-
PDF
- Split View
-
Views
-
Cite
Cite
Jaclyn A Cusumano, Kenneth P Klinker, Angela Huttner, Megan K Luther, Jason A Roberts, Kerry L LaPlante, Towards precision medicine: Therapeutic drug monitoring–guided dosing of vancomycin and β-lactam antibiotics to maximize effectiveness and minimize toxicity, American Journal of Health-System Pharmacy, Volume 77, Issue 14, 15 July 2020, Pages 1104–1112, https://doi.org/10.1093/ajhp/zxaa128
Close - Share Icon Share
Abstract
The goal of this review is to explore the role of antimicrobial therapeutic drug monitoring (TDM), especially in critically ill, obese, and older adults, with a specific focus on β-lactams and vancomycin.
The continued rise of antimicrobial resistance prompts the need to optimize antimicrobial dosing. The aim of TDM is to individualize antimicrobial dosing to achieve antibiotic exposures associated with improved patient outcomes. Initially, TDM was developed to minimize adverse effects during use of narrow therapeutic index agents. Today, patient and organism complexity are expanding the need for precision dosing through TDM services. Alterations of pharmacokinetics and pharmacodynamics (PK/PD) in the critically ill, obese, and older adult populations, in conjunction with declining organism susceptibility, complicate attainment of therapeutic targets. Over the last decade, antimicrobial TDM has expanded with the emergence of literature supporting β-lactam TDM and a shift from monitoring vancomycin trough concentrations to monitoring of the ratio of area under the concentration (AUC) curve to minimum inhibitory concentration (MIC). PK/PD experts should be at the forefront of implementing precision dosing practices.
Precision dosing through TDM is expanding and is especially important in populations with altered PK/PD, including critically ill, obese, and older adults. Due to wide PK/PD variability in these populations, TDM is vital to maximize antimicrobial effectiveness and decrease adverse event rates. However, there is still a need for studies connecting TDM to patient outcomes. Providing patient-specific care through β-lactam TDM and transitioning to vancomycin AUC/MIC monitoring may be challenging, but with experts at the forefront of this initiative, PK-based optimization of antimicrobial therapy can be achieved.
As the older adult population increases and the obesity epidemic continues, patients are at heightened risk for infection and critical illness. Add to the equation altered pharmacokinetics/pharmacodynamics (PK/PD), decreasing organism susceptibility, and unacceptable mortality rates and alternative treatment approaches become essential. Precision medicine provides this alternative and encompasses precision dosing through therapeutic drug monitoring (TDM).
TDM was introduced in the late 1960s to minimize toxicity resulting from use of narrow therapeutic index medications. For antimicrobials, TDM began with aminoglycosides.1 The early literature in this area focused on the practicality of laboratory testing, including methodology, turnaround times, and accuracy of results.1 In the 1980s, antimicrobial TDM studies shifted towards identifying site-specific concentrations and establishing PK/PD indices associated with maximal clinical benefits.1–4 Using these indices, in the 1990s clinicians began to introduce new dosing schemes.1,5,6 For example, introduction of high-dose extended-interval aminoglycoside therapy to achieve desired ratios of maximum concentration (Cmax) to minimum inhibitory concentration (MIC) allowed for maximal drug exposure while minimizing adverse events.5,6 Literature over the last decade has redefined vancomycin PD targets7-11 and characterized the potential for β-lactam TDM12-15 to enable better clinical outcomes, especially in populations at risk for suboptimal antibiotic exposure.
Therapeutic drug monitoring (TDM) is essential due to increased patient and organism complexity, which leads to high variability in pharmacokinetics and pharmacodynamics (PK/PD).
Patient populations with highly variable PK/PD include the critically ill, obese, and older adults.
Precision dosing of β-lactams and vancomycin through TDM is increasingly crucial for these patient populations to improve outcomes and minimize toxicities.
Most antimicrobial regimens approved in clinical trials are designed for the “average patient.” This treatment approach is not successful for all patients. Clinical trials typically enroll patients with less severe infections and more susceptible bacteria, which may necessitate lower dosages. Therefore, package insert–recommended dosing may be insufficient for more severe infection types, including those caused by multidrug-resistant organisms, as well as specific patient populations excluded from trials, such as critically ill, obese, and older adults. TDM provides an opportunity to incorporate precision dosing regimens by considering patient-specific PK and organism variability affecting PD. Doses that ensure achievement of PK/PD targets are especially challenging to predict in critical illness12,16,–19, obesity20,-23, and older adult populations,24–27 making TDM necessary. Expanding antimicrobial TDM services is theorized to be the way of the future for infectious diseases, with PK/PD experts being at the forefront of this initiative. The goal of this review is to describe the evidence for antimicrobial TDM in optimizing therapy, especially in critically ill, obese, and older adult populations, with a specific focus on β-lactams and vancomycin.
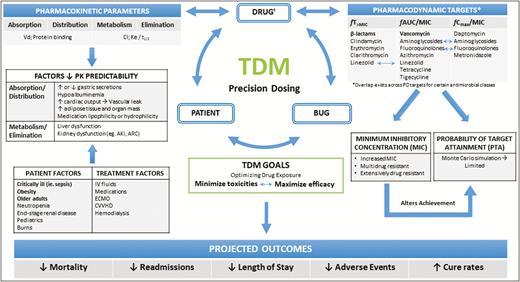
Getting started with TDM: the bug, the drug, and the patient.
Variables impacting antimicrobial PK/PD goals can be broken down into 3 main components: the bug, the drug, and the patient (Figure 1). For microorganisms, the primary variable is the MIC, defined as the lowest antimicrobial concentration needed to inhibit bacterial growth. The MIC serves as a crucial component in determining antimicrobial concentrations needed to achieve therapeutic effectiveness.16 However, the MIC alone does not take into consideration drug concentration fluctuations observed throughout a dosing interval, the concentration achieved at the site of infection, or the concentration required for bactericidal activity.28 Rather, PD indices include the MIC to predict clinical outcomes. Specifically, PD indices describe antimicrobial effects as time-dependent (as reflected by the percentage of the dosing interval during which the free unbound drug concentration is above the MIC [fT>MIC]), concentration-dependent (as indicated by the ratio of peak plasma concentration to MIC [Cmax/MIC]), or concentration-dependent with time dependencies (as reflected by the ratio of 24-hour free unbound drug concentration area under the curve [AUC] to MIC [fAUC/MIC]).20,29 Achieving antibiotic-specific PD targets to prevent over- and underexposure is a vital component of TDM. Selection of ideal PD targets varies by organism and/or patient population. For example, targeting an fAUC/MIC value of >400 in vancomycin therapy is based only on data for invasive Staphylococcus aureus infections (eg, pneumonia or bacteremia).30 Additionally, variability of organism MIC values limits the accuracy of desired PD targets.31 A single MIC reading can range between 1 and 2 dilutions, as methodology can vary by laboratory and laboratory technique.31 Finally, the last component in the equation is the patient. Interpatient variability in PK parameters underscores the challenge in achieving PD therapeutic goals while also minimizing toxicities associated with antimicrobial overexposures; this is observed in various patient populations, especially amongst critically ill, obese, and older adults. In combination, PK/PD parameter variability creates uncertainty and strengthens the argument for individualized antibiotic dosing recommendations.
Therapeutic drug monitoring TDM conceptual model. AKI indicates acute kidney injury; ARC, augmented renal clearance; Cl, clearance; CVVHD, continuous venovenous hemodialysis; ECMO, extracorporeal membrane oxygenation; fT>MIC, free unbound drug concentration time above minimum inhibitory concentration (MIC); fAUC/MIC, ratio of free unbound drug concentration area under the curve to MIC; fCmax/MIC, ratio of free peak plasma concentration to MIC; Ke, elimination rate constant; IV, intravenous; PK, pharmacokinetics; t1/2, half-life; Vd, volume of distribution. Note that optimized drug dosing may reduce the need for TDM.
PK/PD alterations: patient-specific factors.
Antibiotic dosing is determined in early-phase clinical trials wherein the majority of participants are often young, healthy volunteers with minimal PK variability.28 In contrast, patients with severe infections display increased variability in volume of distribution (Vd), protein binding, and drug clearance.16,20,25 Key PK alterations in specific patient populations, including critically ill, obese, and older adults, are further described in Table 1.16,21,23,25 These alterations are multifactorial and may not be present in all patients, further decreasing predictability of PK/PD and supporting the use of TDM (Figure 1).
Key Pharmacokinetic Alterations in Specific Patient Populations
| Populationa . | Volume of Distribution . | Protein Binding . | Clearance . |
|---|---|---|---|
| Critically ill adults | Increased16 | Decreased16 | Increased or decreased16 |
| Obese adults | Increased21 | No change23 | Increased21 |
| Older adults | Increased or decreased25 | Decreased25 | Decreased25 |
| Populationa . | Volume of Distribution . | Protein Binding . | Clearance . |
|---|---|---|---|
| Critically ill adults | Increased16 | Decreased16 | Increased or decreased16 |
| Obese adults | Increased21 | No change23 | Increased21 |
| Older adults | Increased or decreased25 | Decreased25 | Decreased25 |
aAlterations in pharmacokinetic parameters may vary depending on comorbidities and other patient-related factors.
Key Pharmacokinetic Alterations in Specific Patient Populations
| Populationa . | Volume of Distribution . | Protein Binding . | Clearance . |
|---|---|---|---|
| Critically ill adults | Increased16 | Decreased16 | Increased or decreased16 |
| Obese adults | Increased21 | No change23 | Increased21 |
| Older adults | Increased or decreased25 | Decreased25 | Decreased25 |
| Populationa . | Volume of Distribution . | Protein Binding . | Clearance . |
|---|---|---|---|
| Critically ill adults | Increased16 | Decreased16 | Increased or decreased16 |
| Obese adults | Increased21 | No change23 | Increased21 |
| Older adults | Increased or decreased25 | Decreased25 | Decreased25 |
aAlterations in pharmacokinetic parameters may vary depending on comorbidities and other patient-related factors.
Critical illness.
Critical illness is often associated with a rise in cardiac output, which results in increased clearance, endothelial dysfunction, and capillary leak leading to excess extravascular fluid.16,20 These effects result in an increased Vd for hydrophilic antibiotics (eg, β-lactams, vancomycin, aminoglycosides), whereas lipophilic antibiotics (eg, fluoroquinolones, macrolides) are minimally affected.16 Beyond fluid shifts, transcapillary escape of albumin results in hypoalbuminemia, which is reported in 40% to 50% of critically ill patients. Consequently, higher free drug concentrations, larger Vd, and enhanced clearance occur.17,20 For example, in healthy volunteers vs patients with iatrogenically induced hypoalbuminemia due to use of ceftriaxone, a highly (85%-95%) protein-bound antimicrobial, it was found that the hypoalbuminemic patients had significantly higher median fCmax (107 mg/L vs 51 mg/L) and Vd (0.18 L/kg vs 0.15 L/kg).32 Additionally, critically ill patients may have augmented renal clearance (ARC), defined by convention as creatinine clearance of ≥130 mL/min/m2.19 The cause of ARC is poorly characterized but thought to be related to systemic inflammatory response syndrome or other severe inflammatory conditions that can increase cardiac output and decrease vascular resistance, ultimately increasing renal blood flow.19 Conversely, critically ill patients are at high risk for acute kidney injury, adding to the complexity of predicting antimicrobial PK/PD.
Obesity.
The number of individuals with obesity in the United States is expected to reach 65 million by the year 2030.33 PK variability in obese patients is due to alterations in Vd and clearance resulting from increased adipose tissue and lean muscle mass. First, Vd may be overestimated for hydrophilic antimicrobials (eg, β-lactams, vancomycin, aminoglycosides)20 due to poor penetration into adipose tissue.21 For example, in a study comparing obese vs nonobese patients receiving vancomycin, the estimated mean (SD) Vd was significantly higher in obese patients (74.4 [14.5] L vs 50.4 [9.3] L).22 Second, obese patients have increased organ mass, which may lead to increased renal blood flow, thus increasing renal clearance.21 Lastly, patients who have undergone gastric bypass surgery also have significant PK/PD alterations due to the loss of surface area for medication absorption.34 Dosing becomes further complicated as obese patients age, develop chronic kidney disease, or experience acute kidney injury, which can reduce drug clearance.35
Older adults.
The older adult population (ie, individuals 65 years of age or older) is projected to almost double by the year 2050.36 Physiologic alterations in older adult patients include reduced gastric acid secretions, which affect absorption; reduced renal and hepatic blood flow, which slows the rate of clearance; and reduced serum protein concentrations, which increases free drug concentrations.25 For example, ertapenem PK data for older vs younger adult patients reveal a longer half-life (5.2 hours vs 3.8 hours), slower clearance, higher fAUC (39%), and higher unbound ertapenem (55.4 mg/L vs 33.2 mg/L).26 Overexposure to antimicrobials in the older adult population puts them at increased risk for adverse events, and TDM can limit such events.
Role of therapeutic drug monitoring.
Due to extensive PK/PD variability in these populations, leading to either a lack of target therapeutic effectiveness or overexposure leading to toxicity, precision dosing guided by TDM aids patients outside “normal” exposure curves. Further, the presence of multidrug-resistant organisms or less susceptible organisms with elevated MICs may hinder the ability to achieve desired PD targets and highlights the scenario where TDM may be beneficial.
Beta-lactam TDM.
Beta-lactam PD is defined as time-dependent killing (as indicated by fT>MIC).28 Recommended percentage fT>MIC targets are 40% to 50% for penicillins, 60% to 70% for cephalosporins, 40% for carbapenems, and 50% to 60% for monobactams.16,28,37 However, in the literature these targets vary based on the β-lactam and organism targeted and are derived from animal studies, some dating originally from the 1950s.38–41 A target of 100% fT>MIC, as used in the BLING-I trial comparing continuous infusion vs intermittent infusion in critically ill patients42 and in other observational studies,43,44 may be more appropriate in an age of rising MICs. Target concentrations maintained above 4 times the MIC throughout the dosing interval (ie, concentrations of 100% fT>4×MIC) have also been described to improve outcomes.45 Attainment of these PD targets for bactericidal effects depends on the specific pharmacokinetic and pharmacodynamic properties of the β-lactam, the patient, and the infecting organism, thus supporting TDM.28 However, the desired PD targets still remain to be elucidated. Current β-lactam TDM practices are aimed at achieving 100% fT>MIC or 100% fT>4×MIC, but perhaps lower percentage targets (40% to 70%), as described above, are adequate. It is crucial that PK/PD experts begin to take the lead in efforts to discover the ideal PD target for β-lactam TDM that positively impacts patient outcomes.
The use of β-lactam TDM has primarily been studied in the critically ill population, with limited reported experience in obese and older adult patients. Evidence is supported by known alterations in PK/PD. For example, an analysis of critically ill patients receiving β-lactams found that those with ARC had a trough concentration below the MIC 82% of the time.18 In addition, a prospective comparative study assessing critically ill severely obese patients (body mass index [BMI] of >35) vs nonobese patients (BMI of <30) receiving continuous-infusion piperacillin/tazobactam therapy found that measured piperacillin plasma concentrations were significantly lower for obese patients, and the percentage of time during which the piperacillin plasma concentration was above the target of 64 mg/L (4-fold higher than the MIC) was lower (64% vs 93%, P = 0.027).15 It should be noted that assessment of outcomes was dependent on the identified ideal PD target. However, this study highlighted PK/PD variability in this patient population. Trough concentrations of cefotaxime, meropenem, and piperacillin were also assessed in patients over the age of 70.46 The investigators found wide variability in results and concluded that only 36% of patients achieved a trough value above the desired MIC breakpoint. Therefore, the inability to accurately predict serum β-lactam concentrations in these patients emphasizes the importance of β-lactam TDM to ensure adequate exposures associated with improved outcomes.
TDM is also essential in preventing the toxicities of β-lactam overexposure. A retrospective analysis of TDM in patients treated with piperacillin, meropenem, or flucloxacillin found that patients who developed neurotoxicity during use of any of the 3 antibiotics and patients who developed nephrotoxicity during use of piperacillin or meropenem had significantly higher mean trough concentrations.47 The investigators defined threshold trough concentrations associated with a 50% risk of neurotoxicity (piperacillin Cmin of >361.4 mg/L; meropenem Cmin of >64.2 mg/L; and flucloxacillin Cmin of >125.1 mg/L) or nephrotoxicity (piperacillin Cmin of >462.65 mg/L; meropenem Cmin of >44.45 mg/L). Another retrospective study assessing septic intensive care unit (ICU) patients undergoing TDM during treatment with meropenem, piperacillin/tazobactam, ceftazidime, or cefepime found that worsening neurological status was associated with increased trough concentrations of piperacillin/tazobactam and meropenem.48 Conversely, researchers who conducted a retrospective study evaluating critically ill patients receiving either conventional or higher-than-conventional doses of meropenem or piperacillin/tazobactam, with dosing in both groups guided by TDM, concluded that there were no significant between-group differences in rates of various toxicities.49 Similary, a single-center retrospective cohort study of 300 patients (a total of 403 imipenem plasma concentrations were measured) found that 5% of patients experienced toxicity, but those patients did not have higher trough concentrations than patients who did not experience toxicity.50 These findings demonstrated the significance of using β-lactam TDM to optimize dosing and minimize adverse effects but also indicated that validation of toxicity thresholds is still needed.
A recent single-center, retrospective cohort study of 319 patients undergoing cefepime TDM found that a trough concentration less than 7.5 mg/L would limit the incidence of neurotoxicity.51 However, patients who experienced cefepime-associated neurotoxicity had a median plasma trough concentration of 21.6 mg/L (interquartile range [IQR], 17.0–28.6 mg/L.51 Another single-center, retrospective cohort study assessing 161 cefepime trough levels from 93 patients found that a threshold of less than 20 mg/L should be targeted to limit toxicity.52 The mean (SD) trough concentration among patients with toxicity was 52.2 (8.4) mg/L (range, 25.7–59.7 mg/L).52 More research is necessary to define the ideal toxicity threshold for cefepime along with all other β-lactams. An ongoing prospective clinical trial, the OPTIMAL TDM Study (Clinicaltrials.gov identifier, NCT03790631) aims to assess the toxicity of cefepime, imipenem, meropenem, piperacillin, flucloxacillin, amoxicillin, and ceftazidime.
Evidence of improvement of other patient outcomes by means of β-lactam TDM remains limited; however, monitoring of β-lactams is increasingly becoming an area of interest. A 2015 survey of intensivists and infectious diseases physicians in France found that 21% of respondents monitored β-lactams at their institution.53 Another international survey, which targeted clinicians in 9 ICUs that performed β-lactam TDM, indicated that piperacillin and meropenem were the most commonly monitored β-lactams (100% of units), followed by ceftazidime (78%), ceftriaxone (43%), and cefazolin (43%).54
The implications of failure to achieve fT>MIC targets has not been extensively described except by researchers who conducted the Defining Antibiotic Levels in Intensive Care Units (DALI) study.12 This prospective, multinational study evaluated PK/PD target attainment with use of 8 β-lactams in 361 critically ill patients. Of the 361 patients, 248 were treated for active infection; 50% fT>MIC was not achieved in approximately 16% of those patients, and they were 32% less likely to have a positive clinical outcome (odds ratio [OR], 0.68; 95% confidence interval [CI], 0.52–0.91; P = 0.009). The researchers concluded that achieving either 50% fT>MIC or 100% fT>MIC was associated with positive outcomes (ORs of 1.02 [95% CI, 1.01–1.04] and 1.56 [95% CI, 1.5–2.13], respectively). However, 100% fT>MIC was achieved in only 60.4% of the patients, and the researchers’ use of a target of 50% fT>MIC led to potential overstating of the rate of target attainment for patients on a cephalosporin, for which the recommended PD target is 60% to 70% fT>MIC.28 These results highlight the need to elucidate ideal PD targets and indicate that different targets may be needed for different infectious syndromes (this study considered all patients with active infections, without stratification by infection type). Nonetheless, the study is key in describing that not achieving PD targets can lead to poor clinical outcomes and supports the importance of TDM. More clinical outcomes data surrounding TDM are still needed, especially for obese and older adult populations.
Vancomycin TDM.
Vancomycin is a hydrophilic antimicrobial widely distributed throughout body tissues. It is about 55% protein bound and is primarily eliminated by the kidney.30 Consensus guidelines from the American Society of Health-System Pharmacists, Infectious Diseases Society of America, and Society of Infectious Diseases Pharmacists stated that vancomycin PD is best characterized by AUC/MIC.30 The target for therapeutic effectiveness is an AUC/MIC24 of ≥400. However, this AUC/MIC goal is based on total drug concentrations, which may not be generalizable to patients with hypoalbuminemia. Additionally, the described AUC/MIC target only applies to invasive S. aureus infections, with estimates of AUC/MIC values based on the vancomycin MIC for S. aureus.30 Therefore, if estimating AUC alone, vancomycin exposures will increase as the MIC increases (eg, if the reported MIC is 1.5 or 2.0, the AUC targets would be 600 mg · h/L and 800 mg · h/L, respectively, in order to achieve an AUC/MIC of 400).
Although use of vancomycin trough levels for TDM continues, recent research suggests that this is inadequate. In a meta-analysis assessing 14 observational cohort studies involving a total of 1,677 patients with S. aureus bacteremia, higher AUC/MIC values (≥400) were associated with reduced treatment failure, whereas higher trough concentrations (≥15 mg/L) were not.11 In another study, modeling of vancomycin PK in patients with normal renal function and therapeutic AUC/MIC values (ie, ≥400, assuming a MIC of 1 mg/L) predicted that trough concentrations would be below the recommended target for serious infections (<15 mg/L) about 60% of the time.7 This is especially concerning because trough concentrations greater than 15 mg/L were shown to confer a 3-fold increased risk of nephrotoxicity in a multicenter, prospective, observational trial.55 A 3-year prospective serial cohort study also determined that AUC-guided dosing was more likely to achieve therapeutic targets compared to trough-guided dosing, and 31% of AUCs of ≥400 mg · h/L were associated with troughs of <10 mg/L and 68% with troughs of <15 mg/L.8 In addition, implementation of AUC/MIC as the vancomycin PD target leads to fewer blood samples overall,8 shorter durations of therapy,8 and reduced nephrotoxicity.8,9,56 An AUC24 threshold for AKI risk of >563 mg · h/L was identified by classification and regression tree (CART) analysis.9 However, prior publications have demonstrated that an AUC threshold between 600 and 1,300 mg · h/L is predictive of AKI, so there is still a need to determine the optimal upper limit for AUC/MIC for vancomycin to minimize toxicities.7,57,58 Results of a retrospective cohort analysis suggested that a daily AUC range of 400 to 700 mg · h/L may be reasonable based on findings that an AUC24 of ≥677 mg · h/L and an AUC24-48h of ≥683 mg · h/L were associated with higher rates of nephrotoxicity (P < 0.05).59
Vancomycin in the critically ill.
AUC/MIC monitoring is crucial for limiting nephrotoxicity and exposure in at-risk populations. Critically ill patients are often at increased risk for nephrotoxicity due to severity of illness, a higher likelihood of administration of concomitant nephrotoxic agents, and the potential for longer durations of treatment.60,61 In addition, receiving vancomycin in the ICU setting was found to be an independent risk factor for nephrotoxicity;62 although that association did not remain statistically significant on multivariable analysis (OR, 1.90; 95% CI, 0.95–3.8), this population certainly remains at risk. Therefore, due to an increased risk of nephrotoxicity, additional caution is warranted, and integrating AUC/MIC monitoring may mitigate this issue. More research on AUC/MIC TDM in critically ill patients is still required.
Vancomycin in obesity.
Obese patients may also be at increased risk for nephrotoxicity due to higher doses given in an attempt to achieve therapeutic concentrations. In a retrospective cohort study, administration of higher vs lower doses of vancomycin (≥4 g vs <4 g) was associated with a statistically significant increase in nephrotoxicity, and patients with a total body weight of ≥101.4 kg had a higher incidence of nephrotoxicity.63 In 2 different multicenter, retrospective cohort studies of severely ill patients with methicillin-resistant S. aureus (MRSA) bacteremia, vancomycin dosing for patients with a weight of >100 kg was found to be an independent predictor of nephrotoxicity by multivariable analysis.62,64 Conversely, a retrospective cohort analysis comparing dosing for obese vs lean patients found no difference in nephrotoxicity.65 Nonetheless, implementation of AUC/MIC monitoring is crucial to limit vancomycin exposure and toxicity. AUC/MIC monitoring in obese patients should be calculated with peak and trough concentrations or using Bayesian modeling, as use of a single trough concentration may not take into account total body weight and creatinine clearance.66
Vancomycin in older adults.
Older patients may be at increased risk for nephrotoxicity due to reduced vancomycin clearance resulting from impaired renal function. A retrospective analysis of 95 older adult patients (mean [SD] age of 82.3 [6.7] years) found that AUCs and trough concentrations did not correlate.24 More than 30% of cases with a trough concentration of <15 mg/L had an AUC24 of >400 mg · h/L.24 These findings did not account for the MIC but still justify the importance of using AUC to describe exposure. However, other data regarding the relationship between age and risk of vancomycin-associated nephrotoxicity are conflicting, with concomitant nephrotoxic agents playing a role.60,67 A recent matched cohort study, adjusted for risk factors including concomitant nephrotoxic agents, found no difference in the risk of AKI when comparing patients in 3 age groups (18-64, 65-79, and ≥80 years).68 In opposition, 2 different multicenter, retrospective cohort studies of severely ill patients with MRSA bacteremia found that age greater than 52 years and age greater than 65 years were predictive of 2.1- and 2.6-fold increases in the odds of nephrotoxicity, respectively.62,64 Overall, evidence supporting AUC/MIC monitoring is increasing and is consistent with primary TDM tenets of maximizing effectiveness and minimizing adverse events.
Implementing TDM.
Implementation of TDM practices for β-lactams and transitioning vancomycin TDM to target AUC/MIC may present several obstacles, including cost, assay availability, and proper staff education. Due to these constraints, TDM should be reserved for patients with altered PK and/or PD (as discussed in this review) and also for patients requiring longer courses of therapy or patients with difficult-to-eradicate infections such as infective endocarditis, vascular or device-related infections, and osteomyelitis, as well as those with infections caused by multidrug-resistant organisms or organisms with elevated MICs. If the course of therapy is anticipated to extend beyond the hospital stay, outpatient antibiotic therapy (OPAT) should also be considered.
Specific considerations for β-lactam TDM include the limited availability or lack of commercially available β-lactam assays due to strict US Food and Drug Administration (FDA) approval processes.69 Without FDA approval, institutions can collaborate with their laboratory team to develop in-house assays. When developing these assays, teams should focus on the importance of an assay with rapid turnaround time to maximize clinical relevance.70 To minimize costs, institutions may also opt for batch testing. Institutions with limited resources may opt to ship samples for testing; however, personnel need to be aware of the importance of freezing samples upon collection to avoid degradation of certain antibiotics (eg, imipenem).71 When results are not immediately available, providers should also consider the duration of therapy (eg, if results are not available until day 3 of therapy but only a 5-day course is required). Timing of sample collection for drug level determinations and result interpretation will be dependent on the desired PD target. For example, if the goal is 50% fT>MIC, a sample should be collected at 50% of the dosing interval; if the goal is 100% fT>MIC, a trough concentration can be collected.72 If MIC concentrations are not yet available, the clinical breakpoint for the suspected pathogen can be utilized while still considering the toxicities of overexposures.48 The type of infusion should also be considered, as intermittent, extended, and continuous infusions have been shown to assist in PD target attainment.37 Alternative infusion strategies, as well as dosing nomograms and Bayesian software, can supplement β-lactam TDM. However, these strategies may not be applicable to all clinical scenarios and should always be implemented along with education.
For vancomycin, options for TDM may depend on the resources available at the institution. While targeting a trough concentration requires only 1 serum sample and is intuitively simple, measuring the AUC/MIC requires 2 postdose samples and some calculations. Advantageously, the 2 postdose samples do not need to be collected at specific times, but 2 samples are required to calculate patient-specific PK. For ease of implementation, the first sample can be obtained 1 to 2 hours after the end of the vancomycin infusion for a peak level determination, and the second sample can be obtained towards the end of the dosing interval to determine a value similar to a trough value. Once both levels are available, AUC calculations can be performed by software, home-grown Excel (Microsoft Corporation, Redmond, WA) calculations, or even an electronic medical record’s built-in calculators. The alternative to calculating AUC is Bayesian estimation. Software available for Bayesian methodology as well as AUC calculations has been previously summarized.73 Finally, effective implementation of both β-lactam TDM and an AUC/MIC PD target for vancomycin will require persistent education of all involved in patient care, including those without PK/PD expertise. Experts should be at the forefront of this education initiative to ensure effective rollout of monitoring and dosing protocols.
Conclusion
Precision dosing through TDM is expanding and is especially important in populations with altered PK/PD, among them the critically ill and and older adults. Due to wide PK/PD variability in these populations, TDM is vital to maximize antimicrobial effectiveness and decrease adverse event rates. However, there is still a need for studies connecting TDM to patient outcomes. Providing patient-specific care through β-lactam TDM and transitioning to vancomycin AUC/MIC monitoring may be challenging, but with PK/PD experts at the forefront of this initiative, optimization of antimicrobial therapy can be achieved.
Acknowledgments
Graphic design by Karen Cusumano.
Disclosures
This work was supported in part by the Office of Academic Affiliations at the Department of Veterans Affairs, and by COIN: Center of Innovation in Long-Term Services and Supports for Vulnerable Veterans, Providence, RI. Dr. Roberts’ contribution to this work was funded by a Centre of Research Excellence grant (APP1099452) and Practitioner Fellowship (APP1117065) from Australia’s National Health and Medical Research Council. Dr. LaPlante receives research funding or is an advisor and/or consultant for Merck, Pfizer Pharmaceuticals, Ocean Spray Cranberries, Inc., Nabriva Therapeutics US, Inc., Melinta Therapeutics, Inc., and Tetraphase Pharmaceuticals. Dr. Klinker is a current employee of Merck & Co., Inc., which did not provide funding or support for this work; he served on advisory boards for Melinta Therapeutics, Achaogen, and Nabriva therapeutics. Dr. Roberts has received investigator-initiated grants or provided consultancies to Astellas, bioMerieux, Bayer, Accelerate Diagnostics, Melinta Pharmaceuticals, Cardeas Pharma, and Merck, Sharp & Dohme. The other authors have declared no potential conflicts of interest.
Previous affiliations
At the time of writing Dr. Klinker was affiliated with University of Florida College of Pharmacy, Gainesville FL.
Additional information
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs.




Comments