-
PDF
- Split View
-
Views
-
Cite
Cite
Peter J Snyder, Shalender Bhasin, Glenn R Cunningham, Alvin M Matsumoto, Alisa J Stephens-Shields, Jane A Cauley, Thomas M Gill, Elizabeth Barrett-Connor, Ronald S Swerdloff, Christina Wang, Kristine E Ensrud, Cora E Lewis, John T Farrar, David Cella, Raymond C Rosen, Marco Pahor, Jill P Crandall, Mark E Molitch, Susan M Resnick, Matthew Budoff, Emile R Mohler, Nanette K Wenger, Harvey Jay Cohen, Stanley Schrier, Tony M Keaveny, David Kopperdahl, David Lee, Denise Cifelli, Susan S Ellenberg, Lessons From the Testosterone Trials, Endocrine Reviews, Volume 39, Issue 3, June 2018, Pages 369–386, https://doi.org/10.1210/er.2017-00234
Close - Share Icon Share
Abstract
The Testosterone Trials (TTrials) were a coordinated set of seven placebo-controlled, double-blind trials in 788 men with a mean age of 72 years to determine the efficacy of increasing the testosterone levels of older men with low testosterone. Testosterone treatment increased the median testosterone level from unequivocally low at baseline to midnormal for young men after 3 months and maintained that level until month 12. In the Sexual Function Trial, testosterone increased sexual activity, sexual desire, and erectile function. In the Physical Function Trial, testosterone did not increase the distance walked in 6 minutes in men whose walk speed was slow; however, in all TTrial participants, testosterone did increase the distance walked. In the Vitality Trial, testosterone did not increase energy but slightly improved mood and depressive symptoms. In the Cognitive Function Trial, testosterone did not improve cognitive function. In the Anemia Trial, testosterone increased hemoglobin in both men who had anemia of a known cause and in men with unexplained anemia. In the Bone Trial, testosterone increased volumetric bone mineral density and the estimated strength of the spine and hip. In the Cardiovascular Trial, testosterone increased the coronary artery noncalcified plaque volume as assessed using computed tomographic angiography. Although testosterone was not associated with more cardiovascular or prostate adverse events than placebo, a trial of a much larger number of men for a much longer period would be necessary to determine whether testosterone increases cardiovascular or prostate risk.
Testosterone treatment of 1 year for older men with low testosterone improved all aspects of sexual function
Testosterone treatment of 1 year for older men with low testosterone improved walking distance by a small amount
Testosterone treatment of 1 year for older men with low testosterone did not improve vitality but slightly improved mood and depressive symptoms
Testosterone treatment of 1 year for older men with low testosterone improved hemoglobin and corrected mild to moderate anemia
Testosterone treatment of 1 year for older men with low testosterone markedly increased the volumetric bone mineral density and estimated bone strength
Testosterone treatment of 1 year for older men with low testosterone increased the coronary artery plaque volume
Testosterone treatment of 1 year for older men with low testosterone was not associated with more cardiovascular or prostate adverse events; however, the number of men and the duration of treatment were not sufficient to draw definitive conclusions about the risks of this treatment
Previous studies of the effect of testosterone treatment in older men who had low testosterone were based on the observations that as men age their serum testosterone levels decrease and on the parallels in men between the consequences of aging and those of frank hypogonadism. Both cross-sectional and longitudinal studies have demonstrated that as men age from the third to ninth decades, their testosterone levels decrease (1–3). The decrease is gradual, is modest compared with the decrease in estradiol with menopause in women, varies from man to man, and is associated with comorbid conditions (1, 4). The parallels between aging in men and frank hypogonadism due to known pituitary or testicular disease include decreased energy, decreased sexual function, decreased muscle mass and increased fat mass, decreased bone density and an increased incidence of fractures, and decreased hemoglobin. Furthermore, testosterone treatment of men with severe hypogonadism due to recognized pituitary or testicular disease or due to gonadotropin-releasing hormone analog treatment show marked increases in sexual function, energy, and muscle mass (although variably in strength), decrease in fat mass, and increases in bone mineral density and hemoglobin (5–7).
Previous Studies of the Effects of Testosterone in Older Men
The results of previous studies of the effects of testosterone in older men were tantalizing but equivocal. Some of the results were negative, possibly because the men selected had testosterone levels that were only low-normal and not unequivocally low or the testosterone treatment did not raise the serum testosterone sufficiently. Some of the results were positive, possibly because those trials used supraphysiological doses of testosterone. Others were positive, but the primary outcomes were surrogates and not, of themselves, of clinical importance. In one placebo-controlled trial of 108 older men, testosterone treatment for 3 years was not associated with an increase in bone mineral density or increased muscle strength; however, the participants had a mean pretreatment testosterone level that was only low-normal (8, 9). In another trial, testosterone treatment increased both muscle strength and bone mineral density, but the dose used was supraphysiological (10, 11). In two trials reported while the Testosterone Trials (TTrials) were in progress, testosterone treatment of moderately frail older men improved muscle strength but did not clearly or consistently improve physical performance (12, 13).
Institute of Medicine Report of 2003
In 2002, the National Institute on Aging (NIA) and the National Cancer Institute requested that the Institute of Medicine (IOM) (now the National Academy of Medicine) assess the status of clinical research on testosterone therapy for older men. In 2003, the IOM committee concluded that the evidence that testosterone treatment for older men with low testosterone was beneficial was insufficient and recommended that the NIA fund a coordinated set of clinical trials to determine whether this treatment has any benefits and to fund a larger trial to determine the possible risks only if benefits were found (14). The NIA followed this advice and supported the development of the TTrials to implement the IOM recommendation.
Development of TTrials—Overall Considerations
We developed TTrials during intensive discussions for 6 years based on our experience in the many scientific areas to be studied and clinical trial design and analysis under the guidance of the NIA. The eventual design and the rationale have been described previously (15). Our goal was to implement the recommendation of the IOM by selecting older men who had unequivocally low testosterone concentrations compared with young men and symptoms consistent with low testosterone levels, treating them with testosterone or placebo, and determining the efficacy in several postulated areas. We decided to include men aged ≥65 years who had unequivocally low testosterone levels compared with young men. The initial target was <250 ng/dL; however, when initial screening showed that this value was too stringent to accrue participants at an acceptable rate, we chose the mean of two morning values, <275 ng/dL. To allow the results to be widely applicable to older men with low testosterone, we included men with comorbid conditions, unless those conditions might have exposed the men to excessive risk. Thus, we excluded men with a history of prostate cancer and those whose risk, using the prostate cancer risk calculator (16), of any prostate cancer was >35% and that of high-grade prostate cancer was >7%. We also excluded men whose lower urinary tract symptoms were moderately severe, as judged by an International Prostate Symptom Score >19. We also excluded men with any cancer and those with severe cardiac, renal, or hepatic disease.
We designed a placebo-controlled, double-blind trial. The enrollees were allocated to treatment using the technique of minimization (17), which allows for balancing for a greater number of baseline variables than does randomization with stratification. The balancing variables included participation in the three main trials, trial site, screening testosterone level (≤200 or >200 ng/dL), age (≤75 or >75 years, use or nonuse of antidepressants, and use or nonuse of phosphodiesterase type 5 inhibitors. The testosterone preparation was AndroGel 1% (AbbVie, Chicago, IL). The placebo gel was similar in appearance, consistency, and aroma. The initial dose of AndroGel, 5 g daily, was adjusted, according to the serum testosterone concentrations, at months 1, 2, 3, 6, and 9, to keep the serum testosterone concentration within the normal range for young men. To maintain blinding when the dose was adjusted for a participant taking testosterone, the dose was also adjusted in a participant taking placebo.
To qualify for enrollment, a potential participant had to meet the general inclusion and exclusion criteria as stated and also specific entry criteria for at least one of the three main trials: Sexual Function, Physical Function, and Vitality. Those who qualified for one of these could participate in as many of the other two main trials and three other trials (Anemia, Bone, and Cardiovascular) for which he qualified. All participants were tested for cognitive function. The primary outcomes for the three main trials were chosen to be of clinical relevance. The sample sizes for these three trials were set such that each trial would have 90% power to detect a magnitude of difference deemed clinically meaningful for the primary outcome. The analyses were performed by the intention-to-treat approach for all men who had at least one postbaseline result. The treatment and assessments continued for 1 year.
Recruitment, Screening, and Enrollment
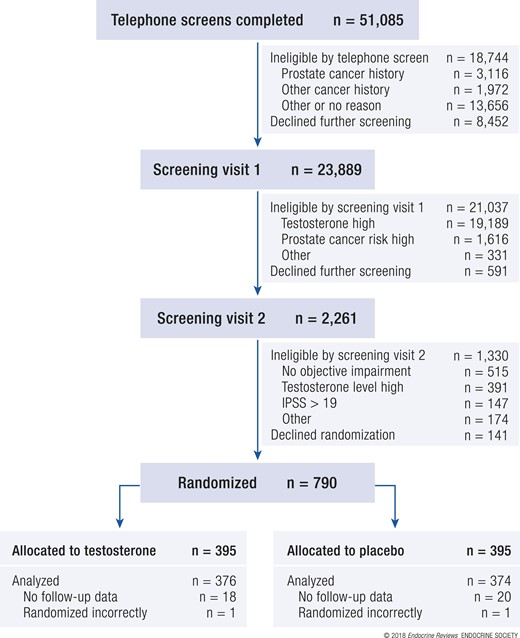
Potential participants were recruited at each of 12 US sites. Most sites recruited primarily by mass mailings to nearby postal codes; some sites used additional methods, including newspaper and radio advertisements (18). In response to the recruiting materials, 51,085 men called a trial site and were screened by telephone. The men who had no symptoms of low testosterone or had disqualifying medical conditions were excluded (Fig. 1). Of the initial responders, 23,889 qualified for and attended the first in-person screening visit, at which fasting blood samples were taken at 8 to 10 am for testosterone and prostate-specific antigen (PSA) levels. Approximately 10% of these men (2261) had a low enough testosterone (<275 ng/dL) level and low enough risk of prostate cancer (<35% risk of any prostate cancer and <7% risk of high-grade prostate cancer) to qualify for and attend the second in-person screening visit. At that visit, blood samples were again taken at 8 to 10 am for a second testosterone determination, and the participants were screened for entry into one of the three main trials. A total of 788 men qualified for and were enrolled in the TTrials (790 men were randomized, but 2 were randomized in error).
Modified CONSORT (Consolidated Standards of Reporting Trials) diagram of participants in TTrials showing the flow of men from initial screening through enrollment, treatment, and completion of the trial. IPSS, International Prostate Symptom Score.
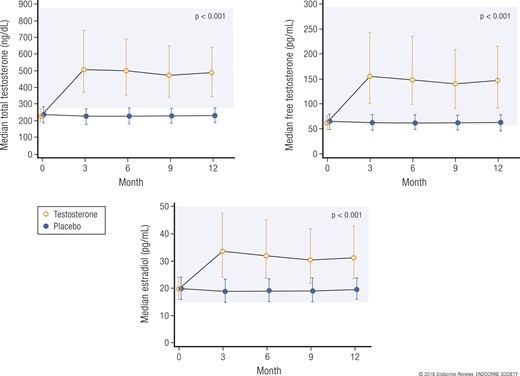
Some important characteristics at baseline of the 788 men enrolled are listed in Table 1. Their mean age was 72 years, and they were predominantly white and overweight or obese. Many had comorbid conditions, including hypertension and diabetes. The median baseline serum testosterone concentration was unequivocally low at 234 ng/dL (Fig. 2). The median baseline serum concentrations of free testosterone and estradiol were also low.
Characteristics of Men Enrolled in Testosterone Trials at Baseline
| Characteristics . | Treatment Group . | |
|---|---|---|
| Placebo . | Testosterone . | |
| Participants, n | 395 | 395 |
| Demographic data | ||
| Age, y | 72.3 ± 5.8 | 72.1 ± 5.7 |
| Race, n | ||
| White | 351 (88.9) | 349 (88.4) |
| Black | 20 (5.1) | 21 (5.3) |
| Other (%) | 24 (6.1) | 25 (6.3) |
| Concomitant conditions | ||
| BMI (kg/m2) | 31.0 ± 3.6 | 31.0 ± 3.5 |
| BMI >30, n (%) | 246 (62.3) | 251 (63.5) |
| Alcohol use, drinks/wk | 3.4 ± 5.0 | 3.0 ± 4.3 |
| Smoking | ||
| Current smoker, n | 34 (8.6) | 30 (7.6) |
| Ever smoker, n | 268 (67.9) | 256 (64.8) |
| Diabetes, n | 144 (36.5) | 148(37.5) |
| Hypertension, n | 280 (70.9) | 286 (72.4) |
| History of myocardial infarction, n | 63 (16.0) | 53 (13.4) |
| History of stroke, n | 17 (4.3) | 16 (4.1) |
| Sleep apnea, n | 76 (19.2) | 78 (19.8) |
| Characteristics . | Treatment Group . | |
|---|---|---|
| Placebo . | Testosterone . | |
| Participants, n | 395 | 395 |
| Demographic data | ||
| Age, y | 72.3 ± 5.8 | 72.1 ± 5.7 |
| Race, n | ||
| White | 351 (88.9) | 349 (88.4) |
| Black | 20 (5.1) | 21 (5.3) |
| Other (%) | 24 (6.1) | 25 (6.3) |
| Concomitant conditions | ||
| BMI (kg/m2) | 31.0 ± 3.6 | 31.0 ± 3.5 |
| BMI >30, n (%) | 246 (62.3) | 251 (63.5) |
| Alcohol use, drinks/wk | 3.4 ± 5.0 | 3.0 ± 4.3 |
| Smoking | ||
| Current smoker, n | 34 (8.6) | 30 (7.6) |
| Ever smoker, n | 268 (67.9) | 256 (64.8) |
| Diabetes, n | 144 (36.5) | 148(37.5) |
| Hypertension, n | 280 (70.9) | 286 (72.4) |
| History of myocardial infarction, n | 63 (16.0) | 53 (13.4) |
| History of stroke, n | 17 (4.3) | 16 (4.1) |
| Sleep apnea, n | 76 (19.2) | 78 (19.8) |
Abbreviation: BMI, body mass index.
Data in parentheses are percentages.
Characteristics of Men Enrolled in Testosterone Trials at Baseline
| Characteristics . | Treatment Group . | |
|---|---|---|
| Placebo . | Testosterone . | |
| Participants, n | 395 | 395 |
| Demographic data | ||
| Age, y | 72.3 ± 5.8 | 72.1 ± 5.7 |
| Race, n | ||
| White | 351 (88.9) | 349 (88.4) |
| Black | 20 (5.1) | 21 (5.3) |
| Other (%) | 24 (6.1) | 25 (6.3) |
| Concomitant conditions | ||
| BMI (kg/m2) | 31.0 ± 3.6 | 31.0 ± 3.5 |
| BMI >30, n (%) | 246 (62.3) | 251 (63.5) |
| Alcohol use, drinks/wk | 3.4 ± 5.0 | 3.0 ± 4.3 |
| Smoking | ||
| Current smoker, n | 34 (8.6) | 30 (7.6) |
| Ever smoker, n | 268 (67.9) | 256 (64.8) |
| Diabetes, n | 144 (36.5) | 148(37.5) |
| Hypertension, n | 280 (70.9) | 286 (72.4) |
| History of myocardial infarction, n | 63 (16.0) | 53 (13.4) |
| History of stroke, n | 17 (4.3) | 16 (4.1) |
| Sleep apnea, n | 76 (19.2) | 78 (19.8) |
| Characteristics . | Treatment Group . | |
|---|---|---|
| Placebo . | Testosterone . | |
| Participants, n | 395 | 395 |
| Demographic data | ||
| Age, y | 72.3 ± 5.8 | 72.1 ± 5.7 |
| Race, n | ||
| White | 351 (88.9) | 349 (88.4) |
| Black | 20 (5.1) | 21 (5.3) |
| Other (%) | 24 (6.1) | 25 (6.3) |
| Concomitant conditions | ||
| BMI (kg/m2) | 31.0 ± 3.6 | 31.0 ± 3.5 |
| BMI >30, n (%) | 246 (62.3) | 251 (63.5) |
| Alcohol use, drinks/wk | 3.4 ± 5.0 | 3.0 ± 4.3 |
| Smoking | ||
| Current smoker, n | 34 (8.6) | 30 (7.6) |
| Ever smoker, n | 268 (67.9) | 256 (64.8) |
| Diabetes, n | 144 (36.5) | 148(37.5) |
| Hypertension, n | 280 (70.9) | 286 (72.4) |
| History of myocardial infarction, n | 63 (16.0) | 53 (13.4) |
| History of stroke, n | 17 (4.3) | 16 (4.1) |
| Sleep apnea, n | 76 (19.2) | 78 (19.8) |
Abbreviation: BMI, body mass index.
Data in parentheses are percentages.
Serum concentrations of testosterone, free testosterone, and estradiol at baseline and during 12 months of treatment with testosterone or placebo. The shaded areas represent the ranges of normal for young men. Values presented as median ± interquartile range. Adapted, with permission, from Snyder et al. (19).
Lessons from screening
Although only 1.5% of men who expressed interest in the TTrials qualified and enrolled, all the TTrials met their enrollment targets, and the median testosterone concentration was unequivocally low, demonstrating that it is possible to recruit older men with low testosterone levels for a clinical trial. The major reason for exclusion was an insufficiently low testosterone level. Only 21.4% of the men had a sufficiently low value at the first screening visit, and only 68.9% of those whose values were low enough at the first screening visit had a low enough value at the second screening visit; thus, only 14.7% of men had sufficiently low values at both visits.
Overall Results During Treatment
Results
Of the 788 enrollees, 89% completed 12 months of treatment and evaluations for efficacy; larger numbers completed intermediate visits (19). Somewhat more men in the placebo group (n = 48) withdrew before month 12 compared with the men in the testosterone group (n = 35). The median serum testosterone concentration of the men treated with testosterone increased from unequivocally low at baseline to midnormal for young men by month 3 and remained at that level during the 12 months of treatment (Fig. 2). Of the 394 men in the testosterone arm, 301 required 504 adjustments of the dose at months 3, 6, and/or 9 to maintain the testosterone level within the target range. The serum concentrations of free testosterone, dihydrotestosterone, and estradiol also increased to midnormal for young men (Fig. 2). The levels of these hormones did not change in the men who used placebo gel. Of the 788 enrollees, 689 participated in more than one of the three main trials (18), and many also participated in one or more of the other trials.
Lessons from treatment
Using a testosterone preparation (gel) that was relatively easy to apply and adjusting the dose according to the serum testosterone levels at months 1, 2, 3, 6, and 9, the serum testosterone concentration could be maintained within the target range for the duration of treatment. By adjusting the dose simultaneously for a placebo-treated man as for a testosterone-treated man, blinding was also maintained.
Sexual Function Trial
Previous studies of the effect of testosterone on sexual function
Testosterone treatment has been shown to improve sexual function in young men who are severely hypogonadal owing to diseases of the pituitary or testes (5, 20) or because of administration of a gonadotropin-releasing hormone analog (6, 21). Testosterone treatment has also been shown to improve sexual function in middle-age men with mildly or moderately low testosterone levels (22). Previous studies of testosterone treatment of older men, however, did not show clear effects on sexual function, possibly owing to an insufficiently low testosterone level, too few participants, or the use of unvalidated instruments.
Specific aims of the Sexual Function Trial
The primary aim of the Sexual Function Trial was to test the hypothesis that testosterone treatment for older men with low testosterone would improve sexual activity as assessed by question 4 of the Psychosexual Daily Questionnaire (23). This question assesses 12 types of sexual activity, from flirting to intercourse (24). Previous studies had shown that sexual activity assessed by this instrument responded to testosterone. This questionnaire was administered by interactive voice response daily for 7 days at baseline and at 3, 6, 9, and 12 months. The scores for the 7 days at each time point were averaged. Question 4 of the Psychosexual Daily Questionnaire was administered to all men in the TTrials; however, the primary outcome was determined only for the men who were enrolled in the Sexual Function Trial. Libido, as assessed by the Derogatis Inventory of Sexual Function–Men–II (25), would have been the primary outcome measure; however, the effect of testosterone on libido using this instrument had not previously been tested. Libido, as assessed by the Derogatis Inventory of Sexual Function–Men–II, and erectile function, as assessed by the International Index of Erectile Function (25), were also evaluated, but only in the men enrolled in the Sexual Function Trial.
Results of the Sexual Function Trial
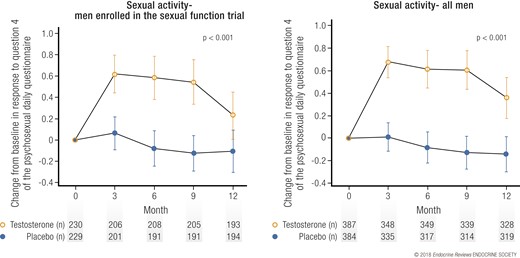
Testosterone treatment, compared with placebo, substantially increased sexual activity, averaged over all follow-up visits, for the men enrolled in this trial and also in all men in the TTrials (Fig. 3) (19). This effect was seen for most types of sexual activity, from flirting to sexual intercourse (24). The clinical significance of this effect can be judged from the effect size of 0.45 (19), which is close to a “moderate” effect of 0.5, and that testosterone increased sexual activity of all types about four times a week. Testosterone also substantially increased libido and, to a lesser degree, erectile function (19). The clinical significance of the effect of testosterone on libido can be judged by the responses to the Patient Global Impression of Change question, in which ~20% of men treated with testosterone reported that their sexual desire was “much better” than before treatment compared with <10% of men treated with placebo (19). Incremental increases in total and free testosterone and estradiol levels were substantially associated with greater improvements in sexual activity and libido but not erectile function (24).
Effect of testosterone on sexual activity. Change from baseline in sexual activity, as assessed by the Psychosexual Daily Questionnaire, question 4, in (left) men taking testosterone or placebo and enrolled in the Sexual Function Trial and (right) all men enrolled in the TTrials. Data presented as means and 95% confidence intervals.
Lessons from the Sexual Function Trial
Testosterone improved most aspects of sexual function in older men with low testosterone, with the effect proportional to the increase in testosterone. The greater effects on sexual activity and libido than on erectile function are consistent with the postulated effects of testosterone and what has been observed in severely hypogonadal men. These results are also consistent with another placebo-controlled study for 16 weeks of a different testosterone gel in 751 men with a mean age 55 years who had low testosterone levels (22).
Physical Function Trial
Previous studies of the effects of testosterone on physical function
Testosterone has long been recognized to stimulate the growth of muscles and increase muscle strength, resulting in greater muscle development during puberty in men than in women. Administration of testosterone to older men also increases muscle mass (9, 11, 13) and, in some studies, increased muscle strength (13). Previous studies of testosterone treatment for older men with mobility limitations, however, have not demonstrated consistent improvement in gait speed and other tests of physical function (12, 13).
Specific aims of the Physical Function Trial
We chose the distance walked in 6 minutes (26) as the primary outcome for the Physical Function Trial, rather than an outcome based on muscle strength, because of the importance of walking in maintaining independence as men age. We specifically postulated that testosterone, compared with placebo, would increase the fraction of men whose walking distance in 6 minutes increased >50 m beyond the baseline distance for the men who qualified for this trial because they had reported difficulty walking and/or climbing stairs and because their gait speed was <1.2 m/s. We took great care to standardize the administration of this test by using a set test course, timing the walk with a stop watch, and training the site personnel initially and retraining them annually. A secondary outcome measure was self-reported mobility and function as assessed using the physical function domain scale of the 36-item Medical Outcomes Short Form Survey (SF-36).
Results of the Physical Function Trial
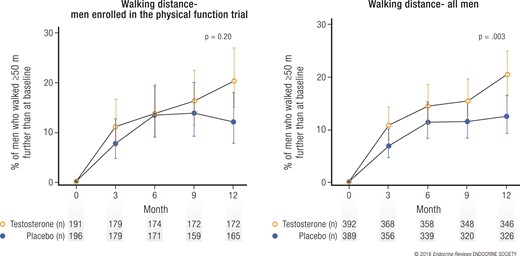
Testosterone, compared with placebo, did not substantially increase the fraction of men whose distance walked in 6 minutes increased >50 m beyond that at baseline or the absolute increase in the distance walked by the 387 men who qualified for this trial (the primary outcome), although the trend was toward greater increases for the men assigned to testosterone treatment (Fig. 4) (19). Testosterone, however, did substantially improve these two parameters when the data from all 788 men in the TTrials were analyzed. Also, the improvement was statistically significant for the men who had not qualified for the Physical Function Trial, demonstrating that this result was not simply a question of increased power. Importantly, both the men who qualified for the trial and all the men who were treated with testosterone perceived that their walking had improved more than did the men treated with placebo, using the physical function domain scale of the SF-36 and a patient global assessment of change question.
Effect of testosterone on walking distance. Graphs showing percentage of (left) men taking testosterone or placebo and enrolled in the Physical Function Trial and (right) all men enrolled in TTrials whose distance walked in 6 minutes increased by ≥50 m greater than the baseline. Data presented as means and 95% confidence intervals.
Lessons from the Physical Function Trial
Testosterone increased both the fraction of all TTrials participants whose distance walked increased >50 m and the absolute increase in distance walked in 6 minutes for all men enrolled in the TTrials and also led to the perception of improved walking. Thus, we have concluded that testosterone treatment for older men with low testosterone does improve walking, although to a small degree. We believe the best explanation for the lack of a statistically significant effect of testosterone on walking for men with self-reported and objectively measured slow walking was an overall small effect plus statistical variability.
Vitality Trial
Previous studies of the effects of testosterone on vitality
It has been a common observation of physicians that testosterone treatment for severely hypogonadal men dramatically improves their energy and initiative. Few previous trials of the effect of testosterone on the energy of older men with low testosterone, however, used validated questionnaires.
Specific aims of the Vitality Trial
We chose the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale as the primary outcome measure of the Vitality Trial (27). This questionnaire distinguishes between energy at one end of the spectrum and fatigue at the other and has been validated for assessing energy vs fatigue in many different diseases. We enrolled men in the Vitality Trial who said that their energy had diminished and who had scored below the midpoint of the FACIT-Fatigue scale.
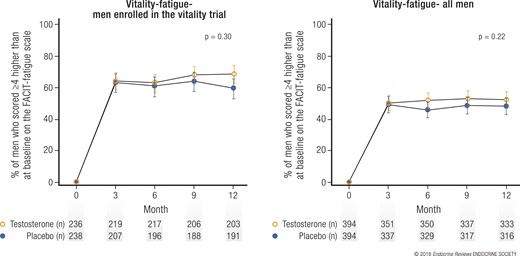
Results of the Vitality Trial
Testosterone, compared with placebo, did not substantially increase vitality, as determined by an increase of ≥4 points on the FACIT-Fatigue scale for the 474 men enrolled in this trial (the primary outcome) (Fig. 5). However, the effect of testosterone on this outcome as a continuous measure was of borderline significance for the men enrolled in the trial and was statistically significant for all 788 TTrials men. Moreover, the effect of testosterone on vitality (determined using the SF-36 vitality subscale), mood (determined using the positive and negative affect scales), and depressive symptoms (determined using the Patient Health Questionnaire-9) was statistically significant. The magnitude of each of these effects, however, was small.
Effect of testosterone on vitality and fatigue. Graphs showing percentage of (left) men taking testosterone or placebo and enrolled in the Vitality Trial and (right) all men enrolled in TTrials whose score on the FACIT-fatigue scale increased by ≥4 points greater than baseline. Data presented as means and 95% confidence intervals.
Lessons from the Vitality Trial
Although testosterone did not improve vitality as assessed by an increase greater than the prespecified threshold value, it did improve vitality, mood, and depressive symptoms as continuous measures using several instruments. The magnitude of each of the improvements, however, was small, perhaps related to the modest degree to which testosterone was low in the participants.
Cognitive Function Trial
Previous studies of the effects of testosterone on cognitive function
Low testosterone levels were associated with cognitive impairment in two epidemiologic studies (28, 29). Also, in a small trial, testosterone improved spatial and verbal memory in healthy older men (30).
Specific aims of the Cognitive Function Trial
The goal of the Cognitive Function Trial was to determine whether testosterone treatment of older men with age-associated memory impairment (AAMI) (31) would improve any aspect of cognitive function. As the primary outcome, we chose delayed paragraph recall, as determined by the Wechsler Memory Scale, Revised, Logical Memory II (32), because of the clinical importance of verbal memory, which declines with age and more rapidly before dementia. To avoid the high cost of screening for AAMI, we administered all the cognitive function tests to all TTrials participants. We then included men considered to have AAMI if they had both subjective memory complaints assessed by their score on the Memory Assessment Clinics Questionnaire and objective memory impairment according to the delayed paragraph recall or visual memory scores (33–35).
Results of the Cognitive Function Trial
For the 493 men with AAMI, testosterone treatment, compared with placebo, did not improve delayed paragraph recall, nor did it improve visual memory, spatial ability, executive function, subjective memory complaints, global cognitive function, or immediate paragraph recall. For all 788 TTrials men, testosterone marginally improved executive function but did not improve any of the other measures of cognitive function.
Lessons from the Cognitive Function Trial
We administered tests that covered a wide range of cognitive functions to all men in the TTrials. However, we found that testosterone did not improve performance for any of them in the men with AAMI nor for virtually all of them in all men in the TTrials. Thus, we have concluded that testosterone treatment for older men with low testosterone does not improve cognitive function.
Anemia Trial
Previous studies of the effects of testosterone on erythropoiesis
Testosterone has long been known to stimulate erythropoiesis, which explains why normal men have higher hemoglobin levels than normal women. Before the availability of erythropoietin, testosterone was used to treat anemia. In men who were severely hypogonadal because of pituitary or testicular disease, testosterone replacement increased hemoglobin levels (5). Also, in previous trials of older men, testosterone treatment increased hemoglobin levels overall (8, 36).
Specific aims of the Anemia Trial
The goal of the Anemia Trial was to determine whether testosterone treatment for older men with low testosterone compared with young men and unexplained mild anemia (those with a hemoglobin <10.0 g/dL were excluded) would increase the hemoglobin by ≥1.0 g/dL and correct the anemia.
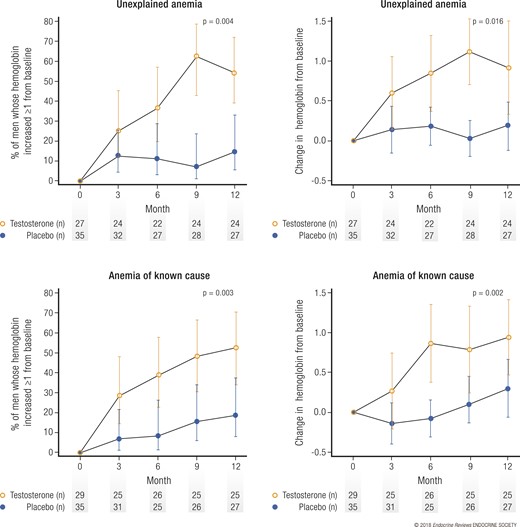
Results of the Anemia Trial
Of the 788 men enrolled in the TTrials, 126 were anemic (hemoglobin <12.7 g/dL) at baseline (37). Of these, 64 were found to have a known cause of the anemia, such as iron, B12, or folate deficiencies or inflammation. The other 62 were considered to have unexplained anemia of aging. In the men with unexplained anemia, testosterone treatment, compared with placebo, substantially increased the hemoglobin concentration by ≥1.0 g/dL (54% vs 15% of men) and corrected the anemia (58.3% vs 22.2% of men; Fig. 6). In the men with anemia of known cause, testosterone also substantially increased the hemoglobin concentration by ≥1 g/dL (52% vs 19%) and corrected the anemia (60% vs 14.8%; Fig. 6). The magnitude of the effect was modest, with a mean increase in hemoglobin to greater than baseline of 0.8 to 1.1 mg/dL at months 6 to 12. This increase might be of clinical significance, because the increase was positively and substantially associated with the patient global impression of change in general health and vitality in these anemic men.
Effect of testosterone on anemia. Men (top) who were anemic at baseline for no known reason (unexplained) or (bottom) who had a known cause were treated with testosterone or placebo. (Left) The percentage of men who experienced an increase in hemoglobin of ≥1.0 g/dL. (Right) Absolute increases in hemoglobin. Data presented as means ± pointwise confidence intervals. Adapted, with permission, from Roy et al. (37).
Lessons from the Anemia Trial
Because testosterone has long been known to stimulate erythropoiesis, it was not surprising that testosterone treatment for older men with low testosterone and a mild degree of anemia (mean hemoglobin concentration of 12.0 g/dL) improved their hemoglobin and corrected their anemia. This effect occurred whether the men had, in addition to testosterone deficiency, another known cause of anemia, such as iron deficiency. This effect shows a clear benefit of testosterone treatment for elderly men with low testosterone and low hemoglobin concentrations.
Bone Trial
Previous studies of the effect of testosterone on bone
Previous studies of the effect of testosterone on bone in men who were severely hypogonadal showed marked increases in areal bone mineral density (aBMD) by dual energy x-ray absorptiometry (5, 38), volumetric bone mineral density (vBMD) by quantitative computed tomography (QCT) (39), and trabecular architecture and estimated bone strength using magnetic resonance imaging (40, 41). Previous studies of the effect of testosterone on bone in older men who had mildly low to low-normal testosterone levels gave mixed results. One study showed no substantial effect of testosterone on aBMD overall (8); however, the men in that study had mean baseline testosterone levels that were low-normal and not frankly low. Another study reported a substantial effect of testosterone on aBMD (10); however, the testosterone doses used were supraphysiological.
Specific aims of the Bone Trial
The specific aim of the Bone Trial was to determine whether testosterone treatment of older men with low testosterone would increase vBMD as assessed using QCT. We chose this outcome because previous studies had shown that testosterone stimulates trabecular bone more than cortical bone (39). Also, QCT, not only can distinguish trabecular from cortical bone, but also is not subject to the artifacts of osteophytes and aortic calcification, as is aBMD using dual energy x-ray absorptiometry. An additional advantage is that bone strength can be estimated from QCT data using finite element analysis. Osteoporosis was not an entry criterion.
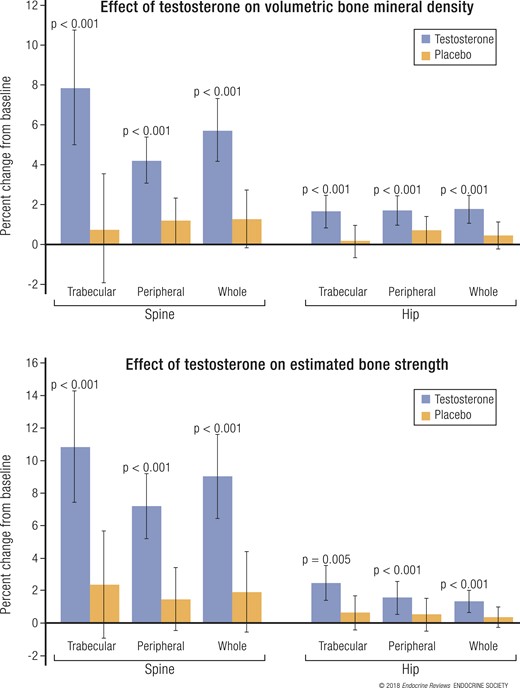
Results of the Bone Trial
In the 211 men in the Bone Trial, testosterone treatment for 1 year increased vBMD of trabecular bone in the spine by 6.8% more than did placebo (P < 0.001) and increased the estimated bone strength of trabecular bone in the spine by 8.5% more than did placebo (P ≤ 0.001; Fig. 7) (42). Testosterone also substantially increased whole bone vBMD, the strength of the spine and trabecular and whole bone vBMD, and the strength of the hip. Increases in aBMD were smaller.
Change from baseline to 12 months in (top) vBMD and (bottom) estimated bone strength, as determined by QCT in trabecular, peripheral, or whole bone of the spine or hip in 211 men treated with testosterone or placebo. Data presented as mean ± standard deviation. Reproduced, with permission, from Snyder et al. (42).
Lessons from the Bone Trial
These striking improvements in vBMD and estimated bone strength are consistent with the effects of testosterone in more severely hypogonadal men and were especially impressive for only 1 year of treatment. These results are also impressive considering that they are at least as great in magnitude to the effects of bisphosphonates on vBMD in women with osteoporosis (43, 44), although these men did not have low aBMD. The effect of testosterone on aBMD was less than that of alendronate in men with osteoporosis (45, 46). These results give impetus to a much larger and longer trial to determine whether testosterone also reduces fracture risk in older men with low testosterone.
Cardiovascular Trial
Previous studies of the effects of testosterone on cardiovascular outcomes
Previous studies of the effects of testosterone on clinical cardiovascular outcomes have given conflicting results. One clinical trial of testosterone in older men with low-normal testosterone levels and mobility limitations was stopped early because of a preponderance of adverse cardiovascular events in the testosterone arm (47). However, another trial of a similar population reported few adverse cardiovascular events (12). Yet another trial showed no difference in the progression of carotid artery intima-media thickness or coronary artery calcification in men with low to low-normal testosterone levels treated with testosterone compared with men treated with placebo for 3 years (48). Meta-analyses of previous clinical trials have not overall shown more adverse events in testosterone-treated than in placebo-treated men (49). None of these previous clinical trials, however, was designed to assess clinical cardiovascular events prospectively or to adjudicate those events. Also, the reliability of the meta-analyses was poor owing to variable entry criteria and testosterone regimens.
Similarly, some retrospective analyses of electronic medical records have reported associations between the use of testosterone and adverse cardiovascular adverse events (50, 51) but others have not (52–54). These studies, by their nature, were uncontrolled for diagnostic criteria, treatment, and monitoring.
The possibility that testosterone treatment increases the risk of venous thromboembolic disease was raised by a case-control study that showed an increased risk during the first 6 months of treatment but not thereafter (55). The Food and Drug Administration, acting on the basis of postmarketing reports, required manufacturers of testosterone products to include in their labeling a warning that testosterone might increase the risk of venous thromboembolic disease unrelated to erythrocytosis.
Specific aims of the Cardiovascular Trial
Knowing that the number of participants in the TTrials would not be nearly sufficient to determine clinical cardiovascular risk, we decided to test the hypothesis that testosterone would improve a surrogate outcome, noncalcified coronary artery plaque volume, as determined by computed tomographic angiography (CTA). This outcome has apparent clinical relevance and good reproducibility (56), making it desirable for a longitudinal study.
Results of the Cardiovascular Trial
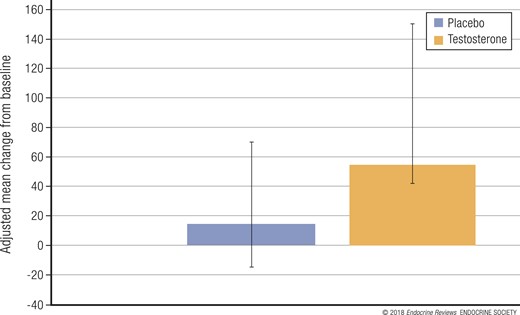
In the 138 men in the Cardiovascular Trial who had undergone CTA at both baseline and 12 months, testosterone treatment was associated with a statistically significant (P < 0.003) greater increase in noncalcified coronary artery plaque volume (median change from 204 to 232 mm3) compared with placebo (median change from 317 to 325 mm3; Fig. 8) (57). The men in both groups at baseline had relatively high rates of obesity, hyperlipidemia, hypertension, diabetes, and prevalence of atherosclerosis, determined by a coronary artery calcification score >300 Agatston units. Men in the placebo group at baseline had both higher mean calcification scores and higher noncalcified coronary artery plaque volume by CTA; however, all analyses were adjusted for baseline values as continuous variables, which should reduce confounding due to this imbalance. Several pieces of evidence suggest that the testosterone treatment effect on noncalcified plaque volume was not likely the result of the imbalance between the treatment arms at baseline. A linear regression model showed no suggestion of an association between the baseline plaque volume and the change from baseline to month 12 (P = 0.9). A scatterplot of the change in coronary artery noncalcified plaque volume against the baseline volume at month 12, which would have shown any type of association, linear or nonlinear, was not indicative of an association. Finally, testosterone had an effect in men whose baseline plaque volume was less than the median that was similar to that in the men whose baseline plaque volume was greater than the median, consistent with the overall effect.
Change from baseline to 12 months in noncalcified coronary artery plaque volume, as determined by CTA, in 138 men treated with testosterone or placebo. Data presented as least square mean ± 95% confidence intervals.
Lessons from the Cardiovascular Trial
The increase in the noncalcified plaque volume in the men treated with testosterone compared with the men treated with placebo is concerning, because any decrease in the coronary artery lumen could be considered deleterious. Whether this effect on the surrogate outcome of plaque volume also increases clinical cardiovascular risk can be determined only by a larger and longer trial.
Adverse Events
Adverse events in previous testosterone trials
In addition to the potential of testosterone to increase the risk of cardiovascular adverse events, testosterone has been postulated to have the potential to increase the risk of prostate adverse events and erythrocytosis. The risks of both adverse events have their basis in the physiological effects of testosterone. The normal functioning of the prostate gland is dependent on testosterone, and administration of testosterone to hypogonadal men has been well documented to increase the serum levels of PSA. Of greater concern, androgen receptor signaling plays an important role in stimulating the growth of metastatic prostate cancer (58), leading to the use of treatments of metastatic prostate cancer aimed at lowering testosterone to castrate levels and/or antagonizing the action of testosterone (59). Meta-analyses of previous testosterone trials, however, did not show that testosterone treatment increases the risk of prostate cancer (60, 61).
The stimulatory effects of testosterone on erythropoiesis are also well known, as illustrated by higher hemoglobin levels in normal men than in normal women. In clinical practice, erythrocytosis is a well-recognized side effect of testosterone treatment, and in previous clinical trials, erythrocytosis occurred much more commonly in testosterone-treated than in placebo-treated men (60).
Goals of the TTrials for adverse events
Although 1 year of treatment to not quite 800 participants did not allow for definitive conclusions regarding the risk of testosterone treatment, we designed the trials to minimize the incidence of adverse events and to monitor prospectively for the adverse events considered most likely. Thus, we excluded men who had a moderately high risk of prostate cancer, as judged by the risk of any prostate cancer of >35% and the risk of high-grade prostate cancer of >7%. We also excluded men who had moderately severe lower urinary tract symptoms, as judged by an International Prostate Symptom Score (IPSS) >19. (The IPSS is a questionnaire used to determine the presence of lower urinary tract symptoms, a possible indication of benign prostatic hyperplasia.) During treatment, we monitored for the possible development of prostate cancer using digital rectal examinations at 3 and 12 months and PSA measurement at 3, 12, and 18 months. We monitored for the development of worsening lower urinary tract symptoms by repeating the International Prostate Symptom Score questionnaire at 3 and 12 months.
We also excluded men whose hemoglobin concentration was >16.0 g/dL and monitored hemoglobin at months 3, 6, 9, and 12. Men whose values increased to >17.5 g/dL were evaluated for other causes of erythropoiesis and had their dose of testosterone lowered.
We further excluded men who had experienced a myocardial infarction or stroke within 3 months and had a blood pressure of >160 mm Hg systolic or 100 mm Hg diastolic. At each visit, a cardiovascular event questionnaire was administered to each participant. All cardiovascular events were adjudicated.
Results of monitoring for adverse events
The 27 men who had a confirmed increase in PSA greater than the trigger value during the 1 year of treatment and the 11 men during the year after treatment were referred for urologic evaluation. However, only 3 men each underwent prostate biopsies during the first year of treatment and during the year after treatment. Prostate cancer was diagnosed in one man in the testosterone arm during the year of treatment and in two men in the testosterone arm and one in the placebo arm during the year after treatment.The IPSS increased during treatment to >19 in 27 men treated with testosterone and 26 men treated with placebo (Table 2). In addition, seven men treated with testosterone, but none treated with placebo, developed erythrocytosis (Table 2). Also, seven men in each treatment arm developed major adverse cardiovascular events; five of the seven in each arm had experienced previous cardiovascular events. Other adverse cardiovascular events were also similar in the two treatment arms.
Adverse Events During 1 Year of Treatment in TTrials
| Event . | Treatment . | |
|---|---|---|
| Placebo . | Testosterone . | |
| Participants, n | 394 | 394 |
| Prostate events, n | ||
| PSA increase ≥1.0 ng/mL | 8 | 23 |
| Prostate cancer | 0 | 1 |
| IPSS >19 | 26 | 27 |
| Hemoglobin ≥17.5 g/dL | 0 | 7 |
| CV events,a n | ||
| MI (definite/probable) | 1 | 2 |
| Stroke (definite/probable) | 5 | 5 |
| CV death | 1 | 0 |
| Total (MI, stroke, CV death) | 7 | 7 |
| Serious adverse events | ||
| Death | 7 | 3 |
| Hospitalization | 78 | 68 |
| Otherb | 6 | 7 |
| Event . | Treatment . | |
|---|---|---|
| Placebo . | Testosterone . | |
| Participants, n | 394 | 394 |
| Prostate events, n | ||
| PSA increase ≥1.0 ng/mL | 8 | 23 |
| Prostate cancer | 0 | 1 |
| IPSS >19 | 26 | 27 |
| Hemoglobin ≥17.5 g/dL | 0 | 7 |
| CV events,a n | ||
| MI (definite/probable) | 1 | 2 |
| Stroke (definite/probable) | 5 | 5 |
| CV death | 1 | 0 |
| Total (MI, stroke, CV death) | 7 | 7 |
| Serious adverse events | ||
| Death | 7 | 3 |
| Hospitalization | 78 | 68 |
| Otherb | 6 | 7 |
Abbreviations: CV, cardiovascular; MI, myocardial infarction.
CV adverse events were collected by a questionnaire at each visit and from the adverse event log and serious adverse event report forms; MI, stroke, and CV death were evaluated by two adjudicators.
Congenital anomaly, disability, or important medical or life-threatening event.
Adverse Events During 1 Year of Treatment in TTrials
| Event . | Treatment . | |
|---|---|---|
| Placebo . | Testosterone . | |
| Participants, n | 394 | 394 |
| Prostate events, n | ||
| PSA increase ≥1.0 ng/mL | 8 | 23 |
| Prostate cancer | 0 | 1 |
| IPSS >19 | 26 | 27 |
| Hemoglobin ≥17.5 g/dL | 0 | 7 |
| CV events,a n | ||
| MI (definite/probable) | 1 | 2 |
| Stroke (definite/probable) | 5 | 5 |
| CV death | 1 | 0 |
| Total (MI, stroke, CV death) | 7 | 7 |
| Serious adverse events | ||
| Death | 7 | 3 |
| Hospitalization | 78 | 68 |
| Otherb | 6 | 7 |
| Event . | Treatment . | |
|---|---|---|
| Placebo . | Testosterone . | |
| Participants, n | 394 | 394 |
| Prostate events, n | ||
| PSA increase ≥1.0 ng/mL | 8 | 23 |
| Prostate cancer | 0 | 1 |
| IPSS >19 | 26 | 27 |
| Hemoglobin ≥17.5 g/dL | 0 | 7 |
| CV events,a n | ||
| MI (definite/probable) | 1 | 2 |
| Stroke (definite/probable) | 5 | 5 |
| CV death | 1 | 0 |
| Total (MI, stroke, CV death) | 7 | 7 |
| Serious adverse events | ||
| Death | 7 | 3 |
| Hospitalization | 78 | 68 |
| Otherb | 6 | 7 |
Abbreviations: CV, cardiovascular; MI, myocardial infarction.
CV adverse events were collected by a questionnaire at each visit and from the adverse event log and serious adverse event report forms; MI, stroke, and CV death were evaluated by two adjudicators.
Congenital anomaly, disability, or important medical or life-threatening event.
Lessons about adverse events
Testosterone treatment was not associated with substantially more diagnoses of prostate cancer compared with placebo. This result might have been influenced by the relatively small number of prostate biopsies, although a much larger number of men were referred for consideration of biopsy because of an increase in PSA. The low percentage of men who underwent biopsy likely reflects the judgment of the site urologists. Only a trial enrolling a much larger number of older men with low testosterone who are then treated and followed up for a much longer period would be able to determine whether testosterone treatment increases the risk of prostate cancer and lower urinary tract symptoms.
Testosterone treatment was also not associated with more men who experienced major adverse cardiovascular events. Just as with prostate adverse events, a trial enrolling many more older men with low testosterone who are followed up for much longer will be able to determine whether testosterone treatment increases cardiovascular risk.
The TTrials results have confirmed that testosterone treatment increases the risk of erythrocytosis, although the relatively low incidence compared with that in previous trials suggests that careful monitoring of the hemoglobin concentration and reduction of the dose of testosterone when appropriate will minimize this risk.
Study Limitations
The major limitation of these trials, although by intent, was that the results apply only to men aged ≥65 years with confirmed testosterone concentrations <275 ng/dL. They do not apply to men with the same symptoms (e.g., decreased libido) or associated findings (e.g., anemia) but with normal testosterone levels. Another limitation was that the duration of treatment was only 1 year; thus, we could not be sure that the efficacy demonstrated during 1 year of treatment would persist if the treatment had been continued for >1 year.
Summary of Lessons
The TTrials were seven, coordinated, placebo-controlled trials that evaluated the efficacy of testosterone treatment for 1 year in 788 older men with low testosterone compared with young men. To the best of our knowledge, they were the first trials of testosterone treatment for older men to enroll men who were unequivocally hypogonadal and in sufficient numbers to provide high power to detect meaningful differences in the efficacy outcomes. Thus, the negative results are reliable in excluding moderate effects. Treatment increased the testosterone levels to midnormal for young men; however, regular monitoring of testosterone levels and adjustment of the testosterone dose was necessary to maintain those levels.
The major efficacy results of the seven TTrials are summarized in Tables 3 and 4. Testosterone treatment improved most aspects of sexual function to a moderate degree. Testosterone also improved the walking distance in all men, but only by a small amount, and improved mood and depressive symptoms to a small degree. For the men who were mildly anemic, testosterone corrected the anemia in the men with unexplained anemia and also in the men who had another, identifiable cause. Testosterone increased the surrogate measure of volumetric bone mineral density and estimated bone strength to similar degrees as standard treatments of osteoporosis. Testosterone did not improve any measure of cognitive function.
Summary of the Results of the Three Main Testosterone Trials (Sexual Function, Physical Function, and Vitality)
| Testosterone Trial . | Participants (n) . | Mean Difference or OR (95% CI)a . | Effect Sizeb . | P Value . | Interpretation of Results . |
|---|---|---|---|---|---|
| Sexual Function Trial | |||||
| Sexual activity, PDQ-Q4 scorec | Enrollees in Sexual Function Trial (459) | 0.58 (0.38–0.78) | 0.45 (0.30–0.60) | <0.001 | Testosterone treatment substantially increased sexual activity and libido and, to a lesser degree, erectile function, in both men enrolled in this trial and all TTrials men |
| Sexual activity, PDQ-Q4 score | All TTrials men (771) | 0.62 (0.45–0.79) | 0.45 (0.33–0.58) | <0.001 | |
| Sexual desire, DISF-M-II score | Enrollees in Sexual Function Trial (470) | 2.93 (2.13–3.74) | 0.44 (0.32–0.56) | <0.001 | |
| Erectile function, IIEF score | Enrollees in Sexual Function Trial (470) | 2.64 (1.68–3.61) | 0.32 (0.20–0.44) | <0.001 | |
| Physical Function Trial | |||||
| Walking; men whose walking distance increased ≥50 m in 6-min walk test, %c | Enrollees in Physical Function Trial (387) | 1.42 (0.83–2.45) | — | 0.20 | Testosterone treatment did not increase distance walked in 6 min by ≥50 m in men enrolled in this trial but did do so in all TTrials men and also increased men’s perception of their walking, suggesting that testosterone treatment probably improves walking to a small degree |
| Walking; men whose walk distance increased ≥50 m in 6-min walk test, % | All TTrials men (781) | 1.76 (1.21–2.57) | — | 0.003 | |
| Walking; 6-min walk distance, m | Enrollees in Physical Function Trial (387) | 4.09 (−3.00 to 11.18) | 0.06 (−0.04 to 0.16) | 0.28 | |
| Men whose PF-10 score increased ≥8, % | Enrollees in Physical Function Trial (365) | 1.34 (0.90–2.00) | 0.15 | ||
| PF-10 score | Enrollees in Physical Function Trial (365) | 2.75 (0.20–5.29) | 0.13 (0.01–0.26) | 0.03 | |
| Vitality Trial | |||||
| Energy, increase ≥4 in FACIT-Fatigue score, %c | Enrollees in Vitality Trial (474) | 1.23 (0.83–1.84) | — | 0.30 | Testosterone treatment did not improve vitality assessed by the FACIT-Fatigue Scale but did improve it slightly by the SF-36 vitality score; testosterone treatment also resulted in small improvements in positive and negative mood and in depressive symptoms |
| Energy, increase ≥ 4 in FACIT-Fatigue score, % | All TTrials men (788) | 1.23 (0.89–1.70) | — | 0.22 | |
| Energy, FACIT-Fatigue score | Enrollees in Vitality Trial (474) | 1.21 (−0.04 to 2.46) | 0.19 (−0.01 to 0.38) | 0.06 | |
| SF-36 vitality score | Enrollees in Vitality Trial (404) | 2.41 (0.31–4.50) | 0.18 (0.02–0.34) | 0.03 | |
| Positive affect, PANAS score | Enrollees in Vitality Trial (463) | 0.47 (0.02–0.92) | 0.14 (0.01–0.27) | 0.04 | |
| Negative affect, PANAS score | Enrollees in Vitality Trial (463) | −0.49 (−0.79 to −0.19) | −0.18 (−0.29 to −0.06) | <0.001 | |
| Depression, PHQ-9 score | Enrollees in Vitality Trial (464) | −0.72 (−1.20 to −0.23) | −0.18 (−0.30 to −0.06) | 0.004 |
| Testosterone Trial . | Participants (n) . | Mean Difference or OR (95% CI)a . | Effect Sizeb . | P Value . | Interpretation of Results . |
|---|---|---|---|---|---|
| Sexual Function Trial | |||||
| Sexual activity, PDQ-Q4 scorec | Enrollees in Sexual Function Trial (459) | 0.58 (0.38–0.78) | 0.45 (0.30–0.60) | <0.001 | Testosterone treatment substantially increased sexual activity and libido and, to a lesser degree, erectile function, in both men enrolled in this trial and all TTrials men |
| Sexual activity, PDQ-Q4 score | All TTrials men (771) | 0.62 (0.45–0.79) | 0.45 (0.33–0.58) | <0.001 | |
| Sexual desire, DISF-M-II score | Enrollees in Sexual Function Trial (470) | 2.93 (2.13–3.74) | 0.44 (0.32–0.56) | <0.001 | |
| Erectile function, IIEF score | Enrollees in Sexual Function Trial (470) | 2.64 (1.68–3.61) | 0.32 (0.20–0.44) | <0.001 | |
| Physical Function Trial | |||||
| Walking; men whose walking distance increased ≥50 m in 6-min walk test, %c | Enrollees in Physical Function Trial (387) | 1.42 (0.83–2.45) | — | 0.20 | Testosterone treatment did not increase distance walked in 6 min by ≥50 m in men enrolled in this trial but did do so in all TTrials men and also increased men’s perception of their walking, suggesting that testosterone treatment probably improves walking to a small degree |
| Walking; men whose walk distance increased ≥50 m in 6-min walk test, % | All TTrials men (781) | 1.76 (1.21–2.57) | — | 0.003 | |
| Walking; 6-min walk distance, m | Enrollees in Physical Function Trial (387) | 4.09 (−3.00 to 11.18) | 0.06 (−0.04 to 0.16) | 0.28 | |
| Men whose PF-10 score increased ≥8, % | Enrollees in Physical Function Trial (365) | 1.34 (0.90–2.00) | 0.15 | ||
| PF-10 score | Enrollees in Physical Function Trial (365) | 2.75 (0.20–5.29) | 0.13 (0.01–0.26) | 0.03 | |
| Vitality Trial | |||||
| Energy, increase ≥4 in FACIT-Fatigue score, %c | Enrollees in Vitality Trial (474) | 1.23 (0.83–1.84) | — | 0.30 | Testosterone treatment did not improve vitality assessed by the FACIT-Fatigue Scale but did improve it slightly by the SF-36 vitality score; testosterone treatment also resulted in small improvements in positive and negative mood and in depressive symptoms |
| Energy, increase ≥ 4 in FACIT-Fatigue score, % | All TTrials men (788) | 1.23 (0.89–1.70) | — | 0.22 | |
| Energy, FACIT-Fatigue score | Enrollees in Vitality Trial (474) | 1.21 (−0.04 to 2.46) | 0.19 (−0.01 to 0.38) | 0.06 | |
| SF-36 vitality score | Enrollees in Vitality Trial (404) | 2.41 (0.31–4.50) | 0.18 (0.02–0.34) | 0.03 | |
| Positive affect, PANAS score | Enrollees in Vitality Trial (463) | 0.47 (0.02–0.92) | 0.14 (0.01–0.27) | 0.04 | |
| Negative affect, PANAS score | Enrollees in Vitality Trial (463) | −0.49 (−0.79 to −0.19) | −0.18 (−0.29 to −0.06) | <0.001 | |
| Depression, PHQ-9 score | Enrollees in Vitality Trial (464) | −0.72 (−1.20 to −0.23) | −0.18 (−0.30 to −0.06) | 0.004 |
Abbreviations: CI, confidence interval; DISF-M-II, Derogatis Inventory of Sexual Function–Men–II, sexual desire domain (range, 0–33); IIEF, International Index of Erectile Function, erectile function domain (range, 0–30); OR, odds ratio; PANAS, Positive and Negative Affect Scale (range, 5–50); PDQ-Q4, Psychosexual Daily Questionnaire, question 4 (range, 0–12); PF-10, physical function scale of the Medical Outcomes Short Form Health Survey (range, 0–100); PHQ-9, Patient Health Questionnaire 9 (range, 0–27; higher scores indicate greater degree of depressive symptoms); SF-36, 36-item Short Form Survey (range, 0–100).
Treatment effect: for continuous outcomes, the treatment effect was the mean change in men allocated to testosterone minus the mean change in men allocated to placebo, adjusted for balancing factors: baseline total testosterone level (≤200 or >200 ng/dL), age (≤75 or >75 years), trial site, participation in the main trials, use or nonuse of antidepressants use or nonuse of phosphodiesterase type 5 inhibitors, and baseline value of the outcome variable; for binary outcomes, the adjusted OR was the ratio of the outcome in men allocated to testosterone to the outcome in men allocated to placebo, adjusted for the same balancing factors.
The effect size for continuous outcomes was calculated from the mean difference divided by the baseline standard deviation pooled across treatment arms; an effect size of 0.2 is considered a small effect, 0.5 a medium effect, and 0.8 a large effect.
The primary outcome of the trial.
Summary of the Results of the Three Main Testosterone Trials (Sexual Function, Physical Function, and Vitality)
| Testosterone Trial . | Participants (n) . | Mean Difference or OR (95% CI)a . | Effect Sizeb . | P Value . | Interpretation of Results . |
|---|---|---|---|---|---|
| Sexual Function Trial | |||||
| Sexual activity, PDQ-Q4 scorec | Enrollees in Sexual Function Trial (459) | 0.58 (0.38–0.78) | 0.45 (0.30–0.60) | <0.001 | Testosterone treatment substantially increased sexual activity and libido and, to a lesser degree, erectile function, in both men enrolled in this trial and all TTrials men |
| Sexual activity, PDQ-Q4 score | All TTrials men (771) | 0.62 (0.45–0.79) | 0.45 (0.33–0.58) | <0.001 | |
| Sexual desire, DISF-M-II score | Enrollees in Sexual Function Trial (470) | 2.93 (2.13–3.74) | 0.44 (0.32–0.56) | <0.001 | |
| Erectile function, IIEF score | Enrollees in Sexual Function Trial (470) | 2.64 (1.68–3.61) | 0.32 (0.20–0.44) | <0.001 | |
| Physical Function Trial | |||||
| Walking; men whose walking distance increased ≥50 m in 6-min walk test, %c | Enrollees in Physical Function Trial (387) | 1.42 (0.83–2.45) | — | 0.20 | Testosterone treatment did not increase distance walked in 6 min by ≥50 m in men enrolled in this trial but did do so in all TTrials men and also increased men’s perception of their walking, suggesting that testosterone treatment probably improves walking to a small degree |
| Walking; men whose walk distance increased ≥50 m in 6-min walk test, % | All TTrials men (781) | 1.76 (1.21–2.57) | — | 0.003 | |
| Walking; 6-min walk distance, m | Enrollees in Physical Function Trial (387) | 4.09 (−3.00 to 11.18) | 0.06 (−0.04 to 0.16) | 0.28 | |
| Men whose PF-10 score increased ≥8, % | Enrollees in Physical Function Trial (365) | 1.34 (0.90–2.00) | 0.15 | ||
| PF-10 score | Enrollees in Physical Function Trial (365) | 2.75 (0.20–5.29) | 0.13 (0.01–0.26) | 0.03 | |
| Vitality Trial | |||||
| Energy, increase ≥4 in FACIT-Fatigue score, %c | Enrollees in Vitality Trial (474) | 1.23 (0.83–1.84) | — | 0.30 | Testosterone treatment did not improve vitality assessed by the FACIT-Fatigue Scale but did improve it slightly by the SF-36 vitality score; testosterone treatment also resulted in small improvements in positive and negative mood and in depressive symptoms |
| Energy, increase ≥ 4 in FACIT-Fatigue score, % | All TTrials men (788) | 1.23 (0.89–1.70) | — | 0.22 | |
| Energy, FACIT-Fatigue score | Enrollees in Vitality Trial (474) | 1.21 (−0.04 to 2.46) | 0.19 (−0.01 to 0.38) | 0.06 | |
| SF-36 vitality score | Enrollees in Vitality Trial (404) | 2.41 (0.31–4.50) | 0.18 (0.02–0.34) | 0.03 | |
| Positive affect, PANAS score | Enrollees in Vitality Trial (463) | 0.47 (0.02–0.92) | 0.14 (0.01–0.27) | 0.04 | |
| Negative affect, PANAS score | Enrollees in Vitality Trial (463) | −0.49 (−0.79 to −0.19) | −0.18 (−0.29 to −0.06) | <0.001 | |
| Depression, PHQ-9 score | Enrollees in Vitality Trial (464) | −0.72 (−1.20 to −0.23) | −0.18 (−0.30 to −0.06) | 0.004 |
| Testosterone Trial . | Participants (n) . | Mean Difference or OR (95% CI)a . | Effect Sizeb . | P Value . | Interpretation of Results . |
|---|---|---|---|---|---|
| Sexual Function Trial | |||||
| Sexual activity, PDQ-Q4 scorec | Enrollees in Sexual Function Trial (459) | 0.58 (0.38–0.78) | 0.45 (0.30–0.60) | <0.001 | Testosterone treatment substantially increased sexual activity and libido and, to a lesser degree, erectile function, in both men enrolled in this trial and all TTrials men |
| Sexual activity, PDQ-Q4 score | All TTrials men (771) | 0.62 (0.45–0.79) | 0.45 (0.33–0.58) | <0.001 | |
| Sexual desire, DISF-M-II score | Enrollees in Sexual Function Trial (470) | 2.93 (2.13–3.74) | 0.44 (0.32–0.56) | <0.001 | |
| Erectile function, IIEF score | Enrollees in Sexual Function Trial (470) | 2.64 (1.68–3.61) | 0.32 (0.20–0.44) | <0.001 | |
| Physical Function Trial | |||||
| Walking; men whose walking distance increased ≥50 m in 6-min walk test, %c | Enrollees in Physical Function Trial (387) | 1.42 (0.83–2.45) | — | 0.20 | Testosterone treatment did not increase distance walked in 6 min by ≥50 m in men enrolled in this trial but did do so in all TTrials men and also increased men’s perception of their walking, suggesting that testosterone treatment probably improves walking to a small degree |
| Walking; men whose walk distance increased ≥50 m in 6-min walk test, % | All TTrials men (781) | 1.76 (1.21–2.57) | — | 0.003 | |
| Walking; 6-min walk distance, m | Enrollees in Physical Function Trial (387) | 4.09 (−3.00 to 11.18) | 0.06 (−0.04 to 0.16) | 0.28 | |
| Men whose PF-10 score increased ≥8, % | Enrollees in Physical Function Trial (365) | 1.34 (0.90–2.00) | 0.15 | ||
| PF-10 score | Enrollees in Physical Function Trial (365) | 2.75 (0.20–5.29) | 0.13 (0.01–0.26) | 0.03 | |
| Vitality Trial | |||||
| Energy, increase ≥4 in FACIT-Fatigue score, %c | Enrollees in Vitality Trial (474) | 1.23 (0.83–1.84) | — | 0.30 | Testosterone treatment did not improve vitality assessed by the FACIT-Fatigue Scale but did improve it slightly by the SF-36 vitality score; testosterone treatment also resulted in small improvements in positive and negative mood and in depressive symptoms |
| Energy, increase ≥ 4 in FACIT-Fatigue score, % | All TTrials men (788) | 1.23 (0.89–1.70) | — | 0.22 | |
| Energy, FACIT-Fatigue score | Enrollees in Vitality Trial (474) | 1.21 (−0.04 to 2.46) | 0.19 (−0.01 to 0.38) | 0.06 | |
| SF-36 vitality score | Enrollees in Vitality Trial (404) | 2.41 (0.31–4.50) | 0.18 (0.02–0.34) | 0.03 | |
| Positive affect, PANAS score | Enrollees in Vitality Trial (463) | 0.47 (0.02–0.92) | 0.14 (0.01–0.27) | 0.04 | |
| Negative affect, PANAS score | Enrollees in Vitality Trial (463) | −0.49 (−0.79 to −0.19) | −0.18 (−0.29 to −0.06) | <0.001 | |
| Depression, PHQ-9 score | Enrollees in Vitality Trial (464) | −0.72 (−1.20 to −0.23) | −0.18 (−0.30 to −0.06) | 0.004 |
Abbreviations: CI, confidence interval; DISF-M-II, Derogatis Inventory of Sexual Function–Men–II, sexual desire domain (range, 0–33); IIEF, International Index of Erectile Function, erectile function domain (range, 0–30); OR, odds ratio; PANAS, Positive and Negative Affect Scale (range, 5–50); PDQ-Q4, Psychosexual Daily Questionnaire, question 4 (range, 0–12); PF-10, physical function scale of the Medical Outcomes Short Form Health Survey (range, 0–100); PHQ-9, Patient Health Questionnaire 9 (range, 0–27; higher scores indicate greater degree of depressive symptoms); SF-36, 36-item Short Form Survey (range, 0–100).
Treatment effect: for continuous outcomes, the treatment effect was the mean change in men allocated to testosterone minus the mean change in men allocated to placebo, adjusted for balancing factors: baseline total testosterone level (≤200 or >200 ng/dL), age (≤75 or >75 years), trial site, participation in the main trials, use or nonuse of antidepressants use or nonuse of phosphodiesterase type 5 inhibitors, and baseline value of the outcome variable; for binary outcomes, the adjusted OR was the ratio of the outcome in men allocated to testosterone to the outcome in men allocated to placebo, adjusted for the same balancing factors.
The effect size for continuous outcomes was calculated from the mean difference divided by the baseline standard deviation pooled across treatment arms; an effect size of 0.2 is considered a small effect, 0.5 a medium effect, and 0.8 a large effect.
The primary outcome of the trial.
Summary of Results of Four Other Testosterone Trials (Cognitive Function, Anemia, Bone, and Cardiovascular)
| Testosterone Trial . | Participants (n) . | Mean Difference or ORa (95% CI) . | Effect Sizeb . | P Value . | Interpretation . |
|---|---|---|---|---|---|
| Cognitive Function Trial | |||||
| Verbal memory; delayed paragraph recallc | Men with age-associated memory impairment (493) | −0.07 (−0.92 to 0.79) | −0.01 (−0.14 to 0.12) | 0.88 | Testosterone treatment did not change several aspects of cognitive function in men who had age-associated memory impairment |
| Verbal memory; delayed paragraph recall | All TTrials men (785) | 0.09 (−0.57 to 0.75) | 0.01 (−0.09 to 0.11) | 0.80 | |
| Visual memory; Benton visual retention test | Men with age-associated memory impairment (492) | −0.28 (−0.76 to 0.19) | −0.09 (−0.24 to 0.06) | 0.24 | |
| Spatial ability; card rotation test | Men with age-associated memory impairment (488) | −0.12 (−1.89 to 1.65) | −0.01 (−0.13 to 0.11) | 0.89 | |
| Executive function; trail-making test B-A, sd | Men with age-associated memory impairment (490) | −5.51 (−12.91 to 1.88) | −0.09 (−0.22 to 0.03) | 0.14 | |
| Anemia Trial | |||||
| Hemoglobin increase from baseline ≥1.0 g/dL,c % | TTrials men with unexplained anemia (62) | 31.5 (3.7–277.8) | — | 0.002 | Testosterone substantially increased hemoglobin and corrected mild anemia in both men who had unexplained anemia and men who had anemia of known cause |
| Hemoglobin, g/dL | TTrials men with unexplained anemia (62) | 0.83 (0.48–1.39) | 1.30 (0.75–2.18) | <0.001 | |
| Hemoglobin increase from baseline ≥1.0 g/dL, % | TTrials men with anemia of known cause (64) | 8.2 (2.1–31.9) | — | 0.003 | |
| Hemoglobin, g/dL | TTrials men with anemia of known (64) | 0.64 (0.12–1.17) | 0.90 (0.17–1.65) | 0.018 | |
| Bone Trial | |||||
| Spine trabecular vBMD,c % change from baseline | Enrollees in Bone Trial (207) | 6.8 (4.8–8.7) | 0.23 (0.17–0.29) | <0.001 | Testosterone treatment substantially increased vBMD, more of trabecular bone than peripheral bone and more in vertebrae than in hip |
| Spine whole bone vBMD, % change from baselined | Enrollees in Bone Trial (207) | 4.2 (3.2–5.3) | 0.12 (0.09–0.15) | <0.001 | |
| Hip trabecular vBMD, % change from baseline | Enrollees in Bone Trial (191) | 1.5 (0.9–2.0) | 0.04 (0.03–0.06) | <0.001 | |
| Hip whole bone vBMD, % change from baseline | Enrollees in Bone Trial (191) | 1.3 (0.8–1.7) | 0.03 (0.02–0.04) | <0.001 | |
| Cardiovascular Trial | |||||
| Noncalcified coronary artery plaque volume,c mm3 | Enrollees in CV Trial (138) | 41 (14–67) | 0.11 (0.04–0.19) | 0.003 | Testosterone was associated with an increase in noncalcified coronary artery plaque volume but not calcified coronary artery calcium score |
| Total coronary artery plaque volume, mm3 | Enrollees in CV Trial (138) | 47 (13–80) | 0.09 (0.02–0.15) | 0.006 | |
| Coronary artery calcium score, Agatston units | Enrollees in CV Trial (138) | −27 (−80 to 26) | −0.03 (−0.07 to 0.02) | 0.31 |
| Testosterone Trial . | Participants (n) . | Mean Difference or ORa (95% CI) . | Effect Sizeb . | P Value . | Interpretation . |
|---|---|---|---|---|---|
| Cognitive Function Trial | |||||
| Verbal memory; delayed paragraph recallc | Men with age-associated memory impairment (493) | −0.07 (−0.92 to 0.79) | −0.01 (−0.14 to 0.12) | 0.88 | Testosterone treatment did not change several aspects of cognitive function in men who had age-associated memory impairment |
| Verbal memory; delayed paragraph recall | All TTrials men (785) | 0.09 (−0.57 to 0.75) | 0.01 (−0.09 to 0.11) | 0.80 | |
| Visual memory; Benton visual retention test | Men with age-associated memory impairment (492) | −0.28 (−0.76 to 0.19) | −0.09 (−0.24 to 0.06) | 0.24 | |
| Spatial ability; card rotation test | Men with age-associated memory impairment (488) | −0.12 (−1.89 to 1.65) | −0.01 (−0.13 to 0.11) | 0.89 | |
| Executive function; trail-making test B-A, sd | Men with age-associated memory impairment (490) | −5.51 (−12.91 to 1.88) | −0.09 (−0.22 to 0.03) | 0.14 | |
| Anemia Trial | |||||
| Hemoglobin increase from baseline ≥1.0 g/dL,c % | TTrials men with unexplained anemia (62) | 31.5 (3.7–277.8) | — | 0.002 | Testosterone substantially increased hemoglobin and corrected mild anemia in both men who had unexplained anemia and men who had anemia of known cause |
| Hemoglobin, g/dL | TTrials men with unexplained anemia (62) | 0.83 (0.48–1.39) | 1.30 (0.75–2.18) | <0.001 | |
| Hemoglobin increase from baseline ≥1.0 g/dL, % | TTrials men with anemia of known cause (64) | 8.2 (2.1–31.9) | — | 0.003 | |
| Hemoglobin, g/dL | TTrials men with anemia of known (64) | 0.64 (0.12–1.17) | 0.90 (0.17–1.65) | 0.018 | |
| Bone Trial | |||||
| Spine trabecular vBMD,c % change from baseline | Enrollees in Bone Trial (207) | 6.8 (4.8–8.7) | 0.23 (0.17–0.29) | <0.001 | Testosterone treatment substantially increased vBMD, more of trabecular bone than peripheral bone and more in vertebrae than in hip |
| Spine whole bone vBMD, % change from baselined | Enrollees in Bone Trial (207) | 4.2 (3.2–5.3) | 0.12 (0.09–0.15) | <0.001 | |
| Hip trabecular vBMD, % change from baseline | Enrollees in Bone Trial (191) | 1.5 (0.9–2.0) | 0.04 (0.03–0.06) | <0.001 | |
| Hip whole bone vBMD, % change from baseline | Enrollees in Bone Trial (191) | 1.3 (0.8–1.7) | 0.03 (0.02–0.04) | <0.001 | |
| Cardiovascular Trial | |||||
| Noncalcified coronary artery plaque volume,c mm3 | Enrollees in CV Trial (138) | 41 (14–67) | 0.11 (0.04–0.19) | 0.003 | Testosterone was associated with an increase in noncalcified coronary artery plaque volume but not calcified coronary artery calcium score |
| Total coronary artery plaque volume, mm3 | Enrollees in CV Trial (138) | 47 (13–80) | 0.09 (0.02–0.15) | 0.006 | |
| Coronary artery calcium score, Agatston units | Enrollees in CV Trial (138) | −27 (−80 to 26) | −0.03 (−0.07 to 0.02) | 0.31 |
Abbreviations: CI, confidence interval; CV, cardiovascular.
Treatment effect: for continuous outcomes, the treatment effect was the mean change in men allocated to testosterone minus the mean change in men allocated to placebo, adjusted for balancing factors: baseline total testosterone level (≤200 or >200 ng/dL), age (≤75 or >75 years), trial site, participation in the main trials, use or nonuse of antidepressants use or nonuse of phosphodiesterase type 5 inhibitors, and baseline value of the outcome variable; for binary outcomes, the adjusted OR was the ratio of the outcome in men allocated to testosterone to the outcome in men allocated to placebo, adjusted for the same balancing factors.
The effect size for continuous outcomes was calculated from the mean difference divided by the baseline standard deviation pooled across treatment arms; an effect size of 0.2 is considered a small effect, 0.5 a medium effect, and 0.8 a large effect.
The primary outcome of the trial.
Lower scores reflect better function.
Summary of Results of Four Other Testosterone Trials (Cognitive Function, Anemia, Bone, and Cardiovascular)
| Testosterone Trial . | Participants (n) . | Mean Difference or ORa (95% CI) . | Effect Sizeb . | P Value . | Interpretation . |
|---|---|---|---|---|---|
| Cognitive Function Trial | |||||
| Verbal memory; delayed paragraph recallc | Men with age-associated memory impairment (493) | −0.07 (−0.92 to 0.79) | −0.01 (−0.14 to 0.12) | 0.88 | Testosterone treatment did not change several aspects of cognitive function in men who had age-associated memory impairment |
| Verbal memory; delayed paragraph recall | All TTrials men (785) | 0.09 (−0.57 to 0.75) | 0.01 (−0.09 to 0.11) | 0.80 | |
| Visual memory; Benton visual retention test | Men with age-associated memory impairment (492) | −0.28 (−0.76 to 0.19) | −0.09 (−0.24 to 0.06) | 0.24 | |
| Spatial ability; card rotation test | Men with age-associated memory impairment (488) | −0.12 (−1.89 to 1.65) | −0.01 (−0.13 to 0.11) | 0.89 | |
| Executive function; trail-making test B-A, sd | Men with age-associated memory impairment (490) | −5.51 (−12.91 to 1.88) | −0.09 (−0.22 to 0.03) | 0.14 | |
| Anemia Trial | |||||
| Hemoglobin increase from baseline ≥1.0 g/dL,c % | TTrials men with unexplained anemia (62) | 31.5 (3.7–277.8) | — | 0.002 | Testosterone substantially increased hemoglobin and corrected mild anemia in both men who had unexplained anemia and men who had anemia of known cause |
| Hemoglobin, g/dL | TTrials men with unexplained anemia (62) | 0.83 (0.48–1.39) | 1.30 (0.75–2.18) | <0.001 | |
| Hemoglobin increase from baseline ≥1.0 g/dL, % | TTrials men with anemia of known cause (64) | 8.2 (2.1–31.9) | — | 0.003 | |
| Hemoglobin, g/dL | TTrials men with anemia of known (64) | 0.64 (0.12–1.17) | 0.90 (0.17–1.65) | 0.018 | |
| Bone Trial | |||||
| Spine trabecular vBMD,c % change from baseline | Enrollees in Bone Trial (207) | 6.8 (4.8–8.7) | 0.23 (0.17–0.29) | <0.001 | Testosterone treatment substantially increased vBMD, more of trabecular bone than peripheral bone and more in vertebrae than in hip |
| Spine whole bone vBMD, % change from baselined | Enrollees in Bone Trial (207) | 4.2 (3.2–5.3) | 0.12 (0.09–0.15) | <0.001 | |
| Hip trabecular vBMD, % change from baseline | Enrollees in Bone Trial (191) | 1.5 (0.9–2.0) | 0.04 (0.03–0.06) | <0.001 | |
| Hip whole bone vBMD, % change from baseline | Enrollees in Bone Trial (191) | 1.3 (0.8–1.7) | 0.03 (0.02–0.04) | <0.001 | |
| Cardiovascular Trial | |||||
| Noncalcified coronary artery plaque volume,c mm3 | Enrollees in CV Trial (138) | 41 (14–67) | 0.11 (0.04–0.19) | 0.003 | Testosterone was associated with an increase in noncalcified coronary artery plaque volume but not calcified coronary artery calcium score |
| Total coronary artery plaque volume, mm3 | Enrollees in CV Trial (138) | 47 (13–80) | 0.09 (0.02–0.15) | 0.006 | |
| Coronary artery calcium score, Agatston units | Enrollees in CV Trial (138) | −27 (−80 to 26) | −0.03 (−0.07 to 0.02) | 0.31 |
| Testosterone Trial . | Participants (n) . | Mean Difference or ORa (95% CI) . | Effect Sizeb . | P Value . | Interpretation . |
|---|---|---|---|---|---|
| Cognitive Function Trial | |||||
| Verbal memory; delayed paragraph recallc | Men with age-associated memory impairment (493) | −0.07 (−0.92 to 0.79) | −0.01 (−0.14 to 0.12) | 0.88 | Testosterone treatment did not change several aspects of cognitive function in men who had age-associated memory impairment |
| Verbal memory; delayed paragraph recall | All TTrials men (785) | 0.09 (−0.57 to 0.75) | 0.01 (−0.09 to 0.11) | 0.80 | |
| Visual memory; Benton visual retention test | Men with age-associated memory impairment (492) | −0.28 (−0.76 to 0.19) | −0.09 (−0.24 to 0.06) | 0.24 | |
| Spatial ability; card rotation test | Men with age-associated memory impairment (488) | −0.12 (−1.89 to 1.65) | −0.01 (−0.13 to 0.11) | 0.89 | |
| Executive function; trail-making test B-A, sd | Men with age-associated memory impairment (490) | −5.51 (−12.91 to 1.88) | −0.09 (−0.22 to 0.03) | 0.14 | |
| Anemia Trial | |||||
| Hemoglobin increase from baseline ≥1.0 g/dL,c % | TTrials men with unexplained anemia (62) | 31.5 (3.7–277.8) | — | 0.002 | Testosterone substantially increased hemoglobin and corrected mild anemia in both men who had unexplained anemia and men who had anemia of known cause |
| Hemoglobin, g/dL | TTrials men with unexplained anemia (62) | 0.83 (0.48–1.39) | 1.30 (0.75–2.18) | <0.001 | |
| Hemoglobin increase from baseline ≥1.0 g/dL, % | TTrials men with anemia of known cause (64) | 8.2 (2.1–31.9) | — | 0.003 | |
| Hemoglobin, g/dL | TTrials men with anemia of known (64) | 0.64 (0.12–1.17) | 0.90 (0.17–1.65) | 0.018 | |
| Bone Trial | |||||
| Spine trabecular vBMD,c % change from baseline | Enrollees in Bone Trial (207) | 6.8 (4.8–8.7) | 0.23 (0.17–0.29) | <0.001 | Testosterone treatment substantially increased vBMD, more of trabecular bone than peripheral bone and more in vertebrae than in hip |
| Spine whole bone vBMD, % change from baselined | Enrollees in Bone Trial (207) | 4.2 (3.2–5.3) | 0.12 (0.09–0.15) | <0.001 | |
| Hip trabecular vBMD, % change from baseline | Enrollees in Bone Trial (191) | 1.5 (0.9–2.0) | 0.04 (0.03–0.06) | <0.001 | |
| Hip whole bone vBMD, % change from baseline | Enrollees in Bone Trial (191) | 1.3 (0.8–1.7) | 0.03 (0.02–0.04) | <0.001 | |
| Cardiovascular Trial | |||||
| Noncalcified coronary artery plaque volume,c mm3 | Enrollees in CV Trial (138) | 41 (14–67) | 0.11 (0.04–0.19) | 0.003 | Testosterone was associated with an increase in noncalcified coronary artery plaque volume but not calcified coronary artery calcium score |
| Total coronary artery plaque volume, mm3 | Enrollees in CV Trial (138) | 47 (13–80) | 0.09 (0.02–0.15) | 0.006 | |
| Coronary artery calcium score, Agatston units | Enrollees in CV Trial (138) | −27 (−80 to 26) | −0.03 (−0.07 to 0.02) | 0.31 |
Abbreviations: CI, confidence interval; CV, cardiovascular.
Treatment effect: for continuous outcomes, the treatment effect was the mean change in men allocated to testosterone minus the mean change in men allocated to placebo, adjusted for balancing factors: baseline total testosterone level (≤200 or >200 ng/dL), age (≤75 or >75 years), trial site, participation in the main trials, use or nonuse of antidepressants use or nonuse of phosphodiesterase type 5 inhibitors, and baseline value of the outcome variable; for binary outcomes, the adjusted OR was the ratio of the outcome in men allocated to testosterone to the outcome in men allocated to placebo, adjusted for the same balancing factors.
The effect size for continuous outcomes was calculated from the mean difference divided by the baseline standard deviation pooled across treatment arms; an effect size of 0.2 is considered a small effect, 0.5 a medium effect, and 0.8 a large effect.
The primary outcome of the trial.
Lower scores reflect better function.
Testosterone treatment caused erythrocytosis in a small number of men. Prostate cancer was diagnosed in only one man during treatment, and the numbers of men who developed moderately severe lower urinary tract symptoms were similar in the two treatment arms. Testosterone treatment was associated with a greater increase than placebo in the volume of noncalcified coronary artery plaque; however, the trial was not large enough to evaluate its effect on major adverse cardiovascular events.
A trial of a much larger number of men treated for a much longer time is necessary to determine whether testosterone treatment for older men with low testosterone increases the risk of major adverse cardiovascular events, lower urinary tract symptoms, and prostate cancer and decreases the risk of osteoporotic fractures. Such a trial would likely also cost much more than the approximately $50 million of the TTrials.
Decisions about treatment depend on the balance between benefits and risks. The results of the TTrials have demonstrated that increasing the testosterone levels in older men with low testosterone to those of young men has clear, although modest, benefits. If a trial of a much larger number of men followed up for a much longer period quantitates the risks of this treatment, the benefits the TTrials have demonstrated should greatly influence the decisions of physicians regarding whether to prescribe testosterone to older men with low testosterone compared with young men.
Abbreviations
- AAMI
age-associated memory impairment
- aBMD
areal bone mineral density
- CTA
computed tomographic angiography
- FACIT
Functional Assessment of Chronic Illness Therapy (range, 0 to 52)
- IOM
Institute of Medicine
- NIA
National Institute on Aging
- PSA
prostate-specific antigen
- QCT
quantitative computed tomography
- TTrials
Testosterone Trials
- vBMD
volumetric bone mineral density
Acknowledgments
Financial Support: The Testosterone Trials (TTrials) were supported by the National Institute on Aging (NIA), National Institutes of Health (NIH) (grant U01 AG030644), which gave advice about the design and conduct of the trial. The TTrials were also supplemented by funds from the National Heart, Lung and Blood Institute, National Institute of Neurologic Diseases and Stroke, and National Institute of Child Health and Human Development. AbbVie (formerly Solvay and Abbott Laboratories) provided funding, AndroGel, and placebo gel. S.B. and the Boston Center were supported, in part, by the Boston Claude D. Pepper Older Americans Independence Center (grant 5P30AG031679). A.M.M. was supported by the Department of Veterans Affairs Puget Sound Health Care System. T.M.G. is the recipient of an Academic Leadership Award (grant K07AG043587) from the NIA. The Yale Field Center was partially supported by the Claude D. Pepper Older Americans Independence Center (grant P30AG021342) and Yale Clinical and Translational Science Award (grant UL1TR000142). S.M.R. was supported by the Intramural Research Program, NIA, NIH. C.E.L. was supported by the National Institute for Diabetes, Digestive and Kidney Diseases, NIH (grant DK079626), to the University of Alabama at Birmingham Diabetes Research and Training Center. J.A.C. was supported by the NIA, NIH (grant R01 AG37679).
Disclosure Summary: P.J.S. reports grants from the National Institute on Aging (NIA) at the National Institutes of Health (NIH) and grants and nonfinancial support from AbbVie during the conduct of the study. S.B. reports grants from the NIA during the conduct of the study, grants and personal fees from Abbvie, Lilly, and Regeneron, and grants from Transition Therapeutics; has a free testosterone calculator patent pending; and has equity interest in FPT, LLC. G.R.C. reports personal fees from AbbVie, Besins, Clarus Therapeutics, Endo Pharma, Ferring, Lilly, and Pfizer outside the submitted work. A.M.M. reports personal fees from AbbVie, Endo, Lilly, Lipocine, and Clarus, outside the submitted work. A.J.S.-S. reports grants from the NIA at the NIH, grants from the NIA, and grants and other support from AbbVie during the conduct of the study. S.S.E. reports grants from the NIH and AbbVie, Inc., during the conduct of the study and grants from AbbVie, Inc., outside the submitted work. D.C. reports grants from the NIA and grants and other support from AbbVie during the conduct of the study. K.E.E. reports grants from the NIA during the conduct of the study. J.T.F. reports grants from the NIA at the NIH and grants and other support from AbbVie during the conduct of the study. C.E.L. reports grants from the NIH and AbbVie during the conduct of the study. M.E.M. reports grants from the NIH and AbbVie during the conduct of the study and personal fees from Abbvie, Eli Lilly & Co., and Pfizer outside the submitted work. S.M.R. reports grants from the NIA at the NIH and grants and other support from AbbVie during the conduct of the study. M.P. reports grants from the NIH during the conduct of the study. R.S.S. reports grants from AbbVie during the conduct of the study and grants and other support from Clarus, Lipocine, and Antares outside the submitted work. C.W. reports grants from Besins Health International, other support from Abbvie during the conduct of the study, and grants from Clarus Therapeutics, Antares, and Lipocine outside the submitted work. T.M.K. reports grants from the NIA and AbbVie during the conduct of the study. D.K. is an employee of, and has equity interest in, O.N. Diagnostics. D.L. is an employee of, and has equity interest, in O.N. Diagnostics. The remaining authors have nothing to disclose.
References
Author notes
These investigators contributed equally to this work.