-
PDF
- Split View
-
Views
-
Cite
Cite
Dana Duricova, Natalia Pedersen, Martin Lenicek, Christian Jakobsen, Milan Lukas, Vibeke Wewer, Pia Munkholm, The clinical implication of drug dependency in children and adults with inflammatory bowel disease: A review, Journal of Crohn's and Colitis, Volume 5, Issue 2, April 2011, Pages 81–90, https://doi.org/10.1016/j.crohns.2010.12.006
Close - Share Icon Share
Abstract
Drug dependency in adult and paediatric patients with inflammatory bowel disease (IBD) is described and the significance of this response pattern in clinical practice discussed in this review. Dependent patients maintain remission while on the treatment, but they relapse shortly after drug cessation or dose decrease. However, a quick restoration of remission and sustained response is achieved when the therapy is re-introduced or dose increased.
Population-based studies have demonstrated that 22–36% of adults and 14–50% of children become corticosteroid dependent. Approximately 1/4–1/3 of treated patients undergo surgery ≤ 1 year after treatment start, although newer paediatric studies reported lower risk of surgery (5–11%), including dependent patients. The frequent use of immunosuppressants (68–80% of children) might explain this favourable outcome and thus reduce importance of the term corticosteroid dependency.
Infliximab dependency was described in 42–66% of children and 29% of adults with Crohn's disease. The risk of surgery 50 and 40 months after treatment start was 10% and 23% in infliximab dependent children and adults, respectively. Maintenance of infliximab in dependent patients was suggested to postpone if not avoid the need of surgery.
Lastly, mesalazine dependency was identified in 23% of adults with Crohn's disease. These patients were characterized by mild disease course and lower surgical risk compared to non-responders to mesalazine (32 vs. 61%).
Identification of drug dependency is useful for prediction of a certain disease course and surgery. An adjustment of medical therapy may alter the prognosis and disease course.
1 Background
Inflammatory bowel disease (IBD) is a chronic disorder of unknown aetiology and various manifestations regarding disease localization, occurrence of complications and frequency of relapses. 1 According to this, different behaviour patterns and disease courses have been identified classifying patients into several subtypes. 2 – 4 Patients, however, differ also in response patterns – response/no response – a phenotype “drug dependency” has been described in children and adults with IBD. 5 – 7 The dependent patients represent a specific population of individuals who maintain remission while on the treatment, but relapse promptly after drug cessation or dose decrease. However, a quick restoration of remission, repeating the way of former response, is achieved and sustained when the therapy is re-introduced or dose increased.
Drug dependency was first described in corticosteroid therapy, 5 later in infliximab treatment 7 – 9 and most recently in the use of mesalazine preparations. 10
The aim of this review is to describe drug dependencies in paediatric and adult patients with IBD and to elucidate the significance of this response pattern in clinical practice.
2 Corticosteroid dependency
2.1 The development of the term corticosteroid dependency
Corticosteroid dependency was first introduced in an inception cohort of adult Crohn's disease (CD) patients from Copenhagen County in 1994. 5 In this study, the first steroid treatment course was evaluated in 109/196 CD patients diagnosed between 1979 and 1987. Later, Faubion et al. 11 published a population-based study assessing the course of the initial corticosteroid treatment in 74/173 CD patients and 63/185 patients with ulcerative colitis (UC) diagnosed in Olmsted county from 1970 to 1993. Furthermore, the outcome of corticosteroid treatment has been studied in several non-population based cohorts. 12 – 16
Four paediatric studies of unselected cohorts have also addressed the term corticosteroid dependency. 6 , 17 - 19 In addition, two paediatric studies based on prospective multicentre database from US and Canada 20 , 21 assessed the outcome of the first steroid course in newly diagnosed children with IBD.
The term corticosteroid dependency was recognized as an important clinical response pattern and the definition has been implemented to the European Crohn's and Colitis Organization (ECCO) guidelines for CD and UC in 2006 and 2008 and has also been accepted by the Food and Drug Administration (FDA) agency as one of the end points for randomized controlled trials. 22 , 23
The different definitions of corticosteroid dependency are summarized in Table 1 .
2.2 How many become corticosteroid dependent?
From the population-based studies it is known that 28–36% of CD adult patients and 22% of UC patients became corticosteroid dependent. 5 , 11 Among paediatric patients, 24–39% of children with CD and 14–50% of those with UC developed corticosteroid dependency 6 , 17 - 19 ( Table 2 ). Similar results were observed in two multicentre studies with 31% of CD and 43% of UC children becoming steroid dependent. 20 , 21
The frequency of steroid dependency in adult studies of selected cohorts ranges from 7.5 to 58% of patients with CD and from 17 to 47% of those with UC. 12 – 16
2.3 What does corticosteroid dependency imply in terms of surgery?
In the study by Munkholm et al. 5 one month after cessation of corticosteroid therapy, 59% of non-responders and 26% of corticosteroid dependent patients, respectively, underwent surgical intervention. None of those with prolonged response had surgery within this time period. However, no significant difference in surgery rate among the response groups has been observed during the following two years.
Faubion et al. 11 described that cumulative probability of surgery 12 months after the start of corticosteroid treatment was 38% in CD and 29% in UC patients. No specification regarding response to corticosteroids was given.
The risk of resection one year after the initial course of corticosteroids was 27% in CD and 29% in UC children as reported by Tung et al. 6 , again without specification as to corticosteroid outcome.
Finally, in the most recent paediatric study 17 the cumulative probability of surgery one year after start of corticosteroid treatment was 11.5% and 7.8% for CD and UC. Interestingly, when stratifying for outcome 30 days after stopping corticosteroids, the risk of surgery was equal for steroid dependent patients and patients with prolonged response in both CD and UC. Similar low risk of surgery was observed in two multicentre studies 20 , 21 where 8% and 5% of children with CD and UC respectively underwent surgery for IBD within one year after start of corticosteroids.
2.4 Are there any predictors for corticosteroid dependency?
No predictor of treatment response has been identified in any of the studies of unselected cohorts assessing adult patient populations, 5 , 11 whereas the paper by Jakobsen et al. identified that CD children with disease localization involving terminal ileum at diagnosis were at increased risk of steroid dependency. 17
3 Infliximab dependency
3.1 The development of the term infliximab dependency
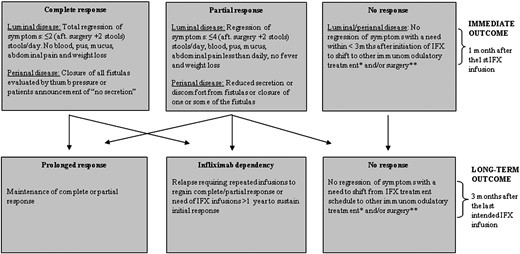
Dependent response pattern was recognized in infliximab treatment first in 2006. 7 Some patients maintained the remission after induction therapy alone or induction and one year maintenance therapy, while others relapsed and required further infusions to sustain the initial response. 24 , 25 This phenotype model was first developed and evaluated in a cohort of 24 children with CD treated in Denmark from 1999 to 2003. 7 Infliximab dependency was assessed 90 days after the intended treatment cessation and patients relapsing within this time period but regaining the initial response after re-introduction of infliximab were considered as infliximab dependent.
The model was later adjusted ( Fig. 1 ) and used in a subsequent study of 82 paediatric CD patients treated with infliximab. 9 The study was an extension of the previous one 7 and included patients from Denmark and the Czech Republic, treated with infliximab from 2000 to 2006.
Furthermore, a national cohort of CD children from the Netherlands has been assessed for infliximab dependency. 8 Sixty-six children, treated from 2002 to 2007, were included and considered as infliximab dependent in case of relapse of symptoms requiring repeated infusions to regain good clinical response.
Lastly, infliximab dependency has been studied in a cohort of adult CD patients. 26 In 132 patients from Denmark and 115 patients from the Czech Republic, treated 1999–2006, infliximab outcome was evaluated according to a phenotype model developed and used in a previous paediatric study. 9
3.2 How many patients developed infliximab dependency?
In paediatric studies 42–66% of children became infliximab dependent, whereas 15–29% maintained initial response as prolonged responders. 7 – 9 In contrast, a study of adult patients found that 49% of the patients were prolonged responders and only 29% became infliximab dependent. 26 The results are summarized in Table 3 .
3.3 What does infliximab dependency imply in term of surgery?
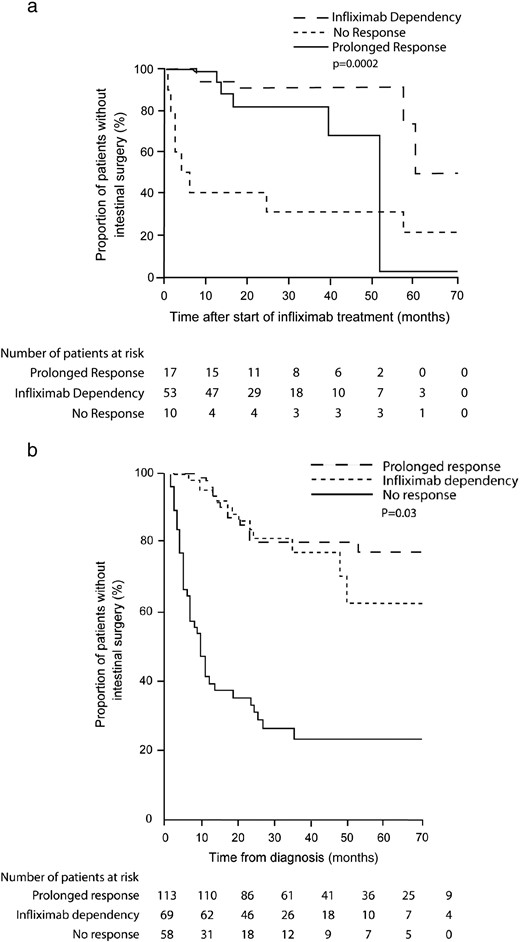
Three articles reported risk of surgery. 8 , 9 , 26 In the Danish-Czech study, 9 the cumulative probability of surgery 50 months after the start of infliximab was 10% in infliximab dependent children, 30% in prolonged responders and 70% in non-responders (p = 0.0002). Similarly, in a study by Pedersen et al. 26 23% of infliximab dependent adult patients, 20% of prolonged responders and 76% of non-responders had surgery 40 months after the start of therapy (p < 0.001) ( Fig. 2 ). Thirty-nine per cent of infliximab treated children required surgery in a study by de Ridder et al., 8 no specification regarding treatment outcome has been described.
3.4 Are there any predictors of infliximab dependency?
An association of dependent phenotype with perianal disease and absence of intestinal surgery prior to infliximab therapy has been found in one paediatric study. 9 Contrary to this, the paper from the Netherlands 8 reported that infliximab dependent response occurred significantly more in children without fistulas than in those with fistulizing disease.
Two genetic variants ( LTA c.207 A>G and CASP9 c.93 C>T) have been suggested as conceivable predictors of long-term and infliximab dependent outcome in a study of adult patients, 26 but needs to be confirmed.
4 5-ASA dependency
4.1 The development of the term 5-ASA dependency
The most recently described dependent pattern has been in therapy with 5-ASA preparations. 17 Five hundred thirty-seven CD patients treated at one centre in Denmark from 1953 to 2007 were retrospectively assessed for the use of 5-ASA preparations. One hundred sixty-five (31%) of them had monotherapy with 5-ASA and were evaluated according to the phenotype model of 5-ASA dependency outlined in Table 4 .
4.2 How many patients became 5-ASA dependent?
Out of 165 CD patients included, 36% obtained prolonged response and 23% developed 5-ASA dependency. 10
4.3 What does 5-ASA dependency imply in term of surgery?
Patients responding to 5-ASA in a dependent way had a lower cumulative probability of surgery during the disease course compared with non-responders (32% vs. 61% at ten years after diagnosis, p = 0.02). 10
4.4 Are there any predictors of 5-ASA dependency?
Female gender was identified as a predictor of prolonged response and 5-ASA dependency. Sixty-eight percent of women achieved prolonged response or developed 5-ASA dependency compared to 51% of men (OR 2.89, 95%CI: 1.08–7.75). Interestingly, patients with longer disease duration (> 3 years) were more likely to become 5-ASA dependent than those with shorter disease course (38% and 18%; OR 4.06, 95%CI: 1.09–15.1). 10
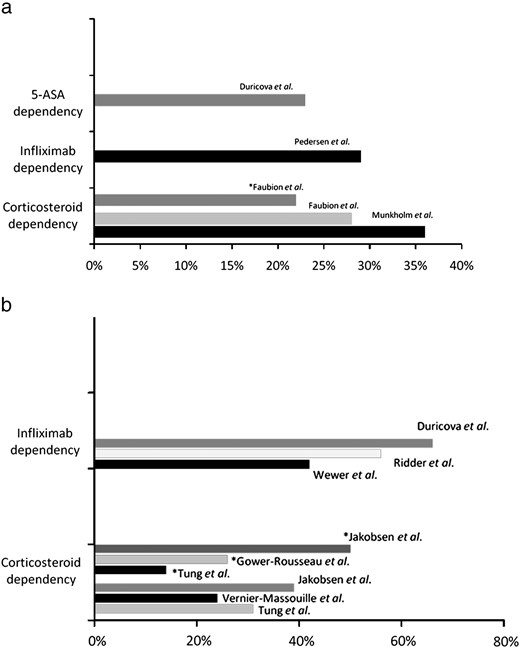
Fig. 3 summarizes the occurrence of drug dependencies in therapy of corticosteroids, infliximab and 5-ASA preparations.
5 Discussion
Up to now, clinical, genetic and serological markers have been of a little help when trying to predict patient's disease course and deciding for long-term treatment management 27 - 29 and a good clinical predictor in daily clinical practice is still lacking.
Drug dependency is dealing with a specific disease phenotype, which to some extent determines patients' prognosis. Hence it should not be understood as a simple description of drug response only, but also as a clinical tool, which can be used for identification of patients' phenotype with a certain disease course and a need for maintenance treatment.
To assess an impact of drug dependency on disease prognosis, one has to consider the efficacy and safety profile of the drug. Corticosteroids are highly efficacious anti-inflammatory agents as the number needed to treat for the induction of remission is three. 30 Nevertheless, corticosteroid dependency is a condition connected with many side effects, mainly growth retardation in children and furthermore associated with a high risk of surgery as obvious from above mentioned studies. 5 , 11 Therefore, early identification of dependent patients, carrying a poor prognosis, is important to introduce early immunomodulator therapy. This has been suggested by results of a recent paediatric population-based study from Denmark17. Despite the high frequency of corticosteroid dependency in this cohort, the cumulative probability of surgery was low for both CD and UC. The frequent use of immunosuppressive preparations among the children (68% of CD and 64% of UC children) could probably explain this favourable outcome. Similar results were found in two multicenter studies; only 8% of children with CD and 5% with UC had a surgery one year after starting steroids; nevertheless 81% and 61% of CD and UC patients received immunomodulators and 28% and 12%, respectively, infliximab within the observed year. 20 , 21
Drug dependency, however, does not need to be only a “negative” response phenotype as apparent from infliximab dependency papers. 9 , 26 The strong supportive evidence has been provided mainly by the findings regarding surgical intervention. One paediatric 9 and one adult 26 study have shown a significantly lower cumulative probability of surgery up to 50 months after treatment start in prolonged responders as well as infliximab dependent patients compared to non-responders. Of note is that infliximab dependent children had a lower risk of undergoing surgery than children with prolonged response. This suggests that in patients responding in a dependent way the need for surgical intervention could be at least postponed, if not avoided. Thus, development of infliximab and corticosteroid dependency might be considered as a useful prognostic marker, which may indicate whether patient will benefit from maintenance therapy (infliximab dependency) or should be shifted to another immunosuppressive or biological treatment (corticosteroid dependency).
So far, the safety profile of infliximab seems to be favourable as outlined by national cohorts 31 , 32 and recently also confirmed by meta-analysis 33 . However, potential adverse events can be very serious and life-threatening 34 - 37 and in recent period, there is an increasing evidence of probably immunopathological adverse events induced by anti-TNF alpha therapy such as various skin eruptions and joint problems. 38 , 39 Therefore, the safety profile is always the matter of concern. Identification of dependent and non-dependent individuals might prevent “unnecessary” use of the drug in non-dependent patients and so limit potential toxicity in this group. Moreover, directing the therapy mainly to dependent individuals could increase overall treatment efficacy and lead to more effective allocation of resources. This speculation might be supported by a recent prospective withdrawal study with infliximab. 40 Infliximab has been discontinued in 115 patients with luminal CD who had been treated with combined infliximab and immunosuppressive therapy for at least one year and obtained a stable corticosteroid-free remission. During the following 12 months 45 patients relapsed while more than half of the patients maintained long-standing remission on immunosuppressors without further need of infliximab treatment. Of note is that re-introduction of infliximab in those relapsing induced a quick remission in all but one patient and was well tolerated. 40
Higher occurrence of infliximab dependency has been observed in children compared to adults, 7 – 9 , 26 whereas no such difference has been seen in case of corticosteroids. Based on previously published data, childhood-onset IBD seems to be a different entity than adult-onset disease, characterized by a more extensive disease and severe phenotype. 18 , 41 , 42 Moreover, higher remission rates have been obtained in studies evaluating efficacy of infliximab in children compared with adults. 24 , 43 Biologicals are nowadays considered the most potent therapy, reserved mainly for patients refractory to conventional treatment including corticosteroids. 44 It can be only speculated whether the observed differences between paediatrics and adults are a consequence of the probably different natural courses of the diseases in these two groups. New studies should confirm or disprove these findings.
The most recent description of dependent response has been in 5-ASA treatment of CD patients. 10 Although the efficacy of 5-ASA in CD is low as compared to above mentioned drugs, 30 the findings have demonstrated that there is a certain, although small population of CD patients – 5-ASA dependent – which benefit from 5-ASA. Advantage of 5-ASA therapy is a high safety profile which is comparable to placebo. 45 , 46 Thus, in case the prescription of 5-ASA was stopped in CD patients; this group would have to be exposed to “stronger” but also more toxic preparations. Therefore, the ongoing use of 5-ASA in CD would be of great benefit.
The future development of dependency definitions should aim at more than 90% of patients would fit into the model, thus leading to the coefficient of variations of < 10%. In practice, a pilot study including small group of patients (about 20 individuals) is performed to evaluate if definitions are generally applicable to patients' disease pattern. The model is then adjusted until the desired goal is reached.
There are several factors having an impact on the rate of drug dependencies, causing the difficulties when trying to compare the outcomes of individual studies. Firstly, there is heterogeneity of the definitions used. An attempt to overcome this problem was made by implementing the unified definition for corticosteroid dependency in the ECCO guidelines. 22 , 23 Nevertheless, the use of the definitions in daily clinical practice is still problematic. Secondly, the recommended treatment policy has been changing over the time. Immunosuppressives are nowadays introduced earlier, mainly in paediatric population, to avoid corticosteroid dependency. 47 , 48 Infliximab is recommended as a long-term maintenance treatment in all patients responding to induction infusions; 47 , 48 hence the frequency of infliximab dependency may be biased. Further, the doctor preference and economical possibilities have to be also considered. Therefore, it is important that as the daily clinical practice is altered over the time; the definitions are also adjusted according to the actual requirements. This might be already observed in the definition of corticosteroid dependency proposed by Jakobsen et al. 17 which has taken in account the use of immunosuppressive or biological drugs in dependent patients.
In the future, there is a need to assess the occurrence of infliximab and 5-ASA dependency also in UC. Furthermore, in addition to already described dependent phenotypes, possible dependencies on other drugs used in IBD, like azathioprine and adalimumab, should be studied and mainly their prognostic value should be assessed.
6 Conclusion
Drug dependency is a specific disease phenotype which may determine patients' disease course and therefore can be used as a prognostic marker when deciding for treatment management. Whereas corticosteroid dependency is considered to be harmful condition, infliximab and 5-ASA dependency seem to be beneficial. In daily clinic, early identification of dependent patients might prevent serious consequences of corticosteroid therapy as well as probably improve patients' disease course by maintaining infliximab treatment.
Disclosure of financial conflict of interest
Dr. Munkholm serves as an advisory board member for Ferring, Tillotts and Shire.
Dr. Elkjaer serves as an advisory board member for Orphan.
Dr. Lukas serves as a consultant for MSD-Schering Plough, Abbott Laboratories and Takeda.
Dr. Duricova and Dr. Pedersen have obtained travel grants for poster presentation from Ferring and Centocor.
Dr. Wewer serves as an advisory board member for MSD.
Statement of authorship
“DD” conceived and drafted the manuscript.
“NP” helped to draft the manuscript, provided a significant advice.
“ML” helped to draft the manuscript, provided a significant advice.
“CJ” helped to draft the manuscript, provided a significant advice.
“ME” helped to draft the manuscript, provided a significant advice.
“ML” helped to draft the manuscript, provided a significant advice.
“VW” helped to draft the manuscript, provided a significant advice.
“PM” conceived the manuscript, helped to draft the manuscript, provided a significant advice.
References
Figures
The phenotype model of infliximab (IFX) dependency.
Footnote:
* corticosteroids, azathioprine or mercaptopurine or methotrexate, other biologicals.
** intestinal (resection, strictureplasty, and colectomy) and/or perianal (fistulotomy, incision of abscess, and advancement flap).
Drug dependency in adults (a) and children (b) with inflammatory bowel disease — summary of the studies.
5-ASA = 5-Aminosalicylic acid, * ulcerative colitis.
Tables
Definitions of corticosteroid dependency.
| Author | Definition |
| Munkholm et al. 5 (1994) | Relapse within 30 days after treatment had finished or relapse at dose reduction impeding discontinuation of prednisolone treatment for more than one year. |
| Faubion et al. 11 (2001) | Continued corticosteroid (CS) therapy at year-end caused by relapse after CSs were discontinued or caused by relapse at dose reduction impeding discontinuation of CS therapy. |
| Tung et al. 6 (2006) | Relapse within 30 days of treatment cessation or relapse when dose reduction was attempted. |
| ECCO guidelines on Crohn's disease 22 (2006) | Relapse when the steroid dose is reduced below 20 mg/day, or within 6 weeks of stopping steroids. |
| ECCO guidelines on ulcerative colitis 23 (2008) | Inability to reduce steroid below the equivalent of prednisolone 10 mg/day within 3 months of starting steroids, without recurrent active disease or relapse within 3 months of stopping steroids. |
| Jakobsen et al. 17 (2010) | Relapse within 30 days after steroid was stopped or the need for azathioprine or 6-mercaptopurine, methotrexate or anti-TNF to end steroid treatment due to relapse during tapering of steroids. |
| Author | Definition |
| Munkholm et al. 5 (1994) | Relapse within 30 days after treatment had finished or relapse at dose reduction impeding discontinuation of prednisolone treatment for more than one year. |
| Faubion et al. 11 (2001) | Continued corticosteroid (CS) therapy at year-end caused by relapse after CSs were discontinued or caused by relapse at dose reduction impeding discontinuation of CS therapy. |
| Tung et al. 6 (2006) | Relapse within 30 days of treatment cessation or relapse when dose reduction was attempted. |
| ECCO guidelines on Crohn's disease 22 (2006) | Relapse when the steroid dose is reduced below 20 mg/day, or within 6 weeks of stopping steroids. |
| ECCO guidelines on ulcerative colitis 23 (2008) | Inability to reduce steroid below the equivalent of prednisolone 10 mg/day within 3 months of starting steroids, without recurrent active disease or relapse within 3 months of stopping steroids. |
| Jakobsen et al. 17 (2010) | Relapse within 30 days after steroid was stopped or the need for azathioprine or 6-mercaptopurine, methotrexate or anti-TNF to end steroid treatment due to relapse during tapering of steroids. |
Definitions of corticosteroid dependency.
| Author | Definition |
| Munkholm et al. 5 (1994) | Relapse within 30 days after treatment had finished or relapse at dose reduction impeding discontinuation of prednisolone treatment for more than one year. |
| Faubion et al. 11 (2001) | Continued corticosteroid (CS) therapy at year-end caused by relapse after CSs were discontinued or caused by relapse at dose reduction impeding discontinuation of CS therapy. |
| Tung et al. 6 (2006) | Relapse within 30 days of treatment cessation or relapse when dose reduction was attempted. |
| ECCO guidelines on Crohn's disease 22 (2006) | Relapse when the steroid dose is reduced below 20 mg/day, or within 6 weeks of stopping steroids. |
| ECCO guidelines on ulcerative colitis 23 (2008) | Inability to reduce steroid below the equivalent of prednisolone 10 mg/day within 3 months of starting steroids, without recurrent active disease or relapse within 3 months of stopping steroids. |
| Jakobsen et al. 17 (2010) | Relapse within 30 days after steroid was stopped or the need for azathioprine or 6-mercaptopurine, methotrexate or anti-TNF to end steroid treatment due to relapse during tapering of steroids. |
| Author | Definition |
| Munkholm et al. 5 (1994) | Relapse within 30 days after treatment had finished or relapse at dose reduction impeding discontinuation of prednisolone treatment for more than one year. |
| Faubion et al. 11 (2001) | Continued corticosteroid (CS) therapy at year-end caused by relapse after CSs were discontinued or caused by relapse at dose reduction impeding discontinuation of CS therapy. |
| Tung et al. 6 (2006) | Relapse within 30 days of treatment cessation or relapse when dose reduction was attempted. |
| ECCO guidelines on Crohn's disease 22 (2006) | Relapse when the steroid dose is reduced below 20 mg/day, or within 6 weeks of stopping steroids. |
| ECCO guidelines on ulcerative colitis 23 (2008) | Inability to reduce steroid below the equivalent of prednisolone 10 mg/day within 3 months of starting steroids, without recurrent active disease or relapse within 3 months of stopping steroids. |
| Jakobsen et al. 17 (2010) | Relapse within 30 days after steroid was stopped or the need for azathioprine or 6-mercaptopurine, methotrexate or anti-TNF to end steroid treatment due to relapse during tapering of steroids. |
Frequency of corticosteroid dependency in population-based studies.
| Author | Crohn's disease | Ulcerative colitis | ||||
| n | Prolonged response (%) | Corticosteroid dependency (%) | n | Prolonged response (%) | Corticosteroid dependency (%) | |
| Adult study | ||||||
| Munkholm et al. 5 | 109 | 48 (44) | 39 (36) | – | – | – |
| Faubion et al. 11 | 74 | 24 (32) | 21 (28) | 63 | 31 (49) | 14 (22) |
| Paediatric study | ||||||
| Tung et al. 6 | 26 | 11 (42) | 8 (31) | 14 | 8 (57) | 2 (14) |
| Vernier-Massouille et al. 18 | 343 | 243 (71) | 83 (24) | – | – | – |
| Gower-Rousseau et al. 19 | – | – | – | 77 | 47 (61) | 20 (26) |
| Jakobsen et al. 17 | 82 | 50 (61) | 32 (39) | 77 | 38 (50) | 38 (50) |
| Author | Crohn's disease | Ulcerative colitis | ||||
| n | Prolonged response (%) | Corticosteroid dependency (%) | n | Prolonged response (%) | Corticosteroid dependency (%) | |
| Adult study | ||||||
| Munkholm et al. 5 | 109 | 48 (44) | 39 (36) | – | – | – |
| Faubion et al. 11 | 74 | 24 (32) | 21 (28) | 63 | 31 (49) | 14 (22) |
| Paediatric study | ||||||
| Tung et al. 6 | 26 | 11 (42) | 8 (31) | 14 | 8 (57) | 2 (14) |
| Vernier-Massouille et al. 18 | 343 | 243 (71) | 83 (24) | – | – | – |
| Gower-Rousseau et al. 19 | – | – | – | 77 | 47 (61) | 20 (26) |
| Jakobsen et al. 17 | 82 | 50 (61) | 32 (39) | 77 | 38 (50) | 38 (50) |
n = number of patients treated with corticosteroids.
Frequency of corticosteroid dependency in population-based studies.
| Author | Crohn's disease | Ulcerative colitis | ||||
| n | Prolonged response (%) | Corticosteroid dependency (%) | n | Prolonged response (%) | Corticosteroid dependency (%) | |
| Adult study | ||||||
| Munkholm et al. 5 | 109 | 48 (44) | 39 (36) | – | – | – |
| Faubion et al. 11 | 74 | 24 (32) | 21 (28) | 63 | 31 (49) | 14 (22) |
| Paediatric study | ||||||
| Tung et al. 6 | 26 | 11 (42) | 8 (31) | 14 | 8 (57) | 2 (14) |
| Vernier-Massouille et al. 18 | 343 | 243 (71) | 83 (24) | – | – | – |
| Gower-Rousseau et al. 19 | – | – | – | 77 | 47 (61) | 20 (26) |
| Jakobsen et al. 17 | 82 | 50 (61) | 32 (39) | 77 | 38 (50) | 38 (50) |
| Author | Crohn's disease | Ulcerative colitis | ||||
| n | Prolonged response (%) | Corticosteroid dependency (%) | n | Prolonged response (%) | Corticosteroid dependency (%) | |
| Adult study | ||||||
| Munkholm et al. 5 | 109 | 48 (44) | 39 (36) | – | – | – |
| Faubion et al. 11 | 74 | 24 (32) | 21 (28) | 63 | 31 (49) | 14 (22) |
| Paediatric study | ||||||
| Tung et al. 6 | 26 | 11 (42) | 8 (31) | 14 | 8 (57) | 2 (14) |
| Vernier-Massouille et al. 18 | 343 | 243 (71) | 83 (24) | – | – | – |
| Gower-Rousseau et al. 19 | – | – | – | 77 | 47 (61) | 20 (26) |
| Jakobsen et al. 17 | 82 | 50 (61) | 32 (39) | 77 | 38 (50) | 38 (50) |
n = number of patients treated with corticosteroids.
Frequency of infliximab dependency in patients with Crohn's disease.
| Author | n | Prolonged response (%) | Infliximab dependency (%) |
| Wewer et al. 7 | 24 | 7 (29) | 10 (42) |
| Duricova et al. 9 | 82 | 18 (22) | 53 (66) |
| de Ridder et al. 8 | 66 | 15 (15) | 37 (56) |
| Pedersen et al. * ,26 | 245 | 114 (47) | 71 (29) |
| Author | n | Prolonged response (%) | Infliximab dependency (%) |
| Wewer et al. 7 | 24 | 7 (29) | 10 (42) |
| Duricova et al. 9 | 82 | 18 (22) | 53 (66) |
| de Ridder et al. 8 | 66 | 15 (15) | 37 (56) |
| Pedersen et al. * ,26 | 245 | 114 (47) | 71 (29) |
n = number of patients treated with infliximab.
* study including adult patients.
Frequency of infliximab dependency in patients with Crohn's disease.
| Author | n | Prolonged response (%) | Infliximab dependency (%) |
| Wewer et al. 7 | 24 | 7 (29) | 10 (42) |
| Duricova et al. 9 | 82 | 18 (22) | 53 (66) |
| de Ridder et al. 8 | 66 | 15 (15) | 37 (56) |
| Pedersen et al. * ,26 | 245 | 114 (47) | 71 (29) |
| Author | n | Prolonged response (%) | Infliximab dependency (%) |
| Wewer et al. 7 | 24 | 7 (29) | 10 (42) |
| Duricova et al. 9 | 82 | 18 (22) | 53 (66) |
| de Ridder et al. 8 | 66 | 15 (15) | 37 (56) |
| Pedersen et al. * ,26 | 245 | 114 (47) | 71 (29) |
n = number of patients treated with infliximab.
* study including adult patients.
Phenotype model of 5-Aminosalicylic acid (5-ASA) dependency.
| Immediate outcome | Long-term outcome |
| Complete response Total regression of symptoms 30 days after 5-ASA initiation. | Prolonged response Still in complete/partial response 1 year after induction of response (maintained on 5-ASA or after cessation of 5-ASA). |
| Partial response Improvement of symptoms 30 days after 5-ASA initiation. | 5-ASA dependency Relapse ≤ 1 year after 5-ASA cessation regaining complete/partial response after 5-ASA re-introduction or relapse on stable or reduced dose of 5-ASA requiring dose escalation to regain response. |
| No response No regression of symptoms ≤ 30 days with a need to shift from 5-ASA to an immunomodulator or surgery. | No response No regression of symptoms with a need to shift from 5-ASA to an immunomodulator or surgery. |
| Immediate outcome | Long-term outcome |
| Complete response Total regression of symptoms 30 days after 5-ASA initiation. | Prolonged response Still in complete/partial response 1 year after induction of response (maintained on 5-ASA or after cessation of 5-ASA). |
| Partial response Improvement of symptoms 30 days after 5-ASA initiation. | 5-ASA dependency Relapse ≤ 1 year after 5-ASA cessation regaining complete/partial response after 5-ASA re-introduction or relapse on stable or reduced dose of 5-ASA requiring dose escalation to regain response. |
| No response No regression of symptoms ≤ 30 days with a need to shift from 5-ASA to an immunomodulator or surgery. | No response No regression of symptoms with a need to shift from 5-ASA to an immunomodulator or surgery. |
Phenotype model of 5-Aminosalicylic acid (5-ASA) dependency.
| Immediate outcome | Long-term outcome |
| Complete response Total regression of symptoms 30 days after 5-ASA initiation. | Prolonged response Still in complete/partial response 1 year after induction of response (maintained on 5-ASA or after cessation of 5-ASA). |
| Partial response Improvement of symptoms 30 days after 5-ASA initiation. | 5-ASA dependency Relapse ≤ 1 year after 5-ASA cessation regaining complete/partial response after 5-ASA re-introduction or relapse on stable or reduced dose of 5-ASA requiring dose escalation to regain response. |
| No response No regression of symptoms ≤ 30 days with a need to shift from 5-ASA to an immunomodulator or surgery. | No response No regression of symptoms with a need to shift from 5-ASA to an immunomodulator or surgery. |
| Immediate outcome | Long-term outcome |
| Complete response Total regression of symptoms 30 days after 5-ASA initiation. | Prolonged response Still in complete/partial response 1 year after induction of response (maintained on 5-ASA or after cessation of 5-ASA). |
| Partial response Improvement of symptoms 30 days after 5-ASA initiation. | 5-ASA dependency Relapse ≤ 1 year after 5-ASA cessation regaining complete/partial response after 5-ASA re-introduction or relapse on stable or reduced dose of 5-ASA requiring dose escalation to regain response. |
| No response No regression of symptoms ≤ 30 days with a need to shift from 5-ASA to an immunomodulator or surgery. | No response No regression of symptoms with a need to shift from 5-ASA to an immunomodulator or surgery. |