-
PDF
- Split View
-
Views
-
Cite
Cite
Emre Bora, Robin M. Murray, Meta-analysis of Cognitive Deficits in Ultra-high Risk to Psychosis and First-Episode Psychosis: Do the Cognitive Deficits Progress Over, or After, the Onset of Psychosis?, Schizophrenia Bulletin, Volume 40, Issue 4, July 2014, Pages 744–755, https://doi.org/10.1093/schbul/sbt085
Close - Share Icon Share
Abstract
Cognitive dysfunction is a well-established feature of schizophrenia, and there is evidence suggesting that cognitive deficits are secondary to abnormal neurodevelopment leading to problems in acquiring such abilities. However, it is not clear whether there is also a decline in cognitive performance over, or after, the onset of psychosis. Our objective was to quantitatively examine the longitudinal changes in cognitive function in patients who presented with first-episode psychosis (FEP), ultra-high risk (UHR) for psychosis, and controls. Electronic databases were searched for the studies published between January 1987 and February 2013. All studies reporting longitudinal cognitive data in FEP and UHR subjects were retrieved. We conducted meta-analyses of 25 studies including 905 patients with FEP, 560 patients at UHR, and 405 healthy controls. The cognitive performances of FEP, UHR, and healthy controls all significantly improved over time. There was no publication bias, and distributions of effect sizes were very homogenous. In FEP, the degree of improvement in verbal working memory and executive functions was significantly associated with reduction in negative symptoms. There was no evidence of cognitive decline in patients with UHR and FEP. In contrast, the cognitive performances of both groups improved at follow-up. These findings suggest that cognitive deficits are already established before the prodromal phases of psychosis. These data support the neurodevelopmental model rather than neurodegenerative and related staging models of schizophrenia.
Introduction
It is well established that cognitive dysfunction is one of the characteristics of schizophrenia.1–3 However, the development and course of the cognitive deficits continues to be a topic of controversy. The main debate regarding the trajectory of cognitive deficits is about whether schizophrenia follows a neurodevelopmental or neurodegenerative course (or some combination of them). Neurodevelopmental theories suggest that core cognitive deficits in schizophrenia are the outcome of abnormal development of the brain leading to problems in acquiring cognitive abilities.4–6 A number of studies have provided evidence indicating that cognitive and intellectual deficits are evident early in neurodevelopment much before the onset of psychosis.7–9
Although most researchers accept that such developmental deficits are evident in schizophrenia, some suggest that there is also evidence for subsequent deterioration of previously acquired cognitive abilities immediately before, and after, the illness onset.10,11 Although there is no pathological evidence for gliosis or neurodegeneration in schizophrenia,12 other neuroregressive processes such as excessive pruning or inflammation in late adolescence have been proposed as possible neurobiological underpinnings of cognitive decline.13–17 The idea of cognitive decline in schizophrenia dates back to early description of the illness as “dementia praecox” by Kraepelin. More recently, clinical observations suggesting functional decline after the onset of illness in subgroups of patients, especially in the late prodrome, and earlier years after the onset of psychosis, have been put forward to support the idea of cognitive deterioration.18,19 In accordance with neurodegenerative views, some authors have proposed applying a clinical staging model to schizophrenia in which there is cognitive decline between stages such that the later stages of illness are associated with increasingly more severe cognitive dysfunction.20
Most evidence supporting cognitive deterioration in schizophrenia is based on indirect comparison of cross-sectional studies of schizophrenia patients in different phases of the illness (chronic, first episode, ultrahigh risk [UHR] to psychosis) with healthy controls. The findings indicate that cognitive deficits in UHR psychosis are substantially less severe than in first-episode psychosis (FEP),21–23 suggesting potential cognitive decline over the transition into psychosis, which might be secondary to the neurobiological changes that lead to the emergence of psychotic symptoms. Although some cross-sectional data suggest that cognitive deficits in first-episode and chronic patients are more similar,23 there is other evidence suggesting progression in selected cognitive domains after the onset of psychosis.11 Overall, findings of cross-sectional cognitive studies can be interpreted as indicating substantial cognitive decline over the onset of psychosis followed by a further more modest and selective deterioration after the onset of the illness.
However, there are many problems in using indirect comparisons of cross-sectional studies as an evidence of progression of cognitive deficits. First, there are methodological problems related to sample differences such as symptom levels, medication, demographical characteristics such as education and gender. Typically, first-episode patients are more symptomatic and take more medications than UHR subjects. Then, UHR is a more heterogeneous concept than either established schizophrenia or FEP: it is likely to include a mixture of true prodromal schizophrenia, affective psychosis and other psychotic disorders, subjects who are in psychotic disorder spectrum but have a favorable outcome, and a majority of subjects who will never develop psychosis. Therefore, it is expected that a lesser percentage of UHR subjects would have cognitive deficits leading to modest effect sizes. In a similar fashion, many individuals who suffered an FEP will recover completely or sufficiently to be cared for by their family doctor leaving psychiatrists to treat those with the most severe and recurrent illnesses. For all these reasons, only the most severe UHR or FEP cases will eventually end up in those samples of chronic schizophrenic patients who are examined for cognitive deficits.6
Longitudinal studies are likely to give a clearer picture. Two meta-analyses of longitudinal cognitive studies in established schizophrenia and also in older patients with this illness did not find evidence of cognitive decline.24,25 In these patient samples, cognitive deficits are stable or even slightly improved at follow-up (possibly due to practice effects and/or clinical stabilization). However, authors of a recent meta-analysis of 8 studies suggested that there is IQ decline in schizophrenia.26 Also McIntosh et al27 who investigated a large birth cohort suggested that schizophrenia susceptibility genes were associated with a greater relative cognitive decline between age 11 and 70.
Follow-up studies of the course of cognitive deficits in FEP and UHR might be more able to detect potential cognitive decline over, and after, the onset of psychosis, especially as it has been argued that most functional decline occurs just before or within few years after the onset of psychosis. A number of studies have investigated the course of cognition in UHR and FEP, and many of these have failed to provide evidence for cognitive decline;28 however, there are contradictory findings suggesting cognitive decline in early psychosis, and a number of authors’ conclusions were indecisive or endorsed cognitive deterioration.26,27,29–32 Overall, the idea of cognitive decline early in psychosis remains the dominant view among clinicians, and the idea of early intervention to prevent cognitive decline remains popular.
Most individual studies have small sample sizes, and reviews are based on vote counting the results of individual studies; thus, they ignore sample size differences and its implications on statistical threshold. Therefore, findings of such reviews might be biased, and they are likely to fail to detect possible modest cognitive decline in selected measures in early psychosis. Meta-analysis go beyond vote counting, and they combine sample size–weighted effect sizes without a statistical threshold. Also confounders such as clinical stabilization/acute presentation, practice effects, and outcome of UHR can be examined in a meta-analysis. Therefore, a formal meta-analysis of the longitudinal course of cognition in UHR and FEP and analysis of the effect of confounding factors would be important to show cognitive changes before and after the onset of psychosis.
Methods
Study Selection
We followed the guidelines of the meta-analysis of observational studies in epidemiology in this study.33 A literature search was conducted (by E.B.) using the databases Pubmed, ScienceDirect, PsycINFO, and Scopus to identify the relevant studies (January 1987 to February 2013). We used the combination of following keywords in this search: schizophrenia, FEP, longitudinal, follow-up, clinical high risk, UHR, prodrome, at risk mental state, psychosis, schizophrenia, cog*, neurops*, memory, attention, and executive function. We also reviewed the reference lists of published studies. The corresponding authors were asked to provide additional data not included in the original report. Inclusion criteria were studies that (1) published in an English language peer-reviewed journal (study quality criterion), (2) reported longitudinal neurocognitive data, and (3) included FEP and/or UHR subjects. Inclusion criteria for follow-up duration for the FEP studies were 1–5 years as we aimed to study possible cognitive changes before or within 5 years after onset of psychosis. Few available early onset schizophrenia studies were not included (3 studies, see online Supplementary Data). For sensitivity analysis of study quality (in addition to inclusion criterion 1), studies were coded as to whether they excluded substance abuse/dependence and whether they used a structured clinical interview. UHR was defined as having 1 or more of 3 psychosis risk syndromes at help seeking youth or young adults: (1) recent onset or worsening of attenuated positive symptoms; (2) recent onset of psychotic symptoms that were significant but not sufficiently sustained to meet the criteria for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, psychotic disorder (brief intermittent psychotic syndrome); (3) genetic/familial risk to psychosis plus deterioration (recent onset or worsening functional decline) syndrome. To qualify as FEP, studies including recent onset psychosis needed to conduct the first cognitive assessment after the psychotic episode (unlike UHR-P). As in UHR sample, FEP included not only schizophrenia cases but also other schizophreniform psychoses and some patients with affective disorders (table 1).
Characteristics of Studies Included in the Meta-analysis of Longitudinal Studies of UHR and FEP
| Study . | Sample (Male) . | Follow-up . | Age . | Characteristics . | Medication . | Cognitive Tasks . |
|---|---|---|---|---|---|---|
| Addingtonet al48 | 124 (83) FEP; 66 (38) HC | 1, 2 y | Age: FEP = 24.6; HC = 22.7 | Substantial improvement in positive symptoms; PANSS; schizophrenia spectrum | 1 y = 82%, 2y = 68%; baseline = 98% on SGA | WCST, TMT A, B, RAVLT, letter and category fluency, Stroop, CPT, logical memory, Rey figure |
| Albuset al50 | 71 (36) FES; 71 (36) HC | 5 y | Age: FES = 29.7; HC = 29.9 | Baseline stable, after admission; PANSS, SANS | 56/71 on AP; 16/71 FGA | WMS, CVLT, TMT, symbol, Stroop, WCST, letter fluency |
| Albus et al34 | 50 (26) FES; 50 (23) HC | 2 y | Age: FES = 29; HC = 31.6 | Baseline stable, after admission; PANSS | 23/50 FGA; 18/50 clozapine | WMS, CVLT, TMT, symbol, Stroop, WCST |
| de Mello Ayreset al57 | 30 FES; 67 HC | 1–3 y | Most taking AP | Digits span, verbal fluency | ||
| Chanet al45 | 34 FEP | 1 and 3 y | Age: FEP = 28.1; HC = 27.6 | 22/34 Schizophrenia, 2/34 schizoaffective; 10/34 schizophreniform; PANSS | Baseline medication naive | WCST, HSCT, monotone counting task |
| Goldet al54 | 54 (41) FEP | 5 y | Age: FEP = 24.0 | FE or recent onset, schizophrenia spectrum; SANS/SAPS | 40/54 AP; 29/54 FGA | TMT B, cancellation task, WCST, RCFT, letter fluency, LM, IQ |
| Noupoulos et al35 | 35 (29) FES | 1–2 y | Age: FES = 23.7 | Baseline at index admission FE or recent onset | 29/35 on AP; 24/35 FGA; baseline all on FGA | RAVLT, PAL, LM, Stroop, letter fluency, TMT B, |
| Hill et al51 | 45 (28) FES; 33 (23) HC | 1, 2 y | Age: FES = 26.1; FES=23.5 | Baseline at index admission | Baseline AP naive; 31/45 on AP at 1 y; 21/45 on AP at 2 y | WCST, Stroop, TMT A, B, Stroop, Symbol coding, digit span, CVLT, WMS-R visual memory, cancellation test |
| Keefeet al44 | 58 FEP | 1, 2 y (26/58 in 2 y) | Olanzapine or haloperidone; at baseline < 4 mo history of AP | Total score based factor; analysis of a cognitive battery | ||
| Kopalaet al52 | 20 FEP | 1, 2 y | Age around 23 | Drawn from sample of 39 consisting of vast majority schizophrenia and 3 schizoaffective | All on quetiapine | RAVLT, Vis memory, letter fluency, design fluency, WCST, CPT, TMT A and B |
| Penaet al47 | 71 (49) FEP; 34 HC | 2 y | Age: FEP = 28.5 | Mixture of schizophreniform and affective psychosis; PANSS | Treated with SGA | Brief test of attention, Digits backwards, LNS, TMT A, Stroop, WCST, letter fluency, categorical fluency, WMS memory |
| Purdonet al56 | 65 (45) FES | 1 y | Age: FES = 28.8 | PANSS | Haloperidol, olanzapine, risperidone | WCST, letter and category fluency, symbol coding, digit span, visual span, trail making, TMT B, Rey figure, story learning, list learning, visual reproduction |
| Rodriguez-Sanchezet al49 | 112 (73) FEP; 22 (9) HC | 1 y; active illness to stabilization | Age: FEP = 2.5 | SAPS | Mostly on haloperidol, olanzapine, risperidone; Baseline AP naive | RAVLT, RCFT, CPT, brief test of attention |
| Rundet al46 | 111 (64) FEP | 1–2 y | Age: FEP = 28.1; HC = 24.1 | PANSS | 78% AP; 73% SGA | CVLT, WCST, TMT A and B, letter fluency, digit span, CPT |
| Scottish Schizophrenia Research Group55 | 27 FES 28 | 1 y | All pimozide or flupenthixol | Digit span, block design, IQ | ||
| Towsendet al53 | 83 (62) FEP | 1 y; baseline after stable | Age: FEP = 24.7 | SAPS/SANS: 67/83 schizophrenia; 14/83 schizoaffective; 2/83 schizophreniform | 40 risperidone, 17 olanzapine, 11 quetiapine, 8 clozapine, 7 FGA | PASAT, WCST, TMT A, B, letter fluency, CPT, Stroop, IQ, symbol coding, WMS |
| UHR | ||||||
| Beckeret al39 | 41(29) UHR; 17 (9) HC | 1–2 y | Age: UHR = 19.8; HC = 19.4 | 24 UHR-NP; 17 UHR-P | UHR-P tested when stabilized on medication | CVLT, letter and category fluency, CPT, SWM, RCFT |
| Bowieet al38 | 53 (40) UHR (3 groups); 17 (9) HC | 6 mo | Age: UHR = 15.8; HC = 16.4 | SIPS; SOPS | 11 UHR with AP; 15 UHR with AD; 27 UHR-no medication | CPT, TMT A and B, LNS, letter fluency |
| Keefeet al37 | 28 UHR | 1 y | 11UHR-P; 17 UHR-NP | No medication; olanzapine using patients excluded | Global cognition | |
| Linet al42 | 230 (99) UHR | 7.2 y | Age: UHR = 18.7 | PACE: 189 UHR good and 41 UHR poor prognosis | VMI, IQ | |
| Wood et al36 | 16 (10) UHR; 17 (14) HC | 1.4 y | Age: UHR = 17.3 and 21; HC = 19.7 | PACE: 7 UHR-NP; 9 UHR-P | AP = 10/16 (9/10 UHR-P) | Digit span, symbol coding, logical memory, visual reproduction, RAVLT, TMT A, B, letter fluency, verbal pair associate |
| Niendamet al41 | 35 (21) UHR | 0.63 y | Age = 17.3 | SIPS; SOPS | Half on AP | TMT A, B, symbol coding letter fluency, matrix reasoning, digit span, visual reproduction, CVLT, logical memory |
| Jahsanet al40 | 48 (28) UHR; 29 (14) HC | 1.1 y | Age: UHR = 18.7; UHR = 19.0 | SIPS/CARE | 10/48 on AP | WCST, Stroop, HVLT, LNS, spatial span |
| Barbatoet al43 | 72 UHR | 6 mo | All UHR-NP | RAVLT, LNS, WCST, Stroop, TMT A and B, CPT, category fluency, SWM, n-back | ||
| Woodberryet al58 | 53 (26) UHR; 32 (16) HC | 1 y | Age: UHR = 16; HC = 16.3 | 43 UHR-NP; 10 UHR-P | AP = 59%; mood stabilizer = 40% | CVLT, WMS, WCST, LNS, CPT, verbal fluency, trail making |
| Study . | Sample (Male) . | Follow-up . | Age . | Characteristics . | Medication . | Cognitive Tasks . |
|---|---|---|---|---|---|---|
| Addingtonet al48 | 124 (83) FEP; 66 (38) HC | 1, 2 y | Age: FEP = 24.6; HC = 22.7 | Substantial improvement in positive symptoms; PANSS; schizophrenia spectrum | 1 y = 82%, 2y = 68%; baseline = 98% on SGA | WCST, TMT A, B, RAVLT, letter and category fluency, Stroop, CPT, logical memory, Rey figure |
| Albuset al50 | 71 (36) FES; 71 (36) HC | 5 y | Age: FES = 29.7; HC = 29.9 | Baseline stable, after admission; PANSS, SANS | 56/71 on AP; 16/71 FGA | WMS, CVLT, TMT, symbol, Stroop, WCST, letter fluency |
| Albus et al34 | 50 (26) FES; 50 (23) HC | 2 y | Age: FES = 29; HC = 31.6 | Baseline stable, after admission; PANSS | 23/50 FGA; 18/50 clozapine | WMS, CVLT, TMT, symbol, Stroop, WCST |
| de Mello Ayreset al57 | 30 FES; 67 HC | 1–3 y | Most taking AP | Digits span, verbal fluency | ||
| Chanet al45 | 34 FEP | 1 and 3 y | Age: FEP = 28.1; HC = 27.6 | 22/34 Schizophrenia, 2/34 schizoaffective; 10/34 schizophreniform; PANSS | Baseline medication naive | WCST, HSCT, monotone counting task |
| Goldet al54 | 54 (41) FEP | 5 y | Age: FEP = 24.0 | FE or recent onset, schizophrenia spectrum; SANS/SAPS | 40/54 AP; 29/54 FGA | TMT B, cancellation task, WCST, RCFT, letter fluency, LM, IQ |
| Noupoulos et al35 | 35 (29) FES | 1–2 y | Age: FES = 23.7 | Baseline at index admission FE or recent onset | 29/35 on AP; 24/35 FGA; baseline all on FGA | RAVLT, PAL, LM, Stroop, letter fluency, TMT B, |
| Hill et al51 | 45 (28) FES; 33 (23) HC | 1, 2 y | Age: FES = 26.1; FES=23.5 | Baseline at index admission | Baseline AP naive; 31/45 on AP at 1 y; 21/45 on AP at 2 y | WCST, Stroop, TMT A, B, Stroop, Symbol coding, digit span, CVLT, WMS-R visual memory, cancellation test |
| Keefeet al44 | 58 FEP | 1, 2 y (26/58 in 2 y) | Olanzapine or haloperidone; at baseline < 4 mo history of AP | Total score based factor; analysis of a cognitive battery | ||
| Kopalaet al52 | 20 FEP | 1, 2 y | Age around 23 | Drawn from sample of 39 consisting of vast majority schizophrenia and 3 schizoaffective | All on quetiapine | RAVLT, Vis memory, letter fluency, design fluency, WCST, CPT, TMT A and B |
| Penaet al47 | 71 (49) FEP; 34 HC | 2 y | Age: FEP = 28.5 | Mixture of schizophreniform and affective psychosis; PANSS | Treated with SGA | Brief test of attention, Digits backwards, LNS, TMT A, Stroop, WCST, letter fluency, categorical fluency, WMS memory |
| Purdonet al56 | 65 (45) FES | 1 y | Age: FES = 28.8 | PANSS | Haloperidol, olanzapine, risperidone | WCST, letter and category fluency, symbol coding, digit span, visual span, trail making, TMT B, Rey figure, story learning, list learning, visual reproduction |
| Rodriguez-Sanchezet al49 | 112 (73) FEP; 22 (9) HC | 1 y; active illness to stabilization | Age: FEP = 2.5 | SAPS | Mostly on haloperidol, olanzapine, risperidone; Baseline AP naive | RAVLT, RCFT, CPT, brief test of attention |
| Rundet al46 | 111 (64) FEP | 1–2 y | Age: FEP = 28.1; HC = 24.1 | PANSS | 78% AP; 73% SGA | CVLT, WCST, TMT A and B, letter fluency, digit span, CPT |
| Scottish Schizophrenia Research Group55 | 27 FES 28 | 1 y | All pimozide or flupenthixol | Digit span, block design, IQ | ||
| Towsendet al53 | 83 (62) FEP | 1 y; baseline after stable | Age: FEP = 24.7 | SAPS/SANS: 67/83 schizophrenia; 14/83 schizoaffective; 2/83 schizophreniform | 40 risperidone, 17 olanzapine, 11 quetiapine, 8 clozapine, 7 FGA | PASAT, WCST, TMT A, B, letter fluency, CPT, Stroop, IQ, symbol coding, WMS |
| UHR | ||||||
| Beckeret al39 | 41(29) UHR; 17 (9) HC | 1–2 y | Age: UHR = 19.8; HC = 19.4 | 24 UHR-NP; 17 UHR-P | UHR-P tested when stabilized on medication | CVLT, letter and category fluency, CPT, SWM, RCFT |
| Bowieet al38 | 53 (40) UHR (3 groups); 17 (9) HC | 6 mo | Age: UHR = 15.8; HC = 16.4 | SIPS; SOPS | 11 UHR with AP; 15 UHR with AD; 27 UHR-no medication | CPT, TMT A and B, LNS, letter fluency |
| Keefeet al37 | 28 UHR | 1 y | 11UHR-P; 17 UHR-NP | No medication; olanzapine using patients excluded | Global cognition | |
| Linet al42 | 230 (99) UHR | 7.2 y | Age: UHR = 18.7 | PACE: 189 UHR good and 41 UHR poor prognosis | VMI, IQ | |
| Wood et al36 | 16 (10) UHR; 17 (14) HC | 1.4 y | Age: UHR = 17.3 and 21; HC = 19.7 | PACE: 7 UHR-NP; 9 UHR-P | AP = 10/16 (9/10 UHR-P) | Digit span, symbol coding, logical memory, visual reproduction, RAVLT, TMT A, B, letter fluency, verbal pair associate |
| Niendamet al41 | 35 (21) UHR | 0.63 y | Age = 17.3 | SIPS; SOPS | Half on AP | TMT A, B, symbol coding letter fluency, matrix reasoning, digit span, visual reproduction, CVLT, logical memory |
| Jahsanet al40 | 48 (28) UHR; 29 (14) HC | 1.1 y | Age: UHR = 18.7; UHR = 19.0 | SIPS/CARE | 10/48 on AP | WCST, Stroop, HVLT, LNS, spatial span |
| Barbatoet al43 | 72 UHR | 6 mo | All UHR-NP | RAVLT, LNS, WCST, Stroop, TMT A and B, CPT, category fluency, SWM, n-back | ||
| Woodberryet al58 | 53 (26) UHR; 32 (16) HC | 1 y | Age: UHR = 16; HC = 16.3 | 43 UHR-NP; 10 UHR-P | AP = 59%; mood stabilizer = 40% | CVLT, WMS, WCST, LNS, CPT, verbal fluency, trail making |
Note: UHR, ultra-high risk; FEP, first-episode psychosis; HC, healthy controls; PANSS, Positive and Negative Syndrome Scale; SGA, second-generation antipsychotics; WCST, Wisconsin Card Sorting Test; TMT, trail making test; RAVLT, Rey Auditory Verbal Learning Test; CPT, continuous performance test; FES, first-episode schizophrenia; SANS, Scale for the Assessment of Negative Symptoms; AP, antipsychotics; FGA, first-generation antipsychotics; CVLT, California verbal learning test; HSCT, Hayling sentence completion test; WMS, Wechsler Memory Scale; SAPS, Schedule for Assessment of Positive Symptoms; SWM, spatial working memory; RCFT, Rey Complex Figure Test; LM, logical memory; PAL, paired associate learning; LNS, letter number sequencing; PASAT, Paced Auditory Serial Addition Test; UHR-P, transition to psychosis in follow-up; UHR-NP, no transition to psychosis in follow-up; SIPS, Structured Interview for Prodromal Syndromes; SOPS, Scale of Prodromal Symptoms; AD, antidepressants; PACE, Personal Assessment and Crisis Evaluation; VMI, Verbal Memory Index; HVLT, Hopkins Verbal Learning Test. Studies with bold characters indicate main samples included in the meta-analysis.
Characteristics of Studies Included in the Meta-analysis of Longitudinal Studies of UHR and FEP
| Study . | Sample (Male) . | Follow-up . | Age . | Characteristics . | Medication . | Cognitive Tasks . |
|---|---|---|---|---|---|---|
| Addingtonet al48 | 124 (83) FEP; 66 (38) HC | 1, 2 y | Age: FEP = 24.6; HC = 22.7 | Substantial improvement in positive symptoms; PANSS; schizophrenia spectrum | 1 y = 82%, 2y = 68%; baseline = 98% on SGA | WCST, TMT A, B, RAVLT, letter and category fluency, Stroop, CPT, logical memory, Rey figure |
| Albuset al50 | 71 (36) FES; 71 (36) HC | 5 y | Age: FES = 29.7; HC = 29.9 | Baseline stable, after admission; PANSS, SANS | 56/71 on AP; 16/71 FGA | WMS, CVLT, TMT, symbol, Stroop, WCST, letter fluency |
| Albus et al34 | 50 (26) FES; 50 (23) HC | 2 y | Age: FES = 29; HC = 31.6 | Baseline stable, after admission; PANSS | 23/50 FGA; 18/50 clozapine | WMS, CVLT, TMT, symbol, Stroop, WCST |
| de Mello Ayreset al57 | 30 FES; 67 HC | 1–3 y | Most taking AP | Digits span, verbal fluency | ||
| Chanet al45 | 34 FEP | 1 and 3 y | Age: FEP = 28.1; HC = 27.6 | 22/34 Schizophrenia, 2/34 schizoaffective; 10/34 schizophreniform; PANSS | Baseline medication naive | WCST, HSCT, monotone counting task |
| Goldet al54 | 54 (41) FEP | 5 y | Age: FEP = 24.0 | FE or recent onset, schizophrenia spectrum; SANS/SAPS | 40/54 AP; 29/54 FGA | TMT B, cancellation task, WCST, RCFT, letter fluency, LM, IQ |
| Noupoulos et al35 | 35 (29) FES | 1–2 y | Age: FES = 23.7 | Baseline at index admission FE or recent onset | 29/35 on AP; 24/35 FGA; baseline all on FGA | RAVLT, PAL, LM, Stroop, letter fluency, TMT B, |
| Hill et al51 | 45 (28) FES; 33 (23) HC | 1, 2 y | Age: FES = 26.1; FES=23.5 | Baseline at index admission | Baseline AP naive; 31/45 on AP at 1 y; 21/45 on AP at 2 y | WCST, Stroop, TMT A, B, Stroop, Symbol coding, digit span, CVLT, WMS-R visual memory, cancellation test |
| Keefeet al44 | 58 FEP | 1, 2 y (26/58 in 2 y) | Olanzapine or haloperidone; at baseline < 4 mo history of AP | Total score based factor; analysis of a cognitive battery | ||
| Kopalaet al52 | 20 FEP | 1, 2 y | Age around 23 | Drawn from sample of 39 consisting of vast majority schizophrenia and 3 schizoaffective | All on quetiapine | RAVLT, Vis memory, letter fluency, design fluency, WCST, CPT, TMT A and B |
| Penaet al47 | 71 (49) FEP; 34 HC | 2 y | Age: FEP = 28.5 | Mixture of schizophreniform and affective psychosis; PANSS | Treated with SGA | Brief test of attention, Digits backwards, LNS, TMT A, Stroop, WCST, letter fluency, categorical fluency, WMS memory |
| Purdonet al56 | 65 (45) FES | 1 y | Age: FES = 28.8 | PANSS | Haloperidol, olanzapine, risperidone | WCST, letter and category fluency, symbol coding, digit span, visual span, trail making, TMT B, Rey figure, story learning, list learning, visual reproduction |
| Rodriguez-Sanchezet al49 | 112 (73) FEP; 22 (9) HC | 1 y; active illness to stabilization | Age: FEP = 2.5 | SAPS | Mostly on haloperidol, olanzapine, risperidone; Baseline AP naive | RAVLT, RCFT, CPT, brief test of attention |
| Rundet al46 | 111 (64) FEP | 1–2 y | Age: FEP = 28.1; HC = 24.1 | PANSS | 78% AP; 73% SGA | CVLT, WCST, TMT A and B, letter fluency, digit span, CPT |
| Scottish Schizophrenia Research Group55 | 27 FES 28 | 1 y | All pimozide or flupenthixol | Digit span, block design, IQ | ||
| Towsendet al53 | 83 (62) FEP | 1 y; baseline after stable | Age: FEP = 24.7 | SAPS/SANS: 67/83 schizophrenia; 14/83 schizoaffective; 2/83 schizophreniform | 40 risperidone, 17 olanzapine, 11 quetiapine, 8 clozapine, 7 FGA | PASAT, WCST, TMT A, B, letter fluency, CPT, Stroop, IQ, symbol coding, WMS |
| UHR | ||||||
| Beckeret al39 | 41(29) UHR; 17 (9) HC | 1–2 y | Age: UHR = 19.8; HC = 19.4 | 24 UHR-NP; 17 UHR-P | UHR-P tested when stabilized on medication | CVLT, letter and category fluency, CPT, SWM, RCFT |
| Bowieet al38 | 53 (40) UHR (3 groups); 17 (9) HC | 6 mo | Age: UHR = 15.8; HC = 16.4 | SIPS; SOPS | 11 UHR with AP; 15 UHR with AD; 27 UHR-no medication | CPT, TMT A and B, LNS, letter fluency |
| Keefeet al37 | 28 UHR | 1 y | 11UHR-P; 17 UHR-NP | No medication; olanzapine using patients excluded | Global cognition | |
| Linet al42 | 230 (99) UHR | 7.2 y | Age: UHR = 18.7 | PACE: 189 UHR good and 41 UHR poor prognosis | VMI, IQ | |
| Wood et al36 | 16 (10) UHR; 17 (14) HC | 1.4 y | Age: UHR = 17.3 and 21; HC = 19.7 | PACE: 7 UHR-NP; 9 UHR-P | AP = 10/16 (9/10 UHR-P) | Digit span, symbol coding, logical memory, visual reproduction, RAVLT, TMT A, B, letter fluency, verbal pair associate |
| Niendamet al41 | 35 (21) UHR | 0.63 y | Age = 17.3 | SIPS; SOPS | Half on AP | TMT A, B, symbol coding letter fluency, matrix reasoning, digit span, visual reproduction, CVLT, logical memory |
| Jahsanet al40 | 48 (28) UHR; 29 (14) HC | 1.1 y | Age: UHR = 18.7; UHR = 19.0 | SIPS/CARE | 10/48 on AP | WCST, Stroop, HVLT, LNS, spatial span |
| Barbatoet al43 | 72 UHR | 6 mo | All UHR-NP | RAVLT, LNS, WCST, Stroop, TMT A and B, CPT, category fluency, SWM, n-back | ||
| Woodberryet al58 | 53 (26) UHR; 32 (16) HC | 1 y | Age: UHR = 16; HC = 16.3 | 43 UHR-NP; 10 UHR-P | AP = 59%; mood stabilizer = 40% | CVLT, WMS, WCST, LNS, CPT, verbal fluency, trail making |
| Study . | Sample (Male) . | Follow-up . | Age . | Characteristics . | Medication . | Cognitive Tasks . |
|---|---|---|---|---|---|---|
| Addingtonet al48 | 124 (83) FEP; 66 (38) HC | 1, 2 y | Age: FEP = 24.6; HC = 22.7 | Substantial improvement in positive symptoms; PANSS; schizophrenia spectrum | 1 y = 82%, 2y = 68%; baseline = 98% on SGA | WCST, TMT A, B, RAVLT, letter and category fluency, Stroop, CPT, logical memory, Rey figure |
| Albuset al50 | 71 (36) FES; 71 (36) HC | 5 y | Age: FES = 29.7; HC = 29.9 | Baseline stable, after admission; PANSS, SANS | 56/71 on AP; 16/71 FGA | WMS, CVLT, TMT, symbol, Stroop, WCST, letter fluency |
| Albus et al34 | 50 (26) FES; 50 (23) HC | 2 y | Age: FES = 29; HC = 31.6 | Baseline stable, after admission; PANSS | 23/50 FGA; 18/50 clozapine | WMS, CVLT, TMT, symbol, Stroop, WCST |
| de Mello Ayreset al57 | 30 FES; 67 HC | 1–3 y | Most taking AP | Digits span, verbal fluency | ||
| Chanet al45 | 34 FEP | 1 and 3 y | Age: FEP = 28.1; HC = 27.6 | 22/34 Schizophrenia, 2/34 schizoaffective; 10/34 schizophreniform; PANSS | Baseline medication naive | WCST, HSCT, monotone counting task |
| Goldet al54 | 54 (41) FEP | 5 y | Age: FEP = 24.0 | FE or recent onset, schizophrenia spectrum; SANS/SAPS | 40/54 AP; 29/54 FGA | TMT B, cancellation task, WCST, RCFT, letter fluency, LM, IQ |
| Noupoulos et al35 | 35 (29) FES | 1–2 y | Age: FES = 23.7 | Baseline at index admission FE or recent onset | 29/35 on AP; 24/35 FGA; baseline all on FGA | RAVLT, PAL, LM, Stroop, letter fluency, TMT B, |
| Hill et al51 | 45 (28) FES; 33 (23) HC | 1, 2 y | Age: FES = 26.1; FES=23.5 | Baseline at index admission | Baseline AP naive; 31/45 on AP at 1 y; 21/45 on AP at 2 y | WCST, Stroop, TMT A, B, Stroop, Symbol coding, digit span, CVLT, WMS-R visual memory, cancellation test |
| Keefeet al44 | 58 FEP | 1, 2 y (26/58 in 2 y) | Olanzapine or haloperidone; at baseline < 4 mo history of AP | Total score based factor; analysis of a cognitive battery | ||
| Kopalaet al52 | 20 FEP | 1, 2 y | Age around 23 | Drawn from sample of 39 consisting of vast majority schizophrenia and 3 schizoaffective | All on quetiapine | RAVLT, Vis memory, letter fluency, design fluency, WCST, CPT, TMT A and B |
| Penaet al47 | 71 (49) FEP; 34 HC | 2 y | Age: FEP = 28.5 | Mixture of schizophreniform and affective psychosis; PANSS | Treated with SGA | Brief test of attention, Digits backwards, LNS, TMT A, Stroop, WCST, letter fluency, categorical fluency, WMS memory |
| Purdonet al56 | 65 (45) FES | 1 y | Age: FES = 28.8 | PANSS | Haloperidol, olanzapine, risperidone | WCST, letter and category fluency, symbol coding, digit span, visual span, trail making, TMT B, Rey figure, story learning, list learning, visual reproduction |
| Rodriguez-Sanchezet al49 | 112 (73) FEP; 22 (9) HC | 1 y; active illness to stabilization | Age: FEP = 2.5 | SAPS | Mostly on haloperidol, olanzapine, risperidone; Baseline AP naive | RAVLT, RCFT, CPT, brief test of attention |
| Rundet al46 | 111 (64) FEP | 1–2 y | Age: FEP = 28.1; HC = 24.1 | PANSS | 78% AP; 73% SGA | CVLT, WCST, TMT A and B, letter fluency, digit span, CPT |
| Scottish Schizophrenia Research Group55 | 27 FES 28 | 1 y | All pimozide or flupenthixol | Digit span, block design, IQ | ||
| Towsendet al53 | 83 (62) FEP | 1 y; baseline after stable | Age: FEP = 24.7 | SAPS/SANS: 67/83 schizophrenia; 14/83 schizoaffective; 2/83 schizophreniform | 40 risperidone, 17 olanzapine, 11 quetiapine, 8 clozapine, 7 FGA | PASAT, WCST, TMT A, B, letter fluency, CPT, Stroop, IQ, symbol coding, WMS |
| UHR | ||||||
| Beckeret al39 | 41(29) UHR; 17 (9) HC | 1–2 y | Age: UHR = 19.8; HC = 19.4 | 24 UHR-NP; 17 UHR-P | UHR-P tested when stabilized on medication | CVLT, letter and category fluency, CPT, SWM, RCFT |
| Bowieet al38 | 53 (40) UHR (3 groups); 17 (9) HC | 6 mo | Age: UHR = 15.8; HC = 16.4 | SIPS; SOPS | 11 UHR with AP; 15 UHR with AD; 27 UHR-no medication | CPT, TMT A and B, LNS, letter fluency |
| Keefeet al37 | 28 UHR | 1 y | 11UHR-P; 17 UHR-NP | No medication; olanzapine using patients excluded | Global cognition | |
| Linet al42 | 230 (99) UHR | 7.2 y | Age: UHR = 18.7 | PACE: 189 UHR good and 41 UHR poor prognosis | VMI, IQ | |
| Wood et al36 | 16 (10) UHR; 17 (14) HC | 1.4 y | Age: UHR = 17.3 and 21; HC = 19.7 | PACE: 7 UHR-NP; 9 UHR-P | AP = 10/16 (9/10 UHR-P) | Digit span, symbol coding, logical memory, visual reproduction, RAVLT, TMT A, B, letter fluency, verbal pair associate |
| Niendamet al41 | 35 (21) UHR | 0.63 y | Age = 17.3 | SIPS; SOPS | Half on AP | TMT A, B, symbol coding letter fluency, matrix reasoning, digit span, visual reproduction, CVLT, logical memory |
| Jahsanet al40 | 48 (28) UHR; 29 (14) HC | 1.1 y | Age: UHR = 18.7; UHR = 19.0 | SIPS/CARE | 10/48 on AP | WCST, Stroop, HVLT, LNS, spatial span |
| Barbatoet al43 | 72 UHR | 6 mo | All UHR-NP | RAVLT, LNS, WCST, Stroop, TMT A and B, CPT, category fluency, SWM, n-back | ||
| Woodberryet al58 | 53 (26) UHR; 32 (16) HC | 1 y | Age: UHR = 16; HC = 16.3 | 43 UHR-NP; 10 UHR-P | AP = 59%; mood stabilizer = 40% | CVLT, WMS, WCST, LNS, CPT, verbal fluency, trail making |
Note: UHR, ultra-high risk; FEP, first-episode psychosis; HC, healthy controls; PANSS, Positive and Negative Syndrome Scale; SGA, second-generation antipsychotics; WCST, Wisconsin Card Sorting Test; TMT, trail making test; RAVLT, Rey Auditory Verbal Learning Test; CPT, continuous performance test; FES, first-episode schizophrenia; SANS, Scale for the Assessment of Negative Symptoms; AP, antipsychotics; FGA, first-generation antipsychotics; CVLT, California verbal learning test; HSCT, Hayling sentence completion test; WMS, Wechsler Memory Scale; SAPS, Schedule for Assessment of Positive Symptoms; SWM, spatial working memory; RCFT, Rey Complex Figure Test; LM, logical memory; PAL, paired associate learning; LNS, letter number sequencing; PASAT, Paced Auditory Serial Addition Test; UHR-P, transition to psychosis in follow-up; UHR-NP, no transition to psychosis in follow-up; SIPS, Structured Interview for Prodromal Syndromes; SOPS, Scale of Prodromal Symptoms; AD, antidepressants; PACE, Personal Assessment and Crisis Evaluation; VMI, Verbal Memory Index; HVLT, Hopkins Verbal Learning Test. Studies with bold characters indicate main samples included in the meta-analysis.
In the case of multiple publications from overlapping samples, the study with the largest sample size was included. Data from 3 other studies were used for duration of follow-up34,35 or UHR-P vs UHR-NP36 analyses or to examine cognitive tasks36 that were not examined by main study. A total of 47 studies met the inclusion criteria. Of these, 22 studies were excluded due to (a) sample overlap, (b) not reporting sufficient data to calculate effect sizes, (c) reassessing FEP sample more than 5 years after, and (d) including early-onset schizophrenia. A complete list of excluded studies and reasons for exclusion for each study and a low diagram are reported in the online Supplementary Data. Finally, 25 studies were included in our meta-analysis (table 1).34–58
Participants
A total of 17 FEP (905 subjects; 14 studies, 65.8% males, age 24.5), 14 UHR (560 subjects; 9 studies, 53.6% males, age 18.0), and 11 control (405 subjects, 11 studies, 52.7% males, age 23.6) main samples were included. UHR subjects were younger, and FEP had higher ratio of males. Data from additional 3 studies with overlapping samples were used for subgroup analyses.41,45,48 A preliminary meta-analysis to examine the effect of outcome on UHR was also conducted based on only 4 studies.
Cognitive Measures
We combined individual tasks into the broader cognitive domains of verbal memory, visual memory, executive functions, fluency, attention, and verbal working memory. This step was undertaken because there were not sufficient studies to perform meta-analyses for all individual tasks (see online Supplementary Data). Visual working memory was not included as there were not sufficient studies in FEP and healthy control groups. In addition to cognitive domain analyses, task-specific analyses were conducted when at least 3 independent studies had employed a given task (e.g., trail making task). Individual tasks that were analyzed separately included list learning, Wisconsin Cart Sorting Test (WCST) perseveration errors, trail making A and B, digit span, symbol coding, Stroop interference, continuous performance test (CPT) d sensitivity score, letter number sequencing, and letter fluency.
Statistical Analyses
Meta-analyses were performed using MIX software version 1.7 on a Windows platform.59 For each cognitive task, an effect size and standard error were estimated. Effect sizes were weighted using the inverse variance method, and a random effects model (DerSimonian–Laird estimate) was used because the distributions of effect sizes were heterogeneous for number of variables. For studies that reported more than 1 cognitive task for each domain, a pooled effect size was calculated. The Q test was used to measure the heterogeneity of the distribution of effect sizes. When the Q test was significant, “I2” (a measure of the degree of inconsistency in the results of the studies) was used to quantify heterogeneity.60I2 estimates the percentage of total variation across studies, which is due to heterogeneity rather than chance. I2 values between 0 and 0.25 suggest small magnitudes of heterogeneity, whereas I2 values in the range 0.25–0.50 suggest medium magnitudes and those > 0.50 indicate large magnitudes. Publication bias was assessed by Egger’s test. We also calculated homogeneity statistics using Qbet to test the differences between cognitive changes in diagnostic groups (FEP, UHR, and controls) and the effect of follow-up duration in FEP (1, 2, and 3–5 y) and the effect of quality measures.
Meta-regression analyses were conducted for age, gender (male ratio), duration of follow-up, education (years), transition rate to psychosis at follow-up (UHR), change in positive and negative symptoms (effect size of change from baseline to reassessment), baseline positive and negative symptoms based on Positive and Negative Syndrome Scale. Meta-regression analyses (weighted generalized least squares regressions) were conducted using SPSS version 11.0 (SPSS Inc). Meta-regression analyses performed with a random effects model were conducted using the restricted-information maximum-likelihood method with a significance level set at P < .05.
Results
Global Cognition
Meta-analysis of global cognition scores showed that performances of all 3 groups (FEP, UHR, and healthy controls) significantly improved over time (table 2). Distribution of effect sizes was very homogenous for each of the 3 groups (I2 = 0). There was no evidence of publication bias either for global cognition or for other cognitive domains (table 2).
Mean Weighted Effect Sizes for Cognitive Changes in FEP, UHR, and HCs
| Test . | Sample . | n . | D . | 95% CI . | Z . | P . | Q Test, P . | I2 . | Bias . |
|---|---|---|---|---|---|---|---|---|---|
| Global | |||||||||
| FEP | 17 | 905 | 0.30 | 0.20–0.39 | 6.11 | <.001 | .54 | 0 | 0.15 |
| UHR | 14 | 560 | 0.23 | 0.11–0.35 | 3.86 | <.001 | .95 | 0 | 0.67 |
| Con | 11 | 405 | 0.38 | 0.24–0.52 | 5.23 | <.001 | .94 | 0 | 0.81 |
| Processing speed | |||||||||
| FEP | 12 | 627 | 0.19 | 0.08–0.30 | 3.48 | <.001 | .84 | 0 | 0.93 |
| UHR | 9 | 242 | 0.18 | 0.0–0.36 | 1.95 | .05 | .64 | 0 | 0.42 |
| Con | 8 | 299 | 0.38 | 0.21–0.54 | 4.48 | <.001 | .85 | 0 | 0.98 |
| Trail A | |||||||||
| FEP | 6 | 447 | 0.25 | 0.11–0.39 | 3.44 | <.001 | .35 | 0 | |
| UHR | 6 | 141 | 0.30 | 0.06–0.54 | 2.46 | .01 | .74 | 0 | |
| Con | 5 | 167 | 0.47 | 0.25–0.69 | 4.13 | <.001 | .58 | 0 | |
| Symbol coding | |||||||||
| FEP | 6 | 254 | 0.05 | −0.34–0.43 | 0.23 | .82 | <.001 | 0.17 | |
| Con | 3 | 84 | 0.21 | −0.10–0.51 | 1.33 | .18 | .95 | 0 | |
| Stroop | |||||||||
| FEP | 3 | 252 | 0.12 | −0.06–0.29 | 1.26 | .21 | .77 | 0 | |
| Con | 3 | 128 | 0.18 | −0.14–0.50 | 1.11 | .27 | .21 | 0.03 | |
| Trail B | |||||||||
| FEP | 9 | 502 | 0.19 | 0.07–0.32 | 2.98 | .003 | .75 | 0 | |
| UHR | 5 | 141 | 0.08 | −0.38–0.53 | 0.33 | .74 | .01 | 0.20 | |
| Con | 4 | 133 | 0.45 | 0.12–0.77 | 2.69 | .007 | .21 | 0.04 | |
| Verbal memory | |||||||||
| FEP | 11 | 702 | 0.33 | 0.19–0.47 | 4.67 | <.001 | .14 | 0.02 | 0.54 |
| UHR | 12 | 532 | 0.31 | 0.12–0.51 | 3.11 | .002 | .02 | 0.06 | 0.60 |
| Con | 10 | 338 | 0.35 | 0.17–0.53 | 3.86 | <.001 | .26 | 0.02 | 0.97 |
| Learning | |||||||||
| FEP | 7 | 365 | 0.26 | 0.11–0.40 | 3.36 | <.001 | .42 | 0 | |
| UHR | 9 | 230 | 0.34 | 0.15–0.52 | 3.52 | <.001 | .52 | 0 | |
| Con | 6 | 179 | 0.30 | 0.09–0.52 | 2.81 | .005 | .85 | 0 | |
| Delayed | |||||||||
| Visual memory | |||||||||
| FEP | 10 | 574 | 0.27 | 0.06–0.48 | 2.54 | .01 | .001 | 0.07 | 0.91 |
| UHR | 5 | 92 | 0.34 | −0.02–0.70 | 1.86 | .06 | .25 | 0.04 | 0.79 |
| Con | 6 | 228 | 0.45 | 0.16–0.73 | 3.03 | .002 | .06 | 0.06 | 0.71 |
| Executive function | |||||||||
| FEP | 12 | 678 | 0.38 | 0.20–0.56 | 4.15 | <.001 | .006 | 0.05 | 0.09 |
| UHR | 5 | 208 | 0.37 | 0.17–0.56 | 3.68 | <.001 | .99 | 0 | 0.31 |
| Con | 6 | 265 | 0.39 | 0.13–0.65 | 2.97 | .003 | .06 | 0.05 | 0.07 |
| WCST per | |||||||||
| FEP | 10 | 553 | 0.43 | 0.18–0.68 | 3.32 | <.001 | <.001 | 0.11 | |
| UHR | 3 | 101 | 0.40 | 0.12–0.69 | 2.76 | .006 | .97 | 0 | |
| Con | 5 | 194 | 0.60 | 0.39–0.80 | 5.72 | <.001 | .78 | 0 | |
| Verbal WM | |||||||||
| FEP | 10 | 503 | 0.13 | −0.03–0.28 | 1.63 | .10 | .20 | 0.02 | 0.27 |
| UHR | 8 | 224 | 0.20 | 0.01–0.39 | 2.10 | .04 | .97 | 0 | 0.97 |
| Con | 7 | 268 | 0.34 | 0.16–0.51 | 3.80 | <.001 | .79 | 0 | 0.62 |
| Digit span | |||||||||
| FEP | 7 | 277 | 0.22 | 0.05–0.39 | 2.51 | .01 | .68 | 0.01 | |
| Con | 3 | 117 | 0.24 | −0.02–0.5 | 1.78 | .07 | .82 | 0 | |
| LNS | |||||||||
| UHR | 5 | 173 | 0.21 | −0.01–0.42 | 1.87 | .06 | .83 | 0 | |
| HC | 3 | 80 | 0.41 | 0.09–0.73 | 2.53 | .01 | .55 | 0 | |
| Attention | |||||||||
| FEP | 8 | 620 | 0.27 | 0.12–0.42 | 3.58 | <.001 | .14 | 0.02 | 0.27 |
| UHR | 8 | 219 | 0.33 | 0.14–0.52 | 2.80 | <.001 | .87 | 0 | 0.48 |
| Con | 7 | 155 | 0.27 | 0.08–0.46 | 2.77 | .006 | .57 | 0 | 0.11 |
| CPT d | |||||||||
| FEP | 4 | 338 | 0.23 | 0.0–0.49 | 1.95 | .05 | .11 | 0.03 | |
| UHR | 8 | 219 | 0.33 | 0.14–0.52 | 3.39 | <.001 | .87 | 0 | |
| Con | 4 | 132 | 0.34 | 0.09–0.58 | 2.70 | .007 | .63 | 0 | |
| Fluency | |||||||||
| FEP | 12 | 575 | 0.14 | 0.01–0.27 | 1.99 | .04 | .15 | 0.02 | 0.05 |
| UHR | 10 | 235 | 0.03 | −0.15–0.20 | 0.30 | .76 | .97 | 0 | 0.99 |
| Con | 9 | 364 | 0.31 | 0.14–0.49 | 3.53 | <.001 | .23 | 0.02 | 0.61 |
| Letter fluency | |||||||||
| FEP | 11 | 545 | 0.08 | −0.05–0.21 | 1.26 | .21 | .25 | 0.01 | |
| UHR | 7 | 110 | 0.07 | −0.19–0.34 | 0.54 | .59 | .94 | 0 | |
| Con | 7 | 265 | 0.26 | 0.08–0.44 | 2.88 | .004 | .60 | 0 | |
| Test . | Sample . | n . | D . | 95% CI . | Z . | P . | Q Test, P . | I2 . | Bias . |
|---|---|---|---|---|---|---|---|---|---|
| Global | |||||||||
| FEP | 17 | 905 | 0.30 | 0.20–0.39 | 6.11 | <.001 | .54 | 0 | 0.15 |
| UHR | 14 | 560 | 0.23 | 0.11–0.35 | 3.86 | <.001 | .95 | 0 | 0.67 |
| Con | 11 | 405 | 0.38 | 0.24–0.52 | 5.23 | <.001 | .94 | 0 | 0.81 |
| Processing speed | |||||||||
| FEP | 12 | 627 | 0.19 | 0.08–0.30 | 3.48 | <.001 | .84 | 0 | 0.93 |
| UHR | 9 | 242 | 0.18 | 0.0–0.36 | 1.95 | .05 | .64 | 0 | 0.42 |
| Con | 8 | 299 | 0.38 | 0.21–0.54 | 4.48 | <.001 | .85 | 0 | 0.98 |
| Trail A | |||||||||
| FEP | 6 | 447 | 0.25 | 0.11–0.39 | 3.44 | <.001 | .35 | 0 | |
| UHR | 6 | 141 | 0.30 | 0.06–0.54 | 2.46 | .01 | .74 | 0 | |
| Con | 5 | 167 | 0.47 | 0.25–0.69 | 4.13 | <.001 | .58 | 0 | |
| Symbol coding | |||||||||
| FEP | 6 | 254 | 0.05 | −0.34–0.43 | 0.23 | .82 | <.001 | 0.17 | |
| Con | 3 | 84 | 0.21 | −0.10–0.51 | 1.33 | .18 | .95 | 0 | |
| Stroop | |||||||||
| FEP | 3 | 252 | 0.12 | −0.06–0.29 | 1.26 | .21 | .77 | 0 | |
| Con | 3 | 128 | 0.18 | −0.14–0.50 | 1.11 | .27 | .21 | 0.03 | |
| Trail B | |||||||||
| FEP | 9 | 502 | 0.19 | 0.07–0.32 | 2.98 | .003 | .75 | 0 | |
| UHR | 5 | 141 | 0.08 | −0.38–0.53 | 0.33 | .74 | .01 | 0.20 | |
| Con | 4 | 133 | 0.45 | 0.12–0.77 | 2.69 | .007 | .21 | 0.04 | |
| Verbal memory | |||||||||
| FEP | 11 | 702 | 0.33 | 0.19–0.47 | 4.67 | <.001 | .14 | 0.02 | 0.54 |
| UHR | 12 | 532 | 0.31 | 0.12–0.51 | 3.11 | .002 | .02 | 0.06 | 0.60 |
| Con | 10 | 338 | 0.35 | 0.17–0.53 | 3.86 | <.001 | .26 | 0.02 | 0.97 |
| Learning | |||||||||
| FEP | 7 | 365 | 0.26 | 0.11–0.40 | 3.36 | <.001 | .42 | 0 | |
| UHR | 9 | 230 | 0.34 | 0.15–0.52 | 3.52 | <.001 | .52 | 0 | |
| Con | 6 | 179 | 0.30 | 0.09–0.52 | 2.81 | .005 | .85 | 0 | |
| Delayed | |||||||||
| Visual memory | |||||||||
| FEP | 10 | 574 | 0.27 | 0.06–0.48 | 2.54 | .01 | .001 | 0.07 | 0.91 |
| UHR | 5 | 92 | 0.34 | −0.02–0.70 | 1.86 | .06 | .25 | 0.04 | 0.79 |
| Con | 6 | 228 | 0.45 | 0.16–0.73 | 3.03 | .002 | .06 | 0.06 | 0.71 |
| Executive function | |||||||||
| FEP | 12 | 678 | 0.38 | 0.20–0.56 | 4.15 | <.001 | .006 | 0.05 | 0.09 |
| UHR | 5 | 208 | 0.37 | 0.17–0.56 | 3.68 | <.001 | .99 | 0 | 0.31 |
| Con | 6 | 265 | 0.39 | 0.13–0.65 | 2.97 | .003 | .06 | 0.05 | 0.07 |
| WCST per | |||||||||
| FEP | 10 | 553 | 0.43 | 0.18–0.68 | 3.32 | <.001 | <.001 | 0.11 | |
| UHR | 3 | 101 | 0.40 | 0.12–0.69 | 2.76 | .006 | .97 | 0 | |
| Con | 5 | 194 | 0.60 | 0.39–0.80 | 5.72 | <.001 | .78 | 0 | |
| Verbal WM | |||||||||
| FEP | 10 | 503 | 0.13 | −0.03–0.28 | 1.63 | .10 | .20 | 0.02 | 0.27 |
| UHR | 8 | 224 | 0.20 | 0.01–0.39 | 2.10 | .04 | .97 | 0 | 0.97 |
| Con | 7 | 268 | 0.34 | 0.16–0.51 | 3.80 | <.001 | .79 | 0 | 0.62 |
| Digit span | |||||||||
| FEP | 7 | 277 | 0.22 | 0.05–0.39 | 2.51 | .01 | .68 | 0.01 | |
| Con | 3 | 117 | 0.24 | −0.02–0.5 | 1.78 | .07 | .82 | 0 | |
| LNS | |||||||||
| UHR | 5 | 173 | 0.21 | −0.01–0.42 | 1.87 | .06 | .83 | 0 | |
| HC | 3 | 80 | 0.41 | 0.09–0.73 | 2.53 | .01 | .55 | 0 | |
| Attention | |||||||||
| FEP | 8 | 620 | 0.27 | 0.12–0.42 | 3.58 | <.001 | .14 | 0.02 | 0.27 |
| UHR | 8 | 219 | 0.33 | 0.14–0.52 | 2.80 | <.001 | .87 | 0 | 0.48 |
| Con | 7 | 155 | 0.27 | 0.08–0.46 | 2.77 | .006 | .57 | 0 | 0.11 |
| CPT d | |||||||||
| FEP | 4 | 338 | 0.23 | 0.0–0.49 | 1.95 | .05 | .11 | 0.03 | |
| UHR | 8 | 219 | 0.33 | 0.14–0.52 | 3.39 | <.001 | .87 | 0 | |
| Con | 4 | 132 | 0.34 | 0.09–0.58 | 2.70 | .007 | .63 | 0 | |
| Fluency | |||||||||
| FEP | 12 | 575 | 0.14 | 0.01–0.27 | 1.99 | .04 | .15 | 0.02 | 0.05 |
| UHR | 10 | 235 | 0.03 | −0.15–0.20 | 0.30 | .76 | .97 | 0 | 0.99 |
| Con | 9 | 364 | 0.31 | 0.14–0.49 | 3.53 | <.001 | .23 | 0.02 | 0.61 |
| Letter fluency | |||||||||
| FEP | 11 | 545 | 0.08 | −0.05–0.21 | 1.26 | .21 | .25 | 0.01 | |
| UHR | 7 | 110 | 0.07 | −0.19–0.34 | 0.54 | .59 | .94 | 0 | |
| Con | 7 | 265 | 0.26 | 0.08–0.44 | 2.88 | .004 | .60 | 0 | |
Note: Abbreviations are explained in the first footnote to table 1. Con, healthy controls; D, Cohen D; Bias, P value of Egger’s test; WM, working memory. Domain names are represented in bold.
Mean Weighted Effect Sizes for Cognitive Changes in FEP, UHR, and HCs
| Test . | Sample . | n . | D . | 95% CI . | Z . | P . | Q Test, P . | I2 . | Bias . |
|---|---|---|---|---|---|---|---|---|---|
| Global | |||||||||
| FEP | 17 | 905 | 0.30 | 0.20–0.39 | 6.11 | <.001 | .54 | 0 | 0.15 |
| UHR | 14 | 560 | 0.23 | 0.11–0.35 | 3.86 | <.001 | .95 | 0 | 0.67 |
| Con | 11 | 405 | 0.38 | 0.24–0.52 | 5.23 | <.001 | .94 | 0 | 0.81 |
| Processing speed | |||||||||
| FEP | 12 | 627 | 0.19 | 0.08–0.30 | 3.48 | <.001 | .84 | 0 | 0.93 |
| UHR | 9 | 242 | 0.18 | 0.0–0.36 | 1.95 | .05 | .64 | 0 | 0.42 |
| Con | 8 | 299 | 0.38 | 0.21–0.54 | 4.48 | <.001 | .85 | 0 | 0.98 |
| Trail A | |||||||||
| FEP | 6 | 447 | 0.25 | 0.11–0.39 | 3.44 | <.001 | .35 | 0 | |
| UHR | 6 | 141 | 0.30 | 0.06–0.54 | 2.46 | .01 | .74 | 0 | |
| Con | 5 | 167 | 0.47 | 0.25–0.69 | 4.13 | <.001 | .58 | 0 | |
| Symbol coding | |||||||||
| FEP | 6 | 254 | 0.05 | −0.34–0.43 | 0.23 | .82 | <.001 | 0.17 | |
| Con | 3 | 84 | 0.21 | −0.10–0.51 | 1.33 | .18 | .95 | 0 | |
| Stroop | |||||||||
| FEP | 3 | 252 | 0.12 | −0.06–0.29 | 1.26 | .21 | .77 | 0 | |
| Con | 3 | 128 | 0.18 | −0.14–0.50 | 1.11 | .27 | .21 | 0.03 | |
| Trail B | |||||||||
| FEP | 9 | 502 | 0.19 | 0.07–0.32 | 2.98 | .003 | .75 | 0 | |
| UHR | 5 | 141 | 0.08 | −0.38–0.53 | 0.33 | .74 | .01 | 0.20 | |
| Con | 4 | 133 | 0.45 | 0.12–0.77 | 2.69 | .007 | .21 | 0.04 | |
| Verbal memory | |||||||||
| FEP | 11 | 702 | 0.33 | 0.19–0.47 | 4.67 | <.001 | .14 | 0.02 | 0.54 |
| UHR | 12 | 532 | 0.31 | 0.12–0.51 | 3.11 | .002 | .02 | 0.06 | 0.60 |
| Con | 10 | 338 | 0.35 | 0.17–0.53 | 3.86 | <.001 | .26 | 0.02 | 0.97 |
| Learning | |||||||||
| FEP | 7 | 365 | 0.26 | 0.11–0.40 | 3.36 | <.001 | .42 | 0 | |
| UHR | 9 | 230 | 0.34 | 0.15–0.52 | 3.52 | <.001 | .52 | 0 | |
| Con | 6 | 179 | 0.30 | 0.09–0.52 | 2.81 | .005 | .85 | 0 | |
| Delayed | |||||||||
| Visual memory | |||||||||
| FEP | 10 | 574 | 0.27 | 0.06–0.48 | 2.54 | .01 | .001 | 0.07 | 0.91 |
| UHR | 5 | 92 | 0.34 | −0.02–0.70 | 1.86 | .06 | .25 | 0.04 | 0.79 |
| Con | 6 | 228 | 0.45 | 0.16–0.73 | 3.03 | .002 | .06 | 0.06 | 0.71 |
| Executive function | |||||||||
| FEP | 12 | 678 | 0.38 | 0.20–0.56 | 4.15 | <.001 | .006 | 0.05 | 0.09 |
| UHR | 5 | 208 | 0.37 | 0.17–0.56 | 3.68 | <.001 | .99 | 0 | 0.31 |
| Con | 6 | 265 | 0.39 | 0.13–0.65 | 2.97 | .003 | .06 | 0.05 | 0.07 |
| WCST per | |||||||||
| FEP | 10 | 553 | 0.43 | 0.18–0.68 | 3.32 | <.001 | <.001 | 0.11 | |
| UHR | 3 | 101 | 0.40 | 0.12–0.69 | 2.76 | .006 | .97 | 0 | |
| Con | 5 | 194 | 0.60 | 0.39–0.80 | 5.72 | <.001 | .78 | 0 | |
| Verbal WM | |||||||||
| FEP | 10 | 503 | 0.13 | −0.03–0.28 | 1.63 | .10 | .20 | 0.02 | 0.27 |
| UHR | 8 | 224 | 0.20 | 0.01–0.39 | 2.10 | .04 | .97 | 0 | 0.97 |
| Con | 7 | 268 | 0.34 | 0.16–0.51 | 3.80 | <.001 | .79 | 0 | 0.62 |
| Digit span | |||||||||
| FEP | 7 | 277 | 0.22 | 0.05–0.39 | 2.51 | .01 | .68 | 0.01 | |
| Con | 3 | 117 | 0.24 | −0.02–0.5 | 1.78 | .07 | .82 | 0 | |
| LNS | |||||||||
| UHR | 5 | 173 | 0.21 | −0.01–0.42 | 1.87 | .06 | .83 | 0 | |
| HC | 3 | 80 | 0.41 | 0.09–0.73 | 2.53 | .01 | .55 | 0 | |
| Attention | |||||||||
| FEP | 8 | 620 | 0.27 | 0.12–0.42 | 3.58 | <.001 | .14 | 0.02 | 0.27 |
| UHR | 8 | 219 | 0.33 | 0.14–0.52 | 2.80 | <.001 | .87 | 0 | 0.48 |
| Con | 7 | 155 | 0.27 | 0.08–0.46 | 2.77 | .006 | .57 | 0 | 0.11 |
| CPT d | |||||||||
| FEP | 4 | 338 | 0.23 | 0.0–0.49 | 1.95 | .05 | .11 | 0.03 | |
| UHR | 8 | 219 | 0.33 | 0.14–0.52 | 3.39 | <.001 | .87 | 0 | |
| Con | 4 | 132 | 0.34 | 0.09–0.58 | 2.70 | .007 | .63 | 0 | |
| Fluency | |||||||||
| FEP | 12 | 575 | 0.14 | 0.01–0.27 | 1.99 | .04 | .15 | 0.02 | 0.05 |
| UHR | 10 | 235 | 0.03 | −0.15–0.20 | 0.30 | .76 | .97 | 0 | 0.99 |
| Con | 9 | 364 | 0.31 | 0.14–0.49 | 3.53 | <.001 | .23 | 0.02 | 0.61 |
| Letter fluency | |||||||||
| FEP | 11 | 545 | 0.08 | −0.05–0.21 | 1.26 | .21 | .25 | 0.01 | |
| UHR | 7 | 110 | 0.07 | −0.19–0.34 | 0.54 | .59 | .94 | 0 | |
| Con | 7 | 265 | 0.26 | 0.08–0.44 | 2.88 | .004 | .60 | 0 | |
| Test . | Sample . | n . | D . | 95% CI . | Z . | P . | Q Test, P . | I2 . | Bias . |
|---|---|---|---|---|---|---|---|---|---|
| Global | |||||||||
| FEP | 17 | 905 | 0.30 | 0.20–0.39 | 6.11 | <.001 | .54 | 0 | 0.15 |
| UHR | 14 | 560 | 0.23 | 0.11–0.35 | 3.86 | <.001 | .95 | 0 | 0.67 |
| Con | 11 | 405 | 0.38 | 0.24–0.52 | 5.23 | <.001 | .94 | 0 | 0.81 |
| Processing speed | |||||||||
| FEP | 12 | 627 | 0.19 | 0.08–0.30 | 3.48 | <.001 | .84 | 0 | 0.93 |
| UHR | 9 | 242 | 0.18 | 0.0–0.36 | 1.95 | .05 | .64 | 0 | 0.42 |
| Con | 8 | 299 | 0.38 | 0.21–0.54 | 4.48 | <.001 | .85 | 0 | 0.98 |
| Trail A | |||||||||
| FEP | 6 | 447 | 0.25 | 0.11–0.39 | 3.44 | <.001 | .35 | 0 | |
| UHR | 6 | 141 | 0.30 | 0.06–0.54 | 2.46 | .01 | .74 | 0 | |
| Con | 5 | 167 | 0.47 | 0.25–0.69 | 4.13 | <.001 | .58 | 0 | |
| Symbol coding | |||||||||
| FEP | 6 | 254 | 0.05 | −0.34–0.43 | 0.23 | .82 | <.001 | 0.17 | |
| Con | 3 | 84 | 0.21 | −0.10–0.51 | 1.33 | .18 | .95 | 0 | |
| Stroop | |||||||||
| FEP | 3 | 252 | 0.12 | −0.06–0.29 | 1.26 | .21 | .77 | 0 | |
| Con | 3 | 128 | 0.18 | −0.14–0.50 | 1.11 | .27 | .21 | 0.03 | |
| Trail B | |||||||||
| FEP | 9 | 502 | 0.19 | 0.07–0.32 | 2.98 | .003 | .75 | 0 | |
| UHR | 5 | 141 | 0.08 | −0.38–0.53 | 0.33 | .74 | .01 | 0.20 | |
| Con | 4 | 133 | 0.45 | 0.12–0.77 | 2.69 | .007 | .21 | 0.04 | |
| Verbal memory | |||||||||
| FEP | 11 | 702 | 0.33 | 0.19–0.47 | 4.67 | <.001 | .14 | 0.02 | 0.54 |
| UHR | 12 | 532 | 0.31 | 0.12–0.51 | 3.11 | .002 | .02 | 0.06 | 0.60 |
| Con | 10 | 338 | 0.35 | 0.17–0.53 | 3.86 | <.001 | .26 | 0.02 | 0.97 |
| Learning | |||||||||
| FEP | 7 | 365 | 0.26 | 0.11–0.40 | 3.36 | <.001 | .42 | 0 | |
| UHR | 9 | 230 | 0.34 | 0.15–0.52 | 3.52 | <.001 | .52 | 0 | |
| Con | 6 | 179 | 0.30 | 0.09–0.52 | 2.81 | .005 | .85 | 0 | |
| Delayed | |||||||||
| Visual memory | |||||||||
| FEP | 10 | 574 | 0.27 | 0.06–0.48 | 2.54 | .01 | .001 | 0.07 | 0.91 |
| UHR | 5 | 92 | 0.34 | −0.02–0.70 | 1.86 | .06 | .25 | 0.04 | 0.79 |
| Con | 6 | 228 | 0.45 | 0.16–0.73 | 3.03 | .002 | .06 | 0.06 | 0.71 |
| Executive function | |||||||||
| FEP | 12 | 678 | 0.38 | 0.20–0.56 | 4.15 | <.001 | .006 | 0.05 | 0.09 |
| UHR | 5 | 208 | 0.37 | 0.17–0.56 | 3.68 | <.001 | .99 | 0 | 0.31 |
| Con | 6 | 265 | 0.39 | 0.13–0.65 | 2.97 | .003 | .06 | 0.05 | 0.07 |
| WCST per | |||||||||
| FEP | 10 | 553 | 0.43 | 0.18–0.68 | 3.32 | <.001 | <.001 | 0.11 | |
| UHR | 3 | 101 | 0.40 | 0.12–0.69 | 2.76 | .006 | .97 | 0 | |
| Con | 5 | 194 | 0.60 | 0.39–0.80 | 5.72 | <.001 | .78 | 0 | |
| Verbal WM | |||||||||
| FEP | 10 | 503 | 0.13 | −0.03–0.28 | 1.63 | .10 | .20 | 0.02 | 0.27 |
| UHR | 8 | 224 | 0.20 | 0.01–0.39 | 2.10 | .04 | .97 | 0 | 0.97 |
| Con | 7 | 268 | 0.34 | 0.16–0.51 | 3.80 | <.001 | .79 | 0 | 0.62 |
| Digit span | |||||||||
| FEP | 7 | 277 | 0.22 | 0.05–0.39 | 2.51 | .01 | .68 | 0.01 | |
| Con | 3 | 117 | 0.24 | −0.02–0.5 | 1.78 | .07 | .82 | 0 | |
| LNS | |||||||||
| UHR | 5 | 173 | 0.21 | −0.01–0.42 | 1.87 | .06 | .83 | 0 | |
| HC | 3 | 80 | 0.41 | 0.09–0.73 | 2.53 | .01 | .55 | 0 | |
| Attention | |||||||||
| FEP | 8 | 620 | 0.27 | 0.12–0.42 | 3.58 | <.001 | .14 | 0.02 | 0.27 |
| UHR | 8 | 219 | 0.33 | 0.14–0.52 | 2.80 | <.001 | .87 | 0 | 0.48 |
| Con | 7 | 155 | 0.27 | 0.08–0.46 | 2.77 | .006 | .57 | 0 | 0.11 |
| CPT d | |||||||||
| FEP | 4 | 338 | 0.23 | 0.0–0.49 | 1.95 | .05 | .11 | 0.03 | |
| UHR | 8 | 219 | 0.33 | 0.14–0.52 | 3.39 | <.001 | .87 | 0 | |
| Con | 4 | 132 | 0.34 | 0.09–0.58 | 2.70 | .007 | .63 | 0 | |
| Fluency | |||||||||
| FEP | 12 | 575 | 0.14 | 0.01–0.27 | 1.99 | .04 | .15 | 0.02 | 0.05 |
| UHR | 10 | 235 | 0.03 | −0.15–0.20 | 0.30 | .76 | .97 | 0 | 0.99 |
| Con | 9 | 364 | 0.31 | 0.14–0.49 | 3.53 | <.001 | .23 | 0.02 | 0.61 |
| Letter fluency | |||||||||
| FEP | 11 | 545 | 0.08 | −0.05–0.21 | 1.26 | .21 | .25 | 0.01 | |
| UHR | 7 | 110 | 0.07 | −0.19–0.34 | 0.54 | .59 | .94 | 0 | |
| Con | 7 | 265 | 0.26 | 0.08–0.44 | 2.88 | .004 | .60 | 0 | |
Note: Abbreviations are explained in the first footnote to table 1. Con, healthy controls; D, Cohen D; Bias, P value of Egger’s test; WM, working memory. Domain names are represented in bold.
Meta-analysis of FEP gave a very similar outcome when this analysis was restricted to samples that include only schizophrenia subjects (d = 0.24, CI = 0.04–0.43, Z = 2.36, P = .02). The magnitude of cognitive improvement observed at 1-year follow-up (d = 0.34, CI = 0.23–0.45, P < .001) was not statistically different from that at 2 years follow-up (d = 0.33, CI = 0.15–0.51, P < .001) (Qbet = 0.04, P = .84) and 3–5 (d = 0.27, CI = 0.07–0.47, P = .001) (Qbet = 0.25, P = .62). Sensitivity analyses did not suggest significant effects of quality measures (substance abuse exclusion criteria, structured clinical interview for diagnosis) on results (see online Supplementary Data).
In meta-regression analyses, there were no effects of the male/female ratio, education, transition rate, or the duration of follow-up on longitudinal cognitive changes (see online Supplementary Data).
Cognitive Domains
First-Episode Psychosis.
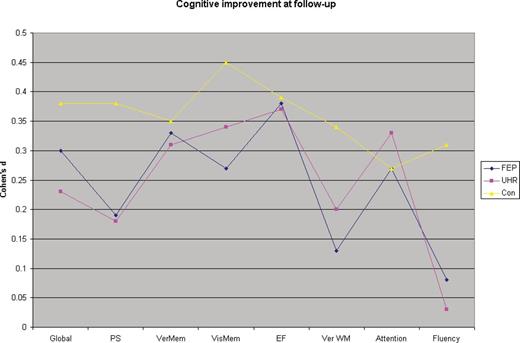
There were significant improvements in performance of FEP in verbal memory (d = 0.33), visual memory (d = 0.27), executive function (d = 0.38), processing speed (d = 0.19), attention (d = 0.26), and fluency (d = 014) (table 2 and figure 1). Only in the verbal working memory domain was improvement (d = 0.13) not significant. Heterogeneity for the distribution of effect sizes was very minimal for each of these domains (I2 = 0–0.07). In individual task analyses, there were significant improvements in list learning (d = 0.26), trail making A (d = 0.25) and B (d = 0.19), WCST per se errors (d = 0.43) and CPT d sensitivity score (d = 0.23). Heterogeneity for the distribution of effect sizes was minimal for each of the individual tasks (I2 = 0–0.17).
Cognitive improvement at follow-up in ultra-high risk (UHR), first-episode psychosis (FEP), and healthy controls.
Ultra-high Risk.
The pattern and magnitude of cognitive improvements were quite similar to FEP (figure 1). These cognitive improvements reached significance in attention (d = 0.31), verbal memory (d = 0.39), verbal working memory (d = 0.20), processing speed (d = 0.22), executive function (d = 0.36) domains (table 1). Heterogeneity for the distribution of effect sizes was very minimal for each of the 7 domains (I2 = 0–0.05). In individual task analyses, there were significant improvements in list learning (d = 0.33) and CPT d (d = 0.31). Distribution of effect sizes was very homogenous for individual tasks (I2 = 0–0.01) except trail making B test (I2 = 0.20).
Healthy Controls.
Performances of healthy controls significantly improved in all cognitive domains (d = 0.27–0.45) (table 2 and figure 1). Heterogeneity for the distribution of effect sizes was very minimal for each of these domains (I2 = 0–0.06). Individual task analyses showed significant (d = 0.26–0.59) improvements for list learning, trail making A and B, WCST per errors, CPT d sensitivity, and letter fluency. Heterogeneity for the distribution of effect sizes was very minimal for each of these domains (I2 = 0–0.04).
Cognitive Improvement in FEP and UHR in Comparison With Healthy Controls
Magnitude of improvement was not significantly different across groups for cognitive domains other than fluency and verbal working memory. In verbal working memory, improvement was significantly more pronounced in healthy controls than FEP (Qbet = 4.10, P = .04). In the fluency domain, improvement in performance was significantly more pronounced in healthy controls than FEP (Qbet = 4.9, P = .03) and UHR (Qbet = 6.2, P = .01).
The Effect of Changes in Symptoms on Cognition
Reduction in negative symptoms was significantly associated with greater improvement in executive functions and verbal working memory at follow-up of FEP. Decrease in positive symptoms was associated with improvement of visual memory performance (see online Supplementary Data). It was not possible to do a similar analysis in UHR as relevant data were not reported in most studies.
Effect of Outcome of UHR on Longitudinal Cognitive Changes
Only 5 published studies reported baseline cognitive performances of UHR-P and UHR-NP samples, and only 1 of these studies reported cognitive decline or less cognitive improvement in UHR-P than in UHR-NP in some domains.36 Four of these studies are included into the current meta-analysis, and there was no significant difference of longitudinal cognitive changes of UHR-P (d = 0.09, CI = −0.33 to 0.50, Z = 0.40, P = .69) and UHR-NP (d = 0.18, CI = −0.11 to 0.47, Z = 1.19, P = .23) (Qbet = 0.13, P = .72). Also transition rate to psychosis had no significant effect on cognitive change (see online Supplementary Data).
Medication Effects
In a meta-regression analysis, the ratio of patients taking antipsychotics was not significantly associated with longitudinal cognitive changes. In 3 studies, FEP patients were antipsychotic naive at the baseline, and these studies had a more pronounced improvement in global cognition at follow-up (d = 0.48, CI = 0.27–0.69, Z = 4.5, P < .001) (Qbet = 4.45, P = .03); however, 2 of these studies also reported the largest scale reductions in positive symptoms among all other studies (d = 3.3 and 4.0).
Meta-regression analyses of the percentage of patients receiving first-generation antipsychotics (FGA) were also not significant. In 3 studies, all patients were treated with FGA, and the magnitude of longitudinal change in cognition was not significantly different from that in other studies (d = 0.38, CI = 0.06–0.72, Z = 2.29, P = .02, Qbet = 0.45, P = .50).
It was not possible to quantitatively analyze effects of medication on cognitive change in the UHR group due to lack of reported information. In one of the UHR studies, patients taking second-generation antipsychotics had no improvement in cognition unlike other patients who took antidepressants or were medication naive.38
Discussion
The aim of this present meta-analysis was to investigate whether there is a cognitive decline over, or after, the onset of FEP. Our findings suggest that there is no evidence of such deterioration in follow-up studies of FEP and UHR. Indeed, as with healthy control subjects, there are improvements in cognitive abilities in both groups. These findings do not support neuroprogressive or staging models of schizophrenia.
Studies of FEP samples clearly showed no decline within 5 years after the onset of psychosis. FEP studies not included in this meta-analysis have shown similar findings, including samples followed for 10 years.61 Analysis of those studies that included only patients with first-episode schizophrenia gave a very similar result, suggesting that cognitive trajectories of different diagnoses within FEP are likely to be similar. However, more studies examining first-episode affective psychoses are necessary. Together with the data in established schizophrenia,24,25 these findings suggest that there is no evidence of the loss of acquired cognitive skills after the onset of psychosis. Many of the studies assessed cognition in multiple points (some also included additional early assessment 6–12 wk after first assessment), and practice effects are likely to play a significant role in cognitive improvements observed in all 3 groups. In accordance with this view, the most consistent improvements across groups were observed in tasks with significant practice effects (i.e., WCST, memory tasks), and less consistent improvements were observed in tasks with poor practice effects (i.e., letter fluency).62,63 Also the pattern of cognitive improvement seem to fit the well-known pattern of practice effects (relatively substantial early improvement followed by a plateau).45,51,52 In addition to practice effects, reductions in symptoms are likely to contribute to cognitive improvements in FEP as, in most FEP studies, there is stabilization of symptoms at follow-up. Our findings showing an association between reduction in negative symptoms (likely to be secondary negative symptoms) and improvement in working memory and executive functions, and relationship between improvement of positive symptoms and visual memory, supports this argument.
Similarly, studies in UHR subjects found no cognitive decline, thus failing to support the idea of a critical cognitive decline before the onset of psychosis. Some argue that “cognitive decline” in UHR might be specific to UHR-P. However, so far only 1 study has supported such an argument, and there were no significant differences in longitudinal cognitive changes between UHR-P and UHR-NP in this meta-analysis.36 It should be noted that in Wood et al,36 all patients in UHR-P but only 1 of UHR-NP subjects were treated with antipsychotics. This might be an important consideration given that Bowie et al38 found that antipsychotic use in UHR was associated with relatively negative effects on cognition at follow-up unlike those UHR subjects with similar characteristics, who were untreated or taking antidepressants. Therefore, current evidence does not support a cognitive decline in UHR-P subjects over the onset of psychosis. However, it should be noted that the current meta-analysis might be underpowered to detect small differences among longitudinal changes in UHR-P and UHR-NP due to the small number of studies included. Therefore, future studies examining longitudinal changes in cognition should include larger sample sizes that control for medication and symptoms.
The evidence regarding stability of cognitive functions before and after onset of psychosis contradicts the idea that schizophrenia is a progressive dementia. Findings of longitudinal brain imaging studies in schizophrenia have been interpreted by many as indicating a progressive brain disorder. However, lack of cognitive decline in prodromal and first-episode patients raises important questions regarding the nature of the supposedly “progressive” brain imaging abnormalities reported in UHR and FEP, as well as in chronic schizophrenia samples.64–67 These structural changes might be related to factors other than neurodegenerative processes intrinsic to the illness. One potential factor is the effect of antipsychotics as long-term treatment with these medications has been associated with cortical gray matter reductions.68 Also these findings might be reflections of normal but delayed cortical changes as gray matter reduction is a normal part of brain development. Other factors such as decreased environmental stimuli related to social isolation might also play a role.
Our findings support a neurodevelopmental model rather than neurodegenerative and related staging models of schizophrenia. Available evidence suggests that cognitive deficits in schizophrenia are already evident before the onset of FEP. However, timing and developmental trajectories of cognitive abnormalities in schizophrenia need to be addressed as there is relative increase of patient-control cognitive differences with age.27 In a substantial minority of the cases, these deficits develop quite early as moderate, and borderline intellectual disabilities are evident in many individuals with schizophrenia.7,69 In others, cognitive abnormalities seem to be more subtle and become evident later in development during late childhood and early adolescence.70,71 Cognitive deficits observed in schizophrenia seem to be best explained by problems in acquisition during neurodevelopment.
Limitations of this meta-analysis include differences in methodology, such as follow-up duration, different versions of cognitive tests used, as well as the small number of studies included in the UHR meta-analysis. Furthermore, many studies did not report variables that might have affected cognition such as positive/negative symptoms, functioning, cannabis, and other drug use. Unlike FEP, we were not able to examine the effect of symptom change on cognition in UHR. Also some might consider that FEP is a heterogeneous group, and cognitive decline might be evident only in first-episode schizophrenia. However, our findings showed no cognitive decline in those studies that only examined schizophrenia either. Advantages of this study include being the first meta-analysis of longitudinal cognitive changes in FEP and UHR, examination of confounding factors, and homogenous distribution of effect sizes.
In conclusion, the present meta-analysis of follow-up studies of cognition in FEP and UHR provided no evidence of cognitive decline. It is likely that cognitive abnormalities in schizophrenia develop long before the onset of the FEP as a result of abnormalities in neurodevelopment.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study. Dr Murray had received honoraria for lectures from Astra-Zenica, Janssen, Lilly, Roche, and BMS.
References