-
PDF
- Split View
-
Views
-
Cite
Cite
Alex de Voux, Kyle T Bernstein, Heather Bradley, Robert D Kirkcaldy, Yunfeng Tie, R Luke Shouse, Syphilis Testing Among Sexually Active Men Who Have Sex With Men and Who Are Receiving Medical Care for Human Immunodeficiency Virus in the United States: Medical Monitoring Project, 2013–2014, Clinical Infectious Diseases, Volume 68, Issue 6, 15 March 2019, Pages 934–939, https://doi.org/10.1093/cid/ciy571
Close - Share Icon Share
Abstract
Guidelines recommend that sexually active men who have sex with men (MSM) including human immunodeficiency virus (HIV)-positive MSM be tested at least annually for syphilis, with testing every 3–6 months for MSM at elevated risk. We examined the proportion of HIV-positive MSM tested for syphilis in the past 3, 6, and 12 months by their HIV care provider during 2013–2014.
Using data from the Medical Monitoring Project, a population-based HIV surveillance system, we evaluated the proportion of MSM who had documentation of being tested for syphilis by their HIV care provider in the past 3, 6, and 12 months.
During 2013–2014, 71% (95% confidence interval [CI]: 69%–73%) of sexually active HIV-positive MSM were tested for syphilis in the past year. This proportion was higher among MSM reporting condomless sex: (75%; 95% CI: 72%–78%), and among MSM reporting ≥ 2 sex partners (77%; 95% CI: 74%–79%), in the past 12 months. Among MSM reporting condomless sex, 49% (95% CI: 45%–53%) were tested in the past 6 months, and 26% (95% CI: 22%–30%) in the past 3 months. Among MSM reporting ≥ 2 sex partners, 49% (95% CI: 44%–54%) were tested in the past 6 months and 26% (95% CI: 22%–29%) in the past 3 months.
Nearly one-third of sexually active HIV-positive MSM were not tested annually, and many at increased risk were not tested at recommended frequencies. Efforts to improve compliance with screening guidelines for high-risk HIV-positive MSM are warranted.
The rate of diagnoses of human immunodeficiency virus (HIV) infection in the United States has decreased between 2010 and 2015 [1]. Although there has been a decreasing trend in the number of diagnoses of HIV infection, 2016 marked the third consecutive year of increases in other reportable sexually transmitted diseases (STDs)—chlamydia, gonorrhea, and syphilis—in the United States [2]. In 2016, 27814 cases of primary and secondary (P&S) syphilis were reported, representing an increase of 17.6% since 2015 [2]. Gay, bisexual, and other men who have sex with men (MSM) accounted for the majority of P&S syphilis cases in 2016, with the greatest increases particularly among young [3] and minority MSM [4]. Reported cases of P&S syphilis were characterized by a high rate of HIV coinfection, particularly among MSM [2]. In 2016 among P&S syphilis cases with known HIV status, 47% of cases among MSM were HIV-positive [2]. Syphilis has been associated with an increased risk of HIV acquisition and transmission [5, 6], whereas HIV-positive persons who become infected with syphilis may be at an increased risk of developing complications including neurologic or ocular complications [7]. Therefore, testing for and treatment of syphilis among HIV-positive MSM is an important part of comprehensive HIV care.
Current guidelines recommend that sexually active HIV-positive MSM be screened for syphilis upon initiation of care and at least annually thereafter [8, 9]. However, the frequency of testing for syphilis is often below recommended levels [10–12]. Although a recent trend analysis of STD testing among HIV-positive MSM in care reported an increasing proportion screened for syphilis between 2010 and 2013, the overall proportion screened at least annually remained below 70% [11]. For MSM at increased risk for syphilis, such as men reporting condomless sex or multiple sex partners, more frequent screening for syphilis, every 3 to 6 months, is recommended. Population-based information on syphilis testing among HIV-positive MSM, particularly the frequency of syphilis screening among MSM at elevated risk, is lacking and of particular importance given the increasing trend in syphilis diagnoses. The objective of this analysis was to examine the proportion of sexually active HIV-positive MSM currently in care tested for syphilis in the past 3, 6, and 12 months by their HIV care provider.
METHODS
We analyzed the most recent data available from the Medical Monitoring Project (MMP; 2013–2014 cycles), an HIV surveillance system designed to produce nationally representative, cross-sectional estimates of behavioral and clinical characteristics of HIV-positive adults receiving medical care in the United States. MMP methods, including sampling, weighting procedures, and response rates, have been described in detail elsewhere [13, 14]. Briefly, during 2013–2014, MMP used a 3-stage, complex sampling design in which US states and territories were sampled, followed by sampling of facilities providing outpatient HIV medical care in those jurisdictions, then sampling of HIV-positive adults (aged ≥18 years) receiving care in those facilities. In the 2013 and 2014 cycles, a total of 23 project areas were funded to conduct data collection—16 states, 1 territory, and 5 separately funded cities. In 2013, 565 eligible facilities were sampled in the 23 project areas, and in 2014, 561 facilities were sampled in 23 project areas. Response rates were similar in 2013 and 2014 for facilities (85% and 86%, respectively) and for patients (55% and 56%, respectively). Eligible persons were HIV-positive, aged ≥18 years, and had received medical care in participating facilities between January and April in the cycle year for which they were sampled. Data were collected from June 2013 through May 2015 using face-to-face or telephone interviews and medical record abstractions. During the MMP 2013–2014 cycles, medical record data from 24 months prior to interview were abstracted. Data were weighted on the basis of known probabilities of selection at state or territory, facilities, and patient levels [14]. In addition, predictors of nonresponse were determined from analysis of data from sampled facilities and patients, and data were weighted to adjust for nonresponse following established methods [15, 16].
In accordance with the federal human subjects protection regulations [17] and guidelines for defining public health research [18], MMP was determined to be a nonresearch, public health surveillance activity used for disease control program or policy purposes. Participating states or territories and facilities obtained local institutional review board approval to be part of MMP if required locally. Informed consent was obtained from all interviewed participants.
DEFINITIONS
We used abstracted medical record data to estimate the prevalence of syphilis testing by an HIV care provider in the 3, 6, and 12 months prior to the interview. Syphilis testing was defined as a result from a non-treponemal or treponemal syphilis test, antibody test, or dark-field microscopy. Because treponemal tests are performed as confirmatory tests soon after a positive non-treponemal test (or vice versa if the reverse testing algorithm is conducted), only syphilis tests documented to occur >30 days apart were considered separate syphilis testing episodes (hereafter referred to as “tests” for simplicity). MSM were defined as men who had sex with men only or with men and women during the past 12 months. We performed stratified analyses according to risky sexual behavioral categories: reporting condomless sex in the past 12 months and reporting ≥2 sex partners in the past 12 months. Race/ethnicity was defined by self-identification as black or African American non-Hispanic, Hispanic or Latino, or white non-Hispanic. Due to small sample sizes, people who reported other or multiple races/ethnicities were combined into a single group (hereafter referred to as other race/ethnicity).
We estimated weighted percentages of MSM tested for syphilis by an HIV care provider during 3 different but overlapping time intervals: anytime in the 3 months prior to the interview, anytime in the 6 months prior to the interview, and anytime in the 12 months prior to the interview. We compared the percentage tested for syphilis by an HIV care provider among MSM who reported risky sexual behavior to MSM who did not; 95% confidence intervals (CIs) were calculated assuming a binomial distribution. Bivariate logistic regression was used to generate prevalence ratios. All analyses accounted for the complex sample design and weights. To measure the frequency of testing we also determined the median number of syphilis tests and the median time between syphilis tests occurring >30 days apart in the 24 months prior to the interview and compared these estimates by reported sexual behavior.
RESULTS
Demographic, Clinical, and Sexual Behavioral Attributes
Most MSM included in the analysis were aged ≥30 years (84%, Table 1) and identified as homosexual (86%); 44% were white, non-Hispanic. Nearly half of MSM reported condomless sex in the past 12 months (48%) and the majority of MSM (58%) reported multiple sex partners in the past 12 months. Overall, 34% (n = 1093) of MSM reported both condomless sex and multiple sex partners in the past 12 months. Most MSM had a clinical disease stage categorized as AIDS or had a nadir CD4 count of <200 cells/mm3. Nearly half of MSM (49%) reported having health insurance other than Ryan White Program (RWP) coverage.
Selected Demographic, Clinical Characteristics, and Sexual Behaviors Among Sexually Active Men Have Sex With Men and Who are Receiving Medical Care for Human Immunodeficiency Virus in the United States—Medical Monitoring Project, 2013–2014
| . | na . | %b . | 95% CI . | % With ≥1 Test in Past 12 Months . | (95% CI) . | Prevalence Ratio . | (95% CI) . |
|---|---|---|---|---|---|---|---|
| 3174 | … | … | 71 | (69–73) | … | … | |
| Age, years | |||||||
| 18–29 | 474 | 16 | (13–19) | 71 | (65–77) | 1.05 | (0.94–1.17) |
| 30–39 | 686 | 22 | (20–24) | 77 | (73–81) | 1.14 | (1.06–1.23) |
| 40–49 | 969 | 30 | (28–33) | 72 | (68–76) | 1.07 | (1.00–1.14) |
| 50+ | 1045 | 32 | (30–34) | 68 | (64–71) | ref | |
| Sexual orientation | |||||||
| Homosexual | 2728 | 86 | (85–88) | 72 | (69–74) | ref | |
| Heterosexual | 27 | 0.8 | (0.5–1) | 74 | (52–95) | 1.03 | (0.77–1.37) |
| Bisexual | 397 | 13 | (11–14) | 70 | (64–75) | 0.97 | (0.89–1.06) |
| Race/ethnicity | |||||||
| White, non-Hispanic | 1374 | 44 | (37–51) | 68 | (64–71) | ref | |
| Black, non-Hispanic | 864 | 28 | (20–35) | 74 | (69–79) | 1.09 | (1.00–1.19) |
| Hispanic or Latino | 783 | 23 | (19–28) | 76 | (72–80) | 1.12 | (1.06–1.19) |
| Other | 153 | 5 | (4–6) | 69 | (61–76) | 1.01 | (0.89–1.16) |
| Disease stage | |||||||
| AIDS or nadir CD4 <200 cells/mm3 | 1843 | 58 | (56–60) | 72 | (69–74) | ref | |
| No AIDS and nadir CD4 200–349 cells/mm3 | 447 | 14 | (13–15) | 74 | (70–78) | 1.03 | (0.97–1.10) |
| No AIDS and nadir CD4 350–500 cells/mm3 | 429 | 14 | (12–15) | 71 | (67–75) | 0.99 | (0.93–1.06) |
| No AIDS and nadir CD4 > 500 cells/mm3 | 449 | 14 | (12–16) | 69 | (65–74) | 0.97 | (0.90–1.04) |
| Reported condomless sex in past 12 months | |||||||
| Yes | 1578 | 48 | (45–52) | 75 | (72–78) | 1.09 | (1.04–1.14) |
| No | 1440 | 52 | (48–55) | 69 | (66–71) | ref | |
| Reported ≥ 2 partners in past 12 months | |||||||
| Yes | 1854 | 58 | (56–60) | 77 | (74–79) | 1.19 | (1.14–1.25) |
| No | 1320 | 42 | (40–44) | 64 | (62–67) | ref | |
| Health insurance | |||||||
| No insurance/coverage | 280 | 9 | (6–11) | 73 | (67–79) | 1.10 | (0.99–1.23) |
| Only have RW | 319 | 10 | (9–12) | 80 | (75–84) | 1.21 | (1.12–1.30) |
| Have insurance (other than RW) | 1508 | 49 | (46–52) | 66 | (63–70) | ref | |
| Have both RW and others | 1044 | 32 | (28–36) | 76 | (73–80) | 1.15 | (1.08–1.23) |
| . | na . | %b . | 95% CI . | % With ≥1 Test in Past 12 Months . | (95% CI) . | Prevalence Ratio . | (95% CI) . |
|---|---|---|---|---|---|---|---|
| 3174 | … | … | 71 | (69–73) | … | … | |
| Age, years | |||||||
| 18–29 | 474 | 16 | (13–19) | 71 | (65–77) | 1.05 | (0.94–1.17) |
| 30–39 | 686 | 22 | (20–24) | 77 | (73–81) | 1.14 | (1.06–1.23) |
| 40–49 | 969 | 30 | (28–33) | 72 | (68–76) | 1.07 | (1.00–1.14) |
| 50+ | 1045 | 32 | (30–34) | 68 | (64–71) | ref | |
| Sexual orientation | |||||||
| Homosexual | 2728 | 86 | (85–88) | 72 | (69–74) | ref | |
| Heterosexual | 27 | 0.8 | (0.5–1) | 74 | (52–95) | 1.03 | (0.77–1.37) |
| Bisexual | 397 | 13 | (11–14) | 70 | (64–75) | 0.97 | (0.89–1.06) |
| Race/ethnicity | |||||||
| White, non-Hispanic | 1374 | 44 | (37–51) | 68 | (64–71) | ref | |
| Black, non-Hispanic | 864 | 28 | (20–35) | 74 | (69–79) | 1.09 | (1.00–1.19) |
| Hispanic or Latino | 783 | 23 | (19–28) | 76 | (72–80) | 1.12 | (1.06–1.19) |
| Other | 153 | 5 | (4–6) | 69 | (61–76) | 1.01 | (0.89–1.16) |
| Disease stage | |||||||
| AIDS or nadir CD4 <200 cells/mm3 | 1843 | 58 | (56–60) | 72 | (69–74) | ref | |
| No AIDS and nadir CD4 200–349 cells/mm3 | 447 | 14 | (13–15) | 74 | (70–78) | 1.03 | (0.97–1.10) |
| No AIDS and nadir CD4 350–500 cells/mm3 | 429 | 14 | (12–15) | 71 | (67–75) | 0.99 | (0.93–1.06) |
| No AIDS and nadir CD4 > 500 cells/mm3 | 449 | 14 | (12–16) | 69 | (65–74) | 0.97 | (0.90–1.04) |
| Reported condomless sex in past 12 months | |||||||
| Yes | 1578 | 48 | (45–52) | 75 | (72–78) | 1.09 | (1.04–1.14) |
| No | 1440 | 52 | (48–55) | 69 | (66–71) | ref | |
| Reported ≥ 2 partners in past 12 months | |||||||
| Yes | 1854 | 58 | (56–60) | 77 | (74–79) | 1.19 | (1.14–1.25) |
| No | 1320 | 42 | (40–44) | 64 | (62–67) | ref | |
| Health insurance | |||||||
| No insurance/coverage | 280 | 9 | (6–11) | 73 | (67–79) | 1.10 | (0.99–1.23) |
| Only have RW | 319 | 10 | (9–12) | 80 | (75–84) | 1.21 | (1.12–1.30) |
| Have insurance (other than RW) | 1508 | 49 | (46–52) | 66 | (63–70) | ref | |
| Have both RW and others | 1044 | 32 | (28–36) | 76 | (73–80) | 1.15 | (1.08–1.23) |
Abbreviations: CI, confidence interval; RW, Ryan White.
aUnweighted frequencies.
bPercentages weighted to adjust for unequal selection probabilities and facility and patient nonresponse.
Selected Demographic, Clinical Characteristics, and Sexual Behaviors Among Sexually Active Men Have Sex With Men and Who are Receiving Medical Care for Human Immunodeficiency Virus in the United States—Medical Monitoring Project, 2013–2014
| . | na . | %b . | 95% CI . | % With ≥1 Test in Past 12 Months . | (95% CI) . | Prevalence Ratio . | (95% CI) . |
|---|---|---|---|---|---|---|---|
| 3174 | … | … | 71 | (69–73) | … | … | |
| Age, years | |||||||
| 18–29 | 474 | 16 | (13–19) | 71 | (65–77) | 1.05 | (0.94–1.17) |
| 30–39 | 686 | 22 | (20–24) | 77 | (73–81) | 1.14 | (1.06–1.23) |
| 40–49 | 969 | 30 | (28–33) | 72 | (68–76) | 1.07 | (1.00–1.14) |
| 50+ | 1045 | 32 | (30–34) | 68 | (64–71) | ref | |
| Sexual orientation | |||||||
| Homosexual | 2728 | 86 | (85–88) | 72 | (69–74) | ref | |
| Heterosexual | 27 | 0.8 | (0.5–1) | 74 | (52–95) | 1.03 | (0.77–1.37) |
| Bisexual | 397 | 13 | (11–14) | 70 | (64–75) | 0.97 | (0.89–1.06) |
| Race/ethnicity | |||||||
| White, non-Hispanic | 1374 | 44 | (37–51) | 68 | (64–71) | ref | |
| Black, non-Hispanic | 864 | 28 | (20–35) | 74 | (69–79) | 1.09 | (1.00–1.19) |
| Hispanic or Latino | 783 | 23 | (19–28) | 76 | (72–80) | 1.12 | (1.06–1.19) |
| Other | 153 | 5 | (4–6) | 69 | (61–76) | 1.01 | (0.89–1.16) |
| Disease stage | |||||||
| AIDS or nadir CD4 <200 cells/mm3 | 1843 | 58 | (56–60) | 72 | (69–74) | ref | |
| No AIDS and nadir CD4 200–349 cells/mm3 | 447 | 14 | (13–15) | 74 | (70–78) | 1.03 | (0.97–1.10) |
| No AIDS and nadir CD4 350–500 cells/mm3 | 429 | 14 | (12–15) | 71 | (67–75) | 0.99 | (0.93–1.06) |
| No AIDS and nadir CD4 > 500 cells/mm3 | 449 | 14 | (12–16) | 69 | (65–74) | 0.97 | (0.90–1.04) |
| Reported condomless sex in past 12 months | |||||||
| Yes | 1578 | 48 | (45–52) | 75 | (72–78) | 1.09 | (1.04–1.14) |
| No | 1440 | 52 | (48–55) | 69 | (66–71) | ref | |
| Reported ≥ 2 partners in past 12 months | |||||||
| Yes | 1854 | 58 | (56–60) | 77 | (74–79) | 1.19 | (1.14–1.25) |
| No | 1320 | 42 | (40–44) | 64 | (62–67) | ref | |
| Health insurance | |||||||
| No insurance/coverage | 280 | 9 | (6–11) | 73 | (67–79) | 1.10 | (0.99–1.23) |
| Only have RW | 319 | 10 | (9–12) | 80 | (75–84) | 1.21 | (1.12–1.30) |
| Have insurance (other than RW) | 1508 | 49 | (46–52) | 66 | (63–70) | ref | |
| Have both RW and others | 1044 | 32 | (28–36) | 76 | (73–80) | 1.15 | (1.08–1.23) |
| . | na . | %b . | 95% CI . | % With ≥1 Test in Past 12 Months . | (95% CI) . | Prevalence Ratio . | (95% CI) . |
|---|---|---|---|---|---|---|---|
| 3174 | … | … | 71 | (69–73) | … | … | |
| Age, years | |||||||
| 18–29 | 474 | 16 | (13–19) | 71 | (65–77) | 1.05 | (0.94–1.17) |
| 30–39 | 686 | 22 | (20–24) | 77 | (73–81) | 1.14 | (1.06–1.23) |
| 40–49 | 969 | 30 | (28–33) | 72 | (68–76) | 1.07 | (1.00–1.14) |
| 50+ | 1045 | 32 | (30–34) | 68 | (64–71) | ref | |
| Sexual orientation | |||||||
| Homosexual | 2728 | 86 | (85–88) | 72 | (69–74) | ref | |
| Heterosexual | 27 | 0.8 | (0.5–1) | 74 | (52–95) | 1.03 | (0.77–1.37) |
| Bisexual | 397 | 13 | (11–14) | 70 | (64–75) | 0.97 | (0.89–1.06) |
| Race/ethnicity | |||||||
| White, non-Hispanic | 1374 | 44 | (37–51) | 68 | (64–71) | ref | |
| Black, non-Hispanic | 864 | 28 | (20–35) | 74 | (69–79) | 1.09 | (1.00–1.19) |
| Hispanic or Latino | 783 | 23 | (19–28) | 76 | (72–80) | 1.12 | (1.06–1.19) |
| Other | 153 | 5 | (4–6) | 69 | (61–76) | 1.01 | (0.89–1.16) |
| Disease stage | |||||||
| AIDS or nadir CD4 <200 cells/mm3 | 1843 | 58 | (56–60) | 72 | (69–74) | ref | |
| No AIDS and nadir CD4 200–349 cells/mm3 | 447 | 14 | (13–15) | 74 | (70–78) | 1.03 | (0.97–1.10) |
| No AIDS and nadir CD4 350–500 cells/mm3 | 429 | 14 | (12–15) | 71 | (67–75) | 0.99 | (0.93–1.06) |
| No AIDS and nadir CD4 > 500 cells/mm3 | 449 | 14 | (12–16) | 69 | (65–74) | 0.97 | (0.90–1.04) |
| Reported condomless sex in past 12 months | |||||||
| Yes | 1578 | 48 | (45–52) | 75 | (72–78) | 1.09 | (1.04–1.14) |
| No | 1440 | 52 | (48–55) | 69 | (66–71) | ref | |
| Reported ≥ 2 partners in past 12 months | |||||||
| Yes | 1854 | 58 | (56–60) | 77 | (74–79) | 1.19 | (1.14–1.25) |
| No | 1320 | 42 | (40–44) | 64 | (62–67) | ref | |
| Health insurance | |||||||
| No insurance/coverage | 280 | 9 | (6–11) | 73 | (67–79) | 1.10 | (0.99–1.23) |
| Only have RW | 319 | 10 | (9–12) | 80 | (75–84) | 1.21 | (1.12–1.30) |
| Have insurance (other than RW) | 1508 | 49 | (46–52) | 66 | (63–70) | ref | |
| Have both RW and others | 1044 | 32 | (28–36) | 76 | (73–80) | 1.15 | (1.08–1.23) |
Abbreviations: CI, confidence interval; RW, Ryan White.
aUnweighted frequencies.
bPercentages weighted to adjust for unequal selection probabilities and facility and patient nonresponse.
Seventy-one percent (95% CI: 69%–73%) (Table 1) of all sexually active HIV-positive MSM had at least 1 syphilis test documented in their medical record in the past 12 months. The proportion tested for syphilis in the past 12 months increased only slightly for MSM reporting condomless sex in the past 12 months (75%; 95% CI: 72%–78%) and for those reporting ≥2 sex partners in the past 12 months (77%; 95% CI: 74%–79%) (Table 1). Compared to MSM ≥ 50 years, MSM aged 30–39 years had a higher proportion with at least 1 syphilis test documented in their medical record in the past 12 months (Table 1). Hispanic or Latino MSM had a higher proportion with documentation of syphilis testing in the past 12 months (Table 1) compared to white non-Hispanics, and MSM with only RWP coverage had a higher proportion with documentation of syphilis testing in the past 12 months (Table 1) compared to MSM with traditional sources of health insurance.
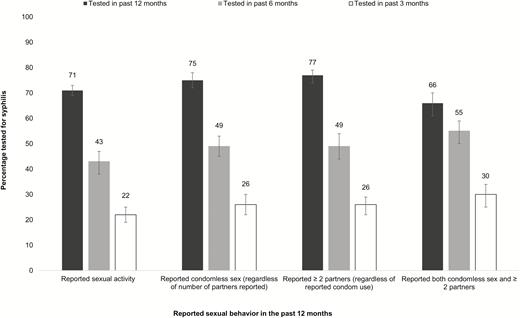
Among sexually active, HIV-positive MSM who reported condomless sex in the past 12 months, only 49% (95% CI: 45%–53%) were tested in the past 6 months, and 26% (95% CI: 22%–30%) were tested in the past 3 months (Figure 1). Among MSM who reported ≥ 2 sex partners in the past 12 months, 49% (95% CI: 44%–54%) were tested in the past 6 months, and 26% (95% CI: 95% CI: 22%–29%) were tested in the past 3 months (Figure 1). Among MSM who reported both ≥2 sex partners and condomless sex in the past 12 months, 66% (95% CI: 61%–70%) were tested in the past 12 months, 55% (95% CI: 50%–59%) in the past 6 months, and 30% (95% CI: 25%–34%) in the past 3 months (Figure 1). MSM who reported either condomless sex or ≥2 sex partners in the past 12 months were 1.2 (95% CI: 1.1–1.3) times as likely to have documentation of syphilis testing in the past 12 months compared to MSM who did not report ≥2 sex partners nor condomless sex in the past 12 months (Table 2). MSM reporting either condomless sex or ≥2 sex partners in the past 12 months also had an increased prevalence of being tested for syphilis in the past 6 months (prevalence ratio = 1.5; 95% CI: 1.3–1.7) and being tested for syphilis in the past 3 months (prevalence ratio = 1.6; 95% CI: 1.3–2.0) compared to MSM who reported neither ≥2 sex partners nor condomless sex in the past 12 months (Table 2).
Percentage of sexually active, HIV-positive men who have sex with men (MSM) and who are receiving medical care for HIV tested for syphilis in the past 3, 6, and 12 months by reported sexual behavior. Note: Sexual behavior categories are not mutually exclusive. Error bars indicate 95% CI. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Prevalence of Testing for Syphilis, by Sexual Behavior Category, Among Sexually Active Men Have Sex with Men and Who are Receiving Medical Care for Human Immunodeficiency Virus in the United States—Medical Monitoring Project, 2013–2014
| . | ≥ 1 Syphilis Test in the: . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Past 12 Months . | Past 6 Months . | Past 3 Months . | ||||||||||
| na . | %b . | 95% CI . | PR (95%) . | na . | %b . | 95% CI . | PR (95%) . | na . | %b . | 95% CI . | PR (95%) . | |
| MSM not reporting ≥ 2 sex partners nor condomless sex in past 12 months | 551 | 63 | (59–68) | ref | 292 | 32 | (27–37) | Ref | 137 | 15 | (12–19) | Ref |
| MSM reporting ≥ 2 sex partners or condomless sex in past 12 months | 1762 | 74 | (72–77) | 1.2 (1.1–1.3) | 1145 | 47 | (42–51) | 1.5 (1.3–1.7) | 603 | 24 | (21–28) | 1.6 (1.3–2.0) |
| . | ≥ 1 Syphilis Test in the: . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Past 12 Months . | Past 6 Months . | Past 3 Months . | ||||||||||
| na . | %b . | 95% CI . | PR (95%) . | na . | %b . | 95% CI . | PR (95%) . | na . | %b . | 95% CI . | PR (95%) . | |
| MSM not reporting ≥ 2 sex partners nor condomless sex in past 12 months | 551 | 63 | (59–68) | ref | 292 | 32 | (27–37) | Ref | 137 | 15 | (12–19) | Ref |
| MSM reporting ≥ 2 sex partners or condomless sex in past 12 months | 1762 | 74 | (72–77) | 1.2 (1.1–1.3) | 1145 | 47 | (42–51) | 1.5 (1.3–1.7) | 603 | 24 | (21–28) | 1.6 (1.3–2.0) |
Abbreviations: CI, confidence interval; PR, prevalence ratio.
aUnweighted frequencies.
bPercentages weighted to adjust for unequal selection probabilities and facility and patient nonresponse.
Prevalence of Testing for Syphilis, by Sexual Behavior Category, Among Sexually Active Men Have Sex with Men and Who are Receiving Medical Care for Human Immunodeficiency Virus in the United States—Medical Monitoring Project, 2013–2014
| . | ≥ 1 Syphilis Test in the: . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Past 12 Months . | Past 6 Months . | Past 3 Months . | ||||||||||
| na . | %b . | 95% CI . | PR (95%) . | na . | %b . | 95% CI . | PR (95%) . | na . | %b . | 95% CI . | PR (95%) . | |
| MSM not reporting ≥ 2 sex partners nor condomless sex in past 12 months | 551 | 63 | (59–68) | ref | 292 | 32 | (27–37) | Ref | 137 | 15 | (12–19) | Ref |
| MSM reporting ≥ 2 sex partners or condomless sex in past 12 months | 1762 | 74 | (72–77) | 1.2 (1.1–1.3) | 1145 | 47 | (42–51) | 1.5 (1.3–1.7) | 603 | 24 | (21–28) | 1.6 (1.3–2.0) |
| . | ≥ 1 Syphilis Test in the: . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Past 12 Months . | Past 6 Months . | Past 3 Months . | ||||||||||
| na . | %b . | 95% CI . | PR (95%) . | na . | %b . | 95% CI . | PR (95%) . | na . | %b . | 95% CI . | PR (95%) . | |
| MSM not reporting ≥ 2 sex partners nor condomless sex in past 12 months | 551 | 63 | (59–68) | ref | 292 | 32 | (27–37) | Ref | 137 | 15 | (12–19) | Ref |
| MSM reporting ≥ 2 sex partners or condomless sex in past 12 months | 1762 | 74 | (72–77) | 1.2 (1.1–1.3) | 1145 | 47 | (42–51) | 1.5 (1.3–1.7) | 603 | 24 | (21–28) | 1.6 (1.3–2.0) |
Abbreviations: CI, confidence interval; PR, prevalence ratio.
aUnweighted frequencies.
bPercentages weighted to adjust for unequal selection probabilities and facility and patient nonresponse.
We also examined the number of syphilis tests occurring > 30 days apart over a 24-month period. The median number of syphilis tests among all sexually active, HIV-positive MSM was 3 (range, 1–12) and this remained unchanged among MSM reporting condomless sex in the past 12 months and for MSM reporting ≥2 sex partners in the past 12 months. The median inter-test interval among all sexually active, HIV-positive MSM was 162 days (range, 35–693). The median inter-test interval decreased slightly among MSM reporting condomless sex in the past 12 months (150 days, range, 36–693) and among MSM reporting ≥2 sex partners in the past 12 months (150 days, range, 35–693).
DISCUSSION
Overall, 71% of sexually active HIV-positive MSM had documentation of a syphilis test in the past 12 months by their HIV care provider. A trend analysis of syphilis testing among MSM receiving medical care for HIV from 2009–2013 showed that 69% (95% CI: 66%–72%) of sexually active HIV-positive MSM were tested for syphilis in 2013 [11]; therefore, our findings show a similar proportion of sexually active HIV-positive MSM tested for syphilis in 2014 compared to 2013. Our findings indicate that nearly one-third of sexually active HIV-positive MSM did not receive recommended syphilis screening in the past 12 months. Frequent syphilis screening has the potential to ensure early detection and treatment, stemming ongoing disease transmission and development of disease sequelae [5–7].
There was variation in the proportion tested for syphilis across subgroups. MSM aged 30–49 years, of Hispanic/Latino ethnicity, and with only RWP coverage were more likely to have been tested for syphilis in the past 12 months. The higher prevalence of syphilis testing among older compared to younger MSM has been reported before [12] and may be reflecting age group disparities in retention in continuous HIV care. Studies have shown lower levels of retention in continuous HIV care among HIV-positive adolescent and young adults [19–21]. Older MSM in this population may have higher levels of retention in continuous HIV care and have a greater number of interactions with their HIV care provider and more opportunities to be screened for syphilis. The lower prevalence of syphilis testing among younger MSM, despite increasing syphilis diagnoses among this population indicates a misalignment between screening practices and the current syphilis epidemiology and could result in missed opportunities to recognize and treat syphilis cases, particularly early, asymptomatic cases.
The higher prevalence of syphilis testing among MSM with only RWP coverage might be explained by these men obtaining comprehensive HIV/STI care at facilities supported through the RWP. The RWP requires supported service providers and facilities to assess the extent to which HIV health services provided to their patients under the grant are consistent with the most recent Public Health Service (PHS) guidelines for the treatment of HIV/AIDS and related opportunistic infections [22]. Studies comparing the quality of HIV care received at RWP-supported facilities to non-RWP supported facilities have shown a higher proportion of patients receiving HIV care according to PHS guidelines at RWP-supported facilities [23, 24].
Looking at time since last test, the prevalence of syphilis testing in the past 3 months (22%) or in the past 6 months (43%) was low, and increased among MSM reporting risky sexual behavior. Given that almost half of MSM in our sample reported at least one risk behavior and may represent a core group at highest risk of syphilis, these missed opportunities for syphilis screening are concerning. Findings from modeling studies suggest that increasing the frequency of syphilis screening among a core group of high-risk MSM to every three months was more effective in reducing syphilis incidence than increasing screening coverage among the general population of MSM [25] and was highly cost-effective when compared to annual syphilis screening [26]. Clinical studies have shown that more frequent syphilis screening among high-risk populations is feasible and increases the detection of asymptomatic infectious syphilis particularly among high risk MSM [27–29]. It is also a practical and inexpensive endeavor to screen for syphilis among HIV-positive MSM in care because these patients undergo routine blood draws and tests for viral load and CD4 count monitoring [26]. Syphilis has been associated with increases in HIV viral load and decreases in CD4 cell count [6]. Given that 58% of MSM in this population had an AIDS diagnosis or a nadir CD4 count of <200 cells/mm3, it further underscores the importance of prompt detection and treatment of syphilis.
Current guidelines for more frequent syphilis screening are based on the presence of sexual risk behaviors and cannot be appropriately implemented without conducting a sexual risk assessment. This speaks to the importance of routinely obtaining sexual histories from patients. Studies have reported low levels of receipt of individual counseling by HIV-positive MSM in care [30]. When an HIV-positive person is diagnosed with syphilis, it is a marker for condomless sex and can be used as an opportunity to engage in behavioral counseling because reducing risk is important for reducing both HIV transmission and the acquisition of other STDs. Novel interventions for enhancing more frequent syphilis screening of MSM, such as health alerts and reminders integrated into electronic health systems, have been shown to be effective at increasing STD screening [31, 32] and are most effective when STD specimens are automatically collected for testing as part of a routine visit [30]. Even though the overall proportion tested in the past 3 months or in the past 6 months was low, MSM who reported sexual risk behavior were more likely to be tested for syphilis, suggesting that some opportunistic risk-based testing may be occurring.
This analysis has a number of limitations. First, we only examined data collected from the medical record of HIV-positive men at their primary HIV care provider. Any syphilis testing occurring outside of this setting may have been missed, so we are likely underestimating syphilis testing rates. A recent analysis looking at local MMP data in San Francisco examining the proportion of sexually active persons screened for STDs at a place other than their primary HIV care provider reported that 8.9% of their MMP sample in 2013 were tested for syphilis, chlamydia, and gonorrhea at an STD clinic only [33]. Furthermore, they found that persons who were MSM, white, 18–39 years of age, and had a private HIV care provider were more likely to be screened for all three STDs at an STD clinic only. Second, we limited our analysis to sexually active MSM based on self-reported sexual behavior. We could therefore be excluding men who did not feel comfortable reporting sexual behaviors including risk behaviors and same-sex sexual behavior. Third, during 2013–2014, MMP only included men who were receiving HIV medical care; therefore, our estimates are likely not generalizable to all HIV-diagnosed MSM in the United States.
Although our findings suggest a continuation of the previously documented increasing trend in syphilis testing among sexually active HIV-positive MSM in care, nearly one-third of MSM did not have any documentation of syphilis testing by their primary HIV care provider in the past 12 months. A lack of timely and regular syphilis screening represents a missed opportunity to identify early, asymptomatic infections, which when adequately treated could have a significant impact on stemming the transmission of syphilis. Given the potentially serious complications that can result from HIV and syphilis coinfection, our findings suggest that improved efforts may be warranted to increase syphilis screening among sexually active HIV-positive MSM.
Notes
Acknowledgments. We thank participating Medical Monitoring Project (MMP) patients, facilities, project areas, and Provider and Community Advisory Board members. We also acknowledge the contributions of the Clinical Outcomes Team and Behavioral and Clinical Surveillance Branch at the Centers for Disease Control and Prevention (CDC) and the MMP Project Area staff. (http://www.cdc.gov/hiv/statistics/systems/mmp/resources.html#StudyGroupMembers).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Funding. This work was supported by the CDC.
Potential conflicts of interest. The authors declare no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Centers for Disease Control and Prevention.
Centers for Disease Control and Prevention.
CDC.
Centers for Disease Control and Prevention (CDC).





Comments