-
PDF
- Split View
-
Views
-
Cite
Cite
Bettina Studer, Alicja Timm, Barbara J Sahakian, Tobias Kalenscher, Stefan Knecht, A decision-neuroscientific intervention to improve cognitive recovery after stroke, Brain, Volume 144, Issue 6, June 2021, Pages 1764–1773, https://doi.org/10.1093/brain/awab128
Close - Share Icon Share
Abstract
Functional recovery after stroke is dose-dependent on the amount of rehabilitative training. However, rehabilitative training is subject to motivational hurdles. Decision neuroscience formalizes drivers and dampers of behaviour and provides strategies for tipping motivational trade-offs and behaviour change. Here, we used one such strategy, upfront voluntary choice restriction (‘precommitment’), and tested if it can increase the amount of self-directed rehabilitative training in severely impaired stroke patients. In this randomized controlled study, stroke patients with working memory deficits (n = 83) were prescribed daily self-directed gamified cognitive training as an add-on to standard therapy during post-acute inpatient neurorehabilitation. Patients allocated to the precommitment intervention could choose to restrict competing options to self-directed training, specifically the possibility to meet visitors. This upfront choice restriction was opted for by all patients in the intervention group and highly effective. Patients in the precommitment group performed the prescribed self-directed gamified cognitive training twice as often as control group patients who were not offered precommitment [on 50% versus 21% of days, Pcorr = 0.004, d = 0.87, 95% confidence interval (CI95%) = 0.31 to 1.42], and, as a consequence, reached a 3-fold higher total training dose (90.21 versus 33.60 min, Pcorr = 0.004, d = 0.83, CI95% = 0.27 to 1.38). Moreover, add-on self-directed cognitive training was associated with stronger improvements in visuospatial and verbal working memory performance (Pcorr = 0.002, d = 0.72 and Pcorr = 0.036, d = 0.62). Our neuroscientific decision add-on intervention strongly increased the amount of effective cognitive training performed by severely impaired stroke patients. These results warrant a full clinical trial to link decision-based neuroscientific interventions directly with clinical outcome.
See Doogan and Leff (doi:10.1093/brain/awab169) for a scientific commentary on this article.
Introduction
Many modern medical interventions are critically dependent on patient behaviour, yet lack effective tools to ensure adherence: patients skip essential medication, only carry out half of their prescribed minimum physical activity, and do not stick to diet recommendations.1-6 This is also true for post-stroke rehabilitation. Each year, 16 million people suffer a first-time stroke.7 Besides physical deficits, impairments in learning, memory and executive functions are common consequences of stroke-induced brain damage, affecting up to 80% of stroke survivors8,9 and significantly weakening patients’ independence, participation, quality of life and long-term outcomes.10,11 Fortunately, high-intensive neurorehabilitative training can alleviate impairments in physical and cognitive functions.12–14 However, patients conduct much less rehabilitative therapy than required for successful recovery.15,16 One reason is that rehabilitative training requires substantial effort and persistence, and these motivational demands are often perceived as almost unconquerable obstacles.17 This is particularly the case when training is performed self-directed, without an encouraging therapist.18,19 Indeed, on three of four occasions, prescribed self-directed rehabilitative training is missed, and those sessions that are initiated are regularly cut short by patients.20
How can we help patients realize self-directed neurorehabilitative training (and other prescribed treatments) more frequently? We posit that models of motivation and tools developed in decision neuroscience and behavioural economics offer promising, yet largely untapped, clinical potential. Specifically, theoretical and empirical research from these fields indicates that the likelihood of conducting a given activity is determined by its subjective value versus its ‘opportunity costs’ in the form of simultaneously available alternatives that have to be forgone.21–23 Failures to conduct prescribed rehabilitative training are thus expected when competing alternatives are more attractive and less effortful. These tempting alternatives are as omnipresent in inpatient rehabilitation as in everyday life, ranging from socializing with visitors to watching TV. We argue that these opportunity costs may be tackled with ‘precommitment’, a strategy where agents voluntarily modify their own choice set ahead of time with the aim to increase the likelihood of a target action.24–26 Theoretically, this self-imposed modification can take two forms: (i) adding punishment for missing targets,27 which enhances the subjective value of the action; or (ii) restricting choice alternatives, which reduces the opportunity costs.22,28 In economic field research, precommitment raises consumers’ rates of healthy food shopping,29 gym attendance,30 retirement saving31 and chances of smoking cessation.32 Moreover, through the computational modelling of laboratory precommitment decisions, we recently demonstrated that precommitment is not only effective when willpower fails, as hitherto theorized, but can also be used to optimize behaviour.28 However, the potential of precommitment to increase health-promoting behaviours and treatment adherence in patients remains entirely unexplored. Furthermore, only punishment-based precommitment, which suffers from high rejection rates, has to date been trialled in real-life settings; whereas choice-restricting precommitment, which might meet higher acceptance, has solely been explored in laboratory experiments.28,33
This randomized controlled intervention study is the first to use precommitment to enhance the effectiveness of a clinical intervention and the first to test an upfront choice-restriction precommitment scheme in a real-life setting. Our trial targeted prescribed self-directed training in severely impaired stroke patients undergoing post-acute inpatient neurorehabilitation and entailed a precommitment intervention and a control group. All patients had deficits in visuospatial working memory and were instructed to conduct 30 min of self-directed restitutive cognitive training each day, using the cognitive training game ‘Wizard’, over a 2-week intervention period, in addition to their standard therapy. Wizard entails a paired-associates learning task in a 2D visual space and has been shown to improve visuospatial working memory performance and everyday life functioning in patients with schizophrenia.34 We chose this cognitive training game because—unlike most other cognitive training software for patients with acquired brain injury—it is suitable for self-directed training and has a motivating gamified nature. Further, this game enabled restitutive training of a cognitive function that is a prerequisite for higher cognitive processes, yet for which no effective interventions have been established to date.35–38
Our main aim was to test whether the adherence of patients to daily self-directed training could be enhanced through precommitment. To this end, we offered patients in the precommitment group two different purpose-designed voluntary precommitment schemes. The first scheme consisted of an upfront restriction of visitors during times designated for self-directed training, thereby removing this tempting alternative from the patients’ choice set and reducing the opportunity costs of training. The second scheme added social punishment to training misses by reporting them to the treating physician. Patients were free to implement one, both or neither precommitment option. We then assessed whether the precommitment group realized the prescribed daily self-directed training more frequently and thereby achieved a significantly higher dose of cognitive rehabilitation than the control group, who were not offered precommitment. We also verified that our stroke patients profited from the self-directed gamified cognitive training, by comparing their improvements in working memory tests to those of matched patients who received standard therapy only.
Materials and methods
Participants, experimental groups and main outcome measures
Adult ischaemic or haemorrhagic stroke patients with visuospatial memory impairments (n = 95) were recruited during inpatient neurorehabilitation at the Mauritius Hospital Meerbusch and randomly allocated to three groups, a precommitment, control and standard therapy-only group. Exclusion criteria were moderate or severe aphasia, dementia, severe deficits in multiple cognitive domains, inability to provide consent and multi-resistant bacteria. A minimization-based randomization algorithm (accounting for memory test scores, age and level of education) created with ‘MinimPy 3’ assigned patients to one of the three experimental groups.
Patients allocated to the precommitment and control groups were prescribed 30 min of self-directed training with the Wizard memory game each day for the 2-week intervention period, in addition to multidisciplinary standard therapy (including physical, language, neuropsychological and occupational therapy, according to individual need). A training reminder was printed on their daily schedules. The precommitment group could additionally choose one, none or both of the two offered precommitment schemes, a choice-restricting visitor ban and a social punishment comprising physician surveillance. Our main aim was to test whether precommitment increases the frequency and total dosage of self-directed gamified cognitive training. For each day, we recorded if and for how long patients trained using the Wizard game and statistically compared these two main outcome measures (training frequency and total training duration) between the precommitment and control groups. The target size was set to n = 33 patients per group a priori, based on effect sizes in previous literature, which range from moderate28,29,33 to very large,31,32 clinical relevance (primarily fulfilled by large effects) and patient availability. This sample size provides a power of 0.89 (at alpha = 0.05, two-tailed) to determine a large effect (d = 0.8) and a power of 0.82 in case of an anticipated drop-out rate of 20%.
A secondary aim of this study was to verify whether the add-on self-directed training with the Wizard game translated to superior recovery of working memory functions. To ensure that we could assess the effectiveness of add-on Wizard training, even if all precommitment and control group patients conducted self-directed training, we also used a standard therapy-only group.
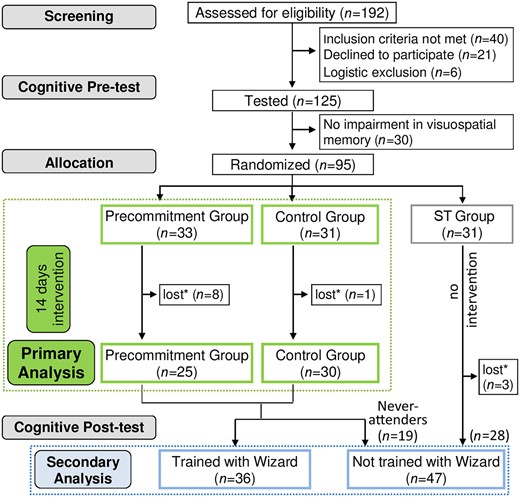
Figure 1 provides detailed information on screening, allocation and end point comparisons.
Study design, recruitment, allocation and analysis. *Patients lost due to being discharged from the hospital during the intervention period.
The Wizard memory game
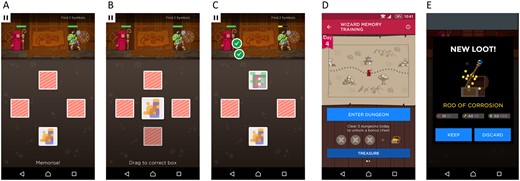
‘Wizard’ (Peak) offers gamified, tablet-based training for visuospatial working memory.34 In this game, geometrical figures are hidden under cards (Fig. 2). In each round, the cards are first turned over one at a time in a randomized order to reveal the hidden figure. Next, one figure at a time is presented, and the player has to indicate the hiding place through touchscreen selection. The task is woven into the narrative of a wizard who requires strength, weapons and trophies to fight monsters. The player collects these tokens through successful trials but loses fights when making too many mistakes. The task adjusts to the skill level of the player and becomes progressively more difficult as performance improves. The Wizard game could be played on tablet computers in a designated room with technical support provided. At the end of each training session, patients rated how much they enjoyed the Wizard game on a Likert scale ranging from 1 (not enjoyable at all) to 7 (extremely enjoyable). For logistical reasons, enjoyment ratings could only be collected from a subset of patients who attended Wizard training (n = 25).
The ‘Wizard’ memory training game. (A–C) Memory task: the positions of hidden geometrical figures have to be memorized and indicated (see main text for further explanation). (D and E) Narrative and example of a reward received after successful completion of a game round.
Cognitive pre- and post-testing
Standardized cognitive testing was performed before randomization and immediately after the intervention period. The testing battery included measures of (i) visuospatial working memory, to test for potential (near-transfer) effects of the visuospatial working memory training inherent to Wizard on an untrained task assessing the same working memory domain; (ii) verbal working memory, to test for potential far-transfer effects of the add-on Wizard training to another working memory domain; and (iii) verbal long-term memory that served as a control for which no training effects were expected.
Visuospatial working memory functions were assessed with the Wechsler Spatial Span Test.39 In this test, patients are shown a board with 10 spatially distributed cubes and asked to reproduce sequences taped by the examiner in the same (forward) or reverse (backward) order. The sequence length begins at two and increases progressively. Two sequences are presented at each length, and the task is terminated once both sequences of a given length are reproduced incorrectly. The number of correct sequences, computed separately for the forward and backward version, indexes visuospatial working memory performance. A percentile rank score of <15.87 for at least one version at pretesting further served as an inclusion criterion.
Verbal working memory and verbal long-term memory were tested with the verbal learning and memory test.40 In this test, patients are asked to memorize a list of 15 words in five consecutive learning trials, each followed by an immediate recall. Recall and recognition are tested again after a 30 min delay. A learning capacity score (quantified as the number of remembered words in the fifth learning trial) serves as a measure of verbal working memory and encoding; delayed recall and recognition scores reflect verbal long-term memory performance.41–44
Ethical approval
This research was approved by the Ethics Committee of the Medical Faculty of the University of Dusseldorf, Germany (protocol no 4835), conducted according to the revised Declaration of Helsinki and prospectively registered in the Clinical Trial Registry at the University of Dusseldorf (registration number: 2017024158). All patients provided written informed consent.
Statistical analyses
Statistical analyses were conducted in JASP and R. First, we compared our two primary outcomes, training frequency and total training duration between the precommitment and control groups with independent two-tailed t-tests. Furthermore, a χ2 contingency test was used to assess whether the proportion of never-attenders differed between these two groups.
Next, we compared pre-post changes (Δ) in visuospatial and verbal working memory measures of patients who performed add-on Wizard training versus those who did not (i.e. those randomized to the standard therapy-only group and never-attenders from the precommitment and control groups) and tested for a positive correlation between attenders’ total Wizard training duration and their improvements (i.e. a dose-effect relationship). We statistically controlled for potential confounding effects of pre-intervention cognitive performance levels in these analyses by using ANCOVAs (with the covariate: pre-intervention raw test score) and partial correlation models, respectively. To ascertain whether cognitive effects of add-on Wizard training were specific to working memory functions, equivalent ANCOVAs and partial correlations were calculated for the verbal long-term memory control measures. Finally, we tested if the outcomes confirmed as being sensitive to add-on Wizard training differed significantly between the precommitment and control groups, again using ANCOVAs controlling for pre-intervention scores.
For all statistical analyses, alpha was set to 0.05 and measures of effect sizes and their 95% confidence intervals (CI95%), in addition to P-values (with Holm-Bonferroni correction for multiple comparisons applied), are reported. In the Supplementary material, we additionally report Bayesian versions of the aforementioned analyses, which provide a direct measure of the likelihood of the identified statistical effects being true.
Data availability
The anonymized datasets of the current study are available from the corresponding author on reasonable request.
Results
Sample characteristics
Twelve of the 95 patients were discharged during the intervention period, resulting in a final sample of 83 patients (precommitment group: n = 25, control group: n = 30 and standard therapy group: n = 28). On average, patients were 73.7 years of age [standard error (SE) = 1.3], were 39.6 days post-stroke (SE = 2.8) and required assistance even in basic activities of daily living [Barthel Index: mean (M) = 60.1, SE = 11.06]. All suffered a marked deficit in visuospatial working memory as assessed using the Wechsler Spatial Span Test (average percentile ranks: forward = 11, SE = 2.62; backward = 6, SE = 2.24). Pairwise t- and contingency tests confirmed that the three groups were matched both in terms of demographic and clinical characteristics and the amount of received conventional therapy (Table 1).
Sample characteristics
| . | Precommitment group (n = 25) . | Control group (n = 30) . | Standard therapy group (n = 28) . | Comparisons, P . | ||
|---|---|---|---|---|---|---|
| . | . | . | . | P-C . | P-ST . | C-ST . |
| Gender, n (%) | 0.921 | 0.145 | 0.156 | |||
| Female | 13 (52) | 16 (53) | 8 (29) | |||
| Male | 12 (48) | 14 (47) | 20 (71) | |||
| Stroke type, n (%) | 0.562 | 0.168 | 0.049 | |||
| Ischaemic | 20 (80) | 22 (73) | 26 (93) | |||
| Haemorrhagic | 5 (20) | 8 (27) | 2 (7) | |||
| Years of education, n (%) | 0.295 | 0.346 | 0.929 | |||
| <12 | 20 (80) | 27 (90) | 25 (89) | |||
| ≥12 | 5 (20) | 3 (10) | 3 (11) | |||
| Age, mean (SE) | 73.72 (2.02) | 72.50 (1.77) | 74.89 (3.01) | 0.718 | 0.743 | 0.375 |
| Days since stroke, mean (SE) | 36.52 (4.36) | 44.87 (5.47) | 36.61 (4.32) | 0.252 | 0.989 | 0.246 |
| Barthel Index, mean (SE) | 65.80 (5.13) | 59.17 (4.91) | 55.89 (4.37) | 0.357 | 0.146 | 0.622 |
| Neuropsychological therapy, mean (SE) | 195.6 (50.1) | 203.0 (40.4) | 166.6 (31.9) | 0.908 | 0.620 | 0.487 |
| Other therapy, mean (SE) | 1194.6 (79.2) | 1202.5 (61.4) | 1164.1 (71.5) | 0.937 | 0.776 | 0.684 |
| . | Precommitment group (n = 25) . | Control group (n = 30) . | Standard therapy group (n = 28) . | Comparisons, P . | ||
|---|---|---|---|---|---|---|
| . | . | . | . | P-C . | P-ST . | C-ST . |
| Gender, n (%) | 0.921 | 0.145 | 0.156 | |||
| Female | 13 (52) | 16 (53) | 8 (29) | |||
| Male | 12 (48) | 14 (47) | 20 (71) | |||
| Stroke type, n (%) | 0.562 | 0.168 | 0.049 | |||
| Ischaemic | 20 (80) | 22 (73) | 26 (93) | |||
| Haemorrhagic | 5 (20) | 8 (27) | 2 (7) | |||
| Years of education, n (%) | 0.295 | 0.346 | 0.929 | |||
| <12 | 20 (80) | 27 (90) | 25 (89) | |||
| ≥12 | 5 (20) | 3 (10) | 3 (11) | |||
| Age, mean (SE) | 73.72 (2.02) | 72.50 (1.77) | 74.89 (3.01) | 0.718 | 0.743 | 0.375 |
| Days since stroke, mean (SE) | 36.52 (4.36) | 44.87 (5.47) | 36.61 (4.32) | 0.252 | 0.989 | 0.246 |
| Barthel Index, mean (SE) | 65.80 (5.13) | 59.17 (4.91) | 55.89 (4.37) | 0.357 | 0.146 | 0.622 |
| Neuropsychological therapy, mean (SE) | 195.6 (50.1) | 203.0 (40.4) | 166.6 (31.9) | 0.908 | 0.620 | 0.487 |
| Other therapy, mean (SE) | 1194.6 (79.2) | 1202.5 (61.4) | 1164.1 (71.5) | 0.937 | 0.776 | 0.684 |
C = control group; WSST = Wechsler Spatial Span Test. Neuropsychological therapy is the total minutes of standard neuropsychological therapy received during the intervention period. Other therapy is the total minutes of standard motor, speech and language, swallowing, occupational, sports and creative therapy received during the intervention period. The groups did not differ significantly in any of these background measures, except that the proportion of ischaemic stroke was higher in the standard therapy than the control group (a contrast that is inconsequential for our outcome analyses).
Sample characteristics
| . | Precommitment group (n = 25) . | Control group (n = 30) . | Standard therapy group (n = 28) . | Comparisons, P . | ||
|---|---|---|---|---|---|---|
| . | . | . | . | P-C . | P-ST . | C-ST . |
| Gender, n (%) | 0.921 | 0.145 | 0.156 | |||
| Female | 13 (52) | 16 (53) | 8 (29) | |||
| Male | 12 (48) | 14 (47) | 20 (71) | |||
| Stroke type, n (%) | 0.562 | 0.168 | 0.049 | |||
| Ischaemic | 20 (80) | 22 (73) | 26 (93) | |||
| Haemorrhagic | 5 (20) | 8 (27) | 2 (7) | |||
| Years of education, n (%) | 0.295 | 0.346 | 0.929 | |||
| <12 | 20 (80) | 27 (90) | 25 (89) | |||
| ≥12 | 5 (20) | 3 (10) | 3 (11) | |||
| Age, mean (SE) | 73.72 (2.02) | 72.50 (1.77) | 74.89 (3.01) | 0.718 | 0.743 | 0.375 |
| Days since stroke, mean (SE) | 36.52 (4.36) | 44.87 (5.47) | 36.61 (4.32) | 0.252 | 0.989 | 0.246 |
| Barthel Index, mean (SE) | 65.80 (5.13) | 59.17 (4.91) | 55.89 (4.37) | 0.357 | 0.146 | 0.622 |
| Neuropsychological therapy, mean (SE) | 195.6 (50.1) | 203.0 (40.4) | 166.6 (31.9) | 0.908 | 0.620 | 0.487 |
| Other therapy, mean (SE) | 1194.6 (79.2) | 1202.5 (61.4) | 1164.1 (71.5) | 0.937 | 0.776 | 0.684 |
| . | Precommitment group (n = 25) . | Control group (n = 30) . | Standard therapy group (n = 28) . | Comparisons, P . | ||
|---|---|---|---|---|---|---|
| . | . | . | . | P-C . | P-ST . | C-ST . |
| Gender, n (%) | 0.921 | 0.145 | 0.156 | |||
| Female | 13 (52) | 16 (53) | 8 (29) | |||
| Male | 12 (48) | 14 (47) | 20 (71) | |||
| Stroke type, n (%) | 0.562 | 0.168 | 0.049 | |||
| Ischaemic | 20 (80) | 22 (73) | 26 (93) | |||
| Haemorrhagic | 5 (20) | 8 (27) | 2 (7) | |||
| Years of education, n (%) | 0.295 | 0.346 | 0.929 | |||
| <12 | 20 (80) | 27 (90) | 25 (89) | |||
| ≥12 | 5 (20) | 3 (10) | 3 (11) | |||
| Age, mean (SE) | 73.72 (2.02) | 72.50 (1.77) | 74.89 (3.01) | 0.718 | 0.743 | 0.375 |
| Days since stroke, mean (SE) | 36.52 (4.36) | 44.87 (5.47) | 36.61 (4.32) | 0.252 | 0.989 | 0.246 |
| Barthel Index, mean (SE) | 65.80 (5.13) | 59.17 (4.91) | 55.89 (4.37) | 0.357 | 0.146 | 0.622 |
| Neuropsychological therapy, mean (SE) | 195.6 (50.1) | 203.0 (40.4) | 166.6 (31.9) | 0.908 | 0.620 | 0.487 |
| Other therapy, mean (SE) | 1194.6 (79.2) | 1202.5 (61.4) | 1164.1 (71.5) | 0.937 | 0.776 | 0.684 |
C = control group; WSST = Wechsler Spatial Span Test. Neuropsychological therapy is the total minutes of standard neuropsychological therapy received during the intervention period. Other therapy is the total minutes of standard motor, speech and language, swallowing, occupational, sports and creative therapy received during the intervention period. The groups did not differ significantly in any of these background measures, except that the proportion of ischaemic stroke was higher in the standard therapy than the control group (a contrast that is inconsequential for our outcome analyses).
Precommitment increased frequency of self-directed training
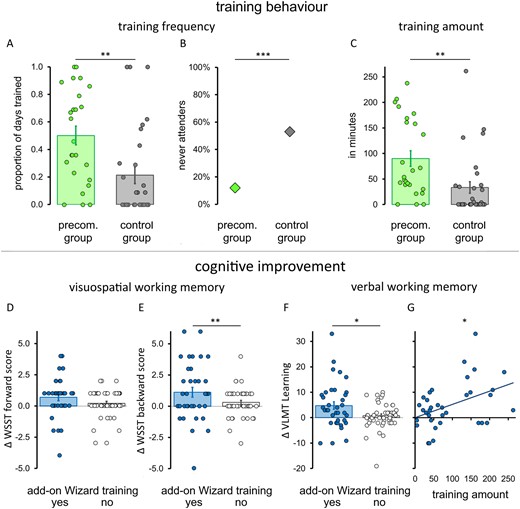
Our patients were highly willing to precommit, with a strong preference for the choice-restricting option (entailing a visitor ban). All patients in the precommitment group opted for this scheme, whereas the physician surveillance option was implemented by only one patient. Most importantly, precommitment significantly enhanced the frequency of performed self-directed cognitive training. On average, patients in the precommitment group trained with the Wizard memory game on every second day (frequency: M = 0.50, SE = 0.068), whereas control group patients only trained on every fifth day [M = 0.21, SE = 0.059, t(53) = 3.206, Pcorr = 0.004; Fig. 3A]. This effect on training frequency was determined to be large (effect size d = 0.868, CI95% = 0.309 to 1.429). The proportion of patients who never performed the prescribed self-directed training was significantly lower in the precommitment group (n = 3, 12%) than in the control group [n = 16; 53%, χ2(1) = 10.303, Pcorr = 0.001, log odds ratio = −2.126, CI95% = −3.529 to −0.723; Fig. 3B].
Precommitment and training effects. Precommitment enhanced training behaviour (A–C). The frequency of training (A), proportion of never-attenders (B) and the total amount of Wizard training performed (C) by the precommitment (green) and control (grey) groups are displayed. Add-on Wizard training was associated with stronger cognitive improvements (D–G). Post-intervention improvements in the Wechsler spatial span test (D–F) and verbal learning and memory test (F) of patients who performed the add-on self-directed training with the Wizard game (blue) and those who underwent standard treatment only (grey) are displayed. Furthermore, the relationship between improvements in verbal learning and the amount of Wizard training performed is shown (G). For all panels, bars (and diamonds in B) indicate the group averages, and circles represent the data-points of the individual patients. Error bars represent the standard error of the mean (SEM). ***Pcorr < 0.001, **Pcorr < 0.01 and *Pcorr < 0.05.
Total training duration likewise differed statistically significantly and strongly between the two groups, with the precommitment group conducting 90.21 min of self-directed training (SE = 15.21) on average, whereas the control group practiced for only 33.60 min [SE = 11.21, t(53) = 3.055, Pcorr = 0.004, d = 0.827, CI95% = 0.270 to 1.377; Fig. 3C]. This effect held only when all patients were included; when never-attenders were removed, the total amount of training by the two groups no longer differed significantly [Mprecom. = 102.51 min, SEM = 15.51; Mcontrol = 72.00 min, SEM = 19.70, t(34) = 1.221, P = 0.471, d = 0.417, CI95% = 0.263 to 1.092]. This indicates that the higher total training dosage achieved by the precommitment group was a direct consequence of their increased training frequency, rather than due to longer play per se (Table 2).
Primary outcomes: frequency and amount of self-directed training
| Training . | Precommitment group (n = 25) Mean (SE) [range] . | Control group (n = 30) Mean (SE) [range] . | Comparison . | ||
|---|---|---|---|---|---|
| . | . | . | Pcorr . | d . | CI95% . |
| Frequency | 0.50 (0.068) [0–100] | 0.21 (0.059) [0–100] | 0.004 | 0.868 | 0.309, to 1.492 |
| Amount, min | 90.21 (15.21) [0–239] | 33.60 (11.21) [0–263] | 0.004 | 0.827 | 0.270, to 1.377 |
| Training . | Precommitment group (n = 25) Mean (SE) [range] . | Control group (n = 30) Mean (SE) [range] . | Comparison . | ||
|---|---|---|---|---|---|
| . | . | . | Pcorr . | d . | CI95% . |
| Frequency | 0.50 (0.068) [0–100] | 0.21 (0.059) [0–100] | 0.004 | 0.868 | 0.309, to 1.492 |
| Amount, min | 90.21 (15.21) [0–239] | 33.60 (11.21) [0–263] | 0.004 | 0.827 | 0.270, to 1.377 |
Pcorr = Holm-Bonferroni corrected P-value.
Primary outcomes: frequency and amount of self-directed training
| Training . | Precommitment group (n = 25) Mean (SE) [range] . | Control group (n = 30) Mean (SE) [range] . | Comparison . | ||
|---|---|---|---|---|---|
| . | . | . | Pcorr . | d . | CI95% . |
| Frequency | 0.50 (0.068) [0–100] | 0.21 (0.059) [0–100] | 0.004 | 0.868 | 0.309, to 1.492 |
| Amount, min | 90.21 (15.21) [0–239] | 33.60 (11.21) [0–263] | 0.004 | 0.827 | 0.270, to 1.377 |
| Training . | Precommitment group (n = 25) Mean (SE) [range] . | Control group (n = 30) Mean (SE) [range] . | Comparison . | ||
|---|---|---|---|---|---|
| . | . | . | Pcorr . | d . | CI95% . |
| Frequency | 0.50 (0.068) [0–100] | 0.21 (0.059) [0–100] | 0.004 | 0.868 | 0.309, to 1.492 |
| Amount, min | 90.21 (15.21) [0–239] | 33.60 (11.21) [0–263] | 0.004 | 0.827 | 0.270, to 1.377 |
Pcorr = Holm-Bonferroni corrected P-value.
Self-directed training with the Wizard game improved working memory functions
Patients rated the Wizard game as highly enjoyable (M = 5.31, SE = 0.194, on a scale of 1–7), and their enjoyment ratings positively predicted their total Wizard training duration (β = 13.034, P = 0.015), confirming that the gamified cognitive training was fun and motivating. Moreover, patients who conducted Wizard training (n = 36) showed a significantly larger pre-post change in the Wechsler spatial span test backward scores than those who were not offered or never executed this add-on self-directed training (n = 47), including 28 standard therapy group patients, 16 never-attenders from the control group and three never-attenders from the precommitment group [F(1,80) = 12.947, Pcorr = 0.002, d = 0.72; Fig. 3E and Table 3]. This stronger visuospatial working memory improvement was found after statistically controlling for pre-intervention performance levels, which were themselves negatively associated with pre-post changes (Supplementary Table 1). Albeit numerically pointing in the same direction, improvements in the Wechsler spatial span test forward score did not significantly differ between those who did and did not undergo Wizard training [F(1,80) = 3.258, Pcorr = 0.225, d = 0.43; Fig. 3D]. Add-on Wizard training was, however, also associated with significantly larger pre-post changes in the verbal learning capacity score on the verbal learning and memory test [F(1,80) = 7.259, Pcorr = 0.036, d = 0.62; Fig. 3F], and the degree of improvement in this verbal working memory index was positively related to the Wizard training dose in training attendees (r = 0.439, CI95% = 0.129 to 0.671, Pcorr = 0.041; Fig. 3G and SupplementaryTable 2). Finally, pre-post change in the two measures of verbal long-term memory, which acted as control measures not expected to improve through Wizard training, did not differ significantly between these two comparison groups [delayed recall: F(1,80) = 0.011, Pcorr = 0.918, d = 0.02; delayed recognition: F(1,80) = 0.739, Pcorr = 0.786, d = 0.16] and neither did the amount of conventional neuropsychological therapy received [MWizard training = 219.6 min, SE = 42.57, MNoWizard training = 164.7 min, SE = 25.2, t(81) = 1.167, P = 0.247].
In a final step, we tested whether the increase in the dose of self-directed training achieved through precommitment was large enough, at the group level, to effectuate stronger improvements in the two measures identified as training-sensitive in the abovementioned main analyses. Such superior improvement could not be confirmed for either cognitive test [Wechsler spatial span test (backward): F(1,52) = 0.679, Pcorr = 0.414, d = 0.21; verbal learning and memory test (verbal learning capacity): F(1,52) = 1.365, Pcorr = 0.596, d = 0.32, respectively; Table 3].
Secondary outcomes: cognitive improvements over the intervention period
| Cognitive outcomes as a function of add-on self-directed training with Wizard . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure . | Wizard training (n = 36) . | No Wizard training (n = 47) . | Comparison improvement (Δ) . | |||||
| . | Pre . | Post . | Pre . | Post . | MD . | CI95% . | Pcorr . | d . |
| WSST | ||||||||
| Forward | 5.64 (0.25)a | 6.33 (0.35)b | 4.38 (0.28)a | 4.59 (0.31)b | 0.58 (0.32) | −0.06 to 1.22 | 0.225 | 0.43 |
| Backward | 3.50 (0.36) | 4.61 (0.37)b | 2.45 (0.28) | 2.75 (0.24)b | 1.26 (0.35) | 0.56 to 1.96 | 0.002 | 0.72 |
| VLMT | ||||||||
| Learning | 28.08 (1.73) | 32.86 (2.37)b | 23.19 (1.45) | 23.91 (1.41)b | 4.33 (1.61) | 1.13 to 7.52 | 0.036 | 0.62 |
| Delayed recall | 5.11 (0.59) | 6.08 (0.89) | 3.40 (0.42) | 4.91 (0.83) | 0.12 (1.19) | −2.26 to 2.51 | 0.918 | 0.02 |
| Delayed recognition | 3.69 (1.27) | 5.72 (1.49) | 2.97 (0.94) | 4.04 (0.97) | 1.27 (1.48) | 1.67 to 4.20 | 0.786 | 0.16 |
| Cognitive outcomes as a function of add-on self-directed training with Wizard . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure . | Wizard training (n = 36) . | No Wizard training (n = 47) . | Comparison improvement (Δ) . | |||||
| . | Pre . | Post . | Pre . | Post . | MD . | CI95% . | Pcorr . | d . |
| WSST | ||||||||
| Forward | 5.64 (0.25)a | 6.33 (0.35)b | 4.38 (0.28)a | 4.59 (0.31)b | 0.58 (0.32) | −0.06 to 1.22 | 0.225 | 0.43 |
| Backward | 3.50 (0.36) | 4.61 (0.37)b | 2.45 (0.28) | 2.75 (0.24)b | 1.26 (0.35) | 0.56 to 1.96 | 0.002 | 0.72 |
| VLMT | ||||||||
| Learning | 28.08 (1.73) | 32.86 (2.37)b | 23.19 (1.45) | 23.91 (1.41)b | 4.33 (1.61) | 1.13 to 7.52 | 0.036 | 0.62 |
| Delayed recall | 5.11 (0.59) | 6.08 (0.89) | 3.40 (0.42) | 4.91 (0.83) | 0.12 (1.19) | −2.26 to 2.51 | 0.918 | 0.02 |
| Delayed recognition | 3.69 (1.27) | 5.72 (1.49) | 2.97 (0.94) | 4.04 (0.97) | 1.27 (1.48) | 1.67 to 4.20 | 0.786 | 0.16 |
| Cognitive outcomes in the precommitment compared to the control group . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure . | Precommitment group (n = 25) . | Control group (n = 30) . | Comparison improvement (Δ) . | |||||
| . | Pre . | Post . | Pre . | Post . | MD . | CI95% . | Pcorr . | d . |
| WSST | ||||||||
| Backward | 2.96 (0.47) | 4.00 (0.51) | 2.73 (0.37) | 3.43 (0.40) | 0.41 (0.50) | 0.59 to 1.42 | 0.414 | 0.21 |
| VLMT | ||||||||
| Learning | 26.76 (2.18) | 31.48 (2.94) | 25.50 (1.86) | 27.67 (2.29) | 2.48 (2.12) | 1.78 to 6.74 | 0.496 | 0.32 |
| Cognitive outcomes in the precommitment compared to the control group . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure . | Precommitment group (n = 25) . | Control group (n = 30) . | Comparison improvement (Δ) . | |||||
| . | Pre . | Post . | Pre . | Post . | MD . | CI95% . | Pcorr . | d . |
| WSST | ||||||||
| Backward | 2.96 (0.47) | 4.00 (0.51) | 2.73 (0.37) | 3.43 (0.40) | 0.41 (0.50) | 0.59 to 1.42 | 0.414 | 0.21 |
| VLMT | ||||||||
| Learning | 26.76 (2.18) | 31.48 (2.94) | 25.50 (1.86) | 27.67 (2.29) | 2.48 (2.12) | 1.78 to 6.74 | 0.496 | 0.32 |
No Wizard training group includes all patients randomized to the standard therapy only group and never-attenders of the precommitment and the control groups. Measures that improved significantly more in patients who conducted add-on training with the Wizard game than in those who did not (after controlling for pre-intervention performance level) are highlighted in bold. C = control; VLMT = Verbal Learning and Memory Test; WSST = Wechsler Spatial Span Test. Pcorr = Holm-Bonferroni corrected P-value.
a,bGroup differences in raw scores at pre-interventiona and post-interventionb testing (conducted separately) where Pcorr ≤ 0.05.
Secondary outcomes: cognitive improvements over the intervention period
| Cognitive outcomes as a function of add-on self-directed training with Wizard . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure . | Wizard training (n = 36) . | No Wizard training (n = 47) . | Comparison improvement (Δ) . | |||||
| . | Pre . | Post . | Pre . | Post . | MD . | CI95% . | Pcorr . | d . |
| WSST | ||||||||
| Forward | 5.64 (0.25)a | 6.33 (0.35)b | 4.38 (0.28)a | 4.59 (0.31)b | 0.58 (0.32) | −0.06 to 1.22 | 0.225 | 0.43 |
| Backward | 3.50 (0.36) | 4.61 (0.37)b | 2.45 (0.28) | 2.75 (0.24)b | 1.26 (0.35) | 0.56 to 1.96 | 0.002 | 0.72 |
| VLMT | ||||||||
| Learning | 28.08 (1.73) | 32.86 (2.37)b | 23.19 (1.45) | 23.91 (1.41)b | 4.33 (1.61) | 1.13 to 7.52 | 0.036 | 0.62 |
| Delayed recall | 5.11 (0.59) | 6.08 (0.89) | 3.40 (0.42) | 4.91 (0.83) | 0.12 (1.19) | −2.26 to 2.51 | 0.918 | 0.02 |
| Delayed recognition | 3.69 (1.27) | 5.72 (1.49) | 2.97 (0.94) | 4.04 (0.97) | 1.27 (1.48) | 1.67 to 4.20 | 0.786 | 0.16 |
| Cognitive outcomes as a function of add-on self-directed training with Wizard . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure . | Wizard training (n = 36) . | No Wizard training (n = 47) . | Comparison improvement (Δ) . | |||||
| . | Pre . | Post . | Pre . | Post . | MD . | CI95% . | Pcorr . | d . |
| WSST | ||||||||
| Forward | 5.64 (0.25)a | 6.33 (0.35)b | 4.38 (0.28)a | 4.59 (0.31)b | 0.58 (0.32) | −0.06 to 1.22 | 0.225 | 0.43 |
| Backward | 3.50 (0.36) | 4.61 (0.37)b | 2.45 (0.28) | 2.75 (0.24)b | 1.26 (0.35) | 0.56 to 1.96 | 0.002 | 0.72 |
| VLMT | ||||||||
| Learning | 28.08 (1.73) | 32.86 (2.37)b | 23.19 (1.45) | 23.91 (1.41)b | 4.33 (1.61) | 1.13 to 7.52 | 0.036 | 0.62 |
| Delayed recall | 5.11 (0.59) | 6.08 (0.89) | 3.40 (0.42) | 4.91 (0.83) | 0.12 (1.19) | −2.26 to 2.51 | 0.918 | 0.02 |
| Delayed recognition | 3.69 (1.27) | 5.72 (1.49) | 2.97 (0.94) | 4.04 (0.97) | 1.27 (1.48) | 1.67 to 4.20 | 0.786 | 0.16 |
| Cognitive outcomes in the precommitment compared to the control group . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure . | Precommitment group (n = 25) . | Control group (n = 30) . | Comparison improvement (Δ) . | |||||
| . | Pre . | Post . | Pre . | Post . | MD . | CI95% . | Pcorr . | d . |
| WSST | ||||||||
| Backward | 2.96 (0.47) | 4.00 (0.51) | 2.73 (0.37) | 3.43 (0.40) | 0.41 (0.50) | 0.59 to 1.42 | 0.414 | 0.21 |
| VLMT | ||||||||
| Learning | 26.76 (2.18) | 31.48 (2.94) | 25.50 (1.86) | 27.67 (2.29) | 2.48 (2.12) | 1.78 to 6.74 | 0.496 | 0.32 |
| Cognitive outcomes in the precommitment compared to the control group . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure . | Precommitment group (n = 25) . | Control group (n = 30) . | Comparison improvement (Δ) . | |||||
| . | Pre . | Post . | Pre . | Post . | MD . | CI95% . | Pcorr . | d . |
| WSST | ||||||||
| Backward | 2.96 (0.47) | 4.00 (0.51) | 2.73 (0.37) | 3.43 (0.40) | 0.41 (0.50) | 0.59 to 1.42 | 0.414 | 0.21 |
| VLMT | ||||||||
| Learning | 26.76 (2.18) | 31.48 (2.94) | 25.50 (1.86) | 27.67 (2.29) | 2.48 (2.12) | 1.78 to 6.74 | 0.496 | 0.32 |
No Wizard training group includes all patients randomized to the standard therapy only group and never-attenders of the precommitment and the control groups. Measures that improved significantly more in patients who conducted add-on training with the Wizard game than in those who did not (after controlling for pre-intervention performance level) are highlighted in bold. C = control; VLMT = Verbal Learning and Memory Test; WSST = Wechsler Spatial Span Test. Pcorr = Holm-Bonferroni corrected P-value.
a,bGroup differences in raw scores at pre-interventiona and post-interventionb testing (conducted separately) where Pcorr ≤ 0.05.
Discussion
Our intervention study attests that choice-restricting precommitment enhances health-restorative training in stroke patients. All patients in our precommitment group chose the choice-restricting and opportunity-costs reducing visitor-block precommitment scheme. Moreover, this upfront choice restriction was highly effective: the precommitment group realized the prescribed daily self-directed cognitive training more than twice as often and attained a 3-fold higher total dosage of the gamified cognitive training than the control group, which was not offered precommitment. This marked increase in the frequency and amount of self-directed cognitive training achieved with the precommitment intervention is of direct clinical relevance.
The degree of functional recovery from stroke is determined by the amount of neurorehabilitative training,45,46 but the amounts of conventional rehabilitative therapy provided fall dramatically short of those required for successful recovery15,16,47—a situation that will continue to worsen due to ongoing demographic change and resulting health-economic pressures. Add-on self-directed training constitutes a resource-efficient solution but suffers from particularly poor adherence.20 Our findings demonstrate that the achieved dosage of self-directed rehabilitative training can be increased through a decision-neuroscientific add-on intervention.
Many of us use precommitment intuitively in everyday life. We impose spending limits, destroy cigarettes and set ourselves costly deadlines.27 Blocking visitors that deter from health-restorative training might likewise appear common sense. However, previous reports of low adherence to self-directed neurorehabilitative therapy20 and our observation that the control group missed their prescribed training on 79% of occasions suggest that patients often fail to employ such seemingly obvious strategies without external prompting. Given that lack of adherence is not unique to stroke rehabilitation, but also observed for pharmacological, lifestyle and dietary interventions,2,48,49 it seems likely that other patient-dependent interventions and secondary prevention therapies would also profit from situation-tailored choice-restricting precommitment schemes.
Our results further indicate that choice-restricting precommitment schemes enjoy higher acceptability than punishment-based precommitment options. Extant field studies have exclusively utilized financial penalties or loss of financial rewards as precommitment options, and these punishment-based schemes were rejected by 74–88% of the participating individuals.29–32 Similarly, our social punishment entailing physician surveillance precommitment scheme was rejected by all but one patient. In stark contrast, all patients accepted the choice-restricting visitor-ban precommitment option. Together, these results call for a shift away from punishment-based to choice-restricting precommitment schemes in future intervention research.
In addition to the precommitment effect, we show that self-directed training with the Wizard game improved working memory functions of our impaired stroke patients. Patients who underwent add-on self-directed Wizard training improved significantly more in the backward version of the Wechsler Spatial Span Test (a measure of visuospatial working memory) and the verbal learning and memory test verbal learning score (a measure of verbal learning) than those who did not, and verbal working memory improvements even scaled linearly with the amount of Wizard training. These results not only concur with other working memory training studies50,51 in refuting doubts over the effectiveness of restitutive training after acquired brain damage,12,37,52 but also indicate that far-transfer effects of training of one working memory domain (visuospatial) to another (verbal) can occur. Working memory has been conceptualized as a multi-component system, where a central control system (‘central executive’) is aided by three temporary storage systems, the visuospatial sketchpad (for visual material), phonological loop (for verbal-auditory material) and episodic buffer.53–56 One plausible mechanism underlying the observed far-transfer effect could be that Wizard training strengthened functioning of the central executive, which is arguably crucial for both visuospatial and verbal working memory performance. Speculatively, such a mechanism of action could also be the reason why improvement differences between those who did and did not perform add-on Wizard training were statistically more robust for the backward Wechsler Spatial Span Test version (with higher central executive demands) than for the forward version, which primarily reflects short-term memory capacity.57–59
Our study further speaks to the potential of gamifying rehabilitative training for stroke and other neurological patients. Gamification uses game design elements such as quests, points, badges, levels, feedback and competition to make serious applications more fun and engaging and can facilitate the realization of established rehabilitation principles.60 Gamification of rehabilitative training is increasingly popular, with the first clinical trials focusing on motor function training providing promising results.61,62 Even so, gamification is not without critics, with the primary concern being that the extrinsic rewards used in gamification might hamper a patient’s autonomy and intrinsic motivation.63 Our results argue against this. Our stroke patients rated the gamified cognitive training as highly enjoyable, echoing prior results in healthy older people64 and patients with schizophrenia.65 Moreover, these enjoyment ratings positively predicted their training amounts, suggesting that the fun-boosting effect of gamification enticed patients to practice for longer. At the same time, gamification alone might not be sufficient because it is only effective during training and therefore likely to fail in prompting patients to choose training over tempting alternatives in the first place. Gamified interventions can thus still profit from precommitment (as shown in this study) and indeed may even need it to become effective.
We note three constraints in our results. First, although the precommitment group conducted the prescribed self-directed training twice as often as the control group, they still skipped it every other day on average and trained shorter than prescribed. This suggests that offering precommitment and gamifying training is still not enough to maximize adherence to self-directed rehabilitative training in every patient. Combination with further behaviour-modification tools derived from decision neuroscience (e.g. competition19 or social groups) and tailoring of these tools to the individual might be interesting avenues for future optimization research. Second, while add-on Wizard training enhanced memory recovery compared to standard therapy alone, at the group level, the cognitive improvements of the precommitment intervention group were not significantly larger than those of the control group. Plausible reasons might be that this group comparison was underpowered and/or that the average training dosage achieved over the 2-week intervention period in the precommitment group—albeit 3-fold higher than that of the control group—was still small. Third, and relatedly, our data do not allow verification of whether the achieved functional improvements in working memory due to the add-on Wizard training generalize to improved functioning in everyday life. It seems likely that larger training doses than those achieved during the 2-week observation period would be required. A follow-up full clinical trial should test if robust improvements in everyday life functioning can be achieved if the precommitment intervention and add-on cognitive training are sustained over longer periods, e.g. over the entire duration of post-stroke rehabilitation.
Conclusions
Our novel approach of using a choice-restricting precommitment scheme successfully and significantly increased the patients’ adherence to prescribed self-directed rehabilitative training. This showcases how decision-neuroscientific strategies can help patients to achieve higher doses of the rehabilitative therapies administered to stroke patients in current clinical practice and thereby create the prerequisites for effective cognitive rehabilitation.
Acknowledgements
We thank George Savulich and Hannah Strenger for assistance and Peak (London, UK) for the provision of the Wizard training game.
Funding
This research was financed by intramural research funds of the Mauritius Hospital Meerbusch. The Wizard Memory Game was invented at the University of Cambridge by Barbara Sahakian and Thomas Piercy, a member of her laboratory, and the Wizard Game was Technology Transferred by Cambridge Enterprise to PEAK. B.J.S. is funded by the Wallitt Foundation and Eton College and her research is conducted within the NIHR MedTech and Invitro Diagnostic Co-operative (MIC) and the NIHR Cambridge Biomedical Research Centre (Neurodegeneration and Mental Health Themes).
Competing interests
B.J.S. consults for Peak. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.