-
PDF
- Split View
-
Views
-
Cite
Cite
Ioannis M. Zacharioudakis, Fainareti N. Zervou, Marios Arvanitis, Panayiotis D. Ziakas, Leonard A. Mermel, Eleftherios Mylonakis, Antimicrobial Lock Solutions as a Method to Prevent Central Line–Associated Bloodstream Infections: A Meta-analysis of Randomized Controlled Trials, Clinical Infectious Diseases, Volume 59, Issue 12, 15 December 2014, Pages 1741–1749, https://doi.org/10.1093/cid/ciu671
Close - Share Icon Share
Antimicrobial lock solutions are effective in preventing catheter-associated bloodstream infections and their effect is additive to standard prevention measures. Nonantibiotic antimicrobial solutions are equally effective to antibiotic solutions and their use may have a lower associated risk of antibiotic resistance.
Background. Antimicrobial lock solutions may be an effective strategy to prevent catheter-associated infections. However, there remains concern about their efficacy and safety.
Methods. To investigate the efficacy of antimicrobial lock therapy to prevent central line–associated bloodstream infections (CLABSIs), we performed a systematic search of PubMed, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov, from the earliest date up to 31 December 2013. Studies were eligible if they were randomized controlled trials comparing antimicrobial lock solutions to heparin and if they provided an appropriate definition of infection.
Results. The 23 included studies reported data on 2896 patients, who were predominantly adult patients undergoing hemodialysis (16/23 studies), but also adult and pediatric oncology patients, critically ill neonates, and patients receiving total parenteral nutrition. The use of antimicrobial lock solutions led to a 69% reduction in CLABSI rate (relative risk [RR], 0.31; 95% confidence interval [CI], .24–.40) and a 32% reduction in the rate of exit site infections (RR, 0.68; 95% CI, .49–.95) compared with heparin, without significantly affecting catheter failure due to noninfectious complications (RR, 0.83; 95% CI, .65–1.06). All-cause mortality was not different between the groups (RR, 0.84; 95% CI .64–1.12). Neither the type of antimicrobial solution nor the population studied, affected the relative reduction in CLABSIs, which also remained significant among studies reporting baseline infection rates of <1.15 per 1000 catheter-days, and studies providing data for catheter-related bloodstream infections. Publication and selective reporting bias are a concern in our study and should be acknowledged.
Conclusions. Antimicrobial lock solutions are effective in reducing risk of CLABSI, and this effect appears to be additive to traditional prevention measures.
Central line–associated bloodstream infections (CLABSIs) are the most costly healthcare-associated infections, averaging $45 814 on a per-case basis [1]. A recent analysis found that 65%–70% of CLABSIs could be prevented with the proper institution of catheter care measures [2]. Despite high compliance with such measures, the incidence of CLABSIs is significantly decreased but not completely eliminated across the United States [2, 3]. As such, additional preventive interventions have been investigated.
An intervention that is the source of much discussion at the national and international level is the use of antimicrobial lock solutions. Indeed, over the past few years, randomized trials have addressed the issue with promising results [4, 5]. However, concerns regarding the emergence of resistant organisms [6], noninfectious complications [7], and the inability of previous studies to prove the additive benefit of lock solutions in conjunction with catheter care bundles [8] have prevented the recommendation to use this strategy as part of routine catheter care in recent guidelines [3, 9, 10]. On the other hand, therapeutic antimicrobial locks have been incorporated into guidelines for the management of catheter-related infections [11]. To evaluate the efficacy and safety of antimicrobial lock therapy, we conducted a meta-analysis of randomized controlled trials (RCTs) comparing antimicrobial lock solutions to heparin.
MATERIALS AND METHODS
Literature Search
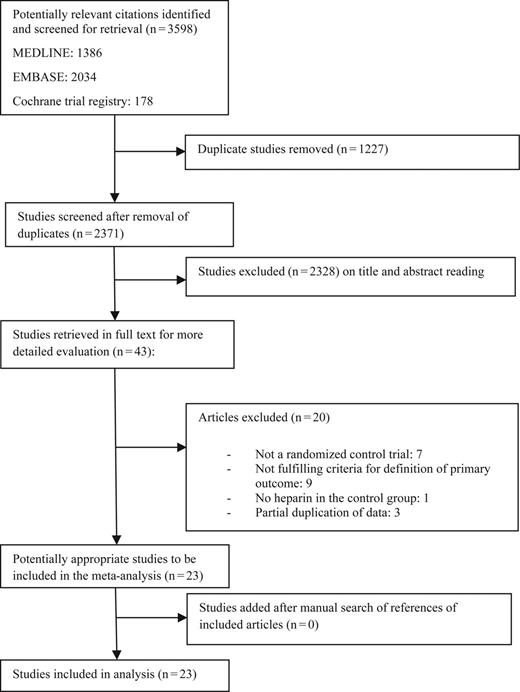
A systematic literature search of PubMed (1951 through December 2013), Embase (1951 through December 2013), the Cochrane Central Register of Controlled Trials (1984 through December 2013), and ClinicalTrials.gov databases was conducted. We used the search term “catheter* AND lock.” We restricted our search to articles written in English.
Three investigators (I. M. Z., F. N. Z., M. A.) independently identified and scrutinized studies for potential inclusion; those that were relevant by title and abstract were retrieved in full text. We complemented our search with the reference lists of eligible articles. Our meta-analysis is in line with Preferred Reporting Items for Systematic Reviews and Meta-analysis recommendations [12].
Eligibility Criteria
To be eligible for inclusion in the meta-analysis, a study had to fulfill the following criteria: (1) be an RCT; (2) use an antimicrobial lock solution in the intervention group; (3) use heparin in the control group; (4) use lock solutions—that is, solutions that were allowed to dwell, rather than simply be flushed through the catheter; and (5) provide an appropriate definition for CLABSI. CLABSI was defined as a primary bloodstream infection (laboratory-confirmed bloodstream infection not related to an infection at another site) in a patient who had a central line or umbilical catheter within a 48-hour period before the development of the bloodstream infection [13]. We excluded studies that did not report the definition used, as well as studies that used a noncompliant definition (eg, studies that defined a CLABSI without the need for microbiologic confirmation of bloodstream infection). In cases when a study separately reported definite, probable, and possible CLABSI, we included only the subgroups that fell within the Centers for Disease Control and Prevention definition. Antimicrobial lock solutions were separated into 2 categories: (1) antibiotic lock solutions (all compounds approved by the US Food and Drug Administration [FDA] and used against human infections), and (2) nonantibiotic antimicrobial lock solutions (antiseptic compounds that, while being active antimicrobial agents, do not have an indication for systemic use against human infections).
Outcomes of Interest
The primary outcome of interest was the incidence of CLABSI. Secondary outcome was the rate of catheter-related bloodstream infection (CRBSI), as defined by the Infectious Diseases Society of America [11]. Specifically, CRBSI was defined as cases with a positive blood culture from a peripheral vein and 1 of the following: (1) positive semiquantitative (>15 colony-forming units [CFU] per catheter segment) or quantitative (102 CFU) culture of a catheter component growing the same organism; (2) positive quantitative blood culture drawn from a central venous catheter (CVC) at a ratio of 5:1 (CVC vs peripheral); or (3) at least 2 hours’ differential time to positivity (ie, positive result of culture from CVC is obtained at least 2 hours earlier than from peripheral blood). Only RCTs that used this definition, or RCTs whose results were detailed enough to be readjudicated according to the aforementioned definition, were included in this subanalysis. We also assessed the rate of catheter failure, exit site infections, and all-cause mortality. Catheter failure was a composite outcome of catheter removal because of noninfectious complications and of persistent inadequate flow rate despite flushing, reposition of the catheter, or use of thrombolytic agents.
Data Extraction
Data from eligible studies were independently extracted by 3 reviewers (I. M. Z., F. N. Z., M. A.) into a spreadsheet (Supplementary Data). Discrepancies between authors were resolved by consensus. The following information was extracted: author, year of publication, country of origin, study period, population, age, number of analyzed patients, number of catheters and catheter-days for each group, site of catheter insertion, interventions used, CLABSIs and CRBSIs per 1000 catheter-days, catheter failures and exit site infections, all-cause mortality, and other adverse events.
Quality Assessment
The methodological quality of each trial was evaluated independently by 3 authors (I. M. Z., F. N. Z., M. A.) using the Cochrane Collaboration tool [14].
Data Analysis
Incidence rate ratios were pooled for CLABSI, and 95% confidence intervals (CIs) were calculated using random-effects DerSimonian and Laird weights. A sensitivity analysis was performed to estimate incidence rate ratio among studies that reported a low rate of CLABSI, defined as a rate of CLABSI <1.15 per 1000 catheter-days [15]. We used the I2 statistic to evaluate the presence of heterogeneity [16]. We evaluated for publication bias using the Harbord–Egger test (along with the corresponding funnel plot) [17], and the trim and fill method was used to adjust for potentially missing studies [18]. Subgroup analyses were performed using a random-effects meta-regression, and an interaction P value was reported to compare the effect size between different subgroups [19]. By using the same random model, we pooled incidence rate ratios for CRBSIs and exit site infections, and risk ratios for catheter failure (per catheter) and all-cause mortality (per patient). Studies that did not report data on secondary outcomes were excluded from the relevant subanalyses. An estimate is considered significant when the 95% CI does not include the unit. All calculations were performed using the Stata version 13 software package (StataCorp, College Station, Texas).
RESULTS
The included studies were published between 2002 and 2013 and reported data on 2896 patients and 383 710 catheter-days. The characteristics of the individual studies (Supplementary Data) are summarized in Table 1. These studies assessed the use of gentamicin (5 studies), vancomycin (2), minocycline (1), cefotaxime (1), cefazolin–gentamicin (1), linezolid (1), and amikacin (1). One study evaluated 2 different antimicrobial agents (vancomycin and linezolid) [20]. Of note, we were unable to include in our analysis the linezolid arm of this study because of missing data on the duration of follow-up for patients in this group. Nonantibiotic antimicrobial lock solutions were assessed in 12 studies. Taurolidine was the most commonly used nonantibiotic lock solution (4/12 studies), followed by ethanol and citrate (2 studies each), and 26% sodium chloride, methylene blue, fusidic acid, and recombinant tissue plasminogen activator (1 study each). The frequency of lock and duration of dwell, as well as the concentration of antimicrobial lock and heparin used in individual studies, are presented in Supplementary Data.
Characteristics of Eligible Studies
| First Author . | Year . | Population . | No. of Patients . | Intervention . | Rate of CLABSIs/1000 Catheter-days I/C . | No. of Exit Site Infections I/C . | No. of Catheter Failures . | No. Deaths I/C . | Adverse Events (I/C) . |
|---|---|---|---|---|---|---|---|---|---|
| Handrup [S1] | 2013 | Pediatric cancer | 113 | Taurolidine, citrate, heparin | 0.4/1.4 | 5/2 | 9/11 | 6/6 | Unpleasant taste (intervention) |
| Broom [S2] | 2012 | HD | 49 | Ethanol, heparin | 0.28/1.64 | 1/0 | 6/4 | 1/0 | 1/0 dry lips, thirst |
| Dumichen [S3] | 2012 | Pediatric cancer | 71 | Taurolidine, citrate | 0.3/1.24 | NR | NR | NR | 1/0 perioral dysesthesia, 2/0 abnormal taste, 2/0 nausea, 1/0 vomiting, 1/0 chest discomfort |
| Moran [S4] | 2012 | HD | 303 | Gentamicin, citrate | 0.28/0.91 | 8/9 | 42/35 | 27/25 | NR |
| Sofroniadou [S5] | 2012 | HD | 135 | Vancomycin, heparin | 0.61/5.48 | 10/9 | 9/11 | 3/1 | No |
| Linezolid, heparin | 0/5.48 | 7/9 | 9/11 | 1/1 | No | ||||
| Oguzhan [S6] | 2012 | HD | 56 | NaCl 26%, heparin | 1.19/0.65 | NR | 4/3 | 0/0 | No |
| Maki [S7] | 2011 | HD | 416 | Sodium citrate, methylene blue, methylparaben, propylparaben | 0.24/0.82 | NR | 0/4 | 2/9 | 2/0 dysgeusia, 1/0 diarrhea, 1/0 unexplained high fever, 1/0 MI, 1/0 pulmonary embolism; 0/2 major hemorrhage, 0/1 interdialytic hypotension |
| Hemmelgarn [S8] | 2011 | HD | 225 | Tissue plasminogen activator, heparin | 0.4/1.37 | NR | 22/40 | 3/5 | 23/34 among which bleeding 13/16 |
| Bisseling [S9] | 2010 | TPN | 30 | Taurolidine | 0.19/2.02 | NR | 0/0 | NR | No |
| Zhang [S10] | 2009 | HD | 140 | Gentamicin, heparin | 0.06/0.67 | 3/3 | 0/0 | 4/6 | 1/0 tinnitus, 1/0 pruritus; 0/1 bleeding |
| Seliem [S11] | 2009 | Critically ill neonates | 97 | Amikacin, heparin | 4.5/19.9 | NR | 0/0 | 4/8 | 5/8 asymptomatic hypoglycemia |
| MacRae [S12] | 2008 | HD | 61 | Citrate | 2.2/3.3 | 5/6 | 8/5 | 4/5 | 25/37 bleeding episodes; 1/2 PLTs < 100 |
| Sanders [S13] | 2008 | Hematology patients | 64 | Ethanol | 5.99/31.2 | 2/1 | 1/0 | 0/0 | 1/0 dyspnea; 0/1 unusual taste and anxiety |
| Filippi [S14] | 2007 | Critically ill neonates | 103 | Fusidic acid, Heparin | 2.2/21.1 | NR | NR | 13/11 | No |
| Kim [S15] | 2006 | HD | 120 | Cefazolin, gentamicin, heparin | 0.44/3.12 | NR | 0/0 | NR | No |
| Saxena [S16] | 2005 | HD | 208 | Cefotaxime, heparin | 1.65/3.13 | 28/11 | 24/17 | NR | NR |
| Weijmer [S17] | 2005 | HD | 291 | Citrate | 1.1/4.1 | 11/32 | 27/29 | 13/18 | 9/2 paresthesia, tingling, metallic taste; 5/16 major bleeding; 6/19 persistent bleeding after insertion; 2/4 unexplained thrombocytopenia |
| Garland [S18] | 2005 | Critically ill neonates | 90 | Vancomycin, heparin | 8.2/24.9 | NR | NR | NR | 8 vs 18 asymptomatic hypoglycemia |
| Bleyer [S19] | 2005 | HD | 60 | Minocycline, EDTA | 0/0.47 | NR | NR | NR | NR |
| McIntyre [S20] | 2004 | HD | 50 | Gentamicin, heparin | 0.31/4.05 | 0/0 | 1/2 | NR | No |
| Betjes [S21] | 2004 | HD | 76 | Taurolidine, citrate | 0/2.12 | 2/4 | 1/2 | NR | No |
| Dogra [S22] | 2002 | HD | 83 | Gentamicin, citrate | 0/1.14 | 1/4 | NR | 0/0 | 4 dizziness without vertigo, deafness, or ataxia (intervention) |
| Pervez [S23] | 2002 | HD | 55 | Gentamicin, tricitrasol | 0.62/3.05 | NR | 4/5 | NR | NR |
| First Author . | Year . | Population . | No. of Patients . | Intervention . | Rate of CLABSIs/1000 Catheter-days I/C . | No. of Exit Site Infections I/C . | No. of Catheter Failures . | No. Deaths I/C . | Adverse Events (I/C) . |
|---|---|---|---|---|---|---|---|---|---|
| Handrup [S1] | 2013 | Pediatric cancer | 113 | Taurolidine, citrate, heparin | 0.4/1.4 | 5/2 | 9/11 | 6/6 | Unpleasant taste (intervention) |
| Broom [S2] | 2012 | HD | 49 | Ethanol, heparin | 0.28/1.64 | 1/0 | 6/4 | 1/0 | 1/0 dry lips, thirst |
| Dumichen [S3] | 2012 | Pediatric cancer | 71 | Taurolidine, citrate | 0.3/1.24 | NR | NR | NR | 1/0 perioral dysesthesia, 2/0 abnormal taste, 2/0 nausea, 1/0 vomiting, 1/0 chest discomfort |
| Moran [S4] | 2012 | HD | 303 | Gentamicin, citrate | 0.28/0.91 | 8/9 | 42/35 | 27/25 | NR |
| Sofroniadou [S5] | 2012 | HD | 135 | Vancomycin, heparin | 0.61/5.48 | 10/9 | 9/11 | 3/1 | No |
| Linezolid, heparin | 0/5.48 | 7/9 | 9/11 | 1/1 | No | ||||
| Oguzhan [S6] | 2012 | HD | 56 | NaCl 26%, heparin | 1.19/0.65 | NR | 4/3 | 0/0 | No |
| Maki [S7] | 2011 | HD | 416 | Sodium citrate, methylene blue, methylparaben, propylparaben | 0.24/0.82 | NR | 0/4 | 2/9 | 2/0 dysgeusia, 1/0 diarrhea, 1/0 unexplained high fever, 1/0 MI, 1/0 pulmonary embolism; 0/2 major hemorrhage, 0/1 interdialytic hypotension |
| Hemmelgarn [S8] | 2011 | HD | 225 | Tissue plasminogen activator, heparin | 0.4/1.37 | NR | 22/40 | 3/5 | 23/34 among which bleeding 13/16 |
| Bisseling [S9] | 2010 | TPN | 30 | Taurolidine | 0.19/2.02 | NR | 0/0 | NR | No |
| Zhang [S10] | 2009 | HD | 140 | Gentamicin, heparin | 0.06/0.67 | 3/3 | 0/0 | 4/6 | 1/0 tinnitus, 1/0 pruritus; 0/1 bleeding |
| Seliem [S11] | 2009 | Critically ill neonates | 97 | Amikacin, heparin | 4.5/19.9 | NR | 0/0 | 4/8 | 5/8 asymptomatic hypoglycemia |
| MacRae [S12] | 2008 | HD | 61 | Citrate | 2.2/3.3 | 5/6 | 8/5 | 4/5 | 25/37 bleeding episodes; 1/2 PLTs < 100 |
| Sanders [S13] | 2008 | Hematology patients | 64 | Ethanol | 5.99/31.2 | 2/1 | 1/0 | 0/0 | 1/0 dyspnea; 0/1 unusual taste and anxiety |
| Filippi [S14] | 2007 | Critically ill neonates | 103 | Fusidic acid, Heparin | 2.2/21.1 | NR | NR | 13/11 | No |
| Kim [S15] | 2006 | HD | 120 | Cefazolin, gentamicin, heparin | 0.44/3.12 | NR | 0/0 | NR | No |
| Saxena [S16] | 2005 | HD | 208 | Cefotaxime, heparin | 1.65/3.13 | 28/11 | 24/17 | NR | NR |
| Weijmer [S17] | 2005 | HD | 291 | Citrate | 1.1/4.1 | 11/32 | 27/29 | 13/18 | 9/2 paresthesia, tingling, metallic taste; 5/16 major bleeding; 6/19 persistent bleeding after insertion; 2/4 unexplained thrombocytopenia |
| Garland [S18] | 2005 | Critically ill neonates | 90 | Vancomycin, heparin | 8.2/24.9 | NR | NR | NR | 8 vs 18 asymptomatic hypoglycemia |
| Bleyer [S19] | 2005 | HD | 60 | Minocycline, EDTA | 0/0.47 | NR | NR | NR | NR |
| McIntyre [S20] | 2004 | HD | 50 | Gentamicin, heparin | 0.31/4.05 | 0/0 | 1/2 | NR | No |
| Betjes [S21] | 2004 | HD | 76 | Taurolidine, citrate | 0/2.12 | 2/4 | 1/2 | NR | No |
| Dogra [S22] | 2002 | HD | 83 | Gentamicin, citrate | 0/1.14 | 1/4 | NR | 0/0 | 4 dizziness without vertigo, deafness, or ataxia (intervention) |
| Pervez [S23] | 2002 | HD | 55 | Gentamicin, tricitrasol | 0.62/3.05 | NR | 4/5 | NR | NR |
The references of eligible studies are provided in the Supplementary Data.
Abbreviations: CLABSI, central line–associated bloodstream infection; EDTA, ethylenediaminetetraacetic acid; HD, hemodialysis patients; I/C, intervention/control group; MI, myocardial infarction; NaCl, sodium chloride; NR, not reported; PLT, platelets; S, list of references in Supplementary Data; TPN, total parenteral nutrition patients.
Characteristics of Eligible Studies
| First Author . | Year . | Population . | No. of Patients . | Intervention . | Rate of CLABSIs/1000 Catheter-days I/C . | No. of Exit Site Infections I/C . | No. of Catheter Failures . | No. Deaths I/C . | Adverse Events (I/C) . |
|---|---|---|---|---|---|---|---|---|---|
| Handrup [S1] | 2013 | Pediatric cancer | 113 | Taurolidine, citrate, heparin | 0.4/1.4 | 5/2 | 9/11 | 6/6 | Unpleasant taste (intervention) |
| Broom [S2] | 2012 | HD | 49 | Ethanol, heparin | 0.28/1.64 | 1/0 | 6/4 | 1/0 | 1/0 dry lips, thirst |
| Dumichen [S3] | 2012 | Pediatric cancer | 71 | Taurolidine, citrate | 0.3/1.24 | NR | NR | NR | 1/0 perioral dysesthesia, 2/0 abnormal taste, 2/0 nausea, 1/0 vomiting, 1/0 chest discomfort |
| Moran [S4] | 2012 | HD | 303 | Gentamicin, citrate | 0.28/0.91 | 8/9 | 42/35 | 27/25 | NR |
| Sofroniadou [S5] | 2012 | HD | 135 | Vancomycin, heparin | 0.61/5.48 | 10/9 | 9/11 | 3/1 | No |
| Linezolid, heparin | 0/5.48 | 7/9 | 9/11 | 1/1 | No | ||||
| Oguzhan [S6] | 2012 | HD | 56 | NaCl 26%, heparin | 1.19/0.65 | NR | 4/3 | 0/0 | No |
| Maki [S7] | 2011 | HD | 416 | Sodium citrate, methylene blue, methylparaben, propylparaben | 0.24/0.82 | NR | 0/4 | 2/9 | 2/0 dysgeusia, 1/0 diarrhea, 1/0 unexplained high fever, 1/0 MI, 1/0 pulmonary embolism; 0/2 major hemorrhage, 0/1 interdialytic hypotension |
| Hemmelgarn [S8] | 2011 | HD | 225 | Tissue plasminogen activator, heparin | 0.4/1.37 | NR | 22/40 | 3/5 | 23/34 among which bleeding 13/16 |
| Bisseling [S9] | 2010 | TPN | 30 | Taurolidine | 0.19/2.02 | NR | 0/0 | NR | No |
| Zhang [S10] | 2009 | HD | 140 | Gentamicin, heparin | 0.06/0.67 | 3/3 | 0/0 | 4/6 | 1/0 tinnitus, 1/0 pruritus; 0/1 bleeding |
| Seliem [S11] | 2009 | Critically ill neonates | 97 | Amikacin, heparin | 4.5/19.9 | NR | 0/0 | 4/8 | 5/8 asymptomatic hypoglycemia |
| MacRae [S12] | 2008 | HD | 61 | Citrate | 2.2/3.3 | 5/6 | 8/5 | 4/5 | 25/37 bleeding episodes; 1/2 PLTs < 100 |
| Sanders [S13] | 2008 | Hematology patients | 64 | Ethanol | 5.99/31.2 | 2/1 | 1/0 | 0/0 | 1/0 dyspnea; 0/1 unusual taste and anxiety |
| Filippi [S14] | 2007 | Critically ill neonates | 103 | Fusidic acid, Heparin | 2.2/21.1 | NR | NR | 13/11 | No |
| Kim [S15] | 2006 | HD | 120 | Cefazolin, gentamicin, heparin | 0.44/3.12 | NR | 0/0 | NR | No |
| Saxena [S16] | 2005 | HD | 208 | Cefotaxime, heparin | 1.65/3.13 | 28/11 | 24/17 | NR | NR |
| Weijmer [S17] | 2005 | HD | 291 | Citrate | 1.1/4.1 | 11/32 | 27/29 | 13/18 | 9/2 paresthesia, tingling, metallic taste; 5/16 major bleeding; 6/19 persistent bleeding after insertion; 2/4 unexplained thrombocytopenia |
| Garland [S18] | 2005 | Critically ill neonates | 90 | Vancomycin, heparin | 8.2/24.9 | NR | NR | NR | 8 vs 18 asymptomatic hypoglycemia |
| Bleyer [S19] | 2005 | HD | 60 | Minocycline, EDTA | 0/0.47 | NR | NR | NR | NR |
| McIntyre [S20] | 2004 | HD | 50 | Gentamicin, heparin | 0.31/4.05 | 0/0 | 1/2 | NR | No |
| Betjes [S21] | 2004 | HD | 76 | Taurolidine, citrate | 0/2.12 | 2/4 | 1/2 | NR | No |
| Dogra [S22] | 2002 | HD | 83 | Gentamicin, citrate | 0/1.14 | 1/4 | NR | 0/0 | 4 dizziness without vertigo, deafness, or ataxia (intervention) |
| Pervez [S23] | 2002 | HD | 55 | Gentamicin, tricitrasol | 0.62/3.05 | NR | 4/5 | NR | NR |
| First Author . | Year . | Population . | No. of Patients . | Intervention . | Rate of CLABSIs/1000 Catheter-days I/C . | No. of Exit Site Infections I/C . | No. of Catheter Failures . | No. Deaths I/C . | Adverse Events (I/C) . |
|---|---|---|---|---|---|---|---|---|---|
| Handrup [S1] | 2013 | Pediatric cancer | 113 | Taurolidine, citrate, heparin | 0.4/1.4 | 5/2 | 9/11 | 6/6 | Unpleasant taste (intervention) |
| Broom [S2] | 2012 | HD | 49 | Ethanol, heparin | 0.28/1.64 | 1/0 | 6/4 | 1/0 | 1/0 dry lips, thirst |
| Dumichen [S3] | 2012 | Pediatric cancer | 71 | Taurolidine, citrate | 0.3/1.24 | NR | NR | NR | 1/0 perioral dysesthesia, 2/0 abnormal taste, 2/0 nausea, 1/0 vomiting, 1/0 chest discomfort |
| Moran [S4] | 2012 | HD | 303 | Gentamicin, citrate | 0.28/0.91 | 8/9 | 42/35 | 27/25 | NR |
| Sofroniadou [S5] | 2012 | HD | 135 | Vancomycin, heparin | 0.61/5.48 | 10/9 | 9/11 | 3/1 | No |
| Linezolid, heparin | 0/5.48 | 7/9 | 9/11 | 1/1 | No | ||||
| Oguzhan [S6] | 2012 | HD | 56 | NaCl 26%, heparin | 1.19/0.65 | NR | 4/3 | 0/0 | No |
| Maki [S7] | 2011 | HD | 416 | Sodium citrate, methylene blue, methylparaben, propylparaben | 0.24/0.82 | NR | 0/4 | 2/9 | 2/0 dysgeusia, 1/0 diarrhea, 1/0 unexplained high fever, 1/0 MI, 1/0 pulmonary embolism; 0/2 major hemorrhage, 0/1 interdialytic hypotension |
| Hemmelgarn [S8] | 2011 | HD | 225 | Tissue plasminogen activator, heparin | 0.4/1.37 | NR | 22/40 | 3/5 | 23/34 among which bleeding 13/16 |
| Bisseling [S9] | 2010 | TPN | 30 | Taurolidine | 0.19/2.02 | NR | 0/0 | NR | No |
| Zhang [S10] | 2009 | HD | 140 | Gentamicin, heparin | 0.06/0.67 | 3/3 | 0/0 | 4/6 | 1/0 tinnitus, 1/0 pruritus; 0/1 bleeding |
| Seliem [S11] | 2009 | Critically ill neonates | 97 | Amikacin, heparin | 4.5/19.9 | NR | 0/0 | 4/8 | 5/8 asymptomatic hypoglycemia |
| MacRae [S12] | 2008 | HD | 61 | Citrate | 2.2/3.3 | 5/6 | 8/5 | 4/5 | 25/37 bleeding episodes; 1/2 PLTs < 100 |
| Sanders [S13] | 2008 | Hematology patients | 64 | Ethanol | 5.99/31.2 | 2/1 | 1/0 | 0/0 | 1/0 dyspnea; 0/1 unusual taste and anxiety |
| Filippi [S14] | 2007 | Critically ill neonates | 103 | Fusidic acid, Heparin | 2.2/21.1 | NR | NR | 13/11 | No |
| Kim [S15] | 2006 | HD | 120 | Cefazolin, gentamicin, heparin | 0.44/3.12 | NR | 0/0 | NR | No |
| Saxena [S16] | 2005 | HD | 208 | Cefotaxime, heparin | 1.65/3.13 | 28/11 | 24/17 | NR | NR |
| Weijmer [S17] | 2005 | HD | 291 | Citrate | 1.1/4.1 | 11/32 | 27/29 | 13/18 | 9/2 paresthesia, tingling, metallic taste; 5/16 major bleeding; 6/19 persistent bleeding after insertion; 2/4 unexplained thrombocytopenia |
| Garland [S18] | 2005 | Critically ill neonates | 90 | Vancomycin, heparin | 8.2/24.9 | NR | NR | NR | 8 vs 18 asymptomatic hypoglycemia |
| Bleyer [S19] | 2005 | HD | 60 | Minocycline, EDTA | 0/0.47 | NR | NR | NR | NR |
| McIntyre [S20] | 2004 | HD | 50 | Gentamicin, heparin | 0.31/4.05 | 0/0 | 1/2 | NR | No |
| Betjes [S21] | 2004 | HD | 76 | Taurolidine, citrate | 0/2.12 | 2/4 | 1/2 | NR | No |
| Dogra [S22] | 2002 | HD | 83 | Gentamicin, citrate | 0/1.14 | 1/4 | NR | 0/0 | 4 dizziness without vertigo, deafness, or ataxia (intervention) |
| Pervez [S23] | 2002 | HD | 55 | Gentamicin, tricitrasol | 0.62/3.05 | NR | 4/5 | NR | NR |
The references of eligible studies are provided in the Supplementary Data.
Abbreviations: CLABSI, central line–associated bloodstream infection; EDTA, ethylenediaminetetraacetic acid; HD, hemodialysis patients; I/C, intervention/control group; MI, myocardial infarction; NaCl, sodium chloride; NR, not reported; PLT, platelets; S, list of references in Supplementary Data; TPN, total parenteral nutrition patients.
Quality Assessment
Based on the Cochrane Collaboration tool, 20 of 23 studies reported appropriate methods for random sequence generation, 12 of 23 had a clearly defined method to conceal allocation of patients, and 13 of 23 were double-blinded (Supplementary Data).
Antimicrobial Locks Reduce the Rate of CLABSI
Summary Estimates of Included Studies
| Study Characteristic . | Combined Effect, RR (95% CI) . | I2, % . | Interaction P Value for Subgroup Analyses . |
|---|---|---|---|
| All studies | 0.31 (.24–.40) | 12.3 | |
| Antibiotic vs nonantibiotic lock solutions | |||
| Antibiotic solutions | 0.29 (.19–.44) | 26.7 | 1.0 |
| Nonantibiotic | 0.29 (.21–.41) | 0.0 | |
| Underlying medical conditions | |||
| Adult hemodialysis | 0.33 (.24–.46) | 21.5 | Ref |
| Nonhemodialysis adult population | 0.16 (.05–.46) | 0.0 | .22 |
| Pediatric population | 0.26 (.16–.43) | 0.0 | .43 |
| Each solution vs heparin | |||
| Gentamicin | 0.23 (.13–.41) | 0.0 | |
| Vancomycin | 0.28 (.13–.62) | 0.0 | |
| Minocyclinea | 0.30 (.01–7.42) | … | |
| Amikacina | 0.23 (.08–.63) | … | |
| Cefotaximea | 0.53 (.38–.73) | … | |
| Cefazolin-gentamicina | 0.14 (.02–1.15) | … | |
| Taurolidine | 0.24 (.12–.46) | 0.0 | |
| Ethanol | 0.19 (.06–.56) | 0.0 | |
| Citrate | 0.37 (.16–.89) | 39.2 | |
| Sodium chloridea | 1.84 (.34–10.04) | … | |
| Sodium citrate, methylene blue, methylparaben, propylparabena | 0.29 (.12–.72) | … | |
| Fusidic acida | 0.10 (.01–.81) | … | |
| rt-PAa | 0.29 (.11–.80) | … | |
| Study Characteristic . | Combined Effect, RR (95% CI) . | I2, % . | Interaction P Value for Subgroup Analyses . |
|---|---|---|---|
| All studies | 0.31 (.24–.40) | 12.3 | |
| Antibiotic vs nonantibiotic lock solutions | |||
| Antibiotic solutions | 0.29 (.19–.44) | 26.7 | 1.0 |
| Nonantibiotic | 0.29 (.21–.41) | 0.0 | |
| Underlying medical conditions | |||
| Adult hemodialysis | 0.33 (.24–.46) | 21.5 | Ref |
| Nonhemodialysis adult population | 0.16 (.05–.46) | 0.0 | .22 |
| Pediatric population | 0.26 (.16–.43) | 0.0 | .43 |
| Each solution vs heparin | |||
| Gentamicin | 0.23 (.13–.41) | 0.0 | |
| Vancomycin | 0.28 (.13–.62) | 0.0 | |
| Minocyclinea | 0.30 (.01–7.42) | … | |
| Amikacina | 0.23 (.08–.63) | … | |
| Cefotaximea | 0.53 (.38–.73) | … | |
| Cefazolin-gentamicina | 0.14 (.02–1.15) | … | |
| Taurolidine | 0.24 (.12–.46) | 0.0 | |
| Ethanol | 0.19 (.06–.56) | 0.0 | |
| Citrate | 0.37 (.16–.89) | 39.2 | |
| Sodium chloridea | 1.84 (.34–10.04) | … | |
| Sodium citrate, methylene blue, methylparaben, propylparabena | 0.29 (.12–.72) | … | |
| Fusidic acida | 0.10 (.01–.81) | … | |
| rt-PAa | 0.29 (.11–.80) | … | |
Abbreviations: CI, confidence interval; Ref, referent subgroup for comparison; RR, relative risk; rt-PA, recombinant tissue plasminogen activator.
a Data are provided by 1 study.
Summary Estimates of Included Studies
| Study Characteristic . | Combined Effect, RR (95% CI) . | I2, % . | Interaction P Value for Subgroup Analyses . |
|---|---|---|---|
| All studies | 0.31 (.24–.40) | 12.3 | |
| Antibiotic vs nonantibiotic lock solutions | |||
| Antibiotic solutions | 0.29 (.19–.44) | 26.7 | 1.0 |
| Nonantibiotic | 0.29 (.21–.41) | 0.0 | |
| Underlying medical conditions | |||
| Adult hemodialysis | 0.33 (.24–.46) | 21.5 | Ref |
| Nonhemodialysis adult population | 0.16 (.05–.46) | 0.0 | .22 |
| Pediatric population | 0.26 (.16–.43) | 0.0 | .43 |
| Each solution vs heparin | |||
| Gentamicin | 0.23 (.13–.41) | 0.0 | |
| Vancomycin | 0.28 (.13–.62) | 0.0 | |
| Minocyclinea | 0.30 (.01–7.42) | … | |
| Amikacina | 0.23 (.08–.63) | … | |
| Cefotaximea | 0.53 (.38–.73) | … | |
| Cefazolin-gentamicina | 0.14 (.02–1.15) | … | |
| Taurolidine | 0.24 (.12–.46) | 0.0 | |
| Ethanol | 0.19 (.06–.56) | 0.0 | |
| Citrate | 0.37 (.16–.89) | 39.2 | |
| Sodium chloridea | 1.84 (.34–10.04) | … | |
| Sodium citrate, methylene blue, methylparaben, propylparabena | 0.29 (.12–.72) | … | |
| Fusidic acida | 0.10 (.01–.81) | … | |
| rt-PAa | 0.29 (.11–.80) | … | |
| Study Characteristic . | Combined Effect, RR (95% CI) . | I2, % . | Interaction P Value for Subgroup Analyses . |
|---|---|---|---|
| All studies | 0.31 (.24–.40) | 12.3 | |
| Antibiotic vs nonantibiotic lock solutions | |||
| Antibiotic solutions | 0.29 (.19–.44) | 26.7 | 1.0 |
| Nonantibiotic | 0.29 (.21–.41) | 0.0 | |
| Underlying medical conditions | |||
| Adult hemodialysis | 0.33 (.24–.46) | 21.5 | Ref |
| Nonhemodialysis adult population | 0.16 (.05–.46) | 0.0 | .22 |
| Pediatric population | 0.26 (.16–.43) | 0.0 | .43 |
| Each solution vs heparin | |||
| Gentamicin | 0.23 (.13–.41) | 0.0 | |
| Vancomycin | 0.28 (.13–.62) | 0.0 | |
| Minocyclinea | 0.30 (.01–7.42) | … | |
| Amikacina | 0.23 (.08–.63) | … | |
| Cefotaximea | 0.53 (.38–.73) | … | |
| Cefazolin-gentamicina | 0.14 (.02–1.15) | … | |
| Taurolidine | 0.24 (.12–.46) | 0.0 | |
| Ethanol | 0.19 (.06–.56) | 0.0 | |
| Citrate | 0.37 (.16–.89) | 39.2 | |
| Sodium chloridea | 1.84 (.34–10.04) | … | |
| Sodium citrate, methylene blue, methylparaben, propylparabena | 0.29 (.12–.72) | … | |
| Fusidic acida | 0.10 (.01–.81) | … | |
| rt-PAa | 0.29 (.11–.80) | … | |
Abbreviations: CI, confidence interval; Ref, referent subgroup for comparison; RR, relative risk; rt-PA, recombinant tissue plasminogen activator.
a Data are provided by 1 study.
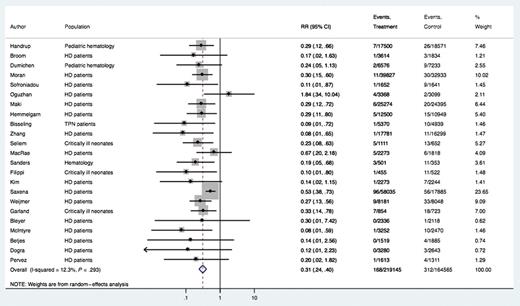
Forest plot of included studies. Individual and combined estimates of relative risk of central line–associated bloodstream infection. Abbreviations: CI, confidence interval; HD, hemodialysis; RR, relative risk; TPN, total parenteral nutrition.
Subgroup analysis of the comparative effectiveness of antibiotic vs nonantibiotic lock solutions revealed a CLABSI relative risk of 0.29 (95% CI, .21–.41) in the nonantibiotic antimicrobial group and 0.29 (95% CI, .19–.44) in the antibiotic group (interaction P = 1.0).
A second subgroup analysis was performed to assess the impact of the underlying medical conditions on the effect size. Sixteen of the 23 studies involved adult patients receiving hemodialysis through CVCs. Two studies included pediatric hematology patients, 3 studies included critically ill neonates, 1 study included adult hematology patients, and 1 included patients receiving total parenteral nutrition. The relative risk for CLABSI was 0.33 (95% CI, .24–.46) in the adult hemodialysis group, 0.16 (95% CI, .05–.46) in the nonhemodialysis adult population (interaction P = .22), and 0.26 (95% CI, .16–.43) in the pediatric population (interaction P = .43).
To measure the effect of lock solutions in centers with a low rate of CLABSI, we performed a sensitivity analysis excluding all studies with a baseline rate >1.15 per 1000 catheter-days, a rate that was suggested by a large multicenter trial to be achievable with optimal implementation of currently used preventive measures [15]. Six trials were included in this analysis. These studies reported full barrier precautions during catheter insertion, routine exit site care using antiseptic agents, and use of masks and sterile gloves when assessing catheter hubs or ports after insertion. We found that the relative reduction in the CLABSI rate remained significant in this subanalysis (RR, 0.32 [95% CI, .17–.60]).
Effect of Antimicrobial Lock on Secondary Outcomes
Using the 12 studies that provided data on exit site infections, we found that antimicrobial lock solutions are effective in reducing the rate of exit site infections (RR, 0.68 [95% CI, .49–.95]; I2 = 0%; Supplementary Data). Eighteen studies reported noninfectious complications necessitating catheter removal, and the pooled effect revealed no statistically significant difference between patients receiving antimicrobial and heparin lock (RR, 0.83 [95% CI, .65–1.06]; Supplementary Data). Furthermore, no statistically significant difference was observed among the 13 studies that provided extractable data on all-cause mortality (RR, 0.84 [95% CI, .64–1.12]; Supplementary Data). Of note, 1 study with data on the mortality rate did not delineate the number of patients per group, and the data were considered nonextractable [20]. Finally, from the analysis of the 4 studies that reported CRBSI, lock therapy proved effective in reducing the rate of CRBSI (RR, 0.12 [95% CI, .03–.47]), an effect consistent across the studies (I2 = 0%) (Supplementary Data).
DISCUSSION
Several basic infection control interventions have been implemented that have led to a reduction in the CLABSI rate in the United States [21]. However, greater reductions in risk of catheterized patients may require additional preventive measures such as use of antimicrobial lock solutions. Our meta-analysis revealed a 69% reduction in the risk of CLABSI among patients using antimicrobial lock therapy and suggests that routine use of antimicrobial lock solutions could prevent 7 of 10 such events. Interestingly, this effect size was relatively consistent across studies that used different antimicrobial agents, different locking regimens, and across studies reporting data on different patient populations.
This finding raises the question of whether antimicrobial lock solutions can reduce the risk of CLABSI in centers that already report a low incidence with traditional prevention measures [8]. By separately analyzing studies that reported a control group event rate of <1.15 per 1000 catheter-days, we found that the effect of antimicrobial locks remains significant. Therefore, it is reasonable to assume that antimicrobial locks have an additive effect in the reduction of CLABSI risk and would be useful as an adjunct to traditional preventive measures. Most studies included in our meta-analysis focused on patients undergoing hemodialysis, which is reasonable given the predominant intraluminal source of catheter-related infections in patients with long-term catheters [22]. However, our findings support that the protective effect of lock solutions is not limited to this patient population (Table 2). Because CLABSI as an outcome indicator may overestimate the beneficial effect of catheter lock, we separately analyzed the effect of lock solutions on the incidence of CRBSI, requiring bloodstream infection to be confirmed by percutaneously drawn blood cultures, and we found that catheter lock use significantly reduced the rate of CRBSI.
Taking into consideration that treatment of CLABSI frequently necessitates catheter removal [11], it is anticipated that antimicrobial lock solutions will reduce the likelihood of premature catheter removal [4]. However, it is important to assess whether antimicrobial lock solutions could have a negative impact on existing catheters, either by directly impairing catheter integrity or by increasing the risk of thrombosis [23, 24]. Importantly, we found that the use of lock solutions did not increase the incidence of catheter malfunction, but different lock solutions, concentrations, and catheter materials should be separately investigated in future studies.
Exit site infections are an additional cause of morbidity in patients with central venous catheters, and these infections can lead to subsequent bloodstream infections, often necessitating catheter removal [25]. Our meta-analysis demonstrated that antimicrobial lock solutions have a significant effect in reducing the risk of these infections, thus suggesting their impact beyond the prevention of bloodstream infections. Although this seems counterintuitive, 2 possible explanations may account for this finding. First, most of the studies of exit site infections were in dialysis patients. Some lock solutions leak from the catheter lumen into the circulation depending on solution density, catheter type, anatomic location of catheter insertion, and body position [26]. As such, at the concentrations used in some lock solutions, leakage into the bloodstream may reach at or near the minimal inhibitory concentration of common pathogens such as staphylococci, and the concentration may be maintained in the blood and subcutaneous tissue at the catheter exit site during the interdialytic period, minimizing the risk of exit site infection. Alternatively, it is known from animal models [27] that microbes directly injected into an implanted port reservoir can, with continued access of the port, eventually lead to finding identical microbes in the surrounding subcutaneous tissue. In this scenario, a lock solution could reduce risk of such fluid leakage causing infection in the surrounding tissue of an implanted port. However, additional laboratory and clinical trials are needed to investigate this association.
Widespread use of antimicrobial compounds inevitably raises concern of antimicrobial resistance. Indeed, gentamicin-resistant bacteria causing CLABSIs have been observed among hemodialysis patients who are receiving gentamicin lock therapy [28], and hence it might not be prudent to be used alone in a lock regimen. To avoid such an outcome, there is interest in use of nonantibiotic antimicrobial solutions such as ethanol [29] and taurolidine [30]. Our meta-analysis shows that these agents have similar influence on risk reduction for CLABSIs as gentamicin or vancomycin. Studies included in our meta-analysis that assessed the effectiveness of ethanol lock were exclusively among hemodialysis patients, compared with studies using taurolidine lock solutions, which were among hematology patients, patients receiving total parenteral nutrition, and patients on hemodialysis. The long dwell time of ethanol lock solution, when used for hemodialysis patients, could potentiate its effectiveness compared with critically ill or oncology patients. Indeed, an RCT that compared the effectiveness of 70% ethanol lock to placebo among adult hematology patients using a dwell time of only 15 minutes did not find a significant reduction in the risk of endoluminal CRBSI [31]. Therefore, further studies should address the optimal dwell time of ethanol lock solutions.
A decision regarding use of an antimicrobial lock solution should be based on potential adverse events, ease of use, interactions with catheter material or medications infused through the same catheter lumens, stability of the product, and cost, as well as the lock dwell time. Gentamicin lock has been linked to ototoxicity [32], and the inadvertent bolus infusion of high-dose citrate has caused the unexpected death of a patient, which led the FDA to remove it from the market as an agent for lock therapy [33]. Ethanol lock solutions have been associated with flushing, dizziness, and syncope; changes in liver function tests; and with structural changes of the catheters [34]. However, among the studies included in our analysis, no debilitating or irreversible side effects were reported (Table 1). Another concern is systemic toxicity from distal catheter leakage of lock solutions used for prolonged periods of time and the idea that some patients (ie, those undergoing hemodialysis) may be maintained on therapeutic or subtherapeutic antimicrobial drug levels due to leakage of lock solutions. This can be minimized by use of non–femoral vein catheter insertion and by changes in density of the lock solution [26, 35]. However, within the catheter lumen, colonizing organisms form a biofilm [36], and thus high concentrations of antimicrobial agents are needed to eradicate these organisms. This is particularly important in patients with catheters placed for a short duration of time, among whom a rapidly effective lock solution is needed.
Previous reports have evaluated the efficacy of antimicrobial lock solutions with promising findings [5]. However, due to the unavailability of relevant data, these studies failed to prove the efficacy of nonantibiotic antimicrobial lock solutions or to suggest an additive effect of antimicrobial locks to currently implemented infection prevention practices. Despite the fact that our study addresses some of these important issues, not all concerns have been eliminated. Specifically, antimicrobial lock therapy may be of limited utility in critically ill and oncology patients in whom catheter lumens are in continuous use, whereas it might be more beneficial for patients who have long-term catheters; in addition, there is still not enough evidence to ascertain the optimum dwell time and concentration for different lock solutions. All these questions should be addressed in future trials. Publication bias is also a concern in our study and this probably indicates that studies with nonsignificant results may have not been published [17]. With regard to secondary outcomes, selective reporting was observed as 9 studies lacked exit site infections as secondary outcome, 5 lacked noninfectious complications necessitating catheter removal, and 10 lacked all-cause mortality.
CONCLUSIONS
Use of antimicrobial lock solutions is an effective prevention strategy to reduce the risk of CVC infections. Although the limited number of prospective, randomized studies in pediatric and hematology patients may preclude an imminent change in policy in these subgroups before larger trials are performed, existing evidence in patients receiving hemodialysis suggests that implementation of antimicrobial lock therapy should be considered.
Notes
Author contributions. I. M. Z. designed the study; performed the literature search; participated in data collection, extraction, statistical analysis, and interpretation; prepared tables and figures; wrote and drafted the initial manuscript, and approved the final manuscript as submitted. F. N. Z. designed the study; performed the literature search; participated in data collection, extraction, statistical analysis, and interpretation; prepared tables and figures; wrote and drafted the manuscript; and approved the final manuscript as submitted.
M. A. designed the study; performed the literature search; participated in data collection, extraction, statistical analysis, and interpretation; prepared tables and figures; wrote and drafted the initial manuscript; and approved the final manuscript as submitted. P. D. Z. designed the study; participated in the statistical analysis and data interpretation; prepared tables and figures; reviewed and revised the manuscript; and approved the final manuscript as submitted. L. A. M. participated in data interpretation; reviewed and revised the manuscript; and approved the final manuscript as submitted. E. M. conceptualized and designed the study; interpreted the data; reviewed and revised the manuscript; and approved the final manuscript as submitted.
Potential conflicts of interest. L. A. M. has been a consultant for Fresenius and Marvao Medical. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
I. M. Z., F. N. Z., and M. A. contributed equally to this work.





Comments