-
PDF
- Split View
-
Views
-
Cite
Cite
Derek M. Huffman, Jamie N. Justice, Michael B. Stout, James L. Kirkland, Nir Barzilai, Steven N. Austad, Evaluating Health Span in Preclinical Models of Aging and Disease: Guidelines, Challenges, and Opportunities for Geroscience, The Journals of Gerontology: Series A, Volume 71, Issue 11, November 2016, Pages 1395–1406, https://doi.org/10.1093/gerona/glw106
Close - Share Icon Share
Abstract
Life extension is no longer considered sufficient evidence of delayed aging in research animals. It must also be demonstrated that a broad swathe of health indicators have been extended. During a retreat of the Geroscience Network, a consortium of basic and clinical aging researchers, potential measures of mouse health were considered for their potential as easily standardized, highly informative metrics. Major health domains considered were neuromuscular, cognitive, cardiovascular, metabolic, and inflammatory functions as well as body composition and energetics and a multitude of assays interrogating these domains. A particularly sensitive metric of health is the ability to respond to, and recover, from stress. Therefore, the Network also considered stresses of human relevance that could be implemented in mouse models to assess frailty and resilience. Mouse models already exist for responses to forced immobility, cancer chemotherapy, infectious diseases, dietary challenges, and surgical stress, and it was felt that these could be employed to determine whether putative senescence-retarding interventions increased and extended organismal robustness. The Network discussed challenges in modeling age-related human chronic diseases and concluded that more attention needs to be paid to developing disease models with later age of onset, models of co- and multimorbidity, diversifying the strains and sexes commonly used in aging research, and considering additional species.
In animals used in preclinical research, an increase in longevity has historically been considered a sufficient indicator of whether an intervention has delayed aging. Although longevity (or life span) remains an important determinant of efficacy, greater emphasis is now being placed on clinically relevant health assessments in addition to longevity metrics. The hope is that identifying models with extended health might help identify strategies capable of compressing morbidity as well as enhancing and extending health span in humans ( 1 ). In order to promote the continued development and standardization of health-span assessment in animal models, which in this context means laboratory mice or rats, a group of leading experts in geriatrics and the biology of aging participated in a conference in Santa Barbara, CA as part of the Geroscience Network ( Table 1 ), to discuss progress and problems in evaluating healthy aging and function in laboratory animals in ways that are relevant to human health.
The Geroscience Network
| Albert Einstein College | University of Alabama at Birmingham |
| Buck Institute | University of Arkansas |
| European Union | University of Connecticut |
| Harvard University | University of Michigan |
| Johns Hopkins University | University of Minnesota |
| Mayo Clinic | University of Texas Health Science Center San Antonio |
| National Institute on Aging | University of Southern California |
| The Scripps Research Institute | University of Washington |
| Stanford University | Wake Forest University |
| Albert Einstein College | University of Alabama at Birmingham |
| Buck Institute | University of Arkansas |
| European Union | University of Connecticut |
| Harvard University | University of Michigan |
| Johns Hopkins University | University of Minnesota |
| Mayo Clinic | University of Texas Health Science Center San Antonio |
| National Institute on Aging | University of Southern California |
| The Scripps Research Institute | University of Washington |
| Stanford University | Wake Forest University |
The Geroscience Network
| Albert Einstein College | University of Alabama at Birmingham |
| Buck Institute | University of Arkansas |
| European Union | University of Connecticut |
| Harvard University | University of Michigan |
| Johns Hopkins University | University of Minnesota |
| Mayo Clinic | University of Texas Health Science Center San Antonio |
| National Institute on Aging | University of Southern California |
| The Scripps Research Institute | University of Washington |
| Stanford University | Wake Forest University |
| Albert Einstein College | University of Alabama at Birmingham |
| Buck Institute | University of Arkansas |
| European Union | University of Connecticut |
| Harvard University | University of Michigan |
| Johns Hopkins University | University of Minnesota |
| Mayo Clinic | University of Texas Health Science Center San Antonio |
| National Institute on Aging | University of Southern California |
| The Scripps Research Institute | University of Washington |
| Stanford University | Wake Forest University |
Among the issues discussed by the panel were advantages and disadvantages of simple versus highly technical health measures commonly employed in aging research, as well as whether consensus could be achieved on recommendations for selecting, prioritizing, and standardizing these measurements across domains and laboratories. In addition, participants discussed the impact of highly artificial, controlled, albeit consistent laboratory conditions in which animal health metrics are typically assessed compared with free-living humans, who commonly deal with predictable and unpredictable, chronic and acute, stresses such as poor health habits, infectious diseases, and invasive medical procedures to which laboratory animals are never exposed. Thus, further development and refinement of models and assays that better characterize scenarios of human relevance were considered to be of high priority.
An enormous investment has been made in animal models to understand the etiology and treatment for numerous diseases in which aging is the major underlying risk factor, including cardiovascular disease (CVD), Alzheimer’s disease (AD), various cancers, and type 2 diabetes mellitus (T2DM). However, most of these diseases do not arise spontaneously in laboratory animals, and none arise in young laboratory animals. As a consequence, most diseases of human relevance must be purposefully initiated in the animals and are typically induced at a relatively young age, leading to pathogenesis that may not entirely resemble the human disorder. Thus, there remains a considerable need for improved disease models in order to recapitulate the etiology of these diseases in humans. These important issues, including opportunities for future emphasis and development, were also discussed and will be highlighted here.
Approach
Health Span Assessment in Animal Models
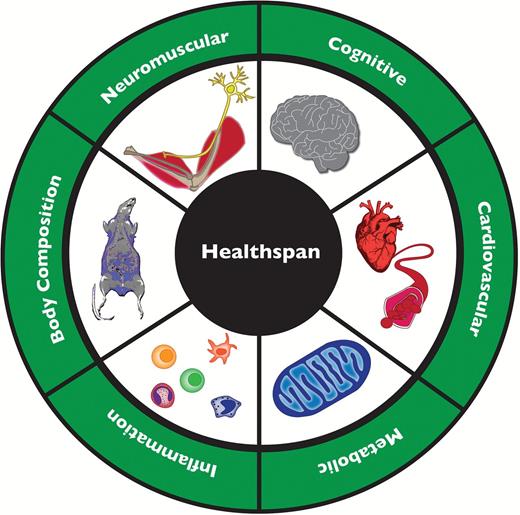
There is a need to develop, prioritize, and standardize non- or minimally invasive tests that can be performed longitudinally on individual animals to assess their functional status and response to interventions in ways relevant to clinical health in humans. This need has already led to several conferences and a working paper on the problem along with recommendations ( 2 ). Our working group of geriatricians and researchers in the basic biology of aging identified the following high-priority translatable, functional domains to characterize health span: (i) neuromuscular, (ii) cognitive, (iii) cardiovascular, (iv) inflammatory, and (v) metabolic functions, as well as (vi) body composition and energetics ( Figure 1 ). This list is clearly not comprehensive, but rather consists of domains considered most obviously important for direct relevance to humans. Here, we will attempt to focus upon those available tests in those domains that are most feasible and widely available to investigators, either within their own laboratories or in common core facilities.
Initial domains of health span considered particularly relevant to human health.
General Considerations
In addition to the assays and paradigms described in the sections below, several features of standard laboratory mouse husbandry and biology should be considered in the context of mouse health metrics with human relevance. First, body mass is a potential confounder in many functional assays. A good example is the rotarod test, in which animals attempt to remain atop an accelerating rotating rod. Lighter mice outperform heavier mice on this test ( 3 ). As mouse weight changes over the course of their lifetime, different mouse strains differ in body weight, and as a number of life-extending interventions reduce body weight, this potential confound is not trivial. It needs to be statistically addressed in all studies as similar body mass–dependent tasks are in human studies. Second, mice are fearful of humans and consequently subject to significant handling and disturbance stress. Any test that requires mice to be handled or disturbed from their normal activity should be assumed to be performed under duress. The impact that stress may have on the outcome of the test should be borne in mind and measures employed to minimize it. Simple methods to minimize handling stress do exist ( 4 , 5 ). However, as some tests (noted below) require handling as part of the assay, these tests will be more challenging to perform while avoiding significant contamination due to handing stress. Third, mice are typically maintained in depauperate environments. Even rudimentary social, sensory, and motor enrichment of standard mouse cages has profound effects on behavior, neuroanatomy, and disease susceptibility—even susceptibility to diseases presumed to have an entirely genetic basis such as Huntington’s disease ( 6 , 7 ). These common features of mouse husbandry should be borne in mind in assessing health span.
Specific Domains
Neuromuscular function
Neuromuscular function and stamina are important clinical outcomes in assessment of overall health in aging adults, as they are highly associated with such adverse health events as falls, depression, hospitalization, nursing home admissions, dependence level, disability, and death ( 8–10 ). Standardized tests have been implemented for assessing strength, locomotor ability, gait speed, endurance capacity, balance, and co-ordination across the human life span ( 9 , 11 ). Of these, handgrip strength and preferred gait speed are most commonly employed and have predictive value for future disability and mortality ( 9 , 11 ). As such, these tests are proposed as primary functional outcomes in clinical trials and have been incorporated into algorithms for defining frailty and sarcopenia ( 12 ).
Neuromuscular function can be reverse translated to clinically relevant subdomains in animal research models ( Table 2 ) ( 13 , 14 ). Indeed, aging in laboratory rodents is characterized by declining strength, spontaneous locomotor activity, and endurance capacity, with a trajectory reminiscent of aging in humans ( 13 ). In mice, strength is most commonly assessed through forelimb grip release using a specialized high-sensitivity force transducer and grip bar or grid. Locomotor behavior is assessed as gait or “scurry” speed on a walking track, the total distance traveled, and speed achieved in a novel open arena at fixed-time intervals, the ability to run on an accelerating rotating rod (“rotarod”), or as spontaneous home cage or wheel running activity. Endurance capacity can be assessed as time to exhaustion or similar performance outcomes using rotarod devices or treadmills at fixed submaximal or maximal speeds. Sensorimotor function can also be efficiently evaluated using a balance beam, whereby the investigator documents the number of slips made by the animal while crossing the beam ( 15 ). Beam difficulty can be increased by decreasing the size of beam, which can help in better discriminating between young and old animals.
Preclinical Tests for Neuromuscular Function and Performance in Aging Mice
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Locomotor activity | Gait speed | Strengths: Standardization & Core lab availability; conservation across species; relevance to human aging |
| Open field distance | ||
| Rotarod ability | ||
| Cage activity | Limitation: Effect of body mass & feeding | |
| Strength | Grip strength | Strengths: Measures of forelimb and/or hindlimb strength; relevance to human aging |
| Limitations: High tester sensitivity | ||
| Endurance | Treadmill/rotarod run to exhaustion | Strengths: Relevance to humans; conservation across species |
| Limitations: Standardization & Core lab availability; effect of body mass & feeding | ||
| Combined functions | Inverted cling | Strength: Simultaneous assessment of strength and endurance |
| Wire suspension | ||
| Limitations: Standardization & Core lab availability; effect of body mass & feeding | ||
| Sensory motor | Balance beam | Strengths: Gross motor co-ordination; fine motor co-ordination |
| Tape removal test | ||
| Limitations: Lack of validation in healthy aging animals; effect of body mass & feeding |
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Locomotor activity | Gait speed | Strengths: Standardization & Core lab availability; conservation across species; relevance to human aging |
| Open field distance | ||
| Rotarod ability | ||
| Cage activity | Limitation: Effect of body mass & feeding | |
| Strength | Grip strength | Strengths: Measures of forelimb and/or hindlimb strength; relevance to human aging |
| Limitations: High tester sensitivity | ||
| Endurance | Treadmill/rotarod run to exhaustion | Strengths: Relevance to humans; conservation across species |
| Limitations: Standardization & Core lab availability; effect of body mass & feeding | ||
| Combined functions | Inverted cling | Strength: Simultaneous assessment of strength and endurance |
| Wire suspension | ||
| Limitations: Standardization & Core lab availability; effect of body mass & feeding | ||
| Sensory motor | Balance beam | Strengths: Gross motor co-ordination; fine motor co-ordination |
| Tape removal test | ||
| Limitations: Lack of validation in healthy aging animals; effect of body mass & feeding |
Preclinical Tests for Neuromuscular Function and Performance in Aging Mice
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Locomotor activity | Gait speed | Strengths: Standardization & Core lab availability; conservation across species; relevance to human aging |
| Open field distance | ||
| Rotarod ability | ||
| Cage activity | Limitation: Effect of body mass & feeding | |
| Strength | Grip strength | Strengths: Measures of forelimb and/or hindlimb strength; relevance to human aging |
| Limitations: High tester sensitivity | ||
| Endurance | Treadmill/rotarod run to exhaustion | Strengths: Relevance to humans; conservation across species |
| Limitations: Standardization & Core lab availability; effect of body mass & feeding | ||
| Combined functions | Inverted cling | Strength: Simultaneous assessment of strength and endurance |
| Wire suspension | ||
| Limitations: Standardization & Core lab availability; effect of body mass & feeding | ||
| Sensory motor | Balance beam | Strengths: Gross motor co-ordination; fine motor co-ordination |
| Tape removal test | ||
| Limitations: Lack of validation in healthy aging animals; effect of body mass & feeding |
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Locomotor activity | Gait speed | Strengths: Standardization & Core lab availability; conservation across species; relevance to human aging |
| Open field distance | ||
| Rotarod ability | ||
| Cage activity | Limitation: Effect of body mass & feeding | |
| Strength | Grip strength | Strengths: Measures of forelimb and/or hindlimb strength; relevance to human aging |
| Limitations: High tester sensitivity | ||
| Endurance | Treadmill/rotarod run to exhaustion | Strengths: Relevance to humans; conservation across species |
| Limitations: Standardization & Core lab availability; effect of body mass & feeding | ||
| Combined functions | Inverted cling | Strength: Simultaneous assessment of strength and endurance |
| Wire suspension | ||
| Limitations: Standardization & Core lab availability; effect of body mass & feeding | ||
| Sensory motor | Balance beam | Strengths: Gross motor co-ordination; fine motor co-ordination |
| Tape removal test | ||
| Limitations: Lack of validation in healthy aging animals; effect of body mass & feeding |
Although the aforementioned tests comprise the group’s recommended assessment of neuromuscular function in rodents, emphasis was also placed on better standardizing the administration of these protocols across laboratories, similarly to dedicated efforts made in clinical investigations ( 16 ). Locomotor behavior should be prioritized, as it is easily assayed and conserved across species thereby improving translational potential. Furthermore, attention should be paid to numerous potential confounders, including changes in body mass with age, training effects, and the timing of tests vis à vis the light–dark cycle or feeding regime in which tests are conducted, as all may have significant effects on performance. Evaluating neuromuscular function and stamina is generally available as a core service at some institutions. All specific tests require specialized equipment and varying degrees of experience and expertise to correctly perform, but training of lab personnel and gaining access or obtaining the equipment to perform these assays “in house” is feasible.
Cognitive function
Cognitive decline is a hallmark of aging in many species. It is a particular concern in humans who depend so thoroughly on cognitive health in many ways. A 2012 survey of American Association of Retired Persons (AARP) members found that staying “mentally sharp” was the top concern of 87% of respondents. Cognitive aging is not confined to memory and learning decay but also includes diminishment of attention, thinking, understanding, problem solving, and decision making ( 17 ). Even narrowly defined as significant memory impairment, as many as approximately 40% of individuals older than 65 years experience some form of cognitive decline ( 18 ). Importantly, age-related cognitive dysfunction is related to numerous adverse health outcomes for individual sufferers, but its adverse impacts extend to family members and the community in the guise of health, public health, and social services required to provide support for the afflicted.
Rodent behavioral batteries have been developed to attempt to reverse translate some cognitive functions from humans ( 19 ), with the majority of tasks demonstrating age-related declines developed to assess learning and memory impairments ( Table 3 ) ( 20 ). Attempts have also been made to model in rodents executive function, a set of general-purpose control mechanisms that regulate the dynamics of human cognition ( 21 ), which are thought to rely strongly on the prefrontal cortex ( 22 ). Although rats may be capable of complex goal-directed behaviors indicative of executive functioning ( 23 ), tests in aging mice are still not validated to general satisfaction. Parenthetically, rats generally are clearly superior models to mice for cognitive assessment, and it is therefore unfortunate that they have fallen out of favor for interventional studies of aging.
Preclinical Tests for Cognitive Function in Aging Mice
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Learning & memory | Novel object recognition | Strength: Relevant to aging humans |
| Stone T maze | Limitations: Standardization & Core lab availability; effect of feeding; length of training and testing time | |
| Modified Barnes maze | ||
| Executive function | Strength: Highly relevant to aging humans | |
| Limitations: Test development and validation needed for mice; requires core availability | ||
| Anxiety | Open field activity (inner compared with outer zones) | Strength: Open field anxiety outcomes are obtained during distance assessment |
| Elevated Plus Maze | Limitations: Standardization & Core lab availability | |
| Depression | Porsolt Forced Swim Test | Strengths: Highly repeatable; requires minimal equipment; complementary to other domains |
| Tail suspension Test | ||
| Anhedonia Test | Limitations: Standardization; failure rate can be high in older models |
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Learning & memory | Novel object recognition | Strength: Relevant to aging humans |
| Stone T maze | Limitations: Standardization & Core lab availability; effect of feeding; length of training and testing time | |
| Modified Barnes maze | ||
| Executive function | Strength: Highly relevant to aging humans | |
| Limitations: Test development and validation needed for mice; requires core availability | ||
| Anxiety | Open field activity (inner compared with outer zones) | Strength: Open field anxiety outcomes are obtained during distance assessment |
| Elevated Plus Maze | Limitations: Standardization & Core lab availability | |
| Depression | Porsolt Forced Swim Test | Strengths: Highly repeatable; requires minimal equipment; complementary to other domains |
| Tail suspension Test | ||
| Anhedonia Test | Limitations: Standardization; failure rate can be high in older models |
Preclinical Tests for Cognitive Function in Aging Mice
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Learning & memory | Novel object recognition | Strength: Relevant to aging humans |
| Stone T maze | Limitations: Standardization & Core lab availability; effect of feeding; length of training and testing time | |
| Modified Barnes maze | ||
| Executive function | Strength: Highly relevant to aging humans | |
| Limitations: Test development and validation needed for mice; requires core availability | ||
| Anxiety | Open field activity (inner compared with outer zones) | Strength: Open field anxiety outcomes are obtained during distance assessment |
| Elevated Plus Maze | Limitations: Standardization & Core lab availability | |
| Depression | Porsolt Forced Swim Test | Strengths: Highly repeatable; requires minimal equipment; complementary to other domains |
| Tail suspension Test | ||
| Anhedonia Test | Limitations: Standardization; failure rate can be high in older models |
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Learning & memory | Novel object recognition | Strength: Relevant to aging humans |
| Stone T maze | Limitations: Standardization & Core lab availability; effect of feeding; length of training and testing time | |
| Modified Barnes maze | ||
| Executive function | Strength: Highly relevant to aging humans | |
| Limitations: Test development and validation needed for mice; requires core availability | ||
| Anxiety | Open field activity (inner compared with outer zones) | Strength: Open field anxiety outcomes are obtained during distance assessment |
| Elevated Plus Maze | Limitations: Standardization & Core lab availability | |
| Depression | Porsolt Forced Swim Test | Strengths: Highly repeatable; requires minimal equipment; complementary to other domains |
| Tail suspension Test | ||
| Anhedonia Test | Limitations: Standardization; failure rate can be high in older models |
A point of particular relevance for cognitive studies of aging animals is that general sensory acuity declines with age. Therefore, any test that depends upon vision, olfaction, hearing, and even experiencing slight pain needs to be performed with attention to making sure that the results are not a consequence of these sensory changes rather than the cognitive properties under study.
Unlike executive function, aging humans, mice, and rats all exhibit significant decrements in behavioral tasks requiring spatial learning, an indication that disorder of the medial temporal lobe is conserved across species in normal aging. Our working group recognizes that there are many shortcomings in rodent models of human cognitive aging, however, it was felt that the Stone T- and modified Barnes maze tasks as well as novel object recognition tasks exhibit some promise as behavioral cognitive outcomes with translational relevance for age-related change in learning and memory ( 24–26 ). The novel object recognition tasks have the distinct advantage of potentially being coupled with open field assessments of neuromuscular function, supporting the ease of their inclusion into broad behavioral batteries of health span. Tests are also available for noncognitive mental states such as anxiety and depression.
From a logistical standpoint, assays to evaluate rodent cognitive function are generally widely available, either in specialized laboratories or in core facilities. Evaluation of cognitive function requires sufficient experience and expertise in order to perform the appropriate tests following standardized procedures. It is advisable that laboratories considering inclusion of these assays for the first time consult with a qualified collaborator or core service. Investigators can consider performing 1–2 representative tests across several domains of function as a screening strategy, and further tests can be considered within domains of interest that warrant additional follow-up. For the purposes of our working group, open field assessment of anxiety and novel object recognition are recommended for conservation of time and equipment, though they may not be the most sensitive to age or intervention.
Cardiovascular function
Despite precipitous drops in age-adjusted mortality rates from heart disease and stroke in recent years ( 27 , 28 ), CVD remains a leading cause of morbidity, disability, and death among older adults in the developed world ( 29 , 30 ). Thus, cardiovascular function represents an important health-span domain that warrants appropriate preclinical attention. Clinically, primary functional assessments can be considered broadly as measures of vascular function, cardiac structure and function, as well as hemodynamic measures, blood pressure, and heart rate variability. Secondary analyses often include measures of lipids (triglycerides, free fatty acids, cholesterol) and the lipoprotein profile (HDL, LDL, VLDL). Although preclinical models can be utilized in most of these approaches, important differences between the mouse and human cardiovascular system and lipid handling do impose important limitations.
The main vascular pathophysiological processes by which aging leads to increased risk of CVD are through the development of systemic vascular endothelial dysfunction as well as stiffening and calcification of the large elastic arteries (aorta and carotid). The most commonly used method of assessing endothelial function in older adults is through endothelium-dependent dilation, or the dilation of blood vessels in response to a mechanical or chemical stimulus, which increases the production of nitric oxide via endothelial nitric oxide synthase (eNOS) ( 31 ).
In rodents, there are no currently validated non- or minimally invasive techniques to translate endothelium-dependent dilation as a longitudinal measure of health. Stiffening of the large elastic arteries can be assessed across species by measuring aortic pulse wave velocity ( 32 ). Clinically, aortic pulse wave velocity, or the delay between pressure waves occurring at proximal and distal sites along the aorta, is considered the gold standard, given its ability to predict future CVD risk ( 33–35 ). Comparable assessment of blood velocity can be performed in rodents using ultrasound echo Doppler velocimetry ( 36 , 37 ). Ultrasonic Doppler methods can be used to derive an index of vessel compliance as well. Note, however, that these measures require mouse anesthesia, the effect of which on subsequent measures either in the cardiovascular or other (eg, cognitive) domains is currently unknown.
Additionally, cardiac function can be measured in rodents with the previous caveat that it requires anesthesia ( Table 4 ). The pressure–volume conductance catheter technique of ventricular function is the optimal measure of cardiac function in mice, but is a terminal measurement that is highly operator sensitive and requires assumptions about ventricle sizes ( 38 , 39 ). Given these difficulties, less invasive measures have been developed in mice that translate to outcomes derived in humans. Echocardiography can be used to assess systolic and diastolic cardiac function including early and late ventricular filling velocities ( E / A ratio) as an index of diastolic function ( 40 ) and fractional shortening (percentage of the end diastolic volume ejected during systolic phase) as an index of systolic function ( 41 ), though these measures may be complicated by the small ventricle size in mice. Alternatively, cardiac function can be easily obtained via Doppler as the Tei index (left ventricular systolic and diastolic function in combination) ( 42 , 43 ).
Preclinical Tests for Cardiovascular Health and Function in Aging Mice
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Vascular function | Endothelial dysfunction: Endothelium- dependent dilation (ex vivo artery isolation) | Strength: Sensitive measure of vascular endothelial function |
| Limitations: Core lab availability; terminal | ||
| Arterial stiffness: Aortic pulse wave velocity | Strength: Sensitive to age and interventions | |
| Limitations: Core lab availability; requires anesthesia | ||
| Cardiac function | Echocardiography: E / A ratio; fractional shortening | Strength: Sensitive to age and interventions |
| Limitations: Core lab availability; requires anesthesia some outcomes affected by small ventricle size | ||
| Doppler: Tei index | ||
| Blood pressure | Telemetry | Strength: Relevant to aging humans |
| Tail cuff plethysmography | Limitations: Invasive surgery to place transmitters; time consuming/Core lab availability; questionable sensitivity of tail cuff measures | |
| Myocardial ischemia | Ischemia–reperfusion by artery ligation | Strength: Relevant to aging humans |
| Limitations: Core lab availability; terminal |
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Vascular function | Endothelial dysfunction: Endothelium- dependent dilation (ex vivo artery isolation) | Strength: Sensitive measure of vascular endothelial function |
| Limitations: Core lab availability; terminal | ||
| Arterial stiffness: Aortic pulse wave velocity | Strength: Sensitive to age and interventions | |
| Limitations: Core lab availability; requires anesthesia | ||
| Cardiac function | Echocardiography: E / A ratio; fractional shortening | Strength: Sensitive to age and interventions |
| Limitations: Core lab availability; requires anesthesia some outcomes affected by small ventricle size | ||
| Doppler: Tei index | ||
| Blood pressure | Telemetry | Strength: Relevant to aging humans |
| Tail cuff plethysmography | Limitations: Invasive surgery to place transmitters; time consuming/Core lab availability; questionable sensitivity of tail cuff measures | |
| Myocardial ischemia | Ischemia–reperfusion by artery ligation | Strength: Relevant to aging humans |
| Limitations: Core lab availability; terminal |
Preclinical Tests for Cardiovascular Health and Function in Aging Mice
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Vascular function | Endothelial dysfunction: Endothelium- dependent dilation (ex vivo artery isolation) | Strength: Sensitive measure of vascular endothelial function |
| Limitations: Core lab availability; terminal | ||
| Arterial stiffness: Aortic pulse wave velocity | Strength: Sensitive to age and interventions | |
| Limitations: Core lab availability; requires anesthesia | ||
| Cardiac function | Echocardiography: E / A ratio; fractional shortening | Strength: Sensitive to age and interventions |
| Limitations: Core lab availability; requires anesthesia some outcomes affected by small ventricle size | ||
| Doppler: Tei index | ||
| Blood pressure | Telemetry | Strength: Relevant to aging humans |
| Tail cuff plethysmography | Limitations: Invasive surgery to place transmitters; time consuming/Core lab availability; questionable sensitivity of tail cuff measures | |
| Myocardial ischemia | Ischemia–reperfusion by artery ligation | Strength: Relevant to aging humans |
| Limitations: Core lab availability; terminal |
| Subdomains . | Specific Tests . | Considerations . |
|---|---|---|
| Vascular function | Endothelial dysfunction: Endothelium- dependent dilation (ex vivo artery isolation) | Strength: Sensitive measure of vascular endothelial function |
| Limitations: Core lab availability; terminal | ||
| Arterial stiffness: Aortic pulse wave velocity | Strength: Sensitive to age and interventions | |
| Limitations: Core lab availability; requires anesthesia | ||
| Cardiac function | Echocardiography: E / A ratio; fractional shortening | Strength: Sensitive to age and interventions |
| Limitations: Core lab availability; requires anesthesia some outcomes affected by small ventricle size | ||
| Doppler: Tei index | ||
| Blood pressure | Telemetry | Strength: Relevant to aging humans |
| Tail cuff plethysmography | Limitations: Invasive surgery to place transmitters; time consuming/Core lab availability; questionable sensitivity of tail cuff measures | |
| Myocardial ischemia | Ischemia–reperfusion by artery ligation | Strength: Relevant to aging humans |
| Limitations: Core lab availability; terminal |
Blood pressure can be assessed through surgically implanted telemetry devices ( 44 ). Alternatively, tail cuff plethysmography can also be used to determine blood pressure and heart rate noninvasively ( 45 ), though handling stress associated with this technique is difficult to minimize and may obscure subtle differences. Surgical models of myocardial ischemia–reperfusion and heart failure also exist for evaluation in rodents, but these tests are highly technical and terminal in nature. In summary, a repertoire of tests exists for preclinical cardiovascular health and function testing, a domain of high relevance to human health. However, most tests require anesthesia and access to sophisticated equipment and skilled investigators with expertise in these procedures. In addition, some tests require invasive surgery and are terminal, and thus are not a viable option for many investigators in search of minimally invasive tests for longitudinal studies.
Inflammation
Modulation of immune function is a common characteristic of the aging process and is characterized by changes in T-cell functionality, B-cell responsiveness, and dampened neutrophil, macrophage, and natural killer cell phagocytosis ( 46–48 ). Aging also results in a chronic, low-grade, systemic, proinflammatory status (sterile inflammation) ( 49 ). Chronic age-related sterile inflammation, or inflammaging ( 50 ), has been linked with several contributors of frailty onset, including osteoporosis, sarcopenia, vascular and/or metabolic dysfunction, CVD, cancer, and AD ( 48 , 51 ). Both local and systemic interleukin-6 (IL-6), IL-1β, IL-8, tumor necrosis factor α (TNFα), C-reactive protein, and several chemokines, including CXC chemokine ligand-2 (CXCL-2) and CXCL-10, are frequently elevated in older humans and believed to be central mediators of inflammaging ( 52–55 ). Studies in mice have also demonstrated the close link between a proinflammatory state and the onset of physical decline, frailty, and diseases of aging ( 56–58 ). Circulating IL-6, IL-1β, and TNFα were strong predictors of frailty and disease onset in these reports, which suggests that mediators of inflammaging are conserved across mammalian species. At a minimum, we suggest that these circulating analytes be considered for inclusion as part of a standardized assessment of health span and response to interventions targeting fundamental aging processes in preclinical models. Several different platforms exist that use flow cytometry, chemiluminescence, or electrochemiluminescence technology. However, the feasibility of such assays has been aided by recent advances in multiplexing technologies, which provide a particularly attractive option over traditional enzyme-linked immunosorbent assays for studies in mice, including the ability to measure a large number of cytokines and chemokines simultaneously ( 20–30 ) in a high-throughput approach, while requiring minimal sample volume along with an associated time and cost savings on a “per analyte” basis ( 59 ). Issues of blood volume required for such assays, particularly when repeated sampling of individual mice is desirable, remain to be experimentally assessed.
Metabolic function
Metabolic dysfunction, including impaired insulin action, hyperlipidemia, hypertension, and other components of the metabolic syndrome, comprise well-established features of aging in humans ( 60 ). Metabolic dysfunction has been linked to a number of age-related clinical comorbidities, including CVD, stroke, cancer, fatty liver disease, cognitive impairment, and microvascular complications. Thus, carefully characterizing the metabolic status of preclinical models is a high-priority domain for evaluating health span and detecting responses to treatments that target basic aging processes ( Table 5 ). Interventions that enhance insulin sensitivity are generally viewed as beneficial, and those that promote insulin resistance are viewed as detrimental, although questions regarding the role of insulin resistance in aging have been raised ( 61 ). In rodents, the least technically demanding and invasive approach to assess metabolic status is to measure fasting insulin and glucose in plasma or serum, which is typically adequate. These assays also enable the calculation of surrogate measures of insulin sensitivity in humans, such as HOMA, although the predictive value of these estimates in rodents has been questioned ( 62 ). As noted earlier, any test that requires handling or other disturbance of mice in their home cages causes significant stress, although methods exist to minimize this stress ( 5 , 63 ). Hemoglobin A1C is another viable and inexpensive variable that can be assayed serially in whole blood and can provide information regarding metabolic status throughout the life course. A significant advantage of this test is that it integrates blood glucose concentration over a long period rather than instantaneously, therefore is not contaminated by procedural stress.
Preclinical Tests of Metabolic Function in Aging Mice
| Subdomains . | Specific Markers . | Action Items/Future Developments . |
|---|---|---|
| Insulin action | Primary: | Strengths: Gold standard; highly informative |
| Hyperglycemic–euglycemic clamps | Limitations: Core lab availability; terminal | |
| Secondary: | Strengths: Simple, fast, safe; can be measured in most labs | |
| Fasting glucose | ||
| Fasting insulin | Limitations: Less informative of mechanism; anesthesia and handling stress can confound fasting measures; fasting length can impact GTT and ITT outcome | |
| ITT | ||
| GTT | ||
| Hyperlipidemia | TG, FFA, HDL, LDL, total cholesterol | Strength: Easy to assay |
| Limitation: Rodents not best model of cholesterol metabolism | ||
| Metabolomics | TCA cycle intermediates, amino acids, acyl carnitines, Redox, bile acids | Strength: Sophisticated and comprehensive |
| Limitations: Requires commercial analysis or Core lab availability; complex analysis of large data sets; can be cost prohibitive; may require terminal bleeding for mouse samples | ||
| Other endocrine | IGF-1, IGFBP-1, IGFBP-3, GH, adiponectin, leptin, cytokines | Strength: Can be measured using commercial kits or Core labs |
| GH | Limitations: Not standardized among different manufacturers; can be limited by sample volume |
| Subdomains . | Specific Markers . | Action Items/Future Developments . |
|---|---|---|
| Insulin action | Primary: | Strengths: Gold standard; highly informative |
| Hyperglycemic–euglycemic clamps | Limitations: Core lab availability; terminal | |
| Secondary: | Strengths: Simple, fast, safe; can be measured in most labs | |
| Fasting glucose | ||
| Fasting insulin | Limitations: Less informative of mechanism; anesthesia and handling stress can confound fasting measures; fasting length can impact GTT and ITT outcome | |
| ITT | ||
| GTT | ||
| Hyperlipidemia | TG, FFA, HDL, LDL, total cholesterol | Strength: Easy to assay |
| Limitation: Rodents not best model of cholesterol metabolism | ||
| Metabolomics | TCA cycle intermediates, amino acids, acyl carnitines, Redox, bile acids | Strength: Sophisticated and comprehensive |
| Limitations: Requires commercial analysis or Core lab availability; complex analysis of large data sets; can be cost prohibitive; may require terminal bleeding for mouse samples | ||
| Other endocrine | IGF-1, IGFBP-1, IGFBP-3, GH, adiponectin, leptin, cytokines | Strength: Can be measured using commercial kits or Core labs |
| GH | Limitations: Not standardized among different manufacturers; can be limited by sample volume |
Note: GTT = glucose tolerance test; ITT = insulin tolerance test; TG = triglycerides; FFA = free fatty acids; TCA = tricarboxylic acid.
Preclinical Tests of Metabolic Function in Aging Mice
| Subdomains . | Specific Markers . | Action Items/Future Developments . |
|---|---|---|
| Insulin action | Primary: | Strengths: Gold standard; highly informative |
| Hyperglycemic–euglycemic clamps | Limitations: Core lab availability; terminal | |
| Secondary: | Strengths: Simple, fast, safe; can be measured in most labs | |
| Fasting glucose | ||
| Fasting insulin | Limitations: Less informative of mechanism; anesthesia and handling stress can confound fasting measures; fasting length can impact GTT and ITT outcome | |
| ITT | ||
| GTT | ||
| Hyperlipidemia | TG, FFA, HDL, LDL, total cholesterol | Strength: Easy to assay |
| Limitation: Rodents not best model of cholesterol metabolism | ||
| Metabolomics | TCA cycle intermediates, amino acids, acyl carnitines, Redox, bile acids | Strength: Sophisticated and comprehensive |
| Limitations: Requires commercial analysis or Core lab availability; complex analysis of large data sets; can be cost prohibitive; may require terminal bleeding for mouse samples | ||
| Other endocrine | IGF-1, IGFBP-1, IGFBP-3, GH, adiponectin, leptin, cytokines | Strength: Can be measured using commercial kits or Core labs |
| GH | Limitations: Not standardized among different manufacturers; can be limited by sample volume |
| Subdomains . | Specific Markers . | Action Items/Future Developments . |
|---|---|---|
| Insulin action | Primary: | Strengths: Gold standard; highly informative |
| Hyperglycemic–euglycemic clamps | Limitations: Core lab availability; terminal | |
| Secondary: | Strengths: Simple, fast, safe; can be measured in most labs | |
| Fasting glucose | ||
| Fasting insulin | Limitations: Less informative of mechanism; anesthesia and handling stress can confound fasting measures; fasting length can impact GTT and ITT outcome | |
| ITT | ||
| GTT | ||
| Hyperlipidemia | TG, FFA, HDL, LDL, total cholesterol | Strength: Easy to assay |
| Limitation: Rodents not best model of cholesterol metabolism | ||
| Metabolomics | TCA cycle intermediates, amino acids, acyl carnitines, Redox, bile acids | Strength: Sophisticated and comprehensive |
| Limitations: Requires commercial analysis or Core lab availability; complex analysis of large data sets; can be cost prohibitive; may require terminal bleeding for mouse samples | ||
| Other endocrine | IGF-1, IGFBP-1, IGFBP-3, GH, adiponectin, leptin, cytokines | Strength: Can be measured using commercial kits or Core labs |
| GH | Limitations: Not standardized among different manufacturers; can be limited by sample volume |
Note: GTT = glucose tolerance test; ITT = insulin tolerance test; TG = triglycerides; FFA = free fatty acids; TCA = tricarboxylic acid.
Somewhat more invasive but highly informative metabolic status are insulin tolerance tests (ITTs) and glucose tolerance tests (GTTs), which involve administering insulin or glucose and monitoring glucose or insulin response over time ( 62 ). Although design considerations for ITTs and GTTs are not trivial, these studies have the distinct advantage of requiring minimal training and being minimally invasive, thus allowing for repeated measurements. Studies of metabolic status in young and old rodents using these approaches have noted age-related changes ( 64 ). It should be pointed out that performing these assays requires special attention to fasting (random fed, short-term fast, overnight fast). The duration of fasting prior to testing can impact response to a metabolic challenge ( 62 ).
Hyperinsulinemic–euglycemic clamps are the “gold standard” approach for assessing insulin sensitivity in humans and rodents and should be strongly considered when metabolic status is a primary endpoint. These studies allow for the evaluation of insulin action in the context of similar insulin and glucose levels and can incorporate the use of radio-labeled tracers to define insulin action at the tissue level and provide information regarding flux of metabolites ( 62 , 65 ). Indeed, clamps have been utilized to demonstrate declines in hepatic and insulin action in aging models ( 66 , 67 ). Their ability to characterize glucose fluxes can reveal specific defects in metabolism that are not captured by GTTs and ITTs ( 67 ) alone. Clamps in rodents and other models do present limitations however. The initial investment in acquiring the necessary equipment and reagents is not trivial and the procedure is highly technical, requiring experienced, trained personnel. Thus, utilizing specialized core facilities is highly recommended. It is also important to keep in mind that clamps in rodents are terminal procedures, so experiments evaluating health span must account for this important limitation.
Body composition and energetics
Changes in body composition occur throughout the human life course. These changes can occur without corresponding alterations in weight or body mass index ( 68 ). Generally, advancing age is associated with progressively increasing fat mass (at least into early old age) and reductions in lean body mass and bone mineral density ( 69 ). In older humans, fat mass is also frequently redistributed from subcutaneous to visceral depots, particularly to the abdomen ( 70 ). Adipocytes in different depots differ in their lipid storage and secretome, with increasing visceral fat being associated with age-related morbidity and mortality as well as diabetes, cancer, and longevity ( 71 ). The mechanisms responsible for age-related lipid redistribution remain unresolved and are likely multifactorial, but appear to be linked to the aging process ( 70 ). Therefore, the establishment of standard methodologies across laboratories to assess changes in body composition longitudinally in animal models is important. For a more detailed description of guidelines, pitfalls, and recommendations, the reader is referred to a very comprehensive report on methodologies to assess energetics and body composition in mice ( 72 ). Here, we will provide a brief overview of available options.
The simplest approach to monitor rodent energy balance across the life course is to monitor body weight and food intake. Rodent patterns of weight change over the lifetime depend on diet, sex, housing conditions, ambient temperature, and genotype. Not unusually, weight gain is rapid during development and slow and steady from early adulthood up to middle age (18–24 months old) without major fluctuations in food intake. It is not unusual for younger ad libitum-fed rodents to nearly double their body mass by 18 months of age—an observation mostly attributed to gains in adiposity (two- to threefold increase). However, similarly to older humans, older rodents lose weight, particularly fat stores, late in life. Food intake can also be monitored in tandem with body weight by measuring the disappearance of food provided over an interval of time. It is important to not rely solely on food disappearance from the hopper, but to take into account food spilled into the bedding. Intake is most reliably determined when animals are singly housed, which may be a challenge for many longitudinal studies in which mice are group housed. Group versus single housing in itself affects food intake, as the thermal environment of a cage is altered by the number of other rodents sharing a living space.
Although body weight and food intake can be informative, they reveal little about body composition or energy expenditure. A number of more sophisticated energetics phenotyping approaches are available ( Table 6 ). Many institutional metabolic/energetic cores are capable of noninvasively assessing body composition in rodents with techniques comparable with human methods, including dual-energy x-ray absorptiometry (DXA), quantitative computed tomography (CT), isotope dilution, electrical conductance, and MRI ( 73 ). In rodents, the gold standard method of assessing body composition is directly by whole-body chemical carcass analysis ( 74 , 75 ), but this is a terminal procedure that is not feasible for many studies. However, more than 15 years ago, specific noninvasive approaches, such as DXA, were adapted and validated against chemical carcass analysis to measure fat mass, lean mass, and bone in rodents ( 76 ). DXA can provide quick (~5 minutes), reliable estimates of body composition, although this technology cannot determine body fat distribution, and the need for anesthesia is a drawback, especially for repeated measurements. The advent of quantitative magnetic resonance (Echo MRI) has dramatically improved the ability to quickly, accurately, and safely assess body composition in rodents without sedation, making it ideal for longitudinal studies ( 75–77 ). Although these instruments require a substantial initial investment, the scans themselves are inexpensive and simple to perform. It is important to note that quantitative magnetic resonance does not provide estimates of bone or body fat distribution, so DXA and CT/MRI may still be needed for these readouts. Alternatively, individual fat pads (and other organs/tissues) can also be dissected and weighed at necropsy by the investigator.
Preclinical Tests for Body Composition and Energetics in Aging Mice
| Subdomains . | Specific Assay . | Considerations . |
|---|---|---|
| Energy balance | Body weight and food intake | Strengths: Simple, safe, and informative |
| Limitations: Provides only general information; impacted by group housing | ||
| Metabolic cages | Strengths: Provide unique insights to energy balance; standard technique | |
| Limitations: Core lab availability; requires expertise in planning and interpreting data | ||
| Body composition | Chemical carcass analysis | Strength: Gold standard technique |
| Limitations: Requires specialized lab; terminal procedure | ||
| Quantitative magnetic resonance | Strengths: Fast, reliable, and does not require anesthesia | |
| Limitations: Requires equipment/Core lab availability; cannot determine fat distribution or bone | ||
| Dual-energy x-ray absorptiometry | Strength: Simultaneously determines fat, lean, and bone | |
| Limitations: Equipment/Core lab availability; requires anesthesia | ||
| Computed tomography and MRI | Strength: Noninvasively measure fat distribution | |
| Limitations: Core lab availability and related expertise; requires anesthesia |
| Subdomains . | Specific Assay . | Considerations . |
|---|---|---|
| Energy balance | Body weight and food intake | Strengths: Simple, safe, and informative |
| Limitations: Provides only general information; impacted by group housing | ||
| Metabolic cages | Strengths: Provide unique insights to energy balance; standard technique | |
| Limitations: Core lab availability; requires expertise in planning and interpreting data | ||
| Body composition | Chemical carcass analysis | Strength: Gold standard technique |
| Limitations: Requires specialized lab; terminal procedure | ||
| Quantitative magnetic resonance | Strengths: Fast, reliable, and does not require anesthesia | |
| Limitations: Requires equipment/Core lab availability; cannot determine fat distribution or bone | ||
| Dual-energy x-ray absorptiometry | Strength: Simultaneously determines fat, lean, and bone | |
| Limitations: Equipment/Core lab availability; requires anesthesia | ||
| Computed tomography and MRI | Strength: Noninvasively measure fat distribution | |
| Limitations: Core lab availability and related expertise; requires anesthesia |
Preclinical Tests for Body Composition and Energetics in Aging Mice
| Subdomains . | Specific Assay . | Considerations . |
|---|---|---|
| Energy balance | Body weight and food intake | Strengths: Simple, safe, and informative |
| Limitations: Provides only general information; impacted by group housing | ||
| Metabolic cages | Strengths: Provide unique insights to energy balance; standard technique | |
| Limitations: Core lab availability; requires expertise in planning and interpreting data | ||
| Body composition | Chemical carcass analysis | Strength: Gold standard technique |
| Limitations: Requires specialized lab; terminal procedure | ||
| Quantitative magnetic resonance | Strengths: Fast, reliable, and does not require anesthesia | |
| Limitations: Requires equipment/Core lab availability; cannot determine fat distribution or bone | ||
| Dual-energy x-ray absorptiometry | Strength: Simultaneously determines fat, lean, and bone | |
| Limitations: Equipment/Core lab availability; requires anesthesia | ||
| Computed tomography and MRI | Strength: Noninvasively measure fat distribution | |
| Limitations: Core lab availability and related expertise; requires anesthesia |
| Subdomains . | Specific Assay . | Considerations . |
|---|---|---|
| Energy balance | Body weight and food intake | Strengths: Simple, safe, and informative |
| Limitations: Provides only general information; impacted by group housing | ||
| Metabolic cages | Strengths: Provide unique insights to energy balance; standard technique | |
| Limitations: Core lab availability; requires expertise in planning and interpreting data | ||
| Body composition | Chemical carcass analysis | Strength: Gold standard technique |
| Limitations: Requires specialized lab; terminal procedure | ||
| Quantitative magnetic resonance | Strengths: Fast, reliable, and does not require anesthesia | |
| Limitations: Requires equipment/Core lab availability; cannot determine fat distribution or bone | ||
| Dual-energy x-ray absorptiometry | Strength: Simultaneously determines fat, lean, and bone | |
| Limitations: Equipment/Core lab availability; requires anesthesia | ||
| Computed tomography and MRI | Strength: Noninvasively measure fat distribution | |
| Limitations: Core lab availability and related expertise; requires anesthesia |
Finally, most energetics cores have rodent metabolic cage systems, such as the Oxymax (CLAMS), for determining energy expenditure, substrate utilization, 24-hour locomotor activity, and feeding behavior. There are many considerations for appropriately conducting and interpreting these studies, but data can be particularly informative about age-related phenotypes and/or complementary to observations of physical activity and glucose and energy homeostasis.
Although the aforementioned areas of preclinical assessment were generally agreed upon as domains of particularly high priority for developing a standardized set of outcomes to characterize health span in clinically relevant models, additional functional areas of importance were recognized. Continued development of a unified “heath-span index,” which incorporates “clinical” and functional assessments that are generally accessible and require minimal training, is viewed as a critical next step toward standardizing the evaluation of rodent frailty (see Frailty) and health status in order to guide translational efforts.
Frailty
Frailty has been conceptualized by geriatricians as “a state of increased vulnerability to stressors due to age-related decline in physiologic reserve across neuromuscular, metabolic, and immune systems” ( 78 ). The concept was developed to attempt to account for variation in peoples’ ability to recover from adverse events. That is, people in superficially similar health can vary dramatically in their ability to recover from surgery, a fall, or a stroke. Without question frailty involves the integrated deterioration of multiple physical systems, such as those discussed earlier. Several assessment panels have been developed to screen for frailty in older people ( 79 , 80 ).
Because of the interest in developing standardized frailty criteria for humans, it would be beneficial to do so for mice as well. This has become a very active area of research with a multitude proposed mouse frailty indices now available ( 58 , 81–83 ). These frailty indices incorporate a number of the health assessment techniques described earlier. A major goal of future research in this area should be a comparative validation across mouse genotypes and sexes. A key aspect of that validation will be evaluating the relation between these frailty indices and resilience to stressors such as mentioned below.
Guidelines for the Implementation of Preclinical Health Assessments
We do not feel that we should recommend specific assays at this point in the development of mouse health assessments. New and better tests are continually being developed. However, we will mention a few general conclusions from the previous discussion. First, health assessments that can be performed longitudinally will always be preferred, everything else being equal. This criterion limits both the amount of physical and cognitive invasiveness that can be imposed during any test. Most tests of neuromuscular function and a number of metabolic and body composition measures fulfill this criterion. For cognitive tests, the major challenge is in limiting training effects. Almost all cardiovascular health assessments are somewhat invasive. Second, evaluations that can be standardized across laboratories should receive particular attention. However, to achieve such standardization is likely to require considerable effort on the part of the participating laboratories. In particular, behavioral tests appear to be difficult to replicate across laboratories, even when considerable efforts are expended to do so ( 84 ). Finally, it would be useful for the field if a standardized and detailed procedural manual or series of training videos for administering these tests were widely available and widely used by researchers in aging biology.
Evaluating Resiliency in Animal Models of Retarded Aging
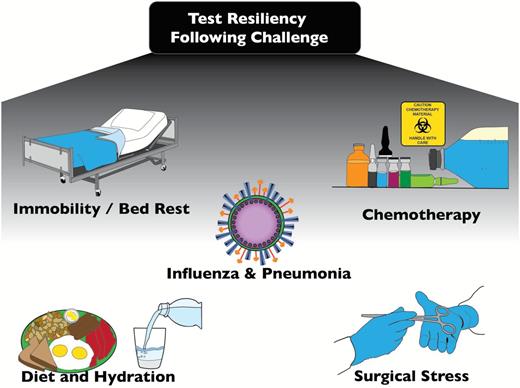
Assays described so far are variably informative about general animal health. As a crude rule of thumb, assays that provide a challenge are more informative than those that rely on baseline measures. For instance, cardiac function is more incisively evaluated with a treadmill test than a resting electrocardiogram. By similar logic, details of generalized health may be more clearly evaluated as responses to generalized challenges. Our working group prioritized the need to expand and develop preclinical studies that address the role of aging and interventions on resiliency against a number of stressors relevant to those encountered by humans ( Figure 2 ). We now propose paradigms that can be implemented in laboratory rodent models, in a way that mimics “real-life” challenges faced by older humans.
Possible assays of resilience for putative senescence-retarding interventions.
Attention should be paid to the appropriate age at which to perform the resilience assays described in the following sections. There is always an urgency to perform tests as early in life as possible, but as these assays are proposed to be informative about the rate of declining resilience, they need to be performed across a range of ages after which the impact of aging can be seen. That is, these resilience assays are not being proposed to obviate the necessity for using older animals. Because these assays are more invasive than most of the health assays mentioned earlier, longitudinal studies are likely not possible. Thus cross-sectional studies will be necessary. Part of developing the following paradigms, which were developed largely to look at the impact of diseases induced at early ages, will be determining the range of ages over which these tests can be most informatively done.
Forced immobility
Bedrest in elderly people leads to greater muscle wasting and slower recovery than in younger people ( 85–87 ). Muscle weakness is an excellent predictor of mortality in older adults. Thus the implementation of experimental paradigms aimed at inducing sarcopenia, and tracking recovery from it, in laboratory rodents is desirable.
Hindlimb unloading (HU) ( 88 ) and partial weight suspension (PWS) ( 89 ) are the most commonly implemented techniques to induce skeletal muscle disuse atrophy in laboratory rodents. As implied by their names, HU and PWS differ in the amount of weight-bearing activity and which limbs are receiving the load. HU consists of complete hindlimb suspension leading to a full ablation of hindlimb use, thus significant atrophy isolated almost exclusively to these limbs. The PWS employs a vest that suspends the entire animal slightly above the base of their cage, thereby reducing weight-bearing activity across all four limbs but to a lesser degree than that of HU. Both HU and PWS elicit declines in skeletal muscle mass and bone mineral density in as little as 20 days ( 90 ). The former appears to be a better model for complete disuse as observed with bedrest, whereas the latter seems more applicable for age-related limitations in mobility. As typically employed, these paradigms do not necessarily include tracking recovery, but for our purposes, that would be the critical metric. The implementation of such techniques requires special technical expertise and equipment relevant to each study design and desired primary outcomes, thus collaboration with experienced laboratories is suggested.
Infectious diseases
Infectious diseases, particularly influenza and pneumonia, are the 8 th leading cause of death in the United States and the 5 th ranking cause for those aged 65 years or older ( 28 ). Regardless of mortality outcomes, infection also has deleterious effects on morbidity in older adults by exacerbating underlying diseases and fostering increased rapidity of functional declines. Increased incidence of infection in older adults is multifactorial and results not only from declining immune function ( 46 ) but also from perturbations in barriers of the skin, lungs, and gastrointestinal tract ( 91 ). Dosing and exposure paradigms exist for influenza ( 92 ) and pneumonia ( 93 ) exposure in aged mice. Thus, this model can be informative of how purported “age-delaying” strategies impact resiliency, which otherwise could not be gleaned from a protected environment.
Chemotherapy
Cancer chemotherapy is frequently associated with phenotypic changes that could be interpreted as accelerated aging ( 94–96 ). These include accelerated neurocognitive decline, increased CVD, reduced cardiovascular function and stamina, sarcopenia and osteoporosis, reduced hearing and vision, and frailty ( 97 , 98 ). Similarly, recent reports indicate that chemotherapy regimens in rodents can elicit declines in cognition ( 99 ), cardiovascular function ( 100 ), and muscle mass ( 101 ) as well. However, experiments eliciting these effects are most often performed in young animals, which are not likely to represent the biological and physiological sequelae of chronological aging. Our group strongly supports the use of aged animals for tests of resiliency to chemotherapeutic treatment in order to recapitulate more closely the reduced functional reserve and increased vulnerability to age-related comorbidities commonly observed in older human cancer patients ( 102 ). Moreover, we feel this paradigm will be informative for assessing the phenotypic impact of interventions that target fundamental aging mechanisms.
Dietary challenges
Older adults are commonly subject to either over- or undernutrition. Overnutrition—that is, obesity—has numerous well-known negative health sequelae, but under- or mal-nutrition does as well, including immune suppression, sarcopenia, retarded wound healing, and cognitive dysfunction ( 103 , 104 ). Evaluating interventions that target fundamental aging mechanisms in the context of obesity and malnutrition may be particularly relevant to human aging. Similarly, dehydration in older adults is associated with numerous negative adverse outcomes including falls, fractures, poor wound healing, heat stress, infections, renal failure, stroke, myocardial infarction, and disability ( 105–107 ). Thus, an experimental paradigm of dehydration stress also seems worth developing for rodent studies to evaluate resilience associated with this clinically relevant problem.
Surgical stress
The global volume of major surgery has been estimated to be between 187 and 281 million annually, and major complications accompany 3–16% of inpatient operations in developed countries ( 108 , 109 ). Therefore, recovery from surgical stress is an especially relevant model that could be modeled in rodents using interventions that target fundamental aging processes. Such paradigms have already been developed ( 110 ). Of additional interest is differentiating the perioperative effects of actual surgery as opposed to anesthesia ( 111 ).
Additional considerations
In addition to the areas described earlier, several other approaches for testing resiliency associated with interventions that target fundamental aging processes were discussed. These included toxin challenges (paraquat, diaquat, lipopolysaccharide), recovery from psychological stress (eg, social isolation), bone fracture, heat or cold stress, and wound healing. Although some of these approaches can be employed following published protocols, other assays need refinement or further development. It is not clear, for instance, whether strategies shown to benefit health span and life span, such as rapamycin, positively or negatively impact resiliency against stressors, such as infection or surgical stress. Indeed, fasting has already been shown to improve chemotherapeutic outcomes ( 112 ), whereas exercise and resveratrol have been shown to confer cardioprotective effects during chemotherapy ( 113 ). However, outcomes from these interventions were all obtained in young animals, and no interventions other than dietary restriction have been evaluated for resiliency against a range of stressors, further highlighting the need for geroscience researchers to advocate for testing resiliency paradigms in aged models. Resilience is considered in depth in the accompanying article in this issue.
Challenges in Modeling Human Age-Related Chronic Diseases
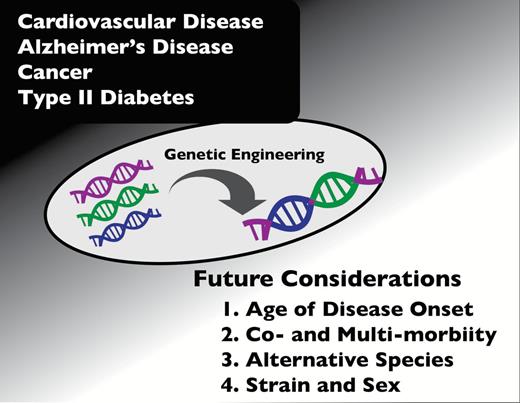
Many major human age-related diseases either do not arise spontaneously in laboratory animals or arise only rarely. In the past several decades, as laboratory mice have become genetically tractable, more and more genetic mouse models of specific human diseases have been developed. Many models now exist for the most common forms of cancer, T2DM, AD, stroke, cardiovascular complications, and a host of other human maladies. However, in virtually all of these models, the desired pathology arises—by design—at a young age, rather than later in life ( Figure 3 ). Although these models may provide convenient and cost-effective research paradigms, it is important to recognize that this can be an inherent limitation in modeling diseases of human aging. Age-related diseases in young animals may differ in many ways from the same disease in aged animals. In fact, the failure to consider age of onset may account for some of the many failures in translating therapeutic success in mice to humans. This translational failure is so common—more than 300 failed translations in AD therapy alone—that each disease group seems to have its own explanation for the failure ( 114–117 ). Those explanations seldom if ever include the issue of aging however. Given the ease with which inducible mouse models can be generated, our group strongly suggests developing more disease models with late age of onset.
Future considerations for modeling chronic, age-related diseases.
A second challenge in modeling chronic age-related diseases in mice is that these diseases rarely occur in isolation. In fact, the presence of one disease often increases the risk of others. For instance, T2DM has been linked to increased risk for CVD, cancers, and dementia ( 118–120 ). Thus, the need for developing preclinical models of multimorbidity, and methods for assessing the interaction among these conditions, is considered an area of high priority. Furthermore, defining multimorbidities in a range of models, including mice, is needed. Advances in this area could be accomplished using existing longitudinal data sets where phenotypic and pathologic data were rigorously collected across ages, to determine the temporal emergence of comorbidities with age and the interrelationships among conditions. Studies in humans have confirmed that a cluster of multimorbidities arise at an accelerated, nonlinear rate at older ages. Animal models could be developed to help define not only associations but causality among these chronic conditions, including mechanisms linking advancing age with the emergence of multimorbidity.
Another consideration suggested by our working group is the creation of progeroid mice harboring age-related diseases as an alternative and innovative approach. Furthermore, although this discussion has focused largely on the mouse for preclinical research, it should also be clear that while generally viewed the most practical option, mice are not necessarily the best model. Other species should also be considered when appropriate. Indeed, dogs remain a superior model for osteoarthritis, rats more closely resemble human glucose metabolism and executive function than mice, baboons spontaneously develop T2DM, and pigs and primates closely share many other manifestations of metabolic syndrome with humans, which are not observed in mouse models. Furthermore, genetic background, as was shown with caloric restriction ( 121 ), can impact the interpretation of mouse data, as can sex differences ( 122 , 123 ). Given this inherent variability among models, validation is imperative and inclusion of a second strain or species should be considered when possible.
Summary
The focus of this retreat as part of the Geroscience Network was to address three broad topics related to preclinical models, including (i) What are the generally agreed upon health-span domains of highest priority, and what are the recommended standardized protocols and minimal data sets, if any, that are accessible among laboratories?; (ii) How can preclinical models be used to test resiliency from stressors of human relevance?; and (iii) What are the challenges of modeling age-related diseases in animals? The discussion of these topics primarily focused on the mouse, due to advantages of practicality and availability of genetically engineered models to address mechanism(s). From a functional perspective, highest priority was given to tests evaluating domains relevant to physical performance and neuromuscular, body composition, cognitive and behavior, cardiovascular, immunological, and metabolic function. We were able to identify several minimally invasive tests, some of which do not require significant training or highly specialized equipment to perform in individual laboratories, whereas other more specialized tests, ranging from minimally invasive to terminal procedures, will likely require assistance from a qualified collaborator or core service. Furthermore, the need to incorporate studies of resiliency was strongly recommended and believed to be informative in ways that cannot be inferred from unprovoked conditions. A particular emphasis was placed on modeling “real-life” challenges commonly encountered by aging humans, including chemotherapy, infection, immobility, overfeeding, and surgical trauma. Finally, several challenges to research on age-related diseases in animals were discussed. Namely, aging is the major underlying risk factor for most comorbidities, but many genetically engineered lines give rise to disease in younger animals, rather than in the context of aging, and thus cannot account for how aging may alter the trajectory of disease pathogenesis or treatment responses. Although appropriate and effective use of preclinical models for aging research is not without its challenges, there is no doubt that this platform is a vital component for continued discovery, and continued development, refinement, and standardization will undoubtedly benefit the progress of translational geroscience.
Funding
This work was supported by the National Institutes of Health grant R24 AG044396 (J.L.K., S.N.A., and N.B.).
Acknowledgments
The authors are grateful for the contributions of the participants in the Geroscience Network retreat at the Four Seasons in Santa Barbara on June 5, 2014. Attendees: Steven Austad, Nir Barzilai, Pinchas Cohen, Ricki Colman, Rafael de Cabo, Mindy Fain, Derek Huffman, Jamie Justice, Brian Kennedy, James Kirkland, George Kuchel, Nathan LeBrasseur, Stephanie Lederman, Bruce Miller, Arlan Richardson, Doug Seals, Felipe Sierra, Stephen Spindler, Michael Stout, Randy Strong, and Jeremy Walston. The authors are also grateful to Ben Ziemer, Alaine Westra, and Jacqueline Armstrong who acted as facilitators and organizers for the retreat and Linda Wadum for administrative assistance.
References
Author notes
Decision Editor: Rafael de Cabo, PhD