-
PDF
- Split View
-
Views
-
Cite
Cite
Arwen M Marker, Amy E Noser, Mark A Clements, Susana R Patton, Shared Responsibility for Type 1 Diabetes Care Is Associated With Glycemic Variability and Risk of Glycemic Excursions in Youth, Journal of Pediatric Psychology, Volume 43, Issue 1, January/February 2018, Pages 61–71, https://doi.org/10.1093/jpepsy/jsx081
Close - Share Icon Share
Abstract
We examined how parent and youth responsibility for type 1 diabetes (T1D) care is related to adherence and glycemic outcomes, namely, glycemic variability and risk of glycemic excursions.
One hundred thirty-five parent–youth dyads (10–16 years old; diagnosed with T1D for at least 6 months) participated in this study. Percent responsibility of T1D care attributed to the youth, parent, or shared was measured using the Diabetes Family Responsibility Questionnaire. We collected youth’s hemoglobin A1c (HbA1c) and glucometer downloads to examine relationships between responsibility and HbA1c, frequency of blood glucose monitoring (self-monitoring blood glucose, SMBG), risk of glycemic excursions, and actual glycemic variability using bivariate correlations and path analysis.
Participants reported shared responsibility for almost half of T1D self-care tasks. Bivariate correlations showed shared responsibility was associated with less variability, whereas parent responsibility was associated with greater glycemic variability and risk for glycemic excursions. Youth responsibility was associated with lower frequency of SMBG. The path analyses confirmed our correlational findings (ps<.05) and better characterized interactions with age for youth-reported responsibility.
Our results support the hypothesis that shared T1D responsibility is associated with better diabetes outcomes in youth.
Introduction
Youth with type 1 diabetes (T1D) consistently display poor glycemic control as measured by hemoglobin A1c (HbA1c). Data suggest that the mean HbA1c among youth is 8.5–9.0% and that only 16% of adolescents achieve the recommended HbA1c level of <7.5% (Miller et al., 2015). Especially as youth progress into adolescence, increasing age is associated with worse glycemic control and increased frequency of diabetic ketoacidosis events (Miller et al., 2015). Poor glycemic control places youth with T1D at an increased risk for diabetes-related complications, such as neuropathy and retinopathy (Chiang, Kirkman, Laffel, & Peters, 2014). Fortunately, shared responsibility between parents and youth for diabetes care is one factor that may be associated with improving these poor outcomes (Young, Lord, Patel, Gruhn, & Jaser, 2014).
The roles of parents and youth in T1D self-care are dynamic and complex. T1D management includes a multitude of tasks, such as checking blood glucose levels, administering insulin, changing pump or injection sites, monitoring food intake and physical activity levels, noticing symptoms of hyper- and hypoglycemia, and attending medical visits. It is anticipated that during early childhood, parents will assume the majority of responsibility for T1D management (Chiang et al., 2014). However, it is also expected that youth with T1D will gradually and appropriately take on more responsibility for T1D care as they move from childhood to adolescence (Chiang et al., 2014; Markowitz, Garvey, & Laffel, 2016). Previous literature suggests that youth should learn and participate in diabetes self-care tasks that are developmentally appropriate (Markowitz et al., 2016), a transition which may be fostered by parental strategies like open communication, warmth, support, and availability for problem-solving (Young, Lord, Patel, Gruhn, & Jaser, 2014).
Several studies have shown positive associations between parental responsibility and monitoring for T1D self-care and better youth glycemic control and adherence (Vesco et al., 2010; Wysocki et al. 2009; Young et al., 2014), while other studies have shown better adherence and lower HbA1c levels in families with shared parent–youth management of T1D (Gruhn, Lord, & Jaser, 2016; Vesco et al., 2010; Young et al., 2014). In a shared responsibility model of T1D management, parents gradually transfer T1D tasks to youth in response to their success in specific tasks, while maintaining a monitoring role and availability to help when youth are unable to complete tasks independently. Evidence suggests that this shared approach to T1D management may be optimal for facilitating youth’s self-efficacy for diabetes management, while also maintaining glycemic control (Palmer et al., 2004; Wiebe et al., 2014). Conversely, disagreement between parents and youth about responsibility for diabetes care tasks is associated with lower achievement of HbA1c target values (Anderson, Auslander, Jung, Miller, & Santiago, 1990), indicating the importance of understanding the roles parents and youth are expected to play in diabetes management. What is not known is if high parental responsibility for T1D or a shared parent–youth management approach also relates to youth glycemic variability and risk for glycemic excursions.
While there is evidence supporting an association between both primary parent responsibility and shared parent–youth responsibility for T1D management and better glycemic control based on youth’s HbA1c (Gruhn et al., 2016; Helgeson, Reynolds, Siminerio, Escobar, & Becker, 2008; Vesco et al., 2010; Wysocki et al., 2009; Young et al., 2014), HbA1c is an imperfect measure of glycemic control, as it may mask variations in daily glucose levels, which have been shown to increase risk of both short- and long-term complications (Gorst et al., 2015; Hoffman, Dye, Huang & Bauer, 2016). Wide variations in blood glucose levels place patients at risk for episodes of hypo- and hyperglycemia, which in turn, may lead to uncomfortable and potentially dangerous counter-regulatory symptoms (Patton et al., 2011; Patton, DeLurgio, & Clements, 2015). Moreover, there is emerging data suggesting that glycemic variability may increase risk for longer term diabetes complications, including microvascular complications (Gorst et al., 2015; Jung, 2015; Škrha, Šoupal, Škrha, & Prázný, 2016). Taken together, these findings suggest that in addition to HbA1c, it may be useful to include glycemic variability as an indicator of glycemic control and risk for diabetes complications.
In the current study, we evaluated associations between shared and unshared responsibility for T1D care and youth outcomes to provide insight into whether primary parent management, primary youth management, or shared care are more strongly linked to youth’s glycemic variability and risk for glycemic excursions. We predicted that shared responsibility for diabetes care tasks (i.e., about equal sharing of responsibility for tasks) would be associated with reduced glycemic variability and lower risk of glycemic excursions, while both high primary parent responsibility and high primary child responsibility (i.e., unshared responsibility) would be associated with increased variability and risk. To replicate and solidify previous findings, we also evaluated associations between parent–youth responsibility, glycemic control, and adherence to self-monitoring blood glucose (SMBG). We predicted that a shared responsibility for diabetes care approach would be associated with better glycemic control and adherence to SMBG, while high primary parent responsibility and high primary child responsibility would predict worse glycemic control and adherence to SMBG (Gruhn et al., 2016; Helgeson et al., 2008; Vesco et al., 2010; Wysocki et al. 2009; Young et al., 2014). Finally, we included age as a moderator in each of these analyses to assess the impact of age on responsibility for T1D care, as increasing age is known to be associated with T1D outcomes such as glycemic control and changes in roles for diabetes management (Chiang et al., 2014; Markowitz et al., 2016; Miller et al., 2015).
Methods
Participants
We recruited youth with T1D and their parents from two hospital-based pediatric diabetes clinics in the Midwestern United States to participate in a larger study about adherence and high blood sugar. To be eligible, youth had to be between the ages of 10 and 16 years and diagnosed with T1D for at least 6 months. The study team identified potential participants via medical chart review and then contacted eligible families about the study via a mailed letter or in-person solicitation during a diabetes clinic visit. Interested families provided written parental consent and youth assent before study participation. Of 152 parent–youth dyads identified as eligible and approached for study participation, 142 enrolled, and 135 completed all study measures, for a participation rate of 89%. Parents were 84.4% mothers. The majority of participants were White (86.6%) and male (54.9%). They ranged in age from 10 to 16 years old (M = 13.5; standard deviation [SD] = 1.8) and had been diagnosed with T1D for a mean of 5.6 (SD = 3.4) years. Additional participant characteristics are listed in Table I.
Descriptive Characteristics of Study Sample (N = 135)
| . | Child . | Parent . | ||
|---|---|---|---|---|
| N . | Mean (SD) . | N . | Mean (SD) . | |
| Gender (% male) | 133 | 54.9% | 133 | 15.6% |
| Age (years) | 133 | 13.51 (1.83) | – | |
| Race/ethnicity | 135 | |||
| White (%) | 86.6% | – | ||
| African American (%) | 2.9% | – | ||
| Hispanic (%) | 0.7% | – | ||
| Mixed race (%) | 6.7% | – | ||
| Other (%) | 0.7% | – | ||
| Not reported (%) | 2.2% | |||
| Marital status | 129 | |||
| Married | – | 73.3% | ||
| Single | – | 5.9% | ||
| Divorced or separated | – | 12.6% | ||
| Engaged | – | 2.2% | ||
| Widowed | – | 1.5% | ||
| Not reported (%) | – | 4.4% | ||
| Diabetes diagnosis duration (years) | 130 | 5.56 (3.43) | – | |
| Treatment regimen | 132 | |||
| Insulin pump (%) | 87.4% | – | ||
| Insulin injection therapy (%) | 10.4% | – | ||
| Not reported (%) | 2.2% | – | ||
| HbA1c | 126 | 9.18 (2.15) | – | |
| Average daily blood glucose (mg/dL) | 123 | 209.43 (55.80) | – | |
| Mean daily blood glucose checks (number of checks) | 123 | 4.22 (2.13) | – | |
| Average daily risk range | 119 | 38.21 (12.59) | – | |
| SD of blood glucose | 123 | 94.39 (23.43) | – | |
| Diabetes responsibility | 133 | 135 | ||
| Total score | 32.99 (5.02) | 33.05 (4.60) | ||
| Parent (%) | 19.15 (12.86) | 27.67 (15.21) | ||
| Child (%) | 36.75 (20.15) | 22.44 (16.28) | ||
| Shared (%) | 44.05 (16.54) | 49.72 (16.30) | ||
| Discord (frequency) | 0.56 (0.91) | – | ||
| Agreement (frequency) | 10.08 (2.90) | – | ||
| . | Child . | Parent . | ||
|---|---|---|---|---|
| N . | Mean (SD) . | N . | Mean (SD) . | |
| Gender (% male) | 133 | 54.9% | 133 | 15.6% |
| Age (years) | 133 | 13.51 (1.83) | – | |
| Race/ethnicity | 135 | |||
| White (%) | 86.6% | – | ||
| African American (%) | 2.9% | – | ||
| Hispanic (%) | 0.7% | – | ||
| Mixed race (%) | 6.7% | – | ||
| Other (%) | 0.7% | – | ||
| Not reported (%) | 2.2% | |||
| Marital status | 129 | |||
| Married | – | 73.3% | ||
| Single | – | 5.9% | ||
| Divorced or separated | – | 12.6% | ||
| Engaged | – | 2.2% | ||
| Widowed | – | 1.5% | ||
| Not reported (%) | – | 4.4% | ||
| Diabetes diagnosis duration (years) | 130 | 5.56 (3.43) | – | |
| Treatment regimen | 132 | |||
| Insulin pump (%) | 87.4% | – | ||
| Insulin injection therapy (%) | 10.4% | – | ||
| Not reported (%) | 2.2% | – | ||
| HbA1c | 126 | 9.18 (2.15) | – | |
| Average daily blood glucose (mg/dL) | 123 | 209.43 (55.80) | – | |
| Mean daily blood glucose checks (number of checks) | 123 | 4.22 (2.13) | – | |
| Average daily risk range | 119 | 38.21 (12.59) | – | |
| SD of blood glucose | 123 | 94.39 (23.43) | – | |
| Diabetes responsibility | 133 | 135 | ||
| Total score | 32.99 (5.02) | 33.05 (4.60) | ||
| Parent (%) | 19.15 (12.86) | 27.67 (15.21) | ||
| Child (%) | 36.75 (20.15) | 22.44 (16.28) | ||
| Shared (%) | 44.05 (16.54) | 49.72 (16.30) | ||
| Discord (frequency) | 0.56 (0.91) | – | ||
| Agreement (frequency) | 10.08 (2.90) | – | ||
Note: Percentages that do not add to 100% are owing to rounding decimal places.
Descriptive Characteristics of Study Sample (N = 135)
| . | Child . | Parent . | ||
|---|---|---|---|---|
| N . | Mean (SD) . | N . | Mean (SD) . | |
| Gender (% male) | 133 | 54.9% | 133 | 15.6% |
| Age (years) | 133 | 13.51 (1.83) | – | |
| Race/ethnicity | 135 | |||
| White (%) | 86.6% | – | ||
| African American (%) | 2.9% | – | ||
| Hispanic (%) | 0.7% | – | ||
| Mixed race (%) | 6.7% | – | ||
| Other (%) | 0.7% | – | ||
| Not reported (%) | 2.2% | |||
| Marital status | 129 | |||
| Married | – | 73.3% | ||
| Single | – | 5.9% | ||
| Divorced or separated | – | 12.6% | ||
| Engaged | – | 2.2% | ||
| Widowed | – | 1.5% | ||
| Not reported (%) | – | 4.4% | ||
| Diabetes diagnosis duration (years) | 130 | 5.56 (3.43) | – | |
| Treatment regimen | 132 | |||
| Insulin pump (%) | 87.4% | – | ||
| Insulin injection therapy (%) | 10.4% | – | ||
| Not reported (%) | 2.2% | – | ||
| HbA1c | 126 | 9.18 (2.15) | – | |
| Average daily blood glucose (mg/dL) | 123 | 209.43 (55.80) | – | |
| Mean daily blood glucose checks (number of checks) | 123 | 4.22 (2.13) | – | |
| Average daily risk range | 119 | 38.21 (12.59) | – | |
| SD of blood glucose | 123 | 94.39 (23.43) | – | |
| Diabetes responsibility | 133 | 135 | ||
| Total score | 32.99 (5.02) | 33.05 (4.60) | ||
| Parent (%) | 19.15 (12.86) | 27.67 (15.21) | ||
| Child (%) | 36.75 (20.15) | 22.44 (16.28) | ||
| Shared (%) | 44.05 (16.54) | 49.72 (16.30) | ||
| Discord (frequency) | 0.56 (0.91) | – | ||
| Agreement (frequency) | 10.08 (2.90) | – | ||
| . | Child . | Parent . | ||
|---|---|---|---|---|
| N . | Mean (SD) . | N . | Mean (SD) . | |
| Gender (% male) | 133 | 54.9% | 133 | 15.6% |
| Age (years) | 133 | 13.51 (1.83) | – | |
| Race/ethnicity | 135 | |||
| White (%) | 86.6% | – | ||
| African American (%) | 2.9% | – | ||
| Hispanic (%) | 0.7% | – | ||
| Mixed race (%) | 6.7% | – | ||
| Other (%) | 0.7% | – | ||
| Not reported (%) | 2.2% | |||
| Marital status | 129 | |||
| Married | – | 73.3% | ||
| Single | – | 5.9% | ||
| Divorced or separated | – | 12.6% | ||
| Engaged | – | 2.2% | ||
| Widowed | – | 1.5% | ||
| Not reported (%) | – | 4.4% | ||
| Diabetes diagnosis duration (years) | 130 | 5.56 (3.43) | – | |
| Treatment regimen | 132 | |||
| Insulin pump (%) | 87.4% | – | ||
| Insulin injection therapy (%) | 10.4% | – | ||
| Not reported (%) | 2.2% | – | ||
| HbA1c | 126 | 9.18 (2.15) | – | |
| Average daily blood glucose (mg/dL) | 123 | 209.43 (55.80) | – | |
| Mean daily blood glucose checks (number of checks) | 123 | 4.22 (2.13) | – | |
| Average daily risk range | 119 | 38.21 (12.59) | – | |
| SD of blood glucose | 123 | 94.39 (23.43) | – | |
| Diabetes responsibility | 133 | 135 | ||
| Total score | 32.99 (5.02) | 33.05 (4.60) | ||
| Parent (%) | 19.15 (12.86) | 27.67 (15.21) | ||
| Child (%) | 36.75 (20.15) | 22.44 (16.28) | ||
| Shared (%) | 44.05 (16.54) | 49.72 (16.30) | ||
| Discord (frequency) | 0.56 (0.91) | – | ||
| Agreement (frequency) | 10.08 (2.90) | – | ||
Note: Percentages that do not add to 100% are owing to rounding decimal places.
Procedure
We collected blood glucose meter data and HbA1c levels from the routine diabetes clinic visit at which the study took place. Both youth and parents completed self-report measures using iPads that linked to a secure, study-specific Internet site at each visit. Study procedures were approved by individual institutional review boards at each of the participating hospitals, and participants could earn up to $100 for completing all study measures.
Measures
The research team collected Demographic Information via medical chart review during the screening process, plus participants self-reported sex, age, race/ethnicity, and parent marital status.
We obtained HbA1c values using standard procedures. In all cases, youth HbA1c levels were processed on either the Tosoh G8 HPLC (Tosoh Bioscience Inc., San Francisco, CA) or the Afinion AS100 Analyzer (Orlando, FL). Both instruments are traceable to the Diabetes Control and Complications standard and report results as percentages (Lenters-Westra & Slingerland, 2014; NGSP, 2016).
Meter downloads completed during the routine clinic appointment during which the study visit took place provided blood glucose values and Self-Monitoring for Blood Glucose (SMBG) frequency, characterized as the mean number of blood glucose checks per day. Across participants, meter data ranged from 8 to 57 days, although the standard clinic download was 14 days. We calculated SMBG by dividing the number of total checks by the number of days for which meter data were provided. SMBG is the most commonly used direct objective measure of adherence to treatment regimen in T1D (Gandhi, Vu, Eshtehardi, Wasserman, & Hilliard, 2015). Notably, while numerous measures of T1D adherence exist, SMBG uses objective data via glucometer downloads, which is shown to be a robust and reliable predictor of health outcomes (Blackwell & Wheeler, 2016; Gandhi et al., 2015). Thus, we preferentially used SMBG as the adherence measure in this study.
We used blood glucose values from meter downloads to examine Glycemic Variability and Risk for Glycemic Excursions. First, we calculated the SD of Blood Glucose to quantify overall glycemic variability. Second, we calculated Average Daily Risk Range (ADRR), or the risk for excursions out of the target blood glucose range, as described in Kovatchev, Otto, Cox, Gonder-Frederick, & Clarke (2006). Following Kovatchev et al.’s (2006) guidelines, we required youth to have at least three blood glucose values per day for 14 days. These 14 days could be nonconsecutive but had to fall within the same 30-day window. Participants who did not have this volume of blood glucose data were excluded from ADRR calculations. If participants had >14 days with ≥3 blood glucose values, the first 14 days with complete data were used. ADRR demonstrates good concurrent validity for identifying hyperglycemia in youth (Patton et al., 2015). ADRR can be interpreted as low (<20), moderate (20–40), or high (>40) risk for glycemic excursions. These measures provide complementary information: ADRR represents risk for glycemic excursions outside of the target blood glucose range, defined as 70–180 mg/dl, while SD reflects variability for both in-range and out-of-range blood glucose values.
Finally, parent–youth dyads completed the Diabetes Family Responsibility Questionnaire (DFRQ). The DFRQ is a 17-item self-report questionnaire, which measures patterns of shared responsibility in T1D care between children and parents (Anderson et al., 1990). Items include responsibility for tasks, such as “Remembering day of clinic appointment” and “Giving insulin injections/boluses.” Parents and youth separately rated each item as a 1 (Parent takes or initiates responsibility for this almost all of the time), 2 (Parent(s) and child share responsibility for this about equally), or 3 (Child takes or initiates responsibility for this almost all of the time). This measure demonstrates adequate internal consistency and concurrent validity (Anderson et al., 1990). Rather than using total scores as described in validation studies, we used an alternate scoring method based on procedures used in Anderson et al. (2009) to calculate percent responsibility attributed to the youth, parent, or shared. The number of items rated as “1” were summed and divided by the total number of items to arrive at the percentage of items an individual reported to be typically completed by the parent. The same procedure was followed to calculate the percentage of items typically completed by the child and the percentage of items typically shared. This scoring method allowed us to independently examine the amount of perceived responsibility for tasks within each category (i.e., 35% youth, 18% parent, and 47% shared), rather than using a total score, which would combine different responsibility attributions into an aggregate score (i.e., total score = 37).
In addition, discord between parent–youth reports of responsibility has previously been found to predict lower achievement of HbA1c target values (Anderson et al., 1990). Discordance was included in post hoc analyses after we found significant discrepancies between reporters for associations with outcome variables. We calculated frequency of agreement and discord between parent and youth reports using calculations described by Anderson et al. (1990, 2009). Each item was assigned a 1 (complete parent–youth dyad agreement) or a 0 (complete parent–youth dyad disagreement). For example, if both parent and youth reported primary parent responsibility for an item, it was counted as a complete agreement. If the parent reported primary parent responsibility and the youth reported primary child responsibility for the same item, it was counted as complete disagreement. The number of complete agreements and complete disagreements were summed to result in a total agreement and a total discord score. Importantly, discord is described as an absolute disagreement between items (i.e., parent-reported primary parent responsibility, while youth-reported primary child responsibility) but not partial disagreement (i.e., one stated they are more responsible for a task, while another reporter described task as shared).
Statistical Analyses
We calculated means, SDs, and frequencies to describe levels of perceived responsibility for diabetes care tasks, parent–youth agreement and discord for responsibility reporting, HbA1c, SMBG, risk of glycemic excursions (ADRR), and glycemic variability (SD). Bivariate Pearson correlations were computed to describe basic relationships between variables. To examine the magnitude of direct relationships between perceived responsibility, adherence to SMBG, and glycemic outcomes simultaneously, we used path analyses (Schumacker & Lomax, 2004). Path analysis is a statistical method similar to multiple regression, but which simultaneously evaluates relationships between all constructs in the model while concurrently accounting for sources of residual error. This method was used to better understand complex relationships between responsibility and outcomes, such as parsing out age effects from our correlational findings, without substantially increasing risk of type 1 error. We analyzed four path models, one assessing patterns of shared responsibility based on parent report, one assessing shared responsibility based on child report, one assessing unshared responsibility based on parent report, and one assessing unshared responsibility based on child report. The shared models measured outcomes associated with diabetes care tasks for which parents and/or youth reported equally shared responsibility. The unshared models measured outcomes based on diabetes care tasks for which participants reported unequal responsibility, meaning that either the parent or youth had primary responsibility for the task most or all of the time. We estimated models using maximum likelihood and reported model parameters as standardized estimates. For all original path models, we examined age-by-responsibility interactions using a bootstrapping procedure (5,000 resamples) with 95% bias-corrected confidence intervals. In models where we found moderation, we used Mplus syntax based on the PROCESS macro to calculate simple slopes (Hayes, 2016). In models where we did not find moderation, we trimmed the models to be more parsimonious by removing unnecessary interaction terms. Analyses were completed using SPSS version 23 (IBM Corporation, Armonk, NY, USA) and Mplus version 7.2 statistical software (Muthen & Muthen, Los Angeles, CA).
Results
Primary Outcome Means
Youth had an average HbA1c of 9.18% (SD = 2.2). Only 23.8% of youth achieved glycemic control within the recommended target range of <7.5%, 30.2% of youth maintained moderate glycemic control from 7.5 to 9.0%, and 46.0% of youth displayed poor glycemic control >9.0%. Families checked blood glucose an average of 4.2 times per day (SD = 2.1) and no family used continuous glucose monitoring during the study. Daily blood glucose values were generally high, with a mean daily value of 209.4 (SD = 55.8), as compared with current standards, which recommend that youth maintain blood glucose between 90 and 150 (American Diabetes Association, 2017). Youth’s mean glycemic variability (SD) for all blood glucose values was 94.4 (SD = 23.4). Youth’s ADRR scores generally fell in the moderate-to-high range (M = 38.2; SD = 12.6) for risk of glycemic excursions. On self-report measures, participants reported shared responsibility for about half of T1D care tasks. Parents and youth reported shared responsibility for 49.7% and 44.1% of T1D tasks, parent-only responsibility for 27.6% and 19.2%, and youth-only responsibility for 22.4% and 36.8%, respectively.
Primary Analyses: Responsibility, Glycemic Variability, Risk for Glycemic Excursions, and Adherence to SMBG
Based on parent reports, parent responsibility for T1D was associated with greater glycemic variability (rSD = .228, p = .011) and risk for glycemic excursions (rADRR = .227, p = .013), while shared responsibility was associated with less variability (rSD = −.245, p = .006). In contrast, youth responsibility was not associated with variability, but did relate to SMBG (rSMBG = −.331, p < .001). Neither parent/youth nor shared responsibility was associated with HbA1c (ps > .05). Bivariate Pearson correlations are listed in Table II. Surprisingly, youth reports of responsibility on the DFRQ were not significantly associated with any of the proposed outcome variables (ps > .05). However, both parent- and child-reported responsibility were included in the final models to fully test for interactions with age, as well as main effects.
Bivariate Pearson Correlations Between Responsibility and Biopsychosocial Outcomes Based on Parent and Youth Report
| Parent report . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . |
|---|---|---|---|---|---|---|---|---|---|
| 1. Responsibility—Parent | 1.000 | ||||||||
| 2. Responsibility—Child | −.465*** | 1.000 | |||||||
| 3. Responsibility—Shared | −.467*** | −.563*** | 1.000 | ||||||
| 4. HbA1c | .020 | .023 | −.044 | 1.000 | |||||
| 5. SMBG | .155 | −.331*** | .201* | −.469*** | 1.000 | ||||
| 6. ADRR | .227* | −.101 | −.107 | .251** | .286*** | 1.000 | |||
| 7. SD | .228* | .031 | −.245** | .323*** | −.115 | .584*** | 1.000 | ||
| 8. Discordance | .601*** | −.252** | −.306*** | .074 | .165 | .127 | .160 | 1.000 | |
| 9. Youth age | −.394*** | .460*** | −.087 | .360*** | −.398*** | −.022 | .020 | −.172* | 1.000 |
Youth report | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1. Responsibility—Parent | 1.000 | ||||||||
| 2. Responsibility—Child | −.577*** | 1.000 | |||||||
| 3. Responsibility—shared | −.078 | −.769*** | 1.000 | ||||||
| 4. HbA1c | −.022 | .010 | .007 | 1.000 | |||||
| 5. SMBG | .009 | −.081 | .088 | −.469*** | 1.000 | ||||
| 6. ADRR | .061 | −.043 | .005 | .251** | .286*** | 1.000 | |||
| 7. SD | .094 | −.036 | −.026 | .323*** | −.115 | .584*** | 1.000 | ||
| 8. Discordance | .130 | .084 | −.202* | .074 | .165 | .127 | .160 | 1.000 | |
| 9. Youth age | −.330*** | .462*** | −.303*** | .360*** | −.398*** | −.022 | .020 | −.172* | 1.000 |
| Parent report . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . |
|---|---|---|---|---|---|---|---|---|---|
| 1. Responsibility—Parent | 1.000 | ||||||||
| 2. Responsibility—Child | −.465*** | 1.000 | |||||||
| 3. Responsibility—Shared | −.467*** | −.563*** | 1.000 | ||||||
| 4. HbA1c | .020 | .023 | −.044 | 1.000 | |||||
| 5. SMBG | .155 | −.331*** | .201* | −.469*** | 1.000 | ||||
| 6. ADRR | .227* | −.101 | −.107 | .251** | .286*** | 1.000 | |||
| 7. SD | .228* | .031 | −.245** | .323*** | −.115 | .584*** | 1.000 | ||
| 8. Discordance | .601*** | −.252** | −.306*** | .074 | .165 | .127 | .160 | 1.000 | |
| 9. Youth age | −.394*** | .460*** | −.087 | .360*** | −.398*** | −.022 | .020 | −.172* | 1.000 |
Youth report | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1. Responsibility—Parent | 1.000 | ||||||||
| 2. Responsibility—Child | −.577*** | 1.000 | |||||||
| 3. Responsibility—shared | −.078 | −.769*** | 1.000 | ||||||
| 4. HbA1c | −.022 | .010 | .007 | 1.000 | |||||
| 5. SMBG | .009 | −.081 | .088 | −.469*** | 1.000 | ||||
| 6. ADRR | .061 | −.043 | .005 | .251** | .286*** | 1.000 | |||
| 7. SD | .094 | −.036 | −.026 | .323*** | −.115 | .584*** | 1.000 | ||
| 8. Discordance | .130 | .084 | −.202* | .074 | .165 | .127 | .160 | 1.000 | |
| 9. Youth age | −.330*** | .462*** | −.303*** | .360*** | −.398*** | −.022 | .020 | −.172* | 1.000 |
p<.05; **p<.01; ***p<.001; frequency of self-monitoring for blood glucose (SMBG); average daily risk range (ADRR); standard deviation of glycemic variability (SD).
Bivariate Pearson Correlations Between Responsibility and Biopsychosocial Outcomes Based on Parent and Youth Report
| Parent report . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . |
|---|---|---|---|---|---|---|---|---|---|
| 1. Responsibility—Parent | 1.000 | ||||||||
| 2. Responsibility—Child | −.465*** | 1.000 | |||||||
| 3. Responsibility—Shared | −.467*** | −.563*** | 1.000 | ||||||
| 4. HbA1c | .020 | .023 | −.044 | 1.000 | |||||
| 5. SMBG | .155 | −.331*** | .201* | −.469*** | 1.000 | ||||
| 6. ADRR | .227* | −.101 | −.107 | .251** | .286*** | 1.000 | |||
| 7. SD | .228* | .031 | −.245** | .323*** | −.115 | .584*** | 1.000 | ||
| 8. Discordance | .601*** | −.252** | −.306*** | .074 | .165 | .127 | .160 | 1.000 | |
| 9. Youth age | −.394*** | .460*** | −.087 | .360*** | −.398*** | −.022 | .020 | −.172* | 1.000 |
Youth report | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1. Responsibility—Parent | 1.000 | ||||||||
| 2. Responsibility—Child | −.577*** | 1.000 | |||||||
| 3. Responsibility—shared | −.078 | −.769*** | 1.000 | ||||||
| 4. HbA1c | −.022 | .010 | .007 | 1.000 | |||||
| 5. SMBG | .009 | −.081 | .088 | −.469*** | 1.000 | ||||
| 6. ADRR | .061 | −.043 | .005 | .251** | .286*** | 1.000 | |||
| 7. SD | .094 | −.036 | −.026 | .323*** | −.115 | .584*** | 1.000 | ||
| 8. Discordance | .130 | .084 | −.202* | .074 | .165 | .127 | .160 | 1.000 | |
| 9. Youth age | −.330*** | .462*** | −.303*** | .360*** | −.398*** | −.022 | .020 | −.172* | 1.000 |
| Parent report . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . |
|---|---|---|---|---|---|---|---|---|---|
| 1. Responsibility—Parent | 1.000 | ||||||||
| 2. Responsibility—Child | −.465*** | 1.000 | |||||||
| 3. Responsibility—Shared | −.467*** | −.563*** | 1.000 | ||||||
| 4. HbA1c | .020 | .023 | −.044 | 1.000 | |||||
| 5. SMBG | .155 | −.331*** | .201* | −.469*** | 1.000 | ||||
| 6. ADRR | .227* | −.101 | −.107 | .251** | .286*** | 1.000 | |||
| 7. SD | .228* | .031 | −.245** | .323*** | −.115 | .584*** | 1.000 | ||
| 8. Discordance | .601*** | −.252** | −.306*** | .074 | .165 | .127 | .160 | 1.000 | |
| 9. Youth age | −.394*** | .460*** | −.087 | .360*** | −.398*** | −.022 | .020 | −.172* | 1.000 |
Youth report | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1. Responsibility—Parent | 1.000 | ||||||||
| 2. Responsibility—Child | −.577*** | 1.000 | |||||||
| 3. Responsibility—shared | −.078 | −.769*** | 1.000 | ||||||
| 4. HbA1c | −.022 | .010 | .007 | 1.000 | |||||
| 5. SMBG | .009 | −.081 | .088 | −.469*** | 1.000 | ||||
| 6. ADRR | .061 | −.043 | .005 | .251** | .286*** | 1.000 | |||
| 7. SD | .094 | −.036 | −.026 | .323*** | −.115 | .584*** | 1.000 | ||
| 8. Discordance | .130 | .084 | −.202* | .074 | .165 | .127 | .160 | 1.000 | |
| 9. Youth age | −.330*** | .462*** | −.303*** | .360*** | −.398*** | −.022 | .020 | −.172* | 1.000 |
p<.05; **p<.01; ***p<.001; frequency of self-monitoring for blood glucose (SMBG); average daily risk range (ADRR); standard deviation of glycemic variability (SD).
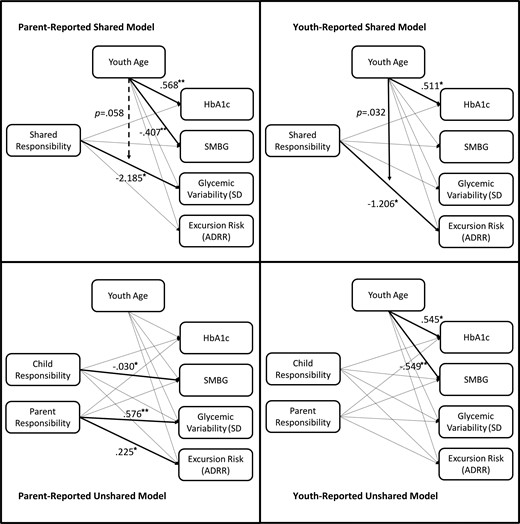
We conducted four path analysis models, two examining associations between parent-reported responsibility (shared and unshared) and outcome variables, and two examining associations between youth-reported responsibility (shared and unshared) and outcomes. All models were fully saturated; therefore, model fit statistics are not reported. In the parent-reported shared responsibility model, shared responsibility predicted lower glycemic variability (βSD = −2.185, p = .020) with a trend toward this relationship being moderated by age (p = .058). In the parent-reported unshared model, there were no significant age-by-responsibility interactions, so we trimmed these moderating variables from the final model for greater parsimony. In this trimmed model, greater parent responsibility predicted greater glycemic variability (βSD = .576, p < .001) and glycemic excursion risk (βADRR = .225, p = .013). Conversely, greater parent-reported youth responsibility predicted lower SMBG (β = −.030, p = .020). In addition, in the parent-reported unshared model, age separately predicted HbA1c (β = .568, p < .001) and SMBG (β = −.407, p < .001).
In the youth-reported shared responsibility model, shared responsibility predicted glycemic excursion risk (βADRR = −1.206, p = .036) and this relationship was moderated by age (p = .032). In addition, we found age to predict HbA1c (β = .511, p = .039). In the youth-reported unshared model, again there were no significant age-by-responsibility interactions, and so, these interactions were trimmed for greater parsimony. After trimming, youth-reported unshared responsibility did not predict any outcome variables (ps > .05); however, age independently predicted HbA1c (β = .545, p < .001) and SMBG (β = −.549, p < .001). Significant pathways for all four models are presented in Figure 1.
Shared and unshared models of parent- and youth-reported responsibility as predictors of physical and adherence outcomes.
*p < .05; **p < .01; only significant results are reported as standardized estimates (beta weights).
Post Hoc Analyses: Parent–Youth Agreement and Discord
Owing to findings that only parent reports were significantly correlated with outcome variables, we more closely examined differences between parent and youth reports of responsibility. We found that parents were more likely to report parent responsibility for nonshared tasks, while youth were more likely to report youth accountability for nonshared tasks. Overall, parent and youth reports significantly differed when compared using paired-samples t-tests (ps < .01), even though discordance between parent and youth reports was low (M = 0.56; SD = 0.91) and agreement was moderately high (M = 10.08; SD = 2.90). Of 17 total items on the DFRQ, the largest number of items on which a parent and youth directly disagreed was five. The majority of the parent–youth dyads (61.5%) did not directly disagree on a single item.
In relation to outcome variables, discordance about responsibility for T1D care tasks was significantly and negatively associated with parent-reported primary youth responsibility for T1D and both parent and youth reports of shared responsibility (all ps < .05); and positively associated with primary parent responsibility based on parent reports only (p < .001). In addition, discordance was significantly associated with age and sex, such that younger youth and males displayed higher discord than older youth and females. Discordance was not directly associated with outcome variables, including HbA1c, SMBG, ADRR, or SD of blood glucose (ps > .05).
Discussion
The study findings extend the literature on parent–child responsibility for T1D management to measure its relation to youth glycemic variability and risk for glycemic excursions. Notably, shared responsibility was found to be associated with lower glycemic variability and risk of glycemic excursions, while primary parent responsibility was associated with greater variability and increased risk of glycemic excursions outside of the target blood glucose range. We used SD and ADRR to quantify variability and risk, respectively, because these variables provide a more accurate measure of an individual’s experience of hypo- and hyperglycemia than mean daily blood glucose or HbA1c (McCall & Kovatchev 2009). In fact, McCall et al. stated that ADRR is “the single best predictor of glucose extremes” (2009, p. 5). The ADRR is also advantageous because it uses transformed blood glucose values, thus making it less vulnerable to the effects of more extreme hyperglycemia than hypoglycemia owing to the inherent asymmetry of blood glucose in persons with T1D (Kovatchev et al., 2006). High glycemic variability and risk for glycemic excursions are associated with altered mood, unpleasant physical symptoms, cognitive dysfunction, increased risk to develop cardiovascular disease, and even death (Kovatchev et al., 2006; McCall & Kovatchev 2009). In addition, glycemic variability is a key barrier to achieving clinical recommendations for glycemic control (McCall & Kovatchev 2009). Our findings suggest that shared parent–youth responsibility for T1D care tasks was associated with lower variability and risk for glycemic excursions, while parent-only T1D management was associated with increased risk. Although the direction of this association cannot be confirmed owing to the cross-sectional nature of this study, clinicians may consider reinforcing recommendations to promote shared approaches to T1D management for preadolescents and adolescents as a link to better youth mood, physical outcomes, and long-term health outcomes.
This study also supports some association between responsibility for T1D care tasks and frequency of SMBG by finding that high youth responsibility was associated with less frequent blood glucose checks. Given documented adherence declines during adolescence, it is intuitive that youth with more primary responsibility may have lower rates of SMBG. Adolescents may have a number of competing interests or a lack of understanding about the long-term consequences of poor adherence behaviors, which may make adherence behaviors difficult to complete independently and highlight the importance of continued parental monitoring to maintain good adherence (Peters, Laffel, & The American Diabetes Association Transitions Working Group, 2011). This finding was further supported by negative associations between age and SMBG, indicating that older adolescents engaged in less frequent SMBG than younger adolescents. However, we did not find expected associations between shared responsibility and more frequent SMBG (Nansel et al., 2009; Young et al., 2014) or high parent responsibility and more frequent SMBG (Ellis et al., 2007; Vesco et al., 2010). These associations may not have been seen in our sample because our study included only one measure of adherence: frequency of SMBG. Thus, our findings may not be directly comparable with previous studies that found associations between responsibility, self-monitoring, and broader adherence behaviors (i.e., Helgeson et al., 2008; Wysocki et al., 2009). In addition, only one item on the DFRQ directly addressed SMBG: “Remembering times when blood sugar or urine should be tested” (Item 17). Only 10% of families reported parent responsibility for this item, which may have resulted in limited variability. Previous studies demonstrate strong links between low adherence, poor glycemic control, and long-term negative health complications. Future studies should continue to explore relationships between responsibility and SMBG, as well as broader adherence behaviors, to pinpoint ways in which adherence to SMBG can be improved during the high-risk period of adolescence.
Interestingly, we found no association between responsibility for T1D care tasks and HbA1c. Our findings were similar to those of Vesco et al. (2010) who also found that caregiver involvement was not associated with glycemic control in adolescents. However, some previous studies have found collaborative parenting or continued high parent involvement for T1D care throughout adolescence to be directly associated with better glycemic control (Gruhn et al., 2016; Wysocki et al., 2009), or indirectly related to HbA1c through adherence (Wu, Hilliard, Rausch, Dolan, & Hood, 2013). One possible explanation for this discrepancy could be that previously observed associations between responsibility and HbA1c were driven by fathers. Indeed, a recent review found that only paternal monitoring, not maternal monitoring, predicted in-range glycemic control in adolescents (Young et al, 2014). Because our parent sample included only 15% fathers, we may have had less ability to find this association even though our overall study was adequately powered to detect a relation between shared responsibility and youth HbA1c (Gruhn et al., 2016). In addition, age was positively associated with HbA1c in three of the four path models, indicating that age effects were the strongest predictor of glycemic control. Researchers should be sure to control for age or test for age as a moderator when examining the effects of responsibility on glycemic control. Owing to mixed findings in the literature, we recommend more father perspectives and adequate age sensitivity should be included in future research to solidify associations between shared parent–youth T1D care and glycemic control.
Additional research is needed to determine whether interventions that experimentally promote shared care can reduce glycemic variability. This study supports the hypothesis that a shared responsibility approach to T1D care is associated with less glycemic variability and risk for glycemic excursions. If this hypothesis is true, then it would support the recommendation that families of preadolescents and adolescents with T1D adopt a shared approach to T1D management versus a parent- or child-only approach. Based on previous literature, to promote successful shared relationships, clinicians may consider: (1) encouraging parents to include their youth in T1D care tasks at a developmentally appropriate level, (2) encouraging active discussions between parents and youth about who should complete tasks, and (3) assessing for changes in youth self-efficacy (Law, Walsh, Queralt, & Nouwen, 2013; Young et al., 2014). Addressing shared responsibility for T1D management with families may also be a way that clinicians can promote resolving diabetes-related conflict, another construct that has been shown to detrimentally impact glycemic control and treatment adherence (Anderson et al., 2002; Anderson et al., 2009; Lancaster, Gadaire, Holman, & LeBlanc, 2015). For families who struggle with overinvolved or absent parenting for T1D management, future studies may wish to investigate family-based behavioral interventions, as these have been shown to improve adherence and glycemic control with moderate effect sizes, reduce family conflict, and may be useful for improving other family processes, as well (Hood, Rohan, Peterson, & Drotar, 2010; Winkley, Landau, Eisler, & Ismail, 2006; Wysocki et al., 2007).
Youth perceptions of shared versus nonshared care have been shown to impact T1D outcomes (Helgeson et al., 2008) and discord between parent–youth reports of responsibility have previously been found to predict worse glycemic control (Anderson et al., 1990). However, in our sample, parent reports on the DFRQ were more associated with outcome variables than were youth reports. Parent and youth reports of responsibility displayed high levels of agreement and low levels of discord, implying that responses were similar across reporters. Other differences between reporters, which may account for these findings, need to be investigated further. One possible explanation could be that youth age had a large impact on self-reported responsibility and youth outcomes. In support of this hypothesis, we found moderation by age for both glycemic variability and risk for glycemic excursions, and age as the only predictor for HbA1c. In addition, we found that younger youth displayed more discord than older youth, which may suggest that preadolescents and their parents have less clearly defined roles for self-care tasks, while older adolescents have developed clearer expectations with their parents about who will complete self-care tasks. This mirrors findings by Anderson et al. (2009), in which they found significantly higher discord for a younger cohort versus an older cohort of adolescents. Future research should continue to examine both parent and youth reports of responsibility for T1D management and identify factors, which may account for discrepancies between parent and youth reports.
The strengths of our study include its focus on the relation between family T1D responsibility and youth glycemic variability and risk for glycemic excursions, a design that has yielded novel results. Our study is also strong because we used an alternative scoring method for the DFRQ, which allowed us to directly quantify the percentage of diabetes management tasks completed by parents, youth, or shared, thus yielding more specific results pertaining to the impact of responsibility on T1D outcomes. However, we acknowledge a few limitations. Our alternate scoring method for the DFRQ has not been validated, and additional studies are needed to validate this scoring algorithm. In addition, we calculated glycemic variability, risk for glycemic excursions, and SMBG from blood glucose meter downloads at a routine clinic visit; however, some families may use multiple meters such that we may not have had access to all blood glucose data from some families. Furthermore, because we calculated youth’s glycemic variability, risk for glycemic excursions, and SMBG scores using data from a single meter download, there is some degree of interdependence among these measures. However, moderate to weak correlations (−0.115–0.586) between these measures suggest that each provides unique information about glycemic control. Likewise, while SMBG is the most commonly used objective measure of adherence to T1D regimens, future studies should consider other measures that could contribute to our understanding of adherence behaviors, such as the mealtime BOLUS score (Patton et al., 2014), self-report questionnaires, structured interviews, or diaries that document the multitude of adherence behaviors required in T1D. Another limitation is that parent and youth responses on the DFRQ may in some cases have been influenced by seeing their meter data and HbA1c at a diabetes clinic visit. While it was typical for the study team to obtain survey responses before meter data and HbA1c were reviewed with the family, there was no protocol requirement to do so; therefore, variation in the timing of survey completion was possible. Similar to previous research in this area, our sample lacked fathers (15% fathers) and racial diversity (83% White). Overall, fathers are underrepresented in the literature on responsibility for pediatric diabetes management (Gruhn et al., 2016; Helgeson et al., 2008; Law et al., 2013; Vesco et al., 2010). Therefore, there is a need for future research to focus on capturing fathers’ perspectives and experiences in having a child with T1D. In youth, T1D remains more common in the non-Hispanic White population than any other racial or ethnic group (SEARCH for Diabetes in Youth Study Group et al., 2006). Even though our sample’s level of diversity closely approximates the U.S. population of youth with T1D and our clinic population, future research should attempt to replicate our results in a more racially or ethnically diverse sample to determine generalizability. This study was also cross-sectional in its design, so directional and causal relationships between responsibility and youth outcomes cannot be inferred. It is possible that families who reported shared responsibility for T1D care displayed better youth outcomes for reasons unaccounted for in the scope of this study.
Conclusions
This study describes novel findings that low reported shared responsibility for T1D management was associated with increased glycemic variability and risk for both hypo- and hyperglycemic excursions, thus extending previous literature, which examined relationships between responsibility, glycemic control, and adherence. These associations may suggest that parents who monopolize T1D care may also have youth who are at increased risk for glycemic excursions, while youth who carry the bulk of responsibility may also not adequately adhere to their treatment regimen. Overall, we provide additional supporting evidence that shared T1D responsibility is associated with better diabetes-related outcomes in youth. However, experimental and prospective studies are needed to test whether shared responsibility for T1D care causes changes in glycemic variability and glycemic excursion risk, which could provide important clinical guidelines about the roles youth and parents should play in diabetes management.
Acknowledgments
The authors wish to thank the parents and children who participated in this study and shared their experiences so that we may learn more about how to help families to successfully manage type 1 diabetes.
Funding
This project was supported in part by a grant from the Diabetes Institute of the University of Kansas Medical Center and by grants R01-DK100779 and DP3-DK108211 (to S.R.P.) from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
Conflicts of interest: None declared.
References