-
PDF
- Split View
-
Views
-
Cite
Cite
Alice C. Levine, Xin-Hua Liu, Pietra D. Greenberg, Mark Eliashvili, Jonathan D. Schiff, Stuart A. Aaronson, James F. Holland, Alexander Kirschenbaum, Androgens Induce the Expression of Vascular Endothelial Growth Factor in Human Fetal Prostatic Fibroblasts
*, Endocrinology, Volume 139, Issue 11, 1 November 1998, Pages 4672–4678, https://doi.org/10.1210/endo.139.11.6303This work was supported by grants-in-aid from the T. J. Martell Foundation for Leukemia, Cancer, and Aids Research; and the Hans E. Schapira, M.D., Foundation for Urologic Research.
Close - Share Icon Share
Abstract
Androgens are known to directly stimulate prostate cancer cell growth. We have previously reported that LNCaP prostate cancer cells were dependent upon stromal coinoculation for growth in nude mice and that the stromal cells secreted a potent angiogenic factor, vascular endothelial growth factor (VEGF), which stimulated tumor angiogenesis. Immunohistochemical staining localized VEGF expression primarily to the stromal cells of human fetal and adult hyperplastic prostates, with both stromal and epithelial cell VEGF expression in prostate cancer. In the present studies, we test the hypothesis that androgens, in addition to their direct effects on prostate epithelial cells, have indirect effects on these cells via up-regulation of stromal VEGF production and angiogenesis. Primary cultures of human prostate fetal fibroblasts were treated with dihydrotestosterone (DHT), and the effects on VEGF messenger RNA (mRNA) expression were determined by Northern blotting. DHT (10 nm) increased VEGF mRNA levels maximally after 2 h. Nuclear run-on transcription assays demonstrated a 2-fold increase in the VEGF transcription rate 2 h after the addition of DHT. VEGF mRNA stability was unaffected by DHT addition. VEGF protein levels were determined by enzyme-linked immunosorbent assay and were increased 2-fold 4 h after DHT addition. These data indicate that androgens increase VEGF transcription and secretion of biologically active VEGF from human prostatic stroma. Androgens, therefore, may indirectly enhance prostate growth via up-regulation of VEGF from the surrounding stroma.
STROMAL-EPITHELIAL interactions, under the influence of androgens, have been demonstrated to play a key role in human prostate development and prostate cancer (1, 2). Androgens are known to directly stimulate the growth of human prostate cancer cells (3, 4). One of the best studied androgen-sensitive human prostate cancer cell lines is LNCaP, originally isolated from the lymph node of a patient with hormone-refractory, metastatic disease (5). The LNCaP cell line is dependent upon coinoculation with stroma for growth in vivo (2, 6). We recently developed tumors in nude mice by the sc coinoculation of LNCaP cells with human prostate fetal fibroblasts. In that system, we found evidence that the stromal cells were producing and secreting vascular endothelial growth factor (VEGF), a potent angiogenic and vascular permeability factor (VPF) known to enhance tumor growth (7). The present studies were undertaken to determine whether androgens, in addition to their known direct effects on prostate cancer cell growth, enhance prostate cancer growth indirectly via up-regulation of stromal VEGF production.
Folkman (8) and colleagues were the first to demonstrate that angiogenesis, the development of new blood vessels, is an essential step in tumor growth. The process of angiogenesis is tightly regulated by a variety of angiogenic and antiangiogenic factors. Several growth factors have been demonstrated to induce angiogenesis, including basic fibroblast growth factor and platelet-derived growth factor; however, these factors are mitogenic to a variety of cell types (9, 10). VEGF is unique among angiogenic molecules, in that it specifically targets endothelial cells and promotes their proliferation and migration, two essential steps in angiogenesis (11).
VEGF itself has been reported to be produced by many normal and neoplastic cells, and it induces both increased vascular permeability and angiogenesis (12). Treatment of tumors with VEGF-neutralizing antibodies effectively inhibits tumor growth in vivo (7, 13). Enhancing local VEGF concentrations, either by transfection of the VEGF gene or by the addition of exogenous VEGF, increases the tumor take rates, growth rates, and vascular density of prostate and breast cancer cells in nude mice (14, 15). VEGF expression has previously been demonstrated to be up-regulated by hypoxia, cytokines, growth factors, estrogen, and progesterone and down-regulated by glucocorticoids (16–22). One report demonstrated that castration inhibited tumor VEGF in two androgen-responsive human prostatic cancer xenograft models (23). The present report is the first to demonstrate androgenic up-regulation of VEGF expression in noncancerous stromal cells. These data suggest that androgen-ablative therapies may partially target stroma, resulting in decreased VEGF, decreased vascular permeability, and decreased edema of primary prostate cancers and metastatic lesions.
Materials and Methods
Tissues
Tissue was obtained from male fetuses (18–24 weeks gestation) at the time of pregnancy interruption and with the approval of the Mount Sinai Medical Center Institutional Review Board. Adult prostatic tissue was obtained from transurethral and retropubic prostatectomies[ performed for benign prostatic hyperplasia (BPH)] and radical prostatectomy specimens performed for prostate cancer, again under the guidelines of the Institutional Review Board. All samples were fixed in formalin.
Immunohistochemistry for VEGF expression
For routine histology, specimens were fixed in 10% neutral buffered formalin and embedded in paraffin, and fixed sections were cut and stained with hematoxylin and eosin. For VEGF immunohistochemistry, all steps were carried out in a humidified chamber at room temperature (except incubation with primary antibody) using reagents from Vector Laboratories, Inc. (Burlingame, CA). Sections were deparaffinized, then treated with 3% hydrogen peroxide in PBS for 20 min, followed by three washes in distilled water for 5 min each. Slides were then treated with 5 μg/ml pepsin in 0.01 n HCl (pH 2.5) for 30 min (to block endogenous peroxidases), followed by incubation with with 5 μg/ml saponin in distilled water for 30 min and three washes in PBS for 5 min each. Slides were then blocked for 20 min in normal goat serum. Incubation with polyclonal antibodies to VEGF (0.5 μg/ml) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was performed overnight at 4 C. Afetr the overnight incubation, slides were washed in PBS twice for 5 min each, then incubated with biotinylated goat antirabbit antibody (dilution 1:200) for 30 min. After two more washes in PBS, an avidin-biotin complex was applied for 30 min, followed by washing in 0.5% Triton X-100 for 30 sec; and application of the chromagen 3,3′-diaminobenzidine was performed for 10 min in the dark. Slides were then counterstained with Harris hematoxylin.
Cell lines and cell culture
With the approval of our Institutional Review Board, fetal fibroblasts were isolated from prostate tissue derived from 18- to 24-week-old gestation fetuses at the time of pregnancy interruption, as previously described (24). Surgical specimens were minced into small cubes and incubated for 48 h in DMEM/Ham’s F12 media (Gibco, Grand Island, NY) with 10% FBS and collagenase type I (1.25 mg/ml; Worthington Biochemical Corp., Freehold, NJ). After collagenase digestion, cells were washed and plated in DMEM/Ham’s F12 with 10% FBS. Only fibroblasts are still visible after the second passage (confirmed by positive immunohistochemical staining for vimentin and negative staining for smooth muscle markers). Thereafter, fibroblast cells were routinely maintained in RPMI 1640 medium with 10% FBS (Gibco). Fibroblast cells from passages 3–5 were used in these studies. For Northern blot, nuclear run-on and actinomycin D (Act D) experiments, subconfluent fibroblast cultures were washed and the medium changed to serum-free, phenol red-free medium containing 0.1% BSA for 24 h. Incubation was continued with and without dihydrotestosterone (DHT) (Sigma Chemical Co., St. Louis, MO) at the doses and time periods indicated.
Isolation of total RNA and Northern blotting
Total RNA extraction from cultured cells and the Northen blot analysis procedures were carried out as described elsewhere (25). Briefly, cells cultured under the desired conditions were harvested, and total RNA was isolated and fractionated on a 1.2% agarose-formaldehyde gel. After transfer to a nitrocellulose membrane, hybridization was performed with an α-32P-labeled VEGF complementary DNA (cDNA) probe (a generous gift from Dr. K. Claffey, Beth Israel Deaconess Medical Center, Boston, MA). The membranes were subsequently hybridized with a glyceraldehyde-phosphate dehydrogenase (GAPDH) cDNA probe (ATCC, Rockville, MD) to monitor RNA loading. The amounts of VEGF messenger RNA (mRNA) were quantified by laser densitometry (Molecular Dynamics, Inc., Sunnyvale, CA).
Nuclei isolation and nuclear run-on
Nuclei were prepared from prostate fetal fibroblasts by the method of Greenberg and Ziff (26). Briefly, cells were lysed in ice-cold nuclear extraction buffer [10 mm Tris-HCl (pH 7.4), 10 mm NaCl, 3 mm MgCl2, and 0.5% Nonidet P-40) and incubated on ice for 10 min. The nuclei were pelleted at 500 × g at 4 C for 5 min, washed once in nuclear extraction buffer, and repelleted. Washed nuclei were resuspended at 2 × 107 nuclei/200 μl in 40% glycerol, 5 mm MgCl2, 0.1 mm EDTA, and 50 mm Tris-HCl at pH 8.3, and frozen in liquid nitrogen and stored at −70 C.
The transcription assay was a modification of the Howard and Ortlepp method (27). An equal volume of transcription buffer (10 mm Tris-HCl at pH 8.0, 5 mm MgCl2, 300 mm KCl, 0.5 mm dithiothreitol) containing 2 mm GTP, cytidine 5′-triphosphate, ATP, and 200 μCi (α-32P) uridine 5′-triphosphate was added to each reaction and incubated at 32 C for 40 min. Nuclear RNA was then isolated by the Chomczynski method (28). The RNA pellet was dissolved in sterile 10 mm Tris-HCl (pH 7.2), 1 mm EDTA, and 0.1% SDS and was used as a probe for hybridization. Radiolabeled RNA was hybridized at 42 C for 40 h to nylon membranes containing 8 μg purified human VEGF cDNA (a gift from Dr. K. Claffey). A plasmid containing the Escherichia coli fragments of GAPDH gene served as a control for noninducible genes. The filters were washed in 2× saline-sodium citrate, 0.1% SDS at 22 C, again at 42 C, and finally in 0.1× saline-sodium citrate, 0.1% SDS at 42 C before autoradiography. The bands identified by autoradiography were analyzed by laser densitometry, and values are reported as the relative increases in transcription rate of VEGF after normalizing to the GAPDH transcriptional rate.
Measurement of VEGF RNA stability
Prostate fetal fibroblasts were routinely maintained in RPMI 1640 with 10% FBS. For treatment with DHT, subconfluent cells were washed and changed to serum-free, phenol red-free medium containing 0.1% BSA for 24 h. Incubation was continued with and without DHT (10 nm) for 2 h. At time zero, Act D (100 μg/ml) was added, and cells were harvested at varying time points. Total RNA was prepared and the level of VEGF transcripts determined by Northern blot analysis.
Preparation of prostate fetal fibroblast-conditioned medium
Prostate fetal fibroblasts were plated at 1 × 105 cells/well in six-well cluster dishes with 2 ml RPMI 1640 medium containing 10% FBS. After 3 days, cells were washed and changed to serum-free, phenol red-free medium containing 0.1% BSA. Incubation was continued with and without DHT (10 nm) for the time periods indicated. The medium was then removed, centrifuged at 1000 g for 10 min to remove suspended cells, and stored at− 20 C for enzyme-linked immunosorbent assay (ELISA) and endothelial cell migration assays.
ELISA for VEGF
Conditioned media, prepared from prostate fetal fibroblasts at the indicated time points after the addition of DHT (10 nm), were assayed for VEGF protein using a sandwich ELISA, according to the manufacturer’s instructions (Intergen Co., Purchase, NY). Results of conditioned medium were expressed as amount of VEGF/106 cells/24 h.
Statistical analysis
Data are expressed as mean ± sem. For time course and dose-response experiments, results were statistically analyzed using ANOVA.
Results
VEGF expression in human prostate specimens by immunohistochemistry
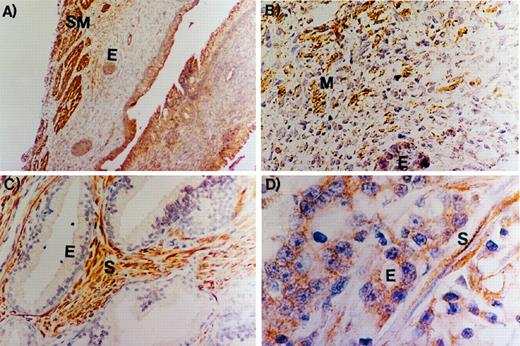
Figure 1 demonstrates the results of immunohistochemical staining for VEGF expression in human prostate specimens. In the fetal prostate, strong VEGF expression was noted in the smooth muscle cells (Fig. 1A). The higher power view of the fetal prostate (Fig. 1B) demonstrates that both the immature mesenchyme (M) and the fetal epithelial (E) cells also demonstrate some VEGF expression. In BPH, there is strong VEGF expression that is limited to the stromal cells, with no appreciable expression in the epithelium (Fig. 1C). In contrast, in prostate cancer, VEGF expression is demonstrable in both the stroma and the cancerous E cells (Fig. 1D). These data demonstrate that VEGF is expressed in the stromal cells of the fetal, adult hyperplastic and cancerous human prostate, whereas E cell VEGF expression is most pronounced in the cancerous prostate.
Immunohistochemical localization of VEGF expression in the human prostate. A and B, Low- and high-power magnifications, respectively, demonstrating VEGF expression in the stromal cells of the human fetal prostate; A, strong VEGF expression is noted in the smooth muscle cells of the human fetal prostate (SM), with less expression in the E cells (magnification, 40×); B, the higher power magnification demonstrates that, in addition to the dense VEGF staining in the smooth muscle cells, there is some VEGF staining in the fetal prostate E cells and the surrounding immature mesenchyme (magnification, 200×); C, in adult BPH, there is strong VEGF expression in the stroma (S), with no expression in the epithelium (E) (magnification, 100×); D, In prostate cancer, both the cancerous E cells and the surrounding stroma (S) stain for VEGF expression (magnification, 200×).
Effect of androgen on VEGF mRNA levels
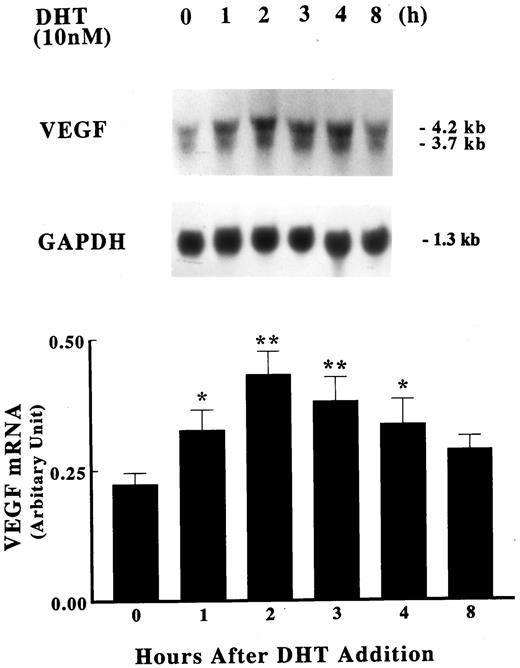
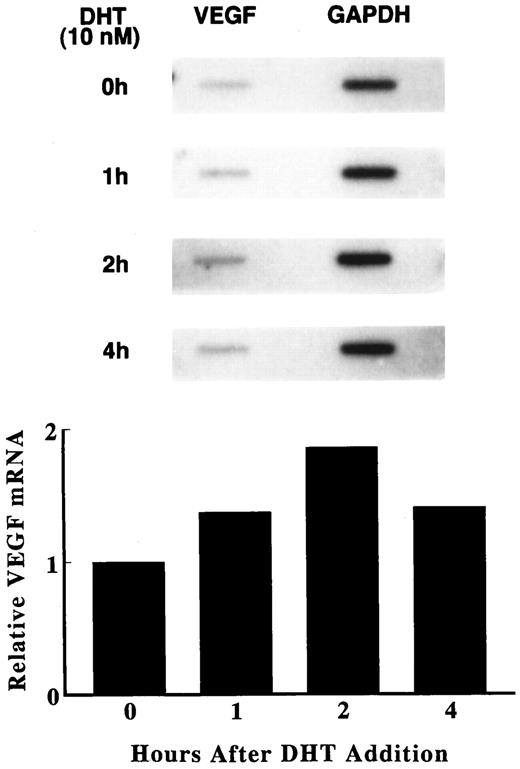
To determine whether androgen modulates the production of VEGF by human fetal prostatic fibroblasts, primary cultures were incubated in serum-free medium with DHT for various times. Fetal prostatic fibroblasts demonstrate a low basal level of VEGF mRNA expression. Two VEGF transcripts (4.2 and 3.7 kb) are discernible, which represent hybridization of the cDNA probe to the known splice variants of VEGF mRNA (29). VEGF mRNA levels were increased as early as 1 h, with a maximum response (approximately twice that demonstrated at time 0) demonstrable 2 h after the addition of 10 nm DHT. VEGF mRNA levels began to decline at 3 h and were almost back to baseline 8 h after DHT addition (Fig. 2).
Induction of VEGF mRNA by DHT. Primary cultures of human fetal prostatic fibroblasts (passage 3) were grown in serum-free medium for 24 h. Incubations were continued with or without 10 nm DHT for various times at 37 C. Upper panel, Expression of VEGF mRNA was assayed by Northern blot analysis. Membranes were also hybridized with a GAPDH probe to assess loading differences. Twenty-five micrograms of total RNA were loaded in each lane. Lower panel, The data in the upper panel were quantified using a densitometer and are presented as a ratio of VEGF mRNA to GPDPH mRNA. Values shown are the mean ± sem (n = 3). *, P < 0.05; **, P < 0.01 vs. control.
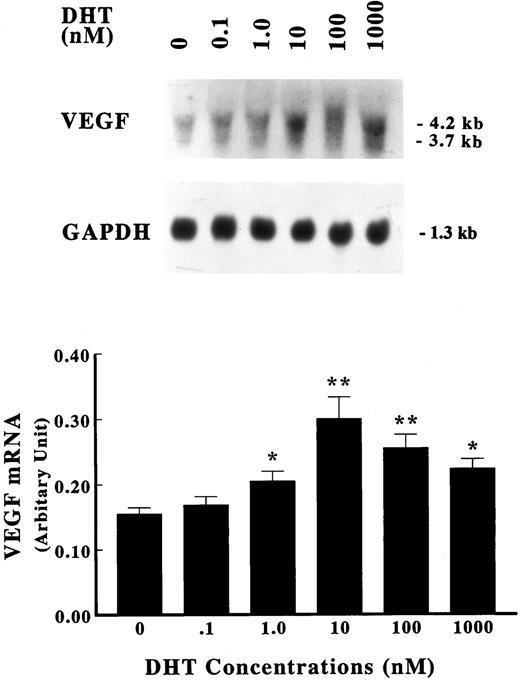
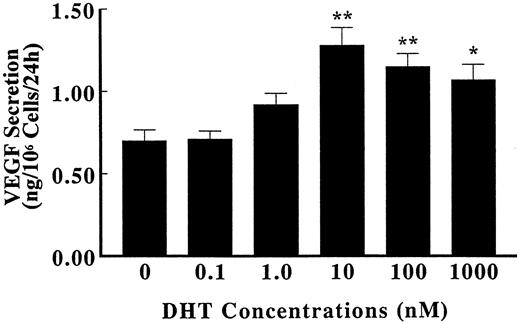
The dose dependence of the DHT effect on VEGF mRNA levels was next characterized. As shown in Fig. 3, a modest enhancement in VEGF mRNA was discernible after treatment with 1 nm DHT for 2 h. Higher concentrations of DHT further increased VEGF mRNA expression, with saturation of this effect noted in the presence of 10 nm DHT, indicating a dose-dependent induction of VEGF transcripts by DHT.
Dose dependence of VEGF induction. Primary cultures of human fetal prostatic fibroblasts at passage 3 were grown in serum-free medium for 24 h. Incubations were continued with or without various concentrations of DHT for an additional 2 h at 37 C. Upper panel, Expression of VEGF mRNA was assayed by Northern blot analysis. Membranes were also hybridized with a GAPDH probe to assess loading differences. Twenty-five micrograms of total RNA were loaded in each lane. Lower panel, The data in the upper panel were quantified using a densitometer and are presented as a ratio of VEGF mRNA to GAPDH mRNA. Values shown are the mean ± sem (n = 3). *, P< 0.05; **, P < 0.01 vs. control.
Effect of androgen on VEGF protein production
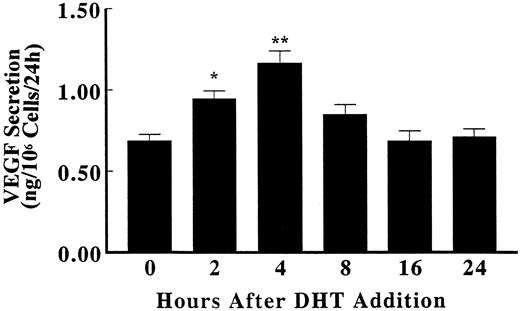
To examine whether the augmented expression of VEGF mRNA was accompanied by an increase in VEGF protein production, an ELISA was performed on fibroblast-conditioned medium. In the presence of 10 nm DHT, VEGF protein secretion was maximal 4 h after DHT addition, began to decline by 8 h, and returned to baseline levels 16 h after androgen addition (Fig. 4).
Up-regulation of VEGF protein secretion by DHT. Primary cultures of human fetal prostatic fibroblasts at passage 3 were grown in serum-free medium for 24 h. Incubations were continued with or without 10 nm DHT for various times at 37 C. The secretion of VEGF protein to the culture medium in triplicate cellular incubates was quantified using ELISA and was normalized by cell number. The data represent the mean values ± sem. *, P < 0.05; **, P < 0.01 vs. control.
The concentration dependence of VEGF protein induction was also examined by ELISA. As shown in Fig. 5, VEGF protein secretion increased progressively with the increased concentration of DHT, starting at 1 nm, with a peak stimulation at 10 nm. Higher doses of DHT had no further effect on VEGF protein production.
Dose dependence of DHT effect on VEGF protein secretion. Primary cultures of human fetal prostatic fibroblasts at passage 3 were grown in serum-free medium for 24 h. Incubations were continued with or without various concentrations of DHT for an additional 4 h at 37 C. The secretion of VEGF protein to the culture medium in triplicate cellular incubates was quantified using ELISA and was normalized by cell number. The data represent the mean values ± sem. *, P < 0.05; **, P < 0.01 vs. control.
Mechanisms of VEGF induction by androgen
The mechanisms by which DHT increases expression of VEGF mRNA were then defined. Nuclear run-on assays were used to determine whether the accumulation of VEGF transcripts is caused by an increased rate of transcription for VEGF mRNA. As shown in Fig. 6, untreated fetal prostatic fibroblasts exhibited detectable levels of VEGF transcription. The addition of 10 nm DHT resulted in an increase in this process by about 40% within 1 h, with a maximal response (doubling over nontreated control) evident 2 h after DHT addition. Thereafter, the rate of transcription diminished, although it remained above control level at 4 h after DHT addition. DHT treatment did not alter the transcription rate of the control GAPDH gene.
Transcriptional regulation of VEGF production. Primary cultures of human fetal prostatic fibroblasts were cultured with 10 nm DHT for various times at 37 C. Transcription was then determined by nuclear run-on assay, as described under Materials and Methods. The data were quantified using a densitometer, and the densities are normalized to GAPDH levels. The results are reported as the percentage increase in VEGF mRNA, relative to the control level in untreated cells.
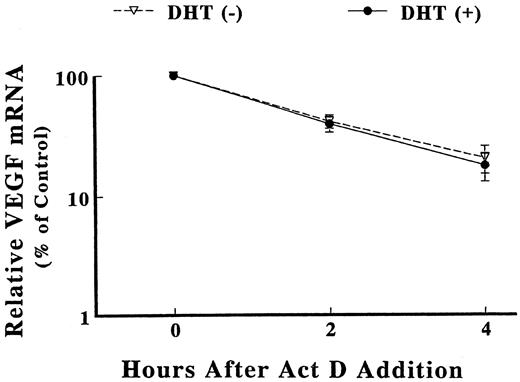
To determine whether the increases in steady-state VEGF mRNA levels were caused by an alteration in transcript stability, we examined the rate of decay of the VEGF mRNA, using Act D to inhibit transcription from DHT-treated and control cells. Fetal prostatic fibroblasts were stimulated with 10 nm DHT for 2 h. Act D was added, and the cells were harvested at various times for Northern blot analysis of VEGF mRNA. As shown in Fig. 7, DHT did not significantly effect the rate of decay of the VEGF mRNA in these fibroblast cells. The estimated half-life of VEGF mRNA was 98 ± 11.6 min vs. 93± 8.1 min in the DHT(−) and DHT(+) cultures, respectively. These results imply that the accumulation of VEGF mRNA in response to DHT was caused by transcriptional activation of the VEGF gene, with no significant effect on VEGF mRNA stability.
Stability of VEGF mRNA. Primary cultures of human fetal prostatic fibroblasts were exposed to 10 nm DHT (•–•) or vehicle (▿–▿) for 2 h at 37 C before the addition of Act D (0.1 mg/ml). Total RNA was extracted from the cells at the indicated times after Act D administration, fractionated by electrophoresis, and transferred to nitrocellulose membranes. The Northern blots were hybridized to a cDNA probe for VEGF. To correct for differences in loading, membranes were also hybridized with a GAPDH cDNA probe. Results were quantified using a densitometer and is presented as a ratio of VEGF mRNA to GAPDH mRNA. The corrected density is plotted as a percentage of time zero value against time. Each point represents mean values ± sem (n = 3).
Discussion
The development of new blood vessels, either through vasculogenesis or angiogenesis, is an essential step in physiologic and pathophysiologic processes. Although there are many known angiogenic factors, recent attention has focused on VEGF because of increasing reports demonstrating its essential role in fetal development and tumor angiogenesis. Knockout of a single VEGF allele (30, 31) or either of the two high-affinity binding VEGF-receptors (32, 33) results in embryonic lethality in transgenic mice. Inhibition of VEGF activity with neutralizing antibodies suppresses the growth of tumors in nude mice (7, 13, 34).
The importance of VEGF in the prostate is beginning to be elucidated. Recent studies have demonstrated VEGF expression in human BPH and prostate cancer specimens (35, 36). In one of those reports (35), there is immunocytochemical evidence of VEGF expression in BPH E cells and noncancerous stroma. This confirms a previous study in which substantial quantities of VEGF were demonstrated in the semen of healthy males (37). In this report, we demonstrate strong VEGF expression in the stroma of the human fetal, adult hyperplastic and cancerous prostate. In contrast, noncancerous fetal and adult prostate E cells express little VEGF, with stronger expression noted in prostate cancer specimens. All human prostate cancer cell lines tested to date express some VEGF (23). There is evidence that VEGF secretion from the androgen-sensitive human prostate cancer cell line LNCaP is increased by androgens (our unpublished results and Ref. 38). Although LNCaP cells do express small amounts of VEGF, we have demonstrated that the dependence of this cell line on stromal coinoculation for growth sc in nude mice may be partially explained by the expression of VEGF in the stromal cells (7).
In this report, we demonstrate androgenic regulation of VEGF expression in human prostate fetal fibroblasts. The androgenic effect on VEGF mRNA levels was rapid, with increased transcription noted as early as 1 h after androgen addition, and peak increases noted 2 h after androgen addition. This is consistent with previous reports demonstrating a rapid induction of VEGF mRNA after treatment with either estrogen or insulin-like growth factor 1 (IGF-1) (19, 39, 40). Our findings differ from those reported with IGF-1, in that androgens primarily increased VEGF mRNA transcription with no effect on VEGF mRNA stability, whereas IGF-1 did increase the VEGF mRNA stability (18). The rapid induction of VEGF mRNA transcription is consistent with a direct effect of androgens on the VEGF promoter. However, to date, a functional androgen response element has not been identified in the VEGF gene. It is possible that androgens have both direct and indirect effects, the latter of which may be mediated by androgenic effects on other growth factors, such as IGF-1.
The increase in VEGF mRNA is maximal at 2 h and was followed shortly thereafter by an increase in VEGF-secreted protein (increased VEGF-secreted protein maximally after 4 h). Androgens induce a doubling of VEGF mRNA transcription rates, with a resultant doubling of VEGF protein levels. The demonstrated increase in VEGF-secreted protein is probably a result of increased synthesis. However, we cannot rule out the possibility that androgens also have an effect on VEGF protein secretion.
The prostate was the first organ system in which the importance of stroma in mediating the effects of androgen on epithelium was demonstrated (1, 2). Prostatic stroma produces the type 2 isozyme of steroid 5α-reductase, which converts testosterone to its more potent metabolite, DHT, intracellularly (41). Prostatic stromal cells also produce and secrete keratinocyte growth factor (KGF) under androgenic regulation, which promotes branching morphogenesis in the mouse seminal vesicle and ductal branching of the rat ventral prostate (42, 43). We recently demonstrated that KGF functions as a stromal andromedin that increases prostate-specific antigen secretion in human fetal prostate organ cultures (44). The present report demonstrates that these same stromal cells, which mediate the effects of androgens on neighboring E cells, also serve as mediators of androgenic effects on local endothelial cells.
Androgens have known direct effects on normal and cancerous prostate E cells. Recent studies have demonstrated that testosterone also targets endothelial cells, increasing the weight of blood vessel walls, blood vessel lumina, and endothelial cells, as well as increasing endothelial cell proliferation rates in the rat ventral prostate (45). The effect of testosterone on the vasculature in that report was more immediate than its effects on E cells. Androgen ablation results in a rapid decrease in blood vessel density in rat prostate cancers (46). We propose that these rapid effects are caused by androgenic effects on VEGF protein levels. VEGF was originally described as VPF and is reported to be 50,000 times more potent than histamine in its ability to enhance vascular permeability (47). Rapid changes in VEGF/VPF secretion would most likely be first manifested clinically as changes in vascular permeability and edema.