-
PDF
- Split View
-
Views
-
Cite
Cite
Florent Hubé, Pascale Reverdiau, Sophie Iochmann, Sylvie Trassard, Gilles Thibault, Yves Gruel, Demonstration of a Tissue Factor Pathway Inhibitor 2 Messenger RNA Synthesis by Pure Villous Cytotrophoblast Cells Isolated from Term Human Placentas, Biology of Reproduction, Volume 68, Issue 5, 1 May 2003, Pages 1888–1894, https://doi.org/10.1095/biolreprod.102.011858
Close - Share Icon Share
Abstract
Tissue factor pathway inhibitor 2 (TFPI-2), a Kunitz-type proteinase inhibitor, might play an important role during placenta growth by regulating trophoblast invasion and differentiation. Many TFPI-2 transcripts have been detected in syncytiotrophoblast cells, but conflicting results have been reported concerning TFPI-2 synthesis by the cytotrophoblast. To address this issue, we developed a method to isolate pure preparations of human villous cytotrophoblast cells from normal term placentas, and the synthesis of tissue factor, TFPI-1, and TFPI-2 mRNAs was then evaluated. Cells were isolated by trypsin-DNase-EDTA digestion, followed by Percoll gradient separation and immunodepletion of human leukocyte antigen-positive cells. The quality of villous cytotrophoblast cells was verified by electron microscopy. Purity of cell preparations was assessed by labeling cells with GB25, a monoclonal antibody specific to villous trophoblast cells, and by checking the absence of contaminating cells using anti-CD9 antibody. The lack of hCG, CD32 mRNA, and tissue factor mRNA also indicated the absence of contaminating cells. Using competitive reverse transcription polymerase chain reaction, we showed that freshly isolated villous cytotrophoblast cells synthesized significant levels of TFPI-1 mRNA and larger amounts of TFPI-2 mRNA. TFPI-1 and TFPI-2 mRNA synthesis remained unchanged when cytotrophoblast cells were cultured in complete medium and evolved as a multinucleated syncytiotrophoblast. These results indicate that the villous cytotrophoblast and syncytiotrophoblast are both important sites of TFPI-2 synthesis in the human placenta. This study also indicates that tissue factor detection should be used systematically to check the purity of cytotrophoblast cell preparations because it allows detection of contamination by monocytes/macrophages and by syncytial fragments.
Introduction
During the development of the human placenta, the extravillous trophoblast invades the uterine wall, whereas the villous trophoblast remains in contact with maternal blood within intervillous spaces [1]. Syncytiotrophoblast cells, derived from the fusion of the underlying villous cytotrophoblast, are involved in maternofetal exchanges and maintenance of the placental hemostatic balance. As found in the endothelial cells lining blood vessels, the syncytiotrophoblast expresses von Willebrand factor and thrombomodulin [2, 3]. Moreover, tissue factor (TF), the transmembrane cell surface receptor of factor VII/VIIa that triggers blood coagulation, has also been detected in human microvilli membranes shed from differentiated multinuclear syncytiotrophoblast cells [4, 5] but not in villous cytotrophoblast cells [3, 6]. As demonstrated by immunohistochemistry, TF pathway inhibitor 1 (TFPI-1), the major inhibitor of TF/VIIa complex, is expressed in both syncytiotrophoblast and cytotrophoblast cells [7] and might regulate TF-mediated thrombin generation in the placenta as in blood vessels. Another inhibitor of the TF/VIIa complex, TFPI-2, also known as placental protein 5 (PP5), has been reported. TFPI-2 was initially isolated from human placenta extracts [8, 9], and its level dramatically increases in maternal blood during pregnancy [9]. TFPI-2 is a Kunitz-type proteinase inhibitor that inhibits the activation of several metalloproteinases and might play a major role during placenta growth by regulating trophoblast invasion and differentiation. TFPI-2 mRNA has been identified by in situ hybridization in the syncytiotrophoblast from the first trimester to term placenta [10], and synthesis of the protein has been consistently demonstrated [11, 12], specifically on the surface of microvilli and the membrane of the endoplasmic reticulum [13]. In contrast, conflicting results have been reported concerning TFPI-2 synthesis by the cytotrophoblast. By immunoperoxidase staining with monoclonal and polyclonal antibodies, Butzow et al. [12] initially showed that PP5 (i.e., TFPI-2) is present in the cytotrophoblast. However, Udagawa et al. [10] reported that no TFPI-2 mRNA was detected in villous cytotrophoblast cells by in situ hybridization and Northern blotting, and no protein was localized by immunochemistry.
The aim of this study was thus to evaluate the levels of TFPI-2, TFPI-1, and TF mRNA in isolated villous cytotrophoblast cells. We first developed a method allowing isolation of pure populations of villous cytotrophoblast cells from normal term human placentas without any contamination by mesenchymal cells and syncytial fragments.
Materials and Methods
Isolation of Villous Cytotrophoblast Cells
Villous cytotrophoblast cells were isolated according to previously published methods [14–18] and modified as follows. Term human placentas were obtained with informed consent after spontaneous vaginal delivery from uncomplicated pregnancies (Prof. G. Body and Prof. F. Perrotin, Département de Gynécologie et Obstétrique, CHU de Tours, France) and processed immediately for isolation of trophoblast cells. Villous tissue (30–40 g) was washed with isotonic saline solution, finely minced, and placed in 100 ml sterile Hanks solution containing 12.5 U/ml trypsin (Invitrogen, Cergy-Pontoise, France), 0.1 mg/ml DNase (Roche Diagnostics, Meylan, France), 25 mM Hepes (Eurobio, les Ulis, France). As a noticeable modification of previously reported methods [14–18], the digestion solution was supplemented with 1 mM EDTA. Supernatants from two to five enzymatic digestions performed in a shaking water bath for 20 min at 37°C were then collected and mixed with 5 ml fetal calf serum (FCS; endotoxin-free, heat-inactivated; ATGC Biotechnologie, Noisy Le Grand, France) to inactivate enzyme activity. After centrifugation at 1000 × g for 5 min, pellets were resuspended in Waymouth MB 752-1 medium (Invitrogen) supplemented with 17% FCS, 106 IU/L penicillin, 100 mg/L streptomycin, and 2.5 μg/L fungizone. Cell suspensions were then filtered and carefully layered on a discontinuous Percoll gradient (5–70% in 5% steps in Hanks balanced salt solution; Amersham Pharmacia Biotech Europe GmbH, France). Cytotrophoblast cells concentrated between densities of 1.062 and 1.048 were collected and then diluted 4-fold with complete medium: Waymouth/Ham F12 medium (v/v) supplemented with 17% FCS, 25 mM Hepes, 106 IU/L penicillin, 100 mg/L streptomycin, 2.5 μg/L fungizone, and 2 mM l-glutamine.
Remaining monocytes/macrophages, fibroblasts, and endothelial cells were removed by immunodepletion using mouse monoclonal antibodies to human leukocyte antigen HLA-ABC, HLA-DR, and CD14 (Immunotech, Marseille, France) not expressed by cytotrophoblast cells. Cytotrophoblast-enriched cell suspension (4 × 106 cells) was incubated at 4°C with 20 μg of each antibody in 1 ml ice-cold medium for 45 min and incubated for 30 min with 1.5 mg magnetic beads coated with goat anti-mouse IgG (Immunotech). Cells positive for HLA-ABC, HLA-DR, and CD14 were then removed using a magnetic separator. Cytotrophoblast cells were then centrifuged for 10 min at 800 × g, resuspended in complete medium, and placed either in air with 5% CO2 at 37°C or in a fixation solution for later observation under electron microscope.
Characterization of Villous Cytotrophoblast Cells
Electron microscopy
For transmission electron microscopy, tissue or trophoblast cells were fixed in 1% glutaraldehyde and 4% paraformaldehyde in 100 mM phosphate buffer, pH 7.4, postfixed in 0.1% osmium tetraoxide, dehydrated in graded ethanol baths, and embedded in epoxy resin. Thin sections were examined in a Jeol 1010 CX electron microscope to identify cytotrophoblast cells and to evaluate how the intercellular junctions between cytotrophoblast and syncytiotrophoblast cells had evolved.
Flow cytometry
Flow cytometry analysis was performed immediately after isolation of cells as previously described [19]. The purity of cell suspensions was checked with GB25, a monoclonal antibody specific for trophoblast cells (generous gift of Dr. Hsi, INSERM U210, France). Contamination by blood and mesenchymal cells was analyzed with a monoclonal anti-CD9 antibody. An isotypic IgG1 was used as a negative control (Immunotech, France).
Reverse transcription polymerase chain reaction for CD32
A reverse transcription polymerase chain reaction (RT-PCR) method specific for CD32 (FcγRIIa) was performed [20] to detect contamination by monocyte/macrophage cells, using a procedure similar to that described below. Monocytes isolated from peripheral blood were used as a positive control.
Measurement of hCG production
Levels of hCG secreted in the 12-h culture of isolated villous cytotrophoblast cells were measured with an immunoenzymatic assay (Total β hCG kit; Beckman-Coulter, Roissy, France). JEG-3 choriocarcinoma cells were used as a positive control [20].
TF, TFPI-1, and TFPI-2 mRNA Synthesis
Total mRNA was isolated from 106 cytotrophoblast cells using the Dynabeads mRNA Direct kit (Dynal France SA, Compiègne, France) according to the manufacturer's instructions. Total mRNA was then reverse-transcribed for 1 h at 42°C in 1× incubation buffer containing 250 μM of each deoxynucleotide triphosphate, 5 μM oligo (dT)20, 25 units of RNase inhibitor, and 20 units of avian myeloblastosis virus reverse transcriptase (Roche Diagnostics).
Standard PCR was then performed as previously described [21] using cDNA obtained from 5 × 104 cells in a total reaction volume of 50 μl containing 10 mM Tris-HCl, pH 9.0, 50 mM KCl, 0.01% (w/v) gelatin, 1.5 mM MgCl2, 0.1% Triton X-100, 50 μM of each deoxynucleoside triphosphate, 1 μM of each forward and reverse synthesized oligonucleotide primer (Genset SA, Paris, France), and 0.5 units of Super Taq DNA polymerase (ATGC Biotechnologies, Croissy-Beaubourg, France). Primers used for specific PCR for TF, TFPI-1, TFPI-2, and glyceraldehyde phosphate dehydrogenase (GAPDH) as a control are presented in Table 1. The primers used for detection of TFPI-2 mRNA were defined taking into account the similarities between TFPI-1 and TFPI-2 to avoid hybridization to homologous sequences [12, 22]. All primers were designed to have melting temperatures in the same range, thus allowing simultaneous amplification, and to span one intron to distinguish genomic DNA from cDNA amplifications.
Primer sequences used in RT-PCR and competitive RT-PCR.
| Transcript . | Primer sequence (5′–3′) . | Target PCR product (bp)a . | Competitor PCR product (bp)a . | Reference . |
|---|---|---|---|---|
| CD32 | ||||
| Forward | CCTGAGAGCGACTCCATTCAGTGGTTCC | 270 | 20 | |
| Reverse | GAAGAATGTGACCTTGACCAGAGGCTTGTCC | |||
| TF | ||||
| Forward | CTACTGTTTCAGTGTTCAAGCAGTGA | 282 | 26 | |
| Reverse | CAGTGCAATATAGCATTGCAGTAC | |||
| TFPI-1 | ||||
| Forward | GGAAGAAGATCCTGGAATATGTCGAG | 230 | 378 | 27 |
| Reverse | CTTGGTTGATTGCGGAGTCAGGGAG | |||
| TFPI-2 | ||||
| Forward | CAGATGAAGCTACTTGTATGGGCTTC | 253 | 398 | 28 |
| Reverse | GGCAAAGCGAAGCTTTGGCATC | |||
| GAPDH | ||||
| Forward | ACAGTCCATGCCATCACTGCC | 265 | 29 | |
| Reverse | GCCTGCTTCACCACCTTCTTG | |||
| hCG | ||||
| Forward | AGACAAGGCAGGGGACGCACCAA | 451 | 30 | |
| Reverse | CTTTGAGGAAGAGGAGTCCTGGAA |
| Transcript . | Primer sequence (5′–3′) . | Target PCR product (bp)a . | Competitor PCR product (bp)a . | Reference . |
|---|---|---|---|---|
| CD32 | ||||
| Forward | CCTGAGAGCGACTCCATTCAGTGGTTCC | 270 | 20 | |
| Reverse | GAAGAATGTGACCTTGACCAGAGGCTTGTCC | |||
| TF | ||||
| Forward | CTACTGTTTCAGTGTTCAAGCAGTGA | 282 | 26 | |
| Reverse | CAGTGCAATATAGCATTGCAGTAC | |||
| TFPI-1 | ||||
| Forward | GGAAGAAGATCCTGGAATATGTCGAG | 230 | 378 | 27 |
| Reverse | CTTGGTTGATTGCGGAGTCAGGGAG | |||
| TFPI-2 | ||||
| Forward | CAGATGAAGCTACTTGTATGGGCTTC | 253 | 398 | 28 |
| Reverse | GGCAAAGCGAAGCTTTGGCATC | |||
| GAPDH | ||||
| Forward | ACAGTCCATGCCATCACTGCC | 265 | 29 | |
| Reverse | GCCTGCTTCACCACCTTCTTG | |||
| hCG | ||||
| Forward | AGACAAGGCAGGGGACGCACCAA | 451 | 30 | |
| Reverse | CTTTGAGGAAGAGGAGTCCTGGAA |
bp, Base pairs.
Primer sequences used in RT-PCR and competitive RT-PCR.
| Transcript . | Primer sequence (5′–3′) . | Target PCR product (bp)a . | Competitor PCR product (bp)a . | Reference . |
|---|---|---|---|---|
| CD32 | ||||
| Forward | CCTGAGAGCGACTCCATTCAGTGGTTCC | 270 | 20 | |
| Reverse | GAAGAATGTGACCTTGACCAGAGGCTTGTCC | |||
| TF | ||||
| Forward | CTACTGTTTCAGTGTTCAAGCAGTGA | 282 | 26 | |
| Reverse | CAGTGCAATATAGCATTGCAGTAC | |||
| TFPI-1 | ||||
| Forward | GGAAGAAGATCCTGGAATATGTCGAG | 230 | 378 | 27 |
| Reverse | CTTGGTTGATTGCGGAGTCAGGGAG | |||
| TFPI-2 | ||||
| Forward | CAGATGAAGCTACTTGTATGGGCTTC | 253 | 398 | 28 |
| Reverse | GGCAAAGCGAAGCTTTGGCATC | |||
| GAPDH | ||||
| Forward | ACAGTCCATGCCATCACTGCC | 265 | 29 | |
| Reverse | GCCTGCTTCACCACCTTCTTG | |||
| hCG | ||||
| Forward | AGACAAGGCAGGGGACGCACCAA | 451 | 30 | |
| Reverse | CTTTGAGGAAGAGGAGTCCTGGAA |
| Transcript . | Primer sequence (5′–3′) . | Target PCR product (bp)a . | Competitor PCR product (bp)a . | Reference . |
|---|---|---|---|---|
| CD32 | ||||
| Forward | CCTGAGAGCGACTCCATTCAGTGGTTCC | 270 | 20 | |
| Reverse | GAAGAATGTGACCTTGACCAGAGGCTTGTCC | |||
| TF | ||||
| Forward | CTACTGTTTCAGTGTTCAAGCAGTGA | 282 | 26 | |
| Reverse | CAGTGCAATATAGCATTGCAGTAC | |||
| TFPI-1 | ||||
| Forward | GGAAGAAGATCCTGGAATATGTCGAG | 230 | 378 | 27 |
| Reverse | CTTGGTTGATTGCGGAGTCAGGGAG | |||
| TFPI-2 | ||||
| Forward | CAGATGAAGCTACTTGTATGGGCTTC | 253 | 398 | 28 |
| Reverse | GGCAAAGCGAAGCTTTGGCATC | |||
| GAPDH | ||||
| Forward | ACAGTCCATGCCATCACTGCC | 265 | 29 | |
| Reverse | GCCTGCTTCACCACCTTCTTG | |||
| hCG | ||||
| Forward | AGACAAGGCAGGGGACGCACCAA | 451 | 30 | |
| Reverse | CTTTGAGGAAGAGGAGTCCTGGAA |
bp, Base pairs.
Competitive PCR was performed on samples containing a constant volume (5.0 μl) of first-strand cDNA obtained from 5 × 104 cells and 2 μl of 2-fold competitor dilutions. Heterologous DNA competitors were previously constructed for every gene studied using the PCR MIMIC kit (Clontech, Palo Alto, CA) according to the manufacturer's instructions and as previously described [23]. PCR was carried out in 1× Taq buffer (ATGC Biotechnologies) containing 200 μM of each deoxynucleotide triphosphate, 0.4 μM of each reverse and forward synthesized oligonucleotide primer specific for human TFPI-1 or TFPI-2, and 0.5 units of Super Taq DNA polymerase.
The PCR was set up in a GeneAmp PCR system 2400 (Applied Biosystems, Courtaboeuf, France) programmed for an initial denaturation step of 3 min at 94°C, followed by 35 cycles at 94°C for 30 sec, 62°C for 30 sec, and 72°C for 30 sec; the final extension step was performed at 72°C for 7 min. PCR products were analyzed by electrophoresis through a 1.6% agarose gel in TBE buffer (90 mM Tris-HCl, 90 mM borate acid, 2.5 mM EDTA) containing 1 μg/ml ethidium bromide and were visualized by ultraviolet transillumination (Gel Doc 1000 system; Bio Rad, Marnes la Coquette, France).
Band intensities of competitive PCR products were measured by the Multi Analyst/Macintosh software and were expressed in arbitrary units corresponding to pixel integration after correction by taking into account the size difference between competitor and target cDNA. The logarithm ratio of target to competitor band intensities was plotted as a function of the logarithm of the amount of competitor added. The amount of target cDNA was determined when the logarithm of the ratio was equal to zero and was expressed in attomoles for 5 × 104 cells.
RT-PCR products specific for TF, TFPI-1, and TFPI-2 were sequenced using the dideoxynucleotide chain termination method [24] on a Perkin Elmer ABI Perkin 377 automat (INSERM U316, Tours, France). Sequencing was performed on both strands with the reverse and forward primers specific for TF, TFPI-1, and TFPI-2 used for RT-PCR.
In separate experiments, TF, TFPI-1, and TFPI-2 mRNA synthesis was also evaluated by RT-PCR in purified cytotrophoblast cells cultured in complete medium for 16 and 60 h to allow them to differentiate into syncytiotrophoblast cells.
The Mann-Whitney U-test was used to compare TFPI-1 and TFPI-2 cDNA levels measured after competitive RT-PCR performed with purified villous cytotrophoblast cells.
Results
Isolation and Characterization of Villous Cytotrophoblast Cells
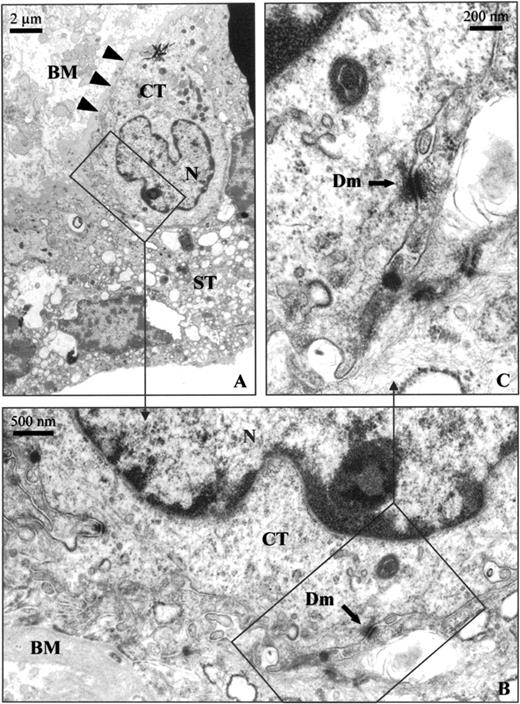
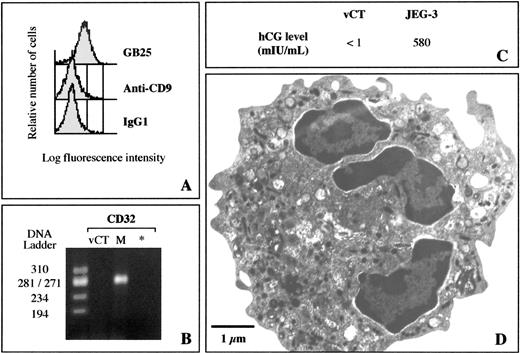
The initial goal of our study was to isolate human villous cytotrophoblast cells from normal term placentas without any contaminating HLA-positive cells such as maternal blood cells, macrophages, endothelial cells, and fibroblastlike cells and with the lowest contamination from syncytial fragments. Two to five trypsin digestions were first performed in combination with DNase treatment; the total amount of trypsin used and digestion times were adjusted for each placenta by checking the basal membrane integrity by light microscopy. As revealed by transmission electron microscopy, desmosomes were numerous between cytotrophoblast and syncytiotrophoblast cells in intact placentas (Fig. 1). Because the integrity of desmosomes is calcium dependent [25], EDTA was added during enzymatic digestion to chelate calcium ions before implementing the immunodepletion procedure using monoclonal antibodies to human HLA-ABC, HLA-DR, and CD14 to remove remaining monocytes/macrophages, fibroblasts, and endothelial cells. Evidence of the trophoblastic origin of isolated cells was revealed by flow cytometry (Fig. 2A). All cells were stained by the trophoblast-specific GB25 antibody. In contrast, cells were not stained by the anti-CD9 monoclonal antibody, confirming the absence of blood and mesenchymal cell contamination. In addition, no CD32 mRNA was detected in cell preparations by RT-PCR (Fig. 2B). Levels of hCG in 12-h culture supernatants were <1 mIU/ml, supporting the absence of contamination by syncytial fragments (Fig. 2C).
Transmission electron micrographs of villous placental tissue. A) Thin section of villous mononuclear cytotrophoblast and multinucleated syncytiotrophoblast cells. B) Evidence of desmosome junctions between cytotrophoblast and syncytiotrophoblast cells. C) Intercellular junction with a desmosome structure. BM, Basal membrane (large arrows); CT, cytotrophoblast cell; ST, syncytiotrophoblast cell; N, nucleus; Dm, desmosome (small arrows)
Characterization of villous cytotrophoblast cells isolated from human term placentas. A) Flow cytometry analysis was performed with GB25 antibody specific for trophoblast cells and with CD9 antibody specific for platelets, lymphocytes, and polynuclear and endothelial cells. Isotypic IgG1 was used as a negative control. B) RT-PCR method specific for CD32. * Negative control; DNA ladder ΦX174 RF/HaeIII DNA ladder; M, monocyte cDNA from 5 × 104 cells, used as a positive control. C) Human CG production in 12-h culture of isolated villous cytotrophoblast cells (vCT) or choriocarcinoma JEG-3 cells, used as positive control. D) Transmission electronic micrograph of purified villous cytotrophoblast cell
Transmission electron microscopic examination of freshly isolated mononuclear cytotrophoblast cells (0.25 × 106 cells/g placental tissue) was also used to check cell integrity and to demonstrate morphological features characteristic of immature villous cytotrophoblast cells, i.e., a poorly developed rough endoplasmic reticulum, few microvilli, and lipid droplets (Fig. 2D).
Detection and Quantification of TF, TFPI-1, and TFPI-2 mRNA in Human Trophoblast Cells
TF, TFPI-1, and TFPI-2 mRNA expression was studied in either human villous cytotrophoblast cells freshly isolated from five normal term placentas or in cells cultured for 16 and 60 h in complete medium. Total mRNA was isolated from 106 cells for each placenta, and RT-PCR specific for TF, TFPI-1, TFPI-2, and GAPDH were then simultaneously performed using single-strand cDNA from about 5 × 104 cells. RT-PCR products were obtained as single bands of 282, 230, 253, and 265 base pairs (bp), the expected sizes of TF, TFPI-1, TFPI-2, and GAPDH cDNA fragments, respectively. The sequences of the RT-PCR products were similar to the previously published sequences [26–30], confirming the specificity of the technique. Cell expression of GAPDH (used as control) was not affected in any experiment.
No TF mRNA was found in villous cytotrophoblast cells freshly isolated with a digestion solution containing EDTA (Fig. 3A), whereas strong TF mRNA synthesis was demonstrated when cells were obtained without EDTA. However, when villous cytotrophoblast cells that had been purified with EDTA were cultured for 16 and 60 h in complete medium, optic microscopy demonstrated that they evolved as a multinucleated syncytiotrophoblast, and significant levels of TF mRNA were detected by RT-PCR (Fig. 3B).
Detection of TF mRNA in villous trophoblast cells by RT-PCR. A) TF mRNA in villous cytotrophoblast cells freshly isolated from human term placentas using a digestion solution without (−) or with (+) 1 mM EDTA. B) TF mRNA in villous cytotrophoblast cells freshly isolated or in cells cultured for 16 and 60 h in complete medium. * Negative control; DNA ladder ΦX174 RF/HaeIII DNA ladder. Data representative of at least two independent experiments
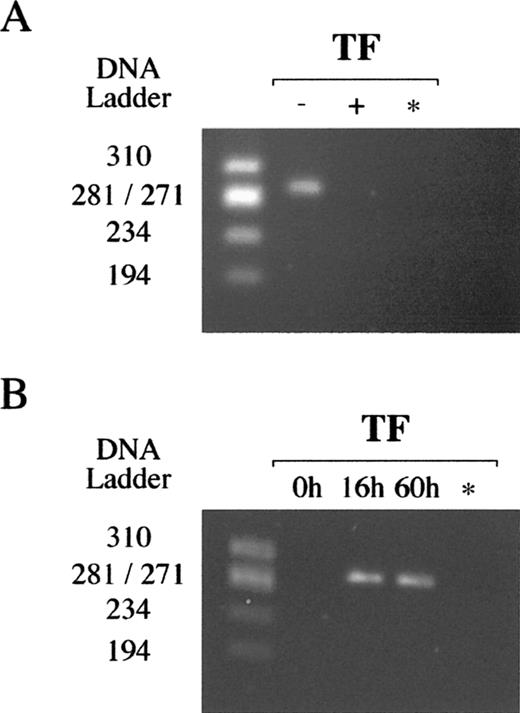
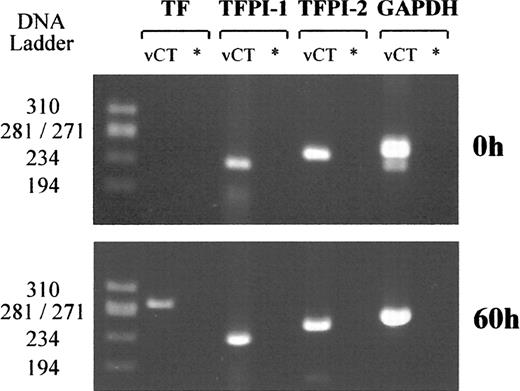
Low levels of TFPI-1 mRNA were detected in freshly isolated villous cytotrophoblast cells (Fig. 4A), but the amount of TFPI-2 mRNA always appeared higher in cells obtained from each placenta. Finally, TFPI-1 and TFPI-2 mRNA synthesis remained unchanged when cytotrophoblast cells were cultured in complete medium for 60 h (Fig. 4B).
Detection of TF, TFPI-1, TFPI-2, and GAPDH mRNA. A) Villous cytotrophoblast cells freshly isolated from human term placentas. B) Cells cultured for 60 h in complete medium. * Negative control; DNA ladder ΦX174 RF/HaeIII DNA ladder. Data representative of five independent experiments
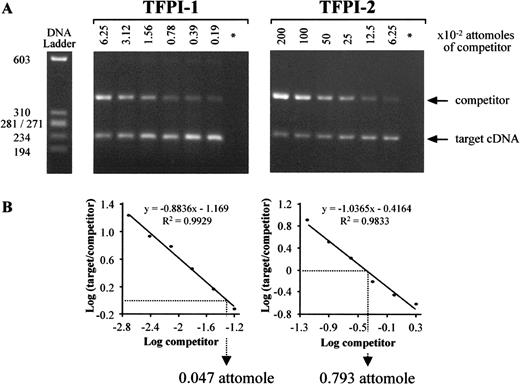
To quantify TFPI-1 and TFPI-2 mRNA expression, competitive RT-PCR assays were performed immediately after isolation of villous cytotrophoblast cells. Serial dilutions of competitors, starting from 0.0625 and 2 attomoles of TFPI-1 and TFPI-2 cDNA competitor, respectively, were coamplified with a constant volume of 5 μl first-strand cDNA obtained from 5 × 104 villous cytotrophoblast cells. Competitors for TFPI-1 and TFPI-2 yielded PCR products 150 bp larger than the target sequence, which were easily distinguished after gel electrophoresis (Table 1). An increase in target PCR products was observed for each gene studied when competitor PCR products decreased (Fig. 5A). Thus, when the intensities of target and competitor PCR products were equal, we could evaluate the amount of target cDNA present in the sample tested (Fig. 5B). Three independent competitive RT-PCR assays were performed with cytotrophoblast cells isolated from two placentas for each gene studied, and results were expressed as mean ± SEM attomoles for 5 × 104 cells. Using the above experimental conditions, villous cytotrophoblast cells isolated from term placentas expressed a low level of TFPI-1 mRNA with 0.041 ± 0.004 attomoles of cDNA. In contrast, the amount of TFPI-2 cDNA measured after competitive RT-PCR was 18 times greater (0.740 ± 0.026 attomoles; U = 16; P = 0.025).
Competitive RT-PCR assays specific for TFPI-1 and TFPI-2 cDNA in villous cytotrophoblast cells isolated from human term placentas. A) Two-fold dilutions of competitor were coamplified with constant aliquots of cDNA from 5 × 104 villous cytotrophoblast cells. B) After measuring target and competitor PCR product intensities, the logarithm of their ratio was plotted as a function of the logarithm of the amount of competitor present in the mixture reaction. Target cDNA levels were then calculated using the linear regression curves obtained, which were all linear with correlation coefficients >0.98. * Negative control; DNA ladder ΦX174 RF/HaeIII DNA ladder. Data representative of two independent experiments
Discussion
The main aim of this study was to evaluate the levels of mRNA synthesis specific to the TFPI-2 gene in human villous cytotrophoblast cells isolated from normal term placentas. Pure cell preparations were necessary to avoid contamination by mesenchymal cells and syncytial fragments. The protocol used to isolate a pure population of villous cytotrophoblast cells was based on mild trypsin-DNase digestion, taking into account lot-to-lot variations, followed by Percoll gradient separation and immunodepletion of contaminating cells using monoclonal antibodies specific for human HLA-ABC, HLA-DR, and CD14. These three steps are rarely combined in the majority of protocols [17, 18]. Most authors have used trypsin-DNase treatments followed by Percoll gradient centrifugation, allowing isolation of homogeneous populations of mononuclear cells containing >95% cytotrophoblast cells [14, 16, 18]. However, remaining contaminating cells (particularly mesenchymal cells) are not usually removed, as was done in the present study in the immunodepletion step as recommended by several investigators [15].
EDTA was added during enzymatic tissue disaggregation, and by chelating the calcium ions, the EDTA presumably altered desmosome organization between cytotrophoblast and syncytiotrophoblast cells [31], thereby contributing to reduced contamination by syncytial fragments.
Several complementary methods were employed to assess the purity of our cell preparations. GB25, a monoclonal antibody specific to the villous trophoblast [32, 33], was used to stain all cells, as demonstrated by flow cytometry analysis. In contrast, no staining was observed with the anti-CD9 monoclonal antibody, confirming the absence of contaminating platelets, B and T lymphocytes, polynuclear cells, endothelial cells, epithelial and vascular smooth muscle cells [33], and extravillous trophoblast cells [34]. The absence of monocyte/macrophage cells was also confirmed using a sensitive RT-PCR method specific for CD32. The lack of hCG, specifically produced by the syncytiotrophoblast of normal placentas [14, 35, 36], also indicated very low contamination with syncytial fragments. Furthermore, purified cells exhibited all typical morphologic features of immature villous cytotrophoblast cells [37, 38].
No TF mRNA was detected in purified villous cytotrophoblast cells. This result was in agreement with those of previous studies, in which TF was detected in only placental macrophages, endothelial cells, and fibroblast cells [6, 39]. This result could be obtained only when cytotrophoblast cells were isolated using a digestion solution containing EDTA. When EDTA was omitted, TF mRNA was detected by PCR, probably as a result of contamination by syncytial fragments, which express large amounts of TF [5]. Strong TF mRNA synthesis occurred when villous cytotrophoblast cells were cultured for 16 or 60 h in complete medium. In vitro differentiation of cells into syncytiotrophoblast has previously been described [14, 33], and TF mRNA synthesis clearly displayed syncytial formation. We therefore propose looking systematically for the presence of TF to assess the purity of villous cytotrophoblast cell preparations. This marker could easily be combined with other parameters [40] to confirm the absence of contaminating cells, syncytial fragments, and syncytiotrophoblast cells.
In this study, we showed that low levels of TFPI-1 mRNA, the major identified inhibitor of TF, are produced by villous cytotrophoblast cells isolated from term placentas and demonstrated that this expression remains unchanged after in vitro cell differentiation into syncytiotrophoblast. Edstrom et al. [7] also showed by immunohistochemical staining that TFPI-1 is localized in the villous cytotrophoblast and in the syncytiotrophoblast, extravillous trophoblast, and vascular endothelium of the human placenta from 10 wk through term. Whether TFPI-1 is functional in the placenta remains to be determined, but this protease inhibitor could contribute to the maintenance of a physiological anticoagulant surface, preventing abnormal formation of thrombi at the maternofetal interface.
The major finding of the present study is that TFPI-2 mRNA is also expressed in large amounts in normal villous cytotrophoblast cells isolated from term placentas. This result is in agreement with those of Bützow et al. [12], who detected PP5 in the cytotrophoblast by immunoperoxidase staining using monoclonal and polyclonal antibodies. In contrast, using in situ hybridization and Northern blotting, Udagawa et al. [10] failed to detect TFPI-2 transcripts in villous cytotrophoblast cells. This apparent discrepancy cannot be explained by contamination of our cytotrophoblast cells by syncytial fragments and/or monocyte/macrophage cells. RT-PCR assays were performed with freshly isolated villous cytotrophoblast cells in which no TF mRNA was detected, and thus such a discrepancy cannot be explained by an in vitro differentiation of cells into a syncytiotrophoblast. The differences more probably resulted from the greater efficiency of the quantitative PCR technique. In a previous study, we measured relatively high levels of TFPI-2 mRNA in BeWo cells [23], whereas Udagawa et al. [10] did not detect TFPI-2 transcripts in this trophoblast cell line. Our study also indicates that TFPI-2 mRNA can be synthesized by differentiated villous cytotrophoblast cells obtained in culture in accordance with the findings of Udagawa et al. [13], who detected transcripts in the syncytiotrophoblast. The normal placenta is thus an important source of TFPI-2, which can be synthesized in both villous cytotrophoblast and syncytiotrophoblast cells, but the physiological role of this protein remains to be clarified.
The amount of TFPI-2 transcript measured in cytotrophoblast cells was 18 times greater than that of TFPI-1. This result suggests that TFPI-2 is involved in the maintenance of hemostatic balance in intervillous spaces, partly compensating for the low level of TFPI-1 synthesis. However, despite structural and sequence similarities to TFPI-1 [28], TFPI-2 weakly inhibits factor Xa and thrombin generation [28, 41], thus poorly regulating TF-mediated coagulation [41]. Therefore, TFPI-2 probably plays another role in the placenta. In this regard, as a Kunitz-type proteinase inhibitor and by inhibiting trypsin and plasmin, TFPI-2 reduces matrix metalloproteinase 1 (MMP-1), MMP-3, and MMP-9 activation [42]. However, the syncytiotrophoblast secretes MMP-9, with polarized release from the basolateral surface of cells, suggesting that this protease has a role in villous tissue remodeling [43]. By inhibiting the generation of activated MMP-9, TFPI-2 could thus regulate trophoblast tissue remodeling or differentiation.
Further studies using appropriate tools are necessary to elucidate the functions of TFPI-2 in the development of the placenta and maintenance of its integrity. For this purpose, we are currently generating recombinant TFPI-2 and monoclonal antibodies. Furthermore, our method for isolating pure preparations of cytotrophoblast cells should be very valuable, especially for studies focusing on differentiation of villous trophoblast, syncytial fusion, and gene expression.
Acknowledgments
We are grateful to Prof. G. Body and Prof. F. Perrotin (Département de Gynecologie et Obstétrique, CHRU de Tours, France) who provided term placentas. We particularly thank S. Marouillat and Prof. C. Andrès (INSERM U316, Laboratoire de Biochimie et de Biologie Moléculaire, Tours, France) for nucleotide sequencing. We also thank D. Raine for editing the English.
References
Author notes
This study was supported by the Institut pour la Recherche sur la Thrombose et l'Hémostase, Tours, France.