-
PDF
- Split View
-
Views
-
Cite
Cite
Shaopeng Xu, Ziping Li, Tianqi Yang, Linjie Li, Xiwen Song, Yongchen Hao, Sidney C Smith, Gregg C Fonarow, Louise Morgan, Jing Liu, Jun Liu, Dong Zhao, Qing Yang, Xin Zhou, Yongle Li, The CCC-ACS Investigators , Association between early oral β-blocker therapy and risk for in-hospital major bleeding after percutaneous coronary intervention for acute coronary syndrome: findings from CCC-ACS project, European Heart Journal - Quality of Care and Clinical Outcomes, Volume 9, Issue 3, April 2023, Pages 293–302, https://doi.org/10.1093/ehjqcco/qcac036
Close - Share Icon Share
Abstract
Information regarding β-blocker use and bleeding risk in patients on antithrombotic therapy in contemporary practice is limited. We examined the association between early (within the first 24 hours) oral β-blocker therapy and major in-hospital bleeds among acute coronary syndrome (ACS) patients treated with percutaneous coronary intervention (PCI).
In the Improving Care for Cardiovascular Disease in China-ACS project, among patients without contraindications to β-blocker, we examined the association between early oral β-blocker exposure [users/non-users, dosing, and type (metoprolol vs. bisoprolol)] and major in-hospital bleeds. Of the 43 640 eligible patients, 36.0% patients received early oral β-blocker and 637 major bleeds were recorded. Compared with non-users, early oral β-blocker was associated reduced risks for major bleeds [odds ratio (OR): 0.48; 95% confidence interval (CI): 0.38–0.61] and in-hospital mortality (OR: 0.47; 95% CI: 0.34–0.64) in multivariable-adjusted logistic regression models. Early oral β-blocker use associated reduction in major bleeding was evident both in high-dose (defined by metoprolol-equivalent dose ≥50 mg/day) users (OR: 0.47; 95% CI: 0.33–0.68) and in low-dose users (metoprolol-equivalent dose <50 mg/day; OR: 0.61; 95% CI: 0.47–0.79). No significant difference was observed between metoprolol and bisoprolol in terms of reductions in major bleeding and mortality. Analyses based on inverse-probability-of-treatment-weighted regression adjustment and propensity-score matching yielded consistent findings.
In this retrospective study based on the nationwide ACS registry, among patients treated by PCI, in addition to a reduction in in-hospital mortality, oral β-blocker therapy initiated within the first 24 hours was associated with a reduced risk for major in-hospital bleeds.
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02306616
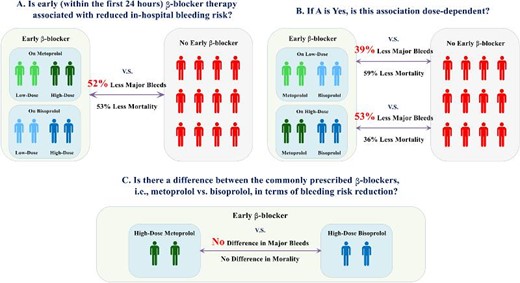
Association of early oral β-blocker therapy and in-hospital bleeding risk after percutaneous coronary intervention for acute coronary syndrome. Low-dose = metoprolol-equivalent dose < 50 mg/day; High-dose = metoprolol-equivalent dose ≥ 50 mg/day.
Introduction
In patients with acute coronary syndrome (ACS), the introduction of percutaneous coronary intervention (PCI) in conjunction with potent antithrombotic therapy has significantly improved cardiovascular outcomes. Meanwhile, during the past 2 decades, there is a doubling of bleeding events in ACS patients following PCI,1 which is the most common and costly non-cardiac complication associated with poor prognosis.2,3 The role of β-blocker in ACS patient management was established in the early 1960s. Despite the recent reports from observational studies questioning the benefit of long-term use of β-blocker following ACS, current guidelines recommend the routine use of early oral β-blockers during ACS hospitalization. Notably, scattered reports from the late 1990s and early 2000s provided clues indicating a potential role of β-blocker therapy in reducing bleeding complications.4,5 For example, an analysis based on the Global Registry of Acute Coronary Events (GRACE) showed that β-blocker use had a univariate association with lower bleeding risk.4 Another study reported that β-blocker use was associated with reduced incident hospitalization for gastrointestinal bleeding among hypertensive patients.5 Furthermore, non-selective oral β-blocker, i.e. propranolol, is recommended for the primary and secondary prevention of gastrointestinal bleeding due to cirrhosis and esophageal varices.6
Given the limited evidence, more information is needed on whether selective oral β1-adrenergic receptor blockers, the most prevalently used β-blockers in contemporary cardiology practice, may serve as a bleeding avoidance approach in ACS patients treated with PCI on standard antithrombotic therapy. We therefore specifically addressed the following three questions in the Improving Care for Cardiovascular Disease in China-ACS (CCC-ACS) project: (1) is early (within the first 24 hours) β-blocker therapy associated with reduced in-hospital bleeding risk? (2) If yes, is this association dose-dependent? and (3) is there a difference between the commonly prescribed β-blockers, i.e. metoprolol vs. bisoprolol, in terms of bleeding risk reduction.
Methods
Study design
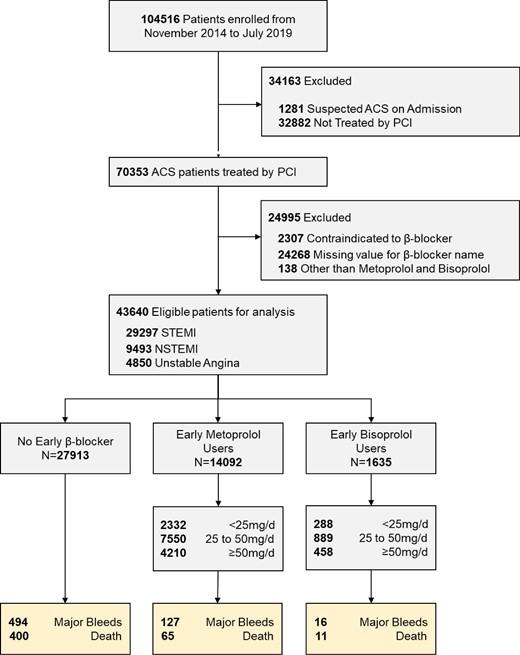
The CCC-ACS project is a collaborative effort by the American Heart Association and the Chinese Society of Cardiology to improve of the quality of clinical management for ACS patients, which is an ongoing nationwide quality improvement project from November 2014 in 150 tertiary hospitals in China. From 2017, the CCC-ACS project was extended to 42 secondary hospitals. The rationale and study design have been published previously.7 The CCC-ACS project was approved by the institutional review board of Beijing Anzhen Hospital, and all sites were granted a waiver of informed consent. This study is registered at https://clinicaltrial.gov (unique identifier: NCT02306616). Our study was observational and retrospective. From November 2014 to July 2019, 104 516 ACS patients were enrolled. We excluded patients not receiving PCI, with contraindications to β-blocker, and with missing value for β-blocker type used. Considering metoprolol and bisoprolol are the most prevalently used β-blockers in ACS patients in China, β-blocker users were defined either on metoprolol or on bisoprolol. Finally, a total of 43 640 patients treated with PCI during the indexed hospitalization was included for analysis. The study diagram according to the exclusion criteria was shown in Figure 1.
Study flow diagram. ACS = acute coronary syndrome; NSTEMI = non-ST-elevation myocardial infarction; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction.
Definitions of early β-blocker exposure for β-blocker therapy
We confined our analysis only in metoprolol and bisoprolol users, which are the most prevalently prescribed β-blockers in ACS patients in China.7 To make these two β-blockers comparable and by taking into account the different formulations of metoprolol (succinate vs. tartrate), the cumulative oral doses of all β-blockers administered within the first 24 hours of medical contact were converted to total daily dose of metoprolol-equivalent dose:8,9 50 mg metoprolol tartrate = 47.5 mg metoprolol succinate = 2.5 mg bisoprolol fumarate. We further classified the cumulative oral dose of β-blockers into low-dose (daily dose of metoprolol-equivalent dose <50 mg) and high-dose (metoprolol-equivalent dose ≥50 mg) groups.
Study covariates
The following 43 variables were treated as covariates for multivariable adjustment and propensity score matching: age, sex, smoking, medical history (hypertension, diabetes, dyslipidaemia, myocardial infarction, PCI, coronary artery bypass grafting, heart failure, atrial fibrillation, renal failure, ischaemic stroke, haemorrhagic stroke, peripheral vascular disease, and chronic obstructive pulmonary disease), admission lipid profile (levels of high density lipoprotein cholesterol, low density lipoprotein cholesterol, and triglycerides), admission systolic and diastolic blood pressure (BP), admission heart rate, Killip class, admission estimated glomerular filtration rate (eGFR), admission haemoglobin, pre-hospital medications in the past 2 weeks [aspirin, P2Y12 inhibitors, statins, β-blockers, angiotensin converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), aldosterone antagonists, oral anticoagulants], dual antiplatelet therapy (DAPT) status within the first 24 hours (see below), anticoagulants after PCI (unfractionated heparin, low molecular weight heparin, and others), platelet glycoprotein IIb/IIIa inhibitor use during hospitalization, other in-hospital medications within the first 24 hours (statins, ACEIs/ARBs, aldosterone antagonists, and oral anticoagulants), route for PCI, and diagnosis on admission [ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction and unstable angina].
The status of DAPT within the first 24 hours was defined as the following four categories: non-DAPT (only one oral antiplatelet agent), non-loading DAPT (none of aspirin and a P2Y12 receptor inhibitor in loading dose), single-loading DAPT (either aspirin or a P2Y12 receptor inhibitor in loading dose), and both-loading DAPT (both aspirin and a P2Y12 receptor inhibitor in loading dose). The loading dose of aspirin was defined as ≥150 mg. The loading dose of P2Y12 receptor inhibitor was defined as ≥300 mg for clopidogrel and ≥180 mg for ticagrelor. eGFR was calculated according to the equation provided by the Chronic Kidney Disease Epidemiology Collaboration.10 These study variables are predefined and are detailed in supplementary material online, Table S1.
Definitions of study outcomes
The CCC-ACS project routinely collected bleeding data as a part of core in-hospital outcomes. Data collected included fatal bleeding, haemorrhagic stroke, bleeding in vital organs/locations (intracranial, spinal canal, retroperitoneal, pericardial, and intra-ocular with compromised vision), bleeding requiring clinical intervention (requiring pressors, surgery, or intravenous vasoactive agents), haemoglobin drop related to bleed (the admission level minus the nadir level), and bleeding requiring blood transfusion and total amount of transfusion. Based on these information, we defined a composite of major bleeds using the following three major bleeding definitions posteriori: (1) Bleeding Academic Research Consortium (BARC) type 3b-3c and type 5, which is defined as a haemoglobin drop of ≥5 g/dL or cardiac tamponade or bleeding requiring surgical intervention or bleeding requiring intravenous vasoactive agents (type 3b), intracranial haemorrhage (type 3c), or fatal bleeding (type 5), respectively;11 (2) Thrombolysis In Myocardial Infarction (TIMI) major bleeding, which is defined as intracranial haemorrhage or clinically overt bleeding associated with a haemoglobin drop of ≥5 g/dL, or fatal bleeding;12 (3) PLATelet inhibition and patient Outcomes (PLATO) life threatening bleeding, which is defined as fatal bleeding, intracranial bleeding, intraoperative bleeding with cardiac tamponade, severe hypotension, hypovolemic shock due to bleeding, and requiring either vasopressor or surgery, a haemoglobin drop of ≥5 g/dL, or the need for transfusion >4 U of whole blood or packed red blood cells.13 Coronary artery bypass grafting related bleeding was excluded. Other study outcomes included in-hospital mortality and less severe but clinically-significant in-hospital bleeds (defined as a haemoglobin drop of 3 to 5 g/dL).
Statistical analysis
Continuous data with a normal distribution are presented as the means and standard deviations. Continuous data with a skewed distribution are presented as the medians with 25th to 75th percentiles. Baseline demographic and clinical information were compared among patients not receiving early β-blockers, metoprolol users and bisoprolol users. The χ2 test was used for categorical variables. One-way ANOVA or Kruskal–Wallis test were used for continuous variables when appropriate. We imputed the missing data using the sequential regression multiple imputation method by IVEware (version 0.2; Survey Research Center, University of Michigan, Ann Arbor, MI) to impute variables with missing value.14 The missing rates of the study variables are shown in supplementary material online, Table S2.
According to the status of early β-blocker exposure and the proportions of patients in each category shown in Figure 1, by using Stata command ‘calipmath’ to perform a greedy matching algorithm with no replacement for all propensity score matching and a caliper width of 0.2 of the standard deviation of the logit of the propensity score was used for all matching,15 we performed the following five matching processes based on the 43 baseline covariates: (1) matching cohort 1: 1-to-1 matching for early β-blockers (without considering the type and dose of β-blockers) vs. no early β-blocker; (2) matching cohort 2: 1-to-1 matching for early low-dose β-blockers vs. no early β-blocker; (3) matching cohort 3: a maximal of 1-to-2 matching for early high-dose β-blockers vs. no early β-blocker; (4) matching cohort 4: a maximal of 1-to-2 matching for early bisoprolol vs. early metoprolol; (5) matching cohort 5: a maximal of 1-to-2 matching for high-dose bisoprolol (≥2.5 mg) vs. high-dose metoprolol (≥50 mg). The absolute standardized differences in baseline covariates were calculated pre- and post-matching; covariates with <10% difference after matching were considered optimally matched. The specifications of exposures, sample sizes and propensity score matching ratios in the above five matching cohorts are summarized in supplementary material online, Table S3.
We performed the following four sensitivity analyses to verify the associations between early β-blocker therapy and the study outcomes: (1) un-adjusted logistic regression models; (2) multivariable-adjusted logistic regression models; (3) a maximal of 1-to-2 matching for early β-blockers vs. no early β-blocker (another matching cohort 1 with a maximal of 1-to-2 matching) (4) inverse-probability-of-treatment-weighted regression adjustment (IPTW-RA) models; (5) excluding in-hospital death based on matching cohort 1; (6) excluding patients receiving unfractionated heparin; (7) excluding haemorrhagic stroke patients. In addition, a 1-to-1 matching for early ACEI/ARB vs. no early ACEI/ARB and the E-values was computed to assess the robustness of the associations to unmeasured or uncontrolled confounders.16
We performed the following interaction tests and subgroup analyses based on matching cohort 3: diagnosis on admission [STEMI and non-ST-elevation acute coronary syndrome (NSTE-ACS)], age (<65 years and ≥ 65 years), sex, hypertension, diabetes, eGFR (<60 mL/min/1.73 m2 and ≥60 mL/min/1.73 m2), DAPT status (full loading or not), platelet glycoprotein IIb/IIIa inhibitor use during hospitalization, and PCI route (femoral PCI or not). We used Stata (version 15.1; StataCorp, College Station, TX) for all analyses. A two-tailed P < 0.05 was considered statistically significant.
Results
Patient characteristics
Among 63 640 eligible patients, less than half (36.0%) received early oral β-blocker therapy (89.6% for early metoprolol and 10.4% for early bisoprolol). During hospitalization, 637 composite major bleeds and 476 deaths were recorded. The Table 1 shows the baseline clinical characteristics of patients by the status of early β-blocker use. Compared with early oral β-blocker users, non-users were more likely to be STEMI with concomitant worsening of Killip Class, with lower levels of BP and heart rate on admission, and lower prevalence of pre-hospital β-blocker use. Moreover, non-users received less in-hospital ACEI/ARB and aldosterone antagonist prescriptions. Notably, among patients on early β-blocker, bisoprolol users had almost the twice of the metoprolol-equivalent dose as received in metoprolol users.
Baseline patient characteristics
| . | . | Status of early β-blockers . | . | ||
|---|---|---|---|---|---|
| . | Total cohort (n = 43 640) . | No β-blocker (n = 27 913) . | Metoprolol (n = 14 092) . | Bisoprolol (n = 1635) . | P for Trend . |
| Age(years) | 62.3 ± 11.9 | 62.7 ± 11.8 | 61.5 ± 12.2 | 62.1 ± 12.2 | <0.001 |
| Male, No. (%) | 33 869 (77.6) | 21 671 (77.6) | 10 926 (77.5) | 1272 (77.8) | 0.95 |
| Smoking, No. (%) | 21 096 (48.3) | 13 348 (47.8) | 6985 (49.6) | 763 (46.7) | 0.001 |
| Acute coronary syndrome subtypes, No. (%) | |||||
| STEMI | 29 297 (67.1) | 19 599 (70.2) | 8776 (62.3) | 922 (56.4) | <0.001 |
| NSTEMI | 9493 (21.8) | 5349 (19.2) | 3686 (26.2) | 458 (28.0) | |
| Unstable angina | 4850 (11.1) | 2965 (10.6) | 1630 (11.6) | 255 (15.6) | |
| Metoprolol-equivalent dose in the first 24 hours of medical contact (mg/day) * | N/A | N/A | 30.4 ± 17.6 | 63.5 ± 48.8 | <0.001 |
| Previous history, No. (%) | |||||
| Myocardial infarction | 2785 (6.40) | 1508 (5.40) | 1099 (7.80) | 178 (10.9) | <0.001 |
| PCI | 3194 (7.30) | 1724 (6.20) | 1259 (8.90) | 211 (12.9) | 0.001 |
| Coronary bypass grafting | 130 (0.30) | 73 (0.30) | 52 (0.40) | 5 (0.30) | 0.075 |
| Diabetes | 9194 (21.1) | 5542 (19.9) | 3226 (22.9) | 426 (26.1) | <0.001 |
| Dyslipidaemia | 2912 (6.70) | 1817 (6.50) | 966 (6.90) | 129 (7.90) | <0.001 |
| Hypertension | 21 874 (50.1) | 13 400 (48.0) | 7560 (53.6) | 914 (55.9) | <0.001 |
| Heart failure | 353 (0.80) | 209 (0.70) | 118 (0.80) | 26 (1.60) | <0.001 |
| Renal failure | 454 (1.00) | 285 (1.00) | 140 (1.00) | 29 (1.80) | 0.011 |
| Atrial fibrillation | 637 (1.50) | 411 (1.50) | 196 (1.40) | 30 (1.80) | 0.35 |
| Ischaemic stroke | 2625 (6.00) | 1791 (6.40) | 744 (5.30) | 90 (5.50) | <0.001 |
| Haemorrhagic stroke | 248 (0.60) | 171 (0.60) | 71 (0.50) | 6 (0.40) | 0.2 |
| Peripheral vascular disease | 306 (0.70) | 200 (0.70) | 88 (0.60) | 18 (1.10) | 0.081 |
| Chronic obstructive lung disease | 471 (1.10) | 336 (1.20) | 111 (0.80) | 24 (1.50) | <0.001 |
| Systolic blood pressure, mmHg | 129.0 ± 23.3 | 126.8 ± 23.5 | 133.0 ± 22.4 | 133.6 ± 22.8 | <0.001 |
| Diastolic blood pressure, mmHg | 77.7 ± 14.3 | 76.2 ± 14.2 | 80.5 ± 14.1 | 81.0 ± 14.2 | <0.001 |
| Heart rate, beats/min | 76.2 ± 15.6 | 74.4 ± 16.0 | 79.4 ± 14.2 | 79.3 ± 14.9 | <0.001 |
| Killip Class > I, No. (%) | 12 445 (28.5) | 8164 (29.2) | 3816 (27.1) | 465 (28.4) | <0.001 |
| Low density lipoprotein cholesterol, mg/dL | 104 (82.0 to 129) | 104 (81.0 to 129) | 105 (82.0 to 130) | 108 (85.0 to 134) | <0.001 |
| High density lipoprotein cholesterol, mg/dL | 40.0 (34.0 to 48.0) | 41.0 (34.0 to 49.0) | 40.0 (34.0 to 48.0) | 41.0 (34.0 to 49.0) | <0.001 |
| Triglycerides, mg/dL | 130 (90.0 to 194) | 127 (88.0 to 189.0) | 136 (95.0 to 202) | 136 (96.0 to 208) | <0.001 |
| eGFR, mL/min/1.73m2 | 84.9 ± 22.9 | 83.9 ± 23.1 | 86.9 ± 22.5 | 84.5 ± 22.8 | <0.001 |
| Haemoglobin on admission, g/dL | 139 (126 to 151) | 138 (126 to 150) | 140 (127 to 152) | 141 (128 to 152) | <0.001 |
| Pre-hospital medication in the past 2 weeks, No. (%) | |||||
| Aspirin | 8180 (18.7) | 5014 (18.0) | 2795 (19.8) | 371 (22.7) | <0.001 |
| P2Y12 inhibitor | 6175 (14.1) | 3819 (13.7) | 2104 (14.9) | 252 (15.4) | <0.001 |
| Statin | 6513 (14.9) | 3826 (13.7) | 2343 (16.6) | 344 (21.0) | <0.001 |
| Oral anticoagulants | 78 (0.20) | 44 (0.20) | 29 (0.20) | 5 (0.30) | 0.25 |
| β-blocker | 2654 (6.10) | 640 (2.30) | 1750 (12.4) | 264 (16.1) | <0.001 |
| ACEI/ARB | 3991 (9.10) | 2069 (7.40) | 1644 (11.7) | 278 (17.0) | <0.001 |
| Aldosterone antagonist | 437 (1.00) | 224 (0.80) | 171 (1.20) | 42 (2.60) | <0.001 |
| Dual Antiplatelet therapy (DAPT) status in the first 24 hours of medical contact, No. (%) | |||||
| Non-DAPT | NA | NA | NA | NA | <0.001 |
| DAPT, neither loading dose | 3943 (9.00) | 2514 (9.00) | 1320 (9.40) | 109 (6.70) | |
| DAPT, either loading dose | 12 848 (29.4) | 9519 (34.1) | 2892 (20.5) | 437 (26.7) | |
| DAPT, both loading dose | 26 849 (61.5) | 15 880 (56.9) | 9880 (70.1) | 1089 (66.6) | |
| Anticoagulation therapy following PCI, No. (%) | |||||
| Unfractionated heparin | 1695 (3.90) | 1235 (4.40) | 412 (2.90) | 48 (2.90) | <0.001 |
| Low molecular weight heparin | 28 707 (65.8) | 18 204 (64.9) | 9542 (67.7) | 1061 (64.9) | <0.001 |
| Others | 720 (1.60) | 497 (1.80) | 204 (1.40) | 19 (1.20) | 0.012 |
| Glycoprotein IIb/IIIa inhibitor, No. (%) | 14 867 (34.1) | 10 119 (36.3) | 4407 (31.3) | 341 (20.9) | <0.001 |
| Other in-hospital medications in the first 24 hours of medical contact, No. (%) | |||||
| Statin | 40 652 (93.2) | 25 245 (90.4) | 13 808 (98.0) | 1599 (97.8) | <0.001 |
| Oral anticoagulant | 197 (0.50) | 91 (0.30) | 85 (0.60) | 21 (1.30) | <0.001 |
| ACEI/ARB | 17 939 (41.1) | 8175 (29.3) | 8728 (61.9) | 1036 (63.4) | <0.001 |
| Aldosterone antagonist | 5657 (13.0) | 3120 (11.2) | 2256 (16.0) | 281 (17.2) | <0.001 |
| Radial route for PCI, No. (%) | 40 935 (93.8) | 25 875 (92.7) | 13 532 (96.0) | 1528 (93.5) | <0.001 |
| . | . | Status of early β-blockers . | . | ||
|---|---|---|---|---|---|
| . | Total cohort (n = 43 640) . | No β-blocker (n = 27 913) . | Metoprolol (n = 14 092) . | Bisoprolol (n = 1635) . | P for Trend . |
| Age(years) | 62.3 ± 11.9 | 62.7 ± 11.8 | 61.5 ± 12.2 | 62.1 ± 12.2 | <0.001 |
| Male, No. (%) | 33 869 (77.6) | 21 671 (77.6) | 10 926 (77.5) | 1272 (77.8) | 0.95 |
| Smoking, No. (%) | 21 096 (48.3) | 13 348 (47.8) | 6985 (49.6) | 763 (46.7) | 0.001 |
| Acute coronary syndrome subtypes, No. (%) | |||||
| STEMI | 29 297 (67.1) | 19 599 (70.2) | 8776 (62.3) | 922 (56.4) | <0.001 |
| NSTEMI | 9493 (21.8) | 5349 (19.2) | 3686 (26.2) | 458 (28.0) | |
| Unstable angina | 4850 (11.1) | 2965 (10.6) | 1630 (11.6) | 255 (15.6) | |
| Metoprolol-equivalent dose in the first 24 hours of medical contact (mg/day) * | N/A | N/A | 30.4 ± 17.6 | 63.5 ± 48.8 | <0.001 |
| Previous history, No. (%) | |||||
| Myocardial infarction | 2785 (6.40) | 1508 (5.40) | 1099 (7.80) | 178 (10.9) | <0.001 |
| PCI | 3194 (7.30) | 1724 (6.20) | 1259 (8.90) | 211 (12.9) | 0.001 |
| Coronary bypass grafting | 130 (0.30) | 73 (0.30) | 52 (0.40) | 5 (0.30) | 0.075 |
| Diabetes | 9194 (21.1) | 5542 (19.9) | 3226 (22.9) | 426 (26.1) | <0.001 |
| Dyslipidaemia | 2912 (6.70) | 1817 (6.50) | 966 (6.90) | 129 (7.90) | <0.001 |
| Hypertension | 21 874 (50.1) | 13 400 (48.0) | 7560 (53.6) | 914 (55.9) | <0.001 |
| Heart failure | 353 (0.80) | 209 (0.70) | 118 (0.80) | 26 (1.60) | <0.001 |
| Renal failure | 454 (1.00) | 285 (1.00) | 140 (1.00) | 29 (1.80) | 0.011 |
| Atrial fibrillation | 637 (1.50) | 411 (1.50) | 196 (1.40) | 30 (1.80) | 0.35 |
| Ischaemic stroke | 2625 (6.00) | 1791 (6.40) | 744 (5.30) | 90 (5.50) | <0.001 |
| Haemorrhagic stroke | 248 (0.60) | 171 (0.60) | 71 (0.50) | 6 (0.40) | 0.2 |
| Peripheral vascular disease | 306 (0.70) | 200 (0.70) | 88 (0.60) | 18 (1.10) | 0.081 |
| Chronic obstructive lung disease | 471 (1.10) | 336 (1.20) | 111 (0.80) | 24 (1.50) | <0.001 |
| Systolic blood pressure, mmHg | 129.0 ± 23.3 | 126.8 ± 23.5 | 133.0 ± 22.4 | 133.6 ± 22.8 | <0.001 |
| Diastolic blood pressure, mmHg | 77.7 ± 14.3 | 76.2 ± 14.2 | 80.5 ± 14.1 | 81.0 ± 14.2 | <0.001 |
| Heart rate, beats/min | 76.2 ± 15.6 | 74.4 ± 16.0 | 79.4 ± 14.2 | 79.3 ± 14.9 | <0.001 |
| Killip Class > I, No. (%) | 12 445 (28.5) | 8164 (29.2) | 3816 (27.1) | 465 (28.4) | <0.001 |
| Low density lipoprotein cholesterol, mg/dL | 104 (82.0 to 129) | 104 (81.0 to 129) | 105 (82.0 to 130) | 108 (85.0 to 134) | <0.001 |
| High density lipoprotein cholesterol, mg/dL | 40.0 (34.0 to 48.0) | 41.0 (34.0 to 49.0) | 40.0 (34.0 to 48.0) | 41.0 (34.0 to 49.0) | <0.001 |
| Triglycerides, mg/dL | 130 (90.0 to 194) | 127 (88.0 to 189.0) | 136 (95.0 to 202) | 136 (96.0 to 208) | <0.001 |
| eGFR, mL/min/1.73m2 | 84.9 ± 22.9 | 83.9 ± 23.1 | 86.9 ± 22.5 | 84.5 ± 22.8 | <0.001 |
| Haemoglobin on admission, g/dL | 139 (126 to 151) | 138 (126 to 150) | 140 (127 to 152) | 141 (128 to 152) | <0.001 |
| Pre-hospital medication in the past 2 weeks, No. (%) | |||||
| Aspirin | 8180 (18.7) | 5014 (18.0) | 2795 (19.8) | 371 (22.7) | <0.001 |
| P2Y12 inhibitor | 6175 (14.1) | 3819 (13.7) | 2104 (14.9) | 252 (15.4) | <0.001 |
| Statin | 6513 (14.9) | 3826 (13.7) | 2343 (16.6) | 344 (21.0) | <0.001 |
| Oral anticoagulants | 78 (0.20) | 44 (0.20) | 29 (0.20) | 5 (0.30) | 0.25 |
| β-blocker | 2654 (6.10) | 640 (2.30) | 1750 (12.4) | 264 (16.1) | <0.001 |
| ACEI/ARB | 3991 (9.10) | 2069 (7.40) | 1644 (11.7) | 278 (17.0) | <0.001 |
| Aldosterone antagonist | 437 (1.00) | 224 (0.80) | 171 (1.20) | 42 (2.60) | <0.001 |
| Dual Antiplatelet therapy (DAPT) status in the first 24 hours of medical contact, No. (%) | |||||
| Non-DAPT | NA | NA | NA | NA | <0.001 |
| DAPT, neither loading dose | 3943 (9.00) | 2514 (9.00) | 1320 (9.40) | 109 (6.70) | |
| DAPT, either loading dose | 12 848 (29.4) | 9519 (34.1) | 2892 (20.5) | 437 (26.7) | |
| DAPT, both loading dose | 26 849 (61.5) | 15 880 (56.9) | 9880 (70.1) | 1089 (66.6) | |
| Anticoagulation therapy following PCI, No. (%) | |||||
| Unfractionated heparin | 1695 (3.90) | 1235 (4.40) | 412 (2.90) | 48 (2.90) | <0.001 |
| Low molecular weight heparin | 28 707 (65.8) | 18 204 (64.9) | 9542 (67.7) | 1061 (64.9) | <0.001 |
| Others | 720 (1.60) | 497 (1.80) | 204 (1.40) | 19 (1.20) | 0.012 |
| Glycoprotein IIb/IIIa inhibitor, No. (%) | 14 867 (34.1) | 10 119 (36.3) | 4407 (31.3) | 341 (20.9) | <0.001 |
| Other in-hospital medications in the first 24 hours of medical contact, No. (%) | |||||
| Statin | 40 652 (93.2) | 25 245 (90.4) | 13 808 (98.0) | 1599 (97.8) | <0.001 |
| Oral anticoagulant | 197 (0.50) | 91 (0.30) | 85 (0.60) | 21 (1.30) | <0.001 |
| ACEI/ARB | 17 939 (41.1) | 8175 (29.3) | 8728 (61.9) | 1036 (63.4) | <0.001 |
| Aldosterone antagonist | 5657 (13.0) | 3120 (11.2) | 2256 (16.0) | 281 (17.2) | <0.001 |
| Radial route for PCI, No. (%) | 40 935 (93.8) | 25 875 (92.7) | 13 532 (96.0) | 1528 (93.5) | <0.001 |
*Bisoprolol dose was converted to a metoprolol-equivalent dose by multiplying a factor of 20. P value between metoprolol and bisoprolol users was calculated based on an unpaired t test.
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; DAPT = dual antiplatelet therapy; eGFR = estimated glomerular filtration rate; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction; NSTEMI = non-ST-elevation myocardial infarction.
Baseline patient characteristics
| . | . | Status of early β-blockers . | . | ||
|---|---|---|---|---|---|
| . | Total cohort (n = 43 640) . | No β-blocker (n = 27 913) . | Metoprolol (n = 14 092) . | Bisoprolol (n = 1635) . | P for Trend . |
| Age(years) | 62.3 ± 11.9 | 62.7 ± 11.8 | 61.5 ± 12.2 | 62.1 ± 12.2 | <0.001 |
| Male, No. (%) | 33 869 (77.6) | 21 671 (77.6) | 10 926 (77.5) | 1272 (77.8) | 0.95 |
| Smoking, No. (%) | 21 096 (48.3) | 13 348 (47.8) | 6985 (49.6) | 763 (46.7) | 0.001 |
| Acute coronary syndrome subtypes, No. (%) | |||||
| STEMI | 29 297 (67.1) | 19 599 (70.2) | 8776 (62.3) | 922 (56.4) | <0.001 |
| NSTEMI | 9493 (21.8) | 5349 (19.2) | 3686 (26.2) | 458 (28.0) | |
| Unstable angina | 4850 (11.1) | 2965 (10.6) | 1630 (11.6) | 255 (15.6) | |
| Metoprolol-equivalent dose in the first 24 hours of medical contact (mg/day) * | N/A | N/A | 30.4 ± 17.6 | 63.5 ± 48.8 | <0.001 |
| Previous history, No. (%) | |||||
| Myocardial infarction | 2785 (6.40) | 1508 (5.40) | 1099 (7.80) | 178 (10.9) | <0.001 |
| PCI | 3194 (7.30) | 1724 (6.20) | 1259 (8.90) | 211 (12.9) | 0.001 |
| Coronary bypass grafting | 130 (0.30) | 73 (0.30) | 52 (0.40) | 5 (0.30) | 0.075 |
| Diabetes | 9194 (21.1) | 5542 (19.9) | 3226 (22.9) | 426 (26.1) | <0.001 |
| Dyslipidaemia | 2912 (6.70) | 1817 (6.50) | 966 (6.90) | 129 (7.90) | <0.001 |
| Hypertension | 21 874 (50.1) | 13 400 (48.0) | 7560 (53.6) | 914 (55.9) | <0.001 |
| Heart failure | 353 (0.80) | 209 (0.70) | 118 (0.80) | 26 (1.60) | <0.001 |
| Renal failure | 454 (1.00) | 285 (1.00) | 140 (1.00) | 29 (1.80) | 0.011 |
| Atrial fibrillation | 637 (1.50) | 411 (1.50) | 196 (1.40) | 30 (1.80) | 0.35 |
| Ischaemic stroke | 2625 (6.00) | 1791 (6.40) | 744 (5.30) | 90 (5.50) | <0.001 |
| Haemorrhagic stroke | 248 (0.60) | 171 (0.60) | 71 (0.50) | 6 (0.40) | 0.2 |
| Peripheral vascular disease | 306 (0.70) | 200 (0.70) | 88 (0.60) | 18 (1.10) | 0.081 |
| Chronic obstructive lung disease | 471 (1.10) | 336 (1.20) | 111 (0.80) | 24 (1.50) | <0.001 |
| Systolic blood pressure, mmHg | 129.0 ± 23.3 | 126.8 ± 23.5 | 133.0 ± 22.4 | 133.6 ± 22.8 | <0.001 |
| Diastolic blood pressure, mmHg | 77.7 ± 14.3 | 76.2 ± 14.2 | 80.5 ± 14.1 | 81.0 ± 14.2 | <0.001 |
| Heart rate, beats/min | 76.2 ± 15.6 | 74.4 ± 16.0 | 79.4 ± 14.2 | 79.3 ± 14.9 | <0.001 |
| Killip Class > I, No. (%) | 12 445 (28.5) | 8164 (29.2) | 3816 (27.1) | 465 (28.4) | <0.001 |
| Low density lipoprotein cholesterol, mg/dL | 104 (82.0 to 129) | 104 (81.0 to 129) | 105 (82.0 to 130) | 108 (85.0 to 134) | <0.001 |
| High density lipoprotein cholesterol, mg/dL | 40.0 (34.0 to 48.0) | 41.0 (34.0 to 49.0) | 40.0 (34.0 to 48.0) | 41.0 (34.0 to 49.0) | <0.001 |
| Triglycerides, mg/dL | 130 (90.0 to 194) | 127 (88.0 to 189.0) | 136 (95.0 to 202) | 136 (96.0 to 208) | <0.001 |
| eGFR, mL/min/1.73m2 | 84.9 ± 22.9 | 83.9 ± 23.1 | 86.9 ± 22.5 | 84.5 ± 22.8 | <0.001 |
| Haemoglobin on admission, g/dL | 139 (126 to 151) | 138 (126 to 150) | 140 (127 to 152) | 141 (128 to 152) | <0.001 |
| Pre-hospital medication in the past 2 weeks, No. (%) | |||||
| Aspirin | 8180 (18.7) | 5014 (18.0) | 2795 (19.8) | 371 (22.7) | <0.001 |
| P2Y12 inhibitor | 6175 (14.1) | 3819 (13.7) | 2104 (14.9) | 252 (15.4) | <0.001 |
| Statin | 6513 (14.9) | 3826 (13.7) | 2343 (16.6) | 344 (21.0) | <0.001 |
| Oral anticoagulants | 78 (0.20) | 44 (0.20) | 29 (0.20) | 5 (0.30) | 0.25 |
| β-blocker | 2654 (6.10) | 640 (2.30) | 1750 (12.4) | 264 (16.1) | <0.001 |
| ACEI/ARB | 3991 (9.10) | 2069 (7.40) | 1644 (11.7) | 278 (17.0) | <0.001 |
| Aldosterone antagonist | 437 (1.00) | 224 (0.80) | 171 (1.20) | 42 (2.60) | <0.001 |
| Dual Antiplatelet therapy (DAPT) status in the first 24 hours of medical contact, No. (%) | |||||
| Non-DAPT | NA | NA | NA | NA | <0.001 |
| DAPT, neither loading dose | 3943 (9.00) | 2514 (9.00) | 1320 (9.40) | 109 (6.70) | |
| DAPT, either loading dose | 12 848 (29.4) | 9519 (34.1) | 2892 (20.5) | 437 (26.7) | |
| DAPT, both loading dose | 26 849 (61.5) | 15 880 (56.9) | 9880 (70.1) | 1089 (66.6) | |
| Anticoagulation therapy following PCI, No. (%) | |||||
| Unfractionated heparin | 1695 (3.90) | 1235 (4.40) | 412 (2.90) | 48 (2.90) | <0.001 |
| Low molecular weight heparin | 28 707 (65.8) | 18 204 (64.9) | 9542 (67.7) | 1061 (64.9) | <0.001 |
| Others | 720 (1.60) | 497 (1.80) | 204 (1.40) | 19 (1.20) | 0.012 |
| Glycoprotein IIb/IIIa inhibitor, No. (%) | 14 867 (34.1) | 10 119 (36.3) | 4407 (31.3) | 341 (20.9) | <0.001 |
| Other in-hospital medications in the first 24 hours of medical contact, No. (%) | |||||
| Statin | 40 652 (93.2) | 25 245 (90.4) | 13 808 (98.0) | 1599 (97.8) | <0.001 |
| Oral anticoagulant | 197 (0.50) | 91 (0.30) | 85 (0.60) | 21 (1.30) | <0.001 |
| ACEI/ARB | 17 939 (41.1) | 8175 (29.3) | 8728 (61.9) | 1036 (63.4) | <0.001 |
| Aldosterone antagonist | 5657 (13.0) | 3120 (11.2) | 2256 (16.0) | 281 (17.2) | <0.001 |
| Radial route for PCI, No. (%) | 40 935 (93.8) | 25 875 (92.7) | 13 532 (96.0) | 1528 (93.5) | <0.001 |
| . | . | Status of early β-blockers . | . | ||
|---|---|---|---|---|---|
| . | Total cohort (n = 43 640) . | No β-blocker (n = 27 913) . | Metoprolol (n = 14 092) . | Bisoprolol (n = 1635) . | P for Trend . |
| Age(years) | 62.3 ± 11.9 | 62.7 ± 11.8 | 61.5 ± 12.2 | 62.1 ± 12.2 | <0.001 |
| Male, No. (%) | 33 869 (77.6) | 21 671 (77.6) | 10 926 (77.5) | 1272 (77.8) | 0.95 |
| Smoking, No. (%) | 21 096 (48.3) | 13 348 (47.8) | 6985 (49.6) | 763 (46.7) | 0.001 |
| Acute coronary syndrome subtypes, No. (%) | |||||
| STEMI | 29 297 (67.1) | 19 599 (70.2) | 8776 (62.3) | 922 (56.4) | <0.001 |
| NSTEMI | 9493 (21.8) | 5349 (19.2) | 3686 (26.2) | 458 (28.0) | |
| Unstable angina | 4850 (11.1) | 2965 (10.6) | 1630 (11.6) | 255 (15.6) | |
| Metoprolol-equivalent dose in the first 24 hours of medical contact (mg/day) * | N/A | N/A | 30.4 ± 17.6 | 63.5 ± 48.8 | <0.001 |
| Previous history, No. (%) | |||||
| Myocardial infarction | 2785 (6.40) | 1508 (5.40) | 1099 (7.80) | 178 (10.9) | <0.001 |
| PCI | 3194 (7.30) | 1724 (6.20) | 1259 (8.90) | 211 (12.9) | 0.001 |
| Coronary bypass grafting | 130 (0.30) | 73 (0.30) | 52 (0.40) | 5 (0.30) | 0.075 |
| Diabetes | 9194 (21.1) | 5542 (19.9) | 3226 (22.9) | 426 (26.1) | <0.001 |
| Dyslipidaemia | 2912 (6.70) | 1817 (6.50) | 966 (6.90) | 129 (7.90) | <0.001 |
| Hypertension | 21 874 (50.1) | 13 400 (48.0) | 7560 (53.6) | 914 (55.9) | <0.001 |
| Heart failure | 353 (0.80) | 209 (0.70) | 118 (0.80) | 26 (1.60) | <0.001 |
| Renal failure | 454 (1.00) | 285 (1.00) | 140 (1.00) | 29 (1.80) | 0.011 |
| Atrial fibrillation | 637 (1.50) | 411 (1.50) | 196 (1.40) | 30 (1.80) | 0.35 |
| Ischaemic stroke | 2625 (6.00) | 1791 (6.40) | 744 (5.30) | 90 (5.50) | <0.001 |
| Haemorrhagic stroke | 248 (0.60) | 171 (0.60) | 71 (0.50) | 6 (0.40) | 0.2 |
| Peripheral vascular disease | 306 (0.70) | 200 (0.70) | 88 (0.60) | 18 (1.10) | 0.081 |
| Chronic obstructive lung disease | 471 (1.10) | 336 (1.20) | 111 (0.80) | 24 (1.50) | <0.001 |
| Systolic blood pressure, mmHg | 129.0 ± 23.3 | 126.8 ± 23.5 | 133.0 ± 22.4 | 133.6 ± 22.8 | <0.001 |
| Diastolic blood pressure, mmHg | 77.7 ± 14.3 | 76.2 ± 14.2 | 80.5 ± 14.1 | 81.0 ± 14.2 | <0.001 |
| Heart rate, beats/min | 76.2 ± 15.6 | 74.4 ± 16.0 | 79.4 ± 14.2 | 79.3 ± 14.9 | <0.001 |
| Killip Class > I, No. (%) | 12 445 (28.5) | 8164 (29.2) | 3816 (27.1) | 465 (28.4) | <0.001 |
| Low density lipoprotein cholesterol, mg/dL | 104 (82.0 to 129) | 104 (81.0 to 129) | 105 (82.0 to 130) | 108 (85.0 to 134) | <0.001 |
| High density lipoprotein cholesterol, mg/dL | 40.0 (34.0 to 48.0) | 41.0 (34.0 to 49.0) | 40.0 (34.0 to 48.0) | 41.0 (34.0 to 49.0) | <0.001 |
| Triglycerides, mg/dL | 130 (90.0 to 194) | 127 (88.0 to 189.0) | 136 (95.0 to 202) | 136 (96.0 to 208) | <0.001 |
| eGFR, mL/min/1.73m2 | 84.9 ± 22.9 | 83.9 ± 23.1 | 86.9 ± 22.5 | 84.5 ± 22.8 | <0.001 |
| Haemoglobin on admission, g/dL | 139 (126 to 151) | 138 (126 to 150) | 140 (127 to 152) | 141 (128 to 152) | <0.001 |
| Pre-hospital medication in the past 2 weeks, No. (%) | |||||
| Aspirin | 8180 (18.7) | 5014 (18.0) | 2795 (19.8) | 371 (22.7) | <0.001 |
| P2Y12 inhibitor | 6175 (14.1) | 3819 (13.7) | 2104 (14.9) | 252 (15.4) | <0.001 |
| Statin | 6513 (14.9) | 3826 (13.7) | 2343 (16.6) | 344 (21.0) | <0.001 |
| Oral anticoagulants | 78 (0.20) | 44 (0.20) | 29 (0.20) | 5 (0.30) | 0.25 |
| β-blocker | 2654 (6.10) | 640 (2.30) | 1750 (12.4) | 264 (16.1) | <0.001 |
| ACEI/ARB | 3991 (9.10) | 2069 (7.40) | 1644 (11.7) | 278 (17.0) | <0.001 |
| Aldosterone antagonist | 437 (1.00) | 224 (0.80) | 171 (1.20) | 42 (2.60) | <0.001 |
| Dual Antiplatelet therapy (DAPT) status in the first 24 hours of medical contact, No. (%) | |||||
| Non-DAPT | NA | NA | NA | NA | <0.001 |
| DAPT, neither loading dose | 3943 (9.00) | 2514 (9.00) | 1320 (9.40) | 109 (6.70) | |
| DAPT, either loading dose | 12 848 (29.4) | 9519 (34.1) | 2892 (20.5) | 437 (26.7) | |
| DAPT, both loading dose | 26 849 (61.5) | 15 880 (56.9) | 9880 (70.1) | 1089 (66.6) | |
| Anticoagulation therapy following PCI, No. (%) | |||||
| Unfractionated heparin | 1695 (3.90) | 1235 (4.40) | 412 (2.90) | 48 (2.90) | <0.001 |
| Low molecular weight heparin | 28 707 (65.8) | 18 204 (64.9) | 9542 (67.7) | 1061 (64.9) | <0.001 |
| Others | 720 (1.60) | 497 (1.80) | 204 (1.40) | 19 (1.20) | 0.012 |
| Glycoprotein IIb/IIIa inhibitor, No. (%) | 14 867 (34.1) | 10 119 (36.3) | 4407 (31.3) | 341 (20.9) | <0.001 |
| Other in-hospital medications in the first 24 hours of medical contact, No. (%) | |||||
| Statin | 40 652 (93.2) | 25 245 (90.4) | 13 808 (98.0) | 1599 (97.8) | <0.001 |
| Oral anticoagulant | 197 (0.50) | 91 (0.30) | 85 (0.60) | 21 (1.30) | <0.001 |
| ACEI/ARB | 17 939 (41.1) | 8175 (29.3) | 8728 (61.9) | 1036 (63.4) | <0.001 |
| Aldosterone antagonist | 5657 (13.0) | 3120 (11.2) | 2256 (16.0) | 281 (17.2) | <0.001 |
| Radial route for PCI, No. (%) | 40 935 (93.8) | 25 875 (92.7) | 13 532 (96.0) | 1528 (93.5) | <0.001 |
*Bisoprolol dose was converted to a metoprolol-equivalent dose by multiplying a factor of 20. P value between metoprolol and bisoprolol users was calculated based on an unpaired t test.
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; DAPT = dual antiplatelet therapy; eGFR = estimated glomerular filtration rate; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction; NSTEMI = non-ST-elevation myocardial infarction.
Associations between early β-blocker exposure and in-hospital bleeding and mortality
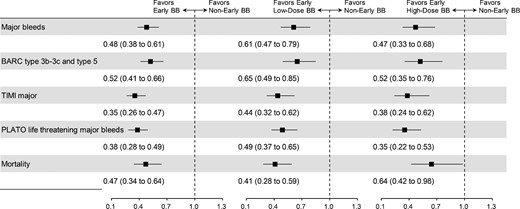
In propensity-score matching-based analyses, the baseline clinical characteristics were well-balanced between patients receiving early β-blocker and no early β-blocker (matching cohort 1, supplementary materials online, Tables S4 and Figure S1), between patients receiving early low-dose β-blocker and no early β-blocker (matching cohort 2 and supplementary material online, Figure S2A), and between patients receiving early high-dose β-blocker and no early β-blocker (matching cohort 3 and supplementary material online, Figure S2B). Based on these three matching cohorts, we confirmed an inverse association between early β-blocker use and major bleeding risk, as well as early low-dose β-blocker and major bleeding risk, and the association was further strengthened by early high-dose β-blocker use, which was associated with a 53% reduction (OR: 0.47, 95% CI: 0.33 to 0.68) in major bleeds. Therefore, the associations between early β-blocker vs. no early β-blocker and major in-hospital bleeds presented a dose-dependent manner. Notably, early β-blocker use, regardless of dosing, was consistently associated with reduced in-hospital mortality (Figure 2). The above results are detailed in supplementary material online, Table S5.
Forest plots for the associations between early β-blocker use and study outcomes in propensity score matching based analyses. The left panel represents early β-blocker vs. no early β-blocker, regardless of dosing, in a 1-to-1 propensity score matched sample. The middle panel represents early low-dose (metoprolol-equivalent dose <50 mg/day) β-blocker vs. no early β-blocker in a 1-to-1 propensity score matched sample. The right panel represents early high-dose (metoprolol-equivalent dose ≥ 50 mg/day) β-blocker vs. no early β-blocker in a maximal of 1-to-2 propensity score matched sample. BARC = Bleeding Academic Research Consortium; TIMI = Thrombolysis In Myocardial Infarction; PLATO = PLATelet inhibition and patient Outcomes.
Early bisoprolol vs. early metoprolol on in-hospital bleeding and mortality
In a 1-to-2 matching based analysis (matching cohort 4), without considering the dosing, early bisoprolol use was not statistically associated with major bleeding as compared with metoprolol (Supplementary material online, Figure S3). Based on a metoprolol dose-equivalent matching (matching cohort 5) of bisoprolol dose ≥2.5 mg/day and metoprolol dose ≥50 mg/day, there was no difference between high-dose bisoprolol and high-dose metoprolol in terms major bleeds and in-hospital death (Supplementary material online, Figure S3). These results can also be found in detail in the supplementary material online, Table S5. Therefore, the bleeding reduction effect of oral β-blocker seems to be a class effect and not dependent on specific type.
Subgroup and interacting analyses
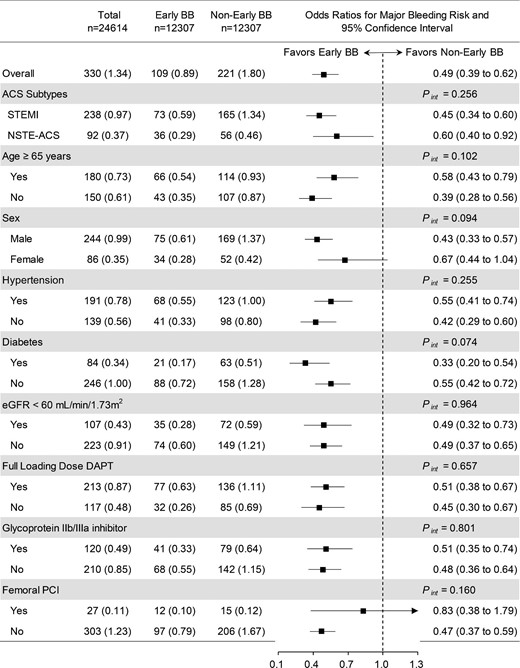
Figure 3 shows that the association between early β-blocker use, and composite major bleeds was consistent across subgroups based on matching cohort 1. Although insignificant results were seen in female and femoral route for PCI subgroups, they should be due to the small sample size. No significant interaction was observed among subgroups.
Subgroup analyses of the association between early β-blocker and composite of major bleeds based on matching cohort 1 (early β-blocker vs. no early β-blocker). ACS = acute coronary syndrome; DAPT = dual antiplatelet therapy; eGFR = estimated glomerular filtration rate; NSTE-ACS = non-ST-elevation acute coronary syndrome; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction. Pint = P value for interaction.
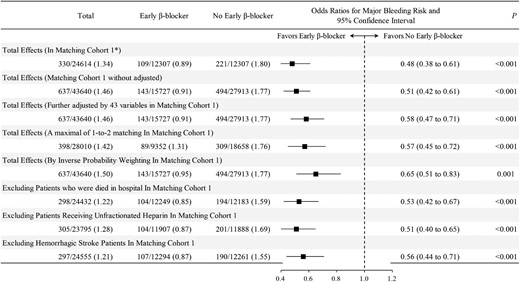
Sensitivity analyses for the associations of early β-blocker vs. no early β-blocker and bleeding risk
The associations between early β-blocker and study outcomes were consistent in un-adjustment, multivariable adjustment, IPTW-RA models, a maximal of 1-to-2 matched sample, and the association remained significant when in-hospital deaths, unfractionated heparin received, and haemorrhagic stroke patients were excluded (Figure 4). In a 1-to-1 matched cohort, although early ACEI/ARB use was associated with reduced in-hospital death, its association with bleeding risk was neutral, indicating that the observed association between high-dose β-blocker and major bleeds was not likely due to a systemic BP-lowering effect. Additionally, the E-values for the associations between early high-dose β-blocker and major bleeds and deaths were 3.68 and 3.59 (Supplementary material online, Figure S4), respectively. To our knowledge, unmeasured/unknown confounders would be less likely to have an association with both high-dose β-blocker and study outcomes (major bleeding/death) to an extent by having an odds ratio exceeding 3.68 and 3.59, respectively.
Sensitivity analyses for the associations of early β-blocker vs. no early β-blocker and bleeding risk
Discussion
In the present study, we compared in-hospital bleeding risk between patients receiving early oral β-blocker vs. no early β-blocker therapy in a large nationwide registry of ACS following PCI. Our main findings are summarized as follows. First, early oral β-blocker therapy was associated with a risk reduction in major in-hospital bleeds, and this association was more evident among patients received metoprolol-equivalent dose of ≥50 mg/day. This finding was consistent by all methods used. Second, no obvious difference was observed between metoprolol and bisoprolol in terms of bleeding and mortality risk reduction, indicating a potential class effect of oral β-blockers. Since the life-saving effect of early oral β-blocker in ACS population was well established, these findings made a step forward by unveiling a previously unrecognized dose-dependent role of early oral β-blockers as a potential bleeding avoidance strategy for ACS patients following PCI. While currently several large randomized trials are underway to confirm the long-term clinical benefit of oral β-blocker following MI,17 the immediate oral β-blocker use, particularly at recommended dosing, should be encouraged and strengthened in the acute management of ACS patients.
In the current reperfusion era, with the wider application of PCI and the advent of more potent antithrombotic agents, the concomitant increase in bleeding complications warrants the implementation of novel cost-effective bleeding avoidance approaches. Enlightened by the following previous reports, we specifically addressed the association between early oral β-blocker use and bleeding risk in ACS patients. First, in one study based on the GRACE registry,4 the use of β-blocker among ACS patients, without having specified the timing and dosing, was associated with reduced major in-hospital bleeds in univariate analysis. Second, in a population-based cohort study of first-time users of antihypertensive medications in the early 1990s, β-blocker use was associated with reduced incident hospitalizations for gastrointestinal bleeding.5 Third, based on the accumulative evidence from 1980s, non-selective β-blockers have been recommended for the primary and secondary prophylaxis of gastrointestinal bleeding in patients with cirrhosis and esophageal varices.6 In the present study, by focusing on ACS patients receiving current high-intensity antithrombotic treatment, we demonstrated a dose-dependent association between early oral β-blocker use and the reduction in major in-hospital bleeding risk by using multiple statistical methods.
The underuse and underdosing of oral β-blockers for ACS patients is common in clinical practice.7,8,18 In our study, although we excluded patients with clear contraindications to β-blocker, among ACS survivors at discharge, the prescription rate for β-blockers was 70.0% (48 162/68 792). This ∼30% increase, as compared with the early β-blocker prescription rate (36.0%), is the manifestation of the clinicians’ major concerns on the side effects of β-blockers, particularly the potential to increase the risk for cardiogenic shock. According to current research, the problem of underuse and underdose of oral β-blocker may be attributed to misleading selection of oral β-blocker based on standard tablet formulations. Clinicians would usually start with an oral β-blocker at lower dose by means of half of the standard tablet formulation. However, the mostly available tablet formulations for metoprolol and bisoprolol are 50 mg and 5 mg, respectively. These two tablet formulations are actually not dose-equivalent, which contributed to the following findings in the present study: in terms of metoprolol-equivalent dose, the bisoprolol users exposed to the twice the dose of metoprolol users (Table 1; due to the limited numbers of bisoprolol users, we did not observe significant differences in outcomes between metoprolol and bisoprolol users). These findings raised an interesting concern about the high prevalence of underdosing when metoprolol was chosen. Moreover, this finding is further supported by a Chinese study showing that bisoprolol (5 mg) provided superior heart rate reduction over metoprolol (47.5 mg in controlled release formulation) in mild-to-moderate hypertension.19 Based on our finding, in most cases without contraindications, it is reasonable to start metoprolol at 50 mg/day, considering the equivalent starting dose of bisoprolol was usually 2.5 mg/day in real-world practice. Notably, this dose is only the 25% of metoprolol target dose (200 mg/day). To maximize the clinical benefit of early oral β-blocker, specific efforts targeting the aforementioned two issues concerning the underuse and underdosing in ACS patients are needed in the future.
Increased heart rate has been identified as an independent risk factor in several prediction models for in-hospital bleeding risk among ACS patients.11,20 Heart rate reduction and reduced cardiac output have been ascribed as the major mechanisms underlying the therapeutic and preventive potential of non-selective and selective β-blockers for variceal bleeding and endoscopic surgery related blood loss.21,22 As the most widely prescribed agents capable of targeting this modifiable risk factor, the role of β-blocker, particularly selective β1-adrenergic receptor blockers, in reducing bleeding risk has not been examined in ACS patients. This may be due to the underuse and underdosing of β-blocker as described earlier. In our study, the systemic BP-lowering capacity of early β-blocker is not likely to play a major role in explaining the observed effects, because early ACEI/ARB use was not associated with any detectable reduction in major bleeds.
Major strengths of the present study are the representativeness of the nationwide population with sufficient statistical power, as well as the robustness of the results derived from multiple statistical methods and sensitivity analyses. Nonetheless, our study has the following limitations. First, the findings are based on an observational study, which cannot establish a causal role of early oral β-blocker therapy in the reduction of major in-hospital bleeding risk, and which requires confirmation in randomized clinical trials. This is a post-hoc analysis of the data available in this large registry, and for this reason the bleeding avoidance benefits of β-blocker can only be considered as hypothesis generating. Given the evidence-based benefit of early oral β-blocker in reducing mortality among ACS patients, which is recommended by current guidelines, early oral β-blocker should be encouraged to be a strategy of ‘killing two birds with one stone’. Second, our findings are exclusively based on the Chinese population. Future studies across other different ethnicities are warranted, especially the comparisons between East Asian and Caucasian populations with difference in the level of body mass index to determine the optimal dosing of β-blockers in terms of bleeding avoidance following PCI for ACS.
Conclusions
In a large nationwide registry of contemporary clinical practice in China, in addition to a reduction in in-hospital mortality, we demonstrated that early oral β-blocker therapy (both bisoprolol and metoprolol) was associated with reduced risk for major in-hospital bleeds after PCI for ACS. Although this finding needs to be confirmed in randomized controlled trial, based on the recommendations from current ACS guidelines, early oral β-blocker therapy with recommended dosing should be strengthened in appropriate ACS patients in the peri-PCI setting.
Acknowledgment
The authors thank all hospitals participating in the CCC-ACS project (Supplementary material online, Table S6) for their invaluable contribution to this work.
Funding
American Heart Association and the Chinese Society of Cardiology. The American Heart Association was funded by Pfizer and AstraZeneca for a quality improvement initiative through an independent grant for learning and change. National Natural Science Foundation of China (81 970 304) and by the National Key R&D Program of China (2020YFC2004706).
Conflicts of interest
Dr F. consulted for Amgen, Bayer, Janssen, and Novartis and served on the AHA's Quality Oversight Committee. The other authors report no conflicts of interest.
Data availability
The data, analytic methods, and study materials will be made available for onsite audit by third parties for purposes of reproducing the results or replicating the procedure.
Notes
A complete list of CCC-ACS Investigators is given at the end of this article.
Reference
Author notes
The first two authors contributed equally to this work.