-
PDF
- Split View
-
Views
-
Cite
Cite
Anne E Willems, Charlotte L Mentzel, Pieter Roberto Bakker, Jim Van Os, Diederik E Tenback, Petra Gelan, Erna Daantjes, Glenn E Matroos, Hans W Hoek, Peter N Van Harten, Movement Disorders and Mortality in Severely Mentally Ill Patients: The Curacao Extrapyramidal Syndromes Study XIV, Schizophrenia Bulletin, Volume 48, Issue 4, July 2022, Pages 766–773, https://doi.org/10.1093/schbul/sbac037
Close - Share Icon Share
Abstract
There is a substantial gap in life expectancy between patients with severe mental illness (SMI) and the general population and it is important to understand which factors contribute to this difference. Research suggests an association between tardive dyskinesia (TD) and mortality; however, results are inconclusive. In addition, studies investigating associations between parkinsonism or akathisia and mortality are rare. We hypothesized that TD would be a risk factor for mortality in patients with SMI.
We studied a cohort of 157 patients diagnosed predominantly with schizophrenia on the former Netherlands Antilles. TD, parkinsonism, and akathisia were assessed with rating scales on eight occasions over a period of 18 years. Twenty-four years after baseline, survival status and if applicable date of death were determined. Associations between movement disorders and survival were analyzed using Cox regression. Sex, age, antipsychotics, antidepressants and benzodiazepines at each measurement occasion were tested as covariates.
Parkinsonism was a significant risk factor with an HR of 1.02 per point on the motor subscale of the Unified Parkinson’s Disease Rating Scale (range 0–56). TD and akathisia were not significantly associated with mortality.
Parkinsonism may be an important risk factor for mortality in SMI patients. This finding calls for more follow-up and intervention studies to confirm this finding and to explore whether treatment or prevention of parkinsonism can reduce excess mortality.
Introduction
Patients with severe mental illness (SMI) are at an increased risk of early death in comparison with the general population.1,2 In schizophrenia and bipolar disorder, diagnoses that are highly prevalent in SMI populations, standardized mortality rates of 2–4.6 have been found,3–5 leading to a substantially reduced life expectancy of 9–25 years.3,6–8
Besides major lifestyle-related problems such as smoking, little physical activity, an unhealthy diet,9 and problems with access to and quality of physical healthcare,10 movement disorders (MD) may also play a role in shortening the lifespan of SMI patients.11,12
In nonaffective psychoses reported MD prevalence ranges from 3% to 70% for tardive dyskinesia (TD), from 17% to 72% for Parkinsonism, and from 9.3% to 31.3% for akathisia.13 MD can be induced by antipsychotics, but also reflect a fundamental aspect of neurodevelopmental pathophysiology involving the sensitization of dopaminergic nigrostriatal circuits.14
Several authors studied a possible link between TD and mortality. Some found higher mortality rates in SMI patients with TD than in those without TD11,12,15–17 but others reported negative findings.18–21 There is a paucity of research on Parkinsonism and akathisia and MD combined.21 Because of the high prevalence of MD and the importance of understanding which factors contribute to the shortened lifespan of patients with SMI, we used data from the Curacao Extrapyramidal Syndromes Study in which we assessed the association between MD and mortality. The Curacao Extrapyramidal Syndromes Study comprises a 24-year follow-up study in which patients with SMI treated at the only psychiatric hospital on the island of Curacao (former Netherlands Antilles) were repeatedly assessed for the presence and severity of MD since 1992.22–26 In the present study, we investigate if TD, Parkinsonism, and akathisia are associated with mortality in a sample of 157 mainly African Caribbean patients.
Methods
Setting and Patients
The present study is part of the Curacao Extrapyramidal Syndromes Study.22,23 Patients from the Klinika Capriles (formerly Dr. D. R. Capriles clinic), the only psychiatric hospital of the former Netherlands Antilles (nowadays Curacao), were assessed 8 times over the course of an 18-year period including assessments for both MD and medication use. The study protocol was approved by the Curacao Institutional Review Board.
Inclusion criteria for the present study were: (1) age of 18 years or older; (2) cumulative history of antipsychotic use of at least three months. Current use of antipsychotics was not required; (3) absence of organic disorders that could cause MD; (4) no diagnosis of dementia; (5) no history of lobotomy; and (6) informed consent.
Patients were mainly of Afro-Caribbean descent and the majority were inpatients. Characteristics of the study design and the cohort are described in more detail in an earlier publication.22
Measurements
Patients were assessed in 1992, 1993, 1994, 1996, 1997, 1998, 2001, and 2009 for TD, Parkinsonism, akathisia, and medication use. All eight assessments were carried out by the same two skilled raters (PvH and GM), simultaneously. TD was assessed with the Abnormal Involuntary Movement Scale (AIMS)27 and case definition was based on Schooler and Kane criteria for probable TD.28 The motor examination part of the Unified Parkinson’s Disease Rating Scale (UPDRS)29 was used to assess Parkinsonism. Since rest-tremor and rigidity are core symptoms of Parkinsonism, cases were assigned to the Parkinsonism group when they scored “mild” on one of those two items. If neither tremor nor rigidity was present, the cut-off point was at least one “moderate” or two “mild” scores on the other items.22 Akathisia was rated with the Barnes Akathisia Rating Scale (BARS)30 and a patient was considered a case when a score of 2 or higher on item 4 was given. Cases, i.e. patients who could be diagnosed with a movement disorder, were used here for illustrative purposes only (Table 2). For the Cox analysis, data about movement disorders were used in a continuous manner.
At baseline and at each follow-up assessment, a trained physician collected current medication use. At baseline, age, sex, DSM III-R diagnosis (schizophrenia or other, where schizophrenia included codes 295.1, 295.2, 295.3, 295.4, 295.6, 296.7, and 295.9), cocaine use, type of treatment (inpatient or day care), age at first admission, duration of illness, total years of admission, duration of last admission, and lifetime intake of anticholinergics were extracted from patients’ files.
Seven years after the final assessment (follow-up seven), on April first, 2016, all-cause mortality was obtained using the patient’s charts and the mortality register of Curacao. For the deceased patients, date of death was recorded.
Data Analysis
The association between MD and mortality was analyzed using a Cox regression with measurement occasion (baseline and 7 follow-ups) (microlevel) clustered in subjects (macrolevel), with the (1) STCOX routine of the STATA 13 statistical program (StataCorp., 2009); and (2) SHARED(ID) procedure for shared frailty, where the random component enters the hazard multiplicatively. Fitting the Cox model with the time-varying covariates was performed with STSPLIT, AT(FAILURES) procedure. Mortality data of each deceased subject were appended to the dataset. Associations were expressed as hazard ratios and proportional-hazard assumptions were tested using the PHTEST procedure.
The total score of the AIMS, the motor part of the UPDRS and the score of item 4 of the BARS at each assessment were included as time-varying variables, as were type of antipsychotic (only first generation antipsychotic (FGA), only second generation antipsychotic (SGA), both FGA and SGA), dose in defined daily dose (DDD)31 of antipsychotics, benzodiazepines, and antidepressants. Age and sex were included as time-independent variables. Variables with a P-value greater than .2 in a univariate analysis were dropped. Extra-linearity was assessed by including quadratic effects for all continuous independent variables. In case of nonlinearity (P < .05 of the quadratic term), a linear-quadratic term remained in the final model as suggested by Cleves.32
Results
Description of the Sample
The original dataset of the Curacao Extrapyramidal Syndromes Study consisted of 222 patients. For the current study, patients who had undergone a lobotomy (N = 23), who had a diagnosis of dementia (N = 13), or a primary diagnosis of mental retardation (N = 3) were excluded as not being representative of current SMI populations, leading to a dataset of 183 patients. Twenty-six patients could not be analyzed due to missing data. Therefore, data of 157 patients were used for the analysis.
Table 1 shows the demographic and clinical characteristics of the sample at baseline.
Sample Characteristics at Baseline
| Characteristics . | n = 157 . |
|---|---|
| Age, mean (SD) | 48.8 (15.6) |
| Males, n (%) | 115 (73.2) |
| Ethnicity, n (%) | |
| African-Caribbean | 109 (74.7) |
| Mixed | 29 (19.9) |
| Caucasian | 6 (4.1) |
| Other | 2 (1.4) |
| Primary diagnosis schizophrenia, n (%) | 130 (82.8) |
| Cocaine use, n (%) | 30 (19.1) |
| Type of treatment, n (%) | |
| Inpatient treatment | 143(91.1) |
| Day care treatment | 14 (8.9) |
| Age at first admission, mean (SD) | 26.2 (10.1) |
| Duration of illness in years, mean (SD) | 23.6 (14.3) |
| Total years of admission, mean (SD) | 16.8 (14.4) |
| Duration of last admission in years, mean (SD) | 12.8 (13.8) |
| Lifetime intake of anticholinergics (g benztropine equivalents), mean (SD) | 23.6 (23.2) |
| Characteristics . | n = 157 . |
|---|---|
| Age, mean (SD) | 48.8 (15.6) |
| Males, n (%) | 115 (73.2) |
| Ethnicity, n (%) | |
| African-Caribbean | 109 (74.7) |
| Mixed | 29 (19.9) |
| Caucasian | 6 (4.1) |
| Other | 2 (1.4) |
| Primary diagnosis schizophrenia, n (%) | 130 (82.8) |
| Cocaine use, n (%) | 30 (19.1) |
| Type of treatment, n (%) | |
| Inpatient treatment | 143(91.1) |
| Day care treatment | 14 (8.9) |
| Age at first admission, mean (SD) | 26.2 (10.1) |
| Duration of illness in years, mean (SD) | 23.6 (14.3) |
| Total years of admission, mean (SD) | 16.8 (14.4) |
| Duration of last admission in years, mean (SD) | 12.8 (13.8) |
| Lifetime intake of anticholinergics (g benztropine equivalents), mean (SD) | 23.6 (23.2) |
Note: Number of cases with missing data; Ethnicity: n = 11; age at first admission: n = 10; duration of Illness: n = 10; total years of admission: n = 11; duration of last admission in years: n = 11; lifetime intake of anticholinergics: n = 42.
Sample Characteristics at Baseline
| Characteristics . | n = 157 . |
|---|---|
| Age, mean (SD) | 48.8 (15.6) |
| Males, n (%) | 115 (73.2) |
| Ethnicity, n (%) | |
| African-Caribbean | 109 (74.7) |
| Mixed | 29 (19.9) |
| Caucasian | 6 (4.1) |
| Other | 2 (1.4) |
| Primary diagnosis schizophrenia, n (%) | 130 (82.8) |
| Cocaine use, n (%) | 30 (19.1) |
| Type of treatment, n (%) | |
| Inpatient treatment | 143(91.1) |
| Day care treatment | 14 (8.9) |
| Age at first admission, mean (SD) | 26.2 (10.1) |
| Duration of illness in years, mean (SD) | 23.6 (14.3) |
| Total years of admission, mean (SD) | 16.8 (14.4) |
| Duration of last admission in years, mean (SD) | 12.8 (13.8) |
| Lifetime intake of anticholinergics (g benztropine equivalents), mean (SD) | 23.6 (23.2) |
| Characteristics . | n = 157 . |
|---|---|
| Age, mean (SD) | 48.8 (15.6) |
| Males, n (%) | 115 (73.2) |
| Ethnicity, n (%) | |
| African-Caribbean | 109 (74.7) |
| Mixed | 29 (19.9) |
| Caucasian | 6 (4.1) |
| Other | 2 (1.4) |
| Primary diagnosis schizophrenia, n (%) | 130 (82.8) |
| Cocaine use, n (%) | 30 (19.1) |
| Type of treatment, n (%) | |
| Inpatient treatment | 143(91.1) |
| Day care treatment | 14 (8.9) |
| Age at first admission, mean (SD) | 26.2 (10.1) |
| Duration of illness in years, mean (SD) | 23.6 (14.3) |
| Total years of admission, mean (SD) | 16.8 (14.4) |
| Duration of last admission in years, mean (SD) | 12.8 (13.8) |
| Lifetime intake of anticholinergics (g benztropine equivalents), mean (SD) | 23.6 (23.2) |
Note: Number of cases with missing data; Ethnicity: n = 11; age at first admission: n = 10; duration of Illness: n = 10; total years of admission: n = 11; duration of last admission in years: n = 11; lifetime intake of anticholinergics: n = 42.
In table 2, age, medication use, prevalence and severity of MD at each assessment, and the numbers of patients that died in each consecutive time interval are presented. Of the 157 included in the Cox regression 84 patients (54%) died during follow-up. Mean age of death was 67.4 years (SD 15.6, range 24–94).
Time Varying Sample Characteristics
| Measurement Year Number of Patients Alive . | Baseline 1992–1993 n = 157 . | FU 1 1993 n = 151 . | FU 2 1994 n = 143 . | FU 3 1996 n = 136 . | FU 4 1997 n = 133 . | FU 5 1998 n = 131 . | FU 6 2001 n = 122 . | FU 7 2009 n = 96 . |
|---|---|---|---|---|---|---|---|---|
| Number of patients that died in subsequent time interval (n) | 6 | 8 | 7 | 3 | 2 | 9 | 26 | 23 |
| Mean age (SD)a | 48.8 (15.6) | 53.0 (14.7) | 50.4 (13.9) | 53.8 (13.2) | 53.0 (12.7) | 54.5(12.7) | 55.5 (13.0) | 61.4 (10.7) |
| Medication | ||||||||
| Antipsychotics | ||||||||
| FGA only, n (%) | 146 (93.0) | 93 (84.5) | 104 (89.7) | 79 (86.8) | 74 (83.1) | 67 (79.8) | 72 (67.9) | 38 (48.1) |
| SGA only, n (%) | 0 | 8 (7.3) | 7 (6.0) | 9 (9.9) | 12 (13.5) | 10 (11.9) | 15(14.2) | 14 (17.7) |
| Combination FGA and SGA n (%) | 0 | 0 | 0 | 0 | 0 | 1 (1.2) | 13 (12.3) | 21 (26.6) |
| No antipsychotic, n (%) | 11 (7.0) | 9 (8.2) | 5 (4.3) | 3 (3.3) | 3 (3.4) | 6 (7.1) | 6 (5.7) | 6 (7.6) |
| DDD antipsychoticsb, mean (SD) | 1.8 (1.4) | 1.9 (1.5) | 2.0(1.3) | 2.0 (1.6) | 2.1 (1.5) | 2.4 (1.7) | 2.1 (1.6) | 2.4 (1.4) |
| Antidepressants, n (%)b | 9 (5.7) | 9 (8.1) | 9 (7.8) | 10 (11.0) | 7 (7.9) | 3 (3.6) | 10 (9.4) | 4 (5.0) |
| DDD antidepressantsb, mean (SD) | 0.9 (0.4) | 0.8 (0.4) | 0.9 (0.3) | 0.9 (0.4) | 0.8 (0.3) | 0.9 (0.3) | 1.1 (0.3) | 1.3 (0.5) |
| Benzodiazepines, n (%) | 29 (18.5) | 23 (20.1) | 34 (29.3) | 31 (34.0) | 31 (34.8) | 35 (41.7) | 52 (49.1) | 41 (51.3) |
| DDD benzodiazepinesb, mean (SD) | 1.4 (1.0) | 1.2 (0.9) | 1.0 (0.7) | 1.0 (0.8) | 1.2 (1.1) | 1.3 (1.2) | 1.2 (1.0) | 1.3 (1.0) |
| Movement disorders | ||||||||
| Tardive dyskinesia, cases (%) | 59 (37.6) | 59 (53.6) | 72 (61.5) | 56 (61.5) | 49 (53.3) | 43 (51.2) | 63 (59.4) | 41 (51.9) |
| Mean score cases AIMS (SD) | 7.5 (3.4) | 7.8 (2.7) | 9.6 (4.3) | 9.6 (4.0) | 9.3 (3.8) | 9.2 (3.8) | 9.9 (4.3) | 8.6 (3.2) |
| Parkinsonism, cases (%) | 56 (35.7) | 44 (40.0) | 37 (31.6) | 30 (33.0) | 26 (28.3) | 23 (27.4) | 37 (34.9) | 24 (30.4) |
| Mean score cases motor part UPDRS (SD) | 18.6 (10.1) | 18.5 (12.2) | 17.9 (11.2) | 22.4 (9.4) | 21.0 (10.2) | 22.7 (12.1) | 19.1 (12.3) | 15.5 (12.1) |
| Akathisia, cases (%) | 16(10.2) | 9 (8.2) | 15 (12.8) | 7 (7.7) | 2 (2.2) | 5 (6.0) | 5 (4.7) | 1 (1.3) |
| Mean score cases item 4 BARS | 2.6 (0.8) | 2.7 (0.7) | 2.8 (0.6) | 2.6 (0.8) | 2.5 (0.7) | 2.6 (0.5) | 2.8 (0.8) | 3.0 |
| Measurement Year Number of Patients Alive . | Baseline 1992–1993 n = 157 . | FU 1 1993 n = 151 . | FU 2 1994 n = 143 . | FU 3 1996 n = 136 . | FU 4 1997 n = 133 . | FU 5 1998 n = 131 . | FU 6 2001 n = 122 . | FU 7 2009 n = 96 . |
|---|---|---|---|---|---|---|---|---|
| Number of patients that died in subsequent time interval (n) | 6 | 8 | 7 | 3 | 2 | 9 | 26 | 23 |
| Mean age (SD)a | 48.8 (15.6) | 53.0 (14.7) | 50.4 (13.9) | 53.8 (13.2) | 53.0 (12.7) | 54.5(12.7) | 55.5 (13.0) | 61.4 (10.7) |
| Medication | ||||||||
| Antipsychotics | ||||||||
| FGA only, n (%) | 146 (93.0) | 93 (84.5) | 104 (89.7) | 79 (86.8) | 74 (83.1) | 67 (79.8) | 72 (67.9) | 38 (48.1) |
| SGA only, n (%) | 0 | 8 (7.3) | 7 (6.0) | 9 (9.9) | 12 (13.5) | 10 (11.9) | 15(14.2) | 14 (17.7) |
| Combination FGA and SGA n (%) | 0 | 0 | 0 | 0 | 0 | 1 (1.2) | 13 (12.3) | 21 (26.6) |
| No antipsychotic, n (%) | 11 (7.0) | 9 (8.2) | 5 (4.3) | 3 (3.3) | 3 (3.4) | 6 (7.1) | 6 (5.7) | 6 (7.6) |
| DDD antipsychoticsb, mean (SD) | 1.8 (1.4) | 1.9 (1.5) | 2.0(1.3) | 2.0 (1.6) | 2.1 (1.5) | 2.4 (1.7) | 2.1 (1.6) | 2.4 (1.4) |
| Antidepressants, n (%)b | 9 (5.7) | 9 (8.1) | 9 (7.8) | 10 (11.0) | 7 (7.9) | 3 (3.6) | 10 (9.4) | 4 (5.0) |
| DDD antidepressantsb, mean (SD) | 0.9 (0.4) | 0.8 (0.4) | 0.9 (0.3) | 0.9 (0.4) | 0.8 (0.3) | 0.9 (0.3) | 1.1 (0.3) | 1.3 (0.5) |
| Benzodiazepines, n (%) | 29 (18.5) | 23 (20.1) | 34 (29.3) | 31 (34.0) | 31 (34.8) | 35 (41.7) | 52 (49.1) | 41 (51.3) |
| DDD benzodiazepinesb, mean (SD) | 1.4 (1.0) | 1.2 (0.9) | 1.0 (0.7) | 1.0 (0.8) | 1.2 (1.1) | 1.3 (1.2) | 1.2 (1.0) | 1.3 (1.0) |
| Movement disorders | ||||||||
| Tardive dyskinesia, cases (%) | 59 (37.6) | 59 (53.6) | 72 (61.5) | 56 (61.5) | 49 (53.3) | 43 (51.2) | 63 (59.4) | 41 (51.9) |
| Mean score cases AIMS (SD) | 7.5 (3.4) | 7.8 (2.7) | 9.6 (4.3) | 9.6 (4.0) | 9.3 (3.8) | 9.2 (3.8) | 9.9 (4.3) | 8.6 (3.2) |
| Parkinsonism, cases (%) | 56 (35.7) | 44 (40.0) | 37 (31.6) | 30 (33.0) | 26 (28.3) | 23 (27.4) | 37 (34.9) | 24 (30.4) |
| Mean score cases motor part UPDRS (SD) | 18.6 (10.1) | 18.5 (12.2) | 17.9 (11.2) | 22.4 (9.4) | 21.0 (10.2) | 22.7 (12.1) | 19.1 (12.3) | 15.5 (12.1) |
| Akathisia, cases (%) | 16(10.2) | 9 (8.2) | 15 (12.8) | 7 (7.7) | 2 (2.2) | 5 (6.0) | 5 (4.7) | 1 (1.3) |
| Mean score cases item 4 BARS | 2.6 (0.8) | 2.7 (0.7) | 2.8 (0.6) | 2.6 (0.8) | 2.5 (0.7) | 2.6 (0.5) | 2.8 (0.8) | 3.0 |
Note: FU: follow-up; FGA: first generations antipsychotic; SGA: second generation antipsychotic; DDD: Defined Daily Dose; AIMS: Abnormal Involuntary Movement Scale; UPDRS: Unified Parkinson Disease Rating Scale; BARS: Barnes Akathisia Rating Scale; AP: antipsychotics; AD: antidepressants; MD: movement disorders; TD: tardive dyskinesia.
aIn case of missing data about age and MD, no measurement had taken place at the respective FU.
Number of patients alive with missing data: FU1: all variables: n = 41; FU2: age and MD n = 26, medication: n = 27; FU3: all variables: n = 45; FU4: age and MD: n = 41, medication: n = 44; FU5: all variables: n = 47; FU6: all variables: n = 16; FU7: age: n = 18, AP: n = 18, AD: n = 17; benzodiazepines: n = 17; TD: n = 18, Parkinsonism: n = 18, akathisia: n = 19.
bMeans and SDs in DDD for the patients who used the respective medications are given.
Time Varying Sample Characteristics
| Measurement Year Number of Patients Alive . | Baseline 1992–1993 n = 157 . | FU 1 1993 n = 151 . | FU 2 1994 n = 143 . | FU 3 1996 n = 136 . | FU 4 1997 n = 133 . | FU 5 1998 n = 131 . | FU 6 2001 n = 122 . | FU 7 2009 n = 96 . |
|---|---|---|---|---|---|---|---|---|
| Number of patients that died in subsequent time interval (n) | 6 | 8 | 7 | 3 | 2 | 9 | 26 | 23 |
| Mean age (SD)a | 48.8 (15.6) | 53.0 (14.7) | 50.4 (13.9) | 53.8 (13.2) | 53.0 (12.7) | 54.5(12.7) | 55.5 (13.0) | 61.4 (10.7) |
| Medication | ||||||||
| Antipsychotics | ||||||||
| FGA only, n (%) | 146 (93.0) | 93 (84.5) | 104 (89.7) | 79 (86.8) | 74 (83.1) | 67 (79.8) | 72 (67.9) | 38 (48.1) |
| SGA only, n (%) | 0 | 8 (7.3) | 7 (6.0) | 9 (9.9) | 12 (13.5) | 10 (11.9) | 15(14.2) | 14 (17.7) |
| Combination FGA and SGA n (%) | 0 | 0 | 0 | 0 | 0 | 1 (1.2) | 13 (12.3) | 21 (26.6) |
| No antipsychotic, n (%) | 11 (7.0) | 9 (8.2) | 5 (4.3) | 3 (3.3) | 3 (3.4) | 6 (7.1) | 6 (5.7) | 6 (7.6) |
| DDD antipsychoticsb, mean (SD) | 1.8 (1.4) | 1.9 (1.5) | 2.0(1.3) | 2.0 (1.6) | 2.1 (1.5) | 2.4 (1.7) | 2.1 (1.6) | 2.4 (1.4) |
| Antidepressants, n (%)b | 9 (5.7) | 9 (8.1) | 9 (7.8) | 10 (11.0) | 7 (7.9) | 3 (3.6) | 10 (9.4) | 4 (5.0) |
| DDD antidepressantsb, mean (SD) | 0.9 (0.4) | 0.8 (0.4) | 0.9 (0.3) | 0.9 (0.4) | 0.8 (0.3) | 0.9 (0.3) | 1.1 (0.3) | 1.3 (0.5) |
| Benzodiazepines, n (%) | 29 (18.5) | 23 (20.1) | 34 (29.3) | 31 (34.0) | 31 (34.8) | 35 (41.7) | 52 (49.1) | 41 (51.3) |
| DDD benzodiazepinesb, mean (SD) | 1.4 (1.0) | 1.2 (0.9) | 1.0 (0.7) | 1.0 (0.8) | 1.2 (1.1) | 1.3 (1.2) | 1.2 (1.0) | 1.3 (1.0) |
| Movement disorders | ||||||||
| Tardive dyskinesia, cases (%) | 59 (37.6) | 59 (53.6) | 72 (61.5) | 56 (61.5) | 49 (53.3) | 43 (51.2) | 63 (59.4) | 41 (51.9) |
| Mean score cases AIMS (SD) | 7.5 (3.4) | 7.8 (2.7) | 9.6 (4.3) | 9.6 (4.0) | 9.3 (3.8) | 9.2 (3.8) | 9.9 (4.3) | 8.6 (3.2) |
| Parkinsonism, cases (%) | 56 (35.7) | 44 (40.0) | 37 (31.6) | 30 (33.0) | 26 (28.3) | 23 (27.4) | 37 (34.9) | 24 (30.4) |
| Mean score cases motor part UPDRS (SD) | 18.6 (10.1) | 18.5 (12.2) | 17.9 (11.2) | 22.4 (9.4) | 21.0 (10.2) | 22.7 (12.1) | 19.1 (12.3) | 15.5 (12.1) |
| Akathisia, cases (%) | 16(10.2) | 9 (8.2) | 15 (12.8) | 7 (7.7) | 2 (2.2) | 5 (6.0) | 5 (4.7) | 1 (1.3) |
| Mean score cases item 4 BARS | 2.6 (0.8) | 2.7 (0.7) | 2.8 (0.6) | 2.6 (0.8) | 2.5 (0.7) | 2.6 (0.5) | 2.8 (0.8) | 3.0 |
| Measurement Year Number of Patients Alive . | Baseline 1992–1993 n = 157 . | FU 1 1993 n = 151 . | FU 2 1994 n = 143 . | FU 3 1996 n = 136 . | FU 4 1997 n = 133 . | FU 5 1998 n = 131 . | FU 6 2001 n = 122 . | FU 7 2009 n = 96 . |
|---|---|---|---|---|---|---|---|---|
| Number of patients that died in subsequent time interval (n) | 6 | 8 | 7 | 3 | 2 | 9 | 26 | 23 |
| Mean age (SD)a | 48.8 (15.6) | 53.0 (14.7) | 50.4 (13.9) | 53.8 (13.2) | 53.0 (12.7) | 54.5(12.7) | 55.5 (13.0) | 61.4 (10.7) |
| Medication | ||||||||
| Antipsychotics | ||||||||
| FGA only, n (%) | 146 (93.0) | 93 (84.5) | 104 (89.7) | 79 (86.8) | 74 (83.1) | 67 (79.8) | 72 (67.9) | 38 (48.1) |
| SGA only, n (%) | 0 | 8 (7.3) | 7 (6.0) | 9 (9.9) | 12 (13.5) | 10 (11.9) | 15(14.2) | 14 (17.7) |
| Combination FGA and SGA n (%) | 0 | 0 | 0 | 0 | 0 | 1 (1.2) | 13 (12.3) | 21 (26.6) |
| No antipsychotic, n (%) | 11 (7.0) | 9 (8.2) | 5 (4.3) | 3 (3.3) | 3 (3.4) | 6 (7.1) | 6 (5.7) | 6 (7.6) |
| DDD antipsychoticsb, mean (SD) | 1.8 (1.4) | 1.9 (1.5) | 2.0(1.3) | 2.0 (1.6) | 2.1 (1.5) | 2.4 (1.7) | 2.1 (1.6) | 2.4 (1.4) |
| Antidepressants, n (%)b | 9 (5.7) | 9 (8.1) | 9 (7.8) | 10 (11.0) | 7 (7.9) | 3 (3.6) | 10 (9.4) | 4 (5.0) |
| DDD antidepressantsb, mean (SD) | 0.9 (0.4) | 0.8 (0.4) | 0.9 (0.3) | 0.9 (0.4) | 0.8 (0.3) | 0.9 (0.3) | 1.1 (0.3) | 1.3 (0.5) |
| Benzodiazepines, n (%) | 29 (18.5) | 23 (20.1) | 34 (29.3) | 31 (34.0) | 31 (34.8) | 35 (41.7) | 52 (49.1) | 41 (51.3) |
| DDD benzodiazepinesb, mean (SD) | 1.4 (1.0) | 1.2 (0.9) | 1.0 (0.7) | 1.0 (0.8) | 1.2 (1.1) | 1.3 (1.2) | 1.2 (1.0) | 1.3 (1.0) |
| Movement disorders | ||||||||
| Tardive dyskinesia, cases (%) | 59 (37.6) | 59 (53.6) | 72 (61.5) | 56 (61.5) | 49 (53.3) | 43 (51.2) | 63 (59.4) | 41 (51.9) |
| Mean score cases AIMS (SD) | 7.5 (3.4) | 7.8 (2.7) | 9.6 (4.3) | 9.6 (4.0) | 9.3 (3.8) | 9.2 (3.8) | 9.9 (4.3) | 8.6 (3.2) |
| Parkinsonism, cases (%) | 56 (35.7) | 44 (40.0) | 37 (31.6) | 30 (33.0) | 26 (28.3) | 23 (27.4) | 37 (34.9) | 24 (30.4) |
| Mean score cases motor part UPDRS (SD) | 18.6 (10.1) | 18.5 (12.2) | 17.9 (11.2) | 22.4 (9.4) | 21.0 (10.2) | 22.7 (12.1) | 19.1 (12.3) | 15.5 (12.1) |
| Akathisia, cases (%) | 16(10.2) | 9 (8.2) | 15 (12.8) | 7 (7.7) | 2 (2.2) | 5 (6.0) | 5 (4.7) | 1 (1.3) |
| Mean score cases item 4 BARS | 2.6 (0.8) | 2.7 (0.7) | 2.8 (0.6) | 2.6 (0.8) | 2.5 (0.7) | 2.6 (0.5) | 2.8 (0.8) | 3.0 |
Note: FU: follow-up; FGA: first generations antipsychotic; SGA: second generation antipsychotic; DDD: Defined Daily Dose; AIMS: Abnormal Involuntary Movement Scale; UPDRS: Unified Parkinson Disease Rating Scale; BARS: Barnes Akathisia Rating Scale; AP: antipsychotics; AD: antidepressants; MD: movement disorders; TD: tardive dyskinesia.
aIn case of missing data about age and MD, no measurement had taken place at the respective FU.
Number of patients alive with missing data: FU1: all variables: n = 41; FU2: age and MD n = 26, medication: n = 27; FU3: all variables: n = 45; FU4: age and MD: n = 41, medication: n = 44; FU5: all variables: n = 47; FU6: all variables: n = 16; FU7: age: n = 18, AP: n = 18, AD: n = 17; benzodiazepines: n = 17; TD: n = 18, Parkinsonism: n = 18, akathisia: n = 19.
bMeans and SDs in DDD for the patients who used the respective medications are given.
Movement Disorders
MD prevalence fluctuated over time (table 2). Across all eight measurements, TD cases were the most prevalent ranging between 37.6 % and 61.5 %. The prevalence of Parkinsonism and akathisia ranged from 27.4–40.0% and 1.3–12.8%, respectively.
Psychotropic Medication
Up until 1992, only FGA were available in Curacao, which is reflected in the percentages of patients using FGA and SGA between 1992 and 2009 (table 2). At baseline in 1992/1993, FGA were used by 93.0% of the sample while the remaining 7.0% did not take antipsychotic medication. Most patients continued to use an FGA, either alone or in combination with an SGA, which was the case for 74.7 % of the patients in 2009. Use of SGA, starting from 0% in 1992/1993, increased to 44.3% in 2009, either alone or in combination with an FGA. The percentage of patients taking no antipsychotics varied between 3.3% and 8.2%. Antidepressants were used by 3.6–11.0% of the patients. The use of benzodiazepines gradually increased from 18.5% at baseline to 51.3% at follow-up 7 in 2009.
Cox Regression
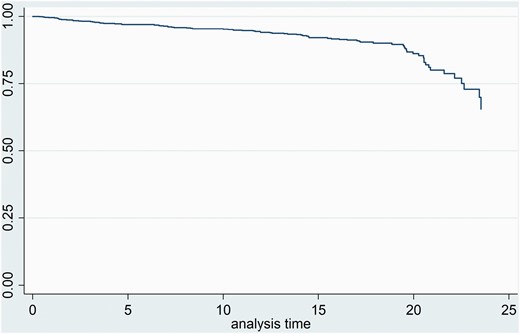
Table 3 shows the results for the possible predictors of mortality from univariate analyses. Age, sex, Parkinsonism, antipsychotic type, and DDD antipsychotics were significant predictors and were included in a multivariate model. TD, akathisia, DDD benzodiazepines, and DDD antidepressants, were not significant as univariate predictors and were therefore not included. Extra-linearity was tested for Parkinsonism, age, and DDD antipsychotics and significance was found for the latter two variables. Linear-quadratic terms for age and DDD antipsychotics were therefore added to the final model (Cleves 2008). Interactions of age and time, and age and Parkinsonism were tested of which only the former was significant and thus included in the final model. Hazard ratios (HR) and significance for the variables in the final multivariate model are presented in table 4. Parkinsonism was positively associated with mortality (HR = 1.020, 95% CI 1.001–1.038, P = .034). Age showed significance as a linear squared predictor (HR = 1.002, 95% CI 1.0004–1.0032, P = .015). DDD antipsychotic displayed a significant linear (HR = 0.792, 95% CI 0.692–0.997 P = .047) and a trend as a linear-squared relation (HR =1.154, 95% CI 0.993–1.340, P = .061). The survival function adjusted for Parkinsonism, age, and DDD antipsychotics is shown in figure 1. Separate survival functions adjusted for Parkinsonism, age and DDD antipsychotics are given in the supplementary materials.
Univariate Tests for Possible Predictors of Mortality
| Variable . | Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Age | 1.058 | 1.038–1.078 | .000* |
| Tardive dyskinesia | 1.024 | 0.981–1.069 | .276 |
| Parkinsonism | 1.029 | 1.014–1.045 | .000* |
| Akathisia | 0.764 | 0.475–1.23 | .267 |
| DDD antipsychotics | 0.727 | 0.600–0.879 | .001* |
| DDD antidepressants | 1.239 | 0.596–2.58 | .566 |
| DDD benzodiazepines | 0.813 | 0.594–1.117 | .203 |
| X2 | df | ||
| Sex | 5.62 | 1 | .018* |
| Type of antipsychotica | 7.83 | 3 | .050* |
| Variable . | Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Age | 1.058 | 1.038–1.078 | .000* |
| Tardive dyskinesia | 1.024 | 0.981–1.069 | .276 |
| Parkinsonism | 1.029 | 1.014–1.045 | .000* |
| Akathisia | 0.764 | 0.475–1.23 | .267 |
| DDD antipsychotics | 0.727 | 0.600–0.879 | .001* |
| DDD antidepressants | 1.239 | 0.596–2.58 | .566 |
| DDD benzodiazepines | 0.813 | 0.594–1.117 | .203 |
| X2 | df | ||
| Sex | 5.62 | 1 | .018* |
| Type of antipsychotica | 7.83 | 3 | .050* |
Note: DDD: Defined Daily Dose.
aType of antipsychotic was one of the following categories: no antipsychotic; first generation antipsychotic(s) (FGA) only; second generation antipsychotic(s)(SGA) only; a combination of FGA and SGA.
*P < .2.
Univariate Tests for Possible Predictors of Mortality
| Variable . | Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Age | 1.058 | 1.038–1.078 | .000* |
| Tardive dyskinesia | 1.024 | 0.981–1.069 | .276 |
| Parkinsonism | 1.029 | 1.014–1.045 | .000* |
| Akathisia | 0.764 | 0.475–1.23 | .267 |
| DDD antipsychotics | 0.727 | 0.600–0.879 | .001* |
| DDD antidepressants | 1.239 | 0.596–2.58 | .566 |
| DDD benzodiazepines | 0.813 | 0.594–1.117 | .203 |
| X2 | df | ||
| Sex | 5.62 | 1 | .018* |
| Type of antipsychotica | 7.83 | 3 | .050* |
| Variable . | Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Age | 1.058 | 1.038–1.078 | .000* |
| Tardive dyskinesia | 1.024 | 0.981–1.069 | .276 |
| Parkinsonism | 1.029 | 1.014–1.045 | .000* |
| Akathisia | 0.764 | 0.475–1.23 | .267 |
| DDD antipsychotics | 0.727 | 0.600–0.879 | .001* |
| DDD antidepressants | 1.239 | 0.596–2.58 | .566 |
| DDD benzodiazepines | 0.813 | 0.594–1.117 | .203 |
| X2 | df | ||
| Sex | 5.62 | 1 | .018* |
| Type of antipsychotica | 7.83 | 3 | .050* |
Note: DDD: Defined Daily Dose.
aType of antipsychotic was one of the following categories: no antipsychotic; first generation antipsychotic(s) (FGA) only; second generation antipsychotic(s)(SGA) only; a combination of FGA and SGA.
*P < .2.
Final Cox Proportional Hazards Model
| Variable . | Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Female sex | 1.267 | 0.742–2.163 | .385 |
| Age | 0.995 | 0.937–1.056 | .863 |
| Age × time | 1.001 | 0.995–1.007 | .765 |
| Age linear-squared | 1.002 | 1.0004–1.0032 | .015* |
| Age linear-squared × time | 1.000 | 0.99975–1.0003 | .117 |
| Parkinsonism | 1.020 | 1.001–1.038 | .034* |
| DDD antipsychotics | 0.792 | 0.629–0.997 | .047* |
| DDD antipsychotics linear-squared | 1.154 | 0.993–1.340 | .061 |
| No antipsychotic** | 0.965 | 0.318–2.933 | .951 |
| FGA only** | 0.984 | 0.442–2.192 | .970 |
| Both FGA and SGA** | 1.021 | 0.254–4.104 | .976 |
| Variable . | Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Female sex | 1.267 | 0.742–2.163 | .385 |
| Age | 0.995 | 0.937–1.056 | .863 |
| Age × time | 1.001 | 0.995–1.007 | .765 |
| Age linear-squared | 1.002 | 1.0004–1.0032 | .015* |
| Age linear-squared × time | 1.000 | 0.99975–1.0003 | .117 |
| Parkinsonism | 1.020 | 1.001–1.038 | .034* |
| DDD antipsychotics | 0.792 | 0.629–0.997 | .047* |
| DDD antipsychotics linear-squared | 1.154 | 0.993–1.340 | .061 |
| No antipsychotic** | 0.965 | 0.318–2.933 | .951 |
| FGA only** | 0.984 | 0.442–2.192 | .970 |
| Both FGA and SGA** | 1.021 | 0.254–4.104 | .976 |
Note: DDD: Defined Daily Dose; FGA: first generation antipsychotics; SGA: second generation antipsychotics.
*P < .05.
**Reference category: SGA only.
Final Cox Proportional Hazards Model
| Variable . | Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Female sex | 1.267 | 0.742–2.163 | .385 |
| Age | 0.995 | 0.937–1.056 | .863 |
| Age × time | 1.001 | 0.995–1.007 | .765 |
| Age linear-squared | 1.002 | 1.0004–1.0032 | .015* |
| Age linear-squared × time | 1.000 | 0.99975–1.0003 | .117 |
| Parkinsonism | 1.020 | 1.001–1.038 | .034* |
| DDD antipsychotics | 0.792 | 0.629–0.997 | .047* |
| DDD antipsychotics linear-squared | 1.154 | 0.993–1.340 | .061 |
| No antipsychotic** | 0.965 | 0.318–2.933 | .951 |
| FGA only** | 0.984 | 0.442–2.192 | .970 |
| Both FGA and SGA** | 1.021 | 0.254–4.104 | .976 |
| Variable . | Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Female sex | 1.267 | 0.742–2.163 | .385 |
| Age | 0.995 | 0.937–1.056 | .863 |
| Age × time | 1.001 | 0.995–1.007 | .765 |
| Age linear-squared | 1.002 | 1.0004–1.0032 | .015* |
| Age linear-squared × time | 1.000 | 0.99975–1.0003 | .117 |
| Parkinsonism | 1.020 | 1.001–1.038 | .034* |
| DDD antipsychotics | 0.792 | 0.629–0.997 | .047* |
| DDD antipsychotics linear-squared | 1.154 | 0.993–1.340 | .061 |
| No antipsychotic** | 0.965 | 0.318–2.933 | .951 |
| FGA only** | 0.984 | 0.442–2.192 | .970 |
| Both FGA and SGA** | 1.021 | 0.254–4.104 | .976 |
Note: DDD: Defined Daily Dose; FGA: first generation antipsychotics; SGA: second generation antipsychotics.
*P < .05.
**Reference category: SGA only.
Survival function adjusted for Parkinsonism, age, and DDD antipsychotics.
Discussion
Our findings indicate that Parkinsonism is a significant risk factor for mortality in patients with SMI whereas TD and akathisia are not.
For Parkinsonism, one point increase on the motor examination subscale of the UPDRS was associated with a HR of 1.02. The motor part of the UPDRS consists of 14 items which can be scored 0–4 leading to a range of possible scores of 0–56. In our sample, the SD for cases with Parkinsonism was close to 10 points and an increase of 10 points would lead to an increase in the HR of mortality of 21%.
Previous studies on the association between MD and mortality in SMI focused on TD2-10 while only two studies also included Parkinsonism.19,29 Modestin et al.19 reported a higher mortality rate in patients with Parkinsonism than in those without, when studying 200 psychiatric patients treated with antipsychotic medication after 9 years follow-up. However, after adjustment, only age remained as a significant risk factor. Important differences with the current study are that the study by Modestin et al. (1) assessed MD only at baseline and (2) had a follow-up of 9 years in contrast with the 24 years of follow-up in the current study. Given the fluctuating nature of MD, assessing MD only at one time might not give enough information to draw conclusions.33 Measuring MD at multiple time points, as we did in the current study, is probably a more valid design to address this research question. A retrospective study by Schoepf et al.34 examined deaths in schizophrenia patients in general hospitals in relation to mental and physical comorbidity.29 Their results showed an odds ratio of 5.0 for the association between the presence of Parkinsonism and hospital mortality, which can be considered a very strong effect. Parkinsonism though, was not measured with formal rating scales which may have diminished the precision of the effect estimate.
Outside the field of psychiatry, two studies reported an association between Parkinsonism and mortality in people aged 65 years and over.30,31 Firstly, in a community sample the presence of Parkinsonism was associated with a twofold increase in the risk of death30 and gait disturbance in particular heightened the risk. Secondly, in a mixed sample of patients with Alzheimer’s disease and subjects without dementia, Parkinsonism was a risk factor for mortality in both subgroups. Interestingly, the latter study focused on spontaneous Parkinsonism as subjects receiving Parkinsonism-inducing medication were excluded which suggest that, along with drug-induced Parkinsonism, spontaneous Parkinsonism may also be a risk factor. Similarly, in Parkinson’s Disease, severity of motor symptoms, especially postural imbalance, and gait disturbance have been found to be associated with mortality.35,36
Considering all these findings of Parkinsonism as a risk factor for mortality in different populations with different underlying causes for Parkinsonian symptoms, it can be hypothesized that Parkinsonism is an independent predictor of all-cause mortality.
It is not directly clear how Parkinsonism increases mortality risk, but Parkinsonism is related to several other factors associated with mortality such as a higher rate of fall incidents and dysphagia which can lead to asphyxia and pneumonia.37 Also it could be hypothesized that the relationship is indirect and, in patients with SMI, might be (partly) based on the association between both spontaneous38,39 and drug-induced40 Parkinsonism with cognitive impairments. Indeed, cognitive deficits may be related to unhealthy lifestyle or less awareness of physical problems and/or access to physical healthcare. Another explanation may be that Parkinsonism reduces physical activity and increases sedentary behavior,41 which in turn increase the mortality rate.
Given the high prevalence of Parkinsonism in SMI patients and several studies suggesting that Parkinsonism may lead to shorter survival, it is important to explore this relationship in more depth, e.g., would reducing Parkinsonism also increase survival?
TD was not significantly associated with risk of death in the present study. Previous studies have reported inconsistent results. Four of eleven studies reported an association between TD and mortality,12,15–17 two reported a trend,42,43 and five no association.18–21,43 In 2000, a meta-analysis by Ballesteros et al. consisting of seven studies demonstrated a significant overall OR of 1.4.11 However, some of the included studies suffered from methodological problems, such as fewer than five years follow-up3,4,6,8 and no control for known confounders such as antipsychotic dose.15–17,19,42 In 2009, three additional studies on the association between TD and mortality were published using the more sophisticated Cox and logistic regression analyses, which are better suited to this type of data than the Chi-square test12,20,21: (1) Dean and Thuras20 used multiple measurements of TD—although they only identified patients with TD at baseline or TD at any time instead of entering TD as a time-varying covariate—and found a significant association between TD and mortality, which disappeared after adjusting for age and antipsychotic drug use; (2) Modestin et al.21 did not find an association; and (3) Chong et al. found an age- and antipsychotic dose-adjusted association with a HR of 1.38 for mild and 1.90 for definite TD which—considering the dose–response effect, represents relatively strong evidence in favor of a real effect of TD on mortality. However, they did not include other MDs and had a relatively short study duration. Evidence shows that severity of TD is positively correlated with symptom severity in schizophrenia44,45 which may, therefore, be a confounding factor in the association between TD and survival. Despite this association, none of the studies up to now including the present study, controlled for psychiatric symptom severity which poses a limitation to the findings.
Akathisia did not show a significant association with all-cause mortality which is consistent with the single previous study.21
We observed a significant effect for DDD antipsychotics as a linear association and a trend for a linear-quadratic effect, which combined reflects a partially U-shaped curve. Well-known metabolic side effects of predominantly second-generation antipsychotics, and in turn their association with adverse health outcomes like type 2 diabetes and cardiovascular disease has let to many studies addressing the question how (cumulative) exposure to antipsychotics affects mortality.46 A robust finding reported by recently published large register-based cohort studies and one systematic review and meta-analysis is that any use is associated with a lower risk compared to no use in schizophrenia.7,46–49 One cohort study (n = 21 492), using nation-wide registers in Sweden, investigated cumulative antipsychotic exposure expressed in DDD and mortality. The relation in chronic patients showed a U-shaped curve in which patients who did not use an antipsychotic had the highest risk, followed by the high dose (>1.5 DDD/day), low dose (<0.5 DDD/day) and moderate dose(0.5–1.5 DDD/day) categories. The shape of the curve we found seems somewhat similar. Nonetheless, above-mentioned studies with large numbers of patients, are better powered to give a precise estimate of the relationship.
An important strength of the study is that it was conducted on the former Netherlands Antilles, which, because they are islands, comprise a well-defined catchment area. Along with the fact that the Klinika Capriles is the only psychiatric hospital on the former Netherlands Antilles, and that almost all of the eligible patients agreed to participate (99%), selection bias is relatively small. On the other hand, at the former Netherlands Antilles, stigma on mental disorders is somewhat stronger than in most western countries and patients with mental illness are to a greater extent taken care of by family members. Thus, patients admitted to the Klinika Capriles consisted generally of those with the most severe psychiatric illnesses which is reflected in our sample. Our results can therefore be generalized to populations with severe psychiatric disorders receiving antipsychotic treatment and might not apply to populations with milder illnesses. A further strength arising from the fact that the Klinika Capriles was the only psychiatric facility on the islands is that our data is close to covering the complete treatment history of the participants. Another major strength of the current study is the use of multiple measurements of MD as time-varying covariates. This is especially important given the fluctuating nature of MD in SMI patients.33 Moreover, the follow-up period of 24 years of the current study is the longest up to now.
Some important factors known to influence mortality were not measured, including access to and adequate use of physical healthcare and metabolic parameters. Indeed one would have to include these variables if the aims were to build a prediction model for mortality. Our aim, however, was to estimate the association of MD and mortality and because the variables mentioned above are not directly related to MD we think that omitting these variables does not bias our results. In contrast, other factors, i.e. symptom severity,44 cognitive functioning,38–40,50 physical activity,41 use of alcohol, illicit drug use,51 and smoking52 have been found to be related to both MD and mortality and the lack of information on those variables puts a limitation to the present findings.
Nevertheless, given the effect size we found for the association between Parkinsonism and mortality, combined with other reports of this association, we think evidence points toward Parkinsonism as a risk factor. This could be confirmed in large well controlled studies with multiple measurements over time with regard to MD, use of psychotropic medication, symptom severity, cognitive functioning, physical activity, smoking, use of alcohol, and illicit drug use. Such studies may give more insight how these variables are interrelated and influence mortality.
Conclusion
In conclusion, Parkinsonism was a significant risk factor for mortality in a cohort of patients with SMI. An increase of 10 points (equals one SD) increased the HR of mortality with 21%. TD and akathisia did not show an association with mortality. To study the complex interplay between MD and mortality more well-controlled follow-up studies are needed. With the knowledge that antipsychotics differ in their potency to induce Parkinsonism, an intervention study is of great clinical importance to find out whether treatment or prevention of Parkinsonism can reduce mortality.
Funding
The Curacao Extrapyramidal Syndromes Studies were supported by a grant from the NASKHO (National Antilles Foundation for Clinical Higher Education).