-
PDF
- Split View
-
Views
-
Cite
Cite
Frederick L. Schuster, Shigeo Yagi, Shilpa Gavali, David Michelson, Ravi Raghavan, Ingrid Blomquist, Christine Glastonbury, Andrew W. Bollen, David Scharnhorst, Sharon L. Reed, Steve Kuriyama, Govinda S. Visvesvara, Carol A. Glaser, Under the Radar: Balamuthia Amebic Encephalitis, Clinical Infectious Diseases, Volume 48, Issue 7, 1 April 2009, Pages 879–887, https://doi.org/10.1086/597260
Close - Share Icon Share
Abstract
Background. We present data from 9 years (1999–2008) of tests for Balamuthia mandrillaris, an agent of amebic encephalitis that were conducted as part of the California Encephalitis Project.
Methods. Specimens obtained from patients with encephalitis were sent to the California Encephalitis Project for diagnostic testing; a subset of these specimens were tested for Balamuthia species. Tests included indirect immunofluorescent staining of sections for amebae, fluorescent antibody staining and enzyme-linked immunosorbent assay for serum titers, and polymerase chain reaction for Balamuthia 16S mitochondrial DNA. Cerebrospinal fluid (CSF) samples obtained from patients with diverse types of encephalitis were also tested for a broad range of cytokines.
Results. Of >3500 cases referred to the California Encephalitis Project, 10 were found to be amebic encephalitis on the basis of serologic and CSF tests and examination of stained tissue sections. Most of these cases would have been described as “encephalitis of unknown origin” if it were not for the California Encephalitis Project. Nine of the 10 patients were male; ages ranged from 1.5 to 72 years. All patients had abnormal neuroimaging findings and abnormal CSF composition. The more common symptoms at presentation included headache, seizures, cranial nerve palsies, and lethargy. CSF specimens from patients with Balamuthia infection had significant elevations in the levels of cytokines IL-6 and IL-8, compared with specimens obtained from persons with viral or noninfectious encephalitides.
Conclusions. Balamuthiasis is difficult to diagnose, and it is likely that cases go unrecognized because clinicians and laboratorians are unfamiliar with the disease. Alerting the medical community to this disease may lead to earlier diagnosis and improve the chances of survival.
Granulomatous amebic encephalitis due to the free-living ameba Balamuthia mandrillaris (BAE) is an often-unrecognized disease. B. mandrillaris has caused, with little attention, >125 cases of BAE since 1975, as determined on the basis of unpublished laboratory results and published reports in the biomedical literature [2]. Relatively few clinicians are familiar with the disease, and diagnosis is frequently made postmortem. Only ∼60 cases of BAE have been diagnosed in the United States (G.S.V., unpublished data), most of which were identified at autopsy [3, 4]. In the 1960s, the rate of autopsy for nonforensic deaths was 30%–40%, but it has since decreased to <6% [5]. With autopsy rates decreasing and without alternative means of diagnosis, even fewer cases of BAE will be identified.
The preliminary diagnosis is often neurotuberculosis or neurocysticercosis, with brain tumor or fungal or viral encephalitides presented as other differential diagnoses. Although it is primarily a disease of the CNS, Balamuthia infection can also manifest as skin lesions prior to the hematogenous spread of amebae to the brain [6].
In addition to causing disease in humans, Balamuthia infection has been identified in other mammalian species, such as nonhuman primates, horses, dogs, and sheep [2, 7]. BAE is difficult to diagnose clinically during the prodromal or acute phases. Only 4 patients in the United States have survived the disease, with varying degrees of neurologic sequelae [8, 9], and 3 patients from Peru have recovered from the cutaneous form of the disease [6]. Successful treatment likely depends on early recognition of the disease. Even so, premortem diagnosis and initiation of antimicrobial therapy do not guarantee that the disease will be prevented from advancing to the terminal stage.
To date, 10 cases of BAE have been diagnosed in California through testing as part of the California Encephalitis Project (table 1). The aims of the study were (1) to identify and evaluate cases of encephalitis due to Balamuthia species and (2) to make clinicians and laboratorians aware of the signs and symptoms of BAE, which otherwise might go undiagnosed.
Demographic, clinical, and laboratory data for patients with balamuthia in the California Encephalitis Project, 1999–2007.
Methods
Patient criteria for the California Encephalitis Project. Physicians throughout the state of California refer cases of encephalitis to the California Encephalitis Project. The purpose of the California Encephalitis Project is to identify etiologic agents in cases of encephalitis that present a diagnostic challenge to hospital laboratory facilities [8]. Severely immunosuppressed patients or patients aged <6 months are excluded from the program. Inclusion in the project also requires the patient to have been hospitalized with encephalopathy or ataxia and to have had ⩾1 of the following symptoms: fever (temperature, ⩾38°C), seizures, focal neurologic findings, CSF pleocytosis, or abnormal electroencephalography or neuroimaging findings [10]. From 1999 to 2008, more than 3500 patients have met these criteria. Specimens are tested for BAE on the basis of a combination of (1) clinical symptoms (e.g., cranial nerve palsies), (2) elevated CSF levels of protein and leukocytes, (3) abnormal neuroimaging findings, and (4) occupational or recreational contact with soil.
Criteria for BAE testing. During 1999–2008, a total of 474 cases were tested for Balamuthia antibodies. In the first 2 years of the project, almost all serum samples (n=208) submitted to the California Encephalitis Project were tested for Balamuthia antibodies, and no cases were identified. As more clinical and laboratory information became available on the characteristics of Balamuthia infection, we became more selective in choosing samples for testing. During the remaining years of the period, specimens from 266 cases were tested for BAE on the basis of the following characteristics: (1) unexplained encephalitis (i.e., no other agent was identified), (2) abnormal CSF findings (especially an elevated protein level [>100 mg/dL] or a low glucose level [<50 mg/dL]) or abnormal neuroimaging findings, and (3) adequate specimen volume.
Clinical data. The referring physician completed a case history form for the patient that included information on exposure, travel, and clinical, demographic, and laboratory characteristics and submitted samples of serum (acute and/or convalescent phase) and CSF. If the initial serologic screening demonstrated evidence of ameba antibodies and if a brain tissue specimen was available, hematoxylin-eosin–stained slides and unstained brain sections were requested for indirect immunofluorescent staining for Balamuthia species. CSF and, if available, brain tissue specimens were tested by PCR.
Immunofluorescent antibody detection. As the initial screening step, serum samples were diluted from 1:2 to 1:4096, and the titer was determined by immunofluorescent staining [11]. ELISA was recently added as an ancillary procedure for corroboration of immunofluorescent stain findings [12].
Immunofluorescent staining of tissue. Deparaffinized tissue sections were treated with anti-Balamuthia–specific serum raised in rabbits, followed by goat anti-rabbit fluorescein isothiocyanate conjugate. Balamuthia amebae, if present, fluoresced apple green in tissues.
PCR. PCR was performed for Balamuthia mitochondrial 16S rRNA gene DNA [13, 14]. DNA was extracted from fresh-frozen or formalin-fixed specimens of brain and other tissues (i.e., kidney, adrenal gland, lung, and thyroid), from centrifuged CSF samples, and from deparaffinized 5-µm–thick brain tissue sections [13–15]. Clinical samples (i.e., brain tissue and serum samples) obtained from 35 patients were tested by PCR, of which 10 tested positive for Balamuthia mitochondrial DNA on the basis of extraction of DNA from fresh brain tissue, deparaffinized tissue sections (brain or otherwise), and/or CSF samples.
All extracts of brain tissue obtained from patients with presumptive BAE tested positive for Balamuthia mitochondrial DNA. Only 2 (25%) of 8 CSF samples obtained from patients with presumptive BAE tested positive for DNA.
Cytokine testing. The California Encephalitis Project initiated CSF testing for cytokines (Biosource Human Cytokine 10-Plex; Biosource) in 2006 for patients with encephalitis, including those with pyogenic bacterial, mycobacterial, viral, and noninfectious encephalitides. A total of 8 CSF samples obtained from patients with BAE were tested for cytokines.
Results
Clinical characteristics. At the onset of the acute phase of the disease, the patients from California presented with abnormal findings from neuroimaging examinations (100% of patients), lethargy (70%), severe headache (60%), generalized seizures (40%), cranial nerve palsies (40%), emesis (30%), and nuchal rigidity (10%). Some patients had low-grade fever (70%); others were afebrile. Typically, young patients were in apparently good health, without predisposing medical conditions; several older patients had health problems, including diabetes, chronic lymphocytic leukemia, gout, and cardiac disease.
In many patients with Balamuthia infection, premonitory signs and symptoms appeared days or weeks before the onset of the acute stage of disease. In some instances, patients had sought medical attention before the onset of fulminant disease and were usually treated with antiviral or antibacterial drugs. Although BAE is often described as a chronic infection, once the acute phase develops, death may occur in a matter of days. The median duration of hospitalization for the California patients with BAE was 16.5 days (range, 3–120 days). Of the 10 patients who were referred to the California Encephalitis Project, 9 died, and 1 was lost to follow-up.
Laboratory testing. Results of cultivation of clinical samples for infectious agents (bacteria or fungi) were routinely negative. Spinal fluid protein levels were elevated (median, 131 mg/dL; range , 64–674 mg/dL), WBC counts were elevated (median, 188 cells/mm>3; range, 11–540 cells/mm>3), with lymphocytic pleocytosis, and glucose levels were normal or low (median, 40 mg/dL; range, 15–74 mg/dL) (table 2).
Comparison of Balamuthia encephalitis with Mycobacterium tuberculosis (MTB) encephalitis and viral encephalitis.
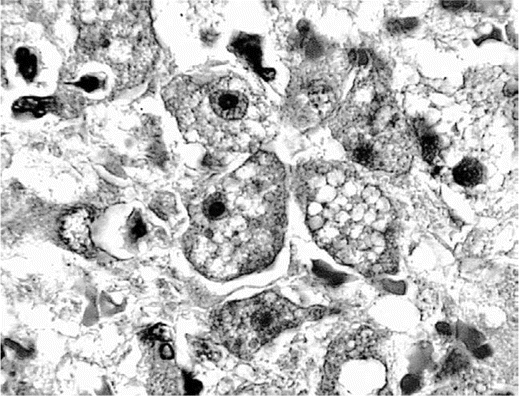
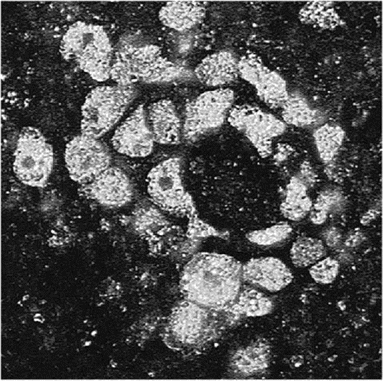
Immunofluorescent staining of tissue sections. Indirect immunofluorescent staining is the definitive test for Balamuthia amebae (antigen) in unstained brain and other tissue sections [16]. Unlike the more investigational techniques (e.g., immunofluorescent staining of serum, PCR, or ELISA), this test is specific for Balamuthia amebae, and in conjunction with hematoxylin-eosin staining, it is precise in identification and location of amebae (figure 1). All samples sent to the California Encephalitis Project that tested positive for Balamuthia antibodies by serum immunostaining (titer, ⩾1:128) or ELISA also tested positive for Balamuthia antibodies by indirect immunofluorescent staining. In immunostained sections, amebae were seen in the perivascular spaces of blood vessels, and they may appear as a ring surrounding the outer wall of a blood vessel (figure 2).
Hematoxylin-eosin–stained section of a necrotic region of brain tissue showing multiple trophic amebae in the brain parenchyma. Note the bull's eye appearance of the nucleus with its central nucleolus, as seen in several of the amebae (original magnification, ×1000).
Indirect immunofluorescent–stained section through a blood vessel in the brain showing fluorescing amebae surrounding the wall of the vessel. The wall of the vessel, however, is not apparent (original magnification, ×400).
Serologic testing. Serum titers positive for Balamuthia antibodies were 1:128 to 1:256 or higher. Negative samples, in contrast, had no detectable antibody titers or titers of <1:64, both for patients with non-Balamuthia encephalitis and for healthy individuals [11]. Samples that yielded positive ELISA results had absorbencies of ⩾2.5.
PCR. PCR has been effective for identification of Balamuthia 16S mitochondrial rRNA gene DNA after amplification and production of a 230-bp amplicon [13, 14]. Thirty-five samples of brain tissue and/or CSF were tested by PCR on the basis of suggestive signs and symptoms; 10 samples were positive for Balamuthia DNA. Samples used for DNA extraction for PCR included fresh or frozen brain tissue, deparaffinized brain or other tissue sections, and CSF that had been centrifuged to collect particulate material in suspension. Amplification products were eluted from gels for sequencing, and the sequences have proven to be identical to the GenBank sequences posted for Balamuthia16S RNA gene DNA [17, 18].
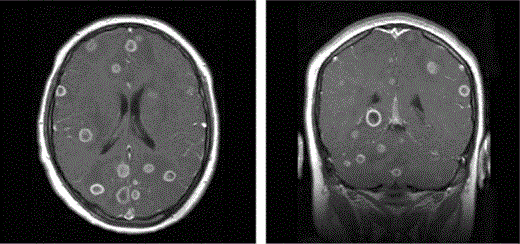
Neuroimaging. All patients in the California Encephalitis Project with Balamuthia infection exhibited abnormal neuroimaging findings. CT and MRI revealed multiple supra- and infratentorial mass lesions. The lesions were of varying size, with the larger rim-enhancing lesions associated with significant edema and local mass effect. Small lesions tended to show solid enhancement and less edema (figure 3). Lesions were seen to involve the deep nuclei (caudate and thalamus), the white matter of the cerebral hemispheres, and the cerebellum, with hydrocephalus in 1 case appearing to be due to mass effect upon the fourth ventricular outlets. Subtle leptomeningeal enhancement was noted in some cases, although cranial nerve enhancement was not identified. Calcification of lesions, however, has been noted in the literature [8] but was not seen in the MRIs or CTs for any of our reported cases.
Axial (left) and coronal (right) T1-weighted images, with contrast, from 2 different patients showing multiple ring-enhancing lesions throughout the supratentorial brain and cerebellum. There is involvement of the frontal, occipital, and temporal lobes, and basal ganglia lesions are also present. Low signal–intensity edema is noted in association with the larger lesions, resulting in mild local mass effect.
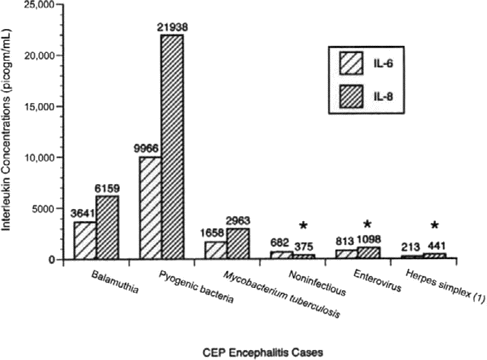
Cytokines in Balamuthia infections. CSF samples sent to the California Encephalitis Project were tested for 10 different cytokines (GM-CSF, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IFN-γ, and TNF-α). Eight of these CSF samples tested for cytokine concentrations were obtained from patients with BAE. These cytokine values were compared with the cytokine profiles of several other CSF specimens, including samples obtained from patients with encephalitides with viral (enteroviruses and herpes viruses), bacterial (Mycobacterium tuberculosis, Neisseria meningitides, and Staphylococcus aureus), and noninfectious (neoplasms, vasculitis, and autoimmune disease) causes. Figure 4 presents the comparative levels of IL-6 and IL-8 in CSF samples obtained from patients with BAE, pyogenic bacterial, mycobacterial, noninfectious, and viral diseases. The levels of IL-6 and IL-8 were significantly higher in the samples obtained from patients with Balamuthia infection than in those obtained from patients with noninfectious and viral encephalitides. No differences were noted for the other 8 cytokines. Cytokine testing was done predominantly on CSF specimens, because the initial tests of cytokines in serum samples in the California Encephalitis Project were uninformative.
Graph showing titers of cytokines IL-6 and IL-8 in CSF samples from patients diagnosed with balamuthiasis (n=8). Other categories included for comparison were pyogenic bacterial encephalitis (n=5), neurotuberculosis (n=7), noninfectious disease (n=15αolm0;18), herpes simplex virus 1 (n=13), and enterovirus (n=15αolm0;16) encephalitidies (specimen volume was inadequate to perform all cytokines for all samples). CEP, California Encephalitis Project. *IL levels that were statistically significantly different (P⩽.05, by Kruskal-Wallace test) from those in patients with balamuthiasis.
Discussion
BAE is an almost invariably fatal disease of the CNS that can result from infection via contact with amebae. A feature common to several of the BAE cases was contact with soil [8, 9, 19]. Disease develops in some persons who contracted infection after contact with soil—for example, playing with flowerpot soil [19], digging a drain [8], gardening in composted soil [9], riding in open vehicles over dusty desert terrain (D.M., unpublished data), and amputation of a leg in a farm accident and contamination of the wound with soil and manure [20]. Despite the numerous opportunities people have for contact with soil, few Balamuthia infections develop, suggesting that B. mandrillaris has a low virulence or that people have some level of protective immunity. Water has been implicated in several cases, including a case involving exposure of an open head wound to swamp water after an accident [3] and cases in 2 immunosuppressed dogs that had access to swampy pond water for exercise (one was in California [17], and the other was in Australia [21]).
Once the disease becomes symptomatic, it is life-threatening, and the patient deteriorates in days or weeks. Symptoms of both neurotuberculosis and neurocysticercosis are similar to those of BAE: tonic-clonic seizures, fever, personality change, hydrocephalus, focal deficits, headache, and emesis. The CSF in patients with neurotuberculosis may demonstrate lymphocytosis (lymphocyte count, ⩽500 cells/mL) and elevated protein level (⩽500 mg/dL) but a normal glucose level, as it does in BAE. A presumptive diagnosis of neurotuberculosis is often made, which can delay treatment because of the time required to confirm presence of the mycobacteria. Testing for neurocysticercosis is problematic; some patients with BAE have had serologic tests positive for cysticercosis.
The protocol that the California Encephalitis Project followed in detecting these 10 cases of Balamuthia infection has limitations. Serologic testing was used as our primary screening method, but the sensitivity and specificity of this method are unknown. In addition to the confirmed cases of Balamuthia infection, there were 12 other cases with positive serologic test results. Subsequent testing with ELISA did not support the initial serologic test result. Brain material was not available from any of these patients to confirm or refute the serologic test results.
Balamuthia amebae are not usually seen in spinal fluid and have yet to be reported in CSF wet mounts. Ameba DNA may be present in CSF centrifugate, and CSF specimens obtained from 2 patients in the California Encephalitis Project tested positive by PCR for Balamuthia DNA [13, 14]. This may result from release of lysed amebae into the CSF from necrotic brain tissue. Not all CSF samples obtained from patients with Balamuthia infection test positive for Balamuthia DNA, suggesting that CSF DNA positivity or negativity may relate to the stage of disease, the degree of necrosis, and the duration of CNS disease. Neuroimaging often reveals brain lesions, but unless a biopsy is performed, the lesions cannot be definitively determined to have been caused by Balamuthia species. Immunosuppressed individuals are vulnerable to infection, as are elderly persons and individuals with concurrent diseases [16, 22, 23]. However, children in good health who do not have overt signs of immune depression have also developed infection. Granulomatous lesions are a hallmark of BAE, but they may be lacking, particularly in immunosuppressed hosts [2].
On the basis of the overall profile of the recognized BAE cases, predisposing factors include youth or old age, chronic health problems, malnutrition, contact with soil or water, and possibly prior treatment with corticosteroids and Hispanic ethnicity [24].
Two of the cases originated from the same hospital in southern California (cases 5 and 7) [19]. A third case, which was diagnosed by the Centers for Disease Control and Prevention, was also previously reported from the same hospital [19]. The occurrence of the 3 cases from the same locale is remarkable, considering the small number of recognized infections worldwide, and is likely a result of increasing awareness of hospital personnel to disease symptomatology. Other factors that may contribute to the relatively high number of cases in California include the facts that agriculture is a major industry in the state and that Hispanics constitute a high proportion of the state's population. Nine of the 10 patients with BAE detected in the California Encephalitis Project study were of Hispanic ethnicity. Thus, Hispanic ethnicity and soil exposure through agriculture have both been suggested as risk factors for disease.
Ring-enhancing lesions in brain tissue were typical of most cases for which MRI was performed [8, 25]. These lesions varied in number and size. Old lesions regressed and new ones developed during the acute phase of the disease. In some instances, calcification of the lesion occurred [8].
Most cases of BAE in North America are first recognized as neurologic disease, whereas those in South America (Peru, in particular) usually present as cutaneous lesions on the trunk, limbs, and face of the patient, with secondary invasion of the CNS [6]. The reason for the difference in the modes of infection is not known. The duration between cutaneous infection and development of neurologic symptoms is, on average, 5–8 months [6].
A strong humoral response develops in the host (IgG titer, ⩾1:128), presumably because the incubation period is relatively prolonged. Most serum specimens obtained from Balamuthia-uninfected individuals have titers ranging from no detectable antibody to ⩽1:8 [11, 12]. The presence of serum antibodies to Balamuthia could mean prior exposure or could be the result of nonspecific cross-reactivity. In our study, a titer of ⩾1:128 was considered a presumptive positive and was selected for further testing by indirect immunofluorescent staining of brain tissue, if available, or PCR of tissue or immunofluorescent staining or ELISA of serum samples. In no case was a diagnosis of BAE based on a single laboratory test.
The elevation of cytokine levels seen in CSF samples obtained from patients with BAE may be a reflection of the prolonged asymptomatic phase of the disease. Higher cytokine levels were found only in patients with pyogenic bacterial encephalitis. Differences were seen in the IL-6 and IL-8 cytokines for patients with Balamuthia encephalitis versus those with encephalitis due to herpes simplex 1 and enteroviruses; in some cases, the differences were statistically significant. These results may be of diagnostic help in distinguishing between viral and Balamuthia encephalitides.
Survivors of Balamuthia infection were treated with a combination of antimicrobials, including pentamidine isothionate, 5-fluorocytosine (flucytosine), fluconazole, sulfadiazine, and a macrolide antibiotic (azithromycin or clarithromycin), with or without a phenothiazine compound (I.B., unpublished data) [7, 8]. Two of the Peruvian patients noted above were treated with albendazol and itraconazole; a third patient with a facial lesion recovered spontaneously without drug therapy [6].
Steroids have been used during the course of disease as anti-inflammatory agents. Use of these agents, however, may be counterproductive, because they can suppress of the host immune response and accelerate the spread of disease [26–28]. Conclusive evidence, however, is lacking. At this time, the prognosis of BAE is poor, with a mortality of ∼98%.
Without the California Encephalitis Project study, most of the BAE cases included in this report would have been identified as “encephalitis of unknown origin.” If BAE is recognized early in the course of disease, there may be an opportunity to halt its insidious progression. To realize this goal, clinicians should be aware of the characteristic profile of disease presentation: the signs and symptoms of disease, neuroimaging findings, CSF composition, and the patient's recent activities, recent travel, and, perhaps, ethnicity.
Acknowledgments
We thank Somayeh Honarmand for providing statistical interpretation of the data and the California physicians who cooperated with the California Encephalitis Program by submitting clinical specimens for testing.
Sadly, Dr. Fred Schuster passed away on 6 January 2009. A renowned parasitologist, he will long be remembered for his contributions to the field. His accomplishments include the discovery and naming of Balamuthia mandrillaris—the topic of this article. Dr. Schuster will be remembered as a wonderful teacher and friend. His generosity, intelligence, unassuming nature, and keen sense of humor will be sorely missed by his colleagues.
Financial support. Emerging Infectious Diseases Program at the Centers for Disease Control and Prevention (U50/CCUP915546–09).
Potential conflicts of interest. All authors: no conflicts.
References
Portions of this article appeared in Morbidity and Mortality Weekly Report [1].
Deceased.
- cytokine
- neuroimaging
- polymerase chain reaction
- encephalitis
- seizures
- enzyme-linked immunosorbent assay
- cerebrospinal fluid analysis
- headache
- amoeba
- diagnostic techniques and procedures
- dna, mitochondrial
- encephalitis, california
- fluorescent antibody technique
- interleukin-8
- radar
- cerebrospinal fluid
- diagnosis
- interleukin-6
- balamuthia
- balamuthia mandrillaris
- lethargy
- cranial nerve palsies
- community
- balamuthia infections





Comments