-
PDF
- Split View
-
Views
-
Cite
Cite
Dong-Sook Kim, Nam Kyung Je, Juhee Park, Sukhyang Lee, Effect of nationwide concurrent drug utilization review program on drug–drug interactions and related health outcome, International Journal for Quality in Health Care, Volume 33, Issue 3, 2021, mzab118, https://doi.org/10.1093/intqhc/mzab118
Close - Share Icon Share
Abstract
A computerized drug utilization review (DUR) program has provided physicians and pharmacists with alerts on drug–drug interactions (DDIs), drug–age precautions and therapeutic duplication in Korea since 2010.
The purpose of this study was to evaluate the impact of the DUR program on health outcomes associated with DDIs.
An uncontrolled before–after study was performed to investigate the impact of the nationwide DUR program on DDIs and related health outcomes. The study population consisted of people who used two types of DDI pairs before DUR implementation (from January 2009 to December 2010) and post-DUR implementation (from January 2012 to December 2013); (i) benzodiazepines with concurrent use of metabolic enzyme inhibitors and (ii) QTc (heart-rate corrected QT interval) prolongation agents. The main outcome measures were all-cause and cause-specific hospitalization admissions or emergency department (ED) visits.
This study included 107 874 people who used benzodiazepines with enzyme inhibitors and 8489 who received co-medication of QTc prolongation agents. For patients receiving a combination of benzodiazepines and enzyme inhibitors, both all-cause hospitalization and cause-specific hospitalization decreased after DUR implementation, from 43.2% to 41.7% and from 4.6% to 4.5% (adjusted odds ratio [OR] = 0.96; 95% confidence interval (CI), 0.93–0.98; OR = 0.89, 95% CI = 0.84–0.99, respectively). For patients receiving co-medication of QTc prolongation agents, all-cause hospitalization (54.2%) was lower than before (54.9%) (OR = 0.87, 95% CI = 0.79–0.96), but no significant change was found for cause-specific hospitalization and ED visits.
Implementation of a DUR program may reduce the adverse health outcomes posed by DDIs in patients on combination of benzodiazepines and enzyme inhibitors potentially QTc-prolongation agents.
Introduction
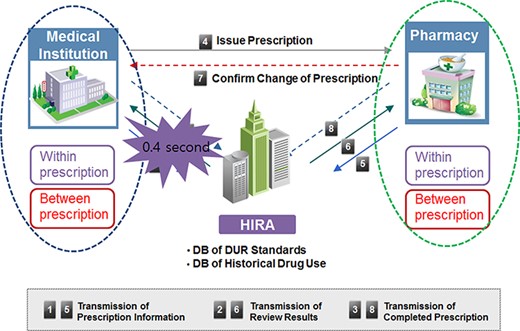
A prospective drug utilization review (DUR) aims to identify drug-related problems (DRPs) such as drug–drug interactions (DDIs) or drug–disease contraindications, therapeutic duplication or other potential adverse drug events. Hence, this approach may prevent hospital admissions and reduce medical expenditures [1–3].The Korean government implemented a nationwide computerized DUR program in real time since December 2010. Once medical institutions and pharmacies transmit prescribing and dispensing information through the program’s web server, the DUR program checks for DRPs. The criteria of DRPs are therapeutic duplication, DDIs, drug–age precautions and drug–pregnancy contraindications [3–6]. Once a DRP happens, the DUR program provides an alert message to physicians or pharmacists at the same time of prescribing and dispensing (Figure 1), and then, physicians or pharmacists are required to either fill out the reason for the prescription or modify prescription [6].
Concurrent DUR program procedure.
Previous studies have evaluated the effects of DUR in Korea [7–12]. However, those studies focused on changes in prescribing patterns using sample data, and few studies have been conducted to investigate the impact of the DUR program on adverse health outcomes in Korea. National health insurance data from a large and representative population could reflect trends in real-world medication utilization. Moreover, national health insurance data constitute a readily available resource to analyze the effects of health policies. However, there were 834 DDI pairs in DUR criteria, and we selected DDI groups with a high frequency of occurrence.
Benzodiazepines with enzyme inhibitors and co-prescription of QTc (heart-rate corrected QT interval) agents were selected as index DDI pairs since these combinations have specific diagnosis of potential adverse event and the highest frequency of occurrence. CYP3A4 (Cytochrome P450 3A4) inhibitors, such as some antifungal agents, macrolide antibiotics and antiviral medications, inhibit the metabolism of benzodiazepines. CYP inhibition increases benzodiazepine exposure, potentially leading to increased sedation and falls [13–18]. Long QT syndrome, in which the QT interval is prolonged on electrocardiography, can cause ventricular tachycardia, ventricular fibrillation or flutter, and, in severe cases, sudden cardiac arrest. Long QT syndrome can occur due to genetic factors, drugs that prolong the QTc interval and other risk factors [13–18].
This study aimed to examine whether the introduction of the DUR program reduced the number of DDIs, prevented all-cause and cause-specific hospitalization or emergency department (ED) visits in patients and reduced the length of stay (LOS) and medical costs in patients who were prescribed DDI pairs.
We hypothesized that the alert message provided by the real-time DUR system would change inappropriate medication use behaviors, thereby potentially preventing the adverse health outcomes associated with DDIs in the nationwide Korean population.
Methods
Data source and study population
The data source was National Health Insurance (NHI) claims data before DUR implementation (from January 2009 to December 2010) and after ~~ DUR implementation (from January 2012 to December 2013), as shown in Figure 1. In Korea, the NHI covers 97% of the Korean population, and claims data are submitted to the Health Insurance Review and Assessment Service (HIRA). The claim record for each prescription includes patients’ demographic characteristics, as well as the diagnosis code of the disease, the international non-proprietary names of the drug, name of the drug manufacturer, route of administration, the prescribed dose per administration and day, the total number of days prescribed and the prescription date. A previous validation study compared claims data with diagnoses in patients’ medical records and found that the overall positive predictive value of claims data for diagnoses was 83.4% [19].
The study subjects were patients older than 18 years who were prescribed two types of DDI pairs as outpatients before (comparison group) and after DUR implementation (intervention group) in the total population. We defined the use of DDI pairs by a concomitant use over at least 1 day, which is the current method of providing an alert in the DUR program. We calculated the number of patients and days of concomitant therapy before and after the introduction of the DUR program.
Selection of DDIs for DUR evaluation study
The Korea Institute of Drug Safety & Risk Management under the Ministry of Food and Drug Safety published DUR criteria based on the approved drug labels, and the Ministry of Health and Welfare and the HIRA implemented the DUR program with 834 DDI pairs. We selected DDI pairs in accordance with the DUR criteria implemented in Korea. The groups of frequent alerts in DDI pairs were statins with enzyme inhibitors, benzodiazepines with enzyme inhibitors and concomitant use of QTc prolongation agents according to the DUR report [20]. However, we excluded statins with enzyme inhibitors among DDI pairs groups, because it has been reported the rhabdomyolysis occurs due to other causes, such as genetic factors, rather than concomitant prescribing statins with enzyme inhibitors. Owing to concomitant prescribing statins with enzyme inhibitors [21]. Finally, study DDI groups were benzodiazepines with enzyme inhibitors and co-medication of QTc prolongation agents considering volume of use and the frequency of adverse drug reactions.
The benzodiazepines analyzed in this study were alprazolam, midazolam and triazolam, which are applied according to the current DUR criteria. The enzyme inhibitors were atazanavir, darunavir, efavirenz, erythromycin, fluconazole, indinavir, itraconazole, josamycin, ketoconazole, lopinavir/ritironavir and nelfinavir. QTc prolongation agents are presented in Appendix 1.
Outcome measures and variables
The outcome measures in the present study were all-cause and cause-specific hospitalizations and all-cause, and cause-specific ED visits. All-cause outcomes were defined as any hospitalization or ED visits within 365 days following the start date of DDI pair prescription during the study period. Patients who had cancer (International Classification of Diseases, 10th Revision [ICD-10]: C*), a pregnancy-related condition (ICD-10: O*, Z37–Z38) or birth defects (ICD-10: Q1–Q8, P0–P9) were excluded to reduce potential bias affecting outcomes.
Cause-specific outcomes were defined as any visit where one of the target outcomes in Appendix 2 was included as a primary diagnosis using ICD-10 codes. The hospital LOS and medical costs were analyzed as secondary outcomes. For medical costs, we did not consider the inflation over time across the entire study duration. We measured the LOS and medical costs of hospitalization for patients who were prescribed DDI pairs.
Falls and fall-related fractures were included as cause-specific outcome indicators in patients who had prescriptions for a combination of a benzodiazepine and an enzyme inhibitor, since this combination increases the effects of sedation. Based on Bauer et al.’s definition of fractures, our study used hospitalization and ED visits due to fractures as our outcome indicators [13]. Fall-related fractures were defined using the following ICD-10 codes: M484-5, S*, T02, T03, T08, T10, T11, T12 and W00-W19. Injuries due to traffic accidents were excluded. In the group with co-prescriptions of QTc prolongation agents, ventricular tachycardia (ICD-10: I472, I479), ventricular fibrillation and flutter (ICD-10: I490), and cardiac arrest (ICD-10: I46) were included as outcome indicators.
Sex, age, comorbidities, type of health insurance, medical institution type and the number of DDIs in two groups are all possible confounders of the association analyzed herein. We defined information on comorbidities according to previous diagnoses and calculated the modified Charlson comorbidity index (CCI) as a score of 0, 1, 2, 3 or higher. We also summed the number of DDI alerts during both study periods (before and after DUR implementation). We selected confounders that might influence the acceptance of DUR alerts and the risks of hospitalization based on previous studies [22].
Statistical analysis
We performed descriptive statistical analyses using the t-test or chi-square test before and after DUR implementation. Multiple logistic regression analysis was conducted to determine the association between DUR program and (i) all-cause hospitalization or ED visits, (ii) all-cause hospitalization, (iii) all-cause ED visits, (iv) cause-specific hospitalization or ED visits, (v) cause-specific hospitalization and (vi) cause-specific ED visits. For the construction of the multivariable models, we included variables that showed statistical significance (P < 0.05) in chi-square test before and after. The final model included age, gender, type health insurance, CCI (2 or higher), medical institution type (tertiary hospital, secondary hospital, hospital, nursing center, public center and clinic) and number of DDIs (three or more) as the adjusting variables. Model calibration was assessed using the Hosmer–Lemeshow goodness-of-fit test. The adjusted odds ratio (aOR) were reported with 95% confidence interval (CI).
Multivariable linear regression models were analyzed to explore the potential difference in (i) all-cause hospitalization LOS, (ii) all-cause hospitalization medical costs, (iii) cause-specific hospitalization LOS and (iv) cause-specific hospitalization cost. Results are expressed as unstandardized regression coefficients (B) with P-values. LOS and medical costs are continuous variables, so normality was assumed according to the central limit theorem.
We considered a two-tailed value of P < 0.05 to indicate statistical significance. The data analysis protocol was approved by the HIRA. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used to perform the analysis.
Results
General characteristics
The number of study subjects who were prescribed benzodiazepines/enzyme inhibitors was 61 882 (mean age: 59.4 ± 15.2) in the pre-DUR period and 45 992 (mean age: 56.4 ± 15.6) in the post-DUR intervention period. The intervention period had fewer male subjects (pre 33.5% vs. post 31.7%), fewer study subjects with a CCI score ≥2 (88.9% vs. 86.0%) and fewer study subjects with number of DDIs more than 3 DDIs (pre 23.3% vs. post 18.0%) than the comparison group. The average number of benzodiazepines and enzyme inhibitor prescriptions per study subject decreased from 2.3 ± 3.0 to 1.9 ± 2.2 (Table 1).
General characteristics of study subjects before and after implementation of the DUR program
| Categories . | Benzodiazepine + enzyme inhibitor . | QTc prolongation agent co-prescription . | ||||
|---|---|---|---|---|---|---|
| Before DUR (n = 61 882) . | After DUR (n = 45 992) . | P-value . | Before DUR (n = 3525) . | After DUR (n = 4964) . | P-value . | |
| Age (mean ± SD, year) | 59.4 ± 15.2 | 56.4 ± 15.6 | <0.0001 | 61.4 ± 19.8 | 58.3 ± 18.9 | 0.0032 |
| Male sex, no. (%) | 20 702 (33.5) | 14 575 (31.7) | <0.0001 | 1793 (50.9) | 2.522 (50.8) | 0.9569 |
| Type of health insurance, no. (%) | <0.0001 | 0.0002 | ||||
| Health insurance | 52 091 (84.2) | 39 924 (87.6) | 3208 (91.0) | 4391 (88.5) | ||
| Medical aid | 9791 (15.8) | 5668 (12.4) | 317 (9.0) | 573 (11.5) | ||
| CCI ≥ 2, no. (%) | 54 996 (88.9) | 39 144 (86.0) | <0.0001 | 2972 (84.3) | 4080 (82.2) | 0.0103 |
| Medical facilities type, no. (%) | <0.0001 | 0.2447 | ||||
| Tertiary hospital | 1961 (3.2) | 1113 (2.4) | 319 (9.0) | 398 (7.8) | ||
| Secondary hospital | 3758 (6.1) | 2829 (6.2) | 370 (10.5) | 580 (11.7) | ||
| Hospital | 4148 (6.7) | 3096 (6.8) | 176 (5.0) | 300 (6.0) | ||
| Nursing center | 501 (0.8) | 319 (0.7) | 19 (0.5) | 36 (0.7) | ||
| Public center | 1581 (2.6) | 886 (1.9) | 109 (3.1) | 112 (2.3) | ||
| Clinic | 49 933 (80.7) | 37 349 (81.9) | 31 (71.8) | 3547 (71.5) | ||
| Use of DDI pairs | ||||||
| Number of DDIs per patient, median (range) | 1 (1–132) | 1 (1–111) | <0.0001 | 3 (1–178) | 2 (1–144) | <0.0001 |
| Number of DDIs ≥3, no. (%) | 14 446 (23.3) | 7887 (17.1) | 0.0737 | 1783 (50.6) | 1230 (24.8) | <0.0001 |
| Categories . | Benzodiazepine + enzyme inhibitor . | QTc prolongation agent co-prescription . | ||||
|---|---|---|---|---|---|---|
| Before DUR (n = 61 882) . | After DUR (n = 45 992) . | P-value . | Before DUR (n = 3525) . | After DUR (n = 4964) . | P-value . | |
| Age (mean ± SD, year) | 59.4 ± 15.2 | 56.4 ± 15.6 | <0.0001 | 61.4 ± 19.8 | 58.3 ± 18.9 | 0.0032 |
| Male sex, no. (%) | 20 702 (33.5) | 14 575 (31.7) | <0.0001 | 1793 (50.9) | 2.522 (50.8) | 0.9569 |
| Type of health insurance, no. (%) | <0.0001 | 0.0002 | ||||
| Health insurance | 52 091 (84.2) | 39 924 (87.6) | 3208 (91.0) | 4391 (88.5) | ||
| Medical aid | 9791 (15.8) | 5668 (12.4) | 317 (9.0) | 573 (11.5) | ||
| CCI ≥ 2, no. (%) | 54 996 (88.9) | 39 144 (86.0) | <0.0001 | 2972 (84.3) | 4080 (82.2) | 0.0103 |
| Medical facilities type, no. (%) | <0.0001 | 0.2447 | ||||
| Tertiary hospital | 1961 (3.2) | 1113 (2.4) | 319 (9.0) | 398 (7.8) | ||
| Secondary hospital | 3758 (6.1) | 2829 (6.2) | 370 (10.5) | 580 (11.7) | ||
| Hospital | 4148 (6.7) | 3096 (6.8) | 176 (5.0) | 300 (6.0) | ||
| Nursing center | 501 (0.8) | 319 (0.7) | 19 (0.5) | 36 (0.7) | ||
| Public center | 1581 (2.6) | 886 (1.9) | 109 (3.1) | 112 (2.3) | ||
| Clinic | 49 933 (80.7) | 37 349 (81.9) | 31 (71.8) | 3547 (71.5) | ||
| Use of DDI pairs | ||||||
| Number of DDIs per patient, median (range) | 1 (1–132) | 1 (1–111) | <0.0001 | 3 (1–178) | 2 (1–144) | <0.0001 |
| Number of DDIs ≥3, no. (%) | 14 446 (23.3) | 7887 (17.1) | 0.0737 | 1783 (50.6) | 1230 (24.8) | <0.0001 |
General characteristics of study subjects before and after implementation of the DUR program
| Categories . | Benzodiazepine + enzyme inhibitor . | QTc prolongation agent co-prescription . | ||||
|---|---|---|---|---|---|---|
| Before DUR (n = 61 882) . | After DUR (n = 45 992) . | P-value . | Before DUR (n = 3525) . | After DUR (n = 4964) . | P-value . | |
| Age (mean ± SD, year) | 59.4 ± 15.2 | 56.4 ± 15.6 | <0.0001 | 61.4 ± 19.8 | 58.3 ± 18.9 | 0.0032 |
| Male sex, no. (%) | 20 702 (33.5) | 14 575 (31.7) | <0.0001 | 1793 (50.9) | 2.522 (50.8) | 0.9569 |
| Type of health insurance, no. (%) | <0.0001 | 0.0002 | ||||
| Health insurance | 52 091 (84.2) | 39 924 (87.6) | 3208 (91.0) | 4391 (88.5) | ||
| Medical aid | 9791 (15.8) | 5668 (12.4) | 317 (9.0) | 573 (11.5) | ||
| CCI ≥ 2, no. (%) | 54 996 (88.9) | 39 144 (86.0) | <0.0001 | 2972 (84.3) | 4080 (82.2) | 0.0103 |
| Medical facilities type, no. (%) | <0.0001 | 0.2447 | ||||
| Tertiary hospital | 1961 (3.2) | 1113 (2.4) | 319 (9.0) | 398 (7.8) | ||
| Secondary hospital | 3758 (6.1) | 2829 (6.2) | 370 (10.5) | 580 (11.7) | ||
| Hospital | 4148 (6.7) | 3096 (6.8) | 176 (5.0) | 300 (6.0) | ||
| Nursing center | 501 (0.8) | 319 (0.7) | 19 (0.5) | 36 (0.7) | ||
| Public center | 1581 (2.6) | 886 (1.9) | 109 (3.1) | 112 (2.3) | ||
| Clinic | 49 933 (80.7) | 37 349 (81.9) | 31 (71.8) | 3547 (71.5) | ||
| Use of DDI pairs | ||||||
| Number of DDIs per patient, median (range) | 1 (1–132) | 1 (1–111) | <0.0001 | 3 (1–178) | 2 (1–144) | <0.0001 |
| Number of DDIs ≥3, no. (%) | 14 446 (23.3) | 7887 (17.1) | 0.0737 | 1783 (50.6) | 1230 (24.8) | <0.0001 |
| Categories . | Benzodiazepine + enzyme inhibitor . | QTc prolongation agent co-prescription . | ||||
|---|---|---|---|---|---|---|
| Before DUR (n = 61 882) . | After DUR (n = 45 992) . | P-value . | Before DUR (n = 3525) . | After DUR (n = 4964) . | P-value . | |
| Age (mean ± SD, year) | 59.4 ± 15.2 | 56.4 ± 15.6 | <0.0001 | 61.4 ± 19.8 | 58.3 ± 18.9 | 0.0032 |
| Male sex, no. (%) | 20 702 (33.5) | 14 575 (31.7) | <0.0001 | 1793 (50.9) | 2.522 (50.8) | 0.9569 |
| Type of health insurance, no. (%) | <0.0001 | 0.0002 | ||||
| Health insurance | 52 091 (84.2) | 39 924 (87.6) | 3208 (91.0) | 4391 (88.5) | ||
| Medical aid | 9791 (15.8) | 5668 (12.4) | 317 (9.0) | 573 (11.5) | ||
| CCI ≥ 2, no. (%) | 54 996 (88.9) | 39 144 (86.0) | <0.0001 | 2972 (84.3) | 4080 (82.2) | 0.0103 |
| Medical facilities type, no. (%) | <0.0001 | 0.2447 | ||||
| Tertiary hospital | 1961 (3.2) | 1113 (2.4) | 319 (9.0) | 398 (7.8) | ||
| Secondary hospital | 3758 (6.1) | 2829 (6.2) | 370 (10.5) | 580 (11.7) | ||
| Hospital | 4148 (6.7) | 3096 (6.8) | 176 (5.0) | 300 (6.0) | ||
| Nursing center | 501 (0.8) | 319 (0.7) | 19 (0.5) | 36 (0.7) | ||
| Public center | 1581 (2.6) | 886 (1.9) | 109 (3.1) | 112 (2.3) | ||
| Clinic | 49 933 (80.7) | 37 349 (81.9) | 31 (71.8) | 3547 (71.5) | ||
| Use of DDI pairs | ||||||
| Number of DDIs per patient, median (range) | 1 (1–132) | 1 (1–111) | <0.0001 | 3 (1–178) | 2 (1–144) | <0.0001 |
| Number of DDIs ≥3, no. (%) | 14 446 (23.3) | 7887 (17.1) | 0.0737 | 1783 (50.6) | 1230 (24.8) | <0.0001 |
The median number of QTc prolongation co-prescriptions per study subject decreased from 3 to 2. For QTc prolongation co-prescriptions, the number of study subjects was 3525 (mean age: 61.4 ± 19.8) before and 4964 (mean age: 58.3 ± 18.9) after implementation of the DUR program. There was no significant difference in the proportion of males. The proportion of study subjects with a CCI ≥2 and DDIs ≥3 was smaller after implementation of the DUR program (pre 84.3% vs. post 82.2% and pre 50.6% vs. post 24.8%, respectively) (Table 1).
The frequent use of DDI pairs
Table 2 shows the days of exposure to the DDI pairs in the two study periods. Overall, the exposure to selected DDIs decreased from 687 427 to 279 549 days in the benzodiazepine and enzyme inhibitor combination group and from 565 861 to 221 413 days in the co-medication of QTc prolongation agents group.
Frequent DDI pairs in benzodiazepines and enzyme inhibitors
| . | Before DUR . | After DUR . | |||
|---|---|---|---|---|---|
| DDI pairs . | (days of therapy (%)) . | (days of therapy (%)) . | |||
| Benzodiazepines and enzyme inhibitors | 683 427 | (100.0) | 279 549 | (100.0) | |
| Alprazolam | Itraconazole | 411 386 | (60.2) | 167 102 | (59.8) |
| Alprazolam | Ketoconazole | 20 837 | (3.0) | 6154 | (2.2) |
| Midazolam | Atazanavir | – | (0.0) | 85 | (0.0) |
| Midazolam | Itraconazole | 2359 | (0.3) | 436 | (0.2) |
| Midazolam | Ketoconazole | 181 | (0.0) | 12 | (0.0) |
| Midazolam | Lopinavir and ritonavir | 8 | (0.0) | 6 | (0.0) |
| Triazolam | Erythromycin | 40 780 | (6.0) | 3245 | (1.2) |
| Triazolam | Fluconazole | 148 400 | (21.7) | 81 095 | (29.0) |
| Triazolam | Itraconazole | 50 135 | (7.3) | 18 911 | (6.8) |
| Triazolam | Josamycin | 3998 | (0.6) | 1225 | (0.4) |
| Triazolam | Ketoconazole | 5343 | (0.8) | 1278 | (0.5) |
| Co-medication of QTc prolongation agents | 565 861 | (100.0) | 221 413 | (100.0) | |
| Amantadine | Mizolastine | 112 | (0.0) | 409 | (0.2) |
| Amantadine | Toremifene | 1023 | (0.2) | 502 | (0.2) |
| Amiodarone | Amantadine | 14 507 | (2.6) | 10 362 | (4.7) |
| Amiodarone | Amisulpride | 532 | (0.1) | 400 | (0.2) |
| Amiodarone | Amitriptyline | 308 445 | (54.5) | 110 836 | (50.1) |
| Amiodarone | Imipramine | 31 864 | (5.6) | 15 922 | (7.2) |
| Amiodarone | Mizolastine | 1475 | (0.3) | 33 | (0.0) |
| Amiodarone | Nortriptyline | 99 704 | (17.6) | 65 526 | (29.6) |
| Amiodarone | Pimozide | 53 | (0.0) | – | (0.0) |
| Amiodarone | Quinidine | 916 | (0.2) | – | (0.0) |
| Amiodarone | Sotalol | 6047 | (1.1) | 7103 | (3.2) |
| Amiodarone | Toremifene | 1353 | (0.2) | – | (0.0) |
| Mizolastine | Flecainide | 704 | (0.1) | 57 | (0.0) |
| Mizolastine | Metronidazole | 955 | (0.2) | 409 | (0.2) |
| Mizolastine | Spiramycin and metronidazole | 603 | (0.1) | 236 | (0.1) |
| Mizolastine | Ziprasidone | 10 | (0.0) | 6 | (0.0) |
| Pimozide | Amantadine | 1829 | (0.3) | 218 | (0.1) |
| Pimozide | Amitriptyline | 7204 | (1.3) | 1323 | (0.6) |
| Pimozide | Azithromycin | 3 | (0.0) | – | (0.0) |
| Pimozide | Citalopram | 67 | (0.0) | – | (0.0) |
| Pimozide | Clarithromycin | 128 | (0.0) | – | (0.0) |
| Pimozide | Erythromycin | 2 | (0.0) | – | (0.0) |
| Pimozide | Escitalopram | 24 919 | (4.4) | 1767 | (0.8) |
| Pimozide | Fluconazole | 152 | (0.0) | – | (0.0) |
| Pimozide | Fluoxetine | 23 035 | (4.1) | 2151 | (1.0) |
| Pimozide | Fluvoxamine | 11 281 | (2.0) | 1456 | (0.7) |
| Pimozide | Imipramine | 614 | (0.1) | 74 | (0.0) |
| Pimozide | Mizolastine | 21 | (0.0) | – | (0.0) |
| Pimozide | Nortriptyline | 234 | (0.0) | 590 | (0.3) |
| Pimozide | Paroxetine | 11 135 | (2.0) | 392 | (0.2) |
| Pimozide | Roxithromycin | 103 | (0.0) | – | (0.0) |
| Pimozide | Sertraline | 11 730 | (2.1) | 1106 | (0.5) |
| Pimozide | Ziprasidone | 5101 | (0.9) | 535 | (0.2) |
| . | Before DUR . | After DUR . | |||
|---|---|---|---|---|---|
| DDI pairs . | (days of therapy (%)) . | (days of therapy (%)) . | |||
| Benzodiazepines and enzyme inhibitors | 683 427 | (100.0) | 279 549 | (100.0) | |
| Alprazolam | Itraconazole | 411 386 | (60.2) | 167 102 | (59.8) |
| Alprazolam | Ketoconazole | 20 837 | (3.0) | 6154 | (2.2) |
| Midazolam | Atazanavir | – | (0.0) | 85 | (0.0) |
| Midazolam | Itraconazole | 2359 | (0.3) | 436 | (0.2) |
| Midazolam | Ketoconazole | 181 | (0.0) | 12 | (0.0) |
| Midazolam | Lopinavir and ritonavir | 8 | (0.0) | 6 | (0.0) |
| Triazolam | Erythromycin | 40 780 | (6.0) | 3245 | (1.2) |
| Triazolam | Fluconazole | 148 400 | (21.7) | 81 095 | (29.0) |
| Triazolam | Itraconazole | 50 135 | (7.3) | 18 911 | (6.8) |
| Triazolam | Josamycin | 3998 | (0.6) | 1225 | (0.4) |
| Triazolam | Ketoconazole | 5343 | (0.8) | 1278 | (0.5) |
| Co-medication of QTc prolongation agents | 565 861 | (100.0) | 221 413 | (100.0) | |
| Amantadine | Mizolastine | 112 | (0.0) | 409 | (0.2) |
| Amantadine | Toremifene | 1023 | (0.2) | 502 | (0.2) |
| Amiodarone | Amantadine | 14 507 | (2.6) | 10 362 | (4.7) |
| Amiodarone | Amisulpride | 532 | (0.1) | 400 | (0.2) |
| Amiodarone | Amitriptyline | 308 445 | (54.5) | 110 836 | (50.1) |
| Amiodarone | Imipramine | 31 864 | (5.6) | 15 922 | (7.2) |
| Amiodarone | Mizolastine | 1475 | (0.3) | 33 | (0.0) |
| Amiodarone | Nortriptyline | 99 704 | (17.6) | 65 526 | (29.6) |
| Amiodarone | Pimozide | 53 | (0.0) | – | (0.0) |
| Amiodarone | Quinidine | 916 | (0.2) | – | (0.0) |
| Amiodarone | Sotalol | 6047 | (1.1) | 7103 | (3.2) |
| Amiodarone | Toremifene | 1353 | (0.2) | – | (0.0) |
| Mizolastine | Flecainide | 704 | (0.1) | 57 | (0.0) |
| Mizolastine | Metronidazole | 955 | (0.2) | 409 | (0.2) |
| Mizolastine | Spiramycin and metronidazole | 603 | (0.1) | 236 | (0.1) |
| Mizolastine | Ziprasidone | 10 | (0.0) | 6 | (0.0) |
| Pimozide | Amantadine | 1829 | (0.3) | 218 | (0.1) |
| Pimozide | Amitriptyline | 7204 | (1.3) | 1323 | (0.6) |
| Pimozide | Azithromycin | 3 | (0.0) | – | (0.0) |
| Pimozide | Citalopram | 67 | (0.0) | – | (0.0) |
| Pimozide | Clarithromycin | 128 | (0.0) | – | (0.0) |
| Pimozide | Erythromycin | 2 | (0.0) | – | (0.0) |
| Pimozide | Escitalopram | 24 919 | (4.4) | 1767 | (0.8) |
| Pimozide | Fluconazole | 152 | (0.0) | – | (0.0) |
| Pimozide | Fluoxetine | 23 035 | (4.1) | 2151 | (1.0) |
| Pimozide | Fluvoxamine | 11 281 | (2.0) | 1456 | (0.7) |
| Pimozide | Imipramine | 614 | (0.1) | 74 | (0.0) |
| Pimozide | Mizolastine | 21 | (0.0) | – | (0.0) |
| Pimozide | Nortriptyline | 234 | (0.0) | 590 | (0.3) |
| Pimozide | Paroxetine | 11 135 | (2.0) | 392 | (0.2) |
| Pimozide | Roxithromycin | 103 | (0.0) | – | (0.0) |
| Pimozide | Sertraline | 11 730 | (2.1) | 1106 | (0.5) |
| Pimozide | Ziprasidone | 5101 | (0.9) | 535 | (0.2) |
Frequent DDI pairs in benzodiazepines and enzyme inhibitors
| . | Before DUR . | After DUR . | |||
|---|---|---|---|---|---|
| DDI pairs . | (days of therapy (%)) . | (days of therapy (%)) . | |||
| Benzodiazepines and enzyme inhibitors | 683 427 | (100.0) | 279 549 | (100.0) | |
| Alprazolam | Itraconazole | 411 386 | (60.2) | 167 102 | (59.8) |
| Alprazolam | Ketoconazole | 20 837 | (3.0) | 6154 | (2.2) |
| Midazolam | Atazanavir | – | (0.0) | 85 | (0.0) |
| Midazolam | Itraconazole | 2359 | (0.3) | 436 | (0.2) |
| Midazolam | Ketoconazole | 181 | (0.0) | 12 | (0.0) |
| Midazolam | Lopinavir and ritonavir | 8 | (0.0) | 6 | (0.0) |
| Triazolam | Erythromycin | 40 780 | (6.0) | 3245 | (1.2) |
| Triazolam | Fluconazole | 148 400 | (21.7) | 81 095 | (29.0) |
| Triazolam | Itraconazole | 50 135 | (7.3) | 18 911 | (6.8) |
| Triazolam | Josamycin | 3998 | (0.6) | 1225 | (0.4) |
| Triazolam | Ketoconazole | 5343 | (0.8) | 1278 | (0.5) |
| Co-medication of QTc prolongation agents | 565 861 | (100.0) | 221 413 | (100.0) | |
| Amantadine | Mizolastine | 112 | (0.0) | 409 | (0.2) |
| Amantadine | Toremifene | 1023 | (0.2) | 502 | (0.2) |
| Amiodarone | Amantadine | 14 507 | (2.6) | 10 362 | (4.7) |
| Amiodarone | Amisulpride | 532 | (0.1) | 400 | (0.2) |
| Amiodarone | Amitriptyline | 308 445 | (54.5) | 110 836 | (50.1) |
| Amiodarone | Imipramine | 31 864 | (5.6) | 15 922 | (7.2) |
| Amiodarone | Mizolastine | 1475 | (0.3) | 33 | (0.0) |
| Amiodarone | Nortriptyline | 99 704 | (17.6) | 65 526 | (29.6) |
| Amiodarone | Pimozide | 53 | (0.0) | – | (0.0) |
| Amiodarone | Quinidine | 916 | (0.2) | – | (0.0) |
| Amiodarone | Sotalol | 6047 | (1.1) | 7103 | (3.2) |
| Amiodarone | Toremifene | 1353 | (0.2) | – | (0.0) |
| Mizolastine | Flecainide | 704 | (0.1) | 57 | (0.0) |
| Mizolastine | Metronidazole | 955 | (0.2) | 409 | (0.2) |
| Mizolastine | Spiramycin and metronidazole | 603 | (0.1) | 236 | (0.1) |
| Mizolastine | Ziprasidone | 10 | (0.0) | 6 | (0.0) |
| Pimozide | Amantadine | 1829 | (0.3) | 218 | (0.1) |
| Pimozide | Amitriptyline | 7204 | (1.3) | 1323 | (0.6) |
| Pimozide | Azithromycin | 3 | (0.0) | – | (0.0) |
| Pimozide | Citalopram | 67 | (0.0) | – | (0.0) |
| Pimozide | Clarithromycin | 128 | (0.0) | – | (0.0) |
| Pimozide | Erythromycin | 2 | (0.0) | – | (0.0) |
| Pimozide | Escitalopram | 24 919 | (4.4) | 1767 | (0.8) |
| Pimozide | Fluconazole | 152 | (0.0) | – | (0.0) |
| Pimozide | Fluoxetine | 23 035 | (4.1) | 2151 | (1.0) |
| Pimozide | Fluvoxamine | 11 281 | (2.0) | 1456 | (0.7) |
| Pimozide | Imipramine | 614 | (0.1) | 74 | (0.0) |
| Pimozide | Mizolastine | 21 | (0.0) | – | (0.0) |
| Pimozide | Nortriptyline | 234 | (0.0) | 590 | (0.3) |
| Pimozide | Paroxetine | 11 135 | (2.0) | 392 | (0.2) |
| Pimozide | Roxithromycin | 103 | (0.0) | – | (0.0) |
| Pimozide | Sertraline | 11 730 | (2.1) | 1106 | (0.5) |
| Pimozide | Ziprasidone | 5101 | (0.9) | 535 | (0.2) |
| . | Before DUR . | After DUR . | |||
|---|---|---|---|---|---|
| DDI pairs . | (days of therapy (%)) . | (days of therapy (%)) . | |||
| Benzodiazepines and enzyme inhibitors | 683 427 | (100.0) | 279 549 | (100.0) | |
| Alprazolam | Itraconazole | 411 386 | (60.2) | 167 102 | (59.8) |
| Alprazolam | Ketoconazole | 20 837 | (3.0) | 6154 | (2.2) |
| Midazolam | Atazanavir | – | (0.0) | 85 | (0.0) |
| Midazolam | Itraconazole | 2359 | (0.3) | 436 | (0.2) |
| Midazolam | Ketoconazole | 181 | (0.0) | 12 | (0.0) |
| Midazolam | Lopinavir and ritonavir | 8 | (0.0) | 6 | (0.0) |
| Triazolam | Erythromycin | 40 780 | (6.0) | 3245 | (1.2) |
| Triazolam | Fluconazole | 148 400 | (21.7) | 81 095 | (29.0) |
| Triazolam | Itraconazole | 50 135 | (7.3) | 18 911 | (6.8) |
| Triazolam | Josamycin | 3998 | (0.6) | 1225 | (0.4) |
| Triazolam | Ketoconazole | 5343 | (0.8) | 1278 | (0.5) |
| Co-medication of QTc prolongation agents | 565 861 | (100.0) | 221 413 | (100.0) | |
| Amantadine | Mizolastine | 112 | (0.0) | 409 | (0.2) |
| Amantadine | Toremifene | 1023 | (0.2) | 502 | (0.2) |
| Amiodarone | Amantadine | 14 507 | (2.6) | 10 362 | (4.7) |
| Amiodarone | Amisulpride | 532 | (0.1) | 400 | (0.2) |
| Amiodarone | Amitriptyline | 308 445 | (54.5) | 110 836 | (50.1) |
| Amiodarone | Imipramine | 31 864 | (5.6) | 15 922 | (7.2) |
| Amiodarone | Mizolastine | 1475 | (0.3) | 33 | (0.0) |
| Amiodarone | Nortriptyline | 99 704 | (17.6) | 65 526 | (29.6) |
| Amiodarone | Pimozide | 53 | (0.0) | – | (0.0) |
| Amiodarone | Quinidine | 916 | (0.2) | – | (0.0) |
| Amiodarone | Sotalol | 6047 | (1.1) | 7103 | (3.2) |
| Amiodarone | Toremifene | 1353 | (0.2) | – | (0.0) |
| Mizolastine | Flecainide | 704 | (0.1) | 57 | (0.0) |
| Mizolastine | Metronidazole | 955 | (0.2) | 409 | (0.2) |
| Mizolastine | Spiramycin and metronidazole | 603 | (0.1) | 236 | (0.1) |
| Mizolastine | Ziprasidone | 10 | (0.0) | 6 | (0.0) |
| Pimozide | Amantadine | 1829 | (0.3) | 218 | (0.1) |
| Pimozide | Amitriptyline | 7204 | (1.3) | 1323 | (0.6) |
| Pimozide | Azithromycin | 3 | (0.0) | – | (0.0) |
| Pimozide | Citalopram | 67 | (0.0) | – | (0.0) |
| Pimozide | Clarithromycin | 128 | (0.0) | – | (0.0) |
| Pimozide | Erythromycin | 2 | (0.0) | – | (0.0) |
| Pimozide | Escitalopram | 24 919 | (4.4) | 1767 | (0.8) |
| Pimozide | Fluconazole | 152 | (0.0) | – | (0.0) |
| Pimozide | Fluoxetine | 23 035 | (4.1) | 2151 | (1.0) |
| Pimozide | Fluvoxamine | 11 281 | (2.0) | 1456 | (0.7) |
| Pimozide | Imipramine | 614 | (0.1) | 74 | (0.0) |
| Pimozide | Mizolastine | 21 | (0.0) | – | (0.0) |
| Pimozide | Nortriptyline | 234 | (0.0) | 590 | (0.3) |
| Pimozide | Paroxetine | 11 135 | (2.0) | 392 | (0.2) |
| Pimozide | Roxithromycin | 103 | (0.0) | – | (0.0) |
| Pimozide | Sertraline | 11 730 | (2.1) | 1106 | (0.5) |
| Pimozide | Ziprasidone | 5101 | (0.9) | 535 | (0.2) |
In the benzodiazepine and enzyme inhibitor combination group, alprazolam + itraconazole was the most commonly used combination, with 411 386 days (60.2% of total DDI pair use) before DUR implementation and 167 102 days (59.8% of total DDI pair use) after DUR implementation, followed by triazolam + fluconazole, which accounted for 148 400 days (21.7%) and 81 095 days (29.0%) before and after DUR implementation, respectively. Most DDIs decreased, but the share of triazolam + fluconazole showed an increase of 8.3% points.
In the patients receiving co-medication of QTc prolongation agents, amiodarone + amitriptyline was the most commonly used drug combination, with 308 445 days (54.5% of total DDI pair use) before DUR implementation and 110 836 days (50.1% of total DDI pair use) after DUR implementation, followed by amiodarone + nortriptyline, with 99 704 (17.6%) and 65 526 days (29.6%), respectively, before and after DUR implementation. The use of amiodarone + sotalol increased from 6047 to 7103 days and that of pimozide + nortriptyline increased from 234 to 590 days.
The risk of hospitalization and ED visits
Benzodiazepine/enzyme inhibitor combination group
The all-cause hospitalization rate was lower after DUR implementation (pre 43.2% vs. post 41.7%), as was the rate of all-cause ED visits (pre 32.6% vs. post 31.3%) (Table 3). Although the rates of fall-related hospitalization and ED visits decreased after DUR implementation, the change was not large (pre 4.6% vs. post 4.5%, and 1.9% vs. 1.7%, respectively).
The risk of hospitalization or ED visits before and after implementation of the DUR program
| Categories . | Before . | After . | Crude OR (95% CI) . | Adjusted OR (95% CI) . |
|---|---|---|---|---|
| Outcome occurrence within 365 days in benzodiazepines and enzyme inhibitors | ||||
| No. of patients | 61 882 | 45 992 | ||
| All-cause outcomes | 34 473 (55.7) | 24 546 (53.8) | 0.93 (0.91–0.95)* | 1.01 (0.98–1.03) |
| All-cause hospitalization | 26 758 (43.2) | 19 173 (41.7) | 0.95 (0.93–0.98)* | 0.96 (0.93–0.98)* |
| All-cause ED visits | 20 185 (32.6) | 14 396 (31.3) | 0.95 (0.93–0.98)* | 1.02 (0.99–1.05) |
| Cause-specific outcomes | 3206 (5.2) | 2308 (5.1) | 0.98 (0.92–1.03) | 0.91 (0.86–0.96)* |
| Cause-specific hospitalization | 2841 (4.6) | 2081 (4.5) | 0.99 (0.94–1.05) | 0.89 (0.84–0.99) |
| Cause-specific ED visits | 1149 (1.9) | 801 (1.7) | 0.95 (0.86–1.04) | 0.94 (0.86–1.03) |
| Outcome occurrence within 365 days in co-medication of QTc prolongation agents | ||||
| No. of patients | 3525 | 4964 | ||
| All-cause outcomes | 2277 (64.6) | 3256 (65.6) | 1.05 (0.95–1.14) | 0.823 (0.75–0.91)* |
| All-cause hospitalization | 1935 (54.9) | 2692 (54.2) | 0.97 (0.89–1.06) | 0. 87 (0.79–0.96)* |
| All-cause ED visits | 1433 (40.7) | 2253 (45.4) | 1.21 (1.11–1.32)* | 0. 75 (0.69–0.83)* |
| Cause-specific outcomes | 78 (2.2) | 111 (2.2) | 1.04 (0.75–1.36) | 0.99 (0.73–1.34) |
| Cause-specific hospitalization | 56 (1.6) | 87 (1.8) | 1.11 (0.79–1.55) | 0.92 (0.65–1.31) |
| Cause-specific ED visits | 68 (1.9) | 80 (1.6) | 0.83 (0.60–1.15) | 1.16 (0.83–1.63) |
| Categories . | Before . | After . | Crude OR (95% CI) . | Adjusted OR (95% CI) . |
|---|---|---|---|---|
| Outcome occurrence within 365 days in benzodiazepines and enzyme inhibitors | ||||
| No. of patients | 61 882 | 45 992 | ||
| All-cause outcomes | 34 473 (55.7) | 24 546 (53.8) | 0.93 (0.91–0.95)* | 1.01 (0.98–1.03) |
| All-cause hospitalization | 26 758 (43.2) | 19 173 (41.7) | 0.95 (0.93–0.98)* | 0.96 (0.93–0.98)* |
| All-cause ED visits | 20 185 (32.6) | 14 396 (31.3) | 0.95 (0.93–0.98)* | 1.02 (0.99–1.05) |
| Cause-specific outcomes | 3206 (5.2) | 2308 (5.1) | 0.98 (0.92–1.03) | 0.91 (0.86–0.96)* |
| Cause-specific hospitalization | 2841 (4.6) | 2081 (4.5) | 0.99 (0.94–1.05) | 0.89 (0.84–0.99) |
| Cause-specific ED visits | 1149 (1.9) | 801 (1.7) | 0.95 (0.86–1.04) | 0.94 (0.86–1.03) |
| Outcome occurrence within 365 days in co-medication of QTc prolongation agents | ||||
| No. of patients | 3525 | 4964 | ||
| All-cause outcomes | 2277 (64.6) | 3256 (65.6) | 1.05 (0.95–1.14) | 0.823 (0.75–0.91)* |
| All-cause hospitalization | 1935 (54.9) | 2692 (54.2) | 0.97 (0.89–1.06) | 0. 87 (0.79–0.96)* |
| All-cause ED visits | 1433 (40.7) | 2253 (45.4) | 1.21 (1.11–1.32)* | 0. 75 (0.69–0.83)* |
| Cause-specific outcomes | 78 (2.2) | 111 (2.2) | 1.04 (0.75–1.36) | 0.99 (0.73–1.34) |
| Cause-specific hospitalization | 56 (1.6) | 87 (1.8) | 1.11 (0.79–1.55) | 0.92 (0.65–1.31) |
| Cause-specific ED visits | 68 (1.9) | 80 (1.6) | 0.83 (0.60–1.15) | 1.16 (0.83–1.63) |
A multiple logistic regression model adjusting for sex, age, the severity of chronic disease, type of health insurance, type of health-care facilities, the number of DDIs and timing (before and after).
P < 0.05.
P < 0.01.
The risk of hospitalization or ED visits before and after implementation of the DUR program
| Categories . | Before . | After . | Crude OR (95% CI) . | Adjusted OR (95% CI) . |
|---|---|---|---|---|
| Outcome occurrence within 365 days in benzodiazepines and enzyme inhibitors | ||||
| No. of patients | 61 882 | 45 992 | ||
| All-cause outcomes | 34 473 (55.7) | 24 546 (53.8) | 0.93 (0.91–0.95)* | 1.01 (0.98–1.03) |
| All-cause hospitalization | 26 758 (43.2) | 19 173 (41.7) | 0.95 (0.93–0.98)* | 0.96 (0.93–0.98)* |
| All-cause ED visits | 20 185 (32.6) | 14 396 (31.3) | 0.95 (0.93–0.98)* | 1.02 (0.99–1.05) |
| Cause-specific outcomes | 3206 (5.2) | 2308 (5.1) | 0.98 (0.92–1.03) | 0.91 (0.86–0.96)* |
| Cause-specific hospitalization | 2841 (4.6) | 2081 (4.5) | 0.99 (0.94–1.05) | 0.89 (0.84–0.99) |
| Cause-specific ED visits | 1149 (1.9) | 801 (1.7) | 0.95 (0.86–1.04) | 0.94 (0.86–1.03) |
| Outcome occurrence within 365 days in co-medication of QTc prolongation agents | ||||
| No. of patients | 3525 | 4964 | ||
| All-cause outcomes | 2277 (64.6) | 3256 (65.6) | 1.05 (0.95–1.14) | 0.823 (0.75–0.91)* |
| All-cause hospitalization | 1935 (54.9) | 2692 (54.2) | 0.97 (0.89–1.06) | 0. 87 (0.79–0.96)* |
| All-cause ED visits | 1433 (40.7) | 2253 (45.4) | 1.21 (1.11–1.32)* | 0. 75 (0.69–0.83)* |
| Cause-specific outcomes | 78 (2.2) | 111 (2.2) | 1.04 (0.75–1.36) | 0.99 (0.73–1.34) |
| Cause-specific hospitalization | 56 (1.6) | 87 (1.8) | 1.11 (0.79–1.55) | 0.92 (0.65–1.31) |
| Cause-specific ED visits | 68 (1.9) | 80 (1.6) | 0.83 (0.60–1.15) | 1.16 (0.83–1.63) |
| Categories . | Before . | After . | Crude OR (95% CI) . | Adjusted OR (95% CI) . |
|---|---|---|---|---|
| Outcome occurrence within 365 days in benzodiazepines and enzyme inhibitors | ||||
| No. of patients | 61 882 | 45 992 | ||
| All-cause outcomes | 34 473 (55.7) | 24 546 (53.8) | 0.93 (0.91–0.95)* | 1.01 (0.98–1.03) |
| All-cause hospitalization | 26 758 (43.2) | 19 173 (41.7) | 0.95 (0.93–0.98)* | 0.96 (0.93–0.98)* |
| All-cause ED visits | 20 185 (32.6) | 14 396 (31.3) | 0.95 (0.93–0.98)* | 1.02 (0.99–1.05) |
| Cause-specific outcomes | 3206 (5.2) | 2308 (5.1) | 0.98 (0.92–1.03) | 0.91 (0.86–0.96)* |
| Cause-specific hospitalization | 2841 (4.6) | 2081 (4.5) | 0.99 (0.94–1.05) | 0.89 (0.84–0.99) |
| Cause-specific ED visits | 1149 (1.9) | 801 (1.7) | 0.95 (0.86–1.04) | 0.94 (0.86–1.03) |
| Outcome occurrence within 365 days in co-medication of QTc prolongation agents | ||||
| No. of patients | 3525 | 4964 | ||
| All-cause outcomes | 2277 (64.6) | 3256 (65.6) | 1.05 (0.95–1.14) | 0.823 (0.75–0.91)* |
| All-cause hospitalization | 1935 (54.9) | 2692 (54.2) | 0.97 (0.89–1.06) | 0. 87 (0.79–0.96)* |
| All-cause ED visits | 1433 (40.7) | 2253 (45.4) | 1.21 (1.11–1.32)* | 0. 75 (0.69–0.83)* |
| Cause-specific outcomes | 78 (2.2) | 111 (2.2) | 1.04 (0.75–1.36) | 0.99 (0.73–1.34) |
| Cause-specific hospitalization | 56 (1.6) | 87 (1.8) | 1.11 (0.79–1.55) | 0.92 (0.65–1.31) |
| Cause-specific ED visits | 68 (1.9) | 80 (1.6) | 0.83 (0.60–1.15) | 1.16 (0.83–1.63) |
A multiple logistic regression model adjusting for sex, age, the severity of chronic disease, type of health insurance, type of health-care facilities, the number of DDIs and timing (before and after).
P < 0.05.
P < 0.01.
The results of multiple logistic regression analysis after adjusting for age, sex, comorbidity index, insurance type and type of medical facilities showed that the aOR of all-cause hospitalization and cause-specific hospitalization after DUR implementation was 0.96 (95% CI 0.93–0.98) and 0.88 (95% CI 0.77–0.99), respectively. However, the risks for all-cause and cause-specific ED visits did not significantly change after DUR implementation.
Co-medication of QTc prolongation agents group
While the all-cause hospitalization rate was lower after DUR implementation (pre 54.9% vs. post 54.2%), the rate of all-cause ED visits was not (pre 40.7% vs. post 45.4%). The rates of arrhythmia-related hospitalizations and ED visits did not show any statistically significant difference.
According to the results of the multiple logistic regression analysis, the aOR of all-cause hospitalization and all-cause ED visits after DUR implementation was 0.87 (95% CI 0.79–0.97) and 0.75 (95% CI 0.69–0.83), respectively.
The length of hospital stay and medical costs
In the benzodiazepine and enzyme inhibitor combination group, hospital LOS and medical costs per patient due to all-cause hospitalization showed a statistically significant decrease from 34.21 ± 50.3 days to 25.32 ± 40.77 days (p < 0.0001), and from 3631 ± 4696 USD to 2991 ± 3951 USD (p < 0.0001), respectively (Table 4). Likewise, hospital LOS and medical costs due to fall-related fractures per patient decreased from 26 ± 26.44 to 23.1 ± 24.93 days (P = 0.0041) and from 2314 ± 2712 USD to 2119 ± 2215 USD (P = 0.00066).
LOS and medical costs before and after implementation of the DUR program
| Categories . | Before . | After . | t-Test (P-value) . | β (P-value) . |
|---|---|---|---|---|
| Outcome occurrence within 365 days in benzodiazepines and enzyme inhibitors | ||||
| No. of patients | 61 882 | 45 992 | ||
| All-cause hospitalization LOS (day) | 34.21 ± 50.3 | 25.32 ± 40.77 | <0.0001 | −8.746 (<0.0001) |
| All-cause hospitalization costs (USD) | 3631 ± 4696 | 2991 ± 3951 | <0.0001 | −621.2 (<0.001) |
| Cause-specific hospitalization LOS (day) | 26 ± 26.44 | 23.1 ± 24.93 | 0.0041 | −2.973 (<0.0001) |
| Cause-specific hospitalization costs (USD) | 2314 ± 2712 | 2119 ± 2215 | <0.0001 | −197.6 (0.0066) |
| Outcome occurrence within 365 days in co-medication of QTc prolongation agents | ||||
| No. of patients | 3525 | 4964 | ||
| All-cause hospitalization LOS (day) | 28.38 ± 41.29 | 29.32 ± 43.22 | 0.0305 | 2.2633 (0.0781) |
| All-cause hospitalization costs (USD) | 4664 ± 5657 | 5215 ± 6275 | 0.0002 | 733.8 (<0.0001) |
| Cause-specific hospitalization LOS (day) | 19 ± 21.06 | 14.38 ± 12.46 | <0.0001 | −4.900 (0.1583) |
| Cause-specific hospitalization costs (USD) | 8422 ± 8378 | 8292 ± 8574 | 0.8831 | −388.3 (0.8216) |
| Categories . | Before . | After . | t-Test (P-value) . | β (P-value) . |
|---|---|---|---|---|
| Outcome occurrence within 365 days in benzodiazepines and enzyme inhibitors | ||||
| No. of patients | 61 882 | 45 992 | ||
| All-cause hospitalization LOS (day) | 34.21 ± 50.3 | 25.32 ± 40.77 | <0.0001 | −8.746 (<0.0001) |
| All-cause hospitalization costs (USD) | 3631 ± 4696 | 2991 ± 3951 | <0.0001 | −621.2 (<0.001) |
| Cause-specific hospitalization LOS (day) | 26 ± 26.44 | 23.1 ± 24.93 | 0.0041 | −2.973 (<0.0001) |
| Cause-specific hospitalization costs (USD) | 2314 ± 2712 | 2119 ± 2215 | <0.0001 | −197.6 (0.0066) |
| Outcome occurrence within 365 days in co-medication of QTc prolongation agents | ||||
| No. of patients | 3525 | 4964 | ||
| All-cause hospitalization LOS (day) | 28.38 ± 41.29 | 29.32 ± 43.22 | 0.0305 | 2.2633 (0.0781) |
| All-cause hospitalization costs (USD) | 4664 ± 5657 | 5215 ± 6275 | 0.0002 | 733.8 (<0.0001) |
| Cause-specific hospitalization LOS (day) | 19 ± 21.06 | 14.38 ± 12.46 | <0.0001 | −4.900 (0.1583) |
| Cause-specific hospitalization costs (USD) | 8422 ± 8378 | 8292 ± 8574 | 0.8831 | −388.3 (0.8216) |
A multivariable linear regression model adjusting for sex, age, the severity of chronic disease, type of health insurance, type of health-care facilities, the number of DDIs and timing (before and after).
Mean ± SD.
LOS and medical costs before and after implementation of the DUR program
| Categories . | Before . | After . | t-Test (P-value) . | β (P-value) . |
|---|---|---|---|---|
| Outcome occurrence within 365 days in benzodiazepines and enzyme inhibitors | ||||
| No. of patients | 61 882 | 45 992 | ||
| All-cause hospitalization LOS (day) | 34.21 ± 50.3 | 25.32 ± 40.77 | <0.0001 | −8.746 (<0.0001) |
| All-cause hospitalization costs (USD) | 3631 ± 4696 | 2991 ± 3951 | <0.0001 | −621.2 (<0.001) |
| Cause-specific hospitalization LOS (day) | 26 ± 26.44 | 23.1 ± 24.93 | 0.0041 | −2.973 (<0.0001) |
| Cause-specific hospitalization costs (USD) | 2314 ± 2712 | 2119 ± 2215 | <0.0001 | −197.6 (0.0066) |
| Outcome occurrence within 365 days in co-medication of QTc prolongation agents | ||||
| No. of patients | 3525 | 4964 | ||
| All-cause hospitalization LOS (day) | 28.38 ± 41.29 | 29.32 ± 43.22 | 0.0305 | 2.2633 (0.0781) |
| All-cause hospitalization costs (USD) | 4664 ± 5657 | 5215 ± 6275 | 0.0002 | 733.8 (<0.0001) |
| Cause-specific hospitalization LOS (day) | 19 ± 21.06 | 14.38 ± 12.46 | <0.0001 | −4.900 (0.1583) |
| Cause-specific hospitalization costs (USD) | 8422 ± 8378 | 8292 ± 8574 | 0.8831 | −388.3 (0.8216) |
| Categories . | Before . | After . | t-Test (P-value) . | β (P-value) . |
|---|---|---|---|---|
| Outcome occurrence within 365 days in benzodiazepines and enzyme inhibitors | ||||
| No. of patients | 61 882 | 45 992 | ||
| All-cause hospitalization LOS (day) | 34.21 ± 50.3 | 25.32 ± 40.77 | <0.0001 | −8.746 (<0.0001) |
| All-cause hospitalization costs (USD) | 3631 ± 4696 | 2991 ± 3951 | <0.0001 | −621.2 (<0.001) |
| Cause-specific hospitalization LOS (day) | 26 ± 26.44 | 23.1 ± 24.93 | 0.0041 | −2.973 (<0.0001) |
| Cause-specific hospitalization costs (USD) | 2314 ± 2712 | 2119 ± 2215 | <0.0001 | −197.6 (0.0066) |
| Outcome occurrence within 365 days in co-medication of QTc prolongation agents | ||||
| No. of patients | 3525 | 4964 | ||
| All-cause hospitalization LOS (day) | 28.38 ± 41.29 | 29.32 ± 43.22 | 0.0305 | 2.2633 (0.0781) |
| All-cause hospitalization costs (USD) | 4664 ± 5657 | 5215 ± 6275 | 0.0002 | 733.8 (<0.0001) |
| Cause-specific hospitalization LOS (day) | 19 ± 21.06 | 14.38 ± 12.46 | <0.0001 | −4.900 (0.1583) |
| Cause-specific hospitalization costs (USD) | 8422 ± 8378 | 8292 ± 8574 | 0.8831 | −388.3 (0.8216) |
A multivariable linear regression model adjusting for sex, age, the severity of chronic disease, type of health insurance, type of health-care facilities, the number of DDIs and timing (before and after).
Mean ± SD.
In the QTc prolongation agent co-prescription group, hospital LOS and medical costs per patient due to all-cause hospitalization increased from 28.38 ± 41.29 to 29.32 ± 43.22 days (P = 0.0305) and from 4664 ± 5657 USD to 5215 ± 6275 USD (P = 0.0002), respectively.
Discussion
Principal findings
In before–after study, we evaluated health outcomes such as all-cause and cause-specific hospitalization, ED visits, hospitalization days and medical costs within 365 days following the selected DDIs pairs. We found that DDIs of both groups decreased. Although the number of patients increased for co-medication of QTc prolongation agents, the days of DDIs decreased. In both groups, the risk of all-cause hospitalization and cause-specific hospitalization-related medical costs decreased after DUR. For benzodiazepine and enzyme inhibitor combination, most of the risk decreased except ED visits. However, for co-medication of QTc prolongation agents, the results of multiple logistic regression analysis adjusting for various confounding factors showed that all-cause hospitalization and ED visits decreased, but this was not the case for cause-specific outcomes. Although there was an increase in the number of patients receiving co-medication of QTC prolongation agents, the numbers and days of DDIs decreased after DUR. Only cause-specific LOS decreased significantly after adjusting for patients’ age, sex, CCI score, type of health insurance and medical institution type.
In accordance with other studies, we found DUR reduced study DDIs [23, 24] and could prevent adverse outcomes related to DDIs [10]. We hypothesized that DUR alert messages can provide feedback to physicians or pharmacists and might change their behavior; therefore, providing DDI alert messages across prescriptions through a DUR program can prevent adverse health outcomes. All benzodiazepines and enzyme inhibitors analyzed are frequently prescribed in the outpatient setting and by different physicians, partly indicating doctor shopping [20]. DUR has been effective in reducing the incidence of DDIs between different doctors [23, 24], thereby also prevented adverse health outcomes from avoidable DPRs. The previous study reported the effect of retrospective DUR associated with the risk of all-cause and cause-specific hospitalization among group which got the intervention letter more frequently compared to the groups who did not [10]. Also, we were unable to identify the effect of DUR on the risk of cause-specific hospitalization for co-medication of QTc prolongation agents. The reason for this might be that the adverse outcomes of QTc prolongation agent co-prescriptions occur infrequently. Moreover, this QTc prolongation agent was used in tertiary hospitals [20] accompanied by patient monitoring, with caution. Another possibility can partly be explained that prescriptions of DDIs after DUR program might be inevitable because physicians keep prescribing with risk–benefit consideration of DDIs despite DUR alert.
We examined the clinical effects of DUR program in numerous practices or across clinicians of different specialties and found that providing DUR alert was clinically useful. However, there is still to address the issues of balance between ~ preventing the clinically relevant adverse outcome by providing DRPs and reducing DUR alert fatigue.
Strengths and limitations
This study is meaningful in that it analyzed real patterns of change after the implementation of a nationwide program by the government. Significantly, the overall decreasing trend of DDIs in both groups should be noted. This is the first study to examine the actual number of DDIs in the total population in Korea.
The DUR program can be evaluated in a number of aspects, including its implementation from a systematic perspective, changes in clinical behavior, clinical outcomes (improvement of health outcomes such as hospitalization), patient satisfaction and economic benefits (reduction in length of hospitalization and medical costs) [25]. Previous studies compared hospital visits, ED visits, hospitalizations and the duration of hospitalization between DDI and non-DDI groups and showed that all outcomes were higher in the DDI group [26]. This study compared the length of all-cause and cause-specific hospitalization and medical costs in two DDI groups to evaluate the DUR program except for user satisfaction. Even when controlling for other factors, in accordance with Wang-Hansen, the nationwide DUR program reduced preventable adverse outcomes associated with DDIs [27].
However, there are several limitations to this study. Because the DUR program was launched nationwide, we had to choose a before–after cohort study design. An observational study cannot include unmeasured confounding factors and thereby might have limited internal validity. For example, the effects of numerous policies were not considered in our study. The study design did not have a comparison group, so we were not able to adjust for many unmeasured confounders (e.g. the impact of interventions to reduce LOS) and had potential selection bias. In addition, the size of cause-specific hospitalization was small, and all these effects might perfectly have been explained by chance or other effects. Also, we did not adjust for other medication use such as z-drug as a confounding variables. In the QTc prolongation agents group because the number of patients was low, the impact of covariates might be large. Because the number of patients was low, it is a limitation that the impact of covariance might be large in the QTc prolongation agents group. We analyzed claims data and can, therefore, only assume that patients actually took their prescribed medicines.
The Korean DUR system issues alerts for DDIs only on a yes/no basis, without a consideration of the dose. So our study population are subjects with at least one prescription for a DDI pair independent of dose, and we did not consider dose response. In addition, we defined cause-specific hospitalization using primary diagnoses from health insurance data; therefore, this outcome measure clearly cannot reflect the patients’ actual health status. Linkage should be conducted between health insurance claims data with medical records from hospitals. Lastly, although we selected two groups considering the frequency and prevalence of adverse reactions, there are many differences between DDI pairs. In the QTc prolongation agents group, the number of patients was low, so the preventive effect of DUR was found to be negligible and not statistically significant. Larger studies are needed to explain this cause and difference between all DDI pairs.
Interpretation within the context of the wider literature
In accordance with previous studies, DUR may have two significant effects on the health system: the direct effect of changing treatment upon a DDI alert and the spillover effect of applying those lessons to future patients when doctors accept the alert.
Implications for policy
Awareness of the risk of potential DDIs can prevent worse adverse outcomes associated with DDIs, especially for LOS and medical costs. Moreover, these results are consistent with a previous study, according to which the nationwide DUR program could reduce preventable adverse outcomes associated with DDIs.
Conclusions
The DUR program checks for potential DRPs, such as DDIs, between prescriptions issued by other physicians, and provides alerts to physicians or pharmacists before prescribing and dispensing. The current study suggested a significant impact of DUR program on health outcomes with DDIs in the total population in Korea. This study should be considered as a starting point for other countries’ policymakers, who may consider introducing a nationwide DUR program for patient safety.
Funding
None declared.
Authors’ contributions
D.-S.K.: design of study and writing draft paper; N.K.J.: revising draft; J.P.: data management and analysis; S.L.: revising draft.
Ethics and other permission
Approved (IRB no. 2014-012-001).
Data availability
The data cannot be made available because of restrictions on who can perform the analysis at our institution.