-
PDF
- Split View
-
Views
-
Cite
Cite
Steven M Silverstein, Joy J Choi, Kyle M Green, Kristen E Bowles-Johnson, Rajeev S Ramchandran, Schizophrenia in Translation: Why the Eye?, Schizophrenia Bulletin, Volume 48, Issue 4, July 2022, Pages 728–737, https://doi.org/10.1093/schbul/sbac050
Close - Share Icon Share
Abstract
Schizophrenia is increasingly recognized as a systemic disease, characterized by dysregulation in multiple physiological systems (eg, neural, cardiovascular, endocrine). Many of these changes are observed as early as the first psychotic episode, and in people at high risk for the disorder. Expanding the search for biomarkers of schizophrenia beyond genes, blood, and brain may allow for inexpensive, noninvasive, and objective markers of diagnosis, phenotype, treatment response, and prognosis. Several anatomic and physiologic aspects of the eye have shown promise as biomarkers of brain health in a range of neurological disorders, and of heart, kidney, endocrine, and other impairments in other medical conditions. In schizophrenia, thinning and volume loss in retinal neural layers have been observed, and are associated with illness progression, brain volume loss, and cognitive impairment. Retinal microvascular changes have also been observed. Abnormal pupil responses and corneal nerve disintegration are related to aspects of brain function and structure in schizophrenia. In addition, studying the eye can inform about emerging cardiovascular, neuroinflammatory, and metabolic diseases in people with early psychosis, and about the causes of several of the visual changes observed in the disorder. Application of the methods of oculomics, or eye-based biomarkers of non-ophthalmological pathology, to the treatment and study of schizophrenia has the potential to provide tools for patient monitoring and data-driven prediction, as well as for clarifying pathophysiology and course of illness. Given their demonstrated utility in neuropsychiatry, we recommend greater adoption of these tools for schizophrenia research and patient care.
Introduction
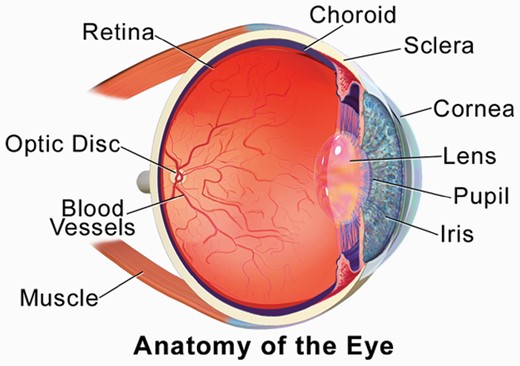
Schizophrenia is increasingly recognized as a systemic disease, characterized by dysregulation in multiple physiological systems (eg, neural, cardiovascular, immune, endocrine).1–9 Many of these changes are observed as early as the first psychotic episode, and in people at risk for the disorder.4,10,11 Expanding the search for biomarkers of schizophrenia beyond genes, blood, and brain may allow for inexpensive, noninvasive, and objective markers of diagnosis, phenotype, treatment response, and prognosis, none of which exist at present. Discovery of schizophrenia-relevant information in the eye is part of the developing field of oculomics, or identification of aspects of eye structure and function that are indicators of disease in other organs and systems.12 This field is especially well developed in the areas of cardiology,13–15 neurology,16–18 and nephrology.19,20 Much of this work is focused on density changes in the retinal neural layers, loss of retinal microvasculature and changes to blood vessel morphology, and altered retinal responses to light. However, other aspects of the eye, as they relate to brain disease, have been studied including the pupil and cornea (see figure 1). More recently, it has become clear that changes in eye physiology and morphology could serve as indicators of biological features of schizophrenia, including loss of neural tissue, neuroinflammation, cardiovascular disease, and metabolic changes. Below, we outline the status, potential, and challenges of this growing field.
Depiction of the components of the eye.Figure reproduced from Blausen.com staff. Medical gallery of Blausen Medical 2014. WikiJ Med. 2014;1(2). doi:10.15347/wjm/2014.010. ISSN 2002-4436, via a creative commons license (CC BY 3.0).
Retina
The retina and brain derive from the same tissue during development and share several key features (eg, layered architecture, neurons, neural circuitry, neurotransmitters, glial cells).21–23 There is also bidirectional communication between the structures. Retinal ganglion cell axons carry information about our visual environment to the lateral geniculate nucleus of the thalamus via the optic nerve, and retrograde transport of neurotransmitters occurs from the brain to the retina.24 In several neurodegenerative disorders changes in the retina are associated with parallel changes in the brain. For example, thinning of retinal neural layers in multiple sclerosis is associated with reduced brain volume as seen on magnetic resonance imaging (MRI), and cognitive decline.18,25–29 Retinal thinning is also associated with forms of dementia,30–37 where retinal changes are associated with gray and white matter loss on MRI, precede cognitive decline,38 and can predict disease progression.35,39–42 In the general population, retinal neural layer thickness (and thinning) can predict gray and white matter volumes and cortical connectivity as revealed by MRI,43,44 as well as concurrent,45 intermediate-38,46 and long-term47 cognitive decline. Based on this growing set of findings, the retina is often referred to as “a window to the brain.” 48
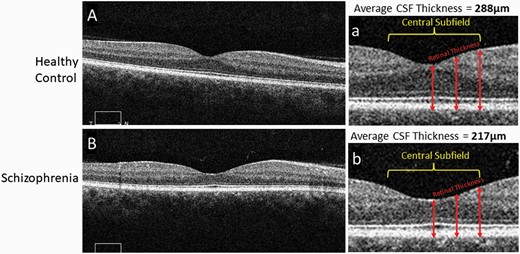
Since 2010, studies using optical coherence tomography (OCT) imaging of the retina have demonstrated thinning of retinal neural layers in schizophrenia (see figure 2). This literature consistently indicates thinning and volume loss in the macula (the retinal region responsible for central vision), with additional but less consistent evidence for thinning of the ganglion cell axonal layer in the region peripheral to the optic disc.49–53 Studies from the past five years have also identified thinning in the photoreceptor layer54 and the choroid,55 the vascular bed between the retina and the sclera. Preliminary evidence suggests that thinning of at least one retinal layer can be observed in unaffected first-degree relatives of schizophrenia patients.56,57 Taken together, OCT findings in schizophrenia suggest a neurodegenerative process occurring in the eye, and some recent evidence suggests that morphological changes in the retina in people with schizophrenia are linked to structural changes in the brain as revealed by MRI, and cognitive impairment.58 There is also a growing literature suggesting the utility of OCT for monitoring neuroinflammatory effects in central nervous system (CNS) diseases.59 While some of these findings need replication with larger samples, overall, the clinical relevance of these data is that OCT could potentially be used as a biomarker for cortical thinning or volume loss, thereby identifying a subgroup of patients at risk for more severe and progressive cortical atrophy, a group that has been characterized by greater cognitive impairment and poorer long-term course.60,61 Such patients could potentially benefit from more structured environments, neuroprotective interventions, and cognitive enhancing interventions such as cognitive training, neurofeedback, and brain stimulation.
Comparison of macular thickness, from OCT images, between a healthy control and subject with schizophrenia.Representative 6 mm OCT scans of the retina in both a healthy control (A) and subject with schizophrenia (B), along with enlarged images of the central portion of the scans (a, b) respectively. Alternating darker and lighter bands seen in the scans represent unique layers in the retina. The central subfield is a 1 mm central section of the scan. Retinal thickness at different points in the scan are measured from the superficial internal limiting membrane to the deep retinal pigment epithelium (arrows). The thickness at multiple corresponding points are appreciably thinner in the schizophrenia scan. The average central subfield thickness is calculated on the OCT device by averaging the retinal thickness at many points in a central circular area of retinal tissue measuring 1 mm in diameter. In these images the thickness of the macula central subfield is 217 microns for the person with schizophrenia and 288 microns for the control subject. OCT, optical coherence tomography.
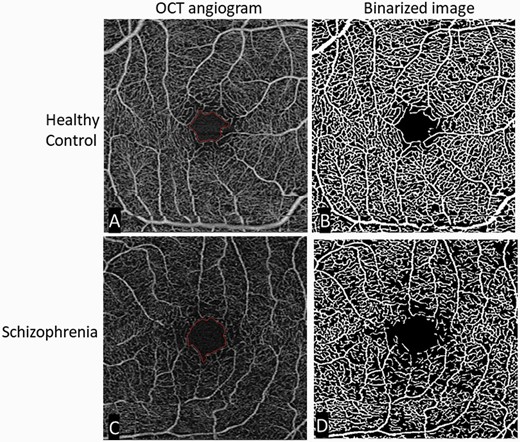
Studies indicating changes to the retinal microvasculature in schizophrenia using fundus photography indicate widening of retinal venules and changes in the appearance (eg, branching, tortuosity) of blood vessels.62–65 A recent machine learning analysis of fundus images demonstrated 95% classification accuracy for schizophrenia patients versus healthy controls, with an area under the curve of 0.98.66 The first schizophrenia studies using OCT angiography (OCTA; see figure 3)) were published in 2021, and all four papers thus far indicate reduced or increased microvasculature density in specific layers.67 This apparent paradox is not unexpected because the loss of vasculature in one layer can be compensated for by increases in angiogenesis in another layer.68 These findings are relevant to schizophrenia for several reasons. One is that schizophrenia has been conceptualized as a neurovascular69 or vascular-ischemic disorder,70 or as a neuroinflammatory condition in which vascular changes are secondary effects and neural effects are sequelae of insufficient blood supply or oxidative injury to neural tissue.10 This suggests that early identification of progressive vascular changes might aid in risk calculation among high risk for psychosis samples. Second, because there is an overlap between candidate genes for schizophrenia and genes regulating vascular function,70 which may account for the increase in cardiometabolic disorders in patients even at the first episode,71 OCTA could be used to screen for early cardiovascular disease in schizophrenia patients or to identify a subgroup which may benefit from primary interventions such as proactive statin use. It is important to note here that antipsychotic medication and lifestyle factors are known to contribute significantly to these problems. This makes it all the more important to assess for early and progressive cardiovascular changes in people with schizophrenia, especially because cardiovascular disease is among the leading causes of death in this population, accounting for approximately 40% of cases.72 Third, because rates of metabolic syndrome and diabetes are increased in schizophrenia, suggesting a shared pathophysiological mechanism,8,11 and because retinal neuronal and vascular degeneration precede the onset of diabetic retinopathy,73 OCT and OCTA could be useful tools to screen for early evidence of insulin resistance-related neural and cardiovascular changes in schizophrenia74 and people at risk for psychosis.
Comparison of retinal microvasculature, from OCTA images, between a healthy control and a subject with schizophrenia.Original and binarized OCT angiograms of a patient with schizophrenia and a healthy control. A standard OCT angiogram of a healthy eye is seen in (A). Vessels are seen in white, with larger arterioles and smaller capillaries represented in the 3 × 3 mm2 window. The foveal avascular zone (the most central region of the macula, where blood vessels are not present) is delineated in red. (B): A binarized version of the same angiogram, allowing for easier qualitative and quantitative assessment of vascular density. The bottom row shows similar images from a patient with schizophrenia. Compared to the healthy control, there is an enlargement of the foveal avascular zone (typically reflecting loss of microvasculature in the surrounding area) and a decrease in vessel density (C) most easily appreciated in the binarized image (D). OCT, optical coherence tomography; OCTA, OCT angiography; Figure reproduced from Green KM, Choi JJ, Ramchandran RS, Silverstein SM. OCT and OCT angiography offer new insights and opportunities in schizophrenia research and treatment. Front Digit Health. 2022;4:836851. doi:10.3389/fdgth.2022.836851 via a creative commons license (CC BY 4.0).
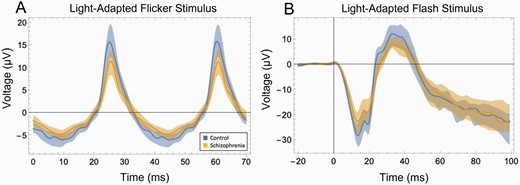
There is consistent evidence for abnormal retinal function in schizophrenia as assessed using electroretinography (ERG), which provides objective measurements of the activity of specific retinal neural layers and cell types (see figure 4).49,53 Some findings are shared with bipolar disorder and some appear to be specific to schizophrenia.75 ERG anomalies have been found in both patients and in unaffected offspring of a parent with schizophrenia or bipolar disorder.76,77 Some ERG changes appear to be trait-related76,77 while others may be state-related and sensitive to treatment,78 while still others appear to share a common basis with negative symptoms79,80 and cortical hypodopaminergia.81 ERG changes are also related to the level of cognitive impairment in people considered to be at high risk for schizophrenia.82 ERG testing can be accomplished using a hand-held portable device and using skin electrodes below the eye, rendering it non-invasive. Testing is brief and practical for clinical settings. The trait- and state-sensitivity of ERG make it potentially useful in risk calculation. State-sensitive aspects suggest that ERG data could be useful in predicting relapse, and response to treatment. ERG findings such as reduced waveform amplitudes and increased latencies of peak responses have been linked to cognitive impairment in people with Alzheimer’s disease,83 suggesting that ERG indices may also have relevance as markers of brain and cognitive function in people with schizophrenia.
Schizophrenia-control differences in strength of retinal cell firing as indicated by ERG.(A) Data from a light-adapted flicker stimulus ERG test, depicting cone photoreceptor response. The stimulus was white light flickering at 28.3 Hz, at an intensity of 85 troland seconds. The difference between the schizophrenia group (tan) and control group (blue) was large: t(48) = −3.58, P = .001, d = 1.01. Shaded areas depict the 25th–75th percentile interquartile range. Substantial non-overlap between groups can be observed. (B) Data from a light-adapted flash stimulus ERG test. The stimulus was light flashing at 1 Hz, at an intensity of 100 troland seconds. The differences between the schizophrenia group (tan) and control group (blue) were large: For the initial negative peak (a-wave), reflecting photoreceptor activity, t(48) = 2.86, P = .006, d = .81. For the subsequent positive peak (b-wave), reflecting bipolar cell and glial cell activity, t(48) = 2.92, P = .005, d = .83. Substantial non-overlap between groups can be observed here as well. Data in both cases from Demmin DL, Davis Q, Roché M, Silverstein SM. Electroretinographic anomalies in schizophrenia. J Abnorm Psychol. 2018;127(4):417–428. doi:10.1037/abn0000347. PMID: 29745706. ERG, electroretinography.
A recent development in noninvasive retinal imaging technology, in vivo adaptive optics (AO) scanning light ophthalmoscopy, has shown the ability, in animal studies, to image microglia in the retina.84 Microglia activation is related to neuroinflammation, and the at-risk mental state and schizophrenia are associated with increases in brain microglia.85–87 Elevated levels of retinal microglia have been identified in animal models of Alzheimer’s disease and Parkinson’s disease.88 This raises the prospect that retinal imaging could be used in schizophrenia to detect neuroinflammation, as well as associated pathologies that correlate with brain impairments, and are found in the disorder, such as mitochondrial dysfunction and impaired glucose metabolism.11,74,89,90 Other advances in AO allow for imaging of single blood cells traveling through retinal vessels in animals,91 and of individual photoreceptors in humans.92 The technique of in-vivo confocal neuroimaging allows for the resolution of single retinal ganglion cells.93 AO methods have yet to be applied to people with schizophrenia, but they will allow for studies of neural, neurovascular, neuro-inflammatory, and stimulus-induced hemodynamic changes at a level beyond what is likely to be possible with brain imaging in the near future.
Pupil
The pupil is the opening in the center of the iris that allows light to enter the eye. Pupil reactivity (ie, increases or decreases in its size) is a reliable indicator of processes relevant to schizophrenia.94,95 For example, pupil dilation is an index of effort and sympathetic nervous system arousal in response to a stimulus,96 and studies indicate that people with schizophrenia show reduced dilation in response to task-related cognitive demands.94,95,97 Reduced pupil dilation is also an index of reduced working memory ability in people with schizophrenia98,99 and of overall reduced cognitive capacity.100 Schizophrenia patients have also demonstrated reduced pupil dilation relative to controls in preparation to perform a task,101 and a reduced pupillary response is related to more negative symptoms.97,101,102
Cornea
The cornea is the transparent tissue in the front of the eye that overlies the iris, lens, and anterior chamber. It has not historically been a focus of psychiatric studies although nerve density in the cornea is higher than in any other organ of the human body,46,103 and corneal nerve atrophy is found in neurodegenerative disorders.104 Basic changes to the cornea, such as reduced overall volume, reduced anterior chamber volume and depth, reduced central thickness, and increased lens thickness have been found in schizophrenia.105 High-resolution imaging of the cornea can be accomplished using OCT and also using corneal confocal microscopy (CCM). Using CCM, Ponirakas et al.106 demonstrated reduced corneal nerve fiber density, length, and branching in schizophrenia, findings similar to what has been observed in the retinal microvasculature. In addition, schizophrenia patients could be distinguished from controls with high levels of accuracy (eg, 88% for corneal nerve fiber length). Moreover, in this study, corneal nerve fiber integrity predicted cingulate gyrus volume.
Several studies have demonstrated elevated corneal temperature in schizophrenia, especially in drug-naïve patients.107–109 These changes are thought to reflect thermal dysregulation secondary to changes in central dopaminergic transmission.109 Consistent with this hypothesis, corneal temperature has been positively correlated with overall symptom severity among both treatment-resistant inpatients and in medication-free outpatients with schizophrenia.108,110
CCM has been proposed as a potentially useful method to detect early signs of diabetic retinopathy in people with schizophrenia.104 This is relevant given the increased rate of diabetes in people with schizophrenia,111 the shared genetics between schizophrenia and insulin resistance,112 the increased risk of diabetes associated with antipsychotic medication,113 and the association of diabetes with vision loss114 and cognitive impairment.115 CCM has already shown an ability to detect diabetic autonomic neuropathy, as well as other small fiber neuropathies.104,116 Therefore, it may prove useful in monitoring for neural changes related to schizophrenia, as well as for medical management of patients.
Eye Disease
The presence of eye disease and other eye anomalies can also be informative about schizophrenia. For example, a recent study demonstrated that a diagnosis of glaucoma preceded a diagnosis of schizophrenia (or bipolar disorder or major depression) to a significant degree.117 Glaucoma is increasingly recognized as a neurodegenerative disease, and its presence is associated with the presence of brain disorders.118 Studies also indicate that impaired visual acuity, which may reflect factors such as retinal anomalies, problems in lens shape and accommodation, and other ocular issues, is related to an increased risk for psychotic symptoms,119–121 and for schizophrenia, based on findings in children122 and young adults.123 Although outside the scope of this review, the deleterious effects of antipsychotic medication on vision and eye health124,125 are important clinical considerations.
Other Reasons to Study the Eye in Schizophrenia
Changes in the eye can be useful for a better understanding of the visual changes noted by people with the disorder. For example, there are impairments in low-level visual processing in schizophrenia (eg, in contrast, sensitivity and visual acuity) that may be retinal in origin.126–129 Also, some of the visual distortions and hallucinations reported by patients (in approximately 65% and over 25% of cases, respectively)130–132 are similar to what is observed in other conditions with retinal impairments.53,126,133–137 Possible effects of retinal disturbances on other aspects of vision in schizophrenia have been reviewed elsewhere.138,139
Issues Arising
Several challenges need to be considered regarding the implementation of retinal methods in psychiatric practice. One can be summarized as: Since psychiatric facilities already have access to brain imaging methods, why would anyone need to conduct a retinal exam? There are several responses to this. First, one of the key advantages of OCT over MRI is its sensitivity to detecting change. OCT can image neural tissue with micrometer (ie, millionths of a meter) resolution, which is substantially better than the resolution of functional MRI (fMRI; which is typically greater than 1 mm).140 As noted, newer methods using AO can achieve resolution at the level of the single retinal neuron or blood vessel, a level not achievable by any human brain imaging method currently. This suggests that OCT could detect changes in neural and vascular structure prior to these changes being observable in brain tissue. Second, OCT is fast and cost-effective, and a full OCT scan of the retina, involving 100 000 to 200 000 individual captured images of portions of the retina, typically takes under one second to obtain. Third, small-footprint or portable forms of ERG, OCT, and pupillometry are already in routine use in other areas of medicine, and so the potential is far greater for these methods, as opposed to brain imaging, to be adopted for use with psychiatric patients in the near future. Fourth, the ability of ERG to measure activity in specific cell types is highly relevant to schizophrenia. For example, activity in short-wavelength (S) cones (those most sensitive to blue light) varies as a function of brain dopaminergic deficiencies126,141–143 and excesses.79,144 This may account for the above-noted relationships with levels of positive and negative symptoms, and suggests utility of ERG in monitoring for relapse and early treatment response.
A second challenge involves the question of whether retinal methods add anything over and above methods such as: (1) collection and analysis of DNA, proteins, cytokines, and hormones; (2) electrocardiogram; and (3) neuropsychological testing. Our view here is that examination of the retina can augment brain- and omics-based methods in several important ways. For questions related to screening and monitoring for CNS volume loss, global cognitive function, and microvasculature pathology, OCT is a faster, high-resolution, noninvasive option. For questions regarding features of psychosis-risk, clinical state, and as a potential diagnostic aid, ERG provides similar advantages. Likewise, pupillometry can provide important information about capacity to engage cognitive resources to effectively attend to and engage in real-world tasks (and effects of treatment on these outcomes). Each of these methods, as well as corneal imaging, is also relevant to detecting and monitoring CNS changes due to lifestyle and medication effects (eg, smoking, activity level, obesity, sleep apnea, diabetes, hypertension). Regarding cardiovascular comorbidity, in particular, recent recommendations in cardiology call for direct examination of microvascular structure and function, as opposed to measurement of only traditional risk factors (eg, body mass, cholesterol, hypertension), as a way to detect and treat cardiovascular illness prior to major events such as myocardial infarction or stroke.145,146 Because microvascular and cardiac changes in schizophrenia can be observed independently of these other risk factors74,147,148 incorporation of OCTA into routine care has obvious advantages.
In short, the retinal measurement techniques we discussed will be useful in the monitoring and treatment of people with schizophrenia to the extent that the syndrome is a multi-system disorder as is increasingly being proposed, and that it involves mechanisms such as mitochondrial dysfunction, neuroinflammation, insulin resistance, and other neurodegenerative mechanisms that are increasingly recognized as operative in schizophrenia. We, therefore, encourage a greater use of these oculomic12 techniques, especially given the success of parallel efforts with other brain disorders. An important area for future research is to determine whether there are ocular markers specific to vulnerability to schizophrenia, or its progression, versus those that are found in multiple conditions affecting the brain, or those caused by medications and lifestyle factors. This is, of course, also a challenge for each of the other methods noted above (eg, cognitive testing, fMRI, etc.), although it is not necessarily a critical issue when monitoring for CNS changes in patients with an established diagnosis.
Conclusion
Schizophrenia is associated with changes in several key structures of the eye (eg, retina, pupil, cornea), and changes in mechanisms inherent to schizophrenia (eg, neural atrophy, vascular insufficiency) are observed in the eyes of those with the disorder. A focus on the eye, which contains the only portion of the CNS that can be viewed using brief, noninvasive measurements, including imaging even down to the single-cell level, can increase our understanding of the pathophysiology, course, and heterogeneity of schizophrenia, and can be useful in monitoring for syndrome- and treatment-related neural and vascular changes in individual patients in our care.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
Supported by a grant from the New York Fund for Innovation in Research and Scientific Talent (NYFIRST), to author S.M.S., as well as an unrestricted grant from Research to Prevent Blindness to R.R.