-
PDF
- Split View
-
Views
-
Cite
Cite
André La Gerche, Andrew T. Burns, Don J. Mooney, Warrick J. Inder, Andrew J. Taylor, Jan Bogaert, Andrew I. MacIsaac, Hein Heidbüchel, David L. Prior, Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes, European Heart Journal, Volume 33, Issue 8, April 2012, Pages 998–1006, https://doi.org/10.1093/eurheartj/ehr397
Close - Share Icon Share
Abstract
Endurance training may be associated with arrhythmogenic cardiac remodelling of the right ventricle (RV). We examined whether myocardial dysfunction following intense endurance exercise affects the RV more than the left ventricle (LV) and whether cumulative exposure to endurance competition influences cardiac remodelling (including fibrosis) in well-trained athletes.
Forty athletes were studied at baseline, immediately following an endurance race (3–11 h duration) and 1-week post-race. Evaluation included cardiac troponin (cTnI), B-type natriuretic peptide, and echocardiography [including three-dimensional volumes, ejection fraction (EF), and systolic strain rate]. Delayed gadolinium enhancement (DGE) on cardiac magnetic resonance imaging (CMR) was assessed as a marker of myocardial fibrosis. Relative to baseline, RV volumes increased and all functional measures decreased post-race, whereas LV volumes reduced and function was preserved. B-type natriuretic peptide (13.1 ± 14.0 vs. 25.4 ± 21.4 ng/L, P = 0.003) and cTnI (0.01 ± .03 vs. 0.14 ± .17 μg/L, P < 0.0001) increased post-race and correlated with reductions in RVEF (r = 0.52, P = 0.001 and r = 0.49, P = 0.002, respectively), but not LVEF. Right ventricular ejection fraction decreased with increasing race duration (r = −0.501, P < 0.0001) and VO2max (r = −0.359, P = 0.011). Right ventricular function mostly recovered by 1 week. On CMR, DGE localized to the interventricular septum was identified in 5 of 39 athletes who had greater cumulative exercise exposure and lower RVEF (47.1 ± 5.9 vs. 51.1 ± 3.7%, P = 0.042) than those with normal CMR.
Intense endurance exercise causes acute dysfunction of the RV, but not the LV. Although short-term recovery appears complete, chronic structural changes and reduced RV function are evident in some of the most practiced athletes, the long-term clinical significance of which warrants further study.
See page 938 for the editorial comment on this article (doi:10.1093/eurheartj/ehr436)
Introduction
Functional and biochemical cardiac abnormalities have been observed following intense endurance exercise, but their significance has been debated.1 The term ‘cardiac fatigue’ has been used to summarize the systolic and diastolic changes in left ventricular (LV) function which are mild and seemingly unrelated to biochemical measures of cardiac injury.2,3 Similarly, chronic LV remodelling resulting from habitual endurance training is most frequently associated with excellent health outcomes.4,5 However, right ventricular (RV) function may be more profoundly affected by intense endurance exercise6–10 and, in some cases, RV recovery may be incomplete.6,11 Moreover, there is speculation that such changes may represent a substrate for proarrhythmic RV remodelling in some highly trained athletes,12,13 even in the absence of a known familial disposition.14 There are also striking similarities between this RV syndrome in athletes and the proarrhythmic RV remodelling induced through endurance training in rats.15 These chronic changes may be the cumulative result of repeated bouts of intense exercise and, if so, a similar RV dominant pattern of acute injury might be expected.
Acute RV impairment has been described in amateur athletes following marathon running7–9 and it has been proposed that limited pre-event training may constitute a relative risk for cardiac injury.9 However, RV arrhythmias and chronic RV remodelling have most commonly been described among highly trained athletes who frequently compete in events of longer duration than a marathon.13,14 Highly trained athletes differ from amateur athletes, in that cardiac remodelling is more profound and may persist despite detraining.16,17 Also, cardiac magnetic resonance imaging (CMR) has been used to identify myocardial fibrosis in athletes with an extensive history of competition in endurance sport18,19 but not among cohorts of relatively novice athletes.7,8,20 Thus, the acute cardiac effects of more prolonged endurance exercise may be most relevant among well-trained athletes.
We hypothesized that intense endurance exercise results in cardiac dysfunction that predominantly affects the RV, may be affected by race duration, and may induce chronic structural changes.
Methods
Study design
Athletes planning to compete in one of four nominated events (a marathon, endurance triathlon, alpine cycling race, and an ultra-triathlon) were sought through advertisements at local triathlon clubs. The first 40 athletes who met the following criteria were enrolled: (i) they were well trained (defined as >10 h of intense training per week) and well performed (having finished within the first 25% of the field in a recent endurance event), (ii) they had no cardiac symptoms or cardiac risk factors, and (iii) they had no resting or inducible structural or electrophysiological abnormalities during stress echocardiography. The four events were selected to represent a progressive increase in exercise duration and the number of athletes studied at each event was adjusted to enable post-race testing to be completed within 1 h. The distances, number of competitors, and completion times for each endurance event are detailed in Table 1.
Baseline demographic and functional measures according to the endurance event completed
| . | Overall . | Marathon run . | Endurance triathlona . | Alpine cycling . | Ultra triathlona . | P-value . |
|---|---|---|---|---|---|---|
| Number of athletes | 40 | 7 | 11 | 9 | 13 | |
| Race distance (km) | 42.2 | 1.9/90/21.1 | 207 | 3.8/180/42.2 | ||
| Race completion time | 2 h 59 min ± 30 min | 5 h 24 min ± 25 min | 8 h 5 min ± 42 min | 10 h 52 min ± 1 h 16 min | ||
| Ambient temperature (°C) | 16–20 | 18–31 | 24–34 | 17–28 | ||
| Age (years) | 37 ± 8 | 38 ± 3 | 33 ± 7 | 44 ± 9 | 34 ± 8 | 0.014 |
| Male (%) | 90 | 86 | 91 | 78 | 100 | 0.378 |
| BMI (kg/m2) | 23.6 ± 1.9 | 22.3 ± 1.6 | 24.0 ± 2.1 | 23.9 ± 2.1 | 23.5 ± 1.3 | 0.306 |
| % of predicted VO2max | 146 ± 18 | 142 ± 8 | 141 ± 20 | 154 ± 20 | 148 ± 18 | 0.36 |
| Training (years) | 10 ± 9 | 13 ± 8 | 6 ± 5 | 12 ± 14 | 11 ± 9 | 0.277 |
| Training (h/week) | 16.3 ± 5.1 | 14 ± 6 | 14 ± 3 | 13 ± 4 | 21 ± 5 | <0.0001 |
| Echocardiographic measures | ||||||
| RVEDV (mL) | 170 ± 30 | 177 ± 31 | 161 ± 38 | 161 ± 24 | 181 ± 20 | 0.258 |
| LVEDV (mL) | 150 ± 23 | 159 ± 11 | 143 ± 26 | 142 ± 21 | 157 ± 26 | 0.237 |
| RVESV (mL) | 83 ± 17 | 85 ± 17 | 80 ± 21 | 80 ± 15 | 90 ± 12 | 0.410 |
| LVESV (mL) | 66 ± 14 | 66 ± 5 | 65 ± 16 | 60 ± 13 | 71 ± 15 | 0.241 |
| RVEF (%) | 51.0 ± 3.6 | 51.9 ± 3.1 | 50.7 ± 5.0 | 50.5 ± 4.0 | 50.3 ± 3.2 | 0.849 |
| LVEF (%) | 56.4 ± 5.2 | 58.2 ± 2.6 | 55.0 ± 5.6 | 58.4 ± 5.8 | 54.8 ± 5.1 | 0.239 |
| IVSd (mm) | 11.0 ± 1.5 | 11.1 ± 1.1 | 10.5 ± 0.9 | 10.9 ± 1.8 | 11.8 ± 1.5 | 0.225 |
| LVIDd (mm) | 56.0 ± 4.9 | 54.5 ± 4.8 | 54.1 ± 3.8 | 57.9 ± 4.5 | 57.7 ± 5.4 | 0.162 |
| RVFAC (%) | 51.5 ± 6.0 | 50.7 ± 5.2 | 51.3 ± 6.0 | 52.6 ± 5.3 | 51.5 ± 7.3 | 0.942 |
| RV strain (%) | −27.2 ± 3.4 | −27.2 ± 1.2 | −26.5 ± 3.8 | −28.8 ± 3.3 | −26.6 ± 3.9 | 0.292 |
| LV strain (%) | −18.4 ± 3.7 | −18.0 ± 3.6 | −17.5 ± 3.8 | −19.8 ± 3.8 | −17.8 ± 2.7 | 0.469 |
| . | Overall . | Marathon run . | Endurance triathlona . | Alpine cycling . | Ultra triathlona . | P-value . |
|---|---|---|---|---|---|---|
| Number of athletes | 40 | 7 | 11 | 9 | 13 | |
| Race distance (km) | 42.2 | 1.9/90/21.1 | 207 | 3.8/180/42.2 | ||
| Race completion time | 2 h 59 min ± 30 min | 5 h 24 min ± 25 min | 8 h 5 min ± 42 min | 10 h 52 min ± 1 h 16 min | ||
| Ambient temperature (°C) | 16–20 | 18–31 | 24–34 | 17–28 | ||
| Age (years) | 37 ± 8 | 38 ± 3 | 33 ± 7 | 44 ± 9 | 34 ± 8 | 0.014 |
| Male (%) | 90 | 86 | 91 | 78 | 100 | 0.378 |
| BMI (kg/m2) | 23.6 ± 1.9 | 22.3 ± 1.6 | 24.0 ± 2.1 | 23.9 ± 2.1 | 23.5 ± 1.3 | 0.306 |
| % of predicted VO2max | 146 ± 18 | 142 ± 8 | 141 ± 20 | 154 ± 20 | 148 ± 18 | 0.36 |
| Training (years) | 10 ± 9 | 13 ± 8 | 6 ± 5 | 12 ± 14 | 11 ± 9 | 0.277 |
| Training (h/week) | 16.3 ± 5.1 | 14 ± 6 | 14 ± 3 | 13 ± 4 | 21 ± 5 | <0.0001 |
| Echocardiographic measures | ||||||
| RVEDV (mL) | 170 ± 30 | 177 ± 31 | 161 ± 38 | 161 ± 24 | 181 ± 20 | 0.258 |
| LVEDV (mL) | 150 ± 23 | 159 ± 11 | 143 ± 26 | 142 ± 21 | 157 ± 26 | 0.237 |
| RVESV (mL) | 83 ± 17 | 85 ± 17 | 80 ± 21 | 80 ± 15 | 90 ± 12 | 0.410 |
| LVESV (mL) | 66 ± 14 | 66 ± 5 | 65 ± 16 | 60 ± 13 | 71 ± 15 | 0.241 |
| RVEF (%) | 51.0 ± 3.6 | 51.9 ± 3.1 | 50.7 ± 5.0 | 50.5 ± 4.0 | 50.3 ± 3.2 | 0.849 |
| LVEF (%) | 56.4 ± 5.2 | 58.2 ± 2.6 | 55.0 ± 5.6 | 58.4 ± 5.8 | 54.8 ± 5.1 | 0.239 |
| IVSd (mm) | 11.0 ± 1.5 | 11.1 ± 1.1 | 10.5 ± 0.9 | 10.9 ± 1.8 | 11.8 ± 1.5 | 0.225 |
| LVIDd (mm) | 56.0 ± 4.9 | 54.5 ± 4.8 | 54.1 ± 3.8 | 57.9 ± 4.5 | 57.7 ± 5.4 | 0.162 |
| RVFAC (%) | 51.5 ± 6.0 | 50.7 ± 5.2 | 51.3 ± 6.0 | 52.6 ± 5.3 | 51.5 ± 7.3 | 0.942 |
| RV strain (%) | −27.2 ± 3.4 | −27.2 ± 1.2 | −26.5 ± 3.8 | −28.8 ± 3.3 | −26.6 ± 3.9 | 0.292 |
| LV strain (%) | −18.4 ± 3.7 | −18.0 ± 3.6 | −17.5 ± 3.8 | −19.8 ± 3.8 | −17.8 ± 2.7 | 0.469 |
Underlined values signify those which differ from the mean. BMI, body mass index; EDV and ESV, end-diastolic and end-systolic volumes determined by 3D echocardiography; IVSd and LVIDd, septal and LV cavity dimensions at end-diastole determined by M-mode echocardiography. The VO2max was performed on an upright cycle ergometer (ER900 and Oxycon Alpha, Jaeger, Germany) using an automated gas analysis system.
aTriathlon comprises a swim, cycle, and run.
Baseline demographic and functional measures according to the endurance event completed
| . | Overall . | Marathon run . | Endurance triathlona . | Alpine cycling . | Ultra triathlona . | P-value . |
|---|---|---|---|---|---|---|
| Number of athletes | 40 | 7 | 11 | 9 | 13 | |
| Race distance (km) | 42.2 | 1.9/90/21.1 | 207 | 3.8/180/42.2 | ||
| Race completion time | 2 h 59 min ± 30 min | 5 h 24 min ± 25 min | 8 h 5 min ± 42 min | 10 h 52 min ± 1 h 16 min | ||
| Ambient temperature (°C) | 16–20 | 18–31 | 24–34 | 17–28 | ||
| Age (years) | 37 ± 8 | 38 ± 3 | 33 ± 7 | 44 ± 9 | 34 ± 8 | 0.014 |
| Male (%) | 90 | 86 | 91 | 78 | 100 | 0.378 |
| BMI (kg/m2) | 23.6 ± 1.9 | 22.3 ± 1.6 | 24.0 ± 2.1 | 23.9 ± 2.1 | 23.5 ± 1.3 | 0.306 |
| % of predicted VO2max | 146 ± 18 | 142 ± 8 | 141 ± 20 | 154 ± 20 | 148 ± 18 | 0.36 |
| Training (years) | 10 ± 9 | 13 ± 8 | 6 ± 5 | 12 ± 14 | 11 ± 9 | 0.277 |
| Training (h/week) | 16.3 ± 5.1 | 14 ± 6 | 14 ± 3 | 13 ± 4 | 21 ± 5 | <0.0001 |
| Echocardiographic measures | ||||||
| RVEDV (mL) | 170 ± 30 | 177 ± 31 | 161 ± 38 | 161 ± 24 | 181 ± 20 | 0.258 |
| LVEDV (mL) | 150 ± 23 | 159 ± 11 | 143 ± 26 | 142 ± 21 | 157 ± 26 | 0.237 |
| RVESV (mL) | 83 ± 17 | 85 ± 17 | 80 ± 21 | 80 ± 15 | 90 ± 12 | 0.410 |
| LVESV (mL) | 66 ± 14 | 66 ± 5 | 65 ± 16 | 60 ± 13 | 71 ± 15 | 0.241 |
| RVEF (%) | 51.0 ± 3.6 | 51.9 ± 3.1 | 50.7 ± 5.0 | 50.5 ± 4.0 | 50.3 ± 3.2 | 0.849 |
| LVEF (%) | 56.4 ± 5.2 | 58.2 ± 2.6 | 55.0 ± 5.6 | 58.4 ± 5.8 | 54.8 ± 5.1 | 0.239 |
| IVSd (mm) | 11.0 ± 1.5 | 11.1 ± 1.1 | 10.5 ± 0.9 | 10.9 ± 1.8 | 11.8 ± 1.5 | 0.225 |
| LVIDd (mm) | 56.0 ± 4.9 | 54.5 ± 4.8 | 54.1 ± 3.8 | 57.9 ± 4.5 | 57.7 ± 5.4 | 0.162 |
| RVFAC (%) | 51.5 ± 6.0 | 50.7 ± 5.2 | 51.3 ± 6.0 | 52.6 ± 5.3 | 51.5 ± 7.3 | 0.942 |
| RV strain (%) | −27.2 ± 3.4 | −27.2 ± 1.2 | −26.5 ± 3.8 | −28.8 ± 3.3 | −26.6 ± 3.9 | 0.292 |
| LV strain (%) | −18.4 ± 3.7 | −18.0 ± 3.6 | −17.5 ± 3.8 | −19.8 ± 3.8 | −17.8 ± 2.7 | 0.469 |
| . | Overall . | Marathon run . | Endurance triathlona . | Alpine cycling . | Ultra triathlona . | P-value . |
|---|---|---|---|---|---|---|
| Number of athletes | 40 | 7 | 11 | 9 | 13 | |
| Race distance (km) | 42.2 | 1.9/90/21.1 | 207 | 3.8/180/42.2 | ||
| Race completion time | 2 h 59 min ± 30 min | 5 h 24 min ± 25 min | 8 h 5 min ± 42 min | 10 h 52 min ± 1 h 16 min | ||
| Ambient temperature (°C) | 16–20 | 18–31 | 24–34 | 17–28 | ||
| Age (years) | 37 ± 8 | 38 ± 3 | 33 ± 7 | 44 ± 9 | 34 ± 8 | 0.014 |
| Male (%) | 90 | 86 | 91 | 78 | 100 | 0.378 |
| BMI (kg/m2) | 23.6 ± 1.9 | 22.3 ± 1.6 | 24.0 ± 2.1 | 23.9 ± 2.1 | 23.5 ± 1.3 | 0.306 |
| % of predicted VO2max | 146 ± 18 | 142 ± 8 | 141 ± 20 | 154 ± 20 | 148 ± 18 | 0.36 |
| Training (years) | 10 ± 9 | 13 ± 8 | 6 ± 5 | 12 ± 14 | 11 ± 9 | 0.277 |
| Training (h/week) | 16.3 ± 5.1 | 14 ± 6 | 14 ± 3 | 13 ± 4 | 21 ± 5 | <0.0001 |
| Echocardiographic measures | ||||||
| RVEDV (mL) | 170 ± 30 | 177 ± 31 | 161 ± 38 | 161 ± 24 | 181 ± 20 | 0.258 |
| LVEDV (mL) | 150 ± 23 | 159 ± 11 | 143 ± 26 | 142 ± 21 | 157 ± 26 | 0.237 |
| RVESV (mL) | 83 ± 17 | 85 ± 17 | 80 ± 21 | 80 ± 15 | 90 ± 12 | 0.410 |
| LVESV (mL) | 66 ± 14 | 66 ± 5 | 65 ± 16 | 60 ± 13 | 71 ± 15 | 0.241 |
| RVEF (%) | 51.0 ± 3.6 | 51.9 ± 3.1 | 50.7 ± 5.0 | 50.5 ± 4.0 | 50.3 ± 3.2 | 0.849 |
| LVEF (%) | 56.4 ± 5.2 | 58.2 ± 2.6 | 55.0 ± 5.6 | 58.4 ± 5.8 | 54.8 ± 5.1 | 0.239 |
| IVSd (mm) | 11.0 ± 1.5 | 11.1 ± 1.1 | 10.5 ± 0.9 | 10.9 ± 1.8 | 11.8 ± 1.5 | 0.225 |
| LVIDd (mm) | 56.0 ± 4.9 | 54.5 ± 4.8 | 54.1 ± 3.8 | 57.9 ± 4.5 | 57.7 ± 5.4 | 0.162 |
| RVFAC (%) | 51.5 ± 6.0 | 50.7 ± 5.2 | 51.3 ± 6.0 | 52.6 ± 5.3 | 51.5 ± 7.3 | 0.942 |
| RV strain (%) | −27.2 ± 3.4 | −27.2 ± 1.2 | −26.5 ± 3.8 | −28.8 ± 3.3 | −26.6 ± 3.9 | 0.292 |
| LV strain (%) | −18.4 ± 3.7 | −18.0 ± 3.6 | −17.5 ± 3.8 | −19.8 ± 3.8 | −17.8 ± 2.7 | 0.469 |
Underlined values signify those which differ from the mean. BMI, body mass index; EDV and ESV, end-diastolic and end-systolic volumes determined by 3D echocardiography; IVSd and LVIDd, septal and LV cavity dimensions at end-diastole determined by M-mode echocardiography. The VO2max was performed on an upright cycle ergometer (ER900 and Oxycon Alpha, Jaeger, Germany) using an automated gas analysis system.
aTriathlon comprises a swim, cycle, and run.
Athletes were studied at three time points: within 2–3 weeks prior to the race (baseline), immediately after (post-race), and 6–11 days after the race (delayed) during which time the athletes did minimal training. Cardiac magnetic resonance imaging was performed at baseline, biochemistry at baseline, and post-race and echocardiography at all time points.
Written informed consent was obtained from all subjects and the protocol was approved by the St Vincent's Hospital Human Research Ethics Committee in accordance with the Declaration of Helsinki.
Echocardiography
A comprehensive resting echocardiography study was performed on a Vivid 7 Dimension echocardiograph (GE Vingmed Ultrasound, Horten, Norway). A full-volume three-dimensional data set was acquired over five cardiac cycles during breath-hold. Left ventricular and RV volumes were then measured off-line using customized software (TomTec software, Germany) as described previously.21 Cardiac dimensions were acquired by two-dimensional and M-mode echocardiography and analysed off-line using commercially available software (Echopac v.108, GE, Norway). These analyses included RV fractional area change (RVFAC), ventricular diameters, LV eccentricity index, and tricuspid annular plane systolic excursion, performed according to contemporary guidelines.22,23 Two-dimensional global strain and peak global systolic strain rate (SRs) were quantified for the LV and RV on apical grey-scale images (60–90 frames/s) as described previously.24
Doppler interrogation of the tricuspid regurgitant jet was used to estimate pulmonary artery systolic pressure (PASP), while the ratio of early diastolic mitral inflow to mitral annular velocity (E/e′) was used as an index of left atrial pressure.
All reported values are the average of three repeated measures from separate cardiac cycles.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging was performed on a 1.5 T scanner (Signa Excite, GE Healthcare, WI, USA) using a dedicated cardiac coil and electrocardiographic gating. Cine imaging was used to obtain a contiguous short-axis stack (8 mm slice thickness without gaps) covering the LV and RV from the apex to a level well above the atrioventricular groove. Twenty images per cardiac cycle were obtained at end-tidal breath-hold. Endo- and epicardial borders were manually traced (Cinetool, Global Applied Science Laboratory, GE Healthcare) to quantify volumes at end-diastole (EDV) and end-systole (ESV).
Subclinical myocardial scarring was evaluated by means of delayed hyperenhancement following the administration of 0.2 mmol/kg gadolinium-DTPA (Magnevist®, Schering, Germany) on T1-weighted images. An inversion-recovery gradient echo technique was applied 10 min following the gadolinium bolus and inversion time was optimized for nulling of normal myocardium. Delayed gadolinium enhancement (DGE) was qualitatively assessed by two experienced clinicians after comparing pre- and post-contrast short-axis slices to exclude artefacts. Delayed gadolinium enhancement within the RV free wall was not assessed because adequate nulling of normal RV free wall myocardium (the reference against which abnormal enhancement is compared) was visualized in only a minority of athletes. To be deemed significant, DGE had to be independently identified by both clinicians and, in addition, mean signal intensities in the region of hyperenhancement were required to be more than two standard deviations (SDs) higher than the mean signal intensity of a region of adjacent myocardium (Osirix Imaging Software, Geneva, Switzerland), as described previously.25
Biochemistry
B-type natriuretic peptide (BNP) was measured using a point-of-care immunoassay (Triage, Biosite Incorporated, San Diego, CA, USA) with a lower detection limit of 5 ng/L. Cardiac troponin I (cTnI) was measured using an AxSYM cTnI assay (Abbott Laboratories, Abbott Park, IL, USA) for which the lower limit of detection was 0.015 and the lower 99th percentile of a normal reference population (upper reference limit) was 0.04 µg/L.
Statistical analysis
Gaussian distribution of all continuous variables was confirmed using a Kolmogorov–Smirnov test and values are reported as mean ± SD. Baseline measures were compared according to the four endurance event groupings using a one-way independent ANOVA with deviation contrasts to assess values differing from the mean. Post-race biochemical measures were compared with baseline using a paired t-test or χ2 test for categorical measures while measures over the three time points were assessed by ANOVA. For analysis of repeated measures by the type of race performed, a factorial repeated-measures ANOVA was used with the endurance event grouping considered as a between-subject effect. A forward stepwise multivariable analysis was used to assess potential predictors of post-race ▵RV ejection fraction (EF). A tolerance of >0.5 was set to avoid collinearity between the five entered variables (race duration, VO2max, age, weekly, and career training). Linear regression was used to assess the relationship between post-race biomarker levels and change in EF.
The sample size of 40 was prospectively determined to have a 90% power of detecting a 10% relative reduction in RVEF averaged across all four endurance events and was larger than had been required in previous studies demonstrating significant post-race cardiac dysfunction.6–8
A two-tailed P-value of <0.05 was considered significant. Statistical analysis was performed using SPSS v.16.0 software.
Results
Baseline measures according to endurance race grouping are presented in Table 1. Athlete characteristics were similar for the four events with the exceptions that athletes competing in the endurance triathlon were younger, and alpine cyclists older, than the mean while ultra-triathlon competitors performed more weekly training.
Post-race changes in ventricular function
The average delays from race finish to blood sample collection and echocardiography were 22 ± 11 and 43 ± 48min, respectively. Functional baseline, post-race, and delayed measures are detailed in Table 2. Heart rate was higher while systemic blood pressure and estimated PASP were lower post-race. When compared with baseline, all measures of systolic RV function were reduced post-race. An example of changes in RV deformation is provided in Supplementary Data. Importantly, all follow-up measures returned to baseline, with the exception of RVSRs which remained depressed. In contrast, all measures of LV function were unchanged.
Haemodynamic and echocardiographic measures at baseline, post-race, and delayed
| . | Baseline . | Post-race . | Delayed . | ANOVA P-value . |
|---|---|---|---|---|
| Heart rate (b.p.m.) | 52 ± 7 | 72 ± 9 | 54 ± 6 | <0.0001 |
| Systolic BP (mmHg) | 147 ± 14 | 117 ± 13 | 134 ± 20 | <0.0001 |
| Diastolic BP (mmHg) | 77 ± 7 | 70 ± 11 | 74 ± 10 | 0.001 |
| PASP (mmHg) | 21.5 ± 3.8 | 18.0 ± 3.3 | 20.0 ± 3.3 | <0.0001 |
| Right ventricular measures | ||||
| RVEF (%) | 51.0 ± 3.6 | 46.4 ± 6.5 | 50.0 ± 3.8 | <0.0001 |
| RVFAC (%) | 51.5 ± 6.0 | 44.3 ± 11.2 | 49.8 ± 6.6 | <0.0001 |
| TAPSE (mm) | 24.9 ± 3.9 | 24.0 ± 4.5 | 26.5 ± 4.1 | 0.035 |
| RV strain (%) | −27.2 ± 3.4 | −23.7 ± 3.7 | −25.6 ± 3.0 | 0.001 |
| RVSRs (s−1) | −1.42 ± 0.24 | −1.26 ± 0.23 | −1.29 ± 0.19 | 0.008 |
| Left ventricular measures | ||||
| LVEF (%) | 56.4 ± 5.2 | 57.5 ± 5.6 | 58.8 ± 5.1 | 0.147 |
| LV strain (%) | −18.4 ± 3.7 | −16.9 ± 2.8 | −17.7 ± 2.3 | 0.071 |
| LVSRs (s−1) | −0.98 ± 0.26 | −0.95 ± 0.15 | −0.89 ± 0.13 | 0.13 |
| Ventricular interaction | ||||
| RV end-systolic diameter (mm) | 20.2 ± 5.2 | 23.8 ± 6.1 | 21.5 ± 5.1 | 0.018 |
| LV end-systolic diameter (mm) | 37.7 ± 3.8 | 35.2 ± 3.2 | 37.5 ± 3.6 | 0.003 |
| RV:LV end-systolic diameter ratio | 0.54 ± 0.14 | 0.69 ± 0.19 | 0.58 ± 0.13 | <0.0001 |
| Eccentricity index | 1.04 ± 0.13 | 1.10 ± 0.15 | 1.01 ± 0.10 | 0.006 |
| . | Baseline . | Post-race . | Delayed . | ANOVA P-value . |
|---|---|---|---|---|
| Heart rate (b.p.m.) | 52 ± 7 | 72 ± 9 | 54 ± 6 | <0.0001 |
| Systolic BP (mmHg) | 147 ± 14 | 117 ± 13 | 134 ± 20 | <0.0001 |
| Diastolic BP (mmHg) | 77 ± 7 | 70 ± 11 | 74 ± 10 | 0.001 |
| PASP (mmHg) | 21.5 ± 3.8 | 18.0 ± 3.3 | 20.0 ± 3.3 | <0.0001 |
| Right ventricular measures | ||||
| RVEF (%) | 51.0 ± 3.6 | 46.4 ± 6.5 | 50.0 ± 3.8 | <0.0001 |
| RVFAC (%) | 51.5 ± 6.0 | 44.3 ± 11.2 | 49.8 ± 6.6 | <0.0001 |
| TAPSE (mm) | 24.9 ± 3.9 | 24.0 ± 4.5 | 26.5 ± 4.1 | 0.035 |
| RV strain (%) | −27.2 ± 3.4 | −23.7 ± 3.7 | −25.6 ± 3.0 | 0.001 |
| RVSRs (s−1) | −1.42 ± 0.24 | −1.26 ± 0.23 | −1.29 ± 0.19 | 0.008 |
| Left ventricular measures | ||||
| LVEF (%) | 56.4 ± 5.2 | 57.5 ± 5.6 | 58.8 ± 5.1 | 0.147 |
| LV strain (%) | −18.4 ± 3.7 | −16.9 ± 2.8 | −17.7 ± 2.3 | 0.071 |
| LVSRs (s−1) | −0.98 ± 0.26 | −0.95 ± 0.15 | −0.89 ± 0.13 | 0.13 |
| Ventricular interaction | ||||
| RV end-systolic diameter (mm) | 20.2 ± 5.2 | 23.8 ± 6.1 | 21.5 ± 5.1 | 0.018 |
| LV end-systolic diameter (mm) | 37.7 ± 3.8 | 35.2 ± 3.2 | 37.5 ± 3.6 | 0.003 |
| RV:LV end-systolic diameter ratio | 0.54 ± 0.14 | 0.69 ± 0.19 | 0.58 ± 0.13 | <0.0001 |
| Eccentricity index | 1.04 ± 0.13 | 1.10 ± 0.15 | 1.01 ± 0.10 | 0.006 |
Underlined values signify P< 0.05 relative to baseline value on Bonferroni's post hoc analysis. BP, blood pressure; PASP, pulmonary artery systolic pressure without addition of right atrial pressure estimate; EF, ejection fraction; FAC, fractional area change; TAPSE, tricuspid annular plane systolic excursion; strain, peak global strain; SRs, peak systolic strain rate.
Haemodynamic and echocardiographic measures at baseline, post-race, and delayed
| . | Baseline . | Post-race . | Delayed . | ANOVA P-value . |
|---|---|---|---|---|
| Heart rate (b.p.m.) | 52 ± 7 | 72 ± 9 | 54 ± 6 | <0.0001 |
| Systolic BP (mmHg) | 147 ± 14 | 117 ± 13 | 134 ± 20 | <0.0001 |
| Diastolic BP (mmHg) | 77 ± 7 | 70 ± 11 | 74 ± 10 | 0.001 |
| PASP (mmHg) | 21.5 ± 3.8 | 18.0 ± 3.3 | 20.0 ± 3.3 | <0.0001 |
| Right ventricular measures | ||||
| RVEF (%) | 51.0 ± 3.6 | 46.4 ± 6.5 | 50.0 ± 3.8 | <0.0001 |
| RVFAC (%) | 51.5 ± 6.0 | 44.3 ± 11.2 | 49.8 ± 6.6 | <0.0001 |
| TAPSE (mm) | 24.9 ± 3.9 | 24.0 ± 4.5 | 26.5 ± 4.1 | 0.035 |
| RV strain (%) | −27.2 ± 3.4 | −23.7 ± 3.7 | −25.6 ± 3.0 | 0.001 |
| RVSRs (s−1) | −1.42 ± 0.24 | −1.26 ± 0.23 | −1.29 ± 0.19 | 0.008 |
| Left ventricular measures | ||||
| LVEF (%) | 56.4 ± 5.2 | 57.5 ± 5.6 | 58.8 ± 5.1 | 0.147 |
| LV strain (%) | −18.4 ± 3.7 | −16.9 ± 2.8 | −17.7 ± 2.3 | 0.071 |
| LVSRs (s−1) | −0.98 ± 0.26 | −0.95 ± 0.15 | −0.89 ± 0.13 | 0.13 |
| Ventricular interaction | ||||
| RV end-systolic diameter (mm) | 20.2 ± 5.2 | 23.8 ± 6.1 | 21.5 ± 5.1 | 0.018 |
| LV end-systolic diameter (mm) | 37.7 ± 3.8 | 35.2 ± 3.2 | 37.5 ± 3.6 | 0.003 |
| RV:LV end-systolic diameter ratio | 0.54 ± 0.14 | 0.69 ± 0.19 | 0.58 ± 0.13 | <0.0001 |
| Eccentricity index | 1.04 ± 0.13 | 1.10 ± 0.15 | 1.01 ± 0.10 | 0.006 |
| . | Baseline . | Post-race . | Delayed . | ANOVA P-value . |
|---|---|---|---|---|
| Heart rate (b.p.m.) | 52 ± 7 | 72 ± 9 | 54 ± 6 | <0.0001 |
| Systolic BP (mmHg) | 147 ± 14 | 117 ± 13 | 134 ± 20 | <0.0001 |
| Diastolic BP (mmHg) | 77 ± 7 | 70 ± 11 | 74 ± 10 | 0.001 |
| PASP (mmHg) | 21.5 ± 3.8 | 18.0 ± 3.3 | 20.0 ± 3.3 | <0.0001 |
| Right ventricular measures | ||||
| RVEF (%) | 51.0 ± 3.6 | 46.4 ± 6.5 | 50.0 ± 3.8 | <0.0001 |
| RVFAC (%) | 51.5 ± 6.0 | 44.3 ± 11.2 | 49.8 ± 6.6 | <0.0001 |
| TAPSE (mm) | 24.9 ± 3.9 | 24.0 ± 4.5 | 26.5 ± 4.1 | 0.035 |
| RV strain (%) | −27.2 ± 3.4 | −23.7 ± 3.7 | −25.6 ± 3.0 | 0.001 |
| RVSRs (s−1) | −1.42 ± 0.24 | −1.26 ± 0.23 | −1.29 ± 0.19 | 0.008 |
| Left ventricular measures | ||||
| LVEF (%) | 56.4 ± 5.2 | 57.5 ± 5.6 | 58.8 ± 5.1 | 0.147 |
| LV strain (%) | −18.4 ± 3.7 | −16.9 ± 2.8 | −17.7 ± 2.3 | 0.071 |
| LVSRs (s−1) | −0.98 ± 0.26 | −0.95 ± 0.15 | −0.89 ± 0.13 | 0.13 |
| Ventricular interaction | ||||
| RV end-systolic diameter (mm) | 20.2 ± 5.2 | 23.8 ± 6.1 | 21.5 ± 5.1 | 0.018 |
| LV end-systolic diameter (mm) | 37.7 ± 3.8 | 35.2 ± 3.2 | 37.5 ± 3.6 | 0.003 |
| RV:LV end-systolic diameter ratio | 0.54 ± 0.14 | 0.69 ± 0.19 | 0.58 ± 0.13 | <0.0001 |
| Eccentricity index | 1.04 ± 0.13 | 1.10 ± 0.15 | 1.01 ± 0.10 | 0.006 |
Underlined values signify P< 0.05 relative to baseline value on Bonferroni's post hoc analysis. BP, blood pressure; PASP, pulmonary artery systolic pressure without addition of right atrial pressure estimate; EF, ejection fraction; FAC, fractional area change; TAPSE, tricuspid annular plane systolic excursion; strain, peak global strain; SRs, peak systolic strain rate.
Interventricular dependence
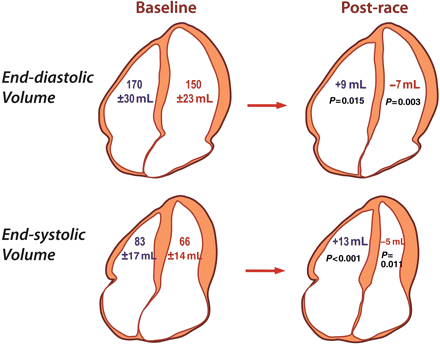
The effect of endurance exercise on cardiac volumes differed according to the ventricle (Figure 1). For the RV, post-race EF decreased by 9% relative to baseline (P < 0.0001) due to an increase in RVESV and RVEDV while LV volumes decreased resulting in a preserved LVEF.
Differential effect of prolonged intense exercise on right and left ventricular volumes. Baseline volumes are shown on the left and the changes in volume post-race are shown on the right. Right ventricular volumes increased in the post-race setting while left ventricular volumes decreased resulting in a decrease in right ventricular ejection fraction but not left ventricular ejection fraction.
Mirroring these volumetric changes, opposing changes in RV and LV end-systolic diameter resulted in an increase in the RV:LV ratio (Table 2). Post-race ventricular interaction is further evidenced by an increase in the LV eccentricity index and the occurrence of an early diastolic ‘septal bounce’ on M-mode echocardiography in 26 of 40 athletes which was not present at baseline (see Supplementary Data).
Post-race LV diastolic filling was impaired, as evidenced by a decrease in the ratio of early (E) to late (A) transmitral flow (EA ratio 1.84 ± 0.35 at baseline vs. 1.34 ± 0.35 post-race, P < 0.0001) and an increase in E deceleration time (187 ± 29 vs. 212 ± 40 ms, P = 0.002), but was not associated with an increase in left atrial pressure estimates. Rather, the E/e′ ratio decreased from 7.5 ± 1.7 at baseline to 6.9 ± 1.6 post-race (P = 0.003).
Correlation between functional and biochemical evidence of myocardial injury
Cardiac troponin was detectable in nine athletes (23%) at baseline (median <0.015 µg/L, range <0.015–0.21 µg/L) and in all athletes post-race (median 0.018 µg/L, range 0.020–0.99 µg/L, P < 0.0001 for increase in proportion and quantity). Relative to baseline, post-race BNP also increased (13.1 ± 14.0 vs. 25.4 ± 21.4 ng/L, P = 0.003).
Significant correlations were evident between the change in RVEF and post-race troponin (r = 0.494, P = 0.002) and BNP levels (r = 0.518, P = 0.001). In the ultra-triathletes, in whom the change in RVEF was greatest, the correlations between RVEF and biomarkers were stronger (r = 0.746, P = 0.003 and r = 0.625, P = 0.022 for cTnI and BNP, respectively). There was no correlation between changes in LVEF and post-race cTnI (r = −0.065, P = 0.702) or BNP (r = 0.250, P = 0.125).
Effect of race duration
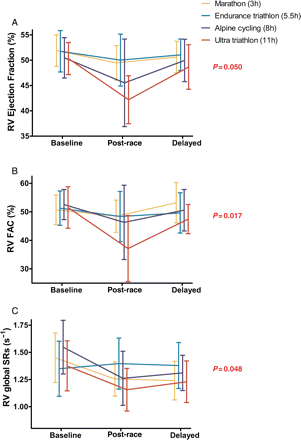
As demonstrated in Figure 2, there was a significant interaction between race duration and changes in RVEF, RVFAC, and RVSRs. For every measure, the greatest reduction in post-race values was in those athletes who completed the longest event (ultra-triathlon).
Duration-dependent increase in right ventricular dysfunction. Ejection fraction (A), fractional area change (B), and systolic strain rate (C) decreased in the post-race setting. There was a significant interaction between event type and time point (P-value) with a greater reduction in function in those completing the longest event (ultra-triathlon).
A multivariable analysis was performed to further assess the relationship between post-race RV function, exercise duration, and other potential confounding influences. The change in RVEF (post-race – baseline) correlated inversely with increasing race completion time (r = −0.501, P < 0.0001) and VO2max (r = −0.359, P= 0.011) but not with age, weekly training volume, or years of endurance sport competition. Race completion time and VO2max remained as independent predictors in the multivariable analysis (β = 0.459, P = 0.002 and β = 0.294, P = 0.036, respectively), together explaining 34% of the variance in ▵RVEF (P = 0.001).
Assessment of cardiac fibrosis by cardiac magnetic resonance imaging
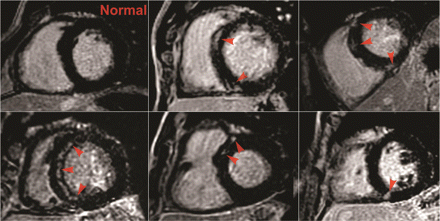
Delayed gadolinium enhancement was identified in five athletes (12.8%) and was confined to the interventricular septum in each case, frequently in the vicinity of the RV attachment (Figure 3).
Delayed gadolinium enhancement in five athletes. Images of five athletes in whom focal delayed gadolinium enhancement (DGE) was identified in the interventricular septum (indicated with arrows) when compared with an athlete with a normal study (top left).
Athletes with and without septal DGE were of similar age (43 ± 13 vs. 35 ± 8 years, P = 0.057), but those athletes with DGE had been competing in endurance sports for longer (20 ± 16 vs. 8 ± 6 years, P = 0.043) and had greater predicted VO2max for age (162 ± 26 vs. 144 ± 16%, P = 0.036). Also, those with DGE had 16% greater RVEDV and 25% greater RVESV than those athletes in whom there was no DGE (P = 0.029 and 0.003, respectively). Right ventricular ejection fraction was lower in athletes with DGE than those without DGE (47.1 ± 5.9 vs. 51.1 ± 3.7%, P = 0.042) while LVEF was similar (56.5 ± 6.8 vs. 59.8 ± 5.6, P = 0.242).
Discussion
Moderate exercise is an important therapy for cardiovascular health, but the effect of more extreme physical exercise is less well defined.26 In a cohort of well-trained athletes, we demonstrated that intense endurance exercise causes an acute reduction in RV function that increases with race duration and correlates with increases in biomarkers of myocardial injury. In contrast, all measures of LV function were preserved and there was no relationship between LV function and biomarker levels. Also, focal gadolinium enhancement and increased RV remodelling were more prevalent in those athletes with a longer history of competitive sport, suggesting that repetitive ultra-endurance exercise may lead to more extensive RV change and possible myocardial fibrosis. Thus, the cardiac impact of both acute and cumulative exercise is greatest on the RV.
Evidence for isolated right ventricular injury
Differing strengths of multiple imaging modalities were utilized in this study; CMR provided optimal morphological characterization of the athletes' hearts, and echocardiography enabled immediate post-race assessment of both the structure and function. Confidence may be placed in the finding that post-race RV function was reduced given the concordance between multiple measures with different principles of functional assessment. Right ventricular end-diastolic volume, area, and dimensions all increased in the post-race setting which, according to the Frank–Starling mechanism, should augment cardiac deformation if contractility is preserved.27 Thus, the post-race reductions in RV deformation (strain and strain rate) are likely to represent true impairment in RV contractility. Similarly, the post-race decrease in PASP and increase in end-systolic RV volume infer a decrease in the end-systolic pressure–volume relationship, another measure of contractility which is relatively load-insensitive.28 Comparisons with the LV are most instructive, given that post-race changes in load, myocardial substrates, and metabolites should confound RV and LV measures similarly. The same measures which were consistently reduced for the RV were unchanged for the LV in the post-race setting.
Greater reductions in RV function occurred in those athletes competing for a longer duration, suggesting that the heart has a finite capacity to maintain the increased work demands of exercise. It has been demonstrated that ventricular load increases with exercise intensity and is greater for the RV than the LV,29 thus potentially explaining why the RV is more susceptible to fatigue after prolonged exercise. However, conclusions regarding the effect of exercise duration need to be tempered by the multiple confounders inherent in the study of endurance exercise. Variability in training levels, exercise modality, race conditions, and motivation are likely to influence cardiac performance. Previous investigators have documented reductions in RV function in less trained subjects over the marathon distance7–9 and have contended that cardiac injury is greatest in the least trained.9 Our results complement these findings by demonstrating more modest post-marathon changes in a highly trained cohort. However, the attenuation of injury as a result of training does not prevent injury when the period of intense exercise is extended further still. We enrolled elite and subelite athletes and found a significant association between fitness (VO2max) and the reduction in post-race RVEF. Perhaps, this may reflect the capacity of the best-trained athletes to maintain the highest level of exercise intensity (and resultant haemodynamic stress) for the longest duration. The focus on well-trained athletes may be of particular relevance, given that they perform exercise of highest intensity and duration most frequently, and, thus, may be at a greater risk of cumulative injury.
Biochemical markers are associated with changes in right ventricular function
The lack of correlation between increases in troponin and changes in LV function seen in this study has been previously interpreted as evidence that post-exercise elevations in cardiac biomarkers are benign.3 However, we observed a significant correlation between changes in RVEF and post-race biomarker levels and this relationship was even stronger in the athletes who completed the race of longest duration, the ultra-triathlon. The correlations with RVEF, but not LVEF, provide further evidence of the differential effects of intense exercise on RV and LV function. We have previously demonstrated that BNP release during intense exercise is associated with greater relative increases in RV systolic pressures, but not LV pressures.30 Thus, BNP may provide a measure of both acute RV load and the resultant fatigue which occurs when this load is sustained. However, care must be taken not to over-interpret these moderate correlations in which much of the variance in biomarker elevations remains unexplained. Some of this variance may relate to the limitations of assessing cTnI and BNP at a single time point31 and independent validation combining repeated biomarker sampling with comprehensive biventricular measures will provide further clarity.
Ventricular interaction and its consequences
The study of ventricular interaction provides important insights into the consequences of RV dysfunction on overall cardiac performance. Intense endurance exercise resulted in an increase in the dimensions of the RV and an opposing decrease in those of the LV.
Potential mechanisms for this occurrence include pericardial constraint which could limit LV filling by the observed right-to-left septal shift (see Supplementary Data). Moreover, the demonstrated RV contractile impairment could explain the reduction in E/e′, whereas impaired relaxation or increased stiffness of the LV myocardium would be expected to increase LV filling pressures. This failure of LV filling from a lack of upstream ‘push’ may provide an insight into the previously unexplained paradox whereby stroke volume decreases during prolonged exercise, despite preserved LV contractility and lusitropy.32 Our data suggest that these reductions in stroke volume may be due to RV limitation which increases with exercise duration.
Chronic myocardial remodelling and the effect of exercise ‘dose’
Even after many years of detraining, cardiac dilation may not completely regress in elite athletes16,17 and this raises the possibility that physiological hypertrophy may be accompanied by expansion of the myocardial extracellular matrix.
Mohlenkamp et al.19 provided support for this premise by demonstrating focal DGE in the myocardium of 12% of veteran runners who had completed a median of 20 marathons over almost 10 years, although the DGE may have been due to subclinical coronary events in at least some of the athletes. More recently, Wilson et al.18 observed DGE in 6 of 12 elite veteran athletes but not in 17 young novice athletes or 20 older non-athletes. Our current finding of DGE in five athletes (13%) who had competed in endurance sports for longer is consistent with these findings. In addition, we demonstrate that the acute injury and chronic remodelling of the myocardium both disproportionately affect the RV and it remains possible that the two are linked.
In all cases, focal DGE was confined to the interventricular septum and commonly at the site of RV attachment. This is similar to the pattern described in patients with pulmonary hypertension in whom increased interventricular wall stress caused through chronic RV pressure overload may cause disruption to the muscle bundle architecture and/or expansion of the interstitial matrix at this site.25,33 The considerable increases in pulmonary pressures demonstrated in trained athletes during intense exercise29,30,34 suggest a similar physiological stress, albeit only transient. However, care must be taken when drawing analogies from other clinical conditions in which DGE is associated with adverse clinical events. Such prognostic implications should not be extrapolated to the healthy cohort of athletes presented here.
There are two significant limitations in the use of CMR to evaluate myocardial fibrosis in athletes. First, the predominance of RV injury following endurance exercise would suggest that the RV free wall may be particularly susceptible to chronic scarring. We found that the normal ‘nulled’ RV free wall myocardium could not be discerned in a majority of athletes. While this could be interpreted as abnormal enhancement, it is more likely to reflect the limitations in spatial resolution of the technique, a sentiment echoed by a panel of experts who deemed that DGE assessment in the RV free wall was too unreliable to be included in the recent revision of diagnostic criteria for arrhythmogenic RV cardiomyopathy.35 Secondly, athletic training results in relatively uniform cardiac remodelling and it is possible that cardiac fibrosis may be under-appreciated with current techniques that assess relative enhancement. Novel techniques which assess global fibrosis36 may be more informative in assessing the degree to which extracellular matrix expansion contributes to the athlete's heart syndrome.
Exercise is a powerful intervention in the prevention and treatment of cardiovascular disease for which compelling evidence exists across a wide range of exercise volumes and intensities, including among competitive athletes.4,26,37 However, there is also emerging evidence that intense endurance exercise may be associated with an excess in arrhythmic disorders, the mechanisms for which remain unexplained.38–41 Studies focussing on the LV of athletes have failed to provide these mechanistic insights,4 but recent data have suggested that exercise-induced proarrhythmic substrates may develop in the RV while sparing the LV.12,13,15 Benito et al.15 recently documented that intense endurance exercise promoted RV-specific fibrosis in rats which increased vulnerability to ventricular tachycardia following programmed stimulation. Extending these hypotheses to human populations, Ector et al.12 demonstrated that RVEF (and not LVEF) was reduced in athletes with complex ventricular arrhythmias when compared with healthy athletes and non-athletes without arrhythmias. Our current study provides further circumstantial evidence for the emerging concept that the RV may be more susceptible to exercise-induced injury. During maximal exercise of short duration, we have previously demonstrated that the RV is placed under greater haemodynamic stress than the LV29 and we extend this finding here by demonstrating that this haemodynamic imbalance, when sustained, may promote transient RV injury with possible long-term structural consequences. However, it is premature to conclude that these changes may represent a proarrhythmic substrate. Our relatively small cohort is underpowered for the assessment of clinical sequelae, and although invasive evaluation of the electrical and histological myocardial changes may have provided important insights, this could not be justified in this healthy athletic cohort. Rather, this study strengthens the rationale for a prospective, multicentre study in which athletes with acute and chronic RV changes are assessed for long-term clinical events.
Conclusion and clinical implications
This study demonstrates, for the first time, an association between endurance exercise of increasing duration and structural, functional, and biochemical markers of cardiac dysfunction in highly trained athletes. Functional abnormalities were confined to the RV and were largely reversible 1 week following the event. However, there remained a significant minority of athletes in whom there was evidence of myocardial fibrosis in the interventricular septum. These athletes had been competing in endurance sport for longer and had greater structural remodelling of the RV. This adds further weight to the proposition that RV abnormalities may be acquired through cumulative bouts of intense exercise and provides direction for prospective investigations aimed at elucidating whether extreme exercise may promote arrhythmias in some athletes.
Supplementary material
Funding
A.L.G. is supported by a postgraduate scholarship (National Health and Medical Research Council, Australia). This project was financed, in part, by a Cardiovascular Lipid Grant (Pfizer, Australia).
Conflict of interest: none declared.
References
- left ventricular ejection fraction
- echocardiography
- aerobic exercise
- right ventricular ejection fraction
- myocardial fibrosis
- ventricular dysfunction, right
- brain natriuretic peptide
- left ventricle
- exercise
- fibrosis
- gadolinium
- systole
- ventricular function, right
- heart
- interventricular septum
- cardiac mri
- cardiac troponin i
- athlete
- strain rate