. However, we recognise that quality of life is not captured in this study, which to some residents may be equally or more desirable. The 4.4% lower mortality risk suggests residents derive benefit over a short period (12-months). This has important implications given the median length of stay per permanent residential care episode is 20.4 months and 84% of exits are due to death [41].
RMMRs are designed to be tailored to resident needs and preferences, and therefore may be operationalised differently. Differences in use of clinical decision tools, processes, interest in collaborative practice, interprofessional relationships, communication strategies (e.g. quality of the pharmacist’s report and any verbal discussions), resident/family engagement and health professional accountability relating to RMMR provision have been reported [42]. This variability at the individual health professional level could potentially limit the ability to observe changes in key health outcomes such as mortality or hospitalisation. This study found no association between RMMR provision and unplanned or falls-related hospitalisations, which is consistent with previous meta-analyses of comprehensive medicines review studies in RACFs [14, 17, 18, 43]. Further building on variability in service provision, co-interventions (e.g. medicines reconciliation or education) may reduce the risk of hospitalisation [44]. Hospitalisation is influenced by a range of factors not necessarily related to medicines use, such as resident preference, access to end-of-life care or mobile radiology services [45, 46]. Future studies should examine RMMR outcomes among individuals with specific health conditions or high-risk medicines use and explore associations between specific changes in medicine use arising from the pharmacist’s RMMR recommendations (e.g. dose reductions, discontinuation, increased monitoring) and health outcomes.
Our finding that RMMRs were provided to only one in five residents (21.8%) who had not previously received this service and accessed RACF care for at least 6 months is consistent with previous research showing low RMMR uptake [20, 23, 28, 47]. Our comprehensive examination of clinical outcomes linked with RMMR provision highlights opportunities for program refinements that could better target unplanned medicines-related hospitalisations. Use of standardised report templates, decision support tools and digital health systems, increased input from RACF medicines advisory committees, remunerating pharmacists to participate in case conferences with GPs and RACF staff and enabling RMMR referrals from nurse practitioners have been previously suggested [47]. At present, there are only two process measures mandated for routine monitoring of the RMMR program (overall number of RMMRs provided and cost). Our findings and previous work [20, 23, 28] indicate opportunities to use aged care registry data for program evaluation, by routinely monitoring a comprehensive set of process, impact, and outcome measures, to guide policy and clinical practice decision-making. The recent Royal Commission in Aged Care Quality and Safety recommended increased uptake of RMMRs, enhanced clinical roles for pharmacists in RACFs and the need to monitor intervention quality and outcomes [48]. The Australian Government has accepted these recommendations and program changes will be implemented in January 2023. This follows changes to RMMR program rules in early 2020 that enabled up to two pharmacist follow-up visits post-RMMR, and temporary delivery via telehealth during the COVID-19 pandemic.
Strengths and limitations
Study strengths include the use of Australia’s only aged care registry to comprehensively examine associations between RMMR provision and clinical outcomes in 57,719 individuals from 1945 RACFs. This is in comparison to clinical trial evidence from a 2016 review of six interventions to optimise prescribing in RACFs for the outcome of mortality that only included 6,805 RCT participants from 188 RACFs [17]. Falls, ED presentations, hospitalisations and all-cause mortality have been prioritised as core outcomes for interventions to optimise medicines use in RACFs [49]. We accounted for the competing risk of death where appropriate and modelled associations in pre-specified sensitivity analyses. Our findings are highly generalisable, with individuals included from RACFs in three Australian states that represent ~69% of older individuals accessing permanent residential care and ~ 73% of RACFs nationally.
Limitations include possible underestimation of RMMR provision due to fewer claims being lodged by GPs (n = 54,803 RMMR, n = 63,872 HMR claims in 2014–2015) than pharmacists (n = 93,517 RMMR, n = 72,607 HMR claims in 2014–2015) [20, 23, 28, 47, 50, 51], with any resulting bias towards the null. We were unable to ascertain RMMR quality, specific pharmacist recommendations or GP implementation of recommendations. Hence, associations between changes to care in response to RMMR recommendations and hospitalisation/mortality could not be examined. Our numerical-based definition of polypharmacy was in keeping with a commonly reported definition in the RACF literature and Australia’s national quality indicator program. However, we acknowledge there are varying definitions reported in the literature, and we could not discern between appropriate and inappropriate polypharmacy [8, 52]. Although admissions to SA private hospitals are not captured in ROSA, most emergency hospitalisations (92%) include public hospital encounters [53] which are included in ROSA. Aged care assessment data and the Rx-risk were used to ascertain dementia. However, the current Australian Rx-risk adaptation [31] does not recognise that risperidone may be used for behavioural and psychological symptoms of dementia, so dementia may be slightly underestimated. Despite the use of robust analytical techniques, residual confounding may still be present. For example, we were unable to account for provider factors that could impact on RMMR referrals and resident outcomes (e.g. strong interprofessional relationships, model of primary care operationalised at the RACF).
Conclusions and implications
Provision of an RMMR in the 6–12 months after RACF entry is associated with a 4.4% lower risk of mortality over 12-months but was not associated with changes in hospitalisations for unplanned events or falls. Hence, although RMMRs may have a meaningful impact for residents and could be implemented more widely, particularly for residents experiencing inappropriate polypharmacy, refinements to the existing RMMR model and/or quality of service delivery are likely needed to curb unplanned hospitalisations from RACFs. Further research is needed to investigate the optimal timing and frequency of RMMR provision in RACFs, together with comprehensive evaluation of the cost-effectiveness and clinical impacts of program refinements on resident outcomes.
Acknowledgements
We acknowledge the Registry of Senior Australians’ (ROSA) Steering Committee and the ROSA South Australian Health and Medical Research Institute (SAHMRI) Research Team for ensuring the success of the ROSA and support with this study. We also acknowledge the South Australian Government Department for Innovation and Skills (2017–2021) who provided us with support to establish ROSA, the Australian Government Medical Research Future Fund (2021–2024, PHRDI000009), and ROSA collaborating partners (SAHMRI, ECH Inc, Silver Chain, Life Care) for its ongoing support, and the Australian Institute of Health and Welfare for the linkage and construction of input data, SA Health, NSW Ministry of Health, and VIC Department of Health and Human Services (DHHS) for the provision of the state-based data used in the ROSA with linkage via the AIHW, Centre for Health Record Linkage (CHeReL), the Centre for Victorian Data Linkage (CVDL), and SA NT DataLink. We also acknowledge Marc Apolloni, previous chair of the Australian Association of Consultant Pharmacy (AACP) board, for comments on the project findings.
Declaration of Conflicts of Interest
J.K.S., S.C., S.J. and A.S. are registered pharmacists and accredited by the Australian Association of Consultant Pharmacy (AACP; the research funder) to perform residential medication management reviews (RMMRs). G.M. is the Chief Executive Officer of the Australian Association of Consultant Pharmacy. S.C. and S.J. are Non-Executive Directors of the AACP. AS is a member of the AACP National Advisory Group.
Declaration of Sources of Funding
This work was supported by funding from the Australian Association of Consultant Pharmacy (AACP), an Australian organisation that accredits pharmacists to provide residential medication management reviews. Members of the AACP Board (S.R.C., S.J.), Advisory Group (ACS) and the Chief Executive Officer (GM) participated in study conceptualisation, read the study protocol prepared by the research team prior to research commencement and participated in discussions about the results. AACP had no role in data collection, data analysis or presentation of results. All authors had final responsibility for the decision to submit the manuscript for publication. J.K.S. is supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1156439). M.C.I. is supported by The Hospital Research Foundation Mid-Career Fellowship (MCF-27-2019) and an NHMRC Investigator Grant (APP119378).




Comments