-
PDF
- Split View
-
Views
-
Cite
Cite
Amber L. Bahorik, Christina E. Newhill, Shaun M. Eack, Neurocognitive Functioning of Individuals With Schizophrenia: Using and Not Using Drugs, Schizophrenia Bulletin, Volume 40, Issue 4, July 2014, Pages 856–867, https://doi.org/10.1093/schbul/sbt099
Close - Share Icon Share
Abstract
Objectives: Research on neurocognition in schizophrenia, using modest samples and self-rated assessments, reports drug use contributes to improved rather than impaired cognitive function. We have sought to replicate these findings in a large sample of patients that had their drug-use status confirmed by laboratory assays and evaluated the potential differences in cognitive function between patients with positive and negative results. Methods: Nine hundred and seventy four schizophrenia patients completed neuropsychological and laboratory tests at screening/baseline of the Clinical Antipsychotic Trials of Intervention Effectiveness study. Radioimmunoassay (RIA) of hair tested for cannabis, cocaine and methamphetamine. Results: Many patients screened positive for drug use (n = 262; 27%), and there were no differences between patients with positive and negative results in terms of cognitive function after adjusting for multiple inference testing, except patients with positive RIA for methamphetamine demonstrated increased processing speed (corrected, P = .024). Moderator models were employed to explore potential subgroup differences in this pattern of results. At low medication dosages, patients with positive RIA for cocaine demonstrated decreased processing speed compared with patients with negative RIA for cocaine (uncorrected, P = .008). And for any other drugs with low psychopathology, patients with positive RIA demonstrated decreased working memory compared with patients with negative RIA (uncorrected, P = .006). Conclusions: No positive effects of cannabis on cognitive function were observed, and drug use was not associated with improved neurocognition across most of the subgroup characteristics explored in this sample of schizophrenia patients.
Introduction
Deficits in neurocognitive function and the use of illicit drugs are both important factors that can affect the recovery outcomes of individuals with schizophrenia.1–3 Estimates indicate more than 50% of adults with schizophrenia use drugs,4–8 that polysubstance use is common and equally prevalent in those with nonaffective and affective presentations of psychosis,9 and more than 80% of these individuals exhibit neurocognitive deficits during the course of their condition.10,11 Not surprisingly, the examination of cognitive deficits has become a primary focus of the current research on drug abuse in schizophrenia.12,13 Although cognitive deficits are already present in first-episode patients,14–17 and drug use results in problematic consequences for adults with schizophrenia,18–24 major research has surprisingly shown that drug use is at times associated with improved, rather than impaired, cognitive function in these individuals.25–36 In fact, findings of recent meta-analyses show schizophrenia patients with histories of cannabis use and/or cannabis-use disorders exhibit superior cognitive function compared with their noncannabis-using counterparts.37–39 Alternatively, other research has failed to find differences in the performance of cognitive tasks when comparing patients with schizophrenia using drugs with those not using drugs,40–43 and fewer investigations have associated drug use with cognitive dysfunction in the condition,44,45except for studies examining cocaine use in the illness.46–48
To date, most of the research in this area paradoxically indicates that individuals with schizophrenia who use drugs, particularly cannabis, exhibit improved cognitive function,25–36 despite evidence that drug use has considerable negative effects on cognition in those without schizophrenia.49–51 Cannabis use is exceedingly common in schizophrenia, with 27.1% of those with the condition experiencing cannabis abuse or dependence in their lifetime,52 compared with only 8.0% of those without schizophrenia.53 Despite the fact that comorbid cannabis-use disorders have been linked with considerable degrees of functional instability in the illness,19 Carey et al25 have shown outpatients with schizophrenia who had (current/former) histories of abuse/dependence exhibited better performance on cognitive tests compared with those without such histories, and more recent research by DeRosse et al12 reported similar findings, where cannabis-use disorders were associated with improved neurocognition. While prior research has associated drug use with superior cognitive function (at times) in first-episode patients,29,31,33,34 inpatients with schizophrenia,26 and patients in acute phases of the illness,28 few studies have employed laboratory assays to detect the use of drugs.
Studies of the residual effects of cannabis in first-episode psychosis have used urine drug screens to confirm abstinence (for the drug-use group), where both Jockers-Scherubl et al36 and Schnell et al35 associated high frequency of cannabis use (prior to illness onset) with improvement on cognitive tasks. Although laboratory tests were not used to confirm drug use, Joyal et al31 examined the impact of negative symptoms on sustaining drug use in males with schizophrenia and revealed cannabis abuse/dependence was associated with less neurological soft signs and improved executive function. The findings reported by Joyal and colleagues validated prior research, indicating a proportion of patients with schizophrenia using drugs exhibit fewer negative symptoms and better premorbid functioning.54 Regardless of the illness-related factors that may aid in sustaining illicit behaviors and drug use in schizophrenia,55,56 prior studies demonstrating improved cognition in these individuals have largely limited their inquiry to cannabis,38,39 utilize modest samples (drug-use group range: n = 12–57)12 from specialty (eg, first-episode, acute psychosis, inpatient) populations,26,28,29,31,34 primarily rely on self-report or interview-based measures of drug use,30–33 and employ laboratory tests to confirm abstinence rather than detect drug use.35,36 The degree to which such findings are specific to cannabis use or persist when laboratory measures of drug use are employed as the confirmatory method for detecting drug use in adequately powered samples is largely unknown.
This study examined neurocognition in a sample of patients with schizophrenia, used laboratory assays to establish drug-use status, and explored subsamples for which drug use may have a greater impact on cognitive function. We selected neurocognitive assessments from 974 patients with schizophrenia who participated in the screening/baseline procedures of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project,57 and examined whether patients who tested positive for drug use showed improved cognitive function compared with those who tested negative for drug use. We also explored whether sample characteristics discriminated subgroups for which drug use had a greater impact on cognitive function.
Methods
Participants
Data were collected as part of the CATIE project, which compared the relative effectiveness of first- and second-generation antipsychotic medications in persons with schizophrenia;2,57 the primary outcomes as well as complete analyses of neurocognitive data are provided in greater detail elsewhere.2,57–59 Individuals were eligible for enrollment if they were between the ages of 18 and 65, had a diagnosis of schizophrenia, as confirmed by the Structured Clinical Interview60 for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), could receive oral antipsychotic medications, did not have refractory illness (treatment nonresponsiveness), had no unstable medical conditions, were not pregnant/breastfeeding, and/or were not in their first episode of psychosis.57,59
This study examined the neurocognitive test performance of 974 CATIE participants who completed radioimmunoassay (RIA) testing during screening, and then completed neuropsychological testing at baseline. Results from RIA were used to confirm drug-use status, and the other methods of substance use detection that were used in the CATIE project are described elsewhere.1 All participants were stabilized as outpatients (100%), most were white (68.5%) and the majority were males (72.1%), who were 40 years of age on average (SD = 10.99), upon enrollment. Further information on sample characteristics is presented in table 1.
Demographic and Clinical Characteristics, and the Prognostic Indicators of Schizophrenia Patients With Negative and Positive RIA Test Results for Drug Use (n = 974)
| Variable . | Drug Use (+) RIA . | No Drug Use (−) RIA . | P . |
|---|---|---|---|
| n = 262 . | n = 712 . | ||
| M (SD) . | M (SD) . | ||
| Demographic | |||
| Age | 39.21 (10.46) | 40.76 (10.95) | .049 |
| Gender | |||
| n (% female) | 58 (22.1%) | 214 (30.0%) | .018 |
| n (% male) | 204 (22.1%) | 498 (70.0%) | |
| Race | |||
| n (% white) | 136 (51.9%) | 531 (66.7%) | <.001 |
| n (% non-white) | 126 (70.6%) | 181 (19.9%) | |
| Marital status | |||
| n (% not married) | 230 (88.0%) | 627 (88.0%) | .995 |
| Employment | |||
| n (% unemployed) | 220 (84.0%) | 594 (84.0%) | .986 |
| Treatment | |||
| Pharmacologic treatment—n (%) | |||
| Antipsychotic | |||
| First generation | 49 (22.0%) | 129 (20.0%) | .697 |
| Second generation | 149 (66.0%) | 485 (75.0%) | .007 |
| First/second | 12 (6.0%) | 45 (8.0%) | .230 |
| Antidepressant | 84 (37.0%) | 247 (38.0%) | .791 |
| Antiepileptic | 34 (15.0%) | 139 (22.0%) | .041 |
| Other medications | 96 (37.0%) | 265 (37.0%) | .986 |
| No medication | 54 (21.0%) | 87 (12.0%) | .001 |
| CPZa | |||
| n (daily dose) | 323.91 (338.31) | 367.63 (384.34) | .170 |
| Treatment/services received | |||
| n (% outpatient) | 262 (100.0%) | 712 (100.0%) | — |
| Psychopathology | |||
| PANSSb | |||
| Total | 76.22 (17.05) | 75.06 (17.43) | .354 |
| General symptomatology | 37.32 (9.14) | 36.88 (9.22) | .511 |
| Negative | 19.68 (6.19) | 20.01 (6.25) | .453 |
| Positive | 19.22 (5.58) | 18.16 (5.59) | .009 |
| Duration of illness | |||
| Duration (average years of illness) | 15.15 (10.35) | 16.35 (10.88) | .133 |
| Duration | |||
| n (% illness <5 years) | 52 (21.0%) | 118 (17.0%) | .299 |
| Drug abuse or dependence | |||
| Illness severity and drug-use severity | |||
| CGIc | |||
| Global illness severity | 4.08 (0.88) | 3.92 (0.91) | .013 |
| DUSd | |||
| Drug-use severity | 1.89 (0.91) | 1.15 (0.48) | <.001 |
| Drug abuse or dependence | |||
| SCIDe | |||
| n (% drug abuse/dependence diagnosis) | 83 (32.0%) | 28 (4.0%) | <.001 |
| Prognostic indicator | |||
| Childhood Adversity Scoref | 2.27 (2.04) | 1.46 (1.69) | <.001 |
| Variable . | Drug Use (+) RIA . | No Drug Use (−) RIA . | P . |
|---|---|---|---|
| n = 262 . | n = 712 . | ||
| M (SD) . | M (SD) . | ||
| Demographic | |||
| Age | 39.21 (10.46) | 40.76 (10.95) | .049 |
| Gender | |||
| n (% female) | 58 (22.1%) | 214 (30.0%) | .018 |
| n (% male) | 204 (22.1%) | 498 (70.0%) | |
| Race | |||
| n (% white) | 136 (51.9%) | 531 (66.7%) | <.001 |
| n (% non-white) | 126 (70.6%) | 181 (19.9%) | |
| Marital status | |||
| n (% not married) | 230 (88.0%) | 627 (88.0%) | .995 |
| Employment | |||
| n (% unemployed) | 220 (84.0%) | 594 (84.0%) | .986 |
| Treatment | |||
| Pharmacologic treatment—n (%) | |||
| Antipsychotic | |||
| First generation | 49 (22.0%) | 129 (20.0%) | .697 |
| Second generation | 149 (66.0%) | 485 (75.0%) | .007 |
| First/second | 12 (6.0%) | 45 (8.0%) | .230 |
| Antidepressant | 84 (37.0%) | 247 (38.0%) | .791 |
| Antiepileptic | 34 (15.0%) | 139 (22.0%) | .041 |
| Other medications | 96 (37.0%) | 265 (37.0%) | .986 |
| No medication | 54 (21.0%) | 87 (12.0%) | .001 |
| CPZa | |||
| n (daily dose) | 323.91 (338.31) | 367.63 (384.34) | .170 |
| Treatment/services received | |||
| n (% outpatient) | 262 (100.0%) | 712 (100.0%) | — |
| Psychopathology | |||
| PANSSb | |||
| Total | 76.22 (17.05) | 75.06 (17.43) | .354 |
| General symptomatology | 37.32 (9.14) | 36.88 (9.22) | .511 |
| Negative | 19.68 (6.19) | 20.01 (6.25) | .453 |
| Positive | 19.22 (5.58) | 18.16 (5.59) | .009 |
| Duration of illness | |||
| Duration (average years of illness) | 15.15 (10.35) | 16.35 (10.88) | .133 |
| Duration | |||
| n (% illness <5 years) | 52 (21.0%) | 118 (17.0%) | .299 |
| Drug abuse or dependence | |||
| Illness severity and drug-use severity | |||
| CGIc | |||
| Global illness severity | 4.08 (0.88) | 3.92 (0.91) | .013 |
| DUSd | |||
| Drug-use severity | 1.89 (0.91) | 1.15 (0.48) | <.001 |
| Drug abuse or dependence | |||
| SCIDe | |||
| n (% drug abuse/dependence diagnosis) | 83 (32.0%) | 28 (4.0%) | <.001 |
| Prognostic indicator | |||
| Childhood Adversity Scoref | 2.27 (2.04) | 1.46 (1.69) | <.001 |
aCPZ, chlorpromazine equivalent dose; CPZ daily dose equivalents were computed based on prescribed typical and/or atypical antipsychotic medications.
bPANSS, Positive and Negative Syndrome Scale74; assessed the presence of psychopathology in the sample.
cCGI, Clinical Global Impression75; global score totals were used as an indicator of illness severity.
dDUS, Drug Use Scale; clinician-rated measure that assessed for drug-use severity; higher DUS scores indicate greater degrees of drug severity.
eSCID, Structured Clinical Interview60 for DSM-IV; assessed the degree of functional impairment associated with 5-year drug-use history.
fChildhood Adversity Score = summed composite of prognostic indicator variables (prognostic variables considered childhood behavioral problems prior to age 15); higher scores indicate worse prognosis.
Demographic and Clinical Characteristics, and the Prognostic Indicators of Schizophrenia Patients With Negative and Positive RIA Test Results for Drug Use (n = 974)
| Variable . | Drug Use (+) RIA . | No Drug Use (−) RIA . | P . |
|---|---|---|---|
| n = 262 . | n = 712 . | ||
| M (SD) . | M (SD) . | ||
| Demographic | |||
| Age | 39.21 (10.46) | 40.76 (10.95) | .049 |
| Gender | |||
| n (% female) | 58 (22.1%) | 214 (30.0%) | .018 |
| n (% male) | 204 (22.1%) | 498 (70.0%) | |
| Race | |||
| n (% white) | 136 (51.9%) | 531 (66.7%) | <.001 |
| n (% non-white) | 126 (70.6%) | 181 (19.9%) | |
| Marital status | |||
| n (% not married) | 230 (88.0%) | 627 (88.0%) | .995 |
| Employment | |||
| n (% unemployed) | 220 (84.0%) | 594 (84.0%) | .986 |
| Treatment | |||
| Pharmacologic treatment—n (%) | |||
| Antipsychotic | |||
| First generation | 49 (22.0%) | 129 (20.0%) | .697 |
| Second generation | 149 (66.0%) | 485 (75.0%) | .007 |
| First/second | 12 (6.0%) | 45 (8.0%) | .230 |
| Antidepressant | 84 (37.0%) | 247 (38.0%) | .791 |
| Antiepileptic | 34 (15.0%) | 139 (22.0%) | .041 |
| Other medications | 96 (37.0%) | 265 (37.0%) | .986 |
| No medication | 54 (21.0%) | 87 (12.0%) | .001 |
| CPZa | |||
| n (daily dose) | 323.91 (338.31) | 367.63 (384.34) | .170 |
| Treatment/services received | |||
| n (% outpatient) | 262 (100.0%) | 712 (100.0%) | — |
| Psychopathology | |||
| PANSSb | |||
| Total | 76.22 (17.05) | 75.06 (17.43) | .354 |
| General symptomatology | 37.32 (9.14) | 36.88 (9.22) | .511 |
| Negative | 19.68 (6.19) | 20.01 (6.25) | .453 |
| Positive | 19.22 (5.58) | 18.16 (5.59) | .009 |
| Duration of illness | |||
| Duration (average years of illness) | 15.15 (10.35) | 16.35 (10.88) | .133 |
| Duration | |||
| n (% illness <5 years) | 52 (21.0%) | 118 (17.0%) | .299 |
| Drug abuse or dependence | |||
| Illness severity and drug-use severity | |||
| CGIc | |||
| Global illness severity | 4.08 (0.88) | 3.92 (0.91) | .013 |
| DUSd | |||
| Drug-use severity | 1.89 (0.91) | 1.15 (0.48) | <.001 |
| Drug abuse or dependence | |||
| SCIDe | |||
| n (% drug abuse/dependence diagnosis) | 83 (32.0%) | 28 (4.0%) | <.001 |
| Prognostic indicator | |||
| Childhood Adversity Scoref | 2.27 (2.04) | 1.46 (1.69) | <.001 |
| Variable . | Drug Use (+) RIA . | No Drug Use (−) RIA . | P . |
|---|---|---|---|
| n = 262 . | n = 712 . | ||
| M (SD) . | M (SD) . | ||
| Demographic | |||
| Age | 39.21 (10.46) | 40.76 (10.95) | .049 |
| Gender | |||
| n (% female) | 58 (22.1%) | 214 (30.0%) | .018 |
| n (% male) | 204 (22.1%) | 498 (70.0%) | |
| Race | |||
| n (% white) | 136 (51.9%) | 531 (66.7%) | <.001 |
| n (% non-white) | 126 (70.6%) | 181 (19.9%) | |
| Marital status | |||
| n (% not married) | 230 (88.0%) | 627 (88.0%) | .995 |
| Employment | |||
| n (% unemployed) | 220 (84.0%) | 594 (84.0%) | .986 |
| Treatment | |||
| Pharmacologic treatment—n (%) | |||
| Antipsychotic | |||
| First generation | 49 (22.0%) | 129 (20.0%) | .697 |
| Second generation | 149 (66.0%) | 485 (75.0%) | .007 |
| First/second | 12 (6.0%) | 45 (8.0%) | .230 |
| Antidepressant | 84 (37.0%) | 247 (38.0%) | .791 |
| Antiepileptic | 34 (15.0%) | 139 (22.0%) | .041 |
| Other medications | 96 (37.0%) | 265 (37.0%) | .986 |
| No medication | 54 (21.0%) | 87 (12.0%) | .001 |
| CPZa | |||
| n (daily dose) | 323.91 (338.31) | 367.63 (384.34) | .170 |
| Treatment/services received | |||
| n (% outpatient) | 262 (100.0%) | 712 (100.0%) | — |
| Psychopathology | |||
| PANSSb | |||
| Total | 76.22 (17.05) | 75.06 (17.43) | .354 |
| General symptomatology | 37.32 (9.14) | 36.88 (9.22) | .511 |
| Negative | 19.68 (6.19) | 20.01 (6.25) | .453 |
| Positive | 19.22 (5.58) | 18.16 (5.59) | .009 |
| Duration of illness | |||
| Duration (average years of illness) | 15.15 (10.35) | 16.35 (10.88) | .133 |
| Duration | |||
| n (% illness <5 years) | 52 (21.0%) | 118 (17.0%) | .299 |
| Drug abuse or dependence | |||
| Illness severity and drug-use severity | |||
| CGIc | |||
| Global illness severity | 4.08 (0.88) | 3.92 (0.91) | .013 |
| DUSd | |||
| Drug-use severity | 1.89 (0.91) | 1.15 (0.48) | <.001 |
| Drug abuse or dependence | |||
| SCIDe | |||
| n (% drug abuse/dependence diagnosis) | 83 (32.0%) | 28 (4.0%) | <.001 |
| Prognostic indicator | |||
| Childhood Adversity Scoref | 2.27 (2.04) | 1.46 (1.69) | <.001 |
aCPZ, chlorpromazine equivalent dose; CPZ daily dose equivalents were computed based on prescribed typical and/or atypical antipsychotic medications.
bPANSS, Positive and Negative Syndrome Scale74; assessed the presence of psychopathology in the sample.
cCGI, Clinical Global Impression75; global score totals were used as an indicator of illness severity.
dDUS, Drug Use Scale; clinician-rated measure that assessed for drug-use severity; higher DUS scores indicate greater degrees of drug severity.
eSCID, Structured Clinical Interview60 for DSM-IV; assessed the degree of functional impairment associated with 5-year drug-use history.
fChildhood Adversity Score = summed composite of prognostic indicator variables (prognostic variables considered childhood behavioral problems prior to age 15); higher scores indicate worse prognosis.
Measures
Neurocognition.
Aspects of neurocognitive function were assessed by 5 cognitive domains,2,58 which are consistent with the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery.2,61,62 A total of 24 individual scores from 11 neurocognitive tests were grouped by 5 composite indexes scored on a z metric (eg, composite means were converted to 0; standard deviations were converted to 1), which included verbal memory, processing speed, vigilance, working memory and reasoning.2 An overall composite index comprised the standardized average of the 5 composite scores.2,58 The reasoning composite was based on measures from the 64-card computerized version of the Wisconsin Card Sorting Test63 and the Wechsler Intelligence Scale Mazes.64 The working memory composite was based on computerized tests of visuospatial memory and letter number sequencing.65 The processing speed composite was based on the Grooved Pegboard,66 the Wechsler Adult Intelligence Scale Revised Digit Symbol Test,67 and verbal fluency measures.68 The verbal memory composite was based on scores from the Hopkins Verbal Learning Test,69 and the vigilance composite was based on the d′ averages from the Continuous Performance Test.70
Drug-use Assays.
Cannabis, cocaine, and methamphetamine were assayed by RIA; a laboratory procedure evaluating drug metabolites that deposit onto growing hair shafts, where each 0.5 inch segment provides a 30-day surveillance window for detecting use.71 Hair specimens of 1.5 inches were procured, which detected drugs ingested by patients for the 90 days preceding RIA procedures. Positive RIA was confirmed by gas chromatography/mass spectrometry, which has detected drugs of abuse in human hair with high degrees of certainty.72,73Individuals who could not provide hair specimens of 1.5 inches were excluded, and positive RIA was defined as (<3 SD) above the mean of a comparison sample of drug-free individuals.57
Psychopathology and Illness Severity.
Psychopathology was measured by the Positive and Negative Syndrome Scale (PANSS);74 illness severity was assessed by the clinician-rated Clinical Global Impression (CGI-S).75 Childhood adversity scores, as a premorbid prognostic indicator of severity, were measured by the clinician-rated SCID for DSM-IV.60 Childhood adversity scores comprised 8 items; participants were asked about the problem behaviors and clinical symptoms (eg, childhood conduct disorder; physical and/or physical abuse) that were present before the age of 15, and endorsed (0 = “no”; 1 = “yes”) whether problems were present at this time. Psychopathology and illness severity were assessed by interviews with participants; higher PANSS scores indicate greater symptomatology; higher CGI-S scores indicate greater illness severity (1= normal, not ill; 7 = among the most severely ill). Deficit schizophrenia syndrome, based on the presence of high negative symptoms and an absence of dysphoria,76–79 was examined by the Proxy for Deficit Syndrome (PDS);77 Schedule for Deficit Syndrome80 was not available. Patients were assigned scores based on PANSS items: PDS = blunted affect − (guilt + anxiety + depression + hostility); cutoff points,76 distinguished deficit (−17 to −5) and nondeficit (−4–1) patients. The PANSS has been widely used in studies of psychosis and has demonstrated good inter-rater reliability for assessments of psychopathology across diverse patient groups.81 The CGI-S has been used in studies of treatment efficacy in schizophrenia82,83 and is a valid and reliable instrument for evaluating the severity of schizophrenia illness and treatment response.84
Pharmacologic Treatment.
Information on current (pre randomized) pharmacologic treatment was collected across 6 separate categories, including first-generation antipsychotics, (2) second-generation antipsychotics, (3) first- and second-generation antipsychotics, (4) antidepressants, (5) anxiolytics, and (6) antiepileptics. Patients not endorsing a pharmacologic treatment were coded as receiving no medications; antipsychotic dosages were converted to chlorpromazine equivalents.85,86
Procedures
The 974 participants were assessed by research staff trained in administering the aforementioned clinical and neuropsychological measures; RIA results were used to confirm the drug use status of those who participated in the neuropsychological testing procedures. Research staff received extensive training in terms of the procedures for each test in the neurocognitive battery.2 Neurocognitive test scores were audited by a review board, and all research staff were required to complete 5 consecutive testing sessions without errors before administering these tests to participants.2 Baseline appointments were scheduled within 21 days of screening appointments. Most participants (90.9%; n = 886) completed all neuropsychological tests (processing speed, working memory, reasoning, and verbal memory) at their baseline appointment, and a small proportion did not complete the tests (9.0%; n = 88) of vigilance. Medication dosages were converted to chlorpromazine equivalents for (89.2%; n = 869) the majority of the sample, and few patients reported receiving no medications (14.4%; n = 141). CATIE was approved and reviewed annually by local Institutional Review Boards, and all patients provided written informed consent prior to enrollment and participating in this research.
Results
As can be seen in table 1, many participants who completed neuropsychological tests screened positive for drug use by RIA, with 262 (27.0%) of the 974 testing positive for cannabis, cocaine, or methamphetamine. Patients with positive RIA tended to be of younger ages, presented with higher degrees of positive symptomatology and more serious degrees of illness severity. Additionally, patients with positive RIA exhibited significantly worse premorbid prognostic indicators (childhood behavioral problems) compared with those who were screened negative for drug use (see table 1).
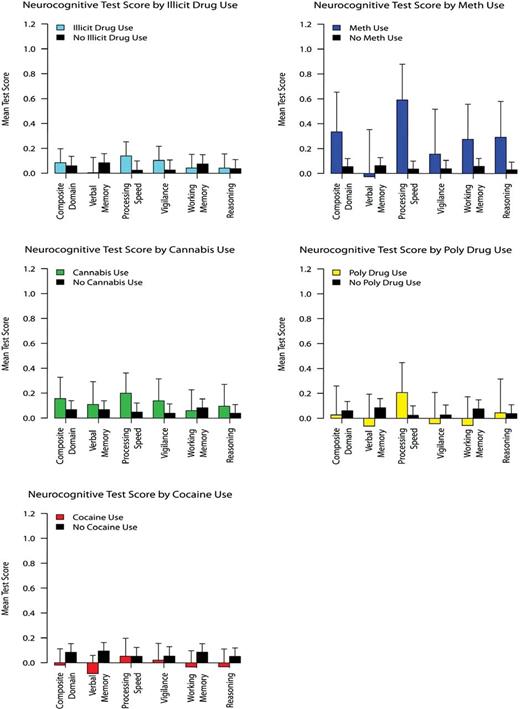
Neurocognitive differences between the groups of patients with positive and negative RIA for drug use were examined by independent sample t tests; Hochberg’s correction87 was used to adjust for multiple inference testing within each category of drug use. No significant differences were observed across the neurocognitive domains that were assessed between the patient groups with positive and negative RIA after adjusting for multiple inference testing, except that patients with positive RIA for methamphetamine demonstrated increased processing speed compared with patients with negative RIA for methamphetamine (see table 2; figure 1). The possible confounding effects of negative symptoms on the relationships (or lack thereof) between positive RIA and cognition were considered as potentially accounting for these findings; however, while negative symptoms were associated with poorer cognition (range of r = −.27 to −.16), they were not associated with positive RIA for drug use (all P > .369), and as such negative symptoms did not appear to confound these results. Confirmatory mixed-effects models adjusting for demographic and clinical (including symptom and illness duration) differences between those with positive and negative RIA indicated the same pattern of results (all P > .108), and only those with positive RIA for methamphetamine demonstrated greater processing speed (P = .035). The presence of deficit syndrome on the associations between positive RIA and cognition was considered as another potentially confounding factor,77 and the same pattern of results was observed in nondeficit schizophrenia patients, where only those with positive RIA for methamphetamine had demonstrated greater processing speed (see Supplementary Data).
Neurocognitive Test Performance Between Patients With Schizophrenia With Negative and Positive RIA Test Results for Drug Use (n = 974)
| Variable . | Drug Use (+) RIA . | No Drug Use (−) RIA . | Between-Group Difference . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | M . | SD . | n . | M . | SD . | t . | df . | P . | Pa . | |
| . | Any drug useb . | |||||||||
| Composite domainc | 262 | .0852 | .9239 | 712 | .0598 | 1.0294 | −0.350 | 972 | .725 | .949 |
| Verbal memory | 262 | .0041 | 1.0127 | 712 | .0847 | .9945 | 1.116 | 972 | .264 | .949 |
| Processing speed | 262 | .1388 | .9393 | 712 | .0242 | 1.0368 | −1.567 | 972 | .117 | .703 |
| Vigilanced | 244 | .1032 | .9105 | 642 | .0265 | 1.0393 | −1.014 | 844 | .310 | .949 |
| Working memory | 262 | .0428 | .9406 | 712 | .0760 | 1.0018 | 0.469 | 972 | .638 | .949 |
| Reasoning | 262 | .0421 | .9450 | 712 | .0377 | .9831 | −0.062 | 972 | .949 | .949 |
| Cannabis use | ||||||||||
| Composite domain | 128 | .1553 | .9917 | 736 | .0669 | 1.0093 | −0.917 | 862 | .359 | .803 |
| Verbal memory | 128 | .1090 | 1.0535 | 736 | .0669 | .9961 | −0.438 | 862 | .661 | .803 |
| Processing speed | 128 | .1196 | .9394 | 736 | .0473 | 1.0201 | −1.577 | 862 | .115 | .690 |
| Vigilance | 119 | .1390 | .9788 | 669 | .0380 | 1.0074 | −1.012 | 786 | .311 | .803 |
| Working memory | 128 | .0592 | .9739 | 736 | .0827 | .9893 | 0.248 | 862 | .803 | .803 |
| Reasoning | 128 | .0955 | 1.0171 | 736 | .0384 | .9755 | −0.608 | 862 | .543 | .803 |
| Cocaine use | ||||||||||
| Composite domain | 162 | −.0197 | .8527 | 809 | .0831 | 1.0299 | 1.191 | 969 | .233 | .934 |
| Verbal memory | 162 | −.0869 | .9526 | 809 | .0935 | 1.0083 | 2.098 | 969 | .036 | .216 |
| Processing speed | 162 | .0531 | .9399 | 809 | .0527 | 1.0271 | −0.003 | 969 | .996 | .996 |
| Vigilance | 149 | −.0215 | .8389 | 734 | .0547 | 1.0381 | 0.366 | 881 | .714 | .996 |
| Working memory | 162 | −.0337 | .8498 | 809 | .0850 | .9994 | 1.414 | 969 | .157 | .788 |
| Reasoning | 162 | −.0317 | .9306 | 809 | .0527 | .9812 | 1.009 | 969 | .312 | .938 |
| Methamphetamine use | ||||||||||
| Composite domain | 28 | .3352 | .8592 | 943 | .0552 | 1.0034 | −1.460 | 969 | .144 | .643 |
| Verbal memory | 28 | −.0251 | 1.0232 | 943 | .0636 | .9986 | 0.463 | 969 | .643 | .643 |
| Processing speed | 28 | .5917 | .7717 | 943 | .0362 | 1.0138 | −2.874 | 969 | .004 | .024 |
| Vigilance | 26 | .1558 | .9380 | 857 | .0387 | 1.0018 | −0.588 | 881 | .556 | .643 |
| Working memory | 28 | .2747 | .7633 | 943 | .0582 | .9798 | −1.158 | 969 | .246 | .643 |
| Reasoning | 28 | .2923 | .7767 | 943 | .0307 | .9782 | −1.140 | 969 | .161 | .643 |
| Poly drug usee | ||||||||||
| Composite domain | 54 | .0265 | .8752 | 712 | .0598 | 1.0294 | 0.231 | 764 | .816 | .963 |
| Verbal memory | 54 | −.0632 | .9620 | 712 | .0847 | 0.9945 | 1.056 | 764 | .291 | .963 |
| Processing speed | 54 | .2067 | .9004 | 712 | .0242 | 1.0368 | −1.257 | 764 | .208 | .963 |
| Vigilance | 48 | −.0427 | .8857 | 642 | .0265 | 1.0393 | 0.449 | 688 | .652 | .963 |
| Working memory | 54 | −.0572 | .8632 | 712 | .0760 | 1.0018 | 0.950 | 764 | .342 | .963 |
| Reasoning | 54 | .0440 | 1.0209 | 712 | .0377 | .9831 | −0.045 | 764 | .963 | .963 |
| Variable . | Drug Use (+) RIA . | No Drug Use (−) RIA . | Between-Group Difference . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | M . | SD . | n . | M . | SD . | t . | df . | P . | Pa . | |
| . | Any drug useb . | |||||||||
| Composite domainc | 262 | .0852 | .9239 | 712 | .0598 | 1.0294 | −0.350 | 972 | .725 | .949 |
| Verbal memory | 262 | .0041 | 1.0127 | 712 | .0847 | .9945 | 1.116 | 972 | .264 | .949 |
| Processing speed | 262 | .1388 | .9393 | 712 | .0242 | 1.0368 | −1.567 | 972 | .117 | .703 |
| Vigilanced | 244 | .1032 | .9105 | 642 | .0265 | 1.0393 | −1.014 | 844 | .310 | .949 |
| Working memory | 262 | .0428 | .9406 | 712 | .0760 | 1.0018 | 0.469 | 972 | .638 | .949 |
| Reasoning | 262 | .0421 | .9450 | 712 | .0377 | .9831 | −0.062 | 972 | .949 | .949 |
| Cannabis use | ||||||||||
| Composite domain | 128 | .1553 | .9917 | 736 | .0669 | 1.0093 | −0.917 | 862 | .359 | .803 |
| Verbal memory | 128 | .1090 | 1.0535 | 736 | .0669 | .9961 | −0.438 | 862 | .661 | .803 |
| Processing speed | 128 | .1196 | .9394 | 736 | .0473 | 1.0201 | −1.577 | 862 | .115 | .690 |
| Vigilance | 119 | .1390 | .9788 | 669 | .0380 | 1.0074 | −1.012 | 786 | .311 | .803 |
| Working memory | 128 | .0592 | .9739 | 736 | .0827 | .9893 | 0.248 | 862 | .803 | .803 |
| Reasoning | 128 | .0955 | 1.0171 | 736 | .0384 | .9755 | −0.608 | 862 | .543 | .803 |
| Cocaine use | ||||||||||
| Composite domain | 162 | −.0197 | .8527 | 809 | .0831 | 1.0299 | 1.191 | 969 | .233 | .934 |
| Verbal memory | 162 | −.0869 | .9526 | 809 | .0935 | 1.0083 | 2.098 | 969 | .036 | .216 |
| Processing speed | 162 | .0531 | .9399 | 809 | .0527 | 1.0271 | −0.003 | 969 | .996 | .996 |
| Vigilance | 149 | −.0215 | .8389 | 734 | .0547 | 1.0381 | 0.366 | 881 | .714 | .996 |
| Working memory | 162 | −.0337 | .8498 | 809 | .0850 | .9994 | 1.414 | 969 | .157 | .788 |
| Reasoning | 162 | −.0317 | .9306 | 809 | .0527 | .9812 | 1.009 | 969 | .312 | .938 |
| Methamphetamine use | ||||||||||
| Composite domain | 28 | .3352 | .8592 | 943 | .0552 | 1.0034 | −1.460 | 969 | .144 | .643 |
| Verbal memory | 28 | −.0251 | 1.0232 | 943 | .0636 | .9986 | 0.463 | 969 | .643 | .643 |
| Processing speed | 28 | .5917 | .7717 | 943 | .0362 | 1.0138 | −2.874 | 969 | .004 | .024 |
| Vigilance | 26 | .1558 | .9380 | 857 | .0387 | 1.0018 | −0.588 | 881 | .556 | .643 |
| Working memory | 28 | .2747 | .7633 | 943 | .0582 | .9798 | −1.158 | 969 | .246 | .643 |
| Reasoning | 28 | .2923 | .7767 | 943 | .0307 | .9782 | −1.140 | 969 | .161 | .643 |
| Poly drug usee | ||||||||||
| Composite domain | 54 | .0265 | .8752 | 712 | .0598 | 1.0294 | 0.231 | 764 | .816 | .963 |
| Verbal memory | 54 | −.0632 | .9620 | 712 | .0847 | 0.9945 | 1.056 | 764 | .291 | .963 |
| Processing speed | 54 | .2067 | .9004 | 712 | .0242 | 1.0368 | −1.257 | 764 | .208 | .963 |
| Vigilance | 48 | −.0427 | .8857 | 642 | .0265 | 1.0393 | 0.449 | 688 | .652 | .963 |
| Working memory | 54 | −.0572 | .8632 | 712 | .0760 | 1.0018 | 0.950 | 764 | .342 | .963 |
| Reasoning | 54 | .0440 | 1.0209 | 712 | .0377 | .9831 | −0.045 | 764 | .963 | .963 |
aP values were adjusted using Hochberg’s (1988) correction for multiple inference testing within each category of drug use.
bAny drug use is the use of cocaine, cannabis, or methamphetamine by radioimmunoassay of (RIA) hair results.
cComposite Domain is the average for neurocognition subdomains (verbal memory, processing speed, vigilance, working memory, and reasoning).
dVigilance tests were not completed by (n = 88) patients.
ePoly drug use is a dichotomous variable consisting of 2 patient groups: the first group included patients (n = 54) with more than 1 positive RIA test; the second group (n = 712) included patients with negative RIA.
Neurocognitive Test Performance Between Patients With Schizophrenia With Negative and Positive RIA Test Results for Drug Use (n = 974)
| Variable . | Drug Use (+) RIA . | No Drug Use (−) RIA . | Between-Group Difference . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | M . | SD . | n . | M . | SD . | t . | df . | P . | Pa . | |
| . | Any drug useb . | |||||||||
| Composite domainc | 262 | .0852 | .9239 | 712 | .0598 | 1.0294 | −0.350 | 972 | .725 | .949 |
| Verbal memory | 262 | .0041 | 1.0127 | 712 | .0847 | .9945 | 1.116 | 972 | .264 | .949 |
| Processing speed | 262 | .1388 | .9393 | 712 | .0242 | 1.0368 | −1.567 | 972 | .117 | .703 |
| Vigilanced | 244 | .1032 | .9105 | 642 | .0265 | 1.0393 | −1.014 | 844 | .310 | .949 |
| Working memory | 262 | .0428 | .9406 | 712 | .0760 | 1.0018 | 0.469 | 972 | .638 | .949 |
| Reasoning | 262 | .0421 | .9450 | 712 | .0377 | .9831 | −0.062 | 972 | .949 | .949 |
| Cannabis use | ||||||||||
| Composite domain | 128 | .1553 | .9917 | 736 | .0669 | 1.0093 | −0.917 | 862 | .359 | .803 |
| Verbal memory | 128 | .1090 | 1.0535 | 736 | .0669 | .9961 | −0.438 | 862 | .661 | .803 |
| Processing speed | 128 | .1196 | .9394 | 736 | .0473 | 1.0201 | −1.577 | 862 | .115 | .690 |
| Vigilance | 119 | .1390 | .9788 | 669 | .0380 | 1.0074 | −1.012 | 786 | .311 | .803 |
| Working memory | 128 | .0592 | .9739 | 736 | .0827 | .9893 | 0.248 | 862 | .803 | .803 |
| Reasoning | 128 | .0955 | 1.0171 | 736 | .0384 | .9755 | −0.608 | 862 | .543 | .803 |
| Cocaine use | ||||||||||
| Composite domain | 162 | −.0197 | .8527 | 809 | .0831 | 1.0299 | 1.191 | 969 | .233 | .934 |
| Verbal memory | 162 | −.0869 | .9526 | 809 | .0935 | 1.0083 | 2.098 | 969 | .036 | .216 |
| Processing speed | 162 | .0531 | .9399 | 809 | .0527 | 1.0271 | −0.003 | 969 | .996 | .996 |
| Vigilance | 149 | −.0215 | .8389 | 734 | .0547 | 1.0381 | 0.366 | 881 | .714 | .996 |
| Working memory | 162 | −.0337 | .8498 | 809 | .0850 | .9994 | 1.414 | 969 | .157 | .788 |
| Reasoning | 162 | −.0317 | .9306 | 809 | .0527 | .9812 | 1.009 | 969 | .312 | .938 |
| Methamphetamine use | ||||||||||
| Composite domain | 28 | .3352 | .8592 | 943 | .0552 | 1.0034 | −1.460 | 969 | .144 | .643 |
| Verbal memory | 28 | −.0251 | 1.0232 | 943 | .0636 | .9986 | 0.463 | 969 | .643 | .643 |
| Processing speed | 28 | .5917 | .7717 | 943 | .0362 | 1.0138 | −2.874 | 969 | .004 | .024 |
| Vigilance | 26 | .1558 | .9380 | 857 | .0387 | 1.0018 | −0.588 | 881 | .556 | .643 |
| Working memory | 28 | .2747 | .7633 | 943 | .0582 | .9798 | −1.158 | 969 | .246 | .643 |
| Reasoning | 28 | .2923 | .7767 | 943 | .0307 | .9782 | −1.140 | 969 | .161 | .643 |
| Poly drug usee | ||||||||||
| Composite domain | 54 | .0265 | .8752 | 712 | .0598 | 1.0294 | 0.231 | 764 | .816 | .963 |
| Verbal memory | 54 | −.0632 | .9620 | 712 | .0847 | 0.9945 | 1.056 | 764 | .291 | .963 |
| Processing speed | 54 | .2067 | .9004 | 712 | .0242 | 1.0368 | −1.257 | 764 | .208 | .963 |
| Vigilance | 48 | −.0427 | .8857 | 642 | .0265 | 1.0393 | 0.449 | 688 | .652 | .963 |
| Working memory | 54 | −.0572 | .8632 | 712 | .0760 | 1.0018 | 0.950 | 764 | .342 | .963 |
| Reasoning | 54 | .0440 | 1.0209 | 712 | .0377 | .9831 | −0.045 | 764 | .963 | .963 |
| Variable . | Drug Use (+) RIA . | No Drug Use (−) RIA . | Between-Group Difference . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | M . | SD . | n . | M . | SD . | t . | df . | P . | Pa . | |
| . | Any drug useb . | |||||||||
| Composite domainc | 262 | .0852 | .9239 | 712 | .0598 | 1.0294 | −0.350 | 972 | .725 | .949 |
| Verbal memory | 262 | .0041 | 1.0127 | 712 | .0847 | .9945 | 1.116 | 972 | .264 | .949 |
| Processing speed | 262 | .1388 | .9393 | 712 | .0242 | 1.0368 | −1.567 | 972 | .117 | .703 |
| Vigilanced | 244 | .1032 | .9105 | 642 | .0265 | 1.0393 | −1.014 | 844 | .310 | .949 |
| Working memory | 262 | .0428 | .9406 | 712 | .0760 | 1.0018 | 0.469 | 972 | .638 | .949 |
| Reasoning | 262 | .0421 | .9450 | 712 | .0377 | .9831 | −0.062 | 972 | .949 | .949 |
| Cannabis use | ||||||||||
| Composite domain | 128 | .1553 | .9917 | 736 | .0669 | 1.0093 | −0.917 | 862 | .359 | .803 |
| Verbal memory | 128 | .1090 | 1.0535 | 736 | .0669 | .9961 | −0.438 | 862 | .661 | .803 |
| Processing speed | 128 | .1196 | .9394 | 736 | .0473 | 1.0201 | −1.577 | 862 | .115 | .690 |
| Vigilance | 119 | .1390 | .9788 | 669 | .0380 | 1.0074 | −1.012 | 786 | .311 | .803 |
| Working memory | 128 | .0592 | .9739 | 736 | .0827 | .9893 | 0.248 | 862 | .803 | .803 |
| Reasoning | 128 | .0955 | 1.0171 | 736 | .0384 | .9755 | −0.608 | 862 | .543 | .803 |
| Cocaine use | ||||||||||
| Composite domain | 162 | −.0197 | .8527 | 809 | .0831 | 1.0299 | 1.191 | 969 | .233 | .934 |
| Verbal memory | 162 | −.0869 | .9526 | 809 | .0935 | 1.0083 | 2.098 | 969 | .036 | .216 |
| Processing speed | 162 | .0531 | .9399 | 809 | .0527 | 1.0271 | −0.003 | 969 | .996 | .996 |
| Vigilance | 149 | −.0215 | .8389 | 734 | .0547 | 1.0381 | 0.366 | 881 | .714 | .996 |
| Working memory | 162 | −.0337 | .8498 | 809 | .0850 | .9994 | 1.414 | 969 | .157 | .788 |
| Reasoning | 162 | −.0317 | .9306 | 809 | .0527 | .9812 | 1.009 | 969 | .312 | .938 |
| Methamphetamine use | ||||||||||
| Composite domain | 28 | .3352 | .8592 | 943 | .0552 | 1.0034 | −1.460 | 969 | .144 | .643 |
| Verbal memory | 28 | −.0251 | 1.0232 | 943 | .0636 | .9986 | 0.463 | 969 | .643 | .643 |
| Processing speed | 28 | .5917 | .7717 | 943 | .0362 | 1.0138 | −2.874 | 969 | .004 | .024 |
| Vigilance | 26 | .1558 | .9380 | 857 | .0387 | 1.0018 | −0.588 | 881 | .556 | .643 |
| Working memory | 28 | .2747 | .7633 | 943 | .0582 | .9798 | −1.158 | 969 | .246 | .643 |
| Reasoning | 28 | .2923 | .7767 | 943 | .0307 | .9782 | −1.140 | 969 | .161 | .643 |
| Poly drug usee | ||||||||||
| Composite domain | 54 | .0265 | .8752 | 712 | .0598 | 1.0294 | 0.231 | 764 | .816 | .963 |
| Verbal memory | 54 | −.0632 | .9620 | 712 | .0847 | 0.9945 | 1.056 | 764 | .291 | .963 |
| Processing speed | 54 | .2067 | .9004 | 712 | .0242 | 1.0368 | −1.257 | 764 | .208 | .963 |
| Vigilance | 48 | −.0427 | .8857 | 642 | .0265 | 1.0393 | 0.449 | 688 | .652 | .963 |
| Working memory | 54 | −.0572 | .8632 | 712 | .0760 | 1.0018 | 0.950 | 764 | .342 | .963 |
| Reasoning | 54 | .0440 | 1.0209 | 712 | .0377 | .9831 | −0.045 | 764 | .963 | .963 |
aP values were adjusted using Hochberg’s (1988) correction for multiple inference testing within each category of drug use.
bAny drug use is the use of cocaine, cannabis, or methamphetamine by radioimmunoassay of (RIA) hair results.
cComposite Domain is the average for neurocognition subdomains (verbal memory, processing speed, vigilance, working memory, and reasoning).
dVigilance tests were not completed by (n = 88) patients.
ePoly drug use is a dichotomous variable consisting of 2 patient groups: the first group included patients (n = 54) with more than 1 positive RIA test; the second group (n = 712) included patients with negative RIA.
Neurocognitive test performance between schizophrenia patients with negative and positive radioimmunoassay results for any drug, cannabis, cocaine, methamphetamine, and poly drug use.
After finding no large difference in neurocognitive function between patients with negative laboratory drug test results and patients with positive laboratory drug test results, an exhaustive series of moderator analyses employing linear mixed-effects models were conducted to explore subsamples for which positive RIA for drug use may have a greater impact on cognitive function. Moderator models were carried out using R version 2.15.0,88,89 and analyses were proceeded by examining potential differences in the association between RIA results and cognitive function by gender, illness duration, drug-use severity, drug abuse and/or dependence, and symptom severity. Results revealed no significant interactions between RIA and gender (uncorrected P > .342), illness duration (uncorrected P > .504), drug-use severity (uncorrected P > .267), drug abuse and/or dependence (uncorrected P > .599) or symptom severity (uncorrected P > .075) on cognitive function. Potential differences in cognitive function between patients with positive RIA and patients with negative RIA were then examined by psychopathology and medication dosages. At low dosages of medication, patients with positive RIA for cocaine demonstrated decreased processing speed (M = −0.063, SE = 0.119) compared with patients with negative RIA for cocaine (M = 0.132, SE = 0.055, F (1, 730) = 6.935, P = .008). For any other drugs with low psychopathology, patients with positive RIA (M = 0.022, SE = 0.820) exhibited decreased working memory function compared with patients with negative RIA (M = 0.233, SE = 0.051, F(1, 970) = 7.407, P = .006). Potential differences in the association between RIA and neurocognition by the amount of time that lapsed between screening and baseline appointments (approximately 21 days) were also examined, and results showed no significant interactions between the amount of time that had lapsed between these appointments (uncorrected P > .226) on cognitive function. The consistency of these findings were maintained for patients who completed neuropsychological testing within 10 days of the RIA procedures (see Supplementary Data), which indicates that those who were drug tested more closely to their neurocognitive-testing session did not show a substantive difference in cognitive function to those screened for drugs farther away from their neurocognitive testing date. Further, sensitivity analyses were conducted for patients with negative RIA who met criteria for drug abuse or dependence, and similar results were observed even after removing those with drug-use comorbidity from the negative RIA group (see Supplementary Data). Lastly, potential differences in the association between RIA and cognitive function by premorbid prognostic indicators (childhood behavioral problems) were examined. Again, results revealed no significant interactions between RIA and these indicators on cognition. In fact, even among patients with the least childhood problems, there were no significant differences in overall cognitive function between patients with positive RIA (M = 0.074, SE = 0.048) and patients with negative RIA (M = 0.040; SE = 0.048) for any drug use. Results of mixed-effects models should be interpreted with caution because no adjustments were made for multiple inference testing. The overall results from this series of moderator analyses do not suggest drug use has a positive effect on cognition, and these findings were largely consistent across most of the treatment, clinical and demographic characteristics observed for this sample of patients with schizophrenia.
Discussion
Neurocognition is one of the strongest long-term predictors of functional outcome and recovery in schizophrenia,14 with cognitive deficits accounting for much of the psychosocial disability that is exacted by the illness.2 Using drugs portends a debilitating course of illness for individuals with schizophrenia across a number of clinical and functional domains.5,9,18–24 Worsened outcomes on cognitive function would be expected for adults with schizophrenia who use drugs, because substance use is known to exacerbate psychotic symptomatology and undermine treatment effectiveness.18,19,21 Cannabis has adverse effects on brain function,90,91 and cocaine has negative effects on learning and memory.92,95Considerable research has yielded results that seem paradoxical to these assumptions and consistently associate drug use, particularly cannabis use, with improved cognitive function in schizophrenia.25–36 This study attempted to replicate such findings in a large sample of adults with schizophrenia that had their drug-use status determined by RIA and evaluated the neurocognitive test performance of patients with positive and negative results.
Contrary to our hypotheses and the findings of prior research on neurocognition and drug use in schizophrenia, our results revealed patients who tested positive for drug use did not exhibit better cognitive function compared with those with negative results. Quite unexpectedly, our findings showed one effect met conventional thresholds for statistical significance after adjusting for multiple inference testing, where patients with positive RIA for methamphetamine demonstrated increased processing speed compared with those with negative RIA for methamphetamine. Patterns of nonsignificant findings were maintained in analyses of nondeficit schizophrenia patients, and no positive effects of cannabis use on cognitive function were observed in any analysis. These patterns of results were largely consistent for men and women, patients in mid- and more chronic phases of illness, and for those treated with lower vs higher doses of antipsychotic medication. At low medication dosages, moderator analyses revealed patients with positive RIA for cocaine demonstrated decreased processing speed compared with those with negative RIA for cocaine. And for any other drugs with low psychopathology, those with positive RIA demonstrated decreased working memory function compared with those with negative RIA. It is not likely that these significant moderator results would have survived correction for multiple inference testing. Taken together, our findings do not suggest a positive effect of drug use, cannabis or otherwise, on cognitive function for this sample.
This research has several implications for investigations of cognitive function and drug use in schizophrenia. First-episode studies have reported improved cognitive function among those who use drugs,13,39 but we did not observe this to be the case for the patients with schizophrenia who were within 5 years of illness (n = 52; 21.0%) and tested positive for drug use. Prior reports suggest that first-episode studies include individuals with cannabis-induced psychosis,13 and we selected patients with a schizophrenia diagnosis, based on the SCID56 for DSM-IV, and confirmed drug-use status by RIA, which may partially account for the our contrary results. Of the investigations reporting improved cognition in patients using drugs, both Stirling et al33 and McCleery et al34 studied first-episode patients, whereas Potvin et al32 and Schnell et al35 included patients with schizoaffective disorder, and these subsamples were excluded from this research. Potvin et al32 and McCleery et al34 studied the effects of multiple drugs on cognitive performance whereas both Schnell et al35 and Stirling et al33 limited their investigations to cannabis, and these studies used diagnostic criteria (current/former substance use disorders) rather than laboratory testing as the basis for selecting their drug-use sample. Løberg et al27 (cannabis) and Thoma et al29 (multiple drugs) both reported improved cognition in schizophrenia patients using drugs, yet made use of modest samples (drug-use group range: n = 13–27, respectively), and indexed their drug-use sample by diagnostic criteria (current/former substance use disorders) rather than laboratory results, which also may in part explain our contrary findings. We did not find that negative symptoms were associated with cognition or with drug use, and thus our results also differ from prior research that has shown improved cognition and less negative symptoms in those using drugs.31,55,56 These investigations indicate that the absence of negative symptoms coupled with improved cognition allows for schizophrenia patients to procure drugs and maintain their use,31,55,56 but this rationale does not explain our results, which could be due to sample differences or to our use of laboratory tests. Studies documenting improved neurocognition in cannabis users with schizophrenia primarily rely on self-rated measures of use, and a recent meta-analysis concluding improved cognitive outcomes in cannabis using patients was largely driven by studies including those with lifetime histories of use rather than current or recent use.39 Due to the potential for considerable underreported drug use by self-report measures in schizophrenia,95,96 it may be difficult to distinguish drug-using groups from drug nonusing groups on cognitive outcomes without employing laboratory tests to confirm use, and studies exclusively relying on self-rated measures may be at risk for reporting biased results. Our results suggest cognitive improvements in drug using patients with schizophrenia may be better explained by study-specific selection factors (eg, cannabis-induced psychosis) biased reporting (eg, modest samples), how drug use is indexed (eg, abuse/dependence vs current use), or other differences, rather than actual increases in neurocognitive test performance in patients who use drugs. Further, neurocognition is one of the strongest predictors of recovery in schizophrenia14 and our results showed that comparative degrees of cognitive deficits were discernible in the sample, indicating patients with negative and positive results for drug use could both benefit from the novel psychosocial therapeutics of cognitive remediation,97 which seeks to improve the social and cognitive functioning of individuals who are affected by the condition. A randomized-controlled trial of Cognitive Enhancement Therapy98 with substance misusing patients with schizophrenia is currently underway, and the results of this trial are eagerly awaited.
Our findings should be interpreted within the context of several limitations. The use of RIA for detecting drug use restricted our ability to examine drugs other than cannabis, cocaine, and methamphetamine, because other drugs (opiates, phencyclidine and stimulants) were excluded due to low base rates and other problems with detection by these methods. RIA detects drugs not found by urine screens,71–73,95,99–101 is (at times) more reliable and valid than self-reports,95 yet studies of neurocognition in schizophrenia rarely use RIA results as the basis for determining drug-use samples.102 Regardless, our inability to detect alcohol levels by RIA is an admitted limitation and calls for the future research that is conducted in this area to incorporate more extensive measures into the assessment to estimate the impact of alcohol use on cognition in schizophrenia. Despite the limitations of RIA, we believe that this study adds important information to the field as well as the existing research on substance use and cognition in schizophrenia. Further, drug-use history, status of current use, and withdrawal are factors that could profoundly impact neurocognition; however, these specific stages of addiction recovery could not be estimated, and the findings reported should be interpreted accordingly. Neurocognitive data were complete for 90.9% (n = 886) of the sample, and missing data were attributable to vigilance tests, which were either incomplete or not collected at baseline. Although common cognitive tests were used, neurocognitive differences may present between patients using drugs and those not using drugs on tests that were not used in this study, and it will be important for future investigations to incorporate more extensive measures into the assessment. Chlorpromazine equivalent dosages were computed for 89.2% (n = 869) of the sample, which limited our examination of the moderating effects of antipsychotic dosage on the relationships between drug use and cognition. Patients with refractory illness were excluded from CATIE because it was thought that their severe illness could impede detection of the differential effectiveness that would be apparent in treatment-responsive patients.55 This exclusion may have precluded drug-using patients from enrollment, which could bias the results reported here, and thus our findings should be interpreted with caution. Our use of dichotomously coded RIA variables and cross-sectional design limits our ability to make causal determinations about the impact drug use has on neurocognition, and longitudinal investigations will need to study the effects of drug use on cognition to further explore these relationships.
While more research is needed to replicate our findings across a greater variety of drugs of abuse, our results showed patients with schizophrenia who use drugs, as determined by positive RIA, do not demonstrate better cognitive function compared with those who do not use drugs, as determined by negative RIA. Our findings are contrary to major research on neurocognition and drug use in schizophrenia, yet were largely consistent across sample characteristics, indicating drug use does not have positive effects on cognition in this population.
Funding
National Institute of Health grants (DA-30763, RR-24154); NIH Contract (N01MH90001 to the University of North Carolina at Chapel Hill).
Acknowledgments
All authors have declared that there are no conflicts of interest in relation to the subject of this study. The data used in the preparation of this article were obtained from the limited access data sets distributed from the National Institute of Health (NIH) supported Clinical Antipsychotic Trials of Intervention Effectiveness in Schizophrenia (CATIE-sz) study. This was a multisite, clinical trial of persons with schizophrenia comparing the effectiveness of randomly assigned medication treatment. The ClinicalTrials.gov identifier is NCT00014001. This manuscript reflects the views of the authors and may not reflect the opinions or views of the CATIE-Sz Study investigators or the NIH.
References