-
PDF
- Split View
-
Views
-
Cite
Cite
R. W. H. Verwer, A. A. Sluiter, R. A. Balesar, J. C. Baayen, D. P. Noske, C. M. F. Dirven, J. Wouda, A. M. van Dam, P. J. Lucassen, D. F. Swaab, Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin, Brain, Volume 130, Issue 12, December 2007, Pages 3321–3335, https://doi.org/10.1093/brain/awm264
Close - Share Icon Share
Abstract
Doublecortin (DCX) is a microtubule-associated protein expressed by migrating neuroblasts and is considered to be a reliable marker of neurogenesis. DCX has been used to study the relation between neurogenesis in adult human brain and neurological and neurodegenerative disease processes in the search for putative therapeutic strategies. Using autopsy and surgically resected tissue from a total of 60 patients, we present evidence that DCX is present in several cellular compartments of differentiated astrocytes in the adult human neocortex. One of these compartments consisted of peripheral processes forming punctate envelopes around mature neuronal cell bodies. Markers of glial activation, such as GFAP and HLA, were not associated with DCX immunoreactivity, however, the presence of cytoarchitectural alterations tended to correlate with reduced DCX staining of astrocytic somata. Interestingly, local Alzheimer pathology that showed no relation with cytoarchitectural abnormalities appeared to correlate negatively with the expression of DCX in the astrocytic somata. In combination with the literature our data support the view that DCX in the adult human neocortex may have a function in glia-to-neuron communication. Furthermore, our results indicate that in the adult human neocortex DCX is neither a reliable nor a selective marker of neurogenesis.
Introduction
Doublecortin is a microtubule-associated protein that is expressed during a limited phase in the development of neuroblasts in both developing and adult mammals (Francis et al., 1999; Gleeson et al., 1999; Brown et al., 2003). As such it is frequently used as a selective marker of migrating neuroblasts and as a reliable indicator of neurogenesis (Arvidsson et al., 2002; Brown et al., 2003; Rao and Shetty, 2004; Couillard-Despres et al., 2005; Darsalia et al., 2005). In adulthood persistent neurogenesis is found in restricted areas along the lateral ventricles (subventricular zone, SVZ) and in the subgranular zone (SGZ) of the dentate gyrus that constitute the adult pools of neural progenitor cells (Lois and Alvarez-Buylla, 1993; Kuhn et al., 1996; Eriksson et al., 1998, Kukekov et al., 1999). It is generally believed that the presence of these adult neural precursor cells holds a promise for therapeutic strategies to reverse neurodegenerative processes.
Evidence from animal models indicates that injury to the brain induces extra production of neural progenitor cells in the discrete zones of neurogenesis (Gould and Tanapat, 1997; Darsalia et al., 2005). Moreover, several studies have reported that the newly generated cells may venture outside these germinal zones. Local-directed migration of DCX+/BrdU+ cells towards the zone of hypoxic cell death in the CA1 area of the adult rat has been observed (Zhou et al., 2004). Epileptic seizures enhance the proliferation of neuroblasts in the adult rat SVZ, which subsequently also migrate into the cortex (Parent, 2002). In spreading depression new neurons arrive in the striatum and injured cortex, but only express DCX while they are still in the SVZ (Yanamoto et al., 2005). In a mechanical lesion study it was shown that DCX+ neuroblasts migrate through the striatum towards, but do not enter the injured cortical areas (Sundholm-Peters et al., 2005). In contrast, endogenous replacement of neurons in adult rodent neocortical areas after ischaemic damage or targeted lesions has been reported by other investigators (Magavi et al., 2000; Jiang et al., 2001; Nakatomi et al., 2002; Jin et al., 2003). Similarly, stroke in the striatum induces extra genesis and migration of DCX+ neuroblasts towards the site of injury where they develop into mature neurons (Arvidsson et al., 2002).
Increased neurogenesis may also occur in response to neurodegenerative disease processes in the adult human brain. In Huntington's disease (Curtis et al., 2003) and Alzheimer's disease (Jin et al., 2004) enhanced neurogenesis has been observed in the subependymal layer and the dentate gyrus, respectively. However, a recent study proposed that the enhanced proliferation of cells in the hippocampus of Alzheimer patients mainly concerns glial and endothelial cells rather than neurons (Boekhoorn et al., 2006). Also, in mesial temporal lobe epilepsy enhanced presence of musashi-1 positive neural progenitors in the dentate gyrus has been reported, but no cells co-expressing DCX or other more mature neuronal cell markers were observed (Crespel et al., 2005). These and more recent observations suggest that there is no extra neurogenesis in epilepsy patients (Crespel et al., 2005; Fahrner et al., 2007).
Isolation of neural stem or precursor cells from adult human neocortical areas at long distances from the established germinal zones (Arsenijevic et al., 2001; Palmer et al., 2001) show that these areas contain precursor cells or mature cells that may transform into such phenotype (Leavitt et al., 1999; Steindler and Laywell, 2003). However, it has been argued that in vivo the adult human neocortex does not allow any such precursors to really differentiate into neurons and integrate into existing functional neuronal networks (Rakic, 2004). Recently, Sanai et al. (2004) explicitly reported that they were unable to culture stem cells from tissue pieces that did not include the subventricular zone. Given these contrasting observations it is important to establish whether cells expressing markers of early developing neurons can be detected in the adult human neocortex and what their properties are.
The aim of this report is to describe the expression of the early neuronal marker doublecortin in adult neocortex of control subjects and patients with various neurological or neurodegenerative diseases. While we did not unambiguously detect DCX+ cells with an undifferentiated morphology, variable amounts of differentiated DCX+ astrocytes and neurons were observed in both controls and diseased patients. In this respect it is also interesting that a few other studies have reported that the endopiriform nucleus and layer II of the piriform cortex of normal adult rats contain differentiated DCX+ neurons (Nacher et al., 2001; Jin et al., 2003). However, in contrast with these studies, the adult human neocortical neurons did not show cytoplasmic DCX expression. This morphological difference might relate to disease processes pertinent to human brain material. Therefore, we tried to correlate the DCX immunoreactivity with markers for glial activation and Alzheimer pathology. It appeared that the staining of neocortical astrocytic somata was negatively correlated with the presence of local Alzheimer pathology. Our observations emphasize the importance of careful interpretation when the expression of neural precursor markers is studied in relation with human neurological or neurodegenerative disorders.
Materials and Methods
This report is based on adult human brain tissue obtained with informed consent at rapid autopsies by the Netherlands Brain Bank or by epilepsy surgery (Department of Neurosurgery, VU University Medical Center, Amsterdam). Permission for the use of the brain tissue for experimental purposes was granted by the Ethical Committee of the VU University Medical Center where both autopsy and surgery took place.
Table 1 summarizes the autopsy material that consisted of neocortex (mainly motor cortex, but also visual and/or prefrontal cortex) from 31 deceased human subjects [14 Alzheimer patients (AD), 7 control (C), 10 other (1 Lewy body disease (LBD) patient, 3 non-Alzheimer demented (NAD) patients, 2 Parkinson patients (PD), 3 Pick's disease (PID) patients, 1 patient with progressive supranuclear palsy (PSP)]. Resected tissue (Table 2) consisted of normal epileptogenic cortex from 29 patients, unresponsive to drug therapy, and was obtained in the frame-work of temporal resections. All tissue specimens were obtained in the frame-work of our adult human brain tissue culture project (cf. Verwer et al., 2002, 2003) but consisted of reference material that was fixed before culture experiments were started.
Clinico-pathological data of autopsy brain tissue
| Patient . | Diseasea . | Ageb (years) . | Sex . | Brain Weightc (g) . | PHd . | PMDe (h) . | Braak stagef . | Cause of death . | Areasg . |
|---|---|---|---|---|---|---|---|---|---|
| AD00-126 | AD | 85 | m | 1020 | 6.1 | 7 | V | Dehydration, cachexia | mot |
| PID03-010 | PID | 72 | f | 929 | 6.5 | 6 | I | Pulmonary embolism | mot |
| AD03-090 | AD | 87 | f | 1007 | 6.5 | 4.5 | V | Mammacarcinoma, infected decubitus, arterial insufficiency | mot |
| AD04-001 | AD | 57 | m | 1092 | 6.5 | 4 | VI | Aspiration pneumonia, toxic hepatitis | mot |
| AD04-011 | AD | 84 | f | 1217 | 6.4 | 4.5 | V | Cachexia | mot, vis |
| NAD04-024 | NAD | 90 | f | 1151 | 6.1 | 5 | III | Heart failure, sepsis by wound infection | mot, vis |
| AD04-025 | AD | 90 | f | 934 | 6.7 | 5.5 | IV | Cachexia, severe decompensatio cordis | mot |
| AD04-029 | AD | 78 | f | 1105 | 6.2 | 3.5 | V | Gastrointestinal bleeding | vis |
| C04-049 | C | 77 | f | 1312 | 6.5 | 6.5 | I | Cachexia, uremia | pfc |
| C04-061 | C | 88 | f | 1168 | 7 | 4 | NA | Old age | mot |
| PD04-067 | PD | 68 | m | 1236 | 6.7 | 4.5 | IV | Dehydration, cachexia | mot, pfc |
| PID04-072 | PID | 68 | f | 752 | 6.5 | 4.5 | NA | Dehydration, cachexia, decubitus with severe dementia | mot |
| C04-074 | C | 55 | f | 1321 | 6.4 | 4.5 | 0 | Small cell lung cancer | mot |
| LBD04-078 | LBD | 66 | m | 1166 | 7 | 5.5 | 0 | Shock, respiratory failure | mot |
| NAD05-003 | NAD | 84 | f | 1109 | 6.8 | 6.5 | V | Dehydration, cachexia | mot |
| AD05-005 | AD | 94 | f | 1041 | 7.3 | 3.5 | V | Cardiovascular accident | mot |
| PSP05-008 | PSP | 67 | m | 1456 | 6.4 | 7.5 | I | Unknown | mot |
| AD05-010 | AD | 93 | m | 1210 | 6.5 | 5 | V | Unknown | mot |
| AD05-011 | AD | 93 | f | 1045 | 6.5 | 2 | IV | Cachexia | mot |
| C05-014 | C | 86 | f | 1127 | 7.1 | 5.5 | III | Heart attack | mot |
| AD05-021 | AD | 89 | f | 1064 | 6.2 | 9 | V | Unknown | mot |
| AD05-026 | AD | 84 | f | 1098 | 6.3 | 4 | V | Dehydration | mot |
| C05-032 | C | 74 | m | 1522 | 6.4 | 4.5 | 0 | Lung carcinoma | mot |
| AD05-040 | AD | 69 | m | 1170 | 6.5 | 4.5 | VI | Pneumonia | mot |
| C05-044 | C | 80 | m | 1376 | 5.8 | 5 | 0 | Cachexia, dehydration | mot |
| C05-063 | C | 93 | f | 1316 | 6.6 | 2 | II | Heart failure | mot |
| PD06-026 | PD | 70 | m | 1451 | 6.5 | 6 | I | Respiratory insufficiency | mot |
| NAD06-039 | NAD | 86 | f | 1359 | 6.5 | 2.5 | 0 | Old age | mot |
| AD06-044 | AD | 86 | f | 950 | 6.9 | 5 | IV | Cachexia, dementia | mot |
| AD06-070 | AD | 86 | f | 951 | 6.7 | 3 | NA | Cachexia, serious heart problems | mot |
| PID07-004 | PID | 66 | m | 1245 | 6.2 | 3.5 | NA | Pneumonia | mot |
| Patient . | Diseasea . | Ageb (years) . | Sex . | Brain Weightc (g) . | PHd . | PMDe (h) . | Braak stagef . | Cause of death . | Areasg . |
|---|---|---|---|---|---|---|---|---|---|
| AD00-126 | AD | 85 | m | 1020 | 6.1 | 7 | V | Dehydration, cachexia | mot |
| PID03-010 | PID | 72 | f | 929 | 6.5 | 6 | I | Pulmonary embolism | mot |
| AD03-090 | AD | 87 | f | 1007 | 6.5 | 4.5 | V | Mammacarcinoma, infected decubitus, arterial insufficiency | mot |
| AD04-001 | AD | 57 | m | 1092 | 6.5 | 4 | VI | Aspiration pneumonia, toxic hepatitis | mot |
| AD04-011 | AD | 84 | f | 1217 | 6.4 | 4.5 | V | Cachexia | mot, vis |
| NAD04-024 | NAD | 90 | f | 1151 | 6.1 | 5 | III | Heart failure, sepsis by wound infection | mot, vis |
| AD04-025 | AD | 90 | f | 934 | 6.7 | 5.5 | IV | Cachexia, severe decompensatio cordis | mot |
| AD04-029 | AD | 78 | f | 1105 | 6.2 | 3.5 | V | Gastrointestinal bleeding | vis |
| C04-049 | C | 77 | f | 1312 | 6.5 | 6.5 | I | Cachexia, uremia | pfc |
| C04-061 | C | 88 | f | 1168 | 7 | 4 | NA | Old age | mot |
| PD04-067 | PD | 68 | m | 1236 | 6.7 | 4.5 | IV | Dehydration, cachexia | mot, pfc |
| PID04-072 | PID | 68 | f | 752 | 6.5 | 4.5 | NA | Dehydration, cachexia, decubitus with severe dementia | mot |
| C04-074 | C | 55 | f | 1321 | 6.4 | 4.5 | 0 | Small cell lung cancer | mot |
| LBD04-078 | LBD | 66 | m | 1166 | 7 | 5.5 | 0 | Shock, respiratory failure | mot |
| NAD05-003 | NAD | 84 | f | 1109 | 6.8 | 6.5 | V | Dehydration, cachexia | mot |
| AD05-005 | AD | 94 | f | 1041 | 7.3 | 3.5 | V | Cardiovascular accident | mot |
| PSP05-008 | PSP | 67 | m | 1456 | 6.4 | 7.5 | I | Unknown | mot |
| AD05-010 | AD | 93 | m | 1210 | 6.5 | 5 | V | Unknown | mot |
| AD05-011 | AD | 93 | f | 1045 | 6.5 | 2 | IV | Cachexia | mot |
| C05-014 | C | 86 | f | 1127 | 7.1 | 5.5 | III | Heart attack | mot |
| AD05-021 | AD | 89 | f | 1064 | 6.2 | 9 | V | Unknown | mot |
| AD05-026 | AD | 84 | f | 1098 | 6.3 | 4 | V | Dehydration | mot |
| C05-032 | C | 74 | m | 1522 | 6.4 | 4.5 | 0 | Lung carcinoma | mot |
| AD05-040 | AD | 69 | m | 1170 | 6.5 | 4.5 | VI | Pneumonia | mot |
| C05-044 | C | 80 | m | 1376 | 5.8 | 5 | 0 | Cachexia, dehydration | mot |
| C05-063 | C | 93 | f | 1316 | 6.6 | 2 | II | Heart failure | mot |
| PD06-026 | PD | 70 | m | 1451 | 6.5 | 6 | I | Respiratory insufficiency | mot |
| NAD06-039 | NAD | 86 | f | 1359 | 6.5 | 2.5 | 0 | Old age | mot |
| AD06-044 | AD | 86 | f | 950 | 6.9 | 5 | IV | Cachexia, dementia | mot |
| AD06-070 | AD | 86 | f | 951 | 6.7 | 3 | NA | Cachexia, serious heart problems | mot |
| PID07-004 | PID | 66 | m | 1245 | 6.2 | 3.5 | NA | Pneumonia | mot |
aAD = Alzheimer's disease, C = control, LDB = Lewy body disease, NAD = non-Alzheimer dementia, PD = Parkinson's disease, PID = Pick's disease, PSP = progressive supranuclear palsy. bAge at death (years). cBrain weight (g). dpH of the cerebrospinal fluid. ePMD = Postmortem delay (h). fCf Braak and Braak (1991), NA = not available. gmot = motor cortex, pfc = prefrontal cortex, vis = visual cortex.
Clinico-pathological data of autopsy brain tissue
| Patient . | Diseasea . | Ageb (years) . | Sex . | Brain Weightc (g) . | PHd . | PMDe (h) . | Braak stagef . | Cause of death . | Areasg . |
|---|---|---|---|---|---|---|---|---|---|
| AD00-126 | AD | 85 | m | 1020 | 6.1 | 7 | V | Dehydration, cachexia | mot |
| PID03-010 | PID | 72 | f | 929 | 6.5 | 6 | I | Pulmonary embolism | mot |
| AD03-090 | AD | 87 | f | 1007 | 6.5 | 4.5 | V | Mammacarcinoma, infected decubitus, arterial insufficiency | mot |
| AD04-001 | AD | 57 | m | 1092 | 6.5 | 4 | VI | Aspiration pneumonia, toxic hepatitis | mot |
| AD04-011 | AD | 84 | f | 1217 | 6.4 | 4.5 | V | Cachexia | mot, vis |
| NAD04-024 | NAD | 90 | f | 1151 | 6.1 | 5 | III | Heart failure, sepsis by wound infection | mot, vis |
| AD04-025 | AD | 90 | f | 934 | 6.7 | 5.5 | IV | Cachexia, severe decompensatio cordis | mot |
| AD04-029 | AD | 78 | f | 1105 | 6.2 | 3.5 | V | Gastrointestinal bleeding | vis |
| C04-049 | C | 77 | f | 1312 | 6.5 | 6.5 | I | Cachexia, uremia | pfc |
| C04-061 | C | 88 | f | 1168 | 7 | 4 | NA | Old age | mot |
| PD04-067 | PD | 68 | m | 1236 | 6.7 | 4.5 | IV | Dehydration, cachexia | mot, pfc |
| PID04-072 | PID | 68 | f | 752 | 6.5 | 4.5 | NA | Dehydration, cachexia, decubitus with severe dementia | mot |
| C04-074 | C | 55 | f | 1321 | 6.4 | 4.5 | 0 | Small cell lung cancer | mot |
| LBD04-078 | LBD | 66 | m | 1166 | 7 | 5.5 | 0 | Shock, respiratory failure | mot |
| NAD05-003 | NAD | 84 | f | 1109 | 6.8 | 6.5 | V | Dehydration, cachexia | mot |
| AD05-005 | AD | 94 | f | 1041 | 7.3 | 3.5 | V | Cardiovascular accident | mot |
| PSP05-008 | PSP | 67 | m | 1456 | 6.4 | 7.5 | I | Unknown | mot |
| AD05-010 | AD | 93 | m | 1210 | 6.5 | 5 | V | Unknown | mot |
| AD05-011 | AD | 93 | f | 1045 | 6.5 | 2 | IV | Cachexia | mot |
| C05-014 | C | 86 | f | 1127 | 7.1 | 5.5 | III | Heart attack | mot |
| AD05-021 | AD | 89 | f | 1064 | 6.2 | 9 | V | Unknown | mot |
| AD05-026 | AD | 84 | f | 1098 | 6.3 | 4 | V | Dehydration | mot |
| C05-032 | C | 74 | m | 1522 | 6.4 | 4.5 | 0 | Lung carcinoma | mot |
| AD05-040 | AD | 69 | m | 1170 | 6.5 | 4.5 | VI | Pneumonia | mot |
| C05-044 | C | 80 | m | 1376 | 5.8 | 5 | 0 | Cachexia, dehydration | mot |
| C05-063 | C | 93 | f | 1316 | 6.6 | 2 | II | Heart failure | mot |
| PD06-026 | PD | 70 | m | 1451 | 6.5 | 6 | I | Respiratory insufficiency | mot |
| NAD06-039 | NAD | 86 | f | 1359 | 6.5 | 2.5 | 0 | Old age | mot |
| AD06-044 | AD | 86 | f | 950 | 6.9 | 5 | IV | Cachexia, dementia | mot |
| AD06-070 | AD | 86 | f | 951 | 6.7 | 3 | NA | Cachexia, serious heart problems | mot |
| PID07-004 | PID | 66 | m | 1245 | 6.2 | 3.5 | NA | Pneumonia | mot |
| Patient . | Diseasea . | Ageb (years) . | Sex . | Brain Weightc (g) . | PHd . | PMDe (h) . | Braak stagef . | Cause of death . | Areasg . |
|---|---|---|---|---|---|---|---|---|---|
| AD00-126 | AD | 85 | m | 1020 | 6.1 | 7 | V | Dehydration, cachexia | mot |
| PID03-010 | PID | 72 | f | 929 | 6.5 | 6 | I | Pulmonary embolism | mot |
| AD03-090 | AD | 87 | f | 1007 | 6.5 | 4.5 | V | Mammacarcinoma, infected decubitus, arterial insufficiency | mot |
| AD04-001 | AD | 57 | m | 1092 | 6.5 | 4 | VI | Aspiration pneumonia, toxic hepatitis | mot |
| AD04-011 | AD | 84 | f | 1217 | 6.4 | 4.5 | V | Cachexia | mot, vis |
| NAD04-024 | NAD | 90 | f | 1151 | 6.1 | 5 | III | Heart failure, sepsis by wound infection | mot, vis |
| AD04-025 | AD | 90 | f | 934 | 6.7 | 5.5 | IV | Cachexia, severe decompensatio cordis | mot |
| AD04-029 | AD | 78 | f | 1105 | 6.2 | 3.5 | V | Gastrointestinal bleeding | vis |
| C04-049 | C | 77 | f | 1312 | 6.5 | 6.5 | I | Cachexia, uremia | pfc |
| C04-061 | C | 88 | f | 1168 | 7 | 4 | NA | Old age | mot |
| PD04-067 | PD | 68 | m | 1236 | 6.7 | 4.5 | IV | Dehydration, cachexia | mot, pfc |
| PID04-072 | PID | 68 | f | 752 | 6.5 | 4.5 | NA | Dehydration, cachexia, decubitus with severe dementia | mot |
| C04-074 | C | 55 | f | 1321 | 6.4 | 4.5 | 0 | Small cell lung cancer | mot |
| LBD04-078 | LBD | 66 | m | 1166 | 7 | 5.5 | 0 | Shock, respiratory failure | mot |
| NAD05-003 | NAD | 84 | f | 1109 | 6.8 | 6.5 | V | Dehydration, cachexia | mot |
| AD05-005 | AD | 94 | f | 1041 | 7.3 | 3.5 | V | Cardiovascular accident | mot |
| PSP05-008 | PSP | 67 | m | 1456 | 6.4 | 7.5 | I | Unknown | mot |
| AD05-010 | AD | 93 | m | 1210 | 6.5 | 5 | V | Unknown | mot |
| AD05-011 | AD | 93 | f | 1045 | 6.5 | 2 | IV | Cachexia | mot |
| C05-014 | C | 86 | f | 1127 | 7.1 | 5.5 | III | Heart attack | mot |
| AD05-021 | AD | 89 | f | 1064 | 6.2 | 9 | V | Unknown | mot |
| AD05-026 | AD | 84 | f | 1098 | 6.3 | 4 | V | Dehydration | mot |
| C05-032 | C | 74 | m | 1522 | 6.4 | 4.5 | 0 | Lung carcinoma | mot |
| AD05-040 | AD | 69 | m | 1170 | 6.5 | 4.5 | VI | Pneumonia | mot |
| C05-044 | C | 80 | m | 1376 | 5.8 | 5 | 0 | Cachexia, dehydration | mot |
| C05-063 | C | 93 | f | 1316 | 6.6 | 2 | II | Heart failure | mot |
| PD06-026 | PD | 70 | m | 1451 | 6.5 | 6 | I | Respiratory insufficiency | mot |
| NAD06-039 | NAD | 86 | f | 1359 | 6.5 | 2.5 | 0 | Old age | mot |
| AD06-044 | AD | 86 | f | 950 | 6.9 | 5 | IV | Cachexia, dementia | mot |
| AD06-070 | AD | 86 | f | 951 | 6.7 | 3 | NA | Cachexia, serious heart problems | mot |
| PID07-004 | PID | 66 | m | 1245 | 6.2 | 3.5 | NA | Pneumonia | mot |
aAD = Alzheimer's disease, C = control, LDB = Lewy body disease, NAD = non-Alzheimer dementia, PD = Parkinson's disease, PID = Pick's disease, PSP = progressive supranuclear palsy. bAge at death (years). cBrain weight (g). dpH of the cerebrospinal fluid. ePMD = Postmortem delay (h). fCf Braak and Braak (1991), NA = not available. gmot = motor cortex, pfc = prefrontal cortex, vis = visual cortex.
Clinico-pathological data of resected brain tissue
| Patient . | Agea (years) . | Sex . | Diagnosisb . | Areac . |
|---|---|---|---|---|
| E03-003 | 17 | f | MTS | tmp |
| E04-001 | 25 | f | MTS | tmp |
| E04-005 | 5 | m | astrocytoma grade II | tmp |
| E04-006 | 36 | f | DNET | tmp |
| E04-007 | 35 | f | oligoastrocytoma grade II | tmp |
| E04-008 | 21 | m | DNET | tmp |
| E04-009 | 52 | f | MTS | tmp |
| E05-001 | 21 | m | MTS | tmp |
| E05-002 | 49 | m | MTS | tmp |
| E05-003 | 10 | f | MTS | tmp |
| E05-004 | 51 | m | cavernous hemangioma | tmp |
| E05-005 | 44 | f | MTS | tmp |
| E05-007 | 35 | f | MTS | tmp |
| E05-008 | 69 | f | MTS | tmp |
| E05-009 | 41 | f | MTS | tmp |
| E05-010 | 41 | m | desmoplasic ganglioglioma | tmp |
| E05-011 | 43 | f | MTS | tmp |
| E05-012 | 58 | m | GBM | tmp |
| E05-013 | 39 | f | MTS | tmp |
| E06-001 | 21 | m | MTS | tmp |
| E06-002 | 20 | f | MTS | tmp |
| E06-003 | 50 | f | no lesion | tmp |
| E06-004 | 58 | m | epidermoid | tmp |
| E06-005 | 33 | f | porencephalic cyste | tmp |
| E06-006 | 56 | m | MTS | tmp |
| E06-007 | 31 | m | MTS | tmp |
| E06-008 | 29 | f | oligodendroglioma | tmp |
| E06-009 | 45 | f | gliosis | tmp |
| E06-010 | 38 | f | MTS | tmp |
| Patient . | Agea (years) . | Sex . | Diagnosisb . | Areac . |
|---|---|---|---|---|
| E03-003 | 17 | f | MTS | tmp |
| E04-001 | 25 | f | MTS | tmp |
| E04-005 | 5 | m | astrocytoma grade II | tmp |
| E04-006 | 36 | f | DNET | tmp |
| E04-007 | 35 | f | oligoastrocytoma grade II | tmp |
| E04-008 | 21 | m | DNET | tmp |
| E04-009 | 52 | f | MTS | tmp |
| E05-001 | 21 | m | MTS | tmp |
| E05-002 | 49 | m | MTS | tmp |
| E05-003 | 10 | f | MTS | tmp |
| E05-004 | 51 | m | cavernous hemangioma | tmp |
| E05-005 | 44 | f | MTS | tmp |
| E05-007 | 35 | f | MTS | tmp |
| E05-008 | 69 | f | MTS | tmp |
| E05-009 | 41 | f | MTS | tmp |
| E05-010 | 41 | m | desmoplasic ganglioglioma | tmp |
| E05-011 | 43 | f | MTS | tmp |
| E05-012 | 58 | m | GBM | tmp |
| E05-013 | 39 | f | MTS | tmp |
| E06-001 | 21 | m | MTS | tmp |
| E06-002 | 20 | f | MTS | tmp |
| E06-003 | 50 | f | no lesion | tmp |
| E06-004 | 58 | m | epidermoid | tmp |
| E06-005 | 33 | f | porencephalic cyste | tmp |
| E06-006 | 56 | m | MTS | tmp |
| E06-007 | 31 | m | MTS | tmp |
| E06-008 | 29 | f | oligodendroglioma | tmp |
| E06-009 | 45 | f | gliosis | tmp |
| E06-010 | 38 | f | MTS | tmp |
aAge at which tissue was resected (years).
bDNET = dysembryoplastic neuroepithelial tumour, GBM = glioblastoma multiforme, MTS = mesiotemporal sclerosis.
ctmp = temporal cortex.
Clinico-pathological data of resected brain tissue
| Patient . | Agea (years) . | Sex . | Diagnosisb . | Areac . |
|---|---|---|---|---|
| E03-003 | 17 | f | MTS | tmp |
| E04-001 | 25 | f | MTS | tmp |
| E04-005 | 5 | m | astrocytoma grade II | tmp |
| E04-006 | 36 | f | DNET | tmp |
| E04-007 | 35 | f | oligoastrocytoma grade II | tmp |
| E04-008 | 21 | m | DNET | tmp |
| E04-009 | 52 | f | MTS | tmp |
| E05-001 | 21 | m | MTS | tmp |
| E05-002 | 49 | m | MTS | tmp |
| E05-003 | 10 | f | MTS | tmp |
| E05-004 | 51 | m | cavernous hemangioma | tmp |
| E05-005 | 44 | f | MTS | tmp |
| E05-007 | 35 | f | MTS | tmp |
| E05-008 | 69 | f | MTS | tmp |
| E05-009 | 41 | f | MTS | tmp |
| E05-010 | 41 | m | desmoplasic ganglioglioma | tmp |
| E05-011 | 43 | f | MTS | tmp |
| E05-012 | 58 | m | GBM | tmp |
| E05-013 | 39 | f | MTS | tmp |
| E06-001 | 21 | m | MTS | tmp |
| E06-002 | 20 | f | MTS | tmp |
| E06-003 | 50 | f | no lesion | tmp |
| E06-004 | 58 | m | epidermoid | tmp |
| E06-005 | 33 | f | porencephalic cyste | tmp |
| E06-006 | 56 | m | MTS | tmp |
| E06-007 | 31 | m | MTS | tmp |
| E06-008 | 29 | f | oligodendroglioma | tmp |
| E06-009 | 45 | f | gliosis | tmp |
| E06-010 | 38 | f | MTS | tmp |
| Patient . | Agea (years) . | Sex . | Diagnosisb . | Areac . |
|---|---|---|---|---|
| E03-003 | 17 | f | MTS | tmp |
| E04-001 | 25 | f | MTS | tmp |
| E04-005 | 5 | m | astrocytoma grade II | tmp |
| E04-006 | 36 | f | DNET | tmp |
| E04-007 | 35 | f | oligoastrocytoma grade II | tmp |
| E04-008 | 21 | m | DNET | tmp |
| E04-009 | 52 | f | MTS | tmp |
| E05-001 | 21 | m | MTS | tmp |
| E05-002 | 49 | m | MTS | tmp |
| E05-003 | 10 | f | MTS | tmp |
| E05-004 | 51 | m | cavernous hemangioma | tmp |
| E05-005 | 44 | f | MTS | tmp |
| E05-007 | 35 | f | MTS | tmp |
| E05-008 | 69 | f | MTS | tmp |
| E05-009 | 41 | f | MTS | tmp |
| E05-010 | 41 | m | desmoplasic ganglioglioma | tmp |
| E05-011 | 43 | f | MTS | tmp |
| E05-012 | 58 | m | GBM | tmp |
| E05-013 | 39 | f | MTS | tmp |
| E06-001 | 21 | m | MTS | tmp |
| E06-002 | 20 | f | MTS | tmp |
| E06-003 | 50 | f | no lesion | tmp |
| E06-004 | 58 | m | epidermoid | tmp |
| E06-005 | 33 | f | porencephalic cyste | tmp |
| E06-006 | 56 | m | MTS | tmp |
| E06-007 | 31 | m | MTS | tmp |
| E06-008 | 29 | f | oligodendroglioma | tmp |
| E06-009 | 45 | f | gliosis | tmp |
| E06-010 | 38 | f | MTS | tmp |
aAge at which tissue was resected (years).
bDNET = dysembryoplastic neuroepithelial tumour, GBM = glioblastoma multiforme, MTS = mesiotemporal sclerosis.
ctmp = temporal cortex.
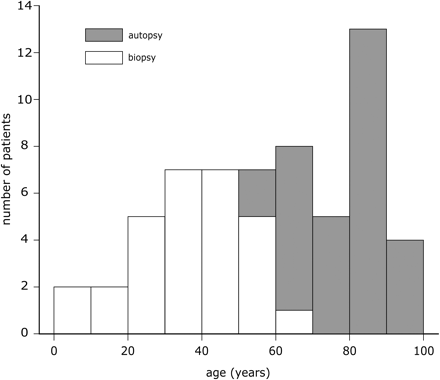
Figure 1 illustrates that the autopsy group contained mainly elderly subjects, aged from 55 to 94 years (median: 85), while the resected tissue was obtained from epilepsy patients which were much younger (age range: 5–69 years, median: 38). There was a relatively small overlap in these age distributions, but together they encompassed the entire age range from juvenile to old. There were 20 females and 11 males in the autopsy group, and 18 females and 11 males in the biopsy group.
The age distributions of patients from whom autopsy or surgically resected tissue was obtained. The ages of autopsied patients were significantly higher than those of operated patients, but taken together the entire age range from juvenile to old was covered.
Immunohistochemistry
Immunohistochemical stainings were performed on 200-μm thick formalin-fixed tissue slices from the pertinent brain areas. Neocortical tissue slices (1–6/patient, depending on availability) from each patient were stained with guinea pig-anti-doublecortin (DCX, Chemicon, Temecula, CA, USA) at a concentration of 1:3000. Routinely, adjacent slices were stained with antibodies against mouse-anti-neuronal nuclear protein (NeuN, Chemicon), 1:200; rabbit-anti-glial fibrillary acidic protein (GFAP, DAKO, Glostrup, Denmark), 1:10 000; mouse-anti-human leukocyte antigen (HLA-DP-DQ-DR, DAKO), 1:100; mouse-anti-amyloid plaques (Aβ4, DAKO), 1:200 or mouse-anti-hyper-phosphorylated tau (AT-8, Immunogenetics, Zwijndrecht, Belgium), 1:300. These stainings were all visualized with diaminobenzidine (DAB) and were used for the reported patient data analysis.
Fluorescent immunocytochemical stainings using material from selected patients were performed with the above-mentioned antibodies and the following antibodies: mouse-anti-glutamine synthetase (Chemicon), 1:400; chicken-anti-vimentin (Chemicon), 1:10 000; rabbit-anti-nestin (Chemicon), 1:1000; goat-anti-doublecortin C18 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), 1:200. For additional information about the DCX antibodies from Chemicon and Santa Cruz we refer to the Supplementary Material.
Of three patients larger tissue pieces (about 0.5 × 2 × 3 cm3) were available from which 100-μm thick vibratome sections were made. The material of one of these patients included hippocampal tissue, which allowed us to study DCX+ cells in the adult human subventricular zone and subgranular zone (Supplementary Material). Cultured human astrocytes and a cell line isolated from brain tissue of an epilepsy patient (R.W.H. Verwer, unpublished data) were used to assess the ability of GFAP containing cells to express DCX (Supplementary Material). Control experiments consisting of the omission of first or second antibodies confirmed that the DCX signal in the human brain tissue pertained to the Chemicon and Santa Cruz antibody, respectively (Supplementary Material) (Table S1, Fig. S1).
Secondary antibodies were either conjugated with biotin (Vector, Burlingame, CA, USA), HRP (DAKO), AlexaFluor 488 or AlexaFluor 594 (Molecular Probes, Leiden, Netherlands), cy-2, or cy-3 (both from Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and were applied at concentrations recommended by the manufacturers. Slices to be visualized using DAB were pretreated with 3% H2O2 and 10% methanol in TBS (50 mM Tris, 150 mM NaCl, pH 7.6) to eliminate endogenous peroxidase activity. First antibodies were incubated overnight at 4°C; secondary antibodies were incubated for 1 h at room temperature. Between incubations slices were washed 3 times with TBS. DAB-stained slices were dehydrated and embedded in entellan (Merck, Darmstadt, Germany). To reduce autofluorescence caused by lipofuscin accumulation in adult human neurons we incubated slices stained with fluorescent antibodies for 7 min with 0.3% sudan black (Brunschwig Chemie, Amsterdam, The Netherlands) in 70% ethanol. Subsequently, the slices were treated for 20 min with 70% ethanol (3 washes), rinsed in TBS and embedded in Vectashield (Molecular Probes, Leiden, NL). Micrographs were made using a Zeiss Axioplan 2 microscope, equipped with an Evolution MP digital camera (Media Cybernetics) or using a Zeiss 410 confocal laser scanning microscope.
To confirm that the DCX protein and its mRNA were actually present in human neocortical tissue we performed Western blotting and reverse transcription polymerase chain reaction (RT-PCR) (Supplementary materials and methods). Western blots with both the Chemicon and the Santa Cruz C18 antibody recognized a weakly stained single band at 37 kDa (Fig. S2). Using the DCX primer pair and the RT-PCR procedure described by Curtis et al. (2007) we were able to detect DCX gene expression in the human neocortex (Fig. S3).
Semi-quantitative data analysis
For semi-quantitative evaluation of DCX+ structures, neurons, astrocytes and fibres five grades were applied: 0, no DCX-positive items present; 1, one or only a few items; 2, several items; 3, a substantial number of items; 4, the majority of the expected number of items is positive. The routine immuno-stainings were also graded, with some slight modifications. The cytoarchitecture was evaluated using NeuN and the following categories were distinguished: 0, apparently normal; –1, minor deviations; –2, some layers show focal absence of NeuN+ neurons; –3, substantial parts of layers lack NeuN+ neurons; –4, global to total absence of NeuN+ neurons. It may be noted that NeuN does not constitute an absolute sign of the presence or absence of neurons (cf ‘Discussion’). Gliotic and inflammatory changes were detected with GFAP and HLA, respectively. Grades for GFAP were: 0, no staining; 1, a few normal astrocytes; 2, many normal astrocytes; 3, many normal and some reactive astrocytes; 4, reactive astrocytes dominate with some aggregates; 5, many aggregates of reactive astrocytes. Grades for HLA were: 0, no staining; 1, few resting/ramifying microglia; 2, many resting/ramifying microglia; 3, widespread microglia; 4, massive (reactive) microglia; 5, excessive reactive microglia. For semi-quantitative grading of local Alzheimer pathology we distinguished the following categories: 0, lack of pathological alterations; 1, mild; 2, moderate; 3, strong; 4, massive pathology. For the routine/pathological grading intermediate scores like 1.5 were used if it was difficult to choose between two categories. In this way we tried to minimize the impact of misclassification.
Statistical analysis was performed using S+ software (Insightful, Seattle, WA). The Kruskal–Wallis test with multiple comparisons (cf Conover, 1980) was applied for comparisons between patient groups. The Mann–Whitney test (2-sample version of the Kruskal–Wallis test) was used to analyse data with respect to sex. The Spearman rank correlation test was used to evaluate possible associations between different factors. Tests were considered to be significant if the level-of-significance (P) was less than 0.01. A test result with 0.01 ≤ P < 0.10 was designated as a tendency.
Results
DCX is present in astrocytic cellular compartments
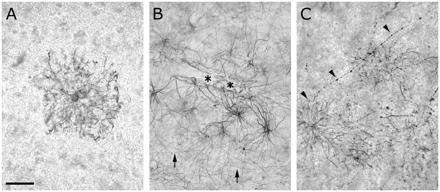
The intensity of the immunocytochemical staining of DCX in the neocortex showed a considerable regional variability. In two small autopsy tissue specimens no DCX+ structures were found. In 90% of the specimens differentiated DCX+ astrocytes were observed. In some specimens it concerned a few solitary cells per slice, whereas in others substantial numbers of DCX+ astrocytes were present. Staining of vibratome sections of much larger (0.5 × 2 × 3 cm3) than normal sized tissue pieces of three patients showed that the differences were due to local variation in astrocytic DCX expression. DCX+ astrocytes could be present in any cortical layer and the white matter and were, often, lightly stained (Fig. 2A). But in the border area between the deep cortical layers and the white matter or near the pia the astrocytic staining was sometimes very intense. Astrocytes with elaborate endfeet contacting blood vessels were visible in the white matter (Fig. 2B), and in layer VI we also observed cells (Fig. 2C and S4A–C) whose morphology was reminiscent of polarized astrocytes (cf. Oberheim et al., 2006). If we considered all patients, relatively few DCX+ astrocytes were found in the middle cortical layers.
Differentiated astrocytes in the adult human neocortex expressed DCX (Chemicon). (A) Solitary astrocyte in layer III. (B) Astrocytes contacting a bloodvessel in the border area of layer VI and white matter. Arrows indicate typical fibres of the white matter and the lower cortical layers. Asterisks indicate astrocytic endfeet. (C) A polarized astrocyte with an ascending fibre (arrow heads) in the border area between layer VI and white matter. Scale bar is 32 μm in A; 50 μm in B and C.
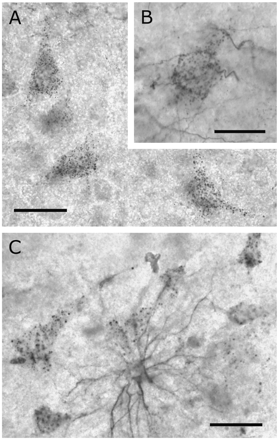
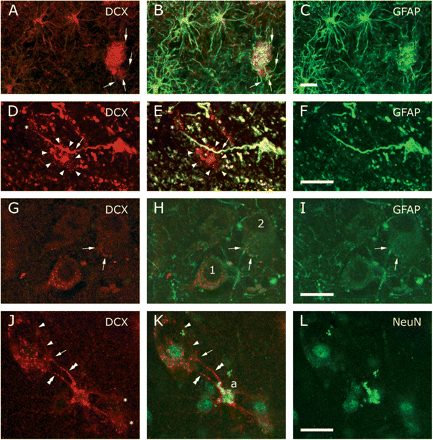
Surprisingly, in 78% of the patients DCX+ neuronal profiles were present in the middle cortical layers. The punctate appearance of the neuronal staining suggested that DCX+ structures formed an envelope around the cell bodies and proximal dendrites (Fig. 3A and S4D–F). This impression was reinforced by the occurrence of tiny DCX+ fibres connecting to the dots (Fig. 3B). Some of the DCX+ fibres contacting the neuronal profiles could be traced directly to neighbouring protoplasmic astrocytes (Fig. 3C). In Fig. 4A–F DCX+/GFAP+ astrocytes and processes are shown to contact DCX+ neuronal profiles. However, the number of DCX+ astrocytic cell bodies present around DCX+ neurons was, usually, very low and it was much more common to find DCX-/GFAP+ astrocytes that contacted DCX+ neuronal profiles in a similar, but not necessarily continuous way (Fig. 4G–I). Double staining of NeuN and DCX confirmed that the punctate DCX+ envelopes consisted of veil-like extensions of astrocytic processes wrapped around the neuronal cell body and were not localized to the neuronal cytoplasm (Fig. 4J–L). Occasionally, very large DCX+ pyramidal neurons (balloon cells) were observed in the neocortex of epilepsy patients (not shown).
DCX+ (Chemicon) neurons were predominantly present in the middle cortical layers. (A) Neuronal doublecortin staining was punctate and appeared to envelope the somata. (B) Some neuronal profiles were contacted by tiny DCX+ fibres. (C) In exceptional cases astrocytes were found to contact several surrounding neurons. Scale bar is 30 μm in A; 20 μm in B; 40 μm in C.
A minority of the neocortical astrocytes was positive for DCX. (A–C) DCX+(Chemicon)/GFAP+ astrocytes and peripheral processes ending in a patch of punctate DCX+/GFAP+ staining. Arrows (A, B) mark afferent processes. (D–F) A predominantly DCX+ (Santa Cruz) neuronal envelope (arrow heads) contacted by a passing process emanating from a DCX+(Santa Cruz)/GFAP+ astrocyte. An asterisk indicates a part of another envelope. (G–I) Often the DCX+ (Chemicon) punctae seemed to be completely segregated from contacting GFAP-containing processes (cf neuron 1), whereas on other neurons (cf neuron 2) part of the processes and punctae contained both GFAP and DCX (arrows). (J–L) Double labelling with DCX (Chemicon) and NeuN supported the idea that DCX is not present in the neuronal cytoplasm but envelopes the soma. Two processes (double arrow heads) emanated from a DCX+ astrocyte (a). One process branched (arrow) where it seemed to touch the neuronal surface, forming a punctated veil and a proceeding branch (arrow heads). Asterisks in J mark other envelopes provided by the astrocyte. B, E, H and K show the merged images. Scale bars are 20 μm.
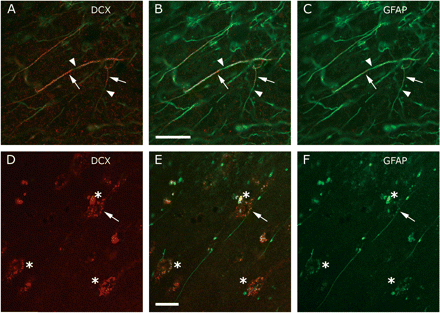
Variable amounts of relatively straight ascending fibres either in the upper cortical layers near the pial surface or in the white matter were found. In other cortical layers the presence of such fibres was much less prominent. Occasionally, fibres running perpendicularly to the predominant ascending orientation were visible in the white matter. Some fibres contained large varicosities, whereas others had a smooth appearance. Double staining with GFAP revealed that the DCX+ fibres constituted a subset of GFAP+ fibres (Fig. 5A–C). Such passing fibres could also be found to contact neuronal cell bodies in the middle cortical layers (Fig. 5D–F). It may be noted that the distinct staining of GFAP and DCX associated with many neuronal envelopes refutes the idea that the DCX signal pertains to lipofuscin (Figs. 4D–I and 5D–F). The outer surface of the cortex beneath the pia was covered with a meshwork of DCX+ fibres containing widely separated spherical varicosities and large blood vessels were often lined by DCX+ fibres (data not shown). Small solitary DCX positive structures in the neocortical neuropil and along smaller blood vessels usually either had the appearance of swellings or endfeet of astrocytic processes. Apart from very rare small spherical cells that were found attached to some large blood vessels, we did not observe any DCX+ structures in the adult human neocortex that had the bipolar elongated morphology of migrating neural precursors. In the motor cortex of only two AD patients we found a few DCX+ neuritic plaques.
A subpopulation of the interlaminar GFAP+ astrocytic fibres (cf Colombo et al., 2002) also contained DCX (Chemicon). (A–C) The relative content of DCX as compared to GFAP varied within fibres. Arrows indicate a relative abundance of DCX, whereas arrow heads point to parts lacking DCX. In A the green signal has only been partially suppressed to better illustrate the relation between GFAP and DCX. (D–F) Some DCX−/GFAP+ interlaminar fibres were observed to contact DCX+ neuronal envelopes. The arrow points to the punctate contact where GFAP and DCX co-localized. B and E show the merged images. Asterisks indicate the presence of a DCX+ neuronal envelopes. Scale bars are 20 μm.
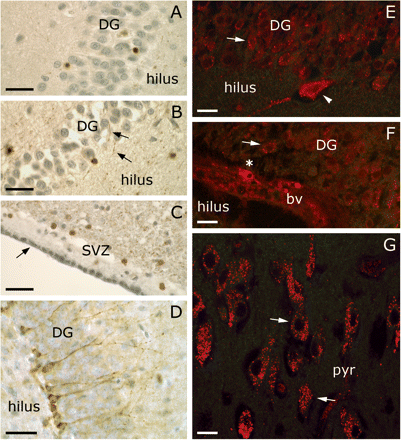
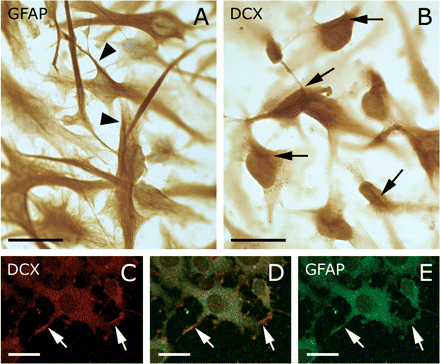
In paraffin sections of the hippocampus of an Alzheimer patient several DCX+ cells were observed in the dentate gyrus (Fig. 6A–B) and subventricular zone (Fig. 6C). These cells had a similar morphology and pattern as shown by Boekhoorn et al. (2006). DCX+ neural precursor cells in the subgranular zone of a 7-week-old mouse served as positive controls (Fig. 6D). Vibratome sections of the human hippocampus showed that neurons in the cornu ammonis area, hilus and dentate gyrus may exhibit similar neuronal envelopes as cortical neurons (Fig. 6E–G). Also, a few small cells attached to a blood vessel in the subgranular zone contained DCX in their cytoplasm (Fig. 6F). Cultured adult human astrocytes and neocortical cells, that have been induced to express GFAP, could also contain DCX (Fig. 7). It was remarkable that the cultured astrocytes did show relatively homogenous presence of DCX in the cytoplasm with stroke-like concentrations (Fig. 7B), unlike the fibrillar appearance of GFAP (Fig. 7A). On the other hand, in the neocortical cells that were induced to ‘differentiate’ by transferral from mitogen-containing medium to fetal calf serum-containing medium, DCX seemed to be relatively abundant in growth cone-like protrusions (Fig. 7C–E).
Doublecortin positive cells in the subventricular zone and hippocampus of an adult human (A–C, E–G; AD07-027) and in the subgranular zone of a 7-week-old mouse (D) as detected by the Chemicon antibody. (A, B) DCX positive cells (brown) in the dentate gyrus (DG) as they appear in paraffin sections with haematoxylin counterstaining. The arrows in B point to DCX+ fibres. (C) In the subventricular zone spherical shaped DCX cells can be found (brown). The arrow in C indicates the ependymal cell layer. (D) Doublecortin positive neural precursor cells in the subgranular zone of a 7-week-old mouse. (A–D) Haematoxylin counterstaining (greyish blue). For DCX staining in paraffin sections of the subgranular zone of the adult human hippocampus by the Santa Cruz antibody we refer to Fig. 8J–N in Boekhoorn et al. (2006). (E–G) In 100 μm vibratome sections of the hippocampus of an Alzheimer patient DCX+ neuronal envelopes were occasionally found (arrows). (E) DCX+ envelopes around granular cells of the dentate gyrus and a hilar cell (arrow head). (F) Infrequently, small cells with a DCX+ cytoplasm were present in the subgranular zone (asterisk). bv denotes blood vessel. (G) Also, several neurons in the pyramidal cell layer of the cornu ammonis of the human hippocampus had a DCX+ envelope. Scale bar is 30 μm in A–D; 20 μm in E–G.
Cultured astrocytes derived from human glioblastoma astrocytoma cell line U373 MG (EACACC No 89081403) and cells isolated from neocortex resected at epilepsy surgery express DCX. (A) GFAP was present as loose fibrils or organized in bundles (arrows). Counterstaining with haematoxylin. (B) The DCX (Chemicon) staining was more diffuse, but also showed a tendency to form structures (arrows) similar to the GFAP bundles. Note that (B) was not counterstained. (C–F) Cells isolated from neocortex resected at epilepsy surgery were cultured in the presence of mitogens. Replacing the mitogens with 10% fetal calf serum induced these cells to express GFAP and DCX. Both GFAP and DCX (Chemicon) were present throughout the cytoplasm, but DCX appeared to be relatively more abundant in small growth cone-like processes (arrows). This is reminiscent of the distribution of DCL in the peripheral processes of cultured mouse astrocytes described by Friocourt et al. (2001). However, in the cultured astrocytes (B) DCX did not display such preference. Scale bar is 30 μm in A, B; 20 μm in C–F.
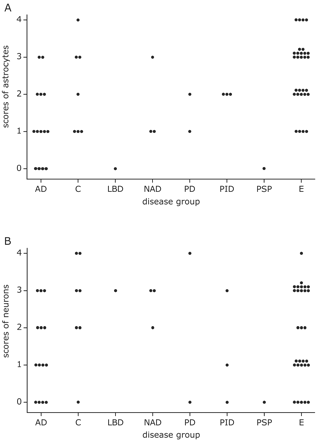
Semi-quantitative evaluation of DCX staining of astrocytic somata (A) and neuronal envelopes (B). Each dot corresponds to data from one patient and denotes the (averaged) score obtained from 1 to 6 neocortex slices. Note that the AD patients have relatively low scores for DCX+ astrocytes (A). Abbreviations: AD: Alzheimer's disease, C: control, E: epilepsy, LBD: Lewy body disease, NAD: non-Alzheimer dementia, PD: Parkinson's disease, PID: Pick's disease, PSP: progressive supranuclear palsy.
Effect of Alzheimer pathology on DCX immunoreactivity
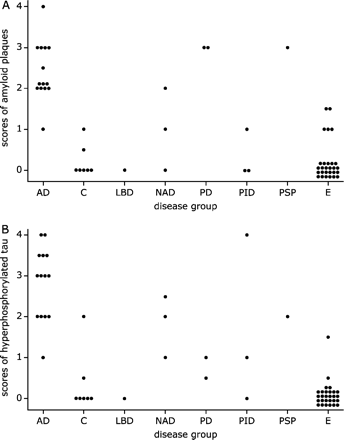
The high variability in the extent and nature of the DCX-stained cellular structures might be related to a number of factors, such as disease, age or sex. Therefore, we have semi-quantitatively graded the presence of DCX+ astrocytes (Fig. 8A), neurons (Fig. 8B) and fibres (not shown). Additionally, the extent of local Alzheimer pathology [plaques (beta-amyloid) and neuritic pathology (AT8), cf. Fig 9A and B], cytoarchitectural integrity based on NeuN staining (not shown) and glial activation using GFAP and HLA staining (not shown) were semi-quantitatively evaluated.
Semi-quantitative evaluation of amyloid (A) and hyperphosphorylated tau pathology, including dystrophic neurites and tangles (B). Each dot corresponds to one patient and denotes the (averaged) score obtained from 1 to 6 neocortex slices. As expected most control and epilepsy patients presented no local AD pathology (A, B). Abbreviations: AD: Alzheimer's disease, C: control, E: epilepsy, LBD: Lewy body disease, NAD: non-Alzheimer dementia, PD: Parkinson's disease, PID: Pick's disease, PSP: progressive supranuclear palsy.
To establish whether there were major differences between different disease categories, we grouped the immunoreactivity scores for the different parameters of the non-Alzheimer neurodegenerative patients (NND: comprising Lewy body disease, non-Alzheimer dementia, Parkinson's disease, Pick's disease and progressive supranuclear palsy) and tested them together with the corresponding scores of the AD patients, control subjects and epilepsy patients. The Kruskal–Wallis test revealed that there were differences among the groups with respect to the DCX+ astrocytic somata scores (overall P-value = 0.001). Figure 6A illustrates that this could be predominantly attributed to the relatively low scores for Alzheimer patients and relatively high scores for epilepsy patients (mutual comparison: P = 0.0001). Also the other neurodegenerative patients had low scores as compared with epilepsy patients (P = 0.002). The other comparisons were insignificant although the difference between AD patients and controls (P = 0.07) hinted at a tendency. The disease categories had no effect on DCX+ neuronal envelope (cf Fig. 8B) and DCX+ fibre scores (P = 0.26 and P = 0.16, respectively). As can be inferred from Figs. 3A and 4G–I, semi-quantitative grading showed that the neuronal and the astrocytic DCX immunoreactivity were not directly correlated (Rho = 0.19, P = 0.13). On the other hand, while only a minority of the GFAP fibres also contained DCX (cf Fig. 5) the DCX+ fibre scores correlated positively with the DCX+ astrocytic somata grades (Rho = 0.56, P < 0.0001).
It appeared that all but one AD patients and four NND patients had moderate to substantial amounts of local amyloid deposits, whereas the plaque load in tissue from one control subject, five epilepsy patients and two NND patients could only be described as mild (Fig. 9A). All but one AD patients, one control and four NND patients had moderate or higher scores for neuritic degeneration (Fig. 9B). Mild hyper-phosphorylated tau pathology was found in one control, two epilepsy patients and four NND patients (Fig. 9B). Overall, both the grades for amyloid deposits (P < 0.0001) and for tau pathology (P < 0.0001) were significantly different among the patient groups. In this sense the NND group constituted an intermediate group between the AD patients on one side and the controls and epilepsy patients on the other. For amyloid pathology grades of control subjects were comparable to those of epilepsy patients (P = 0.76). All other comparisons concerning amyloid pathology were significantly different (all P-values less or equal to 0.01). In the case of hyper-phosphorylated tau pathology (dystrophic neurites, neuropil threads and tangles) the analysis showed that the differences as noted for amyloid were essentially similar (cf Fig. 9B), with comparable scores for control and epilepsy patients (P = 0.19). Using the data from all patients the Spearman rank correlation test established that the two manifestations of local AD pathology exhibited a high mutual correlation (Rho = 0.78, P < 0.0001). More importantly, the scores for DCX+ astrocytes were negatively correlated with the presence of local amyloid (Rho = −0.46, P = 0.0004) and hyperphosphorylated tau pathology (Rho = −0.39, P = 0.002). Additionally, a tendency for a negative effect of age on the DCX-immunoreactivity of astrocytes (Rho = −0.30, P = 0.02) was detected. This finding may be less important since the probability of having Alzheimer pathology in the brain increases with higher age. For instance, the control subjects with non-zero AD pathology scores were 74 and 88 years old and the epilepsy patients with non-zero scores were 38, 49, 50, 51, 58 and 69 years of age, respectively. With the exception of the 38-year-old epilepsy patient who only had very mild AT8 pathology no patient showed any signs of local Alzheimer pathology before the age of 49 years. After that age the variability for both beta-amyloid and AT8 suddenly increased to median values of 1.75 (amyloid) and 2 (tau), respectively. Furthermore, if we only considered the control and epilepsy data, age and DCX+ astrocytic cell body scores were completely unrelated (Rho = 0.02, P = 0.91). Age did not correlate with other immunocytochemical parameters (NeuN, GFAP, HLA, DCX+ neurons and DCX+ fibres). Using the Mann–Whitney test no differences between females and males with respect to any of the parameters studied were found (all P-values larger than 0.14).
For the autopsy tissue data, associated with the death process, were available that are non-existent for resection material. For instance, brain weight may give an indication about atrophic processes, the pH of the cerebrospinal fluid is a useful measure about pre-mortem agonal processes (Hardy et al., 1985), and post-mortem delay may adversely affect detection of substances of interest. However, exploratory graphical examination yielded no indications relating DCX immunoreactivity to these factors (data not shown). For instance, post-mortem delay had no significant effect on DCX+ astrocyte (Rho = −0.19, P = 0.30) and neuron (Rho = 0.23, P = 0.21) scores. Other data such as Braak scores (Braak and Braak, 1991) may provide global information about the extent of Alzheimer pathology in the autopsied brain (cf Table 1). Braak scores were not correlated with DCX+ astrocytic somata (Rho = −0.29, P = 0.14), but there was a tendency that higher Braak scores were associated with lower DCX+ neuronal envelope scores and DCX+ fibre scores (Rho = −0.39, P = 0.05 and Rho = −0.40, P = 0.04, respectively). Braak scores were strongly correlated with local amyloid (Rho = 0.63, P = 0.001) and AT8 (Rho = 0.77, P = 0.0001) grades.
The effect of cytoarchitectural integrity and activated glia on DCX immunoreactivity
When assessed using NeuN, neocortical slices from 61% of all patients displayed noticeable cytoarchitectural alterations varying from partial to global absence of NeuN+ cells. These alterations were most obvious in the Alzheimer (71%) and epilepsy (69%) patients, but also noticeably present in the non-Alzheimer neurodegenerative patients (40%) and surprisingly often in controls (43%). Consequently, the NeuN+ cell scores did not differ between the patient groups. These abnormalities tended to negatively affect the DCX staining of astrocytic somata (Rho = −0.29, P = 0.03), but were not associated with the incidence of DCX immunoreactivity of neuronal envelopes or fibres. Neocortical slices from three epilepsy patients were almost devoid of NeuN+ neurons, while adjacent slices from two of these patients showed substantial numbers of DCX+ neuronal envelopes. The lack of NeuN stained neurons in these epilepsy patients was unrelated to the quality of the NeuN staining. Also Alzheimer pathology or scores for glial activation (GFAP and HLA) did not correlate with irregular appearances of the cytoarchitecture (data not shown). It is important to note that the immunoreactivity of NeuN in neurons as a measure for cytoarchitectural integrity is generally very useful but may, occasionally, give a biased impression (see ‘Discussion’ section).
Astrocytic activation, as assessed by GFAP staining, displayed high variability but was similarly distributed in each disease category (data not shown). Microglia activation and signs of inflammation tended to be more pronounced in patients with a neurodegenerative disorder as reflected by their median HLA scores [median (AD) = 4, median (NND) = 2.25, median (C) = 2, median (E) = 1.75; overall P = 0.08]. When all patients were tested together only a trend between GFAP and HLA could be discerned (Rho = 0.26, P = 0.05). With respect to local Alzheimer pathology, HLA showed a positive correlation (amyloid: Rho = 0.34, P = 0.01 and AT8: Rho = 0.39, P = 0.003), whereas GFAP was not significantly related with amyloid (Rho = 0.22, P = 0.09) or AT8 (Rho = 0.23, P = 0.08). Surprisingly, in autopsy tissue the global pathology as described by the Braak scores was slightly associated with GFAP (Rho = 0.48, P = 0.01) and more definitively with HLA (Rho = 0.59, P = 0.003). Neither GFAP nor HLA immunoreactivity was associated with DCX immunoreactivity (astrocyes, neurons and fibres). It was inferred that the expression of DCX in differentiated astrocytes and their processes is not involved in gliotic or inflammatory processes.
Discussion
We have studied DCX expression in human neocortical tissue from patients with different neurological/neurodegenerative disease processes which entail injury in the form of cell loss, glial activation, AD pathology or a combination of these. Animal models concerning ischaemia (Jin et al., 2003) and targeted lesions (Magavi et al., 2000) show that after adult neocortex injury DCX+ immature neurons may appear in the damaged area. It is at present unclear whether these immature neurons have migrated to the site of injury after a proliferative response in the germinal zones or, alternatively, might have been generated locally in the adult neocortex (cf Björklund and Lindvall, 2000). In support of the latter option, neural stem/precursor cells have been isolated from adult human cortical areas at long distances from the established neurogenic zones (Arsenijevic et al., 2001; Palmer et al., 2001). Considering the animal studies it is conceivable that some precursor cells in the human cortex proceed to the neuronal lineage (cf Jessberger et al., 2005). As a consequence, cells expressing the marker of immature neurons (DCX) might be detectable in adult human neocortex, especially in combination with degenerative injury.
In non-injured adult mammalian brain, DCX expression outside the neurogenic areas of the hippocampal formation and subventricular zone is very rare. A study by Omori et al. (1998) revealed no DCX band in a Northern blot from normal adult human brain tissue and Brown et al. (2003) reportedly found only one DCX positive cell in every 20–25 sections of normal adult rat neocortex. On the other hand, Nacher et al. (2001) found in the dentate gyrus, olfactory bulb, striatum, piriform cortex and endopiriform nucleus of normal adult rats many DCX+ cells which they classified as differentiated neurons. In the temporal cortex of epilepsy patients DCX+ cells have been found in tuberous malformations (Lee et al., 2003) or in various invasive brain tumours (Daou et al., 2005), but not in surrounding control cortex. It is unclear whether the DCX+ cells in tuberous malformations have been newly generated in response to the epileptic damage or are remnants of aberrant development causing the epileptic phenomena (Lee et al., 2003). The epileptogenic tissues we obtained consisted of pieces of non-tumorous normal neocortex that did not display signs of tubers or white matter nodules.
Our data indicate that many differentiated astrocytes in various juvenile and adult human neocortex areas (motor, temporal, visual and prefrontal cortex) with various disease backgrounds may contain DCX at levels around the detection threshold. Regionally, the level of DCX in different astrocytic compartments may exceed the threshold of detection resulting in the observed highly variable immunoreactivity. Thus, parts of peripheral processes and fibres may be visible, whose astrocytic origin may only become apparent after double staining with conventional astrocytic markers, like glial fibrillary acidic protein, glutamine synthetase and vimentin. The DCX immunoreactivity of astrocytic somata did correlate positively with that of fibres in the white matter and interlaminar palisades, which indicates that DCX may be transported to distant targets. The interlaminar palisades consist of astrocytic fibres in the upper cortical layers that are unique to primates and are known to be affected in Alzheimer's disease without a relation with local AD pathology (Colombo et al., 2002). Our observations showed that some DCX−/GFAP+ interlaminar fibres may contact DCX+ envelopes of layer III neurons but did not allow any conclusion about DCX+ interlaminar fibres in relation with AD. The lack of correlation between astrocytic soma and neuronal envelope immunoreactivity may be partly due to the relative sparseness of DCX+ astrocytes in the middle cortical layers, where the neuronal envelopes are, and their relative abundance in the white matter and near the pia. Our data indicate that the punctate and fibrillar DCX immunoreactivity associated with neurons in the adult human neocortex and, possibly, several hippocampal regions consists of thin astrocytic veils supported by passing processes wrapped around the neuronal soma and proximal dendrites. However, at present it cannot be excluded that DCX is also present in the cytoplasm of some neurons.
In principle, the variability of the DCX immunostaining might be explained by cellular changes accompanying the different diseases in our patient cohort. For instance, haematoxylin/eosin staining (Prayson and Estes, 1995) and MAP2 and NeuN immunostaining (Choi et al., 2004) of the neocortex of epilepsy patients often reveal gross cytoarchitectural abnormalities, including absence of cortical layers. Other investigators reported no cell loss, but instead neuropil atrophy and hypertrophy of neurons in the neocortex of temporal lobe epilepsy patients (Bothwell et al., 2001). Using NeuN staining, we observed varying levels of disorganization of the cortical lamination in tissue from many patients, not only epilepsy patients. There was a tendency that DCX immunoreactivity of astrocytic somata was impaired by cytoarchitectural abnormalities, however, neuronal envelopes and fibres were not affected. Remarkably, DCX+ neuronal envelopes were detected in tissue adjacent to slices with very few NeuN+ neurons from two epilepsy patients, suggesting that many neurons were not lost but simply stopped producing the NeuN protein. Neurons that could not be labelled by NeuN in ischaemic rat neocortex (Unal-Cevik et al., 2004) or equivalently by SMI311 or MAP2 in epilepsy patients (Spreafico et al., 1998) have been described before and may indicate impaired cellular health. On the other hand, if lack of NeuN staining indicated bad neuronal health in the tissue this apparently did not prevent the DCX+ astrocytic processes to stay in contact with the affected neuronal cell bodies.
Signs of inflammation (Crespel et al., 2002) and activation of astrocytes (Briellmann et al., 2002) have been reported in the hippocampus of epilepsy patients. In the neocortical white matter of patients with temporal lobe sclerosis a reduction of GFAP+ astrocytes was observed (Kendall et al., 1999). In the neocortex of AD patients both astrocytes and microglia are activated and aggregate around plaques. Apart from exceptionally high microglia scores in six AD patients, the glia markers GFAP and HLA were not statistically different among the disease categories. Furthermore, there was no correlation between DCX immunoreactivity and glia markers. This was emphasized by the fact that plaques were usually not visualized with DCX antibodies. Taken together the above data suggest that although astrocytes with an activated morphology could be DCX+ it is unlikely that DCX has a function in gliotic or inflammatory reactions to disease processes.
Local Alzheimer pathology appeared to be the most influential factor affecting the DCX staining of astrocytic somata in a negative way. However, neither neuronal envelope nor fibre staining was significantly affected by local AD pathology. Although in autopsy tissue global pathological alterations (Braak scores) were strongly correlated with the local Alzheimer pathology (beta-amyloid and AT8 staining) they turned out to be slightly correlated with both DCX+ fibres and neuronal envelopes, but unrelated to the DCX+ astrocytic somata. Thus, the DCX expression in distal fibres and astrocytic terminal fields surrounding neuronal cell bodies may be more affected by aspects of the Alzheimer process that are associated with e.g. general reduced neuronal activity than by local amyloid or hyperphosphorylated tau depositions.
In the adult rat dentate gyrus neurogenesis occurs in vascular niches consisting of clusters of neural, glial and endothelial precursors (Palmer et al., 2000). In the adult human neocortex the majority of DCX+ structures adjoining blood vessels had the appearance of astrocytic endfeet or of fibres with varicosities. DCX+ cells associated with blood vessels displaying an undifferentiated morphology were rare and could not be objectively identified as immature neurons. Nevertheless, in the temporal cortex of epileptic patients (Pincus et al., 1997), as well as in other adult human neocortical areas (Arsenijevic et al., 2001; Palmer et al., 2001) a population of residual precursors with neurogenic potential might persist (see also Björklund and Lindvall, 2000), which in the present study may have been masked by the mature astrocytic DCX immunoreactivity. Adult neural stem cells in the germinal zones have characteristics of astroglia (Alvarez-Buylla et al., 2001; Goldman, 2003) and it might be conceivable that a subpopulation of mature astrocytes has the capacity to de-differentiate and transform into stem-like cells (Leavitt et al., 1999; Steindler and Laywell, 2003). Whether the astrocytes with a DCX+ soma belong to such a subpopulation cannot be inferred from our data and to elucidate this further studies are required.
In brain development DCX is involved in microtubule stabilization (Gleeson et al., 1999) and nuclear translocation during neuronal migration (Koizumi et al., 2006) as well as in growth cone dynamics (Burgess and Reiner, 2000; Friocourt et al., 2003). Cultured cells isolated from the temporal cortex obtained at epilepsy surgery could be induced to express GFAP and DCX. Apart from the homogenous but mutually segregated presence of both GFAP and DCX in the cytoplasm of these ‘differentiated’ cells, the relative abundance of DCX in growth cone-like protrusions seemed to corroborate its original functions (cf Friocourt et al., 2003). Functions like nuclear translocation and growth cone dynamics seem less useful for post-mitotic, differentiated cells outside the germinal zones of the adult human brain. In this respect it is interesting that some but not all cells in mouse astrocyte cultures express DCLK-1C, a doublecortin-like molecule, while these cultured astrocytes did not express doublecortin itself (Friocourt et al., 2003). In these astrocytes DCLK-1C has a preferential localization to the peripheral processes and it was speculated that it might have a function in synaptic modulation (Friocourt et al., 2003). Although it is conceivable that the peripheral distribution of DCX in human neocortical astrocytes in situ subserves a similar function, an obvious preference of DCX for peripheral processes was not seen in cultured adult human astrocytes. Also Nacher et al. (2001) proposed that the DCX+ neurons in the rat piriform cortex were involved in axonal or synaptic plasticity. However, the punctate envelope-like morphology we found in the human brain is in contrast with the clearly cytoplasmic neuronal staining observed in that study (Nacher et al., 2001) suggesting that they may concern different aspects of plasticity.
Both the Santa Cruz and the Chemicon doublecortin antibody could stain astrocytes and neuronal envelopes. However, the difference in staining appearance (cf Fig. 4A–F and S4) and considerations detailed in the immunohistochemistry section of the supplementary material clearly indicate that further studies comparing DCX antibodies from these suppliers (if possible including antibodies from other sources) are required to establish which one is preferred for visualization of DCX in the adult human brain.
In conclusion, the observed DCX expression in differentiated human astrocytes and their cellular compartments was essentially similar over the disease groups studied and there were no indications that it is selectively involved in pathological alterations (including glial activation and inflammation). The presence of local Alzheimer pathology may interfere with the turnover or transport of DCX and, thus, attenuate DCX expression in the astrocytic somata. It is conceivable that DCX punctae of the neuronal envelopes play a role in glia-to-neuron signalling mediating synaptic or metabolic plasticity. Although in the persistent adult neurogenic zones like the subventricular zone, subgranular zone and infundibulum, DCX may be a useful marker of adult neurogenesis our data emphasize that it seems prudent not to extrapolate this notion to the general situation in the adult human brain.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We are grateful to the collaborators of the Netherlands Brain Bank and the epilepsy surgery team of the VU University Medical Center. We thank Dr J. Wijnholds for advice, S.I. Lutgens and P. Chiu for technical assistance and Dr Zhou Min for providing us with cryostat sections of mouse brain. This work was supported by the Internationale Stichting Alzheimer Onderzoek (ISAO), the Jan Dekkerstichting and Dr Lutgardine Bouwmanstichting.