-
PDF
- Split View
-
Views
-
Cite
Cite
Susan C. Tilton, Jerry D. Hendricks, Gayle A. Orner, Cliff B. Pereira, George S. Bailey, David E. Williams, Gene expression analysis during tumor enhancement by the dietary phytochemical, 3,3′-diindolylmethane, in rainbow trout, Carcinogenesis, Volume 28, Issue 7, July 2007, Pages 1589–1598, https://doi.org/10.1093/carcin/bgm017
Close - Share Icon Share
Abstract
Indole-3-carbinol (I3C) and 3,3′-diindolylmethane (DIM), a primary I3C derivative, are known dietary chemopreventive agents also available as supplements. However, I3C has been found to act as a tumor promoter in rat (multi-organ) and trout (liver) models. I3C and DIM were previously found to be estrogenic in trout liver based on toxicogenomic profiles. In this study, we compare the post-initiation effects of DIM and 17β-estradiol (E2) on aflatoxin B1 (AFB1)-induced hepatocarcinogenesis in trout. Trout were initiated as embryos with AFB1 and juvenile fish were fed diets containing 0, 120 or 400 p.p.m. DIM or 5 p.p.m. E2 for 18 weeks. Tumor incidence was determined at 13 months and found to be significantly elevated in AFB1-initiated trout fed either 400 p.p.m. DIM or 5 p.p.m. E2 compared with control animals. To evaluate the mechanism of tumor enhancement, hepatic gene expression profiles were examined in animals fed promotional diets during the course of tumorigenesis and in hepatocellular carcinomas (HCCs) of initiated animals. We demonstrate that DIM alters gene expression profiles similar to E2 in liver samples during tumorigenesis and in HCC tumors. Further, HCCs from animals on DIM and E2 promotional diets had a transcriptional signature indicating decreased invasive or metastatic potential compared with HCCs from control animals. Overall, these findings are the first to demonstrate tumor promotion by DIM. They confirm the importance of estrogenic signaling in the mechanism of promotion by dietary indoles in trout liver and indicate a possible dual effect that enhances tumor incidence and decreases potential for metastasis.
Introduction
3,3′-Diindolylmethane (DIM) is a major in vivo component of the glucobracissin, indole-3-carbinol (I3C), from cruciferous vegetables (1–3). DIM and I3C are also available as dietary supplements and promoted for their well-established chemoprotective effects, particularly in estrogen-sensitive neoplasias such as breast and endometrial cancer (4,5). Both I3C and DIM have been found to inhibit 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis in Sprague–Dawley rats when fed in the diet after initiation (6,7). Additionally, chemoprotection by I3C is consistently observed in various target organs, including mammary and stomach (8), liver (9,10), lung (11) and colon (12), of rodent and rainbow trout models when administered prior to or concurrent with the carcinogen and is thought to block initiation and subsequent adduct formation by modulation of phase I and phase II drug metabolizing enzymes (13,14). Other chemoprotective properties of I3C and DIM determined in vivo and in vitro include the ability to alter cell cycle progression, proliferation, apoptosis and DNA repair suggesting indoles may target other stages of carcinogenesis after initiation (reviewed in refs 15,16). Interestingly, the ability of DIM to act as a cytostatic agent in vitro has been attributed to both anti-estrogenic and estrogenic effects that were not dependent on ligand binding to estrogen receptor (ER), but through crosstalk of ER signaling with aryl hydrocarbon receptor (AhR) or kinase pathways (6,17).
Despite clear evidence for chemoprotection, I3C has been found to enhance tumor formation in multiple organs in rodent models (18–22) and in trout liver (23,24) when fed in the diet long-term post-initiation. Large-scale studies in trout with multiple concentrations of carcinogen and I3C suggest that the promotional potency of I3C is at least as great as its potency as an anti-initiating agent in this model (9,25). Mechanisms for enhancement are not well understood, but have been attributed to altered estrogen metabolism in endometrial adenocarcinoma, inhibition of apoptosis in colon carcinogenesis and biphasic activation of ER- and AhR-mediated responses in trout liver (21,22,25). The ability of DIM to enhance tumor formation in models similar to I3C has not been evaluated until now, but DIM has been increasingly promoted as a chemoprotective agent over I3C due to its chemical stability. Unlike I3C, it does not undergo acid-catalyzed oligomerization to products known to be potent AhR agonists (26) and appears to undergo fairly slow metabolism in target tissue (27). It is not known if the adverse tumor-enhancing effects of I3C are due to these properties. We recently compared the transcriptional profiles of I3C in trout liver with those of two known hepatic tumor promoters, 17β-estradiol (E2) and β-naphthoflavone, ER and AhR agonists, respectively, and found I3C to act similarly to E2 (28). Further, DIM had a more potent estrogenic effect than I3C based on transcriptional profiles suggesting it may also enhance hepatic tumors after initiation in the trout model.
In this study, we determined that the potential for DIM to promote aflatoxin B1 (AFB1)-induced hepatocarcinogenesis in trout is similar to that previously reported for I3C. We further compared the mechanism of enhancement to E2 using a toxicogenomic approach. Hepatic gene expression profiles were examined in liver during the course of tumorigenesis and in hepatocellular carcinoma (HCC) of animals treated with DIM or E2 experimental diets during promotion. Overall, the results show that DIM promotes AFB1-initiated tumors in trout liver when fed in the diet for only 18 weeks and alters transcriptional profiles in liver and HCCs similarly to E2.
Materials and methods
Materials
Analytical grade AFB1 and E2 were purchased from Sigma Chemical (St Louis, MO). DIM was kindly donated by BioResponse (Boulder, CO) and the purity was confirmed by High-performance liquid chromatography. All other compounds were purchased from Sigma chemical unless otherwise stated.
Experimental animals and treatments
Mt Shasta strain rainbow trout (Oncorhynchus mykiss) were hatched and reared at the Oregon State University Sinnhuber Aquatic Research Laboratory in 14°C carbon-filtered flowing well water on a 12:12 h light:dark cycle. All animal protocols were performed in accordance with Oregon State University Institutional Animal Care and Use Committee guidelines. Approximately 4000 embryos were initiated at 21 days post-fertilization with an aqueous exposure to 50 p.p.b. AFB1 (Sigma Chemical) for 30 min. Sham-exposed embryos were exposed to vehicle alone (0.01% ethanol) and served as non-initiated controls for each treatment. After hatching, fry were fed Oregon test diet, a semi-purified casein-based diet, for 3 months (29). Trout were then randomly (within initiator group) divided into experimental treatment groups and fed Oregon test diets containing 0, 120, 400, 800 or 1200 p.p.m. DIM or 5 p.p.m. E2 in 0.1% dimethyl sulfoxide vehicle ad libitum (2.8–5.6% body wt) 5 days/week for 18 weeks. However, due to low survival in the 800 and 1200 p.p.m. groups (<23% in all groups) compared with vehicle control groups (>62%) for both sham- and AFB1-initiated animals, these treatments were removed from analysis in the study. Diets were prepared monthly and stored frozen at −20°C until 2–4 days prior to feeding, when diets were allowed to thaw at 4°C. Duplicate 400 l tanks of 120 trout were maintained for each treatment group under the same conditions described for rearing.
Necropsy and histopathology
Fish were sampled at 3 weeks, 15 weeks and 10 months after start of experimental diets and were euthanized at termination by deep anesthesia with 250 p.p.m. tricaine methanesulfonate. At 3- and 15-week time points, livers were removed (n = 3 fish per tank) and quick-frozen in TRIzol reagent (Invitrogen, Carlsbad, CA) for RNA isolation. At 10 months, trout were sampled for liver tumors over a 5-day period. Fish body weights were recorded and livers were removed, weighed and inspected for neoplasms under a dissecting microscope. After marking the size and location of all surface tumors, a portion of each tumor was collected, placed separately in TRIzol reagent (Invitrogen) and quick-frozen in liquid nitrogen for gene expression analysis. The remaining liver was fixed in Bouin's solution for 2–7 days for histological analysis. Fixed livers were then cut into 1 mm slices with a razor blade to retrieve previously marked surface tumors and allow for examination of deeper tumors. At least one piece of liver from each tumor-bearing fish was then processed by routine histological evaluation and stained with hematoxylin and eosin. Neoplasms were classified by the criteria of Hendricks et al. (30).
RNA isolation
Total RNA was isolated using TRIzol reagent followed by cleanup with RNeasy Mini kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. For the liver samples at 3- and 15-week time points, equal amounts of RNA (μg) were pooled from each of the three fish per tank for every treatment (n = 2). A reference sample was created from RNA pooled from vehicle control fish in both tanks at the appropriate time point. For the HCC tumor samples, RNA was isolated from only those tumors histologically identified as HCC and that were of an adequate size to yield at least 20 μg total RNA. RNA was also isolated from individual livers of 10 sham-initiated trout and aliquots were pooled in equal amounts (μg) for use as a reference sample. RNA quality and quantity were assessed by agarose gel electrophoresis, bioanalyzer trace and spectrophotometric absorbance at 260/280 nm.
Microarray hybridization and analysis
Rainbow trout 70mer oligonucleotide arrays (OSUrbt ver. 2.0) containing 1672 elements representing ∼1400 genes were created at Oregon State University (http://www.science.oregonstate.edu/mfbsc/facility/micro.htm). Microarray construction and quality control have been described previously (31). Hybridizations were performed with the Genisphere Array 350 kit and instructions (Hatfield, PA) using standard reference design with dye swapping. Briefly, 7 μg total RNA were reverse transcribed with Superscript II (Invitrogen) using the Genisphere oligo (dT) primer containing a capture sequence for the Cy3- or Cy5-labeling reagents. Each reaction was spiked with a range of concentrations (0.0049–2.5 ng/μl) of the 10 SpotReport Alien Oligo controls (Stratagene, La Jolla, CA). Each cDNA sample containing the capture sequence for the Cy3 or Cy5 label was combined with equal amounts of reference cDNA (pooled from sham-initiated controls) containing the capture sequence for the opposite label. cDNA from two of the three biological replicates were dye swapped and hybridized to two slides as technical replicates. cDNA from the reference sample was also hybridized to dye-swapped slides (against itself) following the same protocol as experimental samples for use as a negative control. Prior to hybridization, microarrays were processed post-printing by washing twice in 0.1% sodium dodecylsulphate (SDS) for 5 min, 2× standard saline citrate (SSC), 0.1% SDS at 47°C for 20 min, 0.1× SSC for 5 min, water for 3 min, and then dried by centrifugation. The cDNAs (25 μl) were hybridized to arrays in formamide buffer (50% formamide, 8× SSC, 1% SDS, 4× Denhardt's solution) for 16 h at 47°C with 22 × 25 mm Lifterslips (Erie Scientific, Portsmouth, NH). Arrays were then washed once in 2× SSC, 0.1% SDS at 47°C for 10 min, twice in 2× SSC, 0.1% SDS for 5 min, twice in 1× SSC for 5 min, twice in 0.1× SSC for 5 min and dried by centrifugation. Shaded from light, the Cy3 and Cy5 fluorescent molecules (3DNA capture reagent, Genisphere, Hatfield, PA) were hybridized in formamide buffer for 3 h at 49°C to the corresponding capture sequences on cDNAs bound to the arrays. Arrays were washed in the dark with SSC containing 0.1 M dithiothreitol and dried as described earlier.
Scanned images (5 μm) were acquired with ScanArray Express (PerkinElmer, Boston, MA) at an excitation of 543 nm for Cy3 and 633 nm for Cy5 and at 90% power. The photomultiplier tube settings for each fluor were set based on intensity of spiked internal alien controls to normalize among all slides in the experiment. Image files were quantified in QuantArray (PerkinElmer) and raw median signal and background values were exported to BioArray Software Environment for analysis (32). Data were background subtracted and normalized by LOWESS, which is recommended for two-color experiments to eliminate dye-related artifacts and produce ratios that are not affected by signal intensity values. Stringent criteria were used to filter for genes that were regulated at least 1.8-fold consistently in all features from biological replicates and had a P value <0.05 by Welch's t-test (GeneSpring ver.6, Silicon Genetics, Redwood City, CA). The genes that met these criteria were minimally categorized based on function using Gene Ontology and OMIM databases for putative homolog descriptions. Hierarchical clustering of gene expression profiles was performed in GeneSpring and comparisons of microarray and real-time polymerase chain reaction (PCR) gene regulation were performed with GraphPad Prism (GraphPad Software, San Diego, CA).
Real-time reverse transcription–PCR
To assess the authenticity of results from the microarray analyses, messenger RNAs for select genes were also analyzed by real-time reverse transcription–PCR. Genes for confirmation were chosen from each functional category, as determined by Gene Ontology, and were differentially up- or down-regulated or resulted in no change. Total RNA was isolated as described previously and was treated with DNase (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized from 2 μg RNA with an oligo (dT)18 primer using SuperScript II (Invitrogen) following the manufacturer's instructions with a final volume of 100 μl. Synthesized cDNAs (1 μl) were used as templates for amplification of specific gene products in total volumes of 20 μl containing 1× SYBR Green master mix (DyNAmo qPCR kit, Finnzymes, Espoo, Finland) and 0.3 μM of each primer. Primer sequences were as follows: 5′-TAAAAGTTGCACAAGTTTCC-3′ and 5′-AAAGGTCCGTTCTGATCGTC-3′ for cathepsin D (CathD); 5′-GTTGTAGCCCGATTGCCTTT-3′ and 5′-GTTTGTGCTTGTGGTGGAAC-3′ for collagen 2 alpha (COL2A); 5′-GGATCACTTCTCACGTCCAC-3′ and 5′-TTAAACACAGTAAGCCCATC-3′ for chemotaxin (CTX); 5′-CCTGCGGCACGGTCTT-3′ and 5′-CTGACATCTTCATGCATCTCTTG-3′ for differentially regulated trout protein (DRTP); 5′-AGCTCCTGCTCCTGCTCT-3′ and 5′-GGAATGGGCATCTGGTCT-3′ for estrogen receptor alpha (ERα); 5′-CCAACCAAACGCTACCGAAC-3′ and 5′-CCAGATTCCATCTCACCTT-3′ for glyceraldehyde-3-phosphate dehydrogenase; 5′-GATGTCTTTCTCACTGCAACCT-3′ and 5′-GCTGTCTTTTTCCTGTGTCACT-3′ for hepcidin (HAMP); 5′-GAGGGAGATGTTTACTACCG-3′ and 5′-GACACTCAATTGCATACCAG-3′ for Janus kinase 1 (JAK1); 5′-ACAAAGGTGAAGTGTCGTCGG-3′ and 5′-TCATTGGTGGAGTGGCCTCT-3′ for perforin 1 (PER1); 5′-AACTGGAACAGCGTTGTGG-3′ and 5′-GTGGCAATAGCACTGGAGATG-3′ for recombination-activating gene 2 (RAG2); 5′-GGCCAAAGGAGACATCGTTT-3′ and 5′-TCCCAACCTACACCCTGACC-3′ for trout C-polysaccharide-binding protein (TCPBP); 5′-TTAGACCGAACTCCCCCTTG-3′ and 5′-AAATCCCAACAGCATTGCTC-3′ for thioredoxin (THX); 5′-CAGCCACCTGTGGAATGCAC-3′ and 5′-AAAAATGGGATTCAATAGCC-3′ for urokinase-type plasminogen activator receptor (UPAR) and 5′-TTGCCTTTGCCAACATCGAC-3′ and 5′-CGGACATTGACGTATGCTTT-3′ for vitellogenin (VTG). Primer sequences were chosen so that the product contained the 70mer array oligonucleotide sequence to ensure validation of the microarray experiment. Appropriate primers were attempted for OMYOSU127 and OMYOSU506, corresponding to nuclear factor-kappa B p105 and conserved helix–loop–helix ubiquitous kinase by top blast hit (BLASTN or BLASTX), which were both up-regulated by DIM and E2 treatments compared with control on the array. However, primers to the 70mer for specific products yielding consistent results were unobtainable and it is possible that these sequences reveal cross-hybridization on the array. PCR was performed using a DNA Engine Cycler and Opticon 2 Detector (MJ Research, Waltham, MA). PCR was carried out for 35 cycles with denaturation at 94°C for 10 s, annealing at optimum temperature for primers (54–60°C) for 20 s and extension at 72°C for 12 s. DNA amplification was quantified (pg) from the C(T) value based on standard curves to ensure quantification was within a linear range. Standards were created from gel-purified PCR products (QIAX II, Qiagen) for each primer set after quantification with PicoGreen dsDNA Quantification Kit (Molecular Probes, Eugene, OR) and serial dilutions ranging from 0.25 to 100 ng DNA. All signals were normalized against glyceraldehyde-3-phosphate dehydrogenase and ratios were calculated for treated samples compared with sham-initiated control as for the microarray analysis. Expression of glyceraldehyde-3-phosphate dehydrogenase was not altered by treatment based on either microarray analysis or reverse transcription–PCR and so was found to be an appropriate housekeeping gene for normalization in this study.
Statistical analysis of tumor incidence and multiplicity data
Tumor incidence data were modeled by logistic regression (Genmod procedure, SAS 9.1). Variation between tanks was found to be consistent with the binomial assumption through examination of deviance residuals and comparison of the residual deviance with the degrees of freedom (chi-square test, P > 0.69). P-values for incidence differences between pairs of treatments are Wald chi-square tests. Multiplicity results (per tumor bearing animal) were compared between treatments using exact Kruskal–Wallis and Wilcoxon tests (SAS Npar1way procedure, SAS 9.1). Data were pooled over the replicate tanks for the analysis after determining that there was little evidence of differences between the replicate tanks (Wilcoxon test).
Results
Tumor incidence
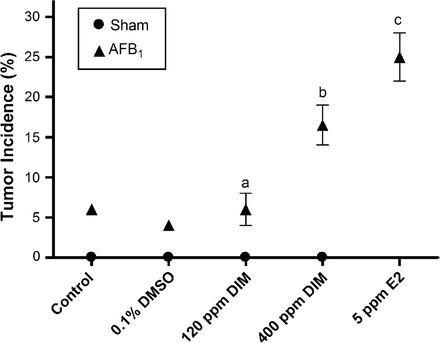
No tumors were observed in sham-initiated animals, whereas those receiving AFB1 had 5% tumor incidence. This incidence was significantly enhanced relative to vehicle controls in trout fed 400 p.p.m. DIM (P = 0.003) or 5 p.p.m. E2 (P < 0.0001) in the diet for 18 weeks post-initiation by AFB1 (Figure 1). Multiplicity was not significantly enhanced by DIM or E2 treatments (P > 0.14, all pairwise comparisons) and the average number of tumors per tumor bearing animal was 1.00, 1.17, 1.20, 1.29 and 1.15 for Oregon test diet control, vehicle control, 120 p.p.m. DIM, 400 p.p.m. DIM and 5 p.p.m. E2, respectively. The spectrum of tumor types determined by histopathological examination was the same as previously observed (33) and included malignant or benign neoplasms of hepatocellular, cholangiocellular or mixed hepatocellular–cholangiocellular origin (Supplementary Table I is available at Carcinogenesis Online). While there was some variation in tumor types among treatments, the overall frequency of different tumor types in this study, including HCC, was consistent with historical observations in AFB1-initiated trout (25,33,34). Overall tumor occurrences were as follows: HCC (38.3%), mixed carcinoma (48.2%), hepatocellular adenoma (11.1%) and cholangiocellular carcinoma (2.4%). Only tumors histologically identified as HCC were further evaluated for gene expression analysis. Histological examination of HCC tumors showed distinct structural differences between HCC and non-tumor tissues [representative images previously published in Tilton et al. (31)]. Non-initiated control liver and non-cancerous liver surrounding HCC showed hepatocytes oriented in tubules with only two hepatocytes between adjacent sinusoids, whereas HCC samples showed both increased basophilia and cellularity between adjacent sinusoids. These structural differences provided distinct borders between the HCC tissue and surrounding liver.
Liver tumor incidence in surviving animals fed control diet, vehicle control (0.1% dimethyl sulfoxide), DIM or E2 for 18 weeks post-initiation by 50 p.p.b. AFB1. Sham animals were initiated with 0.01% EtOH vehicle without AFB1 as a negative control. Each treatment consisted of duplicate tanks with at least 70 animals per tank. Symbols represent pooled overall tumor incidence for each treatment with error bars indicating range of the data. Statistical comparisons of tumor incidence in treated animals compared with vehicle control (0.1% dimethyl sulfoxide) are indicated by aP > 0.50, bP < 0.003 and cP < 0.0001. Sham initiation not applicable for E2-positive control.
Gene expression analysis
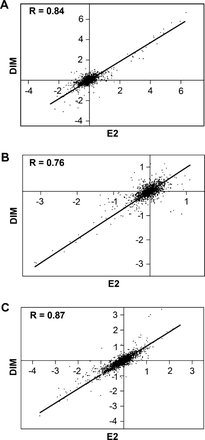
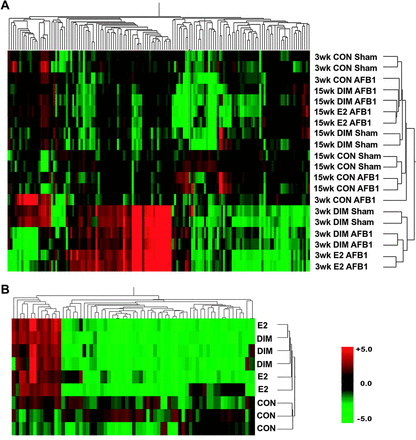
The OSUrbt ver. 2.0 array was used to characterize transcriptional profiles in liver samples and in HCC tumors from animals fed promotional diets of E2 and DIM compared with control animals. Supplemental raw data files are available online through Gene Expression Omnibus accession no. GSE5813 at http://www.ncbi.nlm.nih.gov/geo/. Gene expression was analyzed in liver samples collected from sham and AFB1-initiated trout treated with 0.1% dimethyl sulfoxide vehicle, 5 p.p.m. E2 and 400 p.p.m. DIM at the 3- and 15-week time points during feeding of experimental diets. Each treatment is represented by biological replicates (n = 2) of RNA pooled from three individual liver samples. Array hybridizations were performed with a common reference sample using dye swapping and final fold-change values are calculated as a ratio to appropriate control animals matched for time point and initiation status. Pairwise analysis of all 1672 features on the array indicated strong correlations in transcriptional patterns between E2 and 400 p.p.m. DIM treatments in AFB1-initiated animals at both the 3- and 15-week time points, R = 0.84 and 0.76 (two-tailed P < 0.0001), respectively (Figure 2, panels A and B). Genes were considered differentially expressed if their messenger RNA levels were consistently changed ≥ or ≤1.8-fold compared with appropriate vehicle controls with P < 0.05 (Welch's t-test) among biological replicates. Genes that passed the stringency filter are listed in Table I. Gene descriptions are provided based on sequence homology with the most significant (E < 10−6) BLASTX or BLASTN hit against the current GenBank databases. Genes were categorized by function based on the putative trout homolog using the Gene Ontology and OMIM databases. Bidirectional hierarchical clustering of genes differentially regulated in at least one treatment group (Figure 3, panel A) supported the pairwise analysis by Pearson correlation also indicating there was a high degree of similarity in gene expression patterns between E2 and DIM treatments. Clustering analysis further indicated distinct regulation patterns in all treatments between the two time points. Principal component analysis (PCA) is an exploratory multivariate statistical technique that reduces dimensionality by performing a covariance analysis between factors and was applied on conditions to explore correlations between samples. PCA showed strong similarity between E2 and DIM treatments at both time points. Interestingly, PCA also indicated distinct transcriptional patterns between sham- and AFB1-initiated animals treated with DIM (Supplementary Figure 1 is available at Carcinogenesis Online). Transcripts encoding vitellogenic liver proteins were the most sensitive markers for the estrogenic response in trout at the 3-week time point; however, a number of genes important for cell proliferation, protein transport, immune function and metabolism were also differentially regulated by DIM and E2 treatments. At the 15-week time point, fewer genes indicating an estrogenic response were regulated in liver and most genes were differentially down-regulated, including those important for immune function and metabolism.
Select genes differentially regulated in trout liver during tumorigenesis on promotional diets
| Array ID | TIGR IDa | Gene name (accession number, species)b | Average fold change (P value)c | |||
| Control AFB1 | DIM Sham | DIM AFB1 | E2 AFB1 | |||
| 3-Week time point | ||||||
| Liver-specific proteins (vitellogenesis) | ||||||
| OmyOSU222 | TC47576 | Vitellogenin precursor (X92804, Oncorhynchus mykiss) | 0.68 | 62.78 (0.001) | 99.23 (0.000) | 81.72 (0.000) |
| OmyOSU248 | TC47577 | Vitellogenin (X92804, O.mykiss) | 0.84 | 29.66 (0.000) | 49.49 (0.000) | 42.25 (0.000) |
| OmyOSU1542 | TC85700 | Zona radiata structural protein (AF407574, O.mykiss) | 0.97 | 26.64 (0.000) | 24.67 (0.000) | 29.57 (0.000) |
| OmyOSU203 | TC47576 | Vitellogenin precursor (X92804, O.mykiss) | 0.95 | 27.14 (0.000) | 25.56 (0.000) | 25.66 (0.000) |
| OmyOSU1540 | TC65780 | Vitelline envelope protein alpha (AF231706, O.mykiss) | 1.19 | 19.77 (0.000) | 18.14 (0.000) | 19.00 (0.000) |
| OmyOSU1552 | TC55460 | Vitelline envelope protein gamma (AF231708, O.mykiss) | 0.85 | 23.34 (0.000) | 20.31 (0.000) | 18.68 (0.000) |
| Cell proliferation (signal transduction, growth factors and apoptosis) | ||||||
| OmyOSU212 | TC70106 | TATA-binding protein (AY168633, Danio rerio) | 0.84 | 18.45 (0.000) | 12.89 (0.005) | 15.60 (0.001) |
| OmyOSU244 | NP543968 | Estrogen receptor beta (AJ289883, O.mykiss) | 0.85 | 4.62 (0.000) | 5.88 (0.000) | 5.73 (0.000) |
| OmyOSU151 | TC88754 | Estrogen receptor alpha (M31559, O.mykiss) | 1.04 | 3.01 (0.003) | 1.73 (0.006) | 2.47 (0.000) |
| OmyOSU511 | TC86507 | Ras-like GTPase (BC076026, D.rerio) | 1.26 | 2.20 (0.000) | 1.75 (0.003) | 1.79 (0.003) |
| OmyOSU800 | TC72880 | Non-receptor tyrosine kinase 2 (TYK2) (AF173032, Mus musculus) | 1.03 | 2.45 (0.014) | 1.32 | 2.82 (0.001) |
| OmyOSU915 | TC78497 | Cysteine-rich with EGF-like domains 1 (CR751234, D.rerio) | 1.07 | 2.15 (0.003) | 1.91 (0.005) | 2.29 (0.002) |
| OmyOSU313 | TC76141 | Bone morphogenic protein 7 (S77477, Gallus gallus) | 1.04 | 0.33 (0.005) | 0.33 (0.000) | 0.24 (0.000) |
| OmyOSU285 | CA364711 | Birc4 protein, XIAP (BC055246, D.rerio) | 1.10 | 0.38 (0.015) | 0.41 (0.000) | 0.36 (0.000) |
| Protein stability and transport | ||||||
| OmyOSU139 | TC70102 | Cathepsin D (U90321, O.mykiss) | 0.86 | 1.99 (0.005) | 2.07 (0.007) | 3.05 (0.000) |
| OmyOSU853 | TC81488 | Heat shock protein hsp90 (BC075757, D.rerio) | 1.04 | 2.55 (0.001) | 1.97 | 2.16 (0.004) |
| OmyOSU992 | TC88878 | MAL proteolipid protein 2 (BC078522, Xenopus laevis) | 0.92 | 1.63 (0.001) | 1.70 (0.002) | 2.08 (0.000) |
| Nucleic acid metabolism | ||||||
| OmyOSU1518 | TC80929 | Uridine phosphorylase (D44464, M.musculus) | 0.95 | 7.60 (0.000) | 7.07 (0.000) | 11.59 (0.000) |
| OmyOSU252 | TC70900 | Hypoxanthine–guanine phosphoribosyltransferase (AJ132697, G.gallus) | 0.83 | 5.71 (0.004) | 4.81 (0.009) | 7.37 (0.003) |
| Transcription and translation | ||||||
| OmyOSU1667 | TC78247 | Poly A-binding protein 1 (BC003870, M.musculus) | 1.06 | 1.89 (0.000) | 1.67 (0.007) | 1.90 (0.000) |
| OmyOSU217 | TC86507 | Ribosomal protein L13a (BC047855, D.rerio) | 1.03 | 2.09 (0.000) | 1.75 (0.005) | 1.74 (0.000) |
| Immune function and acute-phase response | ||||||
| OmyOSU1106 | TC77195 | Recombination-activating gene 2 (U31670, O.mykiss) | 0.87 | 1.89 (0.025) | 2.14 (0.029) | 2.26 (0.002) |
| OmyOSU1169 | CA369420 | PER1 (XM_683237, D.rerio) | 1.08 | 2.47 (0.000) | 1.62 | 2.80 (0.001) |
| OmyOSU1615 | TC81096 | Transmembrane 4 superfamily member 5 tumor antigen (AF281357, O.mykiss) | 0.80 | 1.83 (0.002) | 2.19 (0.007) | 2.78 (0.000) |
| OmyOSU852 | TC78880 | MHC-II invariant chain (AY065836, O.mykiss) | 0.93 | 1.19 | 1.15 | 2.28 (0.002) |
| OmyOSU788 | TC89515 | MHC-I heavy chain (AF287487, O.mykiss) | 0.59 | 0.83 | 1.04 | 2.01 (0.017) |
| OmyOSU539 | TC87038 | Precerebellin-like protein (AF192969, O.mykiss) | 0.80 | 0.44 (0.004) | 0.70 (0.002) | 0.35 (0.000) |
| OmyOSU268 | TC71098 | Chemotaxin (AF271114, O.mykiss) | 0.47 | 0.16 (0.049) | 0.43 (0.006) | 0.41 (0.003) |
| Drug, lipid and retinol metabolism/homeostasis | ||||||
| OmyOSU146 | TC63282 | Cytochrome P450 1A (AF059711, O.mykiss) | 1.37 | 2.42 (0.002) | 1.42 (0.043) | 0.95 |
| OmyOSU352 | TC72158 | Cytochrome P450 2K1v2 (L11528, O.mykiss) | 0.95 | 0.59 (0.017) | 0.54 (0.019) | 0.38 (0.001) |
| OmyOSU354 | TC72158 | Cytochrome P450 2K3 (AF043551, O.mykiss) | 0.97 | 0.66 | 0.61 (0.006) | 0.32 (0.001) |
| OmyOSU971 | TC69719 | Glutathione S-transferase (AB026119, O.mykiss) | 0.74 | 0.58 | 0.52 (0.010) | 0.49 (0.009) |
| OmyOSU395 | TC71381 | Arachidonate 5-lipoxygenase (L42198, M.musculus) | 0.86 | 0.52 (0.000) | 0.47 (0.019) | 0.49 (0.012) |
| OmyOSU343 | TC69983 | Biotinidase fragment 2 (AF281333, O.mykiss) | 1.12 | 0.48 (0.016) | 0.45 (0.000) | 0.40 (0.000) |
| 15-Week time point | ||||||
| Acute-phase response | ||||||
| OmyOSU232 | TC91273 | Differentially regulated trout protein (AF281355, O.mykiss) | 2.88 | 0.58 | 0.13 (0.00) | 0.11 (0.001) |
| OmyOSU148 | TC91273 | Differentially regulated trout protein (AF281355, O.mykiss) | 2.75 | 0.66 | 0.13 (0.001) | 0.11 (0.000) |
| OmyOSU268 | TC71098 | Chemotaxin (AF271114, O.mykiss) | 1.09 | 0.16 (0.049) | 0.18 (0.016) | 0.16 (0.005) |
| OmyOSU228 | TC55313 | Hepcidin (AF281354, O.mykiss) | 1.81 | 0.72 | 0.26 (0.001) | 0.27 (0.008) |
| OmyOSU878 | CA367917 | LECT2 neutrophil chemotactic factor (AF363272, O.mykiss) | 0.92 | 0.18 (0.025) | 0.29 (0.002) | 0.26 (0.015) |
| OmyOSU744 | TC71412 | Putative interlectin (AF281350, O.mykiss) | 1.66 | 0.73 | 0.29 (0.013) | 0.37 (0.042) |
| OmyOSU165 | TC90142 | IFN-inducible protein 2 (AJ313031, O.mykiss) | 1.71 (0.026) | 0.90 | 0.39 (0.002) | 0.48 (0.003) |
| Redox regulation | ||||||
| OmyOSU1422 | TC47183 | Thioredoxin (AAH49031, D.rerio) | 1.00 | 0.38 | 0.18 (0.009) | 0.20 (0.010) |
| OmyOSU115 | TC78741 | Glutathione S-transferase class-pi (L40381, Cricetulus longicaudatus) | 2.76 (0.034) | 0.54 | 0.23 (0.010) | 0.22 (0.009) |
| Miscellaneous | ||||||
| OmyOSU153 | TC89948 | Liver fatty acid-binding protein (AF281344, O.mykiss) | 1.03 | 0.31 | 0.23 (0.015) | 0.25 (0.012) |
| Array ID | TIGR IDa | Gene name (accession number, species)b | Average fold change (P value)c | |||
| Control AFB1 | DIM Sham | DIM AFB1 | E2 AFB1 | |||
| 3-Week time point | ||||||
| Liver-specific proteins (vitellogenesis) | ||||||
| OmyOSU222 | TC47576 | Vitellogenin precursor (X92804, Oncorhynchus mykiss) | 0.68 | 62.78 (0.001) | 99.23 (0.000) | 81.72 (0.000) |
| OmyOSU248 | TC47577 | Vitellogenin (X92804, O.mykiss) | 0.84 | 29.66 (0.000) | 49.49 (0.000) | 42.25 (0.000) |
| OmyOSU1542 | TC85700 | Zona radiata structural protein (AF407574, O.mykiss) | 0.97 | 26.64 (0.000) | 24.67 (0.000) | 29.57 (0.000) |
| OmyOSU203 | TC47576 | Vitellogenin precursor (X92804, O.mykiss) | 0.95 | 27.14 (0.000) | 25.56 (0.000) | 25.66 (0.000) |
| OmyOSU1540 | TC65780 | Vitelline envelope protein alpha (AF231706, O.mykiss) | 1.19 | 19.77 (0.000) | 18.14 (0.000) | 19.00 (0.000) |
| OmyOSU1552 | TC55460 | Vitelline envelope protein gamma (AF231708, O.mykiss) | 0.85 | 23.34 (0.000) | 20.31 (0.000) | 18.68 (0.000) |
| Cell proliferation (signal transduction, growth factors and apoptosis) | ||||||
| OmyOSU212 | TC70106 | TATA-binding protein (AY168633, Danio rerio) | 0.84 | 18.45 (0.000) | 12.89 (0.005) | 15.60 (0.001) |
| OmyOSU244 | NP543968 | Estrogen receptor beta (AJ289883, O.mykiss) | 0.85 | 4.62 (0.000) | 5.88 (0.000) | 5.73 (0.000) |
| OmyOSU151 | TC88754 | Estrogen receptor alpha (M31559, O.mykiss) | 1.04 | 3.01 (0.003) | 1.73 (0.006) | 2.47 (0.000) |
| OmyOSU511 | TC86507 | Ras-like GTPase (BC076026, D.rerio) | 1.26 | 2.20 (0.000) | 1.75 (0.003) | 1.79 (0.003) |
| OmyOSU800 | TC72880 | Non-receptor tyrosine kinase 2 (TYK2) (AF173032, Mus musculus) | 1.03 | 2.45 (0.014) | 1.32 | 2.82 (0.001) |
| OmyOSU915 | TC78497 | Cysteine-rich with EGF-like domains 1 (CR751234, D.rerio) | 1.07 | 2.15 (0.003) | 1.91 (0.005) | 2.29 (0.002) |
| OmyOSU313 | TC76141 | Bone morphogenic protein 7 (S77477, Gallus gallus) | 1.04 | 0.33 (0.005) | 0.33 (0.000) | 0.24 (0.000) |
| OmyOSU285 | CA364711 | Birc4 protein, XIAP (BC055246, D.rerio) | 1.10 | 0.38 (0.015) | 0.41 (0.000) | 0.36 (0.000) |
| Protein stability and transport | ||||||
| OmyOSU139 | TC70102 | Cathepsin D (U90321, O.mykiss) | 0.86 | 1.99 (0.005) | 2.07 (0.007) | 3.05 (0.000) |
| OmyOSU853 | TC81488 | Heat shock protein hsp90 (BC075757, D.rerio) | 1.04 | 2.55 (0.001) | 1.97 | 2.16 (0.004) |
| OmyOSU992 | TC88878 | MAL proteolipid protein 2 (BC078522, Xenopus laevis) | 0.92 | 1.63 (0.001) | 1.70 (0.002) | 2.08 (0.000) |
| Nucleic acid metabolism | ||||||
| OmyOSU1518 | TC80929 | Uridine phosphorylase (D44464, M.musculus) | 0.95 | 7.60 (0.000) | 7.07 (0.000) | 11.59 (0.000) |
| OmyOSU252 | TC70900 | Hypoxanthine–guanine phosphoribosyltransferase (AJ132697, G.gallus) | 0.83 | 5.71 (0.004) | 4.81 (0.009) | 7.37 (0.003) |
| Transcription and translation | ||||||
| OmyOSU1667 | TC78247 | Poly A-binding protein 1 (BC003870, M.musculus) | 1.06 | 1.89 (0.000) | 1.67 (0.007) | 1.90 (0.000) |
| OmyOSU217 | TC86507 | Ribosomal protein L13a (BC047855, D.rerio) | 1.03 | 2.09 (0.000) | 1.75 (0.005) | 1.74 (0.000) |
| Immune function and acute-phase response | ||||||
| OmyOSU1106 | TC77195 | Recombination-activating gene 2 (U31670, O.mykiss) | 0.87 | 1.89 (0.025) | 2.14 (0.029) | 2.26 (0.002) |
| OmyOSU1169 | CA369420 | PER1 (XM_683237, D.rerio) | 1.08 | 2.47 (0.000) | 1.62 | 2.80 (0.001) |
| OmyOSU1615 | TC81096 | Transmembrane 4 superfamily member 5 tumor antigen (AF281357, O.mykiss) | 0.80 | 1.83 (0.002) | 2.19 (0.007) | 2.78 (0.000) |
| OmyOSU852 | TC78880 | MHC-II invariant chain (AY065836, O.mykiss) | 0.93 | 1.19 | 1.15 | 2.28 (0.002) |
| OmyOSU788 | TC89515 | MHC-I heavy chain (AF287487, O.mykiss) | 0.59 | 0.83 | 1.04 | 2.01 (0.017) |
| OmyOSU539 | TC87038 | Precerebellin-like protein (AF192969, O.mykiss) | 0.80 | 0.44 (0.004) | 0.70 (0.002) | 0.35 (0.000) |
| OmyOSU268 | TC71098 | Chemotaxin (AF271114, O.mykiss) | 0.47 | 0.16 (0.049) | 0.43 (0.006) | 0.41 (0.003) |
| Drug, lipid and retinol metabolism/homeostasis | ||||||
| OmyOSU146 | TC63282 | Cytochrome P450 1A (AF059711, O.mykiss) | 1.37 | 2.42 (0.002) | 1.42 (0.043) | 0.95 |
| OmyOSU352 | TC72158 | Cytochrome P450 2K1v2 (L11528, O.mykiss) | 0.95 | 0.59 (0.017) | 0.54 (0.019) | 0.38 (0.001) |
| OmyOSU354 | TC72158 | Cytochrome P450 2K3 (AF043551, O.mykiss) | 0.97 | 0.66 | 0.61 (0.006) | 0.32 (0.001) |
| OmyOSU971 | TC69719 | Glutathione S-transferase (AB026119, O.mykiss) | 0.74 | 0.58 | 0.52 (0.010) | 0.49 (0.009) |
| OmyOSU395 | TC71381 | Arachidonate 5-lipoxygenase (L42198, M.musculus) | 0.86 | 0.52 (0.000) | 0.47 (0.019) | 0.49 (0.012) |
| OmyOSU343 | TC69983 | Biotinidase fragment 2 (AF281333, O.mykiss) | 1.12 | 0.48 (0.016) | 0.45 (0.000) | 0.40 (0.000) |
| 15-Week time point | ||||||
| Acute-phase response | ||||||
| OmyOSU232 | TC91273 | Differentially regulated trout protein (AF281355, O.mykiss) | 2.88 | 0.58 | 0.13 (0.00) | 0.11 (0.001) |
| OmyOSU148 | TC91273 | Differentially regulated trout protein (AF281355, O.mykiss) | 2.75 | 0.66 | 0.13 (0.001) | 0.11 (0.000) |
| OmyOSU268 | TC71098 | Chemotaxin (AF271114, O.mykiss) | 1.09 | 0.16 (0.049) | 0.18 (0.016) | 0.16 (0.005) |
| OmyOSU228 | TC55313 | Hepcidin (AF281354, O.mykiss) | 1.81 | 0.72 | 0.26 (0.001) | 0.27 (0.008) |
| OmyOSU878 | CA367917 | LECT2 neutrophil chemotactic factor (AF363272, O.mykiss) | 0.92 | 0.18 (0.025) | 0.29 (0.002) | 0.26 (0.015) |
| OmyOSU744 | TC71412 | Putative interlectin (AF281350, O.mykiss) | 1.66 | 0.73 | 0.29 (0.013) | 0.37 (0.042) |
| OmyOSU165 | TC90142 | IFN-inducible protein 2 (AJ313031, O.mykiss) | 1.71 (0.026) | 0.90 | 0.39 (0.002) | 0.48 (0.003) |
| Redox regulation | ||||||
| OmyOSU1422 | TC47183 | Thioredoxin (AAH49031, D.rerio) | 1.00 | 0.38 | 0.18 (0.009) | 0.20 (0.010) |
| OmyOSU115 | TC78741 | Glutathione S-transferase class-pi (L40381, Cricetulus longicaudatus) | 2.76 (0.034) | 0.54 | 0.23 (0.010) | 0.22 (0.009) |
| Miscellaneous | ||||||
| OmyOSU153 | TC89948 | Liver fatty acid-binding protein (AF281344, O.mykiss) | 1.03 | 0.31 | 0.23 (0.015) | 0.25 (0.012) |
The Institute for Genomic Research (TIGR) ID number of the tentative consensus or singleton expressed sequence tag (EST) sequence corresponding to OSUrbt ver. 2 microarray feature.
The most significant BLASTX is shown. If an EST has no significant (E-value < 10−6) BLASTX hit, and then the most significant BLASTN hit is shown. Genes have been categorized by function based on putative trout homolog using Gene Ontology and OMIM databases.
Average fold change values represent background corrected, LOWESS-normalized signal ratios. Stringent criteria were used to filter for genes that were regulated at least 1.8-fold consistently in all features from biological replicates and had a P value <0.05 by Welch's t-test. Fold-change values for genes that passed stringency criteria are in bold. Animals were initiated with AFB1 or sham initiated (Sham) and then fed DIM or E2 in the diet for 18 weeks after initiation.
Select genes differentially regulated in trout liver during tumorigenesis on promotional diets
| Array ID | TIGR IDa | Gene name (accession number, species)b | Average fold change (P value)c | |||
| Control AFB1 | DIM Sham | DIM AFB1 | E2 AFB1 | |||
| 3-Week time point | ||||||
| Liver-specific proteins (vitellogenesis) | ||||||
| OmyOSU222 | TC47576 | Vitellogenin precursor (X92804, Oncorhynchus mykiss) | 0.68 | 62.78 (0.001) | 99.23 (0.000) | 81.72 (0.000) |
| OmyOSU248 | TC47577 | Vitellogenin (X92804, O.mykiss) | 0.84 | 29.66 (0.000) | 49.49 (0.000) | 42.25 (0.000) |
| OmyOSU1542 | TC85700 | Zona radiata structural protein (AF407574, O.mykiss) | 0.97 | 26.64 (0.000) | 24.67 (0.000) | 29.57 (0.000) |
| OmyOSU203 | TC47576 | Vitellogenin precursor (X92804, O.mykiss) | 0.95 | 27.14 (0.000) | 25.56 (0.000) | 25.66 (0.000) |
| OmyOSU1540 | TC65780 | Vitelline envelope protein alpha (AF231706, O.mykiss) | 1.19 | 19.77 (0.000) | 18.14 (0.000) | 19.00 (0.000) |
| OmyOSU1552 | TC55460 | Vitelline envelope protein gamma (AF231708, O.mykiss) | 0.85 | 23.34 (0.000) | 20.31 (0.000) | 18.68 (0.000) |
| Cell proliferation (signal transduction, growth factors and apoptosis) | ||||||
| OmyOSU212 | TC70106 | TATA-binding protein (AY168633, Danio rerio) | 0.84 | 18.45 (0.000) | 12.89 (0.005) | 15.60 (0.001) |
| OmyOSU244 | NP543968 | Estrogen receptor beta (AJ289883, O.mykiss) | 0.85 | 4.62 (0.000) | 5.88 (0.000) | 5.73 (0.000) |
| OmyOSU151 | TC88754 | Estrogen receptor alpha (M31559, O.mykiss) | 1.04 | 3.01 (0.003) | 1.73 (0.006) | 2.47 (0.000) |
| OmyOSU511 | TC86507 | Ras-like GTPase (BC076026, D.rerio) | 1.26 | 2.20 (0.000) | 1.75 (0.003) | 1.79 (0.003) |
| OmyOSU800 | TC72880 | Non-receptor tyrosine kinase 2 (TYK2) (AF173032, Mus musculus) | 1.03 | 2.45 (0.014) | 1.32 | 2.82 (0.001) |
| OmyOSU915 | TC78497 | Cysteine-rich with EGF-like domains 1 (CR751234, D.rerio) | 1.07 | 2.15 (0.003) | 1.91 (0.005) | 2.29 (0.002) |
| OmyOSU313 | TC76141 | Bone morphogenic protein 7 (S77477, Gallus gallus) | 1.04 | 0.33 (0.005) | 0.33 (0.000) | 0.24 (0.000) |
| OmyOSU285 | CA364711 | Birc4 protein, XIAP (BC055246, D.rerio) | 1.10 | 0.38 (0.015) | 0.41 (0.000) | 0.36 (0.000) |
| Protein stability and transport | ||||||
| OmyOSU139 | TC70102 | Cathepsin D (U90321, O.mykiss) | 0.86 | 1.99 (0.005) | 2.07 (0.007) | 3.05 (0.000) |
| OmyOSU853 | TC81488 | Heat shock protein hsp90 (BC075757, D.rerio) | 1.04 | 2.55 (0.001) | 1.97 | 2.16 (0.004) |
| OmyOSU992 | TC88878 | MAL proteolipid protein 2 (BC078522, Xenopus laevis) | 0.92 | 1.63 (0.001) | 1.70 (0.002) | 2.08 (0.000) |
| Nucleic acid metabolism | ||||||
| OmyOSU1518 | TC80929 | Uridine phosphorylase (D44464, M.musculus) | 0.95 | 7.60 (0.000) | 7.07 (0.000) | 11.59 (0.000) |
| OmyOSU252 | TC70900 | Hypoxanthine–guanine phosphoribosyltransferase (AJ132697, G.gallus) | 0.83 | 5.71 (0.004) | 4.81 (0.009) | 7.37 (0.003) |
| Transcription and translation | ||||||
| OmyOSU1667 | TC78247 | Poly A-binding protein 1 (BC003870, M.musculus) | 1.06 | 1.89 (0.000) | 1.67 (0.007) | 1.90 (0.000) |
| OmyOSU217 | TC86507 | Ribosomal protein L13a (BC047855, D.rerio) | 1.03 | 2.09 (0.000) | 1.75 (0.005) | 1.74 (0.000) |
| Immune function and acute-phase response | ||||||
| OmyOSU1106 | TC77195 | Recombination-activating gene 2 (U31670, O.mykiss) | 0.87 | 1.89 (0.025) | 2.14 (0.029) | 2.26 (0.002) |
| OmyOSU1169 | CA369420 | PER1 (XM_683237, D.rerio) | 1.08 | 2.47 (0.000) | 1.62 | 2.80 (0.001) |
| OmyOSU1615 | TC81096 | Transmembrane 4 superfamily member 5 tumor antigen (AF281357, O.mykiss) | 0.80 | 1.83 (0.002) | 2.19 (0.007) | 2.78 (0.000) |
| OmyOSU852 | TC78880 | MHC-II invariant chain (AY065836, O.mykiss) | 0.93 | 1.19 | 1.15 | 2.28 (0.002) |
| OmyOSU788 | TC89515 | MHC-I heavy chain (AF287487, O.mykiss) | 0.59 | 0.83 | 1.04 | 2.01 (0.017) |
| OmyOSU539 | TC87038 | Precerebellin-like protein (AF192969, O.mykiss) | 0.80 | 0.44 (0.004) | 0.70 (0.002) | 0.35 (0.000) |
| OmyOSU268 | TC71098 | Chemotaxin (AF271114, O.mykiss) | 0.47 | 0.16 (0.049) | 0.43 (0.006) | 0.41 (0.003) |
| Drug, lipid and retinol metabolism/homeostasis | ||||||
| OmyOSU146 | TC63282 | Cytochrome P450 1A (AF059711, O.mykiss) | 1.37 | 2.42 (0.002) | 1.42 (0.043) | 0.95 |
| OmyOSU352 | TC72158 | Cytochrome P450 2K1v2 (L11528, O.mykiss) | 0.95 | 0.59 (0.017) | 0.54 (0.019) | 0.38 (0.001) |
| OmyOSU354 | TC72158 | Cytochrome P450 2K3 (AF043551, O.mykiss) | 0.97 | 0.66 | 0.61 (0.006) | 0.32 (0.001) |
| OmyOSU971 | TC69719 | Glutathione S-transferase (AB026119, O.mykiss) | 0.74 | 0.58 | 0.52 (0.010) | 0.49 (0.009) |
| OmyOSU395 | TC71381 | Arachidonate 5-lipoxygenase (L42198, M.musculus) | 0.86 | 0.52 (0.000) | 0.47 (0.019) | 0.49 (0.012) |
| OmyOSU343 | TC69983 | Biotinidase fragment 2 (AF281333, O.mykiss) | 1.12 | 0.48 (0.016) | 0.45 (0.000) | 0.40 (0.000) |
| 15-Week time point | ||||||
| Acute-phase response | ||||||
| OmyOSU232 | TC91273 | Differentially regulated trout protein (AF281355, O.mykiss) | 2.88 | 0.58 | 0.13 (0.00) | 0.11 (0.001) |
| OmyOSU148 | TC91273 | Differentially regulated trout protein (AF281355, O.mykiss) | 2.75 | 0.66 | 0.13 (0.001) | 0.11 (0.000) |
| OmyOSU268 | TC71098 | Chemotaxin (AF271114, O.mykiss) | 1.09 | 0.16 (0.049) | 0.18 (0.016) | 0.16 (0.005) |
| OmyOSU228 | TC55313 | Hepcidin (AF281354, O.mykiss) | 1.81 | 0.72 | 0.26 (0.001) | 0.27 (0.008) |
| OmyOSU878 | CA367917 | LECT2 neutrophil chemotactic factor (AF363272, O.mykiss) | 0.92 | 0.18 (0.025) | 0.29 (0.002) | 0.26 (0.015) |
| OmyOSU744 | TC71412 | Putative interlectin (AF281350, O.mykiss) | 1.66 | 0.73 | 0.29 (0.013) | 0.37 (0.042) |
| OmyOSU165 | TC90142 | IFN-inducible protein 2 (AJ313031, O.mykiss) | 1.71 (0.026) | 0.90 | 0.39 (0.002) | 0.48 (0.003) |
| Redox regulation | ||||||
| OmyOSU1422 | TC47183 | Thioredoxin (AAH49031, D.rerio) | 1.00 | 0.38 | 0.18 (0.009) | 0.20 (0.010) |
| OmyOSU115 | TC78741 | Glutathione S-transferase class-pi (L40381, Cricetulus longicaudatus) | 2.76 (0.034) | 0.54 | 0.23 (0.010) | 0.22 (0.009) |
| Miscellaneous | ||||||
| OmyOSU153 | TC89948 | Liver fatty acid-binding protein (AF281344, O.mykiss) | 1.03 | 0.31 | 0.23 (0.015) | 0.25 (0.012) |
| Array ID | TIGR IDa | Gene name (accession number, species)b | Average fold change (P value)c | |||
| Control AFB1 | DIM Sham | DIM AFB1 | E2 AFB1 | |||
| 3-Week time point | ||||||
| Liver-specific proteins (vitellogenesis) | ||||||
| OmyOSU222 | TC47576 | Vitellogenin precursor (X92804, Oncorhynchus mykiss) | 0.68 | 62.78 (0.001) | 99.23 (0.000) | 81.72 (0.000) |
| OmyOSU248 | TC47577 | Vitellogenin (X92804, O.mykiss) | 0.84 | 29.66 (0.000) | 49.49 (0.000) | 42.25 (0.000) |
| OmyOSU1542 | TC85700 | Zona radiata structural protein (AF407574, O.mykiss) | 0.97 | 26.64 (0.000) | 24.67 (0.000) | 29.57 (0.000) |
| OmyOSU203 | TC47576 | Vitellogenin precursor (X92804, O.mykiss) | 0.95 | 27.14 (0.000) | 25.56 (0.000) | 25.66 (0.000) |
| OmyOSU1540 | TC65780 | Vitelline envelope protein alpha (AF231706, O.mykiss) | 1.19 | 19.77 (0.000) | 18.14 (0.000) | 19.00 (0.000) |
| OmyOSU1552 | TC55460 | Vitelline envelope protein gamma (AF231708, O.mykiss) | 0.85 | 23.34 (0.000) | 20.31 (0.000) | 18.68 (0.000) |
| Cell proliferation (signal transduction, growth factors and apoptosis) | ||||||
| OmyOSU212 | TC70106 | TATA-binding protein (AY168633, Danio rerio) | 0.84 | 18.45 (0.000) | 12.89 (0.005) | 15.60 (0.001) |
| OmyOSU244 | NP543968 | Estrogen receptor beta (AJ289883, O.mykiss) | 0.85 | 4.62 (0.000) | 5.88 (0.000) | 5.73 (0.000) |
| OmyOSU151 | TC88754 | Estrogen receptor alpha (M31559, O.mykiss) | 1.04 | 3.01 (0.003) | 1.73 (0.006) | 2.47 (0.000) |
| OmyOSU511 | TC86507 | Ras-like GTPase (BC076026, D.rerio) | 1.26 | 2.20 (0.000) | 1.75 (0.003) | 1.79 (0.003) |
| OmyOSU800 | TC72880 | Non-receptor tyrosine kinase 2 (TYK2) (AF173032, Mus musculus) | 1.03 | 2.45 (0.014) | 1.32 | 2.82 (0.001) |
| OmyOSU915 | TC78497 | Cysteine-rich with EGF-like domains 1 (CR751234, D.rerio) | 1.07 | 2.15 (0.003) | 1.91 (0.005) | 2.29 (0.002) |
| OmyOSU313 | TC76141 | Bone morphogenic protein 7 (S77477, Gallus gallus) | 1.04 | 0.33 (0.005) | 0.33 (0.000) | 0.24 (0.000) |
| OmyOSU285 | CA364711 | Birc4 protein, XIAP (BC055246, D.rerio) | 1.10 | 0.38 (0.015) | 0.41 (0.000) | 0.36 (0.000) |
| Protein stability and transport | ||||||
| OmyOSU139 | TC70102 | Cathepsin D (U90321, O.mykiss) | 0.86 | 1.99 (0.005) | 2.07 (0.007) | 3.05 (0.000) |
| OmyOSU853 | TC81488 | Heat shock protein hsp90 (BC075757, D.rerio) | 1.04 | 2.55 (0.001) | 1.97 | 2.16 (0.004) |
| OmyOSU992 | TC88878 | MAL proteolipid protein 2 (BC078522, Xenopus laevis) | 0.92 | 1.63 (0.001) | 1.70 (0.002) | 2.08 (0.000) |
| Nucleic acid metabolism | ||||||
| OmyOSU1518 | TC80929 | Uridine phosphorylase (D44464, M.musculus) | 0.95 | 7.60 (0.000) | 7.07 (0.000) | 11.59 (0.000) |
| OmyOSU252 | TC70900 | Hypoxanthine–guanine phosphoribosyltransferase (AJ132697, G.gallus) | 0.83 | 5.71 (0.004) | 4.81 (0.009) | 7.37 (0.003) |
| Transcription and translation | ||||||
| OmyOSU1667 | TC78247 | Poly A-binding protein 1 (BC003870, M.musculus) | 1.06 | 1.89 (0.000) | 1.67 (0.007) | 1.90 (0.000) |
| OmyOSU217 | TC86507 | Ribosomal protein L13a (BC047855, D.rerio) | 1.03 | 2.09 (0.000) | 1.75 (0.005) | 1.74 (0.000) |
| Immune function and acute-phase response | ||||||
| OmyOSU1106 | TC77195 | Recombination-activating gene 2 (U31670, O.mykiss) | 0.87 | 1.89 (0.025) | 2.14 (0.029) | 2.26 (0.002) |
| OmyOSU1169 | CA369420 | PER1 (XM_683237, D.rerio) | 1.08 | 2.47 (0.000) | 1.62 | 2.80 (0.001) |
| OmyOSU1615 | TC81096 | Transmembrane 4 superfamily member 5 tumor antigen (AF281357, O.mykiss) | 0.80 | 1.83 (0.002) | 2.19 (0.007) | 2.78 (0.000) |
| OmyOSU852 | TC78880 | MHC-II invariant chain (AY065836, O.mykiss) | 0.93 | 1.19 | 1.15 | 2.28 (0.002) |
| OmyOSU788 | TC89515 | MHC-I heavy chain (AF287487, O.mykiss) | 0.59 | 0.83 | 1.04 | 2.01 (0.017) |
| OmyOSU539 | TC87038 | Precerebellin-like protein (AF192969, O.mykiss) | 0.80 | 0.44 (0.004) | 0.70 (0.002) | 0.35 (0.000) |
| OmyOSU268 | TC71098 | Chemotaxin (AF271114, O.mykiss) | 0.47 | 0.16 (0.049) | 0.43 (0.006) | 0.41 (0.003) |
| Drug, lipid and retinol metabolism/homeostasis | ||||||
| OmyOSU146 | TC63282 | Cytochrome P450 1A (AF059711, O.mykiss) | 1.37 | 2.42 (0.002) | 1.42 (0.043) | 0.95 |
| OmyOSU352 | TC72158 | Cytochrome P450 2K1v2 (L11528, O.mykiss) | 0.95 | 0.59 (0.017) | 0.54 (0.019) | 0.38 (0.001) |
| OmyOSU354 | TC72158 | Cytochrome P450 2K3 (AF043551, O.mykiss) | 0.97 | 0.66 | 0.61 (0.006) | 0.32 (0.001) |
| OmyOSU971 | TC69719 | Glutathione S-transferase (AB026119, O.mykiss) | 0.74 | 0.58 | 0.52 (0.010) | 0.49 (0.009) |
| OmyOSU395 | TC71381 | Arachidonate 5-lipoxygenase (L42198, M.musculus) | 0.86 | 0.52 (0.000) | 0.47 (0.019) | 0.49 (0.012) |
| OmyOSU343 | TC69983 | Biotinidase fragment 2 (AF281333, O.mykiss) | 1.12 | 0.48 (0.016) | 0.45 (0.000) | 0.40 (0.000) |
| 15-Week time point | ||||||
| Acute-phase response | ||||||
| OmyOSU232 | TC91273 | Differentially regulated trout protein (AF281355, O.mykiss) | 2.88 | 0.58 | 0.13 (0.00) | 0.11 (0.001) |
| OmyOSU148 | TC91273 | Differentially regulated trout protein (AF281355, O.mykiss) | 2.75 | 0.66 | 0.13 (0.001) | 0.11 (0.000) |
| OmyOSU268 | TC71098 | Chemotaxin (AF271114, O.mykiss) | 1.09 | 0.16 (0.049) | 0.18 (0.016) | 0.16 (0.005) |
| OmyOSU228 | TC55313 | Hepcidin (AF281354, O.mykiss) | 1.81 | 0.72 | 0.26 (0.001) | 0.27 (0.008) |
| OmyOSU878 | CA367917 | LECT2 neutrophil chemotactic factor (AF363272, O.mykiss) | 0.92 | 0.18 (0.025) | 0.29 (0.002) | 0.26 (0.015) |
| OmyOSU744 | TC71412 | Putative interlectin (AF281350, O.mykiss) | 1.66 | 0.73 | 0.29 (0.013) | 0.37 (0.042) |
| OmyOSU165 | TC90142 | IFN-inducible protein 2 (AJ313031, O.mykiss) | 1.71 (0.026) | 0.90 | 0.39 (0.002) | 0.48 (0.003) |
| Redox regulation | ||||||
| OmyOSU1422 | TC47183 | Thioredoxin (AAH49031, D.rerio) | 1.00 | 0.38 | 0.18 (0.009) | 0.20 (0.010) |
| OmyOSU115 | TC78741 | Glutathione S-transferase class-pi (L40381, Cricetulus longicaudatus) | 2.76 (0.034) | 0.54 | 0.23 (0.010) | 0.22 (0.009) |
| Miscellaneous | ||||||
| OmyOSU153 | TC89948 | Liver fatty acid-binding protein (AF281344, O.mykiss) | 1.03 | 0.31 | 0.23 (0.015) | 0.25 (0.012) |
The Institute for Genomic Research (TIGR) ID number of the tentative consensus or singleton expressed sequence tag (EST) sequence corresponding to OSUrbt ver. 2 microarray feature.
The most significant BLASTX is shown. If an EST has no significant (E-value < 10−6) BLASTX hit, and then the most significant BLASTN hit is shown. Genes have been categorized by function based on putative trout homolog using Gene Ontology and OMIM databases.
Average fold change values represent background corrected, LOWESS-normalized signal ratios. Stringent criteria were used to filter for genes that were regulated at least 1.8-fold consistently in all features from biological replicates and had a P value <0.05 by Welch's t-test. Fold-change values for genes that passed stringency criteria are in bold. Animals were initiated with AFB1 or sham initiated (Sham) and then fed DIM or E2 in the diet for 18 weeks after initiation.
Pairwise correlations of gene profiles in (A) liver samples from AFB1-initiated trout fed 5 p.p.m. E2 and 400 p.p.m. DIM for 3 weeks, (B) liver samples from AFB1-initiated trout fed 5 p.p.m. E2 and 400 p.p.m. DIM for 15 weeks and (C) HCC tumors from trout fed 5 p.p.m. E2 and 400 p.p.m. DIM. Values are fold change (log2) compared with appropriate vehicle-treated control samples and were plotted to generate Pearson correlation coefficients (R) among the treatments, P < 0.0001. Line indicates least-squares linear regression.
Bidirectional hierarchical clustering of gene expression in trout liver by Pearson correlation. (A) Correlation in trout liver samples after dietary treatment with 0.1% dimethyl sulfoxide vehicle control (CON), 5 p.p.m. E2 or 400 p.p.m. DIM for 3- and 15-week time points in sham- and AFB1-initiated trout. Results are shown as fold change (n = 2) compared with appropriate vehicle-treated control as follows: CON sham/CON sham, CON AFB1/CON sham, DIM sham/CON sham, DIM AFB1/CON AFB1 and E2 AFB1/CON AFB1. (B) Correlation in trout HCC tumors from AFB1-iniated animals treated with 0.1% dimethyl sulfoxide vehicle control (CON), 5 p.p.m. E2 and 400 p.p.m. DIM. Results are shown as fold change (n = 3) compared with HCC tumors from control trout. Red color, up-regulation; green color, down-regulation; black, unchanged expression and grey, missing values. Heatmap reflects gene expression profiles for genes differentially regulated 1.8-fold up or down (P < 0.05) in at least one treatment group.
Gene expression was also analyzed in HCC tumors of trout from 5 p.p.m. E2 and 400 p.p.m. DIM treatments compared with HCC tumors in 0.1% dimethyl sulfoxide vehicle control animals. Each treatment is represented by biological replicates (n = 3) of RNA isolated from individual HCCs. Pairwise analysis of all 1672 features on the array indicated strong correlation of transcriptional patterns in HCC from E2- and DIM-treated animals, R = 0.87 (two-tailed P < 0.0001; Figure 2, panel C). Genes found to be differentially regulated (≥ or ≤1.8-fold change, P < 0.05 by Welch's t-test) in HCCs from E2 or DIM treatments compared with control animals are listed by functional category in Table II. Bidirectional hierarchical clustering of genes differentially regulated in at least one treatment group (Figure 3, panel B) indicate that there are distinct transcriptional patterns between tumors of animals on promotional diets and control tumors. Clustering also supported the pairwise analysis by Pearson correlation indicating that there was a high degree of similarity in gene expression patterns in HCCs from E2- and DIM-treated animals. Genes differentially regulated in HCC from animals treated with DIM or E2 included those important for the extracellular matrix, vascularization, immune function and redox regulation.
Select genes differentially regulated in AFB1-initiated HCC from animals treated with DIM and E2
| Array ID | TIGR IDa | Gene name (accession number, species)b | Average fold change (P value)c | |
| DIM | E2 | |||
| Extracellular matrix and vascularization factors | ||||
| OmyOSU1502 | TC50691 | Tissue factor, blood coagulation (AJ295167, Oncorhynchus mykiss) | 4.20 (0.006) | 4.50 (0.000) |
| OmyOSU759 | TC79538 | High-mobility group 1 (L32859, O.mykiss) | 0.42 (0.032) | 0.46 (0.041) |
| OmyOSU749 | CA378743 | Fibronectin 1a isoform 1 (XM_691570, Danio rerio) | 0.41 (0.012) | 0.47 (0.026) |
| OmyOSU562 | TC62077 | Collagen 2 alpha (VIII) C1q (AF394686, Salvelinus fontinalis) | 0.47 (0.037) | 0.43 (0.008) |
| OmyOSU380 | TC87593 | CD87, urokinase receptor (AF007789, Rattus norvegicus) | 0.40 (0.027) | 0.31 (0.012) |
| OmyOSU1263 | TC62562 | Plasminogen activator, urokinase receptor (AF007789, R.norvegicus) | 0.08 (0.012) | 0.08 (0.015) |
| OmyOSU33 | TC87593 | Plasminogen activator, urokinase receptor (NM_017350, R.norvegicus) | 0.17 (0.007) | 0.18 (0.017) |
| Redox regulation and metabolism | ||||
| OmyOSU1422 | TC47183 | Thioredoxin (AAH49031, D.rerio) | 0.24 (0.000) | 0.37 (0.000) |
| OmyOSU460 | TC79578 | Aldo-keto reductase family 1 member D1 (BC018333, Mus. musculus) | 2.26 (0.002) | 2.49 (0.000) |
| Immunoregulation | ||||
| OmyOSU1236 | CR375493 | Apopolysialoglycoprotein precursor (J04051, O.mykiss) | 2.71 (0.016) | 3.22 (0.008) |
| OmyOSU1478 | TC79233 | Trout C-polysaccharide-binding protein 1 (AF281345, O.mykiss) | 4.99 (0.009) | 5.53 (0.002) |
| OmyOSU1188 | TC73422 | Transport-associated protein, TAP2B (AF115538, O.mykiss) | 2.21 (0.023) | 2.24 (0.013) |
| OmyOSU1469 | TC8260 | Cathepsin S (AY950578, Paralichthys olivaceus) | 1.85 (0.024) | 2.45 (0.012) |
| OmyOSU1222 | TC69315 | ATPase H+ transporting lysosomal vacuolar proton pump (AY190685, Pagrus major) | 0.49 (0.019) | 0.48 (0.020) |
| OmyOSU882 | TC78878 | MHC-I heavy chain (AF318187, O.mykiss) | 0.35 (0.014) | 0.27 (0.006) |
| OmyOSU175 | TC78877 | MHC-I heavy chain (AF091785, O.mykiss) | 0.20 (0.003) | 0.15 (0.000) |
| OmyOSU790 | TC86587 | MHC-I heavy chain precursor (AF115523, O.mykiss) | 0.25 (0.003) | 0.20 (0.001) |
| OmyOSU401 | NP543817 | Complement component C5 (AF349001, O.mykiss) | 0.57 | 0.33 (0.004) |
| OmyOSU981 | TC78771 | IgM heavy chain (S63348, O.mykiss) | 0.27 (0.026) | 0.26 (0.027) |
| OmyOSU373 | TC81615 | C1q-like adipose-specific protein (AF394686, S.fontinalis) | 0.43 (0.008) | 0.25 |
| Array ID | TIGR IDa | Gene name (accession number, species)b | Average fold change (P value)c | |
| DIM | E2 | |||
| Extracellular matrix and vascularization factors | ||||
| OmyOSU1502 | TC50691 | Tissue factor, blood coagulation (AJ295167, Oncorhynchus mykiss) | 4.20 (0.006) | 4.50 (0.000) |
| OmyOSU759 | TC79538 | High-mobility group 1 (L32859, O.mykiss) | 0.42 (0.032) | 0.46 (0.041) |
| OmyOSU749 | CA378743 | Fibronectin 1a isoform 1 (XM_691570, Danio rerio) | 0.41 (0.012) | 0.47 (0.026) |
| OmyOSU562 | TC62077 | Collagen 2 alpha (VIII) C1q (AF394686, Salvelinus fontinalis) | 0.47 (0.037) | 0.43 (0.008) |
| OmyOSU380 | TC87593 | CD87, urokinase receptor (AF007789, Rattus norvegicus) | 0.40 (0.027) | 0.31 (0.012) |
| OmyOSU1263 | TC62562 | Plasminogen activator, urokinase receptor (AF007789, R.norvegicus) | 0.08 (0.012) | 0.08 (0.015) |
| OmyOSU33 | TC87593 | Plasminogen activator, urokinase receptor (NM_017350, R.norvegicus) | 0.17 (0.007) | 0.18 (0.017) |
| Redox regulation and metabolism | ||||
| OmyOSU1422 | TC47183 | Thioredoxin (AAH49031, D.rerio) | 0.24 (0.000) | 0.37 (0.000) |
| OmyOSU460 | TC79578 | Aldo-keto reductase family 1 member D1 (BC018333, Mus. musculus) | 2.26 (0.002) | 2.49 (0.000) |
| Immunoregulation | ||||
| OmyOSU1236 | CR375493 | Apopolysialoglycoprotein precursor (J04051, O.mykiss) | 2.71 (0.016) | 3.22 (0.008) |
| OmyOSU1478 | TC79233 | Trout C-polysaccharide-binding protein 1 (AF281345, O.mykiss) | 4.99 (0.009) | 5.53 (0.002) |
| OmyOSU1188 | TC73422 | Transport-associated protein, TAP2B (AF115538, O.mykiss) | 2.21 (0.023) | 2.24 (0.013) |
| OmyOSU1469 | TC8260 | Cathepsin S (AY950578, Paralichthys olivaceus) | 1.85 (0.024) | 2.45 (0.012) |
| OmyOSU1222 | TC69315 | ATPase H+ transporting lysosomal vacuolar proton pump (AY190685, Pagrus major) | 0.49 (0.019) | 0.48 (0.020) |
| OmyOSU882 | TC78878 | MHC-I heavy chain (AF318187, O.mykiss) | 0.35 (0.014) | 0.27 (0.006) |
| OmyOSU175 | TC78877 | MHC-I heavy chain (AF091785, O.mykiss) | 0.20 (0.003) | 0.15 (0.000) |
| OmyOSU790 | TC86587 | MHC-I heavy chain precursor (AF115523, O.mykiss) | 0.25 (0.003) | 0.20 (0.001) |
| OmyOSU401 | NP543817 | Complement component C5 (AF349001, O.mykiss) | 0.57 | 0.33 (0.004) |
| OmyOSU981 | TC78771 | IgM heavy chain (S63348, O.mykiss) | 0.27 (0.026) | 0.26 (0.027) |
| OmyOSU373 | TC81615 | C1q-like adipose-specific protein (AF394686, S.fontinalis) | 0.43 (0.008) | 0.25 |
TIGR ID number of the tentative consensus or singleton EST sequence corresponding to OSUrbt ver. 2 microarray feature.
The most significant BLASTX is shown. If an EST has no significant (E-value < 10−6) BLASTX hit, and then the most significant BLASTN hit is shown. Genes have been categorized by function based on putative trout homolog using Gene Ontology and OMIM databases.
Average fold-change values represent background corrected, LOWESS-normalized signal ratios. Stringent criteria were used to filter for genes that were regulated at least 1.8-fold consistently in all features from biological replicates and had a P value <0.05 by Welch's t-test. Fold-change values for genes that passed stringency criteria are in bold.
Select genes differentially regulated in AFB1-initiated HCC from animals treated with DIM and E2
| Array ID | TIGR IDa | Gene name (accession number, species)b | Average fold change (P value)c | |
| DIM | E2 | |||
| Extracellular matrix and vascularization factors | ||||
| OmyOSU1502 | TC50691 | Tissue factor, blood coagulation (AJ295167, Oncorhynchus mykiss) | 4.20 (0.006) | 4.50 (0.000) |
| OmyOSU759 | TC79538 | High-mobility group 1 (L32859, O.mykiss) | 0.42 (0.032) | 0.46 (0.041) |
| OmyOSU749 | CA378743 | Fibronectin 1a isoform 1 (XM_691570, Danio rerio) | 0.41 (0.012) | 0.47 (0.026) |
| OmyOSU562 | TC62077 | Collagen 2 alpha (VIII) C1q (AF394686, Salvelinus fontinalis) | 0.47 (0.037) | 0.43 (0.008) |
| OmyOSU380 | TC87593 | CD87, urokinase receptor (AF007789, Rattus norvegicus) | 0.40 (0.027) | 0.31 (0.012) |
| OmyOSU1263 | TC62562 | Plasminogen activator, urokinase receptor (AF007789, R.norvegicus) | 0.08 (0.012) | 0.08 (0.015) |
| OmyOSU33 | TC87593 | Plasminogen activator, urokinase receptor (NM_017350, R.norvegicus) | 0.17 (0.007) | 0.18 (0.017) |
| Redox regulation and metabolism | ||||
| OmyOSU1422 | TC47183 | Thioredoxin (AAH49031, D.rerio) | 0.24 (0.000) | 0.37 (0.000) |
| OmyOSU460 | TC79578 | Aldo-keto reductase family 1 member D1 (BC018333, Mus. musculus) | 2.26 (0.002) | 2.49 (0.000) |
| Immunoregulation | ||||
| OmyOSU1236 | CR375493 | Apopolysialoglycoprotein precursor (J04051, O.mykiss) | 2.71 (0.016) | 3.22 (0.008) |
| OmyOSU1478 | TC79233 | Trout C-polysaccharide-binding protein 1 (AF281345, O.mykiss) | 4.99 (0.009) | 5.53 (0.002) |
| OmyOSU1188 | TC73422 | Transport-associated protein, TAP2B (AF115538, O.mykiss) | 2.21 (0.023) | 2.24 (0.013) |
| OmyOSU1469 | TC8260 | Cathepsin S (AY950578, Paralichthys olivaceus) | 1.85 (0.024) | 2.45 (0.012) |
| OmyOSU1222 | TC69315 | ATPase H+ transporting lysosomal vacuolar proton pump (AY190685, Pagrus major) | 0.49 (0.019) | 0.48 (0.020) |
| OmyOSU882 | TC78878 | MHC-I heavy chain (AF318187, O.mykiss) | 0.35 (0.014) | 0.27 (0.006) |
| OmyOSU175 | TC78877 | MHC-I heavy chain (AF091785, O.mykiss) | 0.20 (0.003) | 0.15 (0.000) |
| OmyOSU790 | TC86587 | MHC-I heavy chain precursor (AF115523, O.mykiss) | 0.25 (0.003) | 0.20 (0.001) |
| OmyOSU401 | NP543817 | Complement component C5 (AF349001, O.mykiss) | 0.57 | 0.33 (0.004) |
| OmyOSU981 | TC78771 | IgM heavy chain (S63348, O.mykiss) | 0.27 (0.026) | 0.26 (0.027) |
| OmyOSU373 | TC81615 | C1q-like adipose-specific protein (AF394686, S.fontinalis) | 0.43 (0.008) | 0.25 |
| Array ID | TIGR IDa | Gene name (accession number, species)b | Average fold change (P value)c | |
| DIM | E2 | |||
| Extracellular matrix and vascularization factors | ||||
| OmyOSU1502 | TC50691 | Tissue factor, blood coagulation (AJ295167, Oncorhynchus mykiss) | 4.20 (0.006) | 4.50 (0.000) |
| OmyOSU759 | TC79538 | High-mobility group 1 (L32859, O.mykiss) | 0.42 (0.032) | 0.46 (0.041) |
| OmyOSU749 | CA378743 | Fibronectin 1a isoform 1 (XM_691570, Danio rerio) | 0.41 (0.012) | 0.47 (0.026) |
| OmyOSU562 | TC62077 | Collagen 2 alpha (VIII) C1q (AF394686, Salvelinus fontinalis) | 0.47 (0.037) | 0.43 (0.008) |
| OmyOSU380 | TC87593 | CD87, urokinase receptor (AF007789, Rattus norvegicus) | 0.40 (0.027) | 0.31 (0.012) |
| OmyOSU1263 | TC62562 | Plasminogen activator, urokinase receptor (AF007789, R.norvegicus) | 0.08 (0.012) | 0.08 (0.015) |
| OmyOSU33 | TC87593 | Plasminogen activator, urokinase receptor (NM_017350, R.norvegicus) | 0.17 (0.007) | 0.18 (0.017) |
| Redox regulation and metabolism | ||||
| OmyOSU1422 | TC47183 | Thioredoxin (AAH49031, D.rerio) | 0.24 (0.000) | 0.37 (0.000) |
| OmyOSU460 | TC79578 | Aldo-keto reductase family 1 member D1 (BC018333, Mus. musculus) | 2.26 (0.002) | 2.49 (0.000) |
| Immunoregulation | ||||
| OmyOSU1236 | CR375493 | Apopolysialoglycoprotein precursor (J04051, O.mykiss) | 2.71 (0.016) | 3.22 (0.008) |
| OmyOSU1478 | TC79233 | Trout C-polysaccharide-binding protein 1 (AF281345, O.mykiss) | 4.99 (0.009) | 5.53 (0.002) |
| OmyOSU1188 | TC73422 | Transport-associated protein, TAP2B (AF115538, O.mykiss) | 2.21 (0.023) | 2.24 (0.013) |
| OmyOSU1469 | TC8260 | Cathepsin S (AY950578, Paralichthys olivaceus) | 1.85 (0.024) | 2.45 (0.012) |
| OmyOSU1222 | TC69315 | ATPase H+ transporting lysosomal vacuolar proton pump (AY190685, Pagrus major) | 0.49 (0.019) | 0.48 (0.020) |
| OmyOSU882 | TC78878 | MHC-I heavy chain (AF318187, O.mykiss) | 0.35 (0.014) | 0.27 (0.006) |
| OmyOSU175 | TC78877 | MHC-I heavy chain (AF091785, O.mykiss) | 0.20 (0.003) | 0.15 (0.000) |
| OmyOSU790 | TC86587 | MHC-I heavy chain precursor (AF115523, O.mykiss) | 0.25 (0.003) | 0.20 (0.001) |
| OmyOSU401 | NP543817 | Complement component C5 (AF349001, O.mykiss) | 0.57 | 0.33 (0.004) |
| OmyOSU981 | TC78771 | IgM heavy chain (S63348, O.mykiss) | 0.27 (0.026) | 0.26 (0.027) |
| OmyOSU373 | TC81615 | C1q-like adipose-specific protein (AF394686, S.fontinalis) | 0.43 (0.008) | 0.25 |
TIGR ID number of the tentative consensus or singleton EST sequence corresponding to OSUrbt ver. 2 microarray feature.
The most significant BLASTX is shown. If an EST has no significant (E-value < 10−6) BLASTX hit, and then the most significant BLASTN hit is shown. Genes have been categorized by function based on putative trout homolog using Gene Ontology and OMIM databases.
Average fold-change values represent background corrected, LOWESS-normalized signal ratios. Stringent criteria were used to filter for genes that were regulated at least 1.8-fold consistently in all features from biological replicates and had a P value <0.05 by Welch's t-test. Fold-change values for genes that passed stringency criteria are in bold.
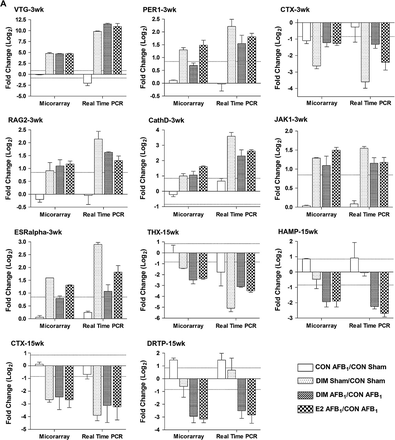
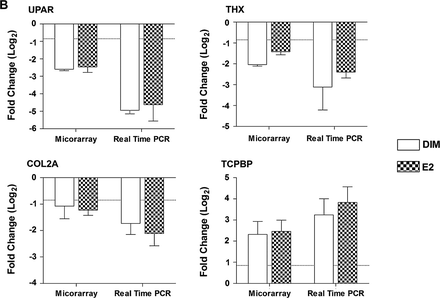
Microarray confirmation by quantitative reverse transcription–PCR
The expression of select genes differentially increased or decreased in the microarray analysis, including CathD, COL2A, CTX, DRTP, ERα, HAMP, JAK1, PER1, RAG2, TCPBP, THX, UPAR and VTG, was confirmed by quantitative reverse transcription–PCR using SYBR Green (Figure 4). The same RNA preparations were used for each technique and the mean expression ratios were compared for all replicates in a treatment. Values for duplicate spots and dye-swapped slides were averaged prior to analysis of biological replicates for microarray data. Overall, we were able to confirm gene expression profiles measured by oligonucleotides microarray analysis using quantitative reverse transcription–PCR for both the liver samples and HCC tumors. These data indicate that our strict criteria for determining differential gene regulation by array, including 1.8-fold change in all biological replicates with P < 0.05, resulted in detection of meaningful changes that could be validated by other methods.
Comparison of gene expression measured by microarray and real-time reverse transcription–PCR. (A) Hepatic gene expression in liver samples from trout treated with 0.1% dimethyl sulfoxide (CON), 5 p.p.m. E2 or 400 p.p.m. DIM for either a 3- or 15-week period post-initiation by 50 p.p.b. AFB1 or 0.01% EtOH (sham), n = 2. (B) Hepatic gene expression in AFB1-initiated HCC from trout treated with 400 p.p.m. DIM and 5 p.p.m. E2, n = 3. Values are expressed as average fold change (log2) with standard deviation compared with appropriate control. Dashed line indicates 1.8-fold change (±0.847 log2).
Discussion
We previously reported that DIM has a transcriptional profile in trout liver similar to two known hepatic tumor promoters, I3C and E2, suggesting DIM may also enhance hepatocarcinogenesis by estrogenic mechanisms in the trout model (28). Further, the data indicated that DIM may be an even more potent tumor promoter than I3C based on this mechanism. In the current study, 400 p.p.m. DIM in the diet greatly enhanced hepatic tumor incidence in animals initiated with 50 p.p.b. AFB1, as did 5 p.p.m. E2, whereas no enhancement was observed at the lower concentration of 120 p.p.m. DIM. Concentrations in the diet are equivalent to treatment with ∼6 and 21 mg/kg/day DIM, 5 days/week for 18 weeks. For comparison, commercially available dietary supplements of DIM recommend a daily dose of 3–6 mg/kg/day. The data presented here are consistent with observations from previous studies in which I3C promoted AFB1-induced hepatocarcinogenesis in trout and glutathione-S-transferase (GST-P) foci in Sprague–Dawley liver (20,25). Comparison of the promotional potency of DIM reported here with previous studies using I3C indicates DIM is more potent as a tumor enhancer in the trout model. Promotional potency is a function of several factors including carcinogen dose, promotional dose and exposure duration (24,25). In the present study, 400 p.p.m. DIM fed in the diet for 18 weeks post-initiation by 50 p.p.b. AFB1, resulted in 16.5% tumor incidence compared with 4% in control animals. In a previous study with I3C, a similar level of tumor incidence (18.9%) was achieved with 1000 p.p.m. I3C fed in the diet for 8 months post-initiation by 50 p.p.b. AFB1 compared with 6.5% in control animals. The relative potency of DIM and I3C are further supported by the conservative nature of the comparison using studies of markedly different exposure duration. Differences in potency may be explained by previous pharmacokinetic studies showing DIM is the primary in vivo component of I3C after oligomerization, absorption and disposition (1–3). DIM was found to comprise >40% of the total hepatic radiolabel after oral gavage with radiolabeled I3C in trout (2). Global hepatic gene profiles further indicate DIM is probably the biologically active I3C component in trout liver with signatures for both indoles showing a strong similarity to E2 (28).
In this study, we investigated whether DIM was also acting through estrogenic mechanisms during tumor enhancement using a toxicogenomic approach. Hepatic gene expression profiles were examined in liver samples from animals fed promotional diets during the course of tumorigenesis and in HCCs of initiated animals using a rainbow trout 70mer oligonucleotide array. Strong transcriptional correlations were observed between DIM and E2 treatments at all time points examined. Gene expression in liver samples from DIM and E2 treatments at the 3-week time point was similar to that described previously for juvenile trout exposed to dietary I3C, DIM and E2 for 2 weeks (28). These included up-regulation of transcripts for known estrogen-responsive proteins, such as vitellogenin, vitelline envelope, ERα/β and cathepsin D, and down-regulation of genes important for acute-phase response and drug, lipid and retinol metabolism. Interestingly, PCA on condition indicated that transcriptional profiles were strongly similar between DIM and E2 treatments in AFB1-initiated animals, but distinct from DIM treatment in sham-initiated trout particularly at the 15-week time point. These data suggest that the effects of DIM on gene expression may be different in animals initiated with a chemical carcinogen compared with control animals. At the 15-week time point, genes associated with the typical ‘estrogenic’ gene signature were no longer differentially regulated and we observed down-regulation of transcripts primarily involved in acute-phase response and redox regulation. These transcriptional patterns indicate that the tumor-enhancing effects of DIM are similar to E2 and are probably mediated through ER-dependent mitogenic signaling in trout liver.
DIM was found to be estrogenic in human breast and endometrial cancer cells in vitro resulting in stimulation of cell proliferation and activation of ER-responsive genes at physiological concentrations through strong ligand-independent activation of ER in the presence of low levels of endogenous E2 (17,35). However, I3C and DIM are commonly reported to have anti-estrogenic properties in estrogen-sensitive cells (6,36,37). Further, there is no evidence for estrogenic activity of DIM in mammalian models in vivo. Trout have previously been shown to be a sensitive model for estrogen-mediated promotion of hepatocarcinogenesis (34), whereas rodent models respond more weakly to oral estrogen treatment (38,39). The reasons for species differences in sensitivity are currently unknown; however, species differences in transactivation of the ER have been reported. Trout ER expressed in bacterial or yeast systems had higher ligand promiscuity compared with other species and also exhibited some basal transcriptional activity in a ligand-independent manner (40,41). While the mechanism for DIM estrogenicity in trout liver needs to be further explored, DIM-induced mitogenic signaling in this study may provide initiated cells having a selective growth advantage an appropriate environment for proliferation and clonal expansion.
We also observed up-regulation of genes at the 3-week time point involved in adaptive immunity, such as RAG2, PER1 and tumor-associated antigen TM4SF5, and in extracellular signaling cascades, including Ras-like GTPase and JAK (similar to JAK1 or TYK2 based on sequence homology). RAG2 and PER are involved in lymphocyte-mediated tumor suppression through interferon-γ (IFNγ) signaling (42). PER has also been found to mediate the potent anti-metastatic effects of natural killer cell cytotoxicity (43). IFNs are a group of immune cytokines with anti-viral and cytostatic functions mediated by JAK activation of JAK/STAT-signaling pathways. DIM was found to have immunomodulatory effects in rat in vivo and in breast tumor cells and Jurkat T cells in vitro (44,45). In those studies, DIM stimulated IFNγ in vitro through an ER-independent mechanism that further induced expression of IFN-inducible genes and major histocompatability complex class-I (MHC-I) tumor-associated antigen-presentation molecules (46). We do not know if IFN expression is induced by DIM and/or E2 in trout liver, but transcriptional changes observed in this study in liver samples of animals treated with DIM and E2 indicate potentiation of adaptive immune function, including some increase in expression of MHC-I and MHC-II transcripts. Enhancement of immune function may be a primary defense against cancer by cancer immunosurveillance. The immune activating properties of DIM previously observed in human cancer cells were associated with inhibition of cellular proliferation.
It is difficult to know what effect enhancement of adaptive immunity may have in trout chemoprotection since it was measured early in the course of tumorigenesis after treatment with concentrations of DIM and E2 that ultimately caused tumor promotion. However, while DIM and E2 increased tumor incidence in initiated animals, transcriptional profiles in HCC tumors from these animals indicated lower invasive and metastatic potential compared with HCCs from control animals. We have previously reported that gene expression in trout HCCs is indicative of tumors of an aggressive nature with high-invasive potential (31). Many of the genes associated with cell migration, invasion or metastasis that were previously found to be differentially regulated in HCC were reversed in this study in HCCs from animals treated with DIM and E2. Transcriptional profiles showed strong down-regulation of transcripts for fibronectin, collagen 2 alpha, urokinase plasminogen activator receptor and high-mobility group 1 and up-regulation of tissue factor. Other factors associated with transport or loading of antigens to MHC-I/II molecules, including transport-associated protein (TAP2B) and cathepsin S, were also up-regulated even though transcripts for MHC-I antigen-presentation molecules were down-regulated in HCC tumors. It is unclear how DIM and E2 treatment may cause decreased HCC tumor invasion in trout, however, early predictors of metastatic potential in liver samples at the 3-week time point include transcriptional up-regulation of RAG2 and PER and down-regulation of bone morphogenic protein 7 (BMP7) and anti-apoptotic XIAP, all of which have been associated with tumors having decreased invasive potential or high prognosis for disease-free survival (42,43,47,48).
In summary, these data are the first to describe tumor enhancement by DIM. Both DIM and E2 promoted AFB1-initiated hepatocarcinogenesis in the rainbow trout model. Toxicogenomic profiling of liver samples collected during the course of tumorigenesis showed strong transcriptional correlations between DIM- and E2-treated animals suggesting that DIM promoted tumors through estrogenic mechanisms. However, HCC tumors from DIM- and E2-treated animals had transcriptional signatures indicating decreased metastatic potential and may describe a novel chemoprotective effect of DIM in tumors in vivo. It is possible that DIM is acting similar to E2 in trout liver and having a dual effect on tumorigenesis in which it may increase tumor incidence through ER-mediated mitogenic signaling and decrease potential for metastasis possibly through immune activation and potentiation, however, these mechanisms need to be further explored. Overall, these data may help explain the dichotomy of chemoprotective and enhancing effects of dietary indoles on cancer development.
Supplementary material
Supplementary table I and figure 1 can be found at http://carcin.oxfordjournals.org/
Abbreviations
- AFB1
aflatoxin B1
- AhR
aryl hydrocarbon receptor
- DIM
3,3′-diindolylmethane
- EST
expressed sequence tag
- ER
estrogen receptor
- E2
17β-estradiol
- HCC
hepatocellular carcinoma
- IFN
interferon
- I3C
indole-3-carbinol
- JAK
Janus kinase
- MHC-I
histocompatability complex class-I
- PCA
principal component analysis
- PCR
polymerase chain reaction
- PER
perforin
- RAG2
recombination-activating gene 2
- SDS
sodium dodecylsulphate
- SSC
standard saline citrate
The authors wish to thank Eric Johnson and Greg Gonnerman for care and maintenance of fish, Sheila Cleveland for histological preparation and Jack Giovanni and Youngai Li for statistical analysis of tumor data. This work was supported by National Institutes of Health grants ES07060, ES03850, ES00210, ES11267, ES013534 and CA90890 and The Linus Pauling Institute.
Conflict of Interest Statement: None declared.