-
PDF
- Split View
-
Views
-
Cite
Cite
Pil-Soon Son, Sin-Aye Park, Hye-Kyung Na, Dae-Myung Jue, Sanghee Kim, Young-Joon Surh, Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-κB activation and cyclooxygenase-2 expression in human breast epithelial cells: cysteine 179 of IKKβ as a potential target, Carcinogenesis, Volume 31, Issue 8, August 2010, Pages 1442–1449, https://doi.org/10.1093/carcin/bgq099
Close - Share Icon Share
Abstract
There are multiple lines of evidence supporting that chronic inflammation is linked to carcinogenesis. Nuclear factor-κB (NF-κB), a major redox-sensitive transcription factor responsible for the induction of a wide array of pro-inflammatory genes, is frequently overactivated in many tumors. Moreover, constitutive activation of IκB kinase (IKK), a key regulator of NF-κB signaling, has been implicated in inflammation-associated tumorigenesis. Piceatannol ( trans -3,4,3′,5′-tetrahydroxystilbene; PIC) derived from grapes, rhubarb and sugarcane exhibits immunosuppressive and antitumorigenic activities in several cell lines, but the underlying mechanisms are poorly understood. In the present study, we found that PIC inhibited migration and anchorage-independent growth of human mammary epithelial cells (MCF-10A) treated with the prototypic tumor promoter, 12- O -tetradecanoylphorbol-13-aceate (TPA). PIC treatment suppressed the TPA-induced activation of NF-κB and expression of cyclooxygenase-2 (COX-2) in MCF-10A cells. We speculate that an electrophilic quinone formed as a consequence of oxidation of PIC bearing the catechol moiety may directly interact with critical cysteine thiols of IKKβ, thereby inhibiting its catalytic activity. In support of this speculation, the reducing agent dithiothreitol abrogated the inhibitory effects of PIC on TPA-induced activation of NF-κB signaling and expression of COX-2. In addition, the inhibitory effects of PIC on NF-κB activation and COX-2 induction were blunted in cells expressing mutant IKKβ (C179A) in which cysteine 179 was replaced by alanine. In conclusion, our results show that direct modification of IKKβ by PIC, presumably at the cysteine 179 residue, blocks NF-κB activation signaling and COX-2 induction in TPA-treated MCF-10A cells and also migration and transformation of these cells.
Introduction
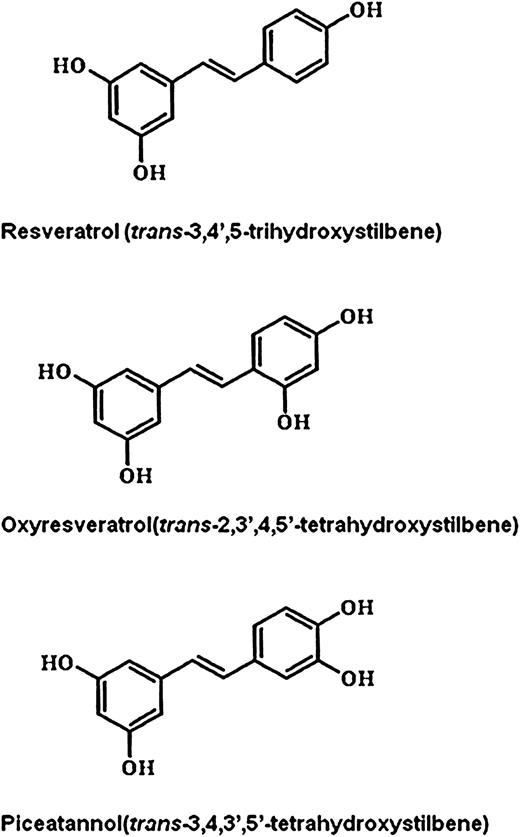
Over the past few years, some naturally occurring stilbene compounds have attracted substantial attention because of a wide range of their biological activities ( 1 ). An example is resveratrol ( trans -3,4′,5-trihydroxystilbene) that is a common phytoalexin found in grapes, mulberries and other edible plants ( 2 ). Resveratrol has a number of health beneficial properties including antioxidant, anti-inflammatory, antiaging, cardioprotective and neuroprotective activities ( 3–5 ). Resveratrol has also been reported to inhibit the process of carcinogenesis in several stages including initiation, promotion and progression ( 6 , 7 ). One of the mechanisms underlying chemopreventive effects of resveratrol is suppression of the inflammatory response by blocking expression or activity of pro-inflammatory enzymes, such as cyclooxygense-2 (COX-2) and inducible nitric oxide synthase that are primarily regulated by nuclear factor-κB (NF-κB) or activator protein-1 ( 8 ).
The bioavailability of resveratrol is relatively poor due to its rapid metabolism ( 9 ), and recent studies focus on chemopreventive potential of other hydroxylated stilbenes. Among them, piceatannol ( trans -3,4,3′,5′-tetrahydroxystilbene; PIC) is a polyphenol found in grapes, rhubarb and sugarcane. It is known as a protein kinase inhibitor that modulates multiple targets in the intracellular signal transduction pathways and elicits antioxidant, immunosuppressive and anti-inflammatory effects as well as antitumorigenic activities ( 10–12 ). PIC was found to exert an inhibitory effect on tumor necrosis factor-induced NF-κB activation and NF-κB-mediated gene expression ( 13 ). However, the mechanisms underlying its chemopreventive as well as anti-inflammatory effects remain largely unresolved.
It has been increasingly evident that chronic inflammation can cause cancer ( 14 , 15 ). COX-2 is an early-response gene product that is inducible by various inflammatory stimuli, including tumor promoters, growth factors, pro-inflammatory cytokines, bacterial toxins and reactive oxygen species ( 15 , 16 ). One of the major transcription factors responsible for regulating cox-2 gene transcription is NF-κB, a ubiquitous eukaryotic transcription factor that is involved in inflammatory responses as well as cell proliferation and survival ( 17 ). NF-κB is sequestered in the cytoplasm of unstimulated cells by a family of inhibitory proteins including inhibitory protein κBα (IκBα). Pro-inflammatory cytokines, such as tumor necrosis factor-α and interleukin-1 as well as the tumor promoter, 12- O -tetradecanoylphorbol-13-acetate (TPA), induce phosphorylation of IκBα at specific serine residues, resulting in ubiquitination and subsequent degradation of this inhibitory protein. Release of NF-κB, quintessentially composed of p65/RelA and p50, allows it to translocate to the nucleus and induce expression of target genes ( 18 , 19 ).
The protein kinases responsible for IκB phosphorylation are IκB kinases (IKKs) that play a central role in coordinating the NF-κB signaling pathway. The IKK complex is composed of two catalytic subunits, IKKα and IKKβ, as well as a non-catalytic subunit, IKKγ or NEMO (NF-κB essential modulator). There are cysteine residues present in the kinase domain of IKKα (Cys178) and IKKβ (Cys179), and they are responsible for redox regulation of NF-κB activation ( 20–22 ). The thiol-modifying agents, such as N -ethylmaleimide and p -hydroxymercuribenzoate, directly restrained IKK activity in vitro that was counteracted by the reducing agents dithiothreitol (DTT) and reduced glutathione (GSH) ( 23 ). In addition, the inhibitory effect of N -ethylmaleimide on the IKK activity was abolished in cells expressing mutant IKKβ in which Cys179 in the activation loop was replaced with alanine, indicating that the redox status of Cys179 of IKKβ is essential for regulating the NF-κB activation ( 21 ). Some chemopreventive and cytoprotective agents have also been found to target cysteine thiols of IKKβ, especially at Cys179, thereby suppressing aberrant overactivation of NF-κB signaling ( 24–28 ).
In an attempt to explore the chemopreventive effects of PIC in relation to its anti-inflammatory activity, we investigated PIC-mediated inhibition of NF-κB signaling in human breast epithelial (MCF-10A) cells stimulated with the tumor promoter TPA. Herein, we present evidence supporting that PIC can block NF-κB signaling by interacting with an IKKβ, presumably at Cys179, thereby inhibiting migration and anchorage-independent growth of these cells.
Materials and methods
Materials
Resveratrol (purity 99%) was purchased from Pharmascience (Montreal, Canada). PIC and TPA were purchased from Alexis Biochemicals (San Diego, CA). Oxyresveratrol was a generous gift from Chulalongkorn University, Thailand. Dulbecco's modified Eagle's medium/F-12, heat-inactivated horse serum, L -glutamine and penicillin/streptomycin/fungizone mixtures were products of Gibco BRL (Grand Island, NY). DTT, insulin, Cholera toxin, hydrocortisone, human epidermal growth factor, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, N -acetyl- L -cysteine and an antibody against actin were purchased from Sigma Chemical Co. (St Louis, MO). Aminobenzotriazole and SKF-525A and dicumarol were purchased from Tocris Bioscience (Ellisville, MO). Rabbit polyclonal anti-COX-2 antibody was the product of Neomarker (Fremont, CA) and rabbit polyclonal anti-pIκBα antibody and IKKβ antibody were obtained from Cell Signaling Technology (Beverly, MA). Antibody against phosphor-IKKα/β, glutathione S -transferase-pIκBα, normal mouse immunoglobulin G, protein G plus-agarose and antibody against pp65 was the product of Santa Cruz Biotechnology (Santa Cruz, CA). Bay 11-7082 was the product of Biomol International LP (Plymouth Meeting, PA). Polyvinylidene difluoride membranes were supplied from Gelman Laboratory (Ann Arbor, MI). [γ- 32 P]ATP was purchased from Amersham Pharmacia Biotech (Acton, MA). The expression plasmid encoding IKKβC179A-Flag was processed according to Jeon et al. ( 23 ).
Cell culture
MCF-10A cells were cultured in Dulbecco's modified Eagle's medium/F-12 supplemented with 5% heat-inactivated horse serum, 10 μg/ml insulin, 100 ng/ml Cholera toxin, 0.5 μg/ml hydrocortisone, 20 ng/ml recombinant human epidermal growth factor, 2 mM L -glutamine and 100 U/ml penicillin/streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 5% CO 2 and 95% air.
Western blot analysis
After treatment, cells were washed with phosphate-buffered saline (PBS) and mixed with lysis buffer [50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 25 mM NaF, 20 mM ethylenediaminetetraacetic acid, 1 mM DTT, 1 mM Na 3 VO 4 , 0.5% Triton X-100 and protease inhibitor cocktail tablets] for 20 min on ice followed by centrifugation at 13 000 g for 15 min. The protein concentration of the supernatant was measured by using the BCA reagents. The protein samples were solubilized with sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis sample loading buffer and boiled for 5 min. Proteins were electrophoresed on 8 or 10% SDS–polyacrylamide gel and transferred to polyvinylidene difluoride membranes. The blots were then blocked with 5% fat-free dry milk-PBST (phosphate-buffered saline containing 0.1% Tween-20) buffer for 1 h at room temperature and incubated with primary antibodies in 3% fat-free dry milk-PBS. Following three washes with PBST, the blots were incubated with horseradish peroxidase-conjugated secondary antibody in 3% fat-free dry milk-PBS for 1 h at room temperature. The blots were rinsed again three times with PBST, and the transferred proteins were incubated with the ECL plus substrate solution (Amersham Pharmacia Biotech, Piscataway, NJ) for 3 min according to the manufacturer's instructions and visualized with an X-ray film.
In vitro IKK activity assay (radioactive)
The IKK activity assay was conducted with slight modification of the protocol described by Bharti et al. ( 29 ). Briefly, the lysed cell extract (200 μg) was precleared using normal mouse immunoglobulin G and protein G agarose beads and subjected to immunoprecipitation by using anti-IKKβ antibody. The resulting immunocomplex was pulled down by mixing with protein G agarose beads. The immunoprecipitate thus obtained was suspended in the reaction mixture containing 47 μl of 1× kinase buffer (25 mmol/l DTT, 0.1 mmol/l Na 3 VO 4 and 10 mmol/l MgCl 2 ), 1 μg glutathione S -transferase-IκBα (1–317) substrate protein and 10 μCi [γ- 32 P]ATP. The mixture was incubated at 30°C for 45 min. The reaction was terminated by adding 15 μl of 5× SDS loading dye, boiled for 5 min at 99°C, vortexed and centrifuged at 13 000 g for 2 min. After separating the phosphorylated proteins contained in the supernatant fraction by 12% SDS–polyacrylamide gel electrophoresis, the gel was stained with Coomassie brilliant blue and treated with a destaining solution (glacial acetic acid/methanol/distilled water, 1:4:5, vol/vol/vol). The destained gel was dried at 80°C for 1 h and exposed to X-ray film to detect the phosphorylated glutathione S -transferase-IκBα in the radiogram. Mouse immunoglobulin G heavy chain band that appeared on the destained gel was used as the loading control to ensure the equal lane loading.
Anchorage-independent growth assay
To prepare the hard agar layer, 3.3% agarose dissolved in PBS was boiled using a microwave oven, and 2.5 ml of the boiled agarose solution was added immediately to 60 mm dishes using a prewarmed pipette and then kept in the 37°C incubator to be solidified. To prepare the soft agar layer containing the cells, MCF-10A cells (1 × 10 5 ) were suspended in the 0.33% agarose solution with gentle mixing, and 2.5 ml of this solution was inoculated on the top of the hard agar layer. After allowing the solution to harden as a soft agar for 4 h, 2.5 ml of the fresh medium was added to the top of the hardened soft agar layer. On the next day, these cells were then exposed to 7,12-dimethylbenz[ a ]anthracene (50 nM). After 6 h incubation with 7,12-dimethylbenz[ a ]anthracene, dimethyl sulfoxide, PIC (30 μM) or Bay 11-7082 (5 μM) were treated together with TPA (10 nm) at 3 days intervals for 3 weeks. After 4 weeks of incubation, anchorage-independent cell growth (spherical formation containing >10 cells) was examined under a light microscope. The total number of foci per 1 × 10 5 cells in a well was counted. The experiments were replicated four times, and a representative set of data were photographed for presentation.
Wound healing assay
To analyze the inhibitory effect of PIC on TPA-induced cell proliferation and migration, the confluent monolayer of cells seeded in six-well growing on tissue culture dishes was wounded by manual scratching of the surface with an 1 ml pipette tip and the scratched surface was washed with PBS to remove cell debris. The dishes containing these cells were then treated with the medium containing vehicle or TPA (10 nM) and incubated at 37°C for 24 h. Phase contrast images of the wound sites were captured at 0 h (control) and 24 h of incubation using an inverted microscope (×10 magnification).
Results
PIC strongly inhibited TPA-induced IKKβ kinase activity and phosphorylation of IκBα
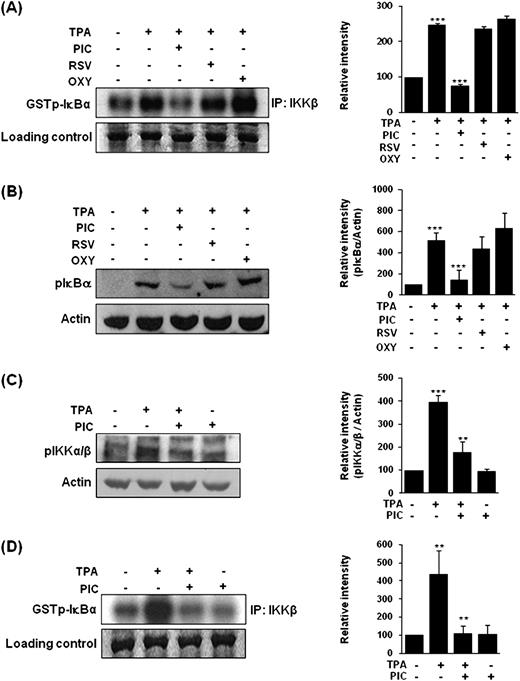
To compare the ability of representative stilbene analogs to inhibit TPA-induced NF-κB signaling activation in MCF-10A cells, the cells were exposed to PIC, resveratrol or oxyresveratrol (30 μM each) for 1 h before TPA (10 nM) treatment. Figure 1 illustrates the chemical structures of these three stilbene analogs. The effects of the aforementioned stilbene analogs on IKKβ activity and phosphorylation of IκBα were determined by an in vitro assay and western blot analysis, respectively. As shown in Figure 2A and B , PIC exerted the most pronounced inhibitory effect on TPA-induced IKKβ kinase activity and phosphorylation of IκBα among the three stilbene compounds tested.
Comparative effects of PIC and its analogs on TPA-induced IKKβ activity ( A ) and phosphorylation of IκBα ( B ). The inhibitory effect of PIC on TPA-induced IKKα/β phosphorylation ( C ) and IKKβ activity ( D ). MCF-10A cells (2 × 10 5 /ml) were pretreated with PIC or its analogs (30 μM each) at 37°C for 1 h, followed by stimulation with 10 nM TPA for additional 2 h. RSV, resveratrol; OXY, oxyresveratrol. Data are representative of three independent experiments. For A and B, *** P < 0.001, solvent control versus TPA alone and TPA versus PIC plus TPA. For C, *** P < 0.001, solvent control versus TPA alone and ** P < 0.01, TPA versus PIC plus TPA. For D, ** P < 0.01, solvent control versus TPA alone and TPA versus PIC plus TPA.
PIC inhibited TPA-induced IKKβ kinase activity and IKKα/β phosphorylation
In stimulated cells, activated IKK phosphorylates IκB proteins. Phosphorylated IκB proteins are rapidly polyubiquitinated and degraded by proteasomes, releasing free NF-κB that translocates to the nucleus and modulates gene expression ( 18 , 19 ). In an attempt to elucidate the mechanism underlying the inhibitory effect of PIC on TPA-induced NF-κB activation by blocking the IKK signaling, IKKα/β phosphorylation and IKKβ activity were determined by western blot analysis and the kinase assay, respectively. Treatment of MCF-10A cells with TPA led to marked phosphorylation of IKKα/β, which was significantly repressed by PIC pretreatment ( Figure 2C ). Under the same experimental conditions, the catalytic activity of IKKβ was suppressed by PIC ( Figure 2D ).
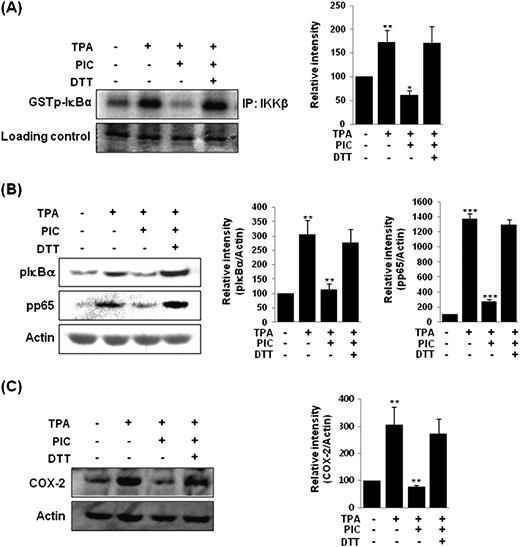
The reducing agent DTT abrogated the PIC-mediated suppression of IKK–NF-κB signaling and COX-2 induction
The subunits of NF-κB such as p50 and p65 have critical cysteine residues ( 20 , 28 ). IKKβ also contains cysteine residues at its active site ( 21 ). It has been shown that the chemical modification of thiol residues in these molecules can inhibit NF-κB activation ( 24–26 , 28 , 30 ). The IKKβ subunit of the IKK complex is essential for activation of NF-κB in response to various pro-inflammatory stimuli. The reducing agent DTT reversed PIC-induced inhibition of IKKβ activation, phosphorylation of IκBα and p65 and COX-2 expression ( Figure 3A–C ).
The abrogation of the inhibitory effect of PIC on TPA-induced IKKβ activity ( A ), phosphorylation of IκBα and p65 ( B ) and COX-2 expression ( C ) by the reducing agent DTT. MCF-10A cells were pretreated with PIC (30 μM) or DTT (500 μM) 1 h before TPA treatment. IKKβ activity was determined by the kinase assay at 2 h. The levels of pIκBα or pp65 as well as that of COX-2 were determined by western blot analysis at 2 and 4 h, respectively. Data are representative of three independent experiments. For A, ** P < 0.01, solvent control versus TPA alone and * P < 0.05, TPA versus PIC plus TPA. For B, ** P < 0.01, solvent control versus TPA alone and TPA versus PIC plus TPA (left) and *** P < 0.001, solvent control versus TPA alone and TPA versus PIC plus TPA B (right). For C, ** P < 0.01, solvent control versus and TPA versus PIC plus TPA.
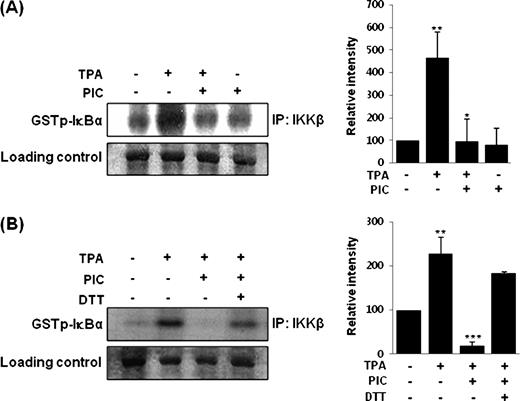
PIC directly inhibited IKKβ activity
To determine whether PIC could directly modify IKK, PIC (30 μM) alone or together with DTT (500 μM) was incubated for 1 h with whole cell lysate from MCF-10A cells stimulated with TPA (10 nM). PIC repressed the IKKβ activity ( Figure 4A ) and this inhibitory effect was reversed when DTT was co-treated ( Figure 4B ). It appears that PIC inhibits NF-κB signaling activation by directly interacting with IKKβ, possibly at a cysteine residue of IKKβ.
Direct inhibition of TPA-induced IKKβ activity in MCF-10A cells by PIC ( A ) and its restoration by treatment with DTT ( B ). After MCF-10A cells (2 × 10 5 /ml) were incubated at 37°C with 10 nM TPA for 2 h, the whole cell lysates were prepared and incubated with PIC (30 μM) or DTT (500 μM) in a test tube for 1 h prior to the kinase assay. Data are representative of three independent experiments. For A, ** P < 0.01, solvent control versus TPA alone and * P < 0.05, TPA versus PIC plus TPA. For B, ** P < 0.01, solvent control versus TPA alone and *** P < 0.001, TPA versus PIC plus TPA.
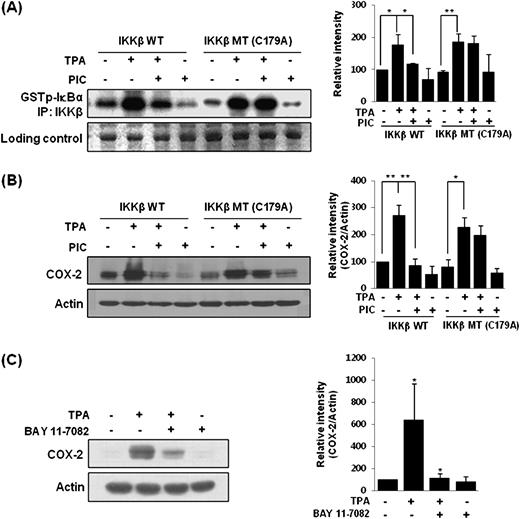
PIC inhibited NF-κB signaling through direct interaction possibly with cysteine 179 in IKKβ
A transient transfection assay was performed with a fusion gene containing pCMV promoter and IKKβ mutant construct. PIC (30 μM) failed to block the IKKβ activity ( Figure 5A ) and subsequent COX-2 expression ( Figure 5B ) in MCF-10A cells transfected with mutant IKKβ (C179A) in which cysteine 179 was replaced by alanine, whereas it substantially suppressed them in IKKβ wild-type cells. The IKK inhibitor, Bay 11-7082 (5 μM) also abrogated TPA-induced COX-2 induction ( Figure 5C ).
Cysteine 179 of IKKβ as a putative target of PIC for suppression of IKKβ activity ( A ) and COX-2 expression ( B ) in MCF-10A cells. The cells were transfected with IKKβ wild-type or IKKβ C179A mutant vector (2.5 μg each). After 48 h, the cells were incubated with PIC (30 μM) for 1 h, followed by stimulation with 10 nM TPA for additional 2 h. The effect of IKK inhibitor Bay 11-7082 on TPA-induced COX-2 expression ( C ). Bay 11-7082, given 1 h prior to TPA, also attenuated the COX-2 expression. For A and B, ** P < 0.01, significantly different between the groups compared; * P < 0.05, significantly different between the groups compared. For C, * P < 0.05, solvent control versus TPA alone and TPA versus BAY 11-7082 plus TPA.
PIC repressed TPA-induced cell migration
We next investigated the inhibitory effect of PIC on TPA-induced cell migration. As shown in Figure 6A , the MCF-10A cells treated with TPA migrated to the center of the wound. PIC (30 μM) markedly suppressed the TPA-induced migration. To confirm that the inhibitory effect of PIC on cell migration was mediated by blocking the IKK activity, the IKK inhibitor, Bay 11-7082 (5 μM) was utilized. The pharmacologic inhibition of IKK activity resulted in suppression of wound closure ( Figure 6A ).
The inhibitory effect of PIC on TPA-induced migration ( A ) and transformation ( B ) of MCF-10A cells. (A) MCF-10A cells were wounded with a micropipette tip. The cells were then treated with TPA (10 nM) for 24 h in the presence or absence of PIC (30 μM) or Bay 11-7082 (5 μM). Wound closure was monitored by photography at 24 h following treatment with each compound. Cell migration (%) was quantified by calculating the wound width. (B) The effect of PIC or Bay 11-7082 (5 μM) on TPA-induced colony formation was determined by the anchorage-independent growth on the soft agar. Cell colonies were scored after incubation for 21 days in 5% CO 2 . Cells (1 × 10 5 /ml) were exposed to 7,12-dimethylbenz[ a ]anthracene (50 nM) for 6 h and subsequently to TPA (10 nM) in the absence or presence of PIC (30 μM) or Bay 11-7082 (5 μM) for 21 days. Columns, means ( n = 3); bars, SD. *** P < 0.001, significantly different between the groups compared; * P < 0.05, significantly different between the groups compared.
PIC abrogated the TPA-induced cell transformation
IKKβ activation has been associated with anchorage-independent growth as well as migration of MCF-10A cells. This prompted us to examine the effects of PIC on transformation of MCF-10A cells ( 31 ). As illustrated in Figure 6B , MCF-10A cells exposed to 7,12-dimethylbenz[ a ]anthracene (50 nM) for 6 h and TPA (10 nM) twice a week for 3 weeks exhibited significantly increased anchorage-independent growth. In the presence of PIC (30 μM) or Bay 11-7082 (5 μM), the TPA-induced colony formation was abolished. Taken together, these results suggest that PIC blocks TPA-induced neoplastic transformation through inhibition of IKK activity.
Discussion
Cancer is an unresolved question of long standing around the world. Even though there is no panacea that can completely cure cancer, the risk of cancer can be reduced by eliminating the identified carcinogens ( 32 ). However, the avoidance of some risk factors normally requires drastic changes in the people's life style. Alternatively, a substantial proportion of many human cancers could be prevented by intaking diets that are known to counteract carcinogens ( 33 ). Evidence has been accumulated, supporting that frequent consumption of fruits and vegetables is inversely associated with the reduced risk of a great number of chronic ailments, including cancer ( 34 ). Substances derived from edible plants, collectively referred to as phytochemicals, are non-nutritive components that possess substantial anticarcinogenic and antimutagenic properties. These dietary phytochemicals have been shown to suppress cancer cell proliferation, growth factor signaling, metastasis, angiogenesis, expression of anti-apoptotic proteins and COX-2, etc. Chemopreventive agents have also been found to reverse chemoresistance and radioresistance in patients under cancer treatment ( 33 , 35 ). Numerous cell culture and animal studies have been conducted to evaluate the ability of specific edible phytochemicals to prevent cancer ( 33 ).
Cysteine sulfhydryls present in various transcription factors or their regulators, such as kelch-like ECH-associated protein 1-NF-E2-related factor2 (Keap1-Nrf2), NF-κB and p53, have been known to be critical for the transcriptional regulation of many genes essential for maintaining cellular homeostasis. Therefore, highly conserved cysteine residues of several redox-sensitive signaling molecules are now emerging as important determinants of their physiological functions. Some chemopreventive and cytoprotective agents have been found to directly or indirectly modify cysteine sulfhydryls present in key transcription factors or their regulators, thereby suppressing the aberrant overactivation of oncogenic signal transduction or restoring or even potentiating cellular defense signaling ( 36 ).
PIC was initially isolated as an antileukemic agent from Euphorbia lagascae ( 37 ). PIC has been shown to exert anti-inflammatory ( 38 , 39 ), antioxidant ( 12 ) and anticancer effects ( 40 , 41 ). When anti-inflammatory effects of PIC were compared with those of two other stilbene analogs, resveratrol and oxyresveratrol in terms of suppression of TPA-induced IKKβ activity and phosphorylation of IκBα ( Figure 2A and B ), PIC bearing a catechol ring structure was found to be more effective in suppressing IKK–NF-κB signaling than the other compounds. Upon oxidation, catechol compounds can be converted to electrophilic quinones ( 42 , 43 ) that can react with nucleophiles, including key protein thiols. Electrophiles can either activate or inhibit specific signal transduction pathways as a consequence of modifying many protein structures by directly reacting with cysteine thiols ( 44 ). For instance, an endogenous electrophile, 15-deoxy-Δ 12,14 -prostaglandin J 2 (15d-PGJ 2 ) ( 45 ) binds to Cys179 of the IKKβ subunit and Cys38 of the NF-κB p65 subunit as well as NF-κB p50 subunit, thereby suppressing the NF-κB signaling ( 25 , 26 , 30 ). IKKβ-dependent phosphorylation of its cytoplasmic IκB partner represents a key event for NF-κB activation, and Cys179 present in the activation loop of IKKβ represents a critical target of anti-inflammatory and anti-proliferative agents ( 18 , 19 , 25 , 27 , 28 , 46 ).
The precise mechanism underlying the oxidation of PIC to its quinone metabolites has not been defined in detail. However, PIC might be oxidized by enzymes such as peroxidases or cytochrome P450s (CYPs) as proposed for the catechol estrogen 4-hydroxyestradiol ( 47–49 ). To examine whether oxidation of PIC by peroxidases or CYPs is involved in the inhibitory effect of PIC on TPA-induced IKKβ activity and COX-2 expression, MCF-10A cells were pretreated with a peroxidase inhibitor aminobenzotriazole or a pan-CYP inhibitor SKF-525A. After 1 h preincubation, the cells were exposed to TPA with PIC. As shown in supplementary Figure 1 (available at Carcinogenesis Online), the inhibitory effects of PIC on TPA-induced IKKβ activity and COX-2 expression were attenuated by treatment with each of these inhibitors, indicative of potential roles of peroxidases and CYPs in conversion of PIC to a reactive species capable of inactivating IKKβ. We speculate that an electrophilic quinone formed as a consequence of oxidation of PIC bearing the catechol moiety ( 49 ) may directly interact with a critical cysteine thiol of IKKβ, especially Cys179, thereby inhibiting its catalytic activity. In support of this supposition, a reducing agent reversed the effect of PIC on inhibition of the levels of pIκBα, pp65 and COX-2 as well as IKKβ activity. Furthermore, mutation of IKKβ by replacing its cysteine 179 with alanine abolished the inhibitory effect of PIC on COX-2 expression and IKKβ activity, lending further support to the possible interaction between PIC and Cys179 of IKKβ. In conclusion, PIC inhibits TPA-induced IKKβ activity in MCF-10A cells presumably by targeting the Cys179 residue located in the activation loop of IKKβ. This results in downregulation of TPA-induced expression of COX-2 and cell migration and transformation induced by TPA. Taken together, our study suggests that PIC has a chemopreventive potential against inflammation-associated carcinogenesis in which the IKK–NF-κB signaling axis plays a predominant role.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
Innovative Drug Research Center (R11-2007-107-01002-0); WCU project, National Research Foundation, Ministry of Education, Science and Technology, Republic of Korea (R31-008-000-10103-0).
Abbreviations
- COX-2
cyclooxygenase-2
- CYPs
cytochrome P450s
- DTT
dithiothreitol
- IKK
IκB kinase
- IκBα
inhibitory protein κBα
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- PIC
piceatannol
- TPA
12- O -tetradecanoylphorbol-13-aceate
Conflict of Interest Statement: None declared.
References
- cyclooxygenase-2
- signal transduction
- immunosuppressive agents
- inflammation
- oxidation
- alanine
- catechols
- cysteine
- dithiothreitol
- esters
- genes
- oxidation-reduction
- phorbols
- phosphorylation
- phosphotransferases
- quinones
- reducing agents
- rhubarb food
- sulfhydryl compounds
- breast
- neoplasms
- transcription factor
- carcinogenesis
- epithelial cells
- chronic inflammation
- polyphenols
- grapes (dietary)
- enzyme activity


![The inhibitory effect of PIC on TPA-induced migration ( A ) and transformation ( B ) of MCF-10A cells. (A) MCF-10A cells were wounded with a micropipette tip. The cells were then treated with TPA (10 nM) for 24 h in the presence or absence of PIC (30 μM) or Bay 11-7082 (5 μM). Wound closure was monitored by photography at 24 h following treatment with each compound. Cell migration (%) was quantified by calculating the wound width. (B) The effect of PIC or Bay 11-7082 (5 μM) on TPA-induced colony formation was determined by the anchorage-independent growth on the soft agar. Cell colonies were scored after incubation for 21 days in 5% CO 2 . Cells (1 × 10 5 /ml) were exposed to 7,12-dimethylbenz[ a ]anthracene (50 nM) for 6 h and subsequently to TPA (10 nM) in the absence or presence of PIC (30 μM) or Bay 11-7082 (5 μM) for 21 days. Columns, means ( n = 3); bars, SD. *** P < 0.001, significantly different between the groups compared; * P < 0.05, significantly different between the groups compared.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/carcin/31/8/10.1093_carcin_bgq099/3/m_carcinbgq099f06_ht.jpeg?Expires=1716415059&Signature=4uYnmwdwGtJuSCXy8UOQuHLcdupT-pRP9mAzT-OUAwFJN5tuiwVmMdxdcIXWzNtsewDsnt7OeUD8Z1~kDg0E5Fvcg0HMBjJvFwltMP4r~4l1AKWxCa3B~YYU0PJr6H91Q72Zya0SDXFr6oZEGCJEi9UHx7PceJwXOA~TNhnmrqkvgKWhe0EV-6VwViJsQEAECa~qGBQTkP3wCYwdb8FD3Zz3SUFKq4dDl6o4WsgLX-FF1MVhIDZacxJE7vlJ7b2PBgSK29YrtyoZWDoRkQHGbQfUH-okIEQAUySEt77XwOWJqv8~6iMbCQ34nc5-i1Y~fpFH9kK84X~PyLo5XzBWsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
