-
PDF
- Split View
-
Views
-
Cite
Cite
LUCIANA BALDONI, NICOLA TOSTI, CLAUDIA RICCIOLINI, ANGJELINA BELAJ, SERGIO ARCIONI, GIORGIO PANNELLI, MARIA ANTONIETTA GERMANA, MAURIZIO MULAS, ANDREA PORCEDDU, Genetic Structure of Wild and Cultivated Olives in the Central Mediterranean Basin, Annals of Botany, Volume 98, Issue 5, November 2006, Pages 935–942, https://doi.org/10.1093/aob/mcl178
Close - Share Icon Share
Abstract
• Background and Aims Olive cultivars and their wild relatives (oleasters) represent two botanical varieties of Olea europaea subsp. europaea (respectively europaea and sylvestris). Olive cultivars have undergone human selection and their area of diffusion overlaps that of oleasters. Populations of genuine wild olives seem restricted to isolated areas of Mediterranean forests, while most other wild-looking forms of olive may include feral forms that escaped cultivation.
• Methods The genetic structure of wild and cultivated olive tree populations was evaluated by amplified fragment length polymorphism (AFLP) markers at a microscale level in one continental and two insular Italian regions.
• Key Results The observed patterns of genetic variation were able to distinguish wild from cultivated populations and continental from insular regions. Island oleasters were highly similar to each other and were clearly distinguishable from those of continental regions. Ancient cultivated material from one island clustered with the wild plants, while the old plants from the continental region clustered with the cultivated group.
• Conclusions On the basis of these results, we can assume that olive trees have undergone a different selection/domestication process in the insular and mainland regions. The degree of differentiation between oleasters and cultivated trees on the islands suggests that all cultivars have been introduced into these regions from the outside, while the Umbrian cultivars have originated either by selection from local oleasters or by direct introduction from other regions.
INTRODUCTION
Olive cultivars and their wild relatives (also named oleasters) represent two botanical varieties of Olea europaea subsp. europaea, respectively var. europaea and var. sylvestris (Green, 2002). Both wild and cultivated olives, are diploid (2n = 2x = 46), predominantly allogamous and distributed along the Mediterranean basin (Green, 2002). Wild olives reproduce sexually by wind pollination and their seeds are mainly dispersed by birds (Herrera, 1995). They are important components of the Mediterranean scrublands (Zohary and Hopf, 2000), even though the presence of isolated populations of oleasters has been reported in a Northern Euro-Siberian region of Spain (Vargas and Kadereit, 2001).
Olive cultivars can be considered as varieties of unknown origin, currently propagated vegetatively by cutting or grafting. Most of them have a very restricted local area of diffusion (Besnard et al., 2001), while others have spread along wide agro-environments (Bronzini de Caraffa et al., 2002; Rotondi et al., 2003). Analysis of nuclear and cytoplasmic DNA polymorphisms in Mediterranean oleaster populations has shown that eastern oleaster populations differ greatly from those of the west Mediterranean (Besnard et al., 2001, 2002a; Lumaret et al., 2004), while the genetic diversity of cultivated populations shows a complex patchy pattern (Besnard et al., 2001,b; Owen et al., 2005). The apparent contradiction regarding the distribution of oleaster and cultivated populations has fuelled the debate on the local origin of cultivated forms.
Lumaret et al. (2004) proposed that populations of genuine wild olives are restricted to a few isolated areas of native Mediterranean forests, where pollen/stones may be distributed by wind/birds, while most other wild-looking forms of olive may include feral forms that escaped cultivation.
Based on the frequency and distribution of polymorphisms, several authors have advanced the hypothesis of multilocal selection of cultivars from naturally cross-bred genotypes (Besnard et al., 2001; Rotondi et al., 2003). Others, highlighting the great genetic distance between populations of wild olives and cultivars, have suggested that large fractions of local sets of cultivars may have an allochthonous origin (Angiolillo et al., 1999; Bronzini de Caraffa et al., 2002). The poor historical documentation of cultivar pedigrees and the fragmentary information available on olive paleobotany have failed to provide definitive conclusions.
The present study evaluated the organization of olive differentiation at a microscale regional level, comparing one continental and two insular Italian regions. The gene contribution of local oleasters to the development of cultivars sharing the same agro-ecological habitats has also been evaluated. The identification of genetically homogeneous groups of individuals has been reached by the implementation of Structure software (Pritchard et al., 2000), and with amplified fragment length polymorphism (AFLP) dominant markers (Angiolillo et al., 1999; Sanz-Cortes et al., 2003; Sensi et al., 2003; Owen et al., 2005), which has been shown to give results as accurate as microsatellites in such analyses (Evanno et al., 2005).
It is important to note that in a few works, very restricted areas have been prospected (Baldoni et al., 2000; Bronzini de Caraffa et al., 2002), while most studies have dealt with wild and cultivated populations sampled from extended areas throughout the Mediterranean (Besnard et al., 2002b; Lumaret et al., 2004). While the validity of the latter approach in describing general patterns of genetic diversity cannot be questioned, it is possible that the pattern of variability distribution at a smaller scale may remain unclear. Analyses at the microscale level are therefore expected to produce new elements for the general debate on olive domestication and to help understand how genetic diversity is partitioned among sets of cultivars (Manel et al., 2003). The long lifespan of the species has allowed 2000- to 3000-year-old olive trees to survive up to now in many Mediterranean regions (Lewington and Parker, 1999). Taking into account that variety substitution has probably occurred continuously during the long history of olive cultivation in the regions of interest, dating at least from the Roman period in Umbria and long before that in the other two regions (at least from the Greek period in Sicily and the Phoenician period in Sardinia), a few samples of ancient cultivated olives (dating currently under evaluation) have been included in the study as representatives of ancient domestication events, in order to verify whether they correspond better to oleasters, to presently cultivated varieties or to remnants of paleo-cultivars.
MATERIALS AND METHODS
Plant material
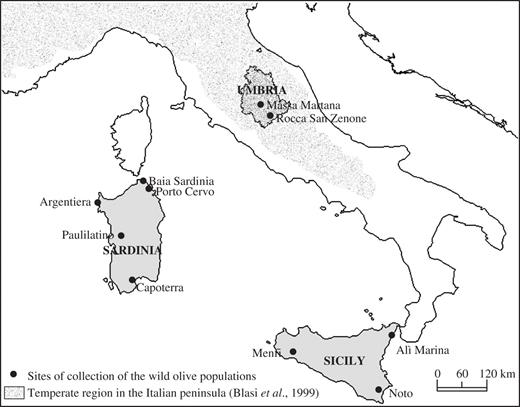
Samples were collected from three Italian regions: Umbria, Sicily and Sardinia (Fig. 1). The sampling sites were chosen because they represent few but very different microclimatic and soil conditions and, due to their central position in the Mediterranean basin, they may sample different wild olive lineages. Umbria represents the mainland area, within the temperate climatic region (Blasi et al., 1999); the occurrence of oleasters has never been reported before in this region, and cultivation of olive is restricted to few micro-areas, made possible by the use of selected and well established local varieties. Sicily and Sardinia have been chosen as representatives of the most favourable conditions for the spread of wild olive and because their geographical position should represent the cut-off between the eastern and western Mediterranean oleaster lineages (Besnard et al., 2002b). Both islands have undergone numerous colonizations that have greatly influenced the introduction of cultivars and cultivation of olive.
Map of the sites where oleaster populations were collected. The regions under study are shown in grey. The temperate region is distinguished from the Mediterranean region by grainy shading.
The list of populations analysed is given in Table 1 (for a detailed list of plant material, see Supplementary Information).
List of the olive cultivars, wild populations and ancient trees analysed
| Populations . | Genotypes . | No. of genotypes . | Region/site of cultivation/diffusion . | Population label . | Source of plant material . |
|---|---|---|---|---|---|
| Cultivated populations | Cultivars | 114 (14 ancient) | Umbria | CV-UM (AT-UM) | ISOl* |
| Cultivars | 46 (4 ancient) | Sicily | CV-SI (AT-SI) | UNIPA+ | |
| Cultivars | 32 (8 ancient) | Sardinia | CV-SA (AT-SI) | CHCON† | |
| Candidate wild populations | RSZ2–RSZ13 | 11 | Umbria/Rocca San Zenone (TR) | W-UM | IGV‡ |
| MMA2–MMA14 | 11 | Umbria/Massa Martana | W-UM | IGV‡ | |
| PAU40–PAU55 | 16 | Sardinia/Paulilatino | W-SA | UNISAS§ | |
| CPT40-CPT46 | 7 | Sardinia/Capoterra | W-SA | UNISAS§ | |
| ARG1–ARG6 | 6 | Sardinia/Argentiera, Alghero | W-SA | IGV‡ | |
| BSA1–BSA5 | 5 | Sardinia/Baia Sardinia | W-SA | IGV‡ | |
| PCE1–PCE3 | 3 | Sardinia/Porto Cervo | W-SA | IGV‡ | |
| ME1–ME18 | 17 | Sicily/Trapani, Menfi | W-SI | IGV‡ | |
| AL1–AL12 | 12 | Sicily/Messina, Ali’ Terme | W-SI | IGV‡ | |
| ROS2–ROS5 | 12 | Sicily/Noto, Siracusa | W-SI | IGV‡ |
| Populations . | Genotypes . | No. of genotypes . | Region/site of cultivation/diffusion . | Population label . | Source of plant material . |
|---|---|---|---|---|---|
| Cultivated populations | Cultivars | 114 (14 ancient) | Umbria | CV-UM (AT-UM) | ISOl* |
| Cultivars | 46 (4 ancient) | Sicily | CV-SI (AT-SI) | UNIPA+ | |
| Cultivars | 32 (8 ancient) | Sardinia | CV-SA (AT-SI) | CHCON† | |
| Candidate wild populations | RSZ2–RSZ13 | 11 | Umbria/Rocca San Zenone (TR) | W-UM | IGV‡ |
| MMA2–MMA14 | 11 | Umbria/Massa Martana | W-UM | IGV‡ | |
| PAU40–PAU55 | 16 | Sardinia/Paulilatino | W-SA | UNISAS§ | |
| CPT40-CPT46 | 7 | Sardinia/Capoterra | W-SA | UNISAS§ | |
| ARG1–ARG6 | 6 | Sardinia/Argentiera, Alghero | W-SA | IGV‡ | |
| BSA1–BSA5 | 5 | Sardinia/Baia Sardinia | W-SA | IGV‡ | |
| PCE1–PCE3 | 3 | Sardinia/Porto Cervo | W-SA | IGV‡ | |
| ME1–ME18 | 17 | Sicily/Trapani, Menfi | W-SI | IGV‡ | |
| AL1–AL12 | 12 | Sicily/Messina, Ali’ Terme | W-SI | IGV‡ | |
| ROS2–ROS5 | 12 | Sicily/Noto, Siracusa | W-SI | IGV‡ |
CV-UM, cultivars from Umbria; CV-SA, cultivars from Sardinia; CV-SI, cultivars from Sicily; W-UM, wild plants from Umbria; W-SI, wild plants from Sicily; W-SA, wild plants from Sardinia; AT-UM, ancient trees from Umbria; AT-SA, ancient trees from Sardinia; AT-SI, ancient trees from Sicily.
* CRA, Experimental Institute of Olives, Spoleto, Perugia.
† Consortium for Horticulture of Cagliari, Oristano and Nuoro.
‡ CNR-Institute of Plant Genetics, Perugia.
§ Department of Economy and Tree Systems, University of Sassari.
+ Dept. S.EN.FI.MI.ZO., University of Palermo.
List of the olive cultivars, wild populations and ancient trees analysed
| Populations . | Genotypes . | No. of genotypes . | Region/site of cultivation/diffusion . | Population label . | Source of plant material . |
|---|---|---|---|---|---|
| Cultivated populations | Cultivars | 114 (14 ancient) | Umbria | CV-UM (AT-UM) | ISOl* |
| Cultivars | 46 (4 ancient) | Sicily | CV-SI (AT-SI) | UNIPA+ | |
| Cultivars | 32 (8 ancient) | Sardinia | CV-SA (AT-SI) | CHCON† | |
| Candidate wild populations | RSZ2–RSZ13 | 11 | Umbria/Rocca San Zenone (TR) | W-UM | IGV‡ |
| MMA2–MMA14 | 11 | Umbria/Massa Martana | W-UM | IGV‡ | |
| PAU40–PAU55 | 16 | Sardinia/Paulilatino | W-SA | UNISAS§ | |
| CPT40-CPT46 | 7 | Sardinia/Capoterra | W-SA | UNISAS§ | |
| ARG1–ARG6 | 6 | Sardinia/Argentiera, Alghero | W-SA | IGV‡ | |
| BSA1–BSA5 | 5 | Sardinia/Baia Sardinia | W-SA | IGV‡ | |
| PCE1–PCE3 | 3 | Sardinia/Porto Cervo | W-SA | IGV‡ | |
| ME1–ME18 | 17 | Sicily/Trapani, Menfi | W-SI | IGV‡ | |
| AL1–AL12 | 12 | Sicily/Messina, Ali’ Terme | W-SI | IGV‡ | |
| ROS2–ROS5 | 12 | Sicily/Noto, Siracusa | W-SI | IGV‡ |
| Populations . | Genotypes . | No. of genotypes . | Region/site of cultivation/diffusion . | Population label . | Source of plant material . |
|---|---|---|---|---|---|
| Cultivated populations | Cultivars | 114 (14 ancient) | Umbria | CV-UM (AT-UM) | ISOl* |
| Cultivars | 46 (4 ancient) | Sicily | CV-SI (AT-SI) | UNIPA+ | |
| Cultivars | 32 (8 ancient) | Sardinia | CV-SA (AT-SI) | CHCON† | |
| Candidate wild populations | RSZ2–RSZ13 | 11 | Umbria/Rocca San Zenone (TR) | W-UM | IGV‡ |
| MMA2–MMA14 | 11 | Umbria/Massa Martana | W-UM | IGV‡ | |
| PAU40–PAU55 | 16 | Sardinia/Paulilatino | W-SA | UNISAS§ | |
| CPT40-CPT46 | 7 | Sardinia/Capoterra | W-SA | UNISAS§ | |
| ARG1–ARG6 | 6 | Sardinia/Argentiera, Alghero | W-SA | IGV‡ | |
| BSA1–BSA5 | 5 | Sardinia/Baia Sardinia | W-SA | IGV‡ | |
| PCE1–PCE3 | 3 | Sardinia/Porto Cervo | W-SA | IGV‡ | |
| ME1–ME18 | 17 | Sicily/Trapani, Menfi | W-SI | IGV‡ | |
| AL1–AL12 | 12 | Sicily/Messina, Ali’ Terme | W-SI | IGV‡ | |
| ROS2–ROS5 | 12 | Sicily/Noto, Siracusa | W-SI | IGV‡ |
CV-UM, cultivars from Umbria; CV-SA, cultivars from Sardinia; CV-SI, cultivars from Sicily; W-UM, wild plants from Umbria; W-SI, wild plants from Sicily; W-SA, wild plants from Sardinia; AT-UM, ancient trees from Umbria; AT-SA, ancient trees from Sardinia; AT-SI, ancient trees from Sicily.
* CRA, Experimental Institute of Olives, Spoleto, Perugia.
† Consortium for Horticulture of Cagliari, Oristano and Nuoro.
‡ CNR-Institute of Plant Genetics, Perugia.
§ Department of Economy and Tree Systems, University of Sassari.
+ Dept. S.EN.FI.MI.ZO., University of Palermo.
Cultivars
Leaf samples were collected from 1-year-old shoots of the tree canopy. The most important cultivars from Sicily, Sardinia and Umbria were collected. In order to verify the relationships among cultivars at a very local level, minor varieties, represented by a few or unique trees, were also sampled in Umbria. Due to their clonal origin (Banilas et al., 2003; Belaj et al., 2004), each cultivar was represented by a single sample.
Very ancient live plants which, from the first dating scores and historical documentation (G. Bongi, CNR, Perugia, Italy, unpubl. res.), may be considered to be around 1500–2000 years old, were sampled from the three regions. Samples of these cultivated trees were collected from the base of the trunk, likely to represent the oldest part of the plant.
Candidate wild olive populations
Two candidate wild olive populations were sampled in Umbria, one from the south of the region (Rocca San Zenone) and one from the centre (Massa Martana). The first one was found in a typical Mediterranean wood, 2–3 km away from cultivated olive fields, and the second one was distributed along the edge of olive orchards. Representatives of three oleaster populations from Sicily have been included in the study, one from the western part (Menfi), one from the east coast (Alì Terme) and a third from the south east area (Noto). All these populations were collected from Mediterranean maquis, quite distant from cultivated olive fields. The wild populations from Sardinia were collected from five undisturbed areas (Paulilatino, Capoterra, Argentiera, Baia Sardinia and Porto Cervo). All wild samples came from shrubs or small trees, whose presumed age does not exceed 100 years. Leaf material was collected from 1-year-old shoots sampled from the upper part of the plants.
AFLP analysis
AFLP analysis was performed as previously described for olive (Angiolillo et al., 1999). Three EcoRI primers (E-AGC, E-ACT and E-AAC) and three MseI primers (M-CAC, M-CAA and M-CTG) with three selective nucleotides were used. A total of five highly polymorphic primer combinations were screened (Table 2) among those previously tested.
AFLP primer combinations and polymorphism rates
| Primer combination . | Total no. of bands . | Polymorphic bands . | Polymorphism (%) . | Scored bands . |
|---|---|---|---|---|
| EAGC/MCAC | 88 | 78 | 88·6 | 31 |
| EACT/MCAC | 83 | 68 | 81·9 | 29 |
| EACT/MCAA | 113 | 92 | 81·4 | 24 |
| EAGC/MCTG | 82 | 68 | 64·1 | 30 |
| EAAC/MCTT | 106 | 82 | 77·3 | 10 |
| Total | 472 | 388 | 124 |
| Primer combination . | Total no. of bands . | Polymorphic bands . | Polymorphism (%) . | Scored bands . |
|---|---|---|---|---|
| EAGC/MCAC | 88 | 78 | 88·6 | 31 |
| EACT/MCAC | 83 | 68 | 81·9 | 29 |
| EACT/MCAA | 113 | 92 | 81·4 | 24 |
| EAGC/MCTG | 82 | 68 | 64·1 | 30 |
| EAAC/MCTT | 106 | 82 | 77·3 | 10 |
| Total | 472 | 388 | 124 |
AFLP primer combinations and polymorphism rates
| Primer combination . | Total no. of bands . | Polymorphic bands . | Polymorphism (%) . | Scored bands . |
|---|---|---|---|---|
| EAGC/MCAC | 88 | 78 | 88·6 | 31 |
| EACT/MCAC | 83 | 68 | 81·9 | 29 |
| EACT/MCAA | 113 | 92 | 81·4 | 24 |
| EAGC/MCTG | 82 | 68 | 64·1 | 30 |
| EAAC/MCTT | 106 | 82 | 77·3 | 10 |
| Total | 472 | 388 | 124 |
| Primer combination . | Total no. of bands . | Polymorphic bands . | Polymorphism (%) . | Scored bands . |
|---|---|---|---|---|
| EAGC/MCAC | 88 | 78 | 88·6 | 31 |
| EACT/MCAC | 83 | 68 | 81·9 | 29 |
| EACT/MCAA | 113 | 92 | 81·4 | 24 |
| EAGC/MCTG | 82 | 68 | 64·1 | 30 |
| EAAC/MCTT | 106 | 82 | 77·3 | 10 |
| Total | 472 | 388 | 124 |
Data analysis
AMOVA
Genetic distances between plants were calculated applying the Euclidean metric of Excoffier et al. (1992). AMOVA (analysis of molecular variance) was used to estimate variance components. The total variance among plants was partitioned into variance among populations and within populations, the populations being defined on the basis of geographic (site of collection) or breeding (wild plants vs. cultivars) criteria. Nested AMOVA was performed to study the effect of population classification criteria. Analyses were done using Arlequin 2·0 software (Schneider and Excoffier, 1999). The significance of fixation indices was tested by a permutation approach with 10 000 permutations.
Structure
To study how breeding or geographic-based groups reflect the interindividual similarity, genetically homogeneous populations were identified by an ad hoc designed clustering approach implemented in the software Structure (Pritchard et al., 2000; Rosenberg et al., 2002; Parker et al., 2004). This software places plants in K clusters that have distinct marker frequencies, where K is chosen in advance and can be varied across different runs. Plants can have memberships in several clusters, with membership coefficients equalling 1 across clusters. Plants were divided into genetic clusters using the software package Structure. AFLP data were analysed by treating each class of genotypes as being, effectively, haploid alleles, according to the software documentation. A no-admixture ancestry model was used and allele frequencies were correlated, with a burnin length of 30 000 followed by 500 000 runs at each K. Five Structure runs produced nearly identical membership coefficients at each K (data not shown).
Principal co-ordinate analysis
PCO analysis, which provides estimates of genetic similarity between individuals, has been used as an alternative way to represent interindividual and intergroup relationships. The analysis was performed with NTSYSpc Version 2·02 (Rohlf, 1998). The pairwise genetic similarities among genotypes were calculated according to the Nei and Li (1979) definition of similarity.
RESULTS
A total of 292 olive trees, sampled from an Italian continental region (Umbria) and two islands (Sardinia and Sicily) (Fig. 1), were genotyped using five AFLP primer combinations. Four hundred and seventy-two bands were amplified, and the percentage of polymorphisms ranged from 64·1 to 88·6 %, depending on the primer combinations. After a very strict selection, based on an unquestionable attribution, 124 of the polymorphic bands were used for the analysis.
First, plants were divided into six different populations (Table 1), according to geographical and breeding criteria. The overall proportion of genetic differentiation among populations was estimated by AMOVA. The interpopulation variance accounted for 23·04 % of the total variance. Pairwise estimates of the proportion of interpopulation molecular variances were taken as an index of differentiation among pairs of populations (Table 3). Cultivated materials showed a similar level of genetic differentiation across regions (see for example 0·103 between CV-UM and CV-SI, 0·107 between CV-SA and CV-SI, and 0·122 between CV-UM and CV-SA). The comparisons between wild populations revealed a strong similarity between the wild plants of the two islands and a significant distance of both from the Umbrian wild varieties (W-SI/W-SA, 0·033; W-UM/W-SI, 0·229; and W-SA/W-UM, 0·253). Comparisons made between breeding groups within each region highlighted two different scenarios: (a) in Sicily and Sardinia, the wild olives were highly differentiated from cultivars but not from ancient trees; and (b) in Umbria, the cultivars were less differentiated from their local oleasters and the ancient trees were close to cultivars and to wild trees.
Pairwise estimated variance
| . | CV-UM . | W-UM . | AT-UM . | W-SA . | CV-SA . | AT-SA . | CV-SI . | W-SI . | AT-SI . |
|---|---|---|---|---|---|---|---|---|---|
| CV-UM | – | ||||||||
| W-UM | 0·165* | – | |||||||
| AT-UM | 0·106* | 0·161* | – | ||||||
| W-SA | 0·305* | 0·253* | 0·362* | – | |||||
| CV-SA | 0·122* | 0·276* | 0·236* | 0·426* | – | ||||
| AT-SA | 0·235* | 0·073* | 0·294* | 0·000 | 0·399* | – | |||
| CV-SI | 0·103* | 0·206* | 0·179* | 0·364* | 0·107* | 0·316* | – | ||
| W-SI | 0·278* | 0·229* | 0·320* | 0·033* | 0·386* | 0·000 | 0·329* | – | |
| AT-SI | 0·184* | 0·216* | 0·251* | 0·227* | 0·283* | 0·238* | 0·219* | 0·188* | – |
| . | CV-UM . | W-UM . | AT-UM . | W-SA . | CV-SA . | AT-SA . | CV-SI . | W-SI . | AT-SI . |
|---|---|---|---|---|---|---|---|---|---|
| CV-UM | – | ||||||||
| W-UM | 0·165* | – | |||||||
| AT-UM | 0·106* | 0·161* | – | ||||||
| W-SA | 0·305* | 0·253* | 0·362* | – | |||||
| CV-SA | 0·122* | 0·276* | 0·236* | 0·426* | – | ||||
| AT-SA | 0·235* | 0·073* | 0·294* | 0·000 | 0·399* | – | |||
| CV-SI | 0·103* | 0·206* | 0·179* | 0·364* | 0·107* | 0·316* | – | ||
| W-SI | 0·278* | 0·229* | 0·320* | 0·033* | 0·386* | 0·000 | 0·329* | – | |
| AT-SI | 0·184* | 0·216* | 0·251* | 0·227* | 0·283* | 0·238* | 0·219* | 0·188* | – |
*P < 0·05.
Pairwise estimated variance
| . | CV-UM . | W-UM . | AT-UM . | W-SA . | CV-SA . | AT-SA . | CV-SI . | W-SI . | AT-SI . |
|---|---|---|---|---|---|---|---|---|---|
| CV-UM | – | ||||||||
| W-UM | 0·165* | – | |||||||
| AT-UM | 0·106* | 0·161* | – | ||||||
| W-SA | 0·305* | 0·253* | 0·362* | – | |||||
| CV-SA | 0·122* | 0·276* | 0·236* | 0·426* | – | ||||
| AT-SA | 0·235* | 0·073* | 0·294* | 0·000 | 0·399* | – | |||
| CV-SI | 0·103* | 0·206* | 0·179* | 0·364* | 0·107* | 0·316* | – | ||
| W-SI | 0·278* | 0·229* | 0·320* | 0·033* | 0·386* | 0·000 | 0·329* | – | |
| AT-SI | 0·184* | 0·216* | 0·251* | 0·227* | 0·283* | 0·238* | 0·219* | 0·188* | – |
| . | CV-UM . | W-UM . | AT-UM . | W-SA . | CV-SA . | AT-SA . | CV-SI . | W-SI . | AT-SI . |
|---|---|---|---|---|---|---|---|---|---|
| CV-UM | – | ||||||||
| W-UM | 0·165* | – | |||||||
| AT-UM | 0·106* | 0·161* | – | ||||||
| W-SA | 0·305* | 0·253* | 0·362* | – | |||||
| CV-SA | 0·122* | 0·276* | 0·236* | 0·426* | – | ||||
| AT-SA | 0·235* | 0·073* | 0·294* | 0·000 | 0·399* | – | |||
| CV-SI | 0·103* | 0·206* | 0·179* | 0·364* | 0·107* | 0·316* | – | ||
| W-SI | 0·278* | 0·229* | 0·320* | 0·033* | 0·386* | 0·000 | 0·329* | – | |
| AT-SI | 0·184* | 0·216* | 0·251* | 0·227* | 0·283* | 0·238* | 0·219* | 0·188* | – |
*P < 0·05.
To summarize the effect of breeding and geographic distribution in partitioning molecular variance, we performed AMOVA with two different hierarchical models, using both criteria as the main classification factors. As shown in Table 4, groups made following the breeding criterion (cultivars vs. wild plants), as well as the geographic groups within breeding groups, turned out to be significantly different. In contrast, when the geographic criterion was used as the main classification factor, only breeding groups within geographic regions were significantly different.
Percentage of variation obtained by hierarchical analysis of variance
| Source of variation . | By region* . | By breeding† . |
|---|---|---|
| Among groups | –4·02 | 14·09 |
| Among populations, within groups | 26·39 | 13·27 |
| Within populations | 77·63 | 72·64 |
| Source of variation . | By region* . | By breeding† . |
|---|---|---|
| Among groups | –4·02 | 14·09 |
| Among populations, within groups | 26·39 | 13·27 |
| Within populations | 77·63 | 72·64 |
*All genotypes grouped by geographical origin (three regions): Umbria, Sardinia and Sicily.
†All genotypes grouped by two breeding criteria: cultivated vs. wild plants.
Percentage of variation obtained by hierarchical analysis of variance
| Source of variation . | By region* . | By breeding† . |
|---|---|---|
| Among groups | –4·02 | 14·09 |
| Among populations, within groups | 26·39 | 13·27 |
| Within populations | 77·63 | 72·64 |
| Source of variation . | By region* . | By breeding† . |
|---|---|---|
| Among groups | –4·02 | 14·09 |
| Among populations, within groups | 26·39 | 13·27 |
| Within populations | 77·63 | 72·64 |
*All genotypes grouped by geographical origin (three regions): Umbria, Sardinia and Sicily.
†All genotypes grouped by two breeding criteria: cultivated vs. wild plants.
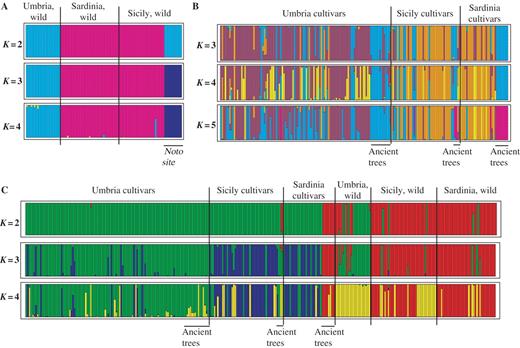
The results of the Structure analysis are reported in Fig. 2A–C. In Fig. 2A, at K = 2 wild genotypes from Sardinia were grouped together with those of west Sicily, while those from Umbria were grouped together with those of east Sicily (at Noto). At K = 3, the Noto group was separated from the Umbrian wild plants. At higher K values, the new groups were composed of individuals belonging to different clusters, making it difficult to identify the underlying classification criterion. These results suggest that genetically defined groups of wild genotypes closely correspond to pre-defined regional groups.
(A) Estimated population structure for oleasters. (B) Estimated population structure for olive cultivars. (C) Estimated population structure for all individuals. Each individual is represented by a vertical coloured line, which is partitioned into K segments that represent the individual's estimated membership fractions in K clusters. Different colours indicate different populations. Colours do not correspond to the same groups in the various figure parts (A, B and C). Long black lines indicate the separation among a priori assigned populations, labelled above each figure. The figure shown for a given K is based on the highest probability run at that K. In all cases, the highest probability runs are shown.
To verify whether this set-up also held true for the cultivated material, the same approach was applied to cultivars (Fig. 2B). In contrast to what was observed for oleasters, not all cultivars clustered according to geographical origin. Nevertheless, as for wild plants, most of the Sicilian and Sardinian cultivated varieties clustered separately from the Umbrian samples. In fact, at K = 3, the overall set of cultivars was divided into three groups, one including most cultivars from Umbria, another with the corresponding genotypes from insular regions, and a third one including ancient olives from Sardinia and most of those from Umbria. At higher K values, only the ancient Sardinian trees differentiated into a new population, together with two ancient Sicilian trees and a local Umbrian cultivar. In some cases, cultivars were not assigned to their original populations but were allocated with other groups, as in the case of some Umbrian varieties, which clustered with the Sicilian–Sardinian cultivated group.
Following these observations, the next important question concerned the relationship between cultivated and wild plants. To obtain this information, the entire set of plants was analysed together (Fig. 2C). At K = 2, one cluster contained the cultivated forms of the three regions, the ancient trees and some of the wild trees from Umbria, while the second included the insular wild plants, the remaining wild plants from Umbria (mainly from the Rocca San Zenone population) and the ancient Sardinian and Sicilian trees. This result confirms that the breeding origin of samples has a stronger effect on population differentiation than the geographic origin. At K = 3, all genotypes were divided into three groups, reflecting an overall breeding (cultivars vs. wild plants) criterion and, at cultivar level, a geographical criterion also (Umbria vs. Sicily–Sardinia). Surprisingly, most of the Umbrian wild trees clustered with cultivars, and the ancient Sardinian cultivated trees clustered with the wild trees. At K = 4, wild Umbrian plants split into a different population together with the Sicilian wild plants from Noto. At K = 5 (data not shown), an additional group was identified, formed by the oleasters from Noto, while, at higher orders of K (from K = 6 to K = 8), the groups did not identify major regions and it was impossible to identify any underlying classification criterion. These results suggest that, when > 3 clusters are allowed, the patterns of genetic diversity reflect both breeding and geographic criteria.
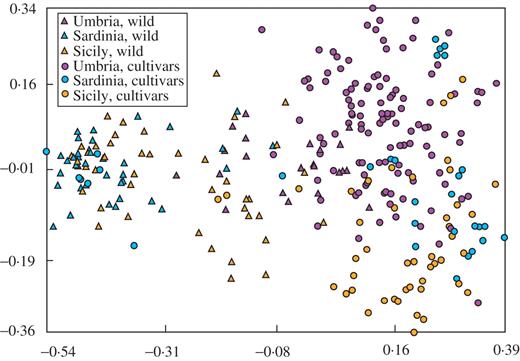
To represent the relationships between individuals graphically, PCO analysis of the complete data set was also performed. The two-dimensional PCO plot (Fig. 3) shows that cultivars and wild plants spread into two distinct groups, as shown by the other tests. The greatest distance between wild plants and cultivars was obtained in the Sardinian set of samples (except the ancient trees). A good differentiation was also shown by the two Sicilian sets, while some of the Umbrian cultivars were intermixed with oleasters. It is noteworthy that some Umbrian wild plants clustered together with the insular oleasters, as revealed by Structure analysis.
Results of the principal co-ordinate analysis on all AFLP data. The different genotypes and regions of diffusion are distinguished.
DISCUSSION
Relationships among wild populations
Two hypotheses can be put forward, based on the low levels of interpopulation genetic variance obtained for the oleasters of Sicily and Sardinia and the high values shown when comparing these populations with the Umbrian wild plants, which better relate to cultivars: (a) the Umbrian populations are not genuine oleasters, but are feral forms deriving from dissemination of cultivars; or (b) only a part of them represent real wild olives, even if clearly distinguishable from those of other areas. The extreme microclimatic conditions of this region, which make the survival of wild olives difficult, and the similarity of some of them to local cultivars, as shown by Structure and PCO analyses, may support the first hypothesis. However, Structure clustering has revealed (Fig. 2C, K = 3) that some Umbrian oleasters grouped with those from the islands and, at K = 4, they all grouped with the Noto population. The Noto population has marker frequencies distinct from those of other insular sites, but is compatible with continental populations sampled in Apulia (data not shown). These considerations contribute to support the contention that at least some of the Umbrian oleasters may be considered as genuine wild olives, which may belong to the same lineage of Noto, while the other Umbrian wild olives represent feral plants, spread in the same uncultivated areas where real oleasters still survive. The differences observed among the Sicilian sites may be due to physical barriers, such as mountains, that isolate the Noto area from the other Sicilian sites.
The low level of differentiation between Sicilian and Sardinian wild plants, as shown by AMOVA and confirmed by Structure and PCO analysis, is difficult to explain for natural populations living in distant, isolated regions. Intermixed variability has also been observed between Corsican and Sardinian wild olives at the mitochondrial DNA level (Bronzini de Caraffa et al., 2002). In that case, the close relationship was explained by the geographical closeness of both islands, but, to justify the low level of differentiation between Sicilian and Sardinian oleasters, other factors must be considered, such as a common ancestral genetic pool, a lack of differential selective pressure and a reduced number of divergent generations between the two populations. The extensive consumption of olive drupes by migrating birds may also have contributed to the long-distance seed dispersal (Herrera, 1995; Alcantara and Rey, 2003).
Relationships among cultivars
Differing from wild plants, only a part of the cultivars clearly clustered according to the geographic origin, while others showed a partial or even total membership of populations of other regions. This finding is probably due to humans moving cultivars to different sites during the past several thousand years of olive cultivation.
It is interesting to note that most of the Umbrian cultivars placed outside the corresponding clusters obtained by Structure and PCO analyses have a very local and restricted diffusion in the most internal and cold areas. These cultivars may represent ancestral varieties first introduced into Umbria during replanting following the recurrent, destructive freezing events in the area.
Relationships between wild and cultivated varieties
Previous reports based on allozyme analysis performed on different groups of wild plants and cultivars have shown that allelic distributions in oleasters and cultivated populations are similar and that heterozygosity in oleasters is higher than in cultivars (Lumaret et al., 2004). Also inter-simple sequence repeats (ISSR) analysis has shown that cultivated olives from different Mediterranean countries are nested within wild populations (Vargas and Kadereit, 2001), indicating either that wild and domesticated olives exchanged genetic material through hybridization or that olive tree domestication took place more than once.
The picture arising from the present work is rather more complex. The results show that cultivated plants in insular regions were domesticated without the contribution of local oleasters, while the olive cultivars of Umbria have both arrived from outside and developed from local wild plants. The high degree of similarity of ancient trees to oleasters in Sardinia is probably due to the propagation system in this island, mainly done by grafting onto wild plants, taking into account that only the tree bases (rootstocks) were considered in this work. Other studies performed on populations from Sardinia and Corsica have also ruled out the possibility that cultivars from these islands were directly selected from local oleasters (Angiolillo et al., 1999; Bronzini de Caraffa et al., 2002). A similar scenario has also been proposed for the olive domestication in Spain, where analyses performed on archeological specimens of olive stones demonstrated that domestication occurred from ancestral wild forms during the Chalcolithic/Bronze age, but, at later times, these cultivated forms were replaced by allochthonous varieties (Terral, 2000; Terral et al., 2004).
With regard to Umbria, the results show that some cultivars were positioned close to local wild plants. Interestingly, these cultivars are widely diffused in the regions of north, central and southern Italy, which suggest their ancient origin and longer time of spread. To establish olive cultivation in Umbria, growers would have selected superior plants from well adapted local wild plants. The selection process may have occurred several times, especially following destructive freezing events that caused extensive olive tree replanting campaigns. The more recent replantings, carried out with material derived from areas with similar microclimatic conditions, would have broadened the genetic pool to the present-day size. The ancient Umbrian genotypes cannot be considered as genuine wild plants but as remnants of primary, locally domesticated, olives.
The results obtained led to the conclusion that olive cultivars of Umbria have undergone a different domestication process compared with that registered in Sardinia and Sicily. In Umbria, part of the cultivars seem to have originated from populations of local oleasters, still present in uncultivated areas, intermixed with feral forms. The contribution of local wild plants to the development of varieties was shown by the presence of wild olives, very ancient cultivated trees and local varieties sharing a large portion of variability. In Sardinia and Sicily, the clear distinction between oleasters and cultivars and the close relationship between the pools of the two islands have confirmed that the island cultivars did not develop from local oleasters but were introduced from abroad and were propagated by grafting on local oleasters. The very close relationships between oleasters of the two islands should confirm that both are likely refugial relicts of the same population.
The authors are grateful to Claudio Munari and to the other people who contributed to plant material collection. Particular thanks are also due to G. G. Vendramin for his critical reading of the manuscript.