-
PDF
- Split View
-
Views
-
Cite
Cite
Yi-Wen Chen, Olivia Surgent, Barkha S. Rana, Francis Lee, Chiye Aoki, Variant BDNF-Val66Met Polymorphism is Associated with Layer-Specific Alterations in GABAergic Innervation of Pyramidal Neurons, Elevated Anxiety and Reduced Vulnerability of Adolescent Male Mice to Activity-Based Anorexia, Cerebral Cortex, Volume 27, Issue 8, August 2017, Pages 3980–3993, https://doi.org/10.1093/cercor/bhw210
Close - Share Icon Share
Abstract
Previously, we determined that rodents’ vulnerability to food restriction (FR)-evoked wheel running during adolescence (activity-based anorexia, ABA) is associated with failures to increase GABAergic innervation of hippocampal and medial prefrontal pyramidal neurons. Since brain-derived neurotrophic factor (BDNF) promotes GABAergic synaptogenesis, we hypothesized that individual differences in this vulnerability may arise from differences in the link between BDNF bioavailability and FR-evoked wheel running. We tested this hypothesis in male BDNF-Val66Met knock-in mice (BDNFMet/Met), known for reduction in the activity-dependent BDNF secretion and elevated anxiety-like behaviors. We found that 1) in the absence of FR or a wheel (i.e., control), BDNFMet/Met mice are more anxious than wild-type (WT) littermates, 2) electron microscopically verified GABAergic innervations of pyramidal neurons of BDNFMet/Met mice are reduced at distal dendrites in hippocampal CA1 and medial prefrontal cortex, 3) following ABA, WT mice exhibit anxiety equal to those of the BDNFMet/Met mice and have lost GABAergic innervation along distal dendrites, 4) BDNFMet/Met mice show blunted ABA vulnerability, and 5) unexpectedly, GABAergic innervation is higher at somata of BDNFMet/Met mice than of WT. We conclude that lamina-specific GABAergic inhibition is important for regulating anxiety, whether arising from environmental stress, such as food deprivation, or genetically, such as BDNFMet/Met single nucleotide polymorphism.
Introduction
Anorexia nervosa (AN) is a mental illness characterized by severe self-imposed food restriction (FR) accompanied by an intense fear of weight gain. Genetic heritability accounts for >50% of its risk (Steinhausen 2002; Boraska et al. 2014), and the disease is co-morbid with anxiety disorders (Kaye et al. 2009). The lifetime prevalence of AN is about 2%, emerging mostly during early adolescence, with about 25% suffering from a chronic and relapsing course (Smink et al. 2014). Mortality associated with AN is >200 times greater than the suicide rate of the general population (Sullivan 1995; Arcelus et al. 2011). The majority of individuals with AN over-exercise, a behavior which exacerbates the severe weight loss associated with FR (Casper 2006). The relationship between anxiety, FR, and exercise remains unclear. One view is that FR and excessive exercise arise from a preexisting condition of anxiety, and that patients deliberately choose these behaviors as anxiolytic agents to abate the intense fear of weight gain. In support of this view, animal models indicate that exercise and FR are anxiolytic, in part, through an increase in brain-derived neurotrophic factor (BDNF) within brain (Duan et al. 2001; Gomez-Pinilla et al. 2002; Lutter et al. 2008; Stranahan et al. 2009; Sciolino and Holmes 2012). Conversely, there is compelling evidence from many species, including humans, pigs, and rodents, that anxiety and hyperactivity are inevitable behaviors stemming from starvation. This pervasive behavior may arise because hyperactivity mimics foraging, an innate behavior that is adaptive for organisms facing insufficient food supply (Guisinger 2003). Caged animals facing FR begin to run excessively on a wheel, even during the limited hours of food access, thereby becoming voluntary food restrictors. This behavior is called activity-based anorexia (ABA) and has been used as an animal model to investigate the neurobiological (i.e., non-social and non-cultural) risk factors and brain circuit changes associated with AN (Gutierrez 2013), both knowledge that are needed for developing pharmacological treatments to prevent relapse.
Prefrontal cortical activity is strongly implicated in individuals’ food choices and decisions to eat or exercise (Kaye et al. 2009; Ehrlich et al. 2015). In contrast, relatively little is known regarding the role of the hippocampus in AN, even though this structure projects directly to the prefrontal cortex. The hippocampus is important for updating memory (Andersen et al. 2007); it is modulated by ghrelin (Lutter et al. 2008), gonadal hormones, and steroidal stress hormones (McEwen et al. 1994; Woolley and McEwen 1994; Hojo et al. 2011); it is where BDNF is expressed at highest levels peri-pubertally (Katoh-Semba et al. 1997); and it controls the amygdala's drive in the prefrontal cortex (Thomases et al. 2014). Moreover, individual differences in ABA vulnerability among C57BL6 adolescent mice correlates with individual differences in GABAergic anatomy of the hippocampus (Chowdhury et al. 2013) and prefrontal cortex (Chen et al. 2015). Specifically, ABA vulnerability is related to failures in the up-regulation of GABAergic innervations upon pyramidal neurons in the hippocampus (Chowdhury et al. 2013) and prefrontal cortex (Chen et al. 2015) and of α4βδ-GABA receptor levels adjacent to excitatory axo-spinous synapses of hippocampal pyramidal cells (Wable et al. 2015a).
Our focus in this study was to address this question: What gives rise to the individual differences in responsiveness of the GABAergic system to ABA induction? We tested the putative role of BDNF bioavailability in the responsiveness of the GABAergic system to ABA induction, since BDNF is a neurotrophic factor promoting GABAergic synaptogenesis (Marty et al. 1996; Jiao et al. 2011). We tested the BDNF bioavailability idea by comparing ABA vulnerability and GABAergic innervation patterns upon hippocampal CA1 and Layer V medial prefrontal pyramidal cells of mice with reduced activity-dependent secretion of BDNF, as well as elevated anxiety-like behaviors due to the knock-in of a human genetic variant, whereby valine (Val) at amino acid 66 in the pro-region of BDNF is substituted by methionine (Met) (BDNFVal66Met) (Chen et al. 2006).
Materials and Methods
Animals
All animals used in the study were bred at New York University's animal facility. All procedures relating to the use of animals were according to the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees of New York University (A3317-01). The generation of BDNFMet/Met mice (homozygous for Val-to-Met substitution at amino acid 66) was described previously (Chen et al. 2006). Male BDNFMet/Met mice and wild-type (WT, BDNFVal/Val) littermates used for this study were derived from heterozygous BDNFVal/Met breeding pairs. All animals were kept on a 12/12 light–dark cycle with food and water available ad libitum. Mice were genotyped as described previously (Chen et al. 2006). After weaning at postnatal day (P) 25 (P25), the animals were group-housed 2–4 per cage with same-sex littermates.
ABA Induction and Controls
On P36, 12th BDNFMet/Met mice and 16th WT (BDNFVal/Val) littermates were randomly assigned to 1 of 2 rearing groups, control or ABA.
Animals of the control rearing group (N = 8 for WT, and N = 6 for BDNFMet/Met) were singly housed in standard home cages with ad libitum access to food (both dry chow, PMI Mouse Diet 5001, 336 kcal per 100 g, and soft food, Clear H2O DietGel 76A, 99.8 kcal per 100 g), and no access to a running wheel for the duration of the study.
Animals of the ABA rearing group (N = 8 for WT, and N = 6 for BDNFMet/Met) were singly housed, starting at noon, in a standard home cage with a running wheel attached (Low-Profile Mouse Wheel, Med Associates, Inc.) and with ad libitum access to food, in order to record baseline running activity. Starting at noon on P41 and until noon on P44, ABA animals’ food access became limited to only the first 2 h of the dark cycle. So as to ensure that food was not available for the remaining 22 h per day, animals were moved to a fresh standard cage on P41, with transfer of some of the bedding from the previous cage, but with no residual food crumbs. Body weight, food intake, and wheel running activity were measured daily within 20 min prior to the start of the dark cycle. Further details of the ABA induction procedure are as reported previously (Chowdhury et al. 2013, 2015).
Elevated Plus Maze
Elevated plus maze (EPM) tests were conducted on P51 for both the CON and ABA groups to measure anxiety-like behaviors. P51 corresponded to the seventh day of recovery from FR for the ABA group. EPM duration was 10 min (Wable et al. 2015b). The time spent and the number of entries into the open arms were recorded and analyzed using the EthoVision tracking system (version 4.1.106, Noldus Information Technology).
Quantitative Ultrastructural Analysis of Contacts Formed by Glutamic Acid Decarboxylase-Immunoreactive Axon Terminals on CA1 Pyramidal Cells of the Dorsal Hippocampus and on Layer V Pyramidal Cells of the Prelimbic Cortex
Animals Used for Electron Microscopic Immunocytochemistry
Mice (N = 5 for control WT, N = 3 for control BDNFMet/Met, N = 5 for ABA WT, and N = 4 for ABA BDNFMet/Met) were euthanized on the morning of P60, between 8:00 and 10:30 AM. The mice were deeply anesthetized with urethane (i.p. 0.34 g/g body weight), then transcardially perfused with 0.1 M phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde (PFA, EM Sciences). Glutaraldehyde-fixation was withheld until after immunocytochemistry, so as to optimize antigen-retention. The brain of each mouse was removed from the skull, stored in 4% paraformaldehyde in 0.1 M PB, and later blocked for slicing and cut on a vibratome (Leica VT1000M) into 50-μm sections (Leica Microsystems GmbH). Coronal sections containing optimal cross sections of the dorsal hippocampus (Bregma −1.58 ~ −1.94 mm) and prelimbic cortex (Bregma 2.10–2.34 mm) were collected.
Glutamic Acid Decarboxylase/3,3′-Diaminobenzidine Tissue Processing
Details of the glutamic acid decarboxylase (GAD) immunocytochemical procedure are similar to the steps described in our previous publication (Chowdhury et al. 2013; Chen et al. 2015). In order to accurately assess the presence of axo-somatic and axo-dendritic GAD terminals in the CA1 region of the dorsal hippocampus and prefrontal cortex in all four groups of mice, coronal sections containing these brain regions were processed for immunocytochemistry and electron microscopy (EM) using the avidin-biotin-HRP complex method (ABC) with 3,3′-diaminobenzidine (DAB) as the chromophore. Tissue was first incubated with 1% hydrogen peroxide in PB for 30 min to minimize background peroxidase reactivity. Blocking was performed with phosphate buffered (0.01 M, pH 7.4) saline (0.9% sodium chloride) (PBS) containing 1% bovine serum albumin (BSA) and 0.05% sodium azide (PBS/BSA/azide) to minimize non-specific labeling of the primary antibody onto sections. The primary antibody, rabbit-anti GAD (Lot 2580693 of Millipore; catalog number AB1511), which recognizes both GAD65 and GAD67 isoforms, was added in a 1:400 ratio with PBS/BSA/azide solution and left to incubate at room temperature under constant agitation for ~65 h (Chen et al. 2015).
After incubation in the buffer containing the primary antibody, sections were rinsed using PBS over a 30-min period. The tissue was then incubated in PBS/BSA/azide solution containing the secondary antibody, consisting of biotinylated goat anti-rabbit IgG (Catalog # BA-1000, Vector, Inc.) at a dilution of 1:200, for 1 h, at room temperature. This step was followed by a 30-min period of rinsing with PBS, and then incubated for 30 mins in PBS containing the avidin-biotinylated horseradish peroxidase (HRP) complex (ABC) (ABC Elite Kit, Catalog # PK-6100, from Vectors). The HRP substrate, DAB (10 mg tablets, Catalog # D5905, Sigma Chem.), becomes brown reaction products and is also osmiophilic, thereby becoming electron-dense markers suitable for both light and EM. Hydrogen peroxide was added to the dissolved tablets to catalyze the reaction and then the DAB-peroxide solution was left to react with the tissue for 9 min and 30 s. The reaction was terminated by rinsing the tissue in PBS.
Electron Microscopic Tissue Preparation
Tissue underwent post-fixation, in preparation for EM, using 2% glutaraldehyde solution in PBS for 16mins. The vibratome sections were then processed by a conventional electron microscopic procedure, consisting of post-fixation by immersion in 1% osmium tetroxide/0.1 M PB for 1 h, then dehydrated using graded concentrations of alcohol, up to 70%, then post-fixed overnight using 1% uranyl acetate (Terzakis 1968; Lozsa 1974), dissolved in 70% ethanol. On the following day, the sections were further dehydrated using 90% ethanol, then 100% ethanol, then were rinsed in acetone, and infiltrated in EPON 812 (EM Sciences). The EPON 812 was later cured by heating the tissue at 60°C, while sandwiched between 2 sheets of Aclar plastic, with lead weights placed on top of the Aclar sheets to ensure flatness of the EPON-embedded sections. These flat-embedded vibratome sections were re-embedded in BEEM capsules (EM Sciences) filled with EPON 812, then ultrathin-sectioned at a plane tangential to the vibratome sections. The ultrathin sections were collected onto formvar-coated, 400 mesh thin-bar nickel grids (EM Sciences). Ultrathin sections were counterstained with Reynolds’ lead citrate before viewing.
Electron Microscopic Image Collection and Analysis
During the image capturing and tracing process, the microscopists were kept entirely blind to the genotype or rearing condition of the animals. Cell bodies in the pyramidal cell layer (PCL), together with distal spiny dendrites in the stratum lacunosum-moleculare (SLM) of the dorsal hippocampal CA1 region (AP Bregma −1.58 ~ −1.94 mm) and cell bodies in Layer V, together with distal spiny dendrites in the Layer I of the prelimbic cortex (AP Bregma 2.10–2.34 mm, DV 1.8–2.2 mm, and ML 0 ± 0.5 mm), identified using the atlas of Paxinos–Franklin (Paxinos and Franklin 2001) were randomly sampled, strictly in the order that they were encountered from the surface-most portions of vibratome sections, exactly as described previously (Chowdhury et al. 2013; Chen et al. 2015). Pyramidal cells were identified using standard morphological criteria (Peters et al. 1991). Pyramidal cell bodies were then distinguished from GABAergic cell bodies by their lack of large indentations in the nuclear membranes and lack of DAB-labeling in the cytoplasm. Images of ~8–15 pyramidal cells bodies, and 15–20 distal spiny dendrites per animal were captured from ultrathin sections with a 1.2-megapixel Hamamtsu CCD camera from Advanced Microscopy Techniques attached to a JEOL 1200XL electron microscope (JEOL Ltd) at magnifications of ×10 000, ×20 000, and ×40 000. Alternatively, the electron microscope (Philips CM12) and digital camera (Gatan 4 × 2.7 k) of the NYU School of Medicine Microscopy Core was used at a magnification of ×25 000. After the images were captured, ImageJ (version 6, an NIH tracing program) was used to measure the perimeter of perikaryal and dendritic plasma membrane and contact lengths of every GAD terminal directly contacting it. These measurements were done using the segmented line tool to trace the entire membrane perimeter once and then re-tracing parts of the plasma membrane directly contacting GAD terminals. Two criteria were used for identifying GAD terminals: the presence of vesicles within the terminal and more electron density than the mitochondria residing inside of the cytoplasm, which reflected accumulation of the HRP-DAB reaction product signifying GAD-immunoreactivity.
Statistical Analysis
Normality of the distribution of measures was tested using the Kruskal–Wallis test. For data that were normally distributed, two-way analysis of variance (ANOVA) was used to evaluate significance of the differences between the four groups, using genotype and rearing conditions as the two factors, followed by Bonferroni's post hoc analysis. For the data set that was not normally distributed, the Mann–Whitney U-test was used to test significance of the difference between the distributions. Bonferroni correction was used for multiple comparisons. All the results are expressed as mean ± standard error of the means (SEM). P values of <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS 22.0.
Results
EPM Open Arm Duration and Entries are Decreased by Variant BDNF-Val66Met and by ABA
Previously, BDNFMet/Met mice (homozygous for the Val-to-Met substitution at amino acid 66) were shown to exhibit elevated anxiety-like behavior in adulthood, compared with WT mice, BDNFVal/Val (homozygous for Val at amino acid 66) (Chen et al. 2006). We sought to determine whether the phenotype anxiety associated with BDNFMet/Met mice emerged by P51, corresponded to late adolescence. We also determined whether ABA induction changed the anxiety-like behavior of animals differently across the two genotypes. To this end, we analyzed the behavior of four groups of animals on the EPM. Two of the groups were reared in control environments (CON), without ABA induction but differed in genotype: BDNFMet/Met versus WT BDNFVal/Val. Two more groups, distributed across the same 2 genotypes, were ABA-induced (ABA), by rearing them in the presence of a wheel from P36 to P44 and food-restricted from P41 to P44. All animals were of postnatal day 51 (P51) on the day tested on the EPM, corresponding to when the ABA animals had undergone 7 days of recovery after the last day of FR.
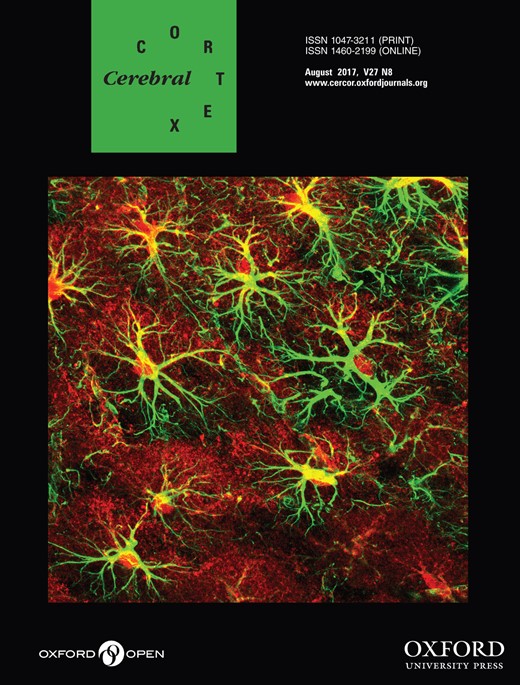
Two-way ANOVA revealed significant genotype × treatment interactions for both ratios of time spent (F(1,24) = 7.483, P = 0.012) (Fig. 1A) and entries into the open arms (F(1,24) = 5.148, P = 0.033) (Fig. 1B). Post hoc analysis revealed that the CON group of the BDNFMet/Met genotype spent less time in the open arms (t(12) = 3.246, P = 0.007) and made less entries into the open arms (t(12) = 3.379, P = 0.005), relative to the CON group of the WT (Val/Val in Fig. 1A) genotype. However, following ABA, this difference across the genotypes was absent (Fig. 1A,B). Post hoc comparison also revealed that the ABA WT mice spent significantly less time in the open arms (t(14) = 3.493, P = 0.004) and made marginally significantly less entries into the open arms, relative to control WT mice (t(14) = 2.76, P = 0.015) (Fig. 1A,B). These findings indicate that the experience of ABA induction during adolescence had a lasting effect upon WT animals’ anxiety-like behavior. Moreover, the BDNF-Met/Met genotype effect was already evident by P51 and did not increase further by ABA induction.
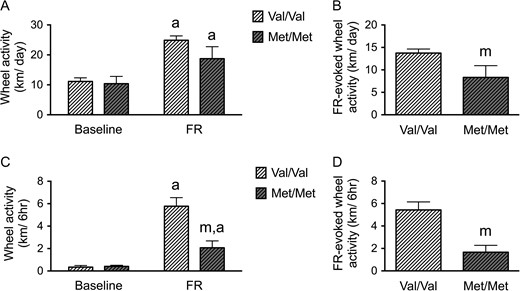
BDNFMet/Met mice show increased anxiety-like behaviors during late adolescence. (A) BDNFMet/Met mice showed decreased time spent in open arms, as a fraction of the total time in the maze, under control condition in late adolescence (P51). The experience of ABA (with FR from P41-44, mid-adolescence) lowered the time spent in the open arms for the WT (BDNFVal/Val, WT) group of mice, but had no further effect on the BDNFMet/Met group of mice. (B) BDNFMet/Met mice showed decreased entries into open arms, as a fraction of total entries, under control condition. The experience of ABA during mid-adolescence lowered the number of entries into the open arms for the WT group, but had no further effect on the BDNFMet/Met group. Bar graphs represent means ± SEM (n = 8 for WT, and 6 for BDNFMet/Met). “m” and “a” indicate statistically significant effects of genotype (Val66Met polymorphism) and ABA treatment, respectively, by comparing to the values of WT controls, as assessed by two-way ANOVA, followed by Bonferroni's test.
GAD Terminal Contacts onto Pyramidal Neurons of the Hippocampal CA1 of Animals with Variant BDNF-Val66Met Following ABA
Previously, we had shown that the extent of GABAergic innervation onto pyramidal neurons in the CA1 and prefrontal cortex was associated with their suppression of ABA-induced hyperactivity (Chowdhury et al. 2013; Chen et al. 2015). Here, we sought to determine whether the variant BDNF, Val66Met, affected the animals’ ABA-induced change in GABAergic innervation of the pyramidal neurons in the CA1. We assessed the extent of axo-somatic synaptic junctions onto pyramidal cell bodies in the PCL and axo-dendritic synaptic junctions in the SLM formed by terminals immunoreactive for GAD, the rate-limiting enzyme for the synthesis of γ-aminobutyric acid (GABA). EM was used to quantify the proportion of the plasma membrane contacted directly by GAD terminals (Figs 2 and 3, A,B). All four groups described earlier for the EPM underwent this EM analysis.
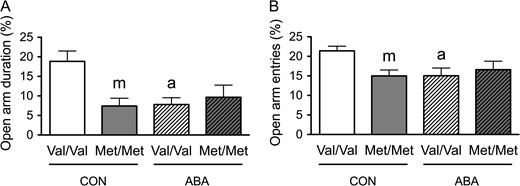
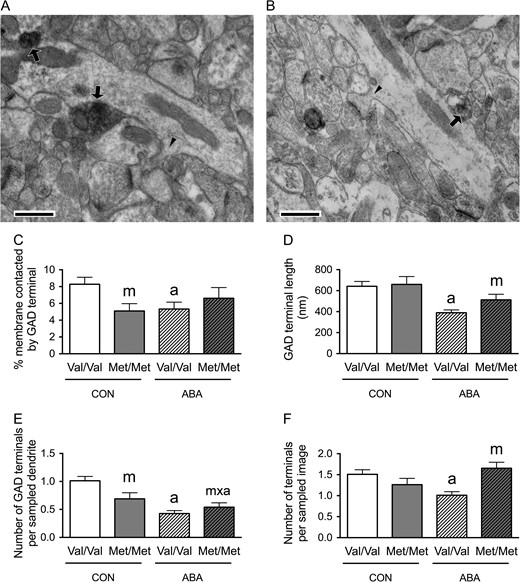
Electron microscopic identification of GAD-immunoreactive axon terminals contacting distal spiny dendrites in SLM of the dorsal hippocampal CA1 of WT (A) and BDNFMet/Met (B) brains at P60. In both Panels A and B, arrows point to portions of the plasma membrane contacted by GAD-immunoreactive axon terminals. Arrowheads point to the spine neck connecting spine heads to dendritic shafts. Calibration bars = 500 nm for both panels. (C) Comparisons of dendritic profiles revealed that BDNFMet/Met reduces the proportions of the plasma membrane contacted by GAD-immunoreactive axon terminals under the control condition. Dendrites from the WT brains following ABA were less covered by GAD-immunoreactive axon terminals, compared with dendrites of WT brains under the control condition. (D) The experience of ABA increased contact lengths of the GAD-immunoreactive axon terminals in both BDNFMet/Met and WT brains. (E) Dendrites of both the BDNFMet/Met and WT ABA brains showed decreased number of GAD-immunoreactive axon terminals per sampled dendrite, compared with dendrites of WT controls. (F) BDNFMet/Met decreased the number of GAD-immunoreactive axon terminals per sampled area under control condition but increased this number under the ABA condition. ABA decreased the number of GAD-immunoreactive axon terminals per sampled area in WT neuropil. Bar graphs represent means ± SEM. “m” indicates statistically significant genotype effect, based on comparisons with WT brains under the same condition (CON or ABA) by the Mann–Whitney U-test. “a” indicates statistically significant effect of ABA, based on comparison to CON brains under the same genotype (WT or BDNFMet/Met) by the Mann–Whitney U-test. “a’” indicates marginally significant genotype effect, by comparing to values of CON brains under the same genotype (WT or BDNFMet/Met) by student-t test (P < 0.06). “m × a” indicates statistically significant effect of ABA, when compared with values from the WT control group by the Mann–Whitney U-test.
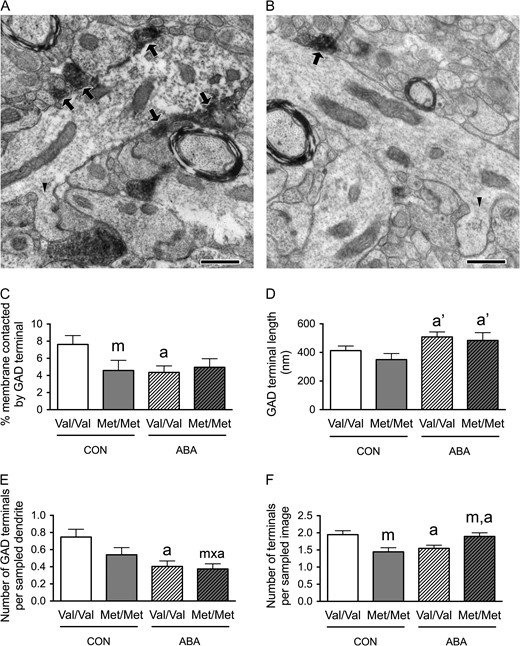
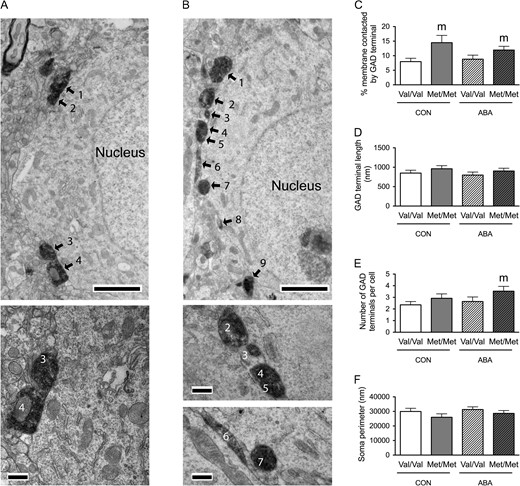
Electron microscopic identification of GAD-immunoreactive axon terminals contacting cell bodies of pyramidal cells in the dorsal hippocampal CA1 of WT (A) and BDNFMet/Met (B) brains. In both top panels A and B, arrows point to portions of the plasma membrane contacted by GAD-immunoreactive axon terminals. ‘s’ indicates the surfaces of vibratome sections that are included in the micrographs to demonstrate proximity of the surface to the analyzed portions of the neuropil. Calibration bars = 2 μm for both panels. Bottom panel of A shows contacts 2 and 3 of the cell in top panel A at a higher magnification of ×25 000 to reveal synaptic specialization. Bottom panel of B shows contacts 5–7 of the cell in top panel B at a higher magnification of ×25 000 to reveal synaptic specialization. Calibration bars = 500 nm. (C) Comparison of the somatic profiles revealed that greater proportions of the plasma membrane of BDNFMet/Met cells are contacted by GAD-immunoreactive axon terminals under both control and ABA conditions. Cell bodies of WT brains that have undergone ABA are more extensively covered by GAD-immunoreactive axon terminals, compared with cell bodies of WT controls. (D) BDNFMet/Met cells showed increased contact length of the GAD-immunoreactive axon terminals under both control and ABA conditions. (E) BDNFMet/Met cells showed increased number of GAD terminals contacting the cell bodies under both control and ABA conditions. Cells from WT mice under ABA has more GAD terminals contacting the cell bodies compared with WT mice in control condition. (F) No significant difference was found in sampled perimeters of the cell bodies among groups. Bar graphs represent means ± SEM. In Panel C, “m” indicates a statistically significant genotype effect, based on comparisons to WT littermates under the same conditions, “a” indicates statistically significant ABA effect, based on comparison to values of WT littermates in control group as assessed by two-way ANOVA followed by Bonferroni's test. In Panels D and E, “m” indicates statistically significant genotype effect, based on comparison to WT littermates under the same rearing condition (CON or ABA), while “a” indicates statistically significant ABA effect, by comparing to values of WT controls by the Mann–Whitney U-test.
In the SLM, Mann–Whitney U-test revealed a significant genotype effect: dendrites from control BDNFMet/Met brains showed decreased proportions of the plasma membrane contacted by GAD-immunoreactive axon terminals (Mann–Whitney U = 2320.5, nCON.WT = 91, nCON.Met/Met = 63, P = 0.031, two-tailed, Fig. 2C). Mann–Whitney U-test also revealed a significant ABA effect upon the WT (Val/Val) animals: ABA decreased the proportions of dendritic plasma membranes contacted by GAD-immunoreactive axon terminals (Mann–Whitney U = 3578, nCON.WT = 91, nABA.WT = 99, P = 0.007, two-tailed, Fig. 2C) to the level observed in BDNFMet/Met brains of both CON and ABA groups.
We evaluated whether these decreases in the proportions of plasma membrane contacted by GAD-immunoreactive axon terminals by the variant BDNF-Val66Met or the ABA experience were associated with changes in the number of GAD terminal contacts onto dendrites, GAD terminal contact lengths onto dendrites or the density of GAD terminals in the surrounding neuropil. The last parameter was assessed by determining the average number of the GABAergic terminals in the sampled area.
Mann–Whitney U-test revealed a significant genotype effect in SLM, with neuropil surrounding distal dendrites from the BDNFMet/Met SLM showing decreased average number of the GABAergic terminals in the sampled area (Mann–Whitney U = 3149.5, nCON.WT = 115, nCON.Met/Met = 72, P = 0.004, two-tailed, Fig. 2F). Mann–Whitney U-test also revealed a significant ABA effect upon the WT group: ABA WT neuropil of SLM show decreased average number of the GABAergic terminals per sampled image (Mann–Whitney U = 5320, nCON.WT = 115, nABA.WT = 115, P = 0.008, two-tailed, Fig. 2F) and decreased average number of GABAergic terminal contacts per dendrite (Mann–Whitney U = 3519, nCON.WT = 91, nABA.WT = 99, P = 0.003, two-tailed, Fig. 2E). Although the genotype effect upon the number of GAD terminals contacting dendrites did not reach statistical significance among the CON brains, this value was significantly reduced for the BDNFMet/Met that experienced ABA, when compared with values from the WT control group by the Mann–Whitney U-test (Mann–Whitney U = 2827, nCON.WT = 91, nABA.Met/Met = 80, P = 0.004, two-tailed, Fig. 2E). The decrease in the number of GAD contacts could not be due to the reduction of their sizes, because that would have caused a reduction in the probability of encountering them within ultrathin sections (the Delesse Principle). Instead, the ABA experience resulted in marginally significant increases in the average GABAergic terminal contact lengths, among both WT (t(79) = −1.975, P = 0.052, Fig. 2D) and BDNFMet/Met cells (t(54) = −1.934, P = 0.058, Fig. 2D), when compared with the CON group of the respective genotypes.
In the PCL, two-way ANOVA revealed marginally significant genotype × treatment interactions (F(1,196) = 3.472, P = 0.064) and significant main effects of genotype (F(1,196) = 56.686, P < 0.001) and of treatment (F(1,196) = 6.342, P = 0.013) for the proportions of the perikaryal plasma membrane contacted by GAD-immunoreactive axon terminals (Fig. 3C). Post hoc analysis revealed that the proportions of perikaryal plasma membrane contacted by GAD-immunoreactive axon terminals was greater for the BDNFMet/Met genotype of both the CON (t(88) = −5.672, P < 0.001) and ABA conditions (t(108) = −4.724, P < 0.001), when compared with the respective treatment groups of the WT genotype (Fig. 3C). Post hoc comparison also revealed that cells from the ABA WT group had significantly increased proportions of the plasma membrane contacted by GAD-immunoreactive axon terminals, relative to cells from the control WT group (t(111) = −3.73, P < 0.001, Fig. 3C). These increases in the percentages of GABAergic terminal contacts by the variant BDNF-Val66Met, under both control and ABA conditions, were associated with two factors: increases in average GABAergic terminal contact lengths (control group, Mann–Whitney U = 722, nCON.WT = 53, nCON.Met/Met = 37, P = 0.034, two-tailed; ABA group, Mann–Whitney U = 1055, nABA.WT = 60, nABA.Met/Met = 50, P = 0.008, two-tailed, Fig. 3D) and increases in the average number of contacts (control group, Mann–Whitney U = 606, nCON.WT = 53, nCON.Met/Met = 37, P = 0.002, two-tailed; ABA group, Mann–Whitney U = 1051, nABA.WT = 60, nABA.Met/Met = 50, P = 0.007, two-tailed, Fig. 3E). The ABA-induced increases in the percentages of terminal contacts upon cells from the WT group were associated with increases in the average number of GABAergic terminals per neuron (Mann–Whitney U = 979.5, nCON.WT = 53, nABA.WT = 60, P < 0.001, two-tailed, Fig. 3E), but not associated with changes in average GABAergic terminal contact lengths (Mann–Whitney U = 1533, nCON.WT = 53, nABA.WT = 60, P = 0.831, two-tailed, Fig. 3D). There was no significant difference between the sizes of sampled neurons’ perimeters in the four groups of mice (Fig. 3F).
Variant BDNF-Met Allele Decreases FR-Evoked Wheel Running Hyperactivity
Previously, we showed that adolescent mice respond to FR with a robust (66%) increase in voluntary wheel running (Chowdhury et al. 2013). In this study, we determined whether the variant BDNF-Met allele changes the FR-evoked increase in wheel running. We compared the wheel running pattern of both the BDNFMet/Met mice and their WT littermates BDNFVal/Val during the first 2 days of their FR phase and the baseline phase (2 days before FR).
Repeated measures ANOVA revealed significant genotype × FR interactions (F(1,12) = 4.856, P = 0.048), and a significant main effect of FR (F(1,12) = 80.777, P < 0.001). Both WT (Val/Val) and BDNFMet/Met mice ran more after the onset of FR compared with baseline (Fig. 4A). However, the extent of the increase in running associated with FR (the difference of running before and during FR) was blunted significantly for the BDNFMet/Met group of animals, when compared with the running by the WT BDNFVal/Val group (t(12) = 2.204, P = 0. 048) (Fig. 4B).
BDNFMet/Met mice show reduced vulnerability to ABA during adolescence. (A) Average wheel running activities of BDNFMet/Met mice (Met/Met) and WT (BDNFVal/Val) littermates (Val/Val) under ABA. Both BDNFMet/Met and WT mice increased wheel running significantly after FR. (B) The difference of wheel running activity between baseline and during FR (FR-elicited wheel running activity) was decreased in BDNFMet/Met mice compared with WT littermates. (C) Both BDNFMet/Met and WT mice increased running during the 6-h preceding feeding (food anticipatory activity, FAA) significantly after FR. BDNFMet/Met mice expressed lower FAA, compared with WT littermates but no difference in baseline running activity. (D) The difference of FAA between baseline and during FR (FR-elicited wheel running activity during FAA) was decreased in the BDNFMet/Met group, compared with the WT littermate group. Bar graphs represent means ± SEM. In Panels A and C, “a” indicates statistically significant effects of ABA, compared with baseline, as assessed by repeated measure ANOVA, followed by Bonferroni's test (n = 8 for WT, and 6 for BDNFMet/Met). In Panels B and D, “m” indicates a statistically significant genotype effect, by comparing to the values of WT littermates by student-t test. In Panel C, “m” indicates statistically significant genotype effect, by comparison to the WT littermates, as assessed by repeated measure ANOVA, followed by Bonferroni's test.
In order to determine whether both genotypes of animals became entrained to the restricted feeding schedule, we measured their FAA (Mistlberger 1994; Chowdhury et al. 2013; Chen et al. 2015), quantified by averaging the extent of wheel running during the 6 h preceding the feeding hours of the food-restricted days, compared with the wheel activity during the same 6 h of the day during the baseline phase. Repeated measures ANOVA revealed significant genotype × FR interactions (F(1,12) = 14.709, P = 0.002) and a main effect of FR for the FAA period (F(1,12) = 52.033, P < 0.001) (Fig. 4C). Both WT and BDNFMet/Met exhibited FAA after the onset of FR (Fig. 4C). However, FR-evoked changes in FAA (measured as the difference in FAA during the FR phase and the baseline phase (Fig. 4D)) were significantly blunted for the BDNFMet/Met group, relative to the WT BDNFVal/Val group (t(12) = 3.835, P = 0.002) (Fig. 4D).
These decreases in FR-evoked wheel running hyperactivity in the BDNFMet/Met mice were neither associated with the body weight differences nor a difference in caloric intake between the two genotypes. Both WT and BDNFMet/Met mice reduced weight during FR to similar degrees. No body weight difference was found during baseline (WT: 17.89 ± 0.69 g; BDNFMet/Met: 18.82 ± 0.61 g; t(12) = −0.973, P = 0.35) or on the last day of FR (WT: 13.91 ± 0.42 g; BDNFMet/Met: 15.10 ± 0.79 g; t(12) = −1.433, P = 0.18). These findings are in agreement with previous findings, namely that the elevated body weight associated with variant BDNF-Val66Met was first evident at 2 months of age (Chen et al. 2006). Also, no genotypic difference in caloric intake was found during baseline or on last day of FR (baseline, WT: 16.75 ± 1.77 kcal; BDNFMet/Met: 16.57 ± 1.39 kcal, t(12) = 0.075, P = 0.94; FR, WT: 4.28 ± 0.40 kcal; BDNFMet/Met: 4.56 ± 0.47 kcal, t(12) = −0.446, P = 0.66).
GAD Terminal Contacts onto Pyramidal Neurons of the Prelimbic Cortex of Animals with Variant BDNF-Val66Met Following ABA
Earlier work had shown that the extent of GABAergic innervation onto pyramidal neurons in the prelimbic cortex was correlated with FAA (Chen et al. 2015). Since we observed decreases in FR-evoked wheel running hyperactivity, especially in FAA, in the BDNFMet/Met mice, we sought to determine whether the variant BDNF, Val66Met, affected the animal's ABA-induced change in GABAergic innervation of the pyramidal neurons in the prelimbic cortex. EM quantifications of the extent of axo-somatic synaptic junctions onto pyramidal cell bodies in Layer V and axo-dendritic synaptic junctions in Layer I formed by terminals immunoreactive for GAD were conducted (Figs 5 and 6, A,B).
Electron microscopic identification of GAD-immunoreactive axon terminals contacting distal spiny dendrites in Layer I of the prelimbic cortex of WT (A) and BDNFMet/Met (B) brains at P60. In both Panels A and B, arrows point to portions of the plasma membrane contacted by GAD-immunoreactive axon terminals. Arrowheads point to the spine neck connecting spine heads to dendritic shafts. Calibration bars = 500 nm for both panels. (C) Comparisons of dendritic profiles revealed that BDNFMet/Met reduces the proportions of the plasma membrane contacted by GAD-immunoreactive axon terminals under the control condition. Dendrites from the WT brains following ABA were less covered by GAD-immunoreactive axon terminals, compared with dendrites of WT brains under the control condition. (D) The experience of ABA decreased contact lengths of the GAD-immunoreactive axon terminals in WT brains but not in BDNFMet/Met brains. (E) Dendrites of both the BDNFMet/Met and WT ABA brains showed decreased number of GAD-immunoreactive axon terminals forming synapses upon sampled dendrite, compared with dendrites of WT controls. (F) BDNFMet/Met increased the number of GAD-immunoreactive axon terminals per sampled area under the ABA condition. ABA decreased the number of GAD-immunoreactive axon terminals per sampled area in WT neuropil. Bar graphs represent means ± SEM. “m” indicates statistically significant genotype effect, based on comparisons with CON brains under the same genotype (WT or BDNFMet/Met) by the Mann–Whitney U-test. “m × a” indicates statistically significant effect of ABA upon BDNFMet/Met brains, when compared with values from the WT control group by the Mann–Whitney U-test.
Electron microscopic identification of GAD-immunoreactive axon terminals contacting cell bodies of pyramidal cells in the prelimbic cortex of WT (A) and BDNFMet/Met (B) brains. In both top panels A and B, arrows point to portions of the plasma membrane contacted by GAD-immunoreactive axon terminals. Calibration bars = 2 μm for both panels. Bottom panel of A shows contacts 3 and 4 of the cell in top panel A at a higher magnification of ×25 000 to reveal synaptic specialization. Bottom panels of B shows contacts 2–7 of the cell in top panel B at a higher magnification of ×25 000 to reveal synaptic specialization. Calibration bars = 500 nm. (C) Comparison of the somatic profiles revealed that greater proportions of the plasma membrane of BDNFMet/Met cells are contacted by GAD-immunoreactive axon terminals under both control and ABA conditions. (D) No significant difference was found in contact length of the GAD-immunoreactive axon terminals under both control and ABA conditions in BDNFMet/Met cells. (E) BDNFMet/Met cells showed increased number of GAD terminals contacting the cell bodies under ABA conditions. (F) No significant difference was found in sampled perimeters of the cell bodies among groups. Bar graphs represent means ± SEM. “m” indicates statistically significant genotype effect, based on comparison to WT littermates under the same rearing condition (CON or ABA) by the Mann–Whitney U-test.
In Layer I of the prelimbic cortex, Mann–Whitney U-test revealed a significant genotype effect: dendrites from control BDNFMet/Met brains showed decreased proportions of the plasma membrane contacted by GAD-immunoreactive axon terminals (Mann–Whitney U = 1356, nCON.WT = 81, nCON.Met/Met = 45, P = 0.016, two-tailed, Fig. 5C). Mann–Whitney U-test also revealed a significant ABA effect upon the WT (Val/Val) animals: ABA decreased the proportions of dendritic plasma membranes contacted by GAD-immunoreactive axon terminals (Mann–Whitney U = 2521, nCON.WT = 81, nABA.WT = 96, P < 0.001, two-tailed, Fig. 5C) to the level observed in BDNFMet/Met brains of CON groups. In contrast, there was no detectable effect of ABA upon the BDNFMet/Met brains (Mann–Whitney U = 1591, nCON.Met/Met = 45; nABA. Met/Met = 74, P = 0.66, two-tailed, Fig. 5C).
We evaluated whether these decreases in the proportions of plasma membrane contacted by GAD-immunoreactive axon terminals by the variant BDNF-Val66Met or the ABA experience were contributed by changes in the number of GAD terminal contacts onto dendrites, GAD terminal contact lengths onto dendrites or the density of GAD terminals in the surrounding neuropil. The last parameter was assessed by determining the average number of the GABAergic terminals in the sampled area. Mann–Whitney U-test revealed a significant genotype effect, with neuropil surrounding distal dendrites from the BDNFMet/Met Layer I showing decreased average number of GABAergic terminal contacts per dendrite (Mann–Whitney U = 1357, nCON.WT = 81, nCON.Met/Met = 45, P = 0.009, two-tailed, Fig. 5E). Mann–Whitney U-test also revealed a significant ABA effect upon the WT group: ABA WT neuropil of Layer I show decreased average number of the GABAergic terminals per sampled image (Mann–Whitney U = 3697, nCON.WT = 108, nABA.WT = 92, P = 0.0009, two-tailed, Fig. 5F) and decreased average number of GABAergic terminal contacts per dendrite (Mann–Whitney U = 2155, nCON.WT = 81, nABA.WT = 96, P < 0.0001, two-tailed, Fig. 5E). The decrease in the number of GAD contacts encountered could be contributed by the reduction of their sizes, where the ABA experience resulted in significant decreases in the average GABAergic terminal contact lengths, among WT cells (t(101) = 4.054, P < 0.0001, Fig. 5D), but not in BDNFMet/Met (t(57) = 1.654, P = 0.104, Fig. 5D) cells, when compared with the CON group of the respective genotypes.
In the Layer V of the prelimbic cortex, Mann–Whitney U-test revealed a significant genotype effect: somata from control BDNFMet/Met brains showed increased proportions of the plasma membrane contacted by GAD-immunoreactive axon terminals (Mann–Whitney U = 244, nCON.WT = 31, nCON.Met/Met = 24, P = 0.029, two-tailed, Fig. 6C), as was observed for the perikarya in the hippocampus of control BDNFMet/Met brains (Fig. 3C); somata from ABA BDNFMet/Met brains also showed increased proportions of the plasma membrane contacted by GAD-immunoreactive axon terminals, when compared with the ABA-treated WT genotype (Mann–Whitney U = 520, nABA,WT = 42, nABA,Met/Met = 34, P = 0.043, two-tailed, Fig. 6C). These increases in the percentages of GABAergic terminal contacts by the variant BDNF-Val66Met, especially under ABA conditions, were contributed by increases in the average number of contacts (Mann–Whitney U = 526.5, nABA,WT = 42, nABA,Met/Met = 34, P = 0.047, two-tailed, Fig. 6E). There was no significant difference between the sizes of sampled neurons’ perimeters in the four groups of mice (Fig. 3F) or contact lengths of axo-somatic synapses formed by GAD terminals (Fig. 6D).
Discussion
GABAergic Neurons Innervating Pyramidal Cells Respond to Local Differences in BDNF Levels
Studies using organotypic slice cultures have shown that the growth-promoting effect of BDNF is confined to 200 μm from its source (Kohara et al. 2007). Our findings contribute to this by revealing that GABAergic innervation pattern can respond to altered local, intracellular levels of BDNF. This is due to the Val66Met-BDNF polymorphism, which disrupts intracellular trafficking of BDNF-mRNA and proteins and results in their accumulation in cell bodies (Chen et al. 2005, 2006; Chiaruttini et al. 2009; Spencer et al. 2010) (Fig. 7). In the hippocampus, parvalbumin (PV) neurons are located nearest to the elevated source of BDNF—the hippocampal pyramidal cell bodies (Sik et al. 1995), making PV cell bodies and axons the most likely sites for elevation of TrkB receptor activation and growth promotion. Conversely, SLM, where distal endings of pyramidal cell dendrites reside, is likely to have undergone the most severe depletion of BDNF within BDNFMet/Met brains, again due to its impaired intracellular trafficking of BDNF-mRNA and BDNF proteins from cell bodies to distal endings, thereby causing the marked reduction of GABAergic synapses there. The GABAergic neurogliaform cells in SLM are likely to have undergone atrophy due to the Val66Met-BDNF polymorphism. These cells are the perforant path-associated interneurons, the Schaffer collateral/commissural pathway-associated INs, and the O-LM cells; all of which have their cell bodies and axon terminals at sites removed from the activity-dependent supply of BDNF in the PCL (Spruston and McBain 2007; Capogna 2011).
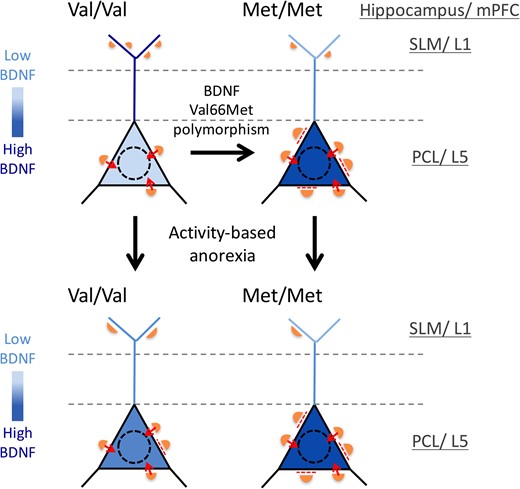
Summary of changes in the GABAergic innervation pattern evoked by variant BDNF, Val66Met, (right-ward arrows) and by ABA (downward arrows) and a hypothesis of the role of BDNF in these effects. We propose that GABAergic axons respond positively to altered local (layer-specific) levels of BDNF within pyramidal cells. Black triangles depict perikarya of CA1 pyramidal neurons in the PCL, while the blue lines depict dendrites of pyramidal neurons traversing stratum radiatum (region between the dashed lines) and forming tufts in SLM. The intensity of blue depicts BDNF levels. Orange semi-circles depict GABAergic axon terminals forming synapses. The sizes and numbers of the orange semi-circles depict differences in the lengths and numbers of GABAergic synapses. Dashed red lines indicate suppression of mature synapses by high levels of perikaryal BDNF; while small red arrows indicate synaptic transmission at perikarya. This model was based on the following published findings: 1) The variant BDNF-Val66Met polymorphism causes disruptions in the intracellular trafficking of BDNF-mRNA and proteins (Chen et al. 2005, 2006; Chiaruttini et al. 2009), which leads to the accumulation of BDNF-Val66Met-mRNA and protein in cell bodies (Spencer et al. 2010); 2) BDNF promotes the initial formation of GABAergic synapses (Mizuno et al. 1994; Marty et al. 1996; Kohara et al. 2007; Jiao et al. 2011); but 3) BDNF also suppresses GABAergic synaptic transmission, once the synapse has matured (Tanaka et al. 1997; Mizoguchi et al. 2003; Wardle and Poo 2003). This ‘suppression of mature synapses by BDNF’ scenario fits well with the greatly depressed GABAergic inhibition observed for the pyramidal neurons in the infralimbic medial prefrontal cortex of BDNFMet/Met mice (Pattwell et al. 2012). Note the decreased number but enlargement of GABAergic terminals in the SLM/Layer 1 of the BDNFMet/Met brains. This is hypothesized to be due to the severe depletion of BDNF in this layer, resulting from impaired intracellular trafficking of BDNF from cell bodies to dendrites. Note the increased number and sizes of GABAergic terminals in the PCL of the BDNFMet/Met brains. This is hypothesized to be due to the accumulation of BDNF within perikarya. The hypothesized relationship between the local concentration of BDNF and GABAergic innervation is extended to the WT brains. Here, BDNF is hypothesized to be elevated in cell bodies and reduced in the SLM/Layer 1 following ABA induction, leading to the observed changes in GABAergic synapses across the proximal versus distal portions of pyramidal neurons. We further hypothesize that the enlargement of GABAergic terminals onto the cell bodies of pyramidal neurons may contribute toward suppression of the FR-evoked hyperactivity. However, the decrease in GABAergic inputs in distal dendrites within the ABA hippocampi of BDNFMet/Met and BDNFVal/Val WT mice, together with the increased perikaryal BDNF that effectively cancels the increased GABAergic innervation of somata, may lead to increase excitability of the hippocampal pyramidal cells, a condition previously shown to contribute toward increased anxiety-like behavior (Bannerman et al. 2004; Shen et al. 2007).
In the frontal cortex, multiple GABAergic cell types reside in Layer V, but among them, PV cells (fast-spiking basket and chandelier cells) are the ones that form axo-somatic GABAergic synapses (Kawaguchi and Kubota 1997). Therefore, the GABAergic cell type most strongly affected by the elevation of local levels of BDNF within Layer V pyramidal neurons are likely to be the PV cells. Axons of PV cells are notably absent in Layer I of cerebral cortex. Instead, axons containing somatostatin arise from Martinotti cells in Layers II/III and V and extensively innervate dendritic tufts of pyramidal neurons in Layer I (Wang et al. 2004; Silberberg and Markram 2007; Xu et al. 2013): these may have been affected relatively more strongly by the depletion of BDNF, because of the reduced intracellular trafficking of BDNF (Fig. 7). Synaptic integration in Layer I is especially important for regulation of pyramidal neurons’ excitability (Spruston 2008) and associative learning (Letzkus et al. 2011).
BDNF has been reported to both promote development (Mizuno et al. 1994; Marty et al. 1996; Jiao et al. 2011), depress synaptic transmission (Tanaka et al. 1997; Mizoguchi et al. 2003; Wardle and Poo 2003) or do neither (Marty et al. 1996) to GABAergic synapses. Some of these differences arise from diversities in the postsynaptic cell type (excitatory vs. inhibitory) (Wardle and Poo 2003), the presynaptic cell type (Marty et al. 1996), and developmental stages (Mizoguchi et al. 2003). A scenario consistent with these observations is that BDNF promotes the initial formation of GABAergic synapses, but suppresses their synaptic transmission, once mature. This ‘promotion of growth’ scenario fits well with our observations. Since the enhanced GABAergic innervation of pyramidal cell bodies and decreased innervation in distal dendrites of both the hippocampus and prelimbic cortex were evident even within BDNFMet/Met mice that experienced no wheel running or FR (Fig. 7), the anatomical difference across the genotypes is independent of the animals’ wheel running or feeding schedule and is contributed by the global knock-in of the BDNF-Met allele.
Suppression of Mature GABAergic Synapses by Perikaryal BDNF
The second, ‘suppression of mature synapses by BDNF’ scenario fits well with the greatly depressed GABAergic inhibition observed electrophysiologically for the pyramidal neurons in the infralimbic medial prefrontal cortex of BDNFMet/Met mice (Pattwell et al. 2012). This suppression is likely to have resulted not only from a loss in the number of GABAergic inputs at distal endings of pyramidal cells but also from the BDNF-mediated suppression at the somata. This could have been particularly strong at axo-somatic GABAergic synapses, which is the subcellular compartment that must have dominated the mIPSC in their whole-cell recordings. Despite hyperinnervation of the perikarya, such perikaryal BDNF-mediated suppression of GABAergic axo-somatic synaptic transmission is likely to have taken place for the BDNFMet/Met mice in our study (Fig. 7).
Single ABA Exposure Increases Perikaryal GABAergic Innervations of Pyramidal Neurons in the Hippocampal CA1 of WT, but Not of Layer V Pyramidal Neurons of the WT Prelimbic Cortex
GABAergic innervations upon cell bodies of pyramidal neurons in both the hippocampus (Chowdhury et al. 2013) and the prelimbic cortex (Chen et al. 2015) of WT adolescent mice increase, following exposures to two episodes of ABA, separated by 7 days of a recovery period. In this study, with a single ABA exposure during adolescence, followed by a similar recovery period, we found increased perikaryal GABAergic innervations in the hippocampal CA1 (Fig 3C) of WT, but not upon Layer V pyramidal neurons of the prelimbic cortex of WT (Figs 6C and 7). This might be due to different roles played by the hippocampus versus the prelimbic cortex during the recovery period.
For the past (Chowdhury et al. 2013) and the present cohorts of mice, almost all of the animals increased running after the first ABA exposure. However, we noted greater heterogeneity in their behavioral responses to the second ABA: some of them continued to run as much as they had during the first ABA, while others ran less (Chowdhury et al. 2013; Wable et al. 2015b), and this individual difference was mirrored by individual differences in the GABAergic innervation of pyramidal cells in the prelimbic cortex. This heterogeneity in the animals’ response to the second ABA may have emerged during the recovery period, as entrainment to the feeding schedule during the first ABA experience was consolidated by some animals and not others. Since the past and present studies both included a recovery period following the first ABA, the anatomical difference that we noted for cell bodies of the WT prelimbic cortex (but not for this study) is likely to have emerged in response to the second ABA, rather than during recovery. It is possible that the GABAergic system of the prelimbic cortex was primed during the recovery period as their entrainment to the feeding schedule became consolidated, but led to synaptogenesis in surrounding perikarya only after experiencing another ABA episode. Conversely, this may explain why, without a second ABA exposure, we find a lack of increase in perikaryal GABAergic innervation in the prelimbic cortex (Figs 6 and 7). In support of the involvement of the prelimbic cortex in entrainment to the feeding schedule, expression of dopamine D1 receptors in the dorsal striatum has been reported to be a requirement (Gallardo et al. 2014): striatum is a major target zone for the prelimbic cortex (Gerfen 1989).
As noted above, the hippocampus exhibited similar anatomical changes, whether or not exposed to a second ABA. The hippocampus is thought to be more responsive than the prelimbic cortex to FR (Lee et al. 2002) and exercise (wheel running) (Oliff et al. 1998) in part by secreting higher levels of BDNF, which directly impacts the survival of dentate gyrus neurons. This heightened level of BDNF secreted by the hippocampus of WT mice with just one ABA may have been sufficient to evoke GABAergic synaptogenesis surrounding cell bodies of hippocampal pyramidal cells.
BDNF-Met Allele Increases Anxiety-Like Behavior, Despite Axo-Somatic GABAergic Hyperinnervation
Reduction of hippocampal pyramidal neuron excitability is linked to reduced anxiety (Bannerman et al. 2004; Shen et al. 2007). This link is supported by our earlier studies, showing correlation of reduced anxiety with reduced FR-evoked hyperactivity in the ABA model (Wable et al. 2015b), especially among animals with greater GABAergic innervation of hippocampal (Chowdhury et al. 2013) and prefrontal cortical (Chen et al. 2015) pyramidal neurons. However, for the BDNFMet/Met mice, this simple link between GABAergic hyperinnervation of cell bodies and reduction of anxiety did not hold. Rather, the BDNFMet/Met mice exhibited levels of anxiety comparable to those of WT littermates that underwent ABA induction, whether or not ABA-induced. This level of anxiety, in the absence of any known stressors during adolescence and in spite of the GABAergic hyperinnervation of cell bodies, is likely to be contributed by BDNF's second effect of suppressing GABAergic synaptic transmission, as discussed earlier. BDNF's suppressive effect would be expected to be the strongest near cell bodies, due to the elevated levels of BDNF present for brains of BDNFMet/Met mice, thereby partially canceling the potential anxiolytic effect associated with enhanced GABAergic innervation of cell bodies. This interpretation fits well with previous reports of elevated anxiety-like measures for the BDNFMet/Met adult mice (Chen et al. 2006; Yu et al. 2012).
Reduction of GABAergic Inhibition in the Distal Dendrites may Contribute to the Anxiety-Like Behavior and have Resulted from FR-Plus-Running
GABAergic synapses were still present within the SLM of BDNFMet/Met mice, but at reduced levels, relative to WTs that did not experience ABA. The increased anxiety elicited by ABA induction in WT mice was accompanied by reductions in GABAergic innervation of the distal dendrites (SLM in the hippocampus and Layer I in the prelimbic cortex) to a level indistinguishable from that of BDNFMet/Met mice. We speculate that the reduction of GABAergic innervation in the SLM, evoked by FR+running among WT mice and by the Val66Met-BDNF polymorphism in the BDNFMet/Met mice, contribute to the emergence of anxiety-like behavior. Moreover, FR+running may have reduced the intracellular trafficking of BDNF-mRNA and BDNF proteins within WT brains, similar to that described for the Val66Met-BDNF polymorphism. This may have resulted in the increase and decrease in GABAergic innervation at cell bodies and distal dendrites, respectively. Although stress evokes reduction of BDNF protein and mRNA levels in the hippocampus of BDNFVal/Met heterozygotes and WT mice, the same treatment evokes an increase in BDNF in the amygdala (Yu et al. 2012). Thus, changes in GABAergic innervation mediated by BDNF may be in opposite directions in the hippocampus, relative to the amygdala.
BDNF-Met Allele Reduces ABA Vulnerability Through Reduced FAA
There are, to date, >500 papers published on the topic of Val66Met-BDNF polymorphism. At least 15 of these have examined the impact of the Val66Met-BDNF polymorphism upon AN vulnerability in the human population (Notaras et al. 2015). This interest in studying the association between Val66Met-BDNF polymorphism with AN is likely to have stemmed from a previous study indicating that BDNFMet/Met mice exhibit increased anxiety-like behavior (Chen et al. 2006). Since anxiety is often co-morbid with AN, one prediction would be that BDNFMet/Met increases AN vulnerability. However, conditional and heterozygous knock-out of BDNF leads to hyperphagia and obesity in mice, suggesting that Val66Met-BDNF may confer resilience to AN. The results from human studies have been equivocal: some studies indicate a link, while others do not (Notaras et al. 2015). Ours is the first to use the animal model to examine ABA vulnerability in BDNFMet/Met mice. Our finding is that BDNFMet/Met reduces ABA vulnerability, measured as reduction in the FR-evoked hyperactivity. However, this reduction is not contributed by hyperphagia, increased body weight, or reduced anxiety.
If axo-somatic GABAergic hyperinnervation is not reducing excitability of the hippocampus or anxiety, what then might explain the reduced wheel running in BDNFMet/Met mice? The reduced running was observed most prominently for the period corresponding to the FAA of WT mice. The reduced FAA by the BDNFMet/Met mice may reflect cognitive impairment, resulting in reduced entrainment to the restrictive feeding schedule. Mice carrying the BDNF-Met allele have been shown to exhibit impaired hippocampus-based learning (Spencer et al. 2010), reduction of hippocampus-based contextual fear conditioning (Chen et al. 2006), as well as reduction of the N-methyl-d-aspartate receptor (NMDAR)-dependent LTP in the hippocampus (Ninan et al. 2010) and prelimbic cortex (Pattwell et al. 2012). Moreover, we have observed an increase in NMDARs at hippocampal synapses of ABA-induced rats that exhibited strong FR-evoked hyperactivity (Klingensmith et al. 2015) (Aoki et al., unpublished data). Conversely, reduced BDNF in dendritic shafts of the BDNFMet/Met mice may contribute to the reduced NMDAR-dependent synaptic plasticity. This could, in turn, impair the animal's ability to form a model of the external world, such as a feeding schedule. FR-induced hyperactivity evokes circuit changes in the striatum and depends on the expression of D1 dopamine (Gallardo et al. 2014) and 5HT4 (Jean et al. 2012) receptors there. Undoubtedly, neurotransmitter systems and brain regions other than those we studied also contribute to the development of FAA, anxiety, and ABA vulnerability of mice.
In conclusion, our findings from the ABA mouse model provide an explanation for the equivocal findings regarding the association of BDNF-Val66Met polymorphism with AN (Notaras et al. 2015). Our findings suggest that carriers of the BDNF-Met allele may exhibit greater vulnerability to anxiety disorders and AN, contributed by increased excitability of pyramidal neurons, due to the combination of decreased GABAergic innervation of distal dendrites and BDNF-mediated suppression of perisomatic inhibitory inputs.
Funding
The Klarman Foundation Grant Program in Eating Disorders Research to C.A., R21MH091445-01 to C.A., R21 MH105846 to C.A., R01NS066019-01A1 to C.A., R01NS047557-07A1 to C.A., NEI Core Grant EY13079 to C.A., NYU's Research Challenge Fund to C.A., NSF-REU 1460880 to O.S., C.A., and Y.W.C., and Fulbright Scholarship to Y.W.C.
Notes
We are thankful to Kathleen Shannon, the Office of Veterinary Resources, NYU, and Dr. Alice Liang and Kristen Dancel-Manning of the Microscopy Core of New York University Langone Medical Center for their technical assistance with use of the EM. We are grateful to Hannah Actor-Engel for helpful discussions on the manuscript. Conflict of Interest: None declared.
References
Author notes
Equal contributions